Abstract
Background:
Prenatal exposure to a mixture of endocrine disrupting chemicals (EDC) has the potential to disrupt human metabolism. Prenatal periods are especially sensitive as many developmental processes are regulated by hormones. Prenatal exposure to EDCs has inconsistently been associated with children’s body mass index (BMI) and obesity. The objective of this study was to investigate if prenatal exposure to a mixture of EDCs was associated with children’s BMI and overweight (ISO-BMI ≥ 25) at 5.5 years of age, and if there were sex-specific effects.
Methods:
A total of 1,105 mother-child pairs with complete data on prenatal EDCs concentrations (e.g., phthalates, non-phthalate plasticizers, phenols, PAH, pesticides, PFAS, organochlorine pesticides, and PCBs), children’s measured height and weight, and selected covariates in the Swedish Environmental Longitudinal, Mother and child, Asthma and allergy (SELMA) study were included in this analysis. The mixture effect of EDCs with children’s BMI and overweight was assessed using WQS regression with 100 repeated holdouts. A positively associated WQS index with higher BMI and odds of overweight was derived. Models with interaction term and stratified weights by sex was applied in order to evaluate sex-specific associations.
Results:
A significant WQS*sex interaction term was identified and associations for boys and girls were in opposite directions. Higher prenatal exposure to a mixture of EDCs was associated with lower BMI (Mean β = −0.19, 95%CI: −0.40, 0.01) and lower odds of overweight (Mean OR = 0.72, 95%CI: 0.48, 1.04) among girls with borderline significance. However, the association among boys did not reach statistical significance. Among girls, the possible chemicals of concern were MEP, 2-OHPH, BPF, BPS, DPP and PFNA.
Conclusion:
Prenatal exposure to a mixture of EDCs was associated with lower BMI and overweight among girls, and non-significant associations among boys. Chemicals of concern for girls included phthalates, non-phthalate plasticizers, bisphenols, PAHs, and PFAS.
Keywords: Endocrine disrupting chemicals, EDC, BMI, overweight, child, pregnancy
1. Introduction
Childhood overweight and obesity affects approximately one of every three children in Europe (29% of boys and 27% of girls) (Spinelli et al. 2021). Childhood obesity is a concern for health outcomes in adulthood and has been associated with a higher risk of type 2 diabetes, hypertension and cardiovascular disease (Park et al. 2012). In Sweden, the prevalence of overweight and obesity among the adult population is 50% of which 16% are obese (Public Health Agency of Sweden 2022). An increasing trend of children having overweight and obesity can be observed among children 4 years of age as well as children at 6 to 9 years of age. Among 4-year old’s, the prevalence of overweight and obesity increased from 11% to 13% between the years 2018 and 2020 (Miregård et al. 2023). Similarly, among children 6–9 years of age overweight and obesity increased from 18% to 21% between the school years 2015/2016 and 2018/2019 (Public Health Agency of Sweden 2021). The prevalence differs by sex and age group, with girls having a higher prevalence of overweight at ages 4 to 9, and boys having a higher prevalence after 9 years of age (Public Health Agency of Sweden 2021; 2023). In the county of Värmland, county of residence of the current study population, 16% prevalence of overweight and obesity was reported among 4-year-olds and 20% among 6-year-olds over the school year 2018/2019 (Region Värmland 2019). Among 4-year-olds the prevalence is higher than the national statistics (13%) and although for 6- to 9-year-olds it has plateaued over the last six years and similar to the national statistics (21%), it is still high and a public health concern (Public Health Agency of Sweden 2021; 2023; Region Värmland 2019).
Risk factors for childhood overweight and obesity include hereditary and environmental factors. About 40–70% of the variation in BMI has been estimated to be influenced by genetic factors, however, environmental factors also play a role in the phenotypic expression of overweight and obesity (Heindel et al. 2017a). Among environmental factors, endocrine disrupting chemicals (EDCs) are of special interest (Heindel and Blumberg 2019). EDCs have several utilities such as plasticizers, solvents, and pesticides and are therefore present in commonly used products (i.e., building materials, electronics, personal care products, textiles) (Kabir et al. 2015). These chemicals can interact with human hormones through various mechanisms either imitating, blocking or enhancing the natural hormones (Kabir et al. 2015). Some EDCs such as bisphenol-A (BPA), pesticides and perfluorinated compounds (PFAS) have been identified as potentially obesogenic due to their ability to promote obesity in humans and animals (Amato et al. 2021; Veiga-Lopez et al. 2018).
Exposure to EDCs during pregnancy is of special concern as many EDCs may pass the placental barrier and can reach the developing fetus (Kabir et al. 2015). During pregnancy, several developmental processes are steered by hormones and are therefore sensitive to any disruption of the hormonal balance (Hoffman et al. 2021). The Developmental Origins of Health and Disease (DOHaD) hypothesize that early exposure to environmental factors (e.g., EDCs) during this critical period of development may have long-term health effects in childhood and adulthood (Gore et al. 2015; Heindel et al. 2017b). Prenatal exposure to EDCs has inconsistently been associated with BMI and overweight in children (Lee et al. 2022; Stratakis et al. 2022). Specifically, bisphenols have been detected in children and adolescents in cross-sectional studies, and higher concentrations have been associated with higher odds of obesity at 6 to 19 years of age (Jacobson et al. 2019). Furthermore, longitudinal studies show that prenatal phthalate exposure has been associated with higher adiposity among children between 3 and 12 years of age (Ferguson et al. 2022; Kupsco et al. 2022), and also with higher BMI trajectory in girls during the first 6 years of life (Gao et al. 2022). However, humans are simultaneously exposed to a large number of EDCs in low concentrations (Bornehag et al. 2019), and therefore it is vital to evaluate health associations with a mixtures approach considering different classes of EDCs. To date, there are a handful of epidemiological studies evaluating prenatal exposure to mixtures of EDCs with children’s weight. A previous analysis in the SELMA study, showed that higher exposure to EDC mixture was associated with lower birthweight, and slower growth rate and tempo of peak growth velocity from birth until 5.5 years of age. However, the association between EDC mixture and childhood BMI and overweight remains to explore further as previous studies show varied results. Two other cohort studies found that prenatal exposure to a mixture of EDCs were associated with higher BMI at 5 and 7 years of age (Agay-Shay et al. 2015; Berger et al. 2021), whereas three other cohort studies have found the opposite association with lower weight (Marks et al. 2021) or no association (Kupsco et al. 2022; Vrijheid et al. 2020). These studies did not report or were not able to evaluate sex differences due to study design. Consequently, more research is needed with a mixture approach to investigate if prenatal exposure to several classes of EDCs has an effect on children’s weight and if they differ by sex.
In this study, we evaluated the association between prenatal exposure to a mixture of 26 EDCs of both short-lived and persistent chemicals with higher BMI and odds of overweight among children 5.5 years of age, and if these associations differed by sex. The analysis was performed on data from 1,105 mother-child pairs participating in the Swedish Environmental Longitudinal, Mother and child, Asthma and allergy (SELMA) study.
2. Material and Methods
2.1. Study Population
The study population for this study comprises women and their children who are participants of an ongoing pregnancy cohort, the SELMA study. During the period from November 2007 to April 2010, a total of 2,582 pregnant women were recruited with a 39% participation rate during their first trimester of pregnancy at approximately 10 weeks of gestation (Figure S1). Women registered at one of the 25 prenatal care centers in the county of Värmland in Sweden were invited to the study. A more detailed description of the recruitment protocol has been described elsewhere (Bornehag et al. 2012). A total of 1,279 women-child pairs had available information on children’s height and weight at 5.5 years of age and measured EDCs concentrations from urine and serum samples from early pregnancy. Those participants with complete data for a set of selected covariates (e.g., maternal BMI, education, smoking, parity, children’s age and sex) were included in the analysis for a total of 1,105 mother-child pairs. A total of 170 mother-child pairs were excluded due to missing data from either maternal age (n=39), education (n=42) or maternal BMI (n=92). One child was excluded due to much older age than the rest (8.7 years). This study complies with the Declaration of Helsinki and has been approved by the Regional Ethical Review Board in Uppsala, Sweden (Dnr: 2007/062 and Dnr: 2015/177). All participating women signed written informed consent and parental consent was obtained for the children’s participation.
2.2. Collection of samples and assessment of prenatal exposure to EDCs
Serum samples and a first morning void urine samples were collected during the enrollment visit, which took place at a prenatal care center at approximately 10 weeks of gestation. Samples were kept frozen until analysis at −80°C for serum, and −20°C for urine samples. Urinary metabolites of nonpersistent chemicals with short biological half-life were analyzed using liquid chromatography coupled to a triple quadrupole mass spectrometer (LC-MS/MS; QTRAP 5500, AB Sciex, Framingham, MA, USA) according to the method by Gyllenhammar et al., (Gyllenhammar et al. 2017). Serum was analyzed for PFAS and cotinine using LC-MS/MS according to the method by Norén et al., (Norén et al. 2021). These analyses were conducted at the Laboratory of Occupational and Environmental Medicine (OEM) at Lund University in Lund, Sweden. In-house prepared quality control samples and chemical blank samples were analyzed twice within each sample batch in a 96-well plate. The laboratory participates in G-EQUAS inter-laboratory program and has qualified as HBM4EU laboratory for several of the analytes in both urine and serum. Serum samples were further analyzed for persistent organic pollutants at the Finnish Institute for Health and Welfare in Finland. The analytes were quantified using gas chromatography - high triple quadrupole mass spectrometry (Agilent 7010 GC–MS/MS system (Wilmington, DE, USA), GC column DB-5MS UI (J&W Scientific, 20 m, ID 0.18 mm, 0.18 μm)) according to the method by Koponen et al., (Koponen et al. 2013).
A total of 54 chemicals/analytes were analyzed and 41 had detectable values in at least 75% of the samples. Those 41 chemicals included 14 phthalates and non-phthalate plasticizers: monoethyl phthalate (MEP), monobutyl phthalate (MBP), monobenzyl phthalate (MBzP), DEHP metabolites (mono-2-ethylhexyl phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP)), DINP metabolites (mono(hydroxyisononyl) phthalate (MHiNP), mono(oxoisononyl) phthalate (MOiNP), mono(carboxyisooctyl) phthalate (MCiOP)), monohydroxyisodecyl phthalate (MHiDP), monocarboxyisononyl phthalate (MCiNP), 2–4-methyl-7-oxyooctyl-oxycarbonyl-cyclohexane carboxylic acid (MOiNCH), and diphenylphosphate (DPP). Chemicals also included four phenols: 2,4,4′-trichloro-2′-hydroxydiphenyl ether (Triclosan), bisphenol A (BPA), 4,4-bisphenol F (BPF), bisphenol S (BPA); one PAH: 2-hydroxyphenanthrene (2-OHPH), and two pesticides: 3,5,6-trichloro-2-pyridinol (TCP), and 3-phenoxybenzoic acid (3-PBA). Also, six PFAS: perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorohexanesulfonic acid (PFHxS), three organochlorine pesticides: hexachlorobenzene (HCB), trans-nonachlor, dichlorodiphenyltrichloroethane (DDT) and its metabolite dichlorodiphenyldichloroethylene (DDE), and ten polychlorinated biphenyls (PCBs) congeners 74, 99, 118, 138, 153, 156, 170, 180, 183, and 187. We calculated the sum of DEHP and DINP metabolites on a molar basis, DDT with its metabolite DDE and all the PCB congeners for total exposure variables. After calculating the summed variables, it resulted in a total of 26 chemicals together with the non-summed chemicals, which were included in the analysis (Table 2). Chemical concentrations were above the level of detection (LOD) in 90–100% of the samples for all chemicals, except for Trans-Nonachlor that was detected in 77.5% of the samples. Values below the level of detection (LOD) were replaced by the value of LOD/√(2) for the chemicals analyzed in serum. For PFASs, also analyzed in serum, and chemicals analyzed in urine we were able to use the machine read values. We did not adjust lipophilic persistent chemicals (e.g., PCBs and organochlorine pesticides) by serum lipids as the literature is conflicting on whether or not it introduces bias (Schisterman et al. 2005). For regression analyses, all the urinary metabolites were creatinine adjusted in units of nmol per mmol creatinine to account for urinary dilution. Spearman correlation coefficients between all chemicals in the EDC mixture ranged from low to moderately correlated (−0.16 to 0.75) (Figure S3); previously published by Tanner et al., (Tanner et al. 2020).
Table 2.
Concentrations of 26 compounds (ng/mL) of the EDC mixture in prenatal urine (not creatine adjusted) and serum samples, n=1,105
| Components of the EDC mixture | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Matrix | Chemical Type | Parent Compound (if applicable) | Analyte | % ≥ LODa | 25th percentile | GM (GSD) | 75th percentile |
| Urine | Phthalates | DEP | MEP | 100 | 29.8 | 65.2 (3.0) | 126.2 |
| DBP | MBP | 100 | 40.2 | 66.4 (2.2) | 113.1 | ||
| BBzP | MBzP | 100 | 7.73 | 15.5 (2.9) | 32.6 | ||
| DEHP | ΣDEHPb | --- | 36.2 | 63.7 (2.4) | 102.8 | ||
| DiNP | ΣDiNPc | --- | 11.8 | 25.7 (3.0) | 46.2 | ||
| DiDP/DPHP | MHiDP | 100 | 0.64 | 1.22 (2.8) | 2.17 | ||
| MCiNP | 99.9 | 0.38 | 0.66 (2.4) | 1.08 | |||
| Plasticizer | DiNCH | MOiNCH | 99.0 | 0.13 | 0.31 (4.1) | 0.60 | |
| TPP | DPP | 100 | 0.70 | 1.37 (2.6) | 2.37 | ||
| Antibacterial | Triclosan | 92.4 | 0.29 | 1.24 (9.9) | 2.73 | ||
| Bisphenols | BPA | 100 | 0.85 | 1.50 (2.4) | 2.60 | ||
| BPF | 90.3 | 0.05 | 0.15 (5.1) | 0.35 | |||
| BPS | 97.5 | 0.03 | 0.07 (3.0) | 0.12 | |||
| PAH | 2OHPH | 100 | 0.11 | 0.20 (2.3) | 0.34 | ||
| Pesticide | Chlorpyrifos | TCP | 100 | 0.70 | 1.27 (2.5) | 2.21 | |
| Pyrethroids | 3-PBA | 99.0 | 0.08 | 0.16 (2.7) | 0.29 | ||
|
| |||||||
| Serum | PFAS | PFOA | 100 | 1.11 | 1.60 (1.7) | 2.28 | |
| PFOS | 100 | 3.94 | 5.33 (1.7) | 7.58 | |||
| PFNA | 100 | 0.39 | 0.54 (1.7) | 0.73 | |||
| PFDA | 100 | 0.19 | 0.26 (1.6) | 0.34 | |||
| PFUnDA | 99.7 | 0.15 | 0.21 (1.9) | 0.33 | |||
| PFHxS | 100 | 0.86 | 1.29 (1.8) | 1.89 | |||
| Organo-chlorine pesticide | HCB | 100 | 0.04 | 0.04 (1.4) | 0.05 | ||
| Trans-Nonachlor | 77.5 | 0.01 | 0.01 (1.8) | 0.01 | |||
| DDT | ΣDDT/DDEd | --- | 0.12 | 0.19 (2.1) | 0.28 | ||
| PCB | ΣPCBe | --- | 0.26 | 0.36 (1.7) | 0.50 | ||
Abbreviations: GM = Geometric mean, GSD = Geometric standard deviation
Notes: Values<LOD retained the machine read value for urine and PFASs, values<LOQ were substituted with LOQ/2 for other serum compounds.
LOD reported for all urine and PFASs, LOQ reported for other serum compounds.
Molar sum of metabolites: mono-2-ethylhexyl, mono(2-ethyl-5-hydroxyhexyl), mono(2-ethyl-5-oxohexyl), and mono(2-ethyl-5-carboxypentyl) phthalates.
Molar sum of metabolites: mono(hydroxyisononyl), mono(oxoisononyl), and mono(carboxyisooctyl) phthalates.
Sum of DDT and its metabolite dichlorodiphenyldichloroethylene.
Sum of PCB congeners 74, 99, 118, 138, 153, 156, 170, 180, 183, 187.
2.3. Children’s anthropometric measures
Children’s height and weight were measured at birth and then up to 15 times during routine health care visits at a Child Health Center (CHC) from two weeks until 66 months of age. For this analysis we selected the measurements at 5.5 years of age (66 months) representing the longest possible follow-up at CHSs. We calculated BMI and BMI z-scores according to the WHO child growth standards from 0–59 months and 5–19 years of age as children’s age ranged from 4.2–7.6 years (mean (SD): 5.6 (0.3) (Onis et al. 2007; WHO Multicentre Growth Reference Study Group 2006). A total of three values were flagged as biologically implausible and excluded from the analysis (value above ± 5SD for BMI-for-age z-scores). The proportion of children with overweight was calculated according to the age and sex specific cut-off level in children; ISO-BMI ≥ 25 developed by the International Obesity Task Force, which represents the centile curves that at 18 years passes through the cut-off points of 25 kg/m2 for adult overweight (Cole et al. 2000).
2.4. Selected covariates
The selected covariates for analysis, maternal BMI, education, smoking, parity, and children’s age and sex, were selected based on a directed acyclic graph (DAG) (Greenland et al. 1999) (Figure S2). Sociodemographic characteristics including maternal education, smoking and maternal height were collected through self-administered questionnaires. Information on maternal age, weight, parity and child’s sex was extracted from the Swedish national birth medical registry. Maternal height and weight (collected at the enrollment visit) were used to calculate maternal BMI (kg/m2). Level of education was categorized as less than college / university, and college / university or more. Mothers were classified as active or passive smokers if cotinine levels were above or equal to 0.2 ng/mL, and as non-smokers if cotinine levels were below 0.2 ng/mL. Cotinine levels were measured in serum samples collected during the enrollment visit (median of 10 weeks of gestation). Parity was dichotomized as primiparous and multiparous. Maternal BMI in early pregnancy has been associated with children’s BMI in childhood and adolescence (Önnestam et al. 2022). Likewise, smoking during pregnancy has been associated with lower birthweight (Zaren et al. 1996) and higher BMI in childhood (Vrijheid et al. 2020). Parity, specifically nulliparity, is related to lower birthweight (Shah et al. 2010) and therefore, also a cofounder for body composition in childhood. Both education and smoking are indicators of lifestyle factors that are associated with health outcomes (Johansson and Sundquist 1999). All these factors are also related to exposure to EDCs during pregnancy (Pacyga et al. 2022).
2.4. Statistical analysis
Descriptive statistics were used to calculate central tendency measures and frequencies of sociodemographic characteristics, EDC concentrations, children’s BMI and covariates of the study population overall and by sex. The association between prenatal exposure to EDC and children’s BMI at 5.5 years of age was evaluated using a weighted quantile sum (WQS) regression model (Carrico et al. 2015; Czarnota et al. 2015). This model derives a weighted index estimating the mixture effect associated with a specific outcome, in this case, children’s BMI and overweight. The prenatal urine and serum concentrations of 26 chemicals, including non-summed chemicals (i.e., PFAS, bisphenols) and summed chemicals (i.e., DEHP, DINP), were included in the mixture (Table 2). The term represents the index that weights and sums the components included in the mixture; where wi is the weight associated with the ith component, and qi is the quantile (here, deciles) of the ith component. The model also identifies chemicals of concern through estimated weights. The weights associated with each component in the mixture are estimated as the average from 100 bootstrap samples. The higher the estimated weight, the higher the contribution of that chemical to the weighted index. We decided a priori to derive a positively associated index with children’s BMI and overweight as we were interested in assessing the association with increased BMI and overweight among children. The weighted index was assessed in a linear regression model for BMI and a logistic regression for overweight. In order to get as robust estimates as possible we applied a validation technique currently available for the WQS regression, namely 100 repeated holdouts (Tanner et al. 2019). The validation step randomly partitions the data (40/60%) with replacement 100 times and runs a WQS regression each time. The results provide a distribution of the estimates and chemical weights from the 100 WQS regressions with different partitions of the data. The mean estimates with 95% confidence intervals and the mean chemical weights are then reported. A p-value of the estimates is not provided. Instead, the criteria for a significant estimate were if at least 97.5% of the beta coefficients were above or below the null effect as shown by the 95%CI (Tanner et al. 2019). We also identified how many of the holdouts resulted in an estimate above or below the null effect. If the mean estimate was significant or borderline significant then the next step was applied to interpret the chemical weights and identify chemicals of concern. For each chemical we applied the “Busgang criteria”; if at least 90% of the holdouts were above the 1/c threshold (e.g., equal weighting), where c is the number of chemicals in the mixture, then the chemical was defined as a “probable” contributor, and if 50% of the holdouts were above the threshold it was considered a “possible” contributor to the mixture effect (Bennett et al. 2022; Busgang et al. 2022).
Our objective was to evaluate sex-specific differences in the association and chemicals of concern related to BMI and overweight. Therefore, we performed WQS regression models with an interaction term for WQS*sex and stratified sex-specific weights. By adding the interaction term, we allow the effect of one-unit increase in the WQS index to differ by sex. This modelling approach allows for different slopes as well as different ranks and magnitudes for the estimated weights for boys and girls (Busgang et al. 2022). This means that although we derived a positively associated index with children’s BMI and overweight, the direction of the association could vary by sex. The relative weights were calculated by dividing each average chemical weight by the total weight within the strata (e.g., boys or girls). Therefore, the total weight percentage sum to 100% and the relative weights within the strata also sum to 100%. The relative weights indicate, on average, how much each chemical contribute to the total weight within the strata. The 1/c threshold (e.g., equal weighting) represents 3.9% of the relative weight within the strata (1/26*100) and chemicals with a relative weight above this threshold in at least 50% of the repeated holdouts was considered chemicals of concern. All models were adjusted for the following selected covariates: maternal BMI, education, smoking parity, and children’s age and sex. The R package “gWQS: generalized weighted quantile sum regression” version 3.0.4 was used to conduct the WQS regression models (Renzetti et al. 2021).
As sensitivity analysis, we performed single-compound analysis with one linear regression model for each chemical and children’s BMI or overweight as outcome, adjusted by covariates. For these models, the chemicals were log10 transformed to diminish the effect of skewed distributions of the concentrations. These models were run for all children as well as stratified by sex. A second sensitivity analysis was performed to explore birthweight as potential mediator of the association between EDC mixture and children’s BMI. The model was run with bootstrap sampling and 10,000 simulations, adjusting for the same covariates as in the WQS regression. The average causal mediation effect (ACME), average direct effect (ADE) and the average proportion of mediation was calculated. As initial step, a WQS regression with BMI as outcome and adjusted by covariates was run to extract the WQS index and apply as the exposure variable in the mediation model. This WQS regression model was run without splitting the data or repeated holdout validation in order to get a WQS index from the full sample. Interaction between the EDC mixture and birthweight was also evaluated in the mediation analysis. The model was run using the R package “mediation” version 4.5.0 (Tingley et al. 2014). All the statistical analyses were performed using the statistical software R version 4.1.2.
3. Results
The women included in this analysis had a mean (± SD) age of 30.6 ± 4.7 years and BMI of 24.7 ± 4.4 kg/m2 (Table 1). More than half (58%) had a college/university education level or higher, 11% percent were active or passive smokers, and almost half of the women were primiparous (47%). The children had a birthweight of 3.6 ± 0.5 kg, with boys having a higher birthweight as compared to girls (3.7 ± 0.6 kg vs. 3.6 ± 0.5 kg). At follow-up, the children were 5.6 ± 0.2 years of age, and had a BMI of 15.9 ± 1.7 (kg/m2). A total of 16.1% were overweight (ISO-BMI ≥ 25) of which 4.1% were obese, and this proportion was higher among girls as compared to boys (overweight: 19.5% vs. 13.0%, and obesity: 5.4% vs. 2.9%).
Table 1.
Sociodemographic characteristics of the study population, overall and by sex, n=1,105
| Overall (n=1,105) | Boys (n=583) | Girls (n=522) | P-value * | |
|---|---|---|---|---|
|
| ||||
| Maternal characteristics | Mean (SD) | Mean (SD) | Mean (SD) | |
|
|
||||
| Maternal age (years) | 30.6 (4.7) | 30.5 (4.6) | 30.6 (4.8) | 0.669 |
| Maternal BMI (kg/m2) | 24.7 (4.4) | 24.7 (4.3) | 24.7 (4.4) | 0.956 |
| Maternal Education (n (%)) | ||||
| Less than college/ university | 464 (42.0) | 239 (41.0) | 225 (43.1) | |
| College/ university or more | 641 (58.0) | 344 (59.0) | 297 (56.9) | 0.517 |
| Smoking (n (%)) | ||||
| Non-smoker | 986 (89.2) | 527 (90.4) | 459 (87.9) | |
| Passive or active smoker | 119 (10.8) | 56 (9.6) | 63 (12.1) | 0.222 |
| Parity (n (%)) | ||||
| Primiparous | 517 (46.8) | 271 (46.5) | 246 (47.1) | |
| Multiparous | 588 (53.2) | 312 (53.5) | 276 (52.9) | 0.878 |
| Children’s characteristics | ||||
| Age at 5.5 years visit | 5.6 (0.2) | 5.6 (0.3) | 5.6 (0.2) | 0.048 |
| Birthweight (kg) | 3.622 (0.554) | 3.665 (0.560) | 3.574 (0.545) | 0.007 |
| BMI (kg/m2) | 15.9 (1.72) | 15.9 (1.56) | 15.9 (1.89) | 0.868 |
| BMI z-score | 0.33 (1.06) | 0.36 (1.06) | 0.30 (1.06) | 0.317 |
| Weight-for age z-score | 0.57 (1.02) | 0.65 (1.04) | 0.49 (0.98) | 0.008 |
| Height-for-age z-score | 0.55 (0.97) | 0.63 (1.00) | 0.46 (0.93) | 0.004 |
| Overweight and obese (ISO-BMI ≥ 25) (n (%)) | 178 (16.1) | 76 (13.0) | 102 (19.5) | 0.004 |
| Obese (ISO-BMI ≥ 30 kg/m2) (n (%)) | 45 (4.1) | 17 (2.9) | 28 (5.4) | 0.057 |
Abbreviations: BMI = Body mass index, SD = Standard deviation
P-value from Student t-test or Chi-square test
Prenatal EDC measurements in urine samples showed highest concentrations of MBP, MEP, and ΣDEHP metabolites with geometric means (GM) of 66.4, 65.2 and 63.7 ng/mL, respectively (Table 2). The chemicals with highest GM in serum samples were PFOS (5.3 ng/mL) and PFOA (1.6 ng/mL). Prenatal concentrations of EDCs did not differ by child’s sex, except for ΣDEHP and 2-OHPH, which were slightly higher if the child was a girl (GM ± GSD: 64.9 ± 2.5 vs. 62.8 ± 2.3 for boys; and 0.21 ± 2.4 vs. 0.20 ± 2.3 for boys; p-value <0.05) (Table S1).
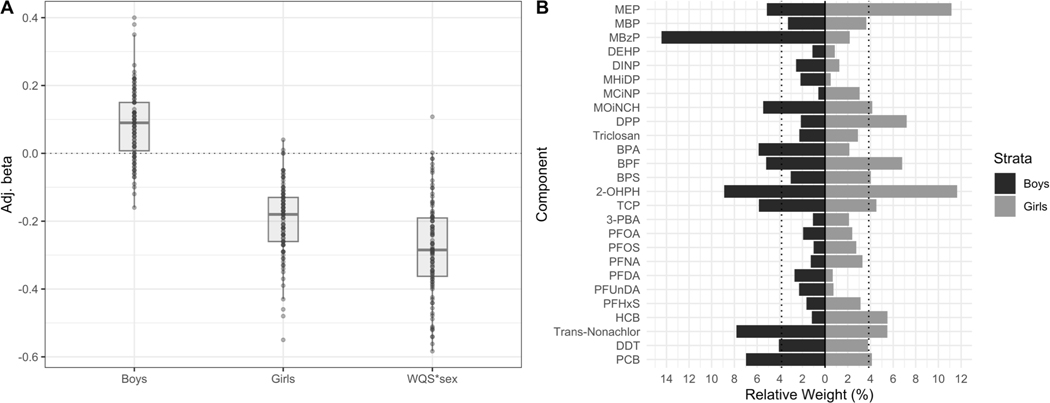
The WQS regression analysis resulted in a distribution of betas and chemical weights that indicates there are sex-specific differences (Figure 1A and 1B). The exact values of the estimated associations are shown in Table S2. This was observed by a significant WQS*sex interaction term (Mean β = −0.28, 95%CI: −0.55, −0.01) and a negative distribution with 98 out of 100 negative betas. Thus, even though a positive WQS index was assumed for the reference group (i.e., the boys), the slope for boys and girls was in the opposite direction. Among boys, the mean beta was positive (Mean β = 0.08, 95%CI: −0.12, 0.29) but did not reach significance (76/100 positive betas). Among girls, the association was negative and borderline significant, suggesting that higher prenatal EDC exposure was associated with lower BMI (Mean β = −0.19, 95%CI: −0.40, 0.01), with 98 out of 100 negative betas.
Figure 1.
Mean adjusted betas (A) and sex-specific relative weights (B) from a WQS linear regression model with 100 repeated holdouts between prenatal EDC mixture analysis and children’s BMI, n = 1,105. The model was adjusted for maternal BMI, education, age, smoking, parity and child’s sex and age. A) Illustrates the distribution of the adjusted betas across the 100 repeated holdouts where each dot represents the estimate from each holdout. B) Illustrates the mean estimated relative weight for each chemical of the EDC mixture across the 100 repeated holdouts. The relative weight is the percentage of weight attributable to each chemical in the EDC mixture within the total weight of each strata (boys and girls). The dotted line represents the threshold (3.9%) for chemicals of concern. Chemicals with relative weights above this threshold in at least 50% of the repeated holdouts were considered chemicals of concern.
Abbreviations: BMI = Body mass index
Notes: Chemicals assessed in urine were creatinine adjusted. All chemicals were log10 transformed to reduce skewness in the distribution of the concentrations.
The estimated weights for each chemical showed a different pattern for boys and girls. For boys, as the association did not reach significance, we did not go further into interpreting the weights of the chemicals to identify chemicals of concern. The chemicals associated with lower BMI among girls had slightly different rankings and magnitudes as compared to boys (Figure 1B). The possible chemicals of concern for girls were 2-OHPH, MEP, DPP, and BPF, with weights above the threshold in 56%−80% of the holdouts.
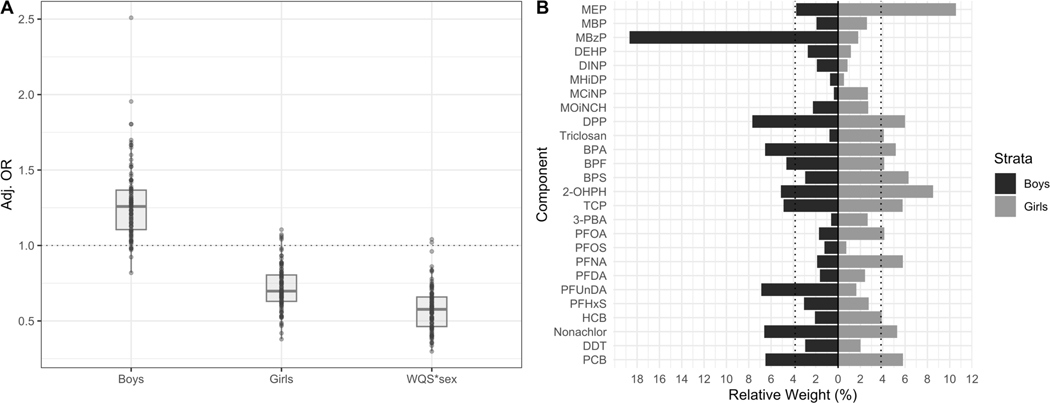
In regards to the association with overweight (ISO-BMI ≥ 25) at age 5.5 years, we also found sex-specific associations and chemical weights distribution (Figure 2A and 2B). The exact numbers of the estimated associations are shown in Table S2. The WQS*sex interaction term was significant (Mean OR = 0.56, 95%CI: 0.34, 0.91) with 98 out of 100 negative betas, suggesting the slopes were different for boys and girls. The odds of overweight were lower for girls (Mean OR = 0.72, 95%CI: 0.48, 1.04) and higher for boys (Mean OR = 1.26, 95%CI: 0.89, 1.78). The distribution of the ORs were in opposite directions for boys and girls, as 92 out of 100 betas were positive for boys and 95 out of 100 betas were negative for girls. However, the association were borderline significant for girls and did not reach significance for boys. The chemical weights showed similar sex-specific pattern as in the linear regression model. The possible chemicals of concern for the girls were MEP, 2-OHPH, BPS, DPP, and PFNA with weights above the threshold in 50–77% of the holdouts.
Figure 2.
Mean adjusted betas (A) and sex-specific relative weights (B) from a WQS logistic regression model with 100 repeated holdouts between prenatal EDC mixture analysis and overweight (ISO-BMI ≥ 25), n = 1,105. The model was adjusted for maternal BMI, education, age, smoking, parity and child’s sex and age. A) Illustrates the distribution of the adjusted betas across the 100 repeated holdouts where each dot represents the estimate from each holdout. B) Illustrates the mean estimated relative weight for each chemical of the EDC mixture across the 100 repeated holdouts. The relative weight is the percentage of weight attributable to each chemical in the EDC mixture within the total weight of each strata (boys and girls). The dotted line represents the threshold (3.9%) for chemicals of concern. Chemicals with relative weights above this threshold in at least 50% of the repeated holdouts were considered chemicals of concern.
Abbreviations: BMI = Body mass index
Notes: Chemicals assessed in urine were creatinine adjusted. All chemicals were log10 transformed to reduce skewness in the distribution of the concentrations.
The sensitivity analysis of single compounds with BMI and overweight did not show any significant associations in the overall study population (Table S3, S4). When stratifying by sex, there were positive associations between MBzP and higher BMI among boys (β = 0.35, 95%CI: 0.03, 0.68), as well as higher odds of overweight (OR = 1.11, 95%CI: 1.03, 1.19). However, no significant associations were found for girls. The second sensitivity analysis, explored birthweight as potential mediator of the association between the EDC mixture and children’s BMI. The results showed a trend of higher exposure to EDC mixture associated with lower birthweight z-score (β = −0.05, p-value = 0.059), and lower birthweight z-score associated with higher BMI (β = 0.27, p-value < 0.001). The ADE between EDC mixture and higher BMI (β = 0.10, p-value = 0.011), and the total effect (β = 0.09, p-value = 0.024) were significant. However, the proportion of mediation was not significant (p-value = 0.092) (Figure S4).
4. Discussion
Contrary to our hypothesis, our results suggest that higher concentrations of a mixture of EDCs during early pregnancy was associated with lower BMI and lower odds of overweight among girls. The chemicals of concern driving this association were a mixture of phthalates (MEP), non-phthalates plasticizers (DPP), bisphenols (BPF, BPS), PAHs (2-OHPH), and PFASs (PFNA). We did not find significant association between the EDC mixture with BMI and overweight among boys. However, in the single chemical model higher MBzP levels were associated with more BMI and overweight among boys. Even though the effect of the mixture was not significant, the estimated weight for MBzP was the highest among boys in the WQS regression. The main difference is the WQS estimates the mixture effect as compared to the single-chemical models evaluating the effect of one chemical at the time. Also, we added the additional validation step of repeated holdout calculating a more robust estimate. Still, the distribution of the estimated betas showed a positive trend, especially for overweight with 92 out of 100 positive betas. So, even though our results did not reach significance among boys, future studies may want to consider MBzP when evaluating child overweight.
Five previous studies have evaluated prenatal exposure to EDC mixtures with children’s weight and body fat measures with varied results. These studies differ from our study in terms of the selected EDCs in the mixture and also modelling approaches. Therefore, our results may not be directly comparable but they are still informative in regards to EDC mixtures and children’s weight. The first study, comprised of 425 mothers and their female child living in the United Kingdom and evaluated prenatal exposure to a total of 31 EDCs (e.g., PFASs, PCBs and OCPs) using a BKMR analysis (Marks et al. 2021). They found that higher concentrations of all the three classes of EDCs at the 75th percentile compared to the 50th percentile was associated with lower weight-for-age z-score among girls longitudinally from birth to 9 months of age. A Spanish birth cohort of 470 mother-child pairs assessed prenatal exposure to a group of 27 chemicals (e.g., bisphenols, phthalates, metals, OCPs, PCB, and PBDEs) using a Principal Component analysis (PCA) with children’s BMI z-scores and odds of overweight at seven years of age (Agay-Shay et al. 2015). They found different associations with different classes of EDCs. The organochlorine chemicals (e.g., DDE, HCB, PCBs) were associated with higher BMI z-scores and odds of overweight, whereas, the opposite was found for several phthalates (e.g., MEP, MBP, MBzP, and DEHP metabolites). The latter being consistent with our findings of an association with lower odds of overweight. A third birth cohort of 309 mother-child pairs in the US assessed prenatal exposure to 15 EDCs (e.g., phthalates, phenols, and parabens) using a Bayesian Kernel Machine regression (BKMR), and found an overall effect of the mixture, where MEP and MCiNP were distinct in the association with higher BMI z-score and overweight/obesity at five years of age (Berger et al. 2021). Forth, a large human early life exposome (HELIX) study assessed prenatal exposure to 77 pollutants from different sources (e.g., air pollutants, built environment, smoking) in six European birth cohorts (Vrijheid et al. 2020). They used a deletion/substitution/addition (DSA) method, which is an iterative process that adds, replaces, and removes variables until the final model is selected. The EDCs assessed in this study were persistent organic pollutants, metals, phthalates, phenols and pesticides, and yet, only smoking during pregnancy was associated with higher BMI z-score among children 6 to 11 years of age. In contrast, the fifth birth cohort, among 514 Mexican children from 4 to 12 years of age, did not find any significant overall effect of the prenatal phthalate mixture on children’s body fat measures over time using a Quantile G computation analysis (Kupsco et al. 2022). These studies did not find and/or report differences by sex. Also, one study only comprised mother-daughter pairs and were therefore not able to evaluate sex differences (Marks et al. 2021). The inconsistency of the direction and significance of the association may be due to several factors such as different analytical methods, the chemicals assessed in the EDC mixture, timing of the exposure during pregnancy as well as the age of the body fat measure in childhood, but also sample size, population characteristics and lifestyles.
In Sweden, the prevalence of obesity and overweight increase by age for children between 6 and 9 years of age in Sweden with the prevalence among boys more specifically increases from the age of 9 years (Public Health Agency of Sweden 2021). It may be possible the effect of EDCs on children’s weight is only identified at specific periods of growth and development and not in others. In our study, the mean age of the children was 5.5 years of age (range: 4.2–7.6 years), which represents a prepubertal time before sex steroids increase and influence body composition and fat distribution (Roemmich and Rogol 1999). At approximately 3 to 6 years of age the BMI trajectory is generally at its lowest point and children are expected to be slimmer (Roemmich and Rogol 1999; Wen et al. 2012). The linear growth velocities, specifically of height, for boys and girls converge after four years of age until the age of nine when girls begin a pubertal growth spurt (Veldhuis et al. 2005). It is possible that the effect of prenatal exposure to EDCs vary at different periods in childhood and adolescence. For example, a Mexican birth cohort found that higher prenatal phthalate exposure to MECPP was associated with the highest BMI trajectory at 14 years of age but with the lowest BMI trajectory before the age of nine, and varied by level of phthalate exposure and was modified by sex (Yang et al. 2018).
A possible explanation to the observed sex-specific association between EDCs and children’s BMI may be through disruption of the naturally occurring hormones and their influence on body fat distribution. In prepubertal children, higher estradiol concentrations are associated with more percent body fat and higher testosterone is associated with more percent abdominal fat (Garnett et al. 2004). Androgenic or antiandrogenic effects on the human endocrine system have been associated with exposure to different EDCs (Wuttke et al. 2010), and a few previous studies have reported sex-specific associations with children’s BMI. Pooled analysis from three birth cohorts in the US, found that prenatal exposure to MEP and summed DEHP metabolites were associated with lower BMI among girls between 4–7 years of age (Buckley et al. 2016). This is consistent with our findings where MEP was one of the chemicals identified as driving the inverse association with BMI and less odds of overweight among girls. In contrast there have been different findings in other cohort studies. A Spanish study found that higher prenatal exposure to phthalates of high molecular weight (i.e., DEHP metabolites and MBzP) was associated with lower BMI z-score in boys and higher BMI z-score in girls at 4 and 7 years of age (Valvi et al. 2015). Also, in a cohort study in the US, non-DEHP metabolites (e.g., MEP, MBP, MiBP, MBzP and MCPP) was associated with lower BMI in boys of 5 and 7 years of age (Maresca et al. 2016). In addition, a Chinese cohort, found that prenatal exposure to MEP and DEHP was associated with higher BMI trajectories in girls from birth to 6 years of age (Gao et al. 2022). It is further possible that the associations may be different depending on the timing of exposure during pregnancy. These studies described above, have all measured EDC levels during third trimester of pregnancy except for Valvi et al., (2015), which calculated an average from first and third trimester. In the SELMA study, the EDC levels were assessed during first trimester. Critical time windows of specific sensitivity to EDCs during fetal development are still important to assess in a sex-specific manner in future studies.
Of the six PFAS, and also other persistent chemicals (e.g., OCPs, PCBs) included in the mixture, we only found PFNA to be a chemical of concern among girls. An American cohort study, showed that prenatal exposure to PFAS was associated with fat mass in a sex-specific fashion among younger children at 5 months of age (Starling et al. 2019). Prenatal exposure to PFOS and PFHxS was associated with lower weight -for-age and weight-for-length z-scores among girls and PFOA and PFNA was associated with higher fat mass among boys. The direction of the association is in agreement with our results, however, the specific PFAS compounds driving these associations differ. In contrast to our findings, a meta-analysis summarized that prenatal exposure to PFOA was associated with a small increase in BMI z-score and odds of overweight in childhood (Liu et al. 2018). Also, prenatal PFOS and PFOA serum concentrations among Norwegian mothers have been associated with higher BMI z-scores and odds of overweight/obesity among their children at 5 years of age (Lauritzen et al. 2018).
In regards to DPP, the metabolite of TPP an organophosphate flame retardant, at the moment the epidemiological studies evaluating prenatal exposure to DPP and weight in childhood are scarce. A previous EDC mixture analysis in the SELMA study, identified DPP as chemical of concern in the association with slower infant weight increase (Svensson et al. 2021). In addition, animal studies suggest a sex-specific metabolic disturbance. A low dose of TPP was associated with up-regulated lipid-related metabolites among male mice and no significance among female mice (Wang et al. 2018). In addition, exposure to TPP in adult zebrafish was associated with disruption of the thyroid hormones with different effects for males and females (Liu et al. 2019).
In contrast to our findings, prenatal exposure to polycyclic aromatic hydrocarbons (PAHs) have been associated with higher BMI and greater odds of overweight/obesity at 5 and 7 years of age in the US (Rundle et al. 2012). Also, among mice offspring, prenatal exposure to PAHs was associated with greater weight and fat mass (Yan et al. 2014). However, in line with our results sex-specific associations have been found with greater weight-for-age among boys but not among girls in Bangladesh (Rahman et al. 2023).
In terms of persistent organic pollutants (POPs), we did not find DDT or PCB to be chemicals of concern for children’s weight in our analysis, which is consistent with two previous studies on prenatal exposure of DDT, PCBs and HCB with overweight and obesity at 7 years of age (Cupul-Uicab et al. 2013; Warner et al. 2013). However, a follow-up study by Warner et al., found significant association between DDT and higher BMI z-scores among boys at 12 years of age (Warner et al. 2017), and a meta-analysis of 33 epidemiological studies found that prenatal levels of DDE and HCB was associated with higher BMI z-score among children (Stratakis et al. 2022). Also, animal studies, suggest that prenatal exposure to DDE and HCB alters metabolic markers in mice and rat offspring (Al-Obaidi et al. 2022; La Merrill et al. 2014; vonderEmbse et al. 2021). Follow-up analysis in our study population would be informative to whether associations can be found later in childhood or puberty.
In early life, EDCs may influence developmental programming of adipogenesis through epigenetic modifications, such as DNA methylation, histone modifications and non-coding RNAs, which may alter gene expression (Bianco-Miotto et al. 2017; Hanson et al. 2011). In addition, EDCs present in plastic consumer products (e.g., phthalates, organophosphates) have in vitro demonstrated to induce adipogenic activity, such as increasing the number of adipocytes and increased lipid droplet counts (Völker et al. 2022). Similarly, a mixture of EDCs in low concentrations associated with lower birthweight and relevant to human exposure based on the SELMA study, was tested in vitro on human mesenchymal stem cells (MSCs). The results suggest that the EDC mixture induced adipogenesis by increasing lipid droplet accumulation (Lizunkova et al. 2022). These results are consistent with our previous analysis showing that higher exposure to EDC mixture was associated with lower birthweight (Svensson et al. 2021). However, it differs with our current results as we find sex-specific associations of lower BMI among girls. As, we found a non-significant trend of higher BMI among boys, follow-up analysis later in childhood and puberty would be informative. The most commonly suggested mechanism for EDC to induce adipogenesis is through activation of the peroxisome proliferator-activated receptor γ (PPARγ), which is a key regulator of adipogenesis (Heindel et al. 2017a). Although, as EDCs are a diverse group of chemicals, it is also possible that EDCs may influence adipogenesis through several nuclear receptors and multiple mechanisms (Völker et al. 2022). However, experimental studies are needed to find possible mechanisms on the sex-specific effect of EDCs, especially to confirm our findings on lower BMI among girls. Also, further studies are still needed to identify plausible mechanisms by which EDC may influence fetal growth and development and potentially also children’s weight long term.
The mediation analysis did not show significant results of birthweight as a mediator of the association between EDC mixture. However, there was a trend of higher EDC mixture associated with lower birthweight and lower birthweight with higher BMI. Even though we did not find significant results, there are previous research showing that lower birthweight or SGA infants have higher risk for metabolic disorders (Martín-Calvo et al. 2022), and we have previously found that higher exposure to EDC mixture is associated with lower birthweight (Svensson et al. 2021). Therefore, future studies may want to explore this question further.
This study has several strengths. The SELMA study is a pregnancy cohort with a large and comprehensive battery of data from the time of pregnancy until school-age. The EDCs exposure assessment is early in pregnancy, which is a critical period for several developing processes sensitive to endocrine disruption. The biological samples were collected at a median of 10 week’s gestation, which is just before the appearance and development of adipose tissue in the fetus (Desoye and Herrera 2021; Poissonnet et al. 1984). Children’s anthropometric measures were collected by trained personnel in a systematic and standardized way in health care centers where routine health visits are conducted. Finally, the EDC classes considered in this study is a mixture of chemicals of short half-life (i.e., phthalates) and persistent chemicals (i.e., PFASs) with suspect or proven endocrine disrupting properties of common exposure in the general population as has been reported by biomonitoring studies (Norström et al. 2020).
It is important to also account for some limitations. Even though we were able to adjust for maternal BMI, we did not have information on gestational weight gain and could therefore, not adjust for it in our models. Both maternal BMI and gestational weight gain are important risk factors for children’s body fat and important for future studies to consider. The assessment of EDCs is based on a single sample collection and limits the possibility to evaluate differences in exposure throughout pregnancy. Persistent chemicals, such as PFASs, are bioaccumulating and therefore, the day-to-day variability is lower than compared to chemicals with short half-life, such as phthalates and bisphenols (Agier et al. 2020; Buck Louis et al. 2019). The exposure is more variable for chemicals with short half-life and the possibility of misclassification exist (Casas et al. 2018; Fisher et al. 2015). This raises the possibility that our results for the non-persistent chemicals are due to chance findings. However, a one-time sample may represent the exposure of a short period of time although it may be biomarker specific with less reproducibility for chemicals that vary with diet (Fisher et al. 2015). In the SELMA study, the collection of urine samples was conducted in a standardized way collecting the first morning void urine sample. Finally, the selected EDCs is not comprehensive of all chemicals that humans are exposed to in real life. There are other obesogenic chemicals we did not have the possibility to evaluate in this analysis (e.g., PBDEs), or more recently introduced replacement chemicals with potential obesogenic properties. Therefore, the generalizability of this study is limited to the EDCs selected and only represents a small portion of the total chemical burden that populations may be exposed to.
5. Conclusions
Prenatal exposure to a mixture of EDCs is in the current study associated with BMI and overweight at 5.5 years of age in a statistically significant sex-specific manner. The overall EDC mixture effect, with borderline significance, was associated with lower BMI and lower odds of overweight among girls and non-significant associations among boys. The chemicals of concern for girls included a mixture of phthalate (MEP), non-phthalate plasticizer (DPP), bisphenols (BPF, BPS), PAH (2-OHPH), and PFAS (PFNA). Based on our findings of prenatal EDCs and lower BMI among girls, we cannot make any final conclusions on the long-term effects on an individual or population level. We can only highlight that our results suggest that prenatal exposure to EDCs may influence children’s BMI, and possibly in a sex-specific manner. Future studies are warranted evaluating the long-term effect of prenatal exposure to EDCs on children’s BMI until adulthood, and evaluate if the associations found in our study remain or change over time.
Supplementary Material
Highlights:
Sex-specific associations were found between prenatal EDC and BMI and overweight
Higher prenatal EDC exposure was associated with lower BMI among girls.
Higher prenatal EDC exposure was associated with less overweight among girls.
Chemicals of concern included a mixture of short half-life and persistent EDCs.
No significant association was found among boys.
Acknowledgements
The authors thank the participating families, staff of the SELMA study, and the antenatal care in the County Council of Värmland. We also posthumously thank Professor Bo Jönsson of Lund University, Sweden for his work with the chemical analyses in the present study. We also thank Åsa Amilon, Margareta Maxe and Agneta Kristensen for their work with chemical analysis. We thank Dr. Aimon Niklasson for computational support.
Funding:
The study was funded by grants from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas), the EDC-MixRisk European Union’s Horizon 2020 Research and Innovation Programme (634880), and the County Council of Värmland. C.G. were supported by Powering Research Through Innovative Methods for Mixtures in Epidemiology (PRIME) Program (R01ES028811-01).
Abbreviations:
- EDC
endocrine disrupting chemicals
- DOHaD
Developmental Origins of Health and Disease
- BMI
Body mass index
- CHC
Child Health Center
- 2OHPH
2-hydroxyphenanthrene
- BPA
bisphenol A
- BPF
bisphenol F
- BPS
bisphenol S
- DEHP
di-(2-ethylhexyl) phthalate
- DDT
dichlorodiphenyltrichloroethane
- DINP
diisononyl phthalate
- DPP
diphenylphosphate
- HCB
hexachlorobenzene
- MBP
monobutyl phthalate
- MBzP
monobenzyl phthalate
- MCiNP
monocarboxyisononyl phthalate
- MEP
monoethyl phthalate
- MHiDP
monohydroxyisodecyl phthalate
- MOiNCH
2–4-methyl-7-oxyooctyl-oxycarbonyl-cyclohexane carboxylic acid
- PBA
3-phenoxybenzoic acid
- PCB
polychlorinated biphenyl
- PFAS
perfluoroalkyl substance
- PFDA
perfluorodecanoic acid
- PFHxS
perfluorohexane sulfonic acid
- PFNA
perfluorononanoic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctane sulfonic acid
- PFUnDA
perfluoroundecanoic acid
- TCP
3,5,6-trichloro-2-pyridinol
Footnotes
Declaration of interest: The authors have no conflict of interest to declare.
Data availability statement
According to the Ethical Review Board decision and obtained personal consent, data on participating children or their mothers cannot be made freely available. This since they constitute clinical data subject to secrecy in accordance with the Swedish Public Access to Information and Secrecy Act [OSL 2009:400] (Ministry of Justice 2009). Unique combinations of clinical data could make a study participant identifiable, and consequently a review of secrecy may result in restrictions regarding data availability.
References
- Agay-Shay K; Martinez D; Valvi D; Garcia-Esteban R; Basagaña X; Robinson O; Casas M; Sunyer J; Vrijheid M. Exposure to endocrine-disrupting chemicals during pregnancy and weight at 7 years of age: A multi-pollutant approach. Environmental Health Perspectives 2015;123:1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agier L; Slama R; Basagaña X. Relying on repeated biospecimens to reduce the effects of classical-type exposure measurement error in studies linking the exposome to health. Environ Res 2020;186:109492 [DOI] [PubMed] [Google Scholar]
- Al-Obaidi ZAF; Erdogan CS; Sümer E; Özgün HB; Gemici B; Sandal S; Yilmaz B. Investigation of obesogenic effects of hexachlorobenzene, DDT and DDE in male rats. Gen Comp Endocrinol 2022;327:114098 [DOI] [PubMed] [Google Scholar]
- Amato AA; Wheeler HB; Blumberg B. Obesity and endocrine-disrupting chemicals. Endocrine connections 2021;10:R87–R105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DH; Busgang SA; Kannan K; Parsons PJ; Takazawa M; Palmer CD; Schmidt RJ; Doucette JT; Schweitzer JB; Gennings C; Hertz-Picciotto I. Environmental exposures to pesticides, phthalates, phenols and trace elements are associated with neurodevelopment in the CHARGE study. Environ Int 2022;161:107075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K; Hyland C; Ames JL; Mora AM; Huen K; Eskenazi B; Holland N; Harley KG Prenatal Exposure to Mixtures of Phthalates, Parabens, and Other Phenols and Obesity in Five-Year-Olds in the CHAMACOS Cohort. Int J Environ Res Public Health 2021;18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco-Miotto T; Craig JM; Gasser YP; van Dijk SJ; Ozanne SE Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis 2017;8:513–519 [DOI] [PubMed] [Google Scholar]
- Bornehag CG; Kitraki E; Stamatakis A; Panagiotidou E; Rudén C; Shu H; Lindh C; Ruegg J; Gennings C. A Novel Approach to Chemical Mixture Risk Assessment-Linking Data from Population-Based Epidemiology and Experimental Animal Tests. Risk analysis : an official publication of the Society for Risk Analysis 2019;39:2259–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag CG; Moniruzzaman S; Larsson M; Lindström CB; Hasselgren M; Bodin A; von Kobyletzkic LB; Carlstedt F; Lundin F; Nånberg E. The SELMA study: a birth cohort study in Sweden following more than 2000 mother–child pairs. Paediatric and perinatal epidemiology 2012;26:456–467 [DOI] [PubMed] [Google Scholar]
- Buck Louis GM; Yeung E; Kannan K; Maisog J; Zhang C; Grantz KL; Sundaram R. Patterns and Variability of Endocrine-disrupting Chemicals During Pregnancy: Implications for Understanding the Exposome of Normal Pregnancy. Epidemiology 2019;30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP; Engel SM; Braun JM; Whyatt RM; Daniels JL; Mendez MA; Richardson DB; Xu Y; Calafat AM; Wolff MS; Lanphear BP; Herring AH; Rundle AG Prenatal Phthalate Exposures and Body Mass Index Among 4- to 7-Year-old Children: A Pooled Analysis. Epidemiology 2016;27:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busgang SA; Spear EA; Andra SS; Narasimhan S; Bragg JB; Renzetti S; Curtin P; Bates M; Arora M; Gennings C. Application of growth modeling to assess the impact of hospital-based phthalate exposure on preterm infant growth parameters during the neonatal intensive care unit hospitalization. Science of The Total Environment 2022;850:157830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C; Gennings C; Wheeler DC; Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. Journal of agricultural, biological, and environmental statistics 2015;20:100–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M; Basagaña X; Sakhi AK; Haug LS; Philippat C; Granum B; Manzano-Salgado CB; Brochot C; Zeman F; de Bont J; Andrusaityte S; Chatzi L; Donaire-Gonzalez D; Giorgis-Allemand L; Gonzalez JR; Gracia-Lavedan E; Grazuleviciene R; Kampouri M; Lyon-Caen S; Pañella P; Petraviciene I; Robinson O; Urquiza J; Vafeiadi M; Vernet C; Waiblinger D; Wright J; Thomsen C; Slama R; Vrijheid M. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environment International 2018;121:561–573 [DOI] [PubMed] [Google Scholar]
- Cole TJ; Bellizzi MC; Flegal KM; Dietz WH Establishing a standard definition for child overweight and obesity worldwide: international survey. Bmj 2000;320:1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupul-Uicab LA; Klebanoff MA; Brock JW; Longnecker MP Prenatal exposure to persistent organochlorines and childhood obesity in the US collaborative perinatal project. Environ Health Perspect 2013;121:1103–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnota J; Gennings C; Wheeler DC Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer informatics 2015;14:CIN. S17295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoye G; Herrera E. Adipose tissue development and lipid metabolism in the human fetus: The 2020 perspective focusing on maternal diabetes and obesity. Progress in Lipid Research 2021;81:101082 [DOI] [PubMed] [Google Scholar]
- Ferguson KK; Bommarito PA; Arogbokun O; Rosen EM; Keil AP; Zhao S; Barrett ES; Nguyen RHN; Bush NR; Trasande L; McElrath TF; Swan SH; Sathyanarayana S. Prenatal Phthalate Exposure and Child Weight and Adiposity from in Utero to 6 Years of Age. Environ Health Perspect 2022;130:47006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M; Arbuckle TE; Mallick R; LeBlanc A; Hauser R; Feeley M; Koniecki D; Ramsay T; Provencher G; Bérubé R; Walker M. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. Journal of exposure science & environmental epidemiology 2015;25:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H; Geng M.-l.; Gan H; Huang K; Zhang C; Zhu B.-b.; Sun L; Wu X; Zhu P; Tao F.-b. Prenatal single and combined exposure to phthalates associated with girls’ BMI trajectory in the first six years. Ecotoxicology and Environmental Safety 2022;241:113837 [DOI] [PubMed] [Google Scholar]
- Garnett SP; Högler W; Blades B; Baur LA; Peat J; Lee J; Cowell CT Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr 2004;80:966–972 [DOI] [PubMed] [Google Scholar]
- Gore AC; Chappell VA; Fenton SE; Flaws JA; Nadal A; Prins GS; Toppari J; Zoeller RT. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 2015;36:E1–e150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S; Pearl J; Robins JM Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48 [PubMed] [Google Scholar]
- Gyllenhammar I; Glynn A; Jönsson BA; Lindh CH; Darnerud PO; Svensson K; Lignell S. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs? Environmental research 2017;153:48–54 [DOI] [PubMed] [Google Scholar]
- Hanson M; Godfrey KM; Lillycrop KA; Burdge GC; Gluckman PD Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms. Prog Biophys Mol Biol 2011;106:272–280 [DOI] [PubMed] [Google Scholar]
- Heindel JJ; Blumberg B. Environmental obesogens: mechanisms and controversies. Annual review of pharmacology and toxicology 2019;59:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ; Blumberg B; Cave M; Machtinger R; Mantovani A; Mendez MA; Nadal A; Palanza P; Panzica G; Sargis R; Vandenberg LN; Vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 2017a;68:3–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ; Skalla LA; Joubert BR; Dilworth CH; Gray KA Review of developmental origins of health and disease publications in environmental epidemiology. Reprod Toxicol 2017b;68:34–48 [DOI] [PubMed] [Google Scholar]
- Hoffman DJ; Powell TL; Barrett ES; Hardy DB Developmental origins of metabolic diseases. Physiol Rev 2021;101:739–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MH; Woodward M; Bao W; Liu B; Trasande L. Urinary Bisphenols and Obesity Prevalence Among U.S. Children and Adolescents. J Endocr Soc 2019;3:1715–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson SE; Sundquist J. Change in lifestyle factors and their influence on health status and all-cause mortality. International Journal of Epidemiology 1999;28:1073–1080 [DOI] [PubMed] [Google Scholar]
- Kabir ER; Rahman MS; Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environmental toxicology and pharmacology 2015;40:241–258 [DOI] [PubMed] [Google Scholar]
- Koponen J; Rantakokko P; Airaksinen R; Kiviranta H. Determination of selected perfluorinated alkyl acids and persistent organic pollutants from a small volume human serum sample relevant for epidemiological studies. Journal of Chromatography A 2013;1309:48–55 [DOI] [PubMed] [Google Scholar]
- Kupsco A; Wu H; Calafat AM; Kioumourtzoglou M-A; Cantoral A; Tamayo-Ortiz M; Pantic I; Pizano-Zárate ML; Oken E; Braun JM; Deierlein AL; Wright RO; Téllez-Rojo MM; Baccarelli AA; Just AC Prenatal maternal phthalate exposures and trajectories of childhood adiposity from four to twelve years. Environmental Research 2022;204:112111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M; Karey E; Moshier E; Lindtner C; La Frano MR; Newman JW; Buettner C. Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PLoS One 2014;9:e103337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen HB; Larose TL; Øien T; Sandanger TM; Odland J; van de Bor M; Jacobsen GW Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: a prospective cohort study. Environmental health : a global access science source 2018;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW; Lim HM; Lee JY; Min KB; Shin CH; Lee YA; Hong YC Prenatal exposure to phthalate and decreased body mass index of children: a systematic review and meta-analysis. Sci Rep 2022;12:8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P; Yang F; Wang Y; Yuan Z. Perfluorooctanoic Acid (PFOA) Exposure in Early Life Increases Risk of Childhood Adiposity: A Meta-Analysis of Prospective Cohort Studies. Int J Environ Res Public Health 2018;15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X; Cai Y; Wang Y; Xu S; Ji K; Choi K. Effects of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and triphenyl phosphate (TPP) on sex-dependent alterations of thyroid hormones in adult zebrafish. Ecotoxicol Environ Saf 2019;170:25–32 [DOI] [PubMed] [Google Scholar]
- Lizunkova P; Engdahl E; Borbély G; Gennings C; Lindh C; Bornehag CG; Rüegg J. A Mixture of Endocrine Disrupting Chemicals Associated with Lower Birth Weight in Children Induces Adipogenesis and DNA Methylation Changes in Human Mesenchymal Stem Cells. Int J Mol Sci 2022;23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca MM; Hoepner LA; Hassoun A; Oberfield SE; Mooney SJ; Calafat AM; Ramirez J; Freyer G; Perera FP; Whyatt RM; Rundle AG Prenatal Exposure to Phthalates and Childhood Body Size in an Urban Cohort. Environ Health Perspect 2016;124:514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KJ; Howards PP; Smarr MM; Flanders WD; Northstone K; Daniel JH; Sjödin A; Calafat AM; Hartman TJ Prenatal exposure to mixtures of persistent endocrine disrupting chemicals and postnatal body size in British girls. Early Hum Dev 2021;161:105450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Calvo N; Goni L; Tur JA; Martínez JA Low birth weight and small for gestational age are associated with complications of childhood and adolescence obesity: Systematic review and meta-analysis. Obesity reviews 2022;23:e13380 [DOI] [PubMed] [Google Scholar]
- Ministry of Justice. Public access to information and secrecy - the legislation in brief. 2009 [Google Scholar]
- Miregård J; Nowicka P; Nylander C. National data showed an increased prevalence of overweight and obesity among four-year-old Swedish children during the first year of COVID-19. Acta Paediatrica 2023;112:1269–1274 [DOI] [PubMed] [Google Scholar]
- Norén E; Lindh C; Glynn A; Rylander L; Pineda D; Nielsen C. Temporal trends, 2000–2017, of perfluoroalkyl acid (PFAA) concentrations in serum of Swedish adolescents. Environment International 2021;155:106716 [DOI] [PubMed] [Google Scholar]
- Norström K; Hellström A; Latvala S; Andersson Å; Nyberg E; Linderholm L. Gifter & miljö [2020]: Nya utmaningar och gamla synder. 2020 [Google Scholar]
- Onis M.d.; Onyango AW; Borghi E; Siyam A; Nishida C; Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World health Organization 2007;85:660–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga DC; Haggerty DK; Nicol M; Henning M; Calafat AM; Braun JM; Schantz SL; Strakovsky RS Identification of profiles and determinants of maternal pregnancy urinary biomarkers of phthalates and replacements in the Illinois Kids Development Study. Environ Int 2022;162:107150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH; Falconer C; Viner RM; Kinra S. The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obesity reviews 2012;13:985–1000 [DOI] [PubMed] [Google Scholar]
- Poissonnet CM; Burdi AR; Garn SM The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev 1984;10:1–11 [DOI] [PubMed] [Google Scholar]
- Public Health Agency of Sweden. Overweight and obesity are common and increase with age in 6–9 year olds. Public Health Agency of Sweden,; 2021 [Google Scholar]
- Public Health Agency of Sweden. Annual Report 2022. Public Health Agency of Sweden; 2022 [Google Scholar]
- Public Health Agency of Sweden. Statistik om övervikt och fetma hos barn 0–5 år. . 2023 [Google Scholar]
- Rahman SM; Malin Igra A; Essig JY; Ekström E-C; Dreij K; Trask M; Lindh C; Arifeen SE; Rahman A; Krais AM; Kippler M. Polycyclic aromatic hydrocarbon (PAH) exposure during pregnancy and child anthropometry from birth to 10 years of age: Sex-specific evidence from a cohort study in rural Bangladesh. Environmental Research 2023;227:115787 [DOI] [PubMed] [Google Scholar]
- Region Värmland. Barnhälsovården i Värmland-Årsrapport 2019. Karlstad: Region Värmland,; 2019 [Google Scholar]
- Renzetti S; Curtin P; Just AC; Bello G; Gennings C. How to use gWQS package. 2021 [Google Scholar]
- Roemmich JN; Rogol AD Hormonal changes during puberty and their relationship to fat distribution. American journal of human biology : the official journal of the Human Biology Council 1999;11:209–224 [DOI] [PubMed] [Google Scholar]
- Rundle A; Hoepner L; Hassoun A; Oberfield S; Freyer G; Holmes D; Reyes M; Quinn J; Camann D; Perera F; Whyatt R. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol 2012;175:1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF; Whitcomb BW; Louis GM; Louis TA Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect 2005;113:853–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS; ; Knowledge Synthesis Group on Determinants of LBW/PT births. Parity and low birth weight and preterm birth: a systematic review and meta-analyses. Acta obstetricia et gynecologica Scandinavica 2010;89:862–875 [DOI] [PubMed] [Google Scholar]
- Spinelli A; Buoncristiano M; Nardone P; Starc G; Hejgaard T; Júlíusson PB; Fismen AS; Weghuber D; Musić Milanović S; García-Solano M. Thinness, overweight, and obesity in 6-to 9-year-old children from 36 countries: The World Health Organization European Childhood Obesity Surveillance Initiative—COSI 2015–2017. Obesity Reviews 2021;22:e13214 [DOI] [PubMed] [Google Scholar]
- Starling AP; Adgate JL; Hamman RF; Kechris K; Calafat AM; Dabelea D. Prenatal exposure to per- and polyfluoroalkyl substances and infant growth and adiposity: the Healthy Start Study. Environ Int 2019;131:104983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis N; Rock S; La Merrill MA; Saez M; Robinson O; Fecht D; Vrijheid M; Valvi D; Conti DV; McConnell R; Chatzi VL Prenatal exposure to persistent organic pollutants and childhood obesity: A systematic review and meta-analysis of human studies. Obesity Reviews 2022;23:e13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson K; Tanner E; Gennings C; Lindh C; Kiviranta H; Wikström S; Bornehag CG Prenatal exposures to mixtures of endocrine disrupting chemicals and children’s weight trajectory up to age 5.5 in the SELMA study. Sci Rep 2021;11:11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner EM; Bornehag C-G; Gennings C. Repeated holdout validation for weighted quantile sum regression. MethodsX 2019;6:2855–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner EM; Hallerbäck MU; Wikström S; Lindh C; Kiviranta H; Gennings C; Bornehag C-G Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven. Environment international 2020;134:105185 [DOI] [PubMed] [Google Scholar]
- Tingley D; Yamamoto T; Hirose K; Keele L; Imai K. Mediation: R package for causal mediation analysis. 2014; [Google Scholar]
- Valvi D; Casas M; Romaguera D; Monfort N; Ventura R; Martinez D; Sunyer J; Vrijheid M. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect 2015;123:1022–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A; Pu Y; Gingrich J; Padmanabhan V. Obesogenic endocrine disrupting chemicals: identifying knowledge gaps. Trends in Endocrinology & Metabolism 2018;29:607–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD; Roemmich JN; Richmond EJ; Rogol AD; Lovejoy JC; Sheffield-Moore M; Mauras N; Bowers CY Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev 2005;26:114–146 [DOI] [PubMed] [Google Scholar]
- vonderEmbse AN; Elmore SE; Jackson KB; Habecker BA; Manz KE; Pennell KD; Lein PJ; La Merrill MA Developmental exposure to DDT or DDE alters sympathetic innervation of brown adipose in adult female mice. Environmental health : a global access science source 2021;20:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M; Fossati S; Maitre L; Márquez S; Roumeliotaki T; Agier L; Andrusaityte S; Cadiou S; Casas M; de Castro M; Dedele A; Donaire-Gonzalez D; Grazuleviciene R; Haug LS; McEachan R; Meltzer HM; Papadopouplou E; Robinson O; Sakhi AK; Siroux V; Sunyer J; Schwarze PE; Tamayo-Uria I; Urquiza J; Vafeiadi M; Valentin A; Warembourg C; Wright J; Nieuwenhuijsen MJ; Thomsen C; Basagaña X; Slama R; Chatzi L. Early-Life Environmental Exposures and Childhood Obesity: An Exposome-Wide Approach. Environ Health Perspect 2020;128:67009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völker J; Ashcroft F; Vedøy Å; Zimmermann L; Wagner M. Adipogenic Activity of Chemicals Used in Plastic Consumer Products. Environmental science & technology 2022;56:2487–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D; Zhu W; Chen L; Yan J; Teng M; Zhou Z. Neonatal triphenyl phosphate and its metabolite diphenyl phosphate exposure induce sex- and dose-dependent metabolic disruptions in adult mice. Environ Pollut 2018;237:10–17 [DOI] [PubMed] [Google Scholar]
- Warner M; Aguilar Schall R; Harley KG; Bradman A; Barr D; Eskenazi B. In utero DDT and DDE exposure and obesity status of 7-year-old Mexican-American children in the CHAMACOS cohort. Environ Health Perspect 2013;121:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M; Ye M; Harley K; Kogut K; Bradman A; Eskenazi B. Prenatal DDT exposure and child adiposity at age 12: The CHAMACOS study. Environmental Research 2017;159:606–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X; Kleinman K; Gillman MW; Rifas-Shiman SL; Taveras EM Childhood body mass index trajectories: modeling, characterizing, pairwise correlations and socio-demographic predictors of trajectory characteristics. BMC Med Res Methodol 2012;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta paediatrica 2006;95:76–85 [DOI] [PubMed] [Google Scholar]
- Wuttke W; Jarry H; Seidlova-Wuttke D. Definition, classification and mechanism of action of endocrine disrupting chemicals. Hormones (Athens) 2010;9:9–15 [DOI] [PubMed] [Google Scholar]
- Yan Z; Zhang H; Maher C; Arteaga-Solis E; Champagne FA; Wu L; McDonald JD; Yan B; Schwartz GJ; Miller RL Prenatal polycyclic aromatic hydrocarbon, adiposity, peroxisome proliferator-activated receptor (PPAR) γ methylation in offspring, grand-offspring mice. PLoS One 2014;9:e110706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TC; Peterson KE; Meeker JD; Sánchez BN; Zhang Z; Cantoral A; Solano M; Tellez-Rojo MM Exposure to Bisphenol A and phthalates metabolites in the third trimester of pregnancy and BMI trajectories. Pediatr Obes 2018;13:550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaren B; Lindmark G; Gebre-Medhin M. Maternal smoking and body composition of the newborn. Acta Paediatrica 1996;85:213–219 [DOI] [PubMed] [Google Scholar]
- Önnestam L; Vad OH; Andersson T; Jolesjö Å; Sandegård J; Bengtsson Boström K. Maternal body mass index in early pregnancy is associated with overweight and obesity in children up to 16 years of age. PLoS One 2022;17:e0275542 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
According to the Ethical Review Board decision and obtained personal consent, data on participating children or their mothers cannot be made freely available. This since they constitute clinical data subject to secrecy in accordance with the Swedish Public Access to Information and Secrecy Act [OSL 2009:400] (Ministry of Justice 2009). Unique combinations of clinical data could make a study participant identifiable, and consequently a review of secrecy may result in restrictions regarding data availability.