Abstract
The optical densities (ODs) of 216 dried venous blood (DVB) samples submitted to the Victorian Infectious Diseases Reference Laboratory as part of enhanced measles surveillance were compared to the ODs of the corresponding serum samples collected at the same time. DVB samples, stored for up to 24 months at 4°C, were tested by the Dade Behring Enzygnost Anti-Measles-Virus/IgM immunoassay. Elution and testing conditions were optimized with the use of spiked DVB samples. The assay showed an overall sensitivity of 90.2% and a specificity of 98.8% for DVB samples compared to the results for serum. When the results were analyzed according to the length of time that the DVB sample had been stored, the assay was 100% sensitive and 97% specific according to the ODs for those samples stored for less than 6 months compared to the results for the corresponding serum samples, with 97.7% agreement between the results for the two sample types. These results demonstrate the potential for the use of DVB samples for the diagnosis of measles in routine diagnostic laboratories.
The diagnosis of many viral diseases has historically relied on the detection of antibodies in serum or plasma. However, venipuncture is often resisted by parents and children and may require specially trained staff. Transport of serum and/or plasma specimens and their long-term storage may present logistical problems (10). The use of dried blood spots for the investigation of measles virus hemagglutination inhibition antibodies was reported in the early 1980s (9, 15). Since then several other laboratories have demonstrated the feasibility of using dried blood samples in an enzyme immunoassay (EIA) format for the detection of measles virus-specific immunoglobulin G (IgG) (2, 10) and IgM (3). In a small study, Novello et al. (10) demonstrated the use of dried blood samples with the Dade Behring measles virus-specific IgG EIA for the investigation of immunity to measles virus. However other studies have used in-house EIAs, restricting the use of such samples to more specialized laboratories.
Here, we report on the use of dried venous blood (DVB) samples for the detection of measles virus-specific IgM by the Dade Behring Enzygnost Anti-Measles-Virus/IgM immunoassay. This commercial EIA has been extensively evaluated and is widely used for the detection of measles virus-specific IgM and IgG (11). Moreover, this EIA performed with high degrees of sensitivity and specificity compared to the results of the Centers for Diseases Control and Prevention IgM capture assay, considered to be the “gold standard” for the detection of measles virus-specific IgM (12).
MATERIALS AND METHODS
Patients and samples.
As part of the enhanced measles surveillance program in the state of Victoria, Australia, 216 DVB samples were prepared from venous blood drawn from 211 patients clinically suspected of having measles and reported to the Victorian Department of Human Services between March 1999 and March 2001 (7). All patients or guardians of patients younger than 18 years gave informed consent for collection of blood. Approximately 100 μl of venous blood was applied to each of three 13-mm-diameter circles on Schleicher & Schuell no. 903 filter paper at the time that a sample of whole blood was taken from each patient. The filter paper was allowed to dry at room temperature (RT) and was then transferred to plastic resealable specimen bags and stored at 4°C for up to 24 months before testing.
Serum was separated on arrival at the Victorian Infectious Diseases Reference Laboratory (VIDRL) and stored at 4°C until completion of testing, after which the sera were stored at −20°C. All sera were tested for IgM and IgG antibodies specific for measles virus (Dade Behring Enzygnost, Marburg, Germany), parvovirus type B19 (Biotrin, Dublin, Ireland), and rubella virus (Beckman Access; Beckman Instruments, Chaska, Minn.), usually within 2 to 3 days, as described previously (7).
Preparation of control samples and validation criteria.
The DVB samples used for assay optimization were prepared in the manner described by Meredith and Hannon (8), with minor modifications. Briefly, blood from two healthy volunteers negative by EIA for measles virus-specific IgG and IgM was collected and placed into EDTA-containing tubes. Although neither volunteer was blood group O positive, as recommended, these samples were used since the prevalence of antibodies to measles virus in Victoria is high (6) and the detection of group O-positive blood donors negative for measles virus antibodies may have required substantial screening. Packed cells were washed as described previously (8), and 400 μl of cells was combined with 600 μl of serum with known measles virus-specific IgG and IgM reactivities by EIA. Subsequently, 100 μl of spiked blood was delivered to the center of each 13-mm circle and allowed to dry at RT.
EDTA-anticoagulated blood samples from volunteers negative for measles virus IgM (n = 3) and from a patient confirmed to be positive for measles virus-specific IgM by PCR were prepared for use as DVB negative and positive controls for inclusion in all assays. To ensure that each plate met the validity criteria provided with each kit batch number, the positive control provided with the kit was diluted in accordance with the instructions and was included on each test plate.
Serum and DVB samples which returned a test optical density (OD) ≥0.2 were considered positive for measles virus-specific IgM, as were all samples which repeatedly returned 0.1 ≥ OD < 0.2 (nominally equivocal, but positive according to the interpretation provided by kit manufacturer). All samples for which the OD was <0.1 were deemed to be negative for measles virus-specific IgM.
Optimization of elution conditions.
Sample buffer with or without rheumatoid factor (RF) absorbent (both reagents were supplied with the kit), 1 to 10% dry milk powder (blotting grade, nonfat dry milk; Bio-Rad, Hercules, Calif.) diluted in RF absorbent or phosphate-buffered saline-Tween 20 (PBST) (0.5%), undiluted RF absorbent, and PBST (0.5%) alone were compared as elution buffers.
Disks of 6 mm in diameter with DVB were cut with a metal paper hole punch and placed into microplate wells. Different elution dilutions were investigated in conjunction with final sample dilutions under the assumption that a 6-mm disk contains the equivalent of approximately 5 μl of serum (14). To ensure thorough “wetting” of the DVB samples, the plate was agitated for 30 min at RT, after which the plate was sealed and incubated overnight at 4°C.
Optimization of EIA conditions.
Serum and DVB samples were tested by the Dade Behring Enzygnost Anti-Measles-Virus/IgM immunoassay for measles virus-specific IgM. Serum samples were processed and validated according to the manufacturer’s instructions with an automated enzyme-linked immunosorbent assay processor (ETI-LAB; Sorin Biomedica). All serum and DVB samples with equivocal results were retested individually, and the result of the repeat test was recorded.
DVB samples were manually tested independently of the serum samples, and the ODs for the DVB samples were compared to the ODs for the corresponding serum samples, determined during the initial investigation of the rash illness.
Conditions investigated for adaptation of the assay to the testing of DVB samples included sample incubation time (1 or 1.5 h), final sample dilution (1:23 or 1:46), plate incubation (still or shaking), and wash conditions (three to six washes with PBST [0.05%]). ODs were determined at 450 nm (reference wavelength, 620 nm; Multiscan Ascent plate reader; Labysystems).
Data analysis.
Data were analyzed with Stata Statistical Software (release 6.0, 1999; Stata Corporation, College Station, Tex.). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated by comparison of the ODs for the DVB sample to the ODs for the serum samples. The cutoff and validity requirements for each plate were the same for the serum and DVB samples and were as recommended in the assay kit protocol. The IgM-positive control provided with the kit, in addition to known IgM-negative and -positive DVB samples, was used to calculate coefficients of variation (CVs) to assess interassay variations. The kappa and R2 statistics were calculated to measure the level of agreement between the results for the two sample types. Samples were categorized by the duration of DVB sample storage and were aggregated into collections of samples from 6-month blocks. For each of these blocks, R2 was determined by analysis of covariance by regression of the OD for the DVB sample on the OD for the serum sample, with the regression lines constrained to pass through the origin. The dependence of the regression coefficient (slope) on storage time was assessed by including a term for the interaction of storage >6 months with the OD for the serum sample.
RESULTS
Table 1 describes the laboratory and epidemiological investigations undertaken at the time of notification of all serum samples and corresponding DVB samples. Two samples tested within 6 months of storage had discordant results; one consisted of a serum sample from a measles-mumps-rubella vaccine recipient negative for IgM, and the other was a DVB sample from a patient with a borderline equivocal result (OD = 0.102), not consistent with either recent infection or vaccination.
TABLE 1.
Anti-measles virus IgM results for serum and DVB samples collected as part of enhanced measles surveillance
| Patient condition | No. (%) of samples IgM positive
|
||
|---|---|---|---|
| Total | Serum samples | DVB samples | |
| Measles | |||
| Clinical infection | 45 | 45 (100) | 42 (93)a |
| Receipt of measles-mumps-rubella vaccine within past 45 days | 5 | 4 (80)b | 4 (80%)b |
| Total | 50 | 49 (98) | 46 (92) |
| Undiagnosed rash illness | 148 | 2 (1.4)c | 2 (1.4)d |
| Parvovirus type B19 infection | 12 | 0 | 0 |
| Rubella virus infection | 6 | 0 | 0 |
| Total | 216 | 51 (23.6) | 48 (22.2) |
Three DVB samples had false-negative results; all samples had been stored for >6 months before testing.
Measles virus-specific IgM failed to be detected in serum and DVB samples from one of five vaccine recipients. Only three of five vaccine recipients had concordant measles virus-specific IgM-positive results.
Two serum samples had false-positive results; the patients who provided the samples were tested for immune status but had no clinical symptoms; the serum sample from patient 1 was rubella and measles virus IgM positive and the patient’s DVB sample was measles virus IgM positive; the serum sample from patient 2 was measles virus IgM positive, and the patient’s DVB sample was measles virus IgM negative.
Two DVB samples had false-positive results. The results for patient 1 were as described in footnote c for patient 1; the serum sample from patient 2 was measles virus IgM negative, and the DVB sample had a borderline equivocal result for IgM (OD = 0.102; the DVB sample had been stored for <6 months before testing).
The results for 23% (50 of 216) of all samples collected were clinically and epidemiologically consistent with recent measles virus infection or vaccination.
Elution conditions.
Spiked DVB samples were used to determine the optimal elution conditions (data not shown). Elution with 5% dry milk powder-PBST (0.5%) allowed prolonged incubation of the eluted specimen and antigen, increasing the rate of detection of low levels of IgM without increased reactivity in the control antigen well. Elution in buffer without dry milk powder resulted in increased control antigen reactivity (data not shown). Optimal elution was achieved by the addition of 220 μl of 5% dry milk-PBST (0.5%) to two DVB sample disks. Since each 6-mm disk contained approximately 5 μl of serum, this represented a 1:23 dilution of the sample.
EIA conditions.
Optimal assay conditions were achieved after overnight elution at 4°C of the DVB samples in the microtiter plate, followed by further agitation the next day for 15 min at RT. The plate was then centrifuged (2,200 × g; 15 min) before 170 μl of eluate was incubated with 170 μl of RF absorbent, prepared as specified by the manufacturer, and absorbed by agitation for 15 to 30 min at RT. The final sample dilution was 1:46.
Once absorbed, 150 μl of the eluate-RF mixture were added to each control and antigen well and the assay was completed in accordance with the manufacturer’s protocol, with minor modifications. The incubation times for both the sample and the conjugate were increased to 1.5 h, and the plate was washed five times (Wellwash Ascent plate washer; Labysystems) at each wash step with PBST (0.05%). The incubation that occurred while the plate was being agitated increased the reactivities in the control wells (data not shown).
The IgM-positive control (P/P) provided with the kit was included on every assay plate to ensure the validity of the results for the plate, in accordance with the manufacturer’s requirements. The CV for the P/P was 8.1% (n = 7). In addition, known positive and negative DVB samples were included on each test plate, and the CVs for these samples were 6.0 and 20.1%, respectively (data not shown). These results indicate acceptable interassay variations, particularly when the results for the P/P and DVB positive control samples are considered.
Comparison of serum and DVB samples.
The sensitivity and specificity of the Dade Behring Enzygnost Anti-Measles-Virus/IgM, as reported by the manufacturers, are each 100% when serum specimens are tested for measles virus-specific IgM. The sensitivity, specificity, PPV, NPV, and kappa statistics for the DVB samples compared to the results for the serum samples are reported in Table 2. Since positive and equivocal results are considered suggestive of clinical infection with measles virus, DVB samples stored for 12 to 17 months appear to perform optimally with regard to sensitivity and specificity. However, an increase in the number of DVB samples with equivocal results compared to the number of paired serum samples with equivocal results was observed in all groups of DVB samples stored for >6 months (data not shown). The sensitivity was 100% and the kappa value was 0.93 for samples tested within 6 months of collection, whereas the values were 90.3% and 0.89, respectively, for samples stored for 6 to 24 months, suggesting that long-term storage at 4°C may affect the stability of the antibody.
TABLE 2.
Sensitivities, specificities, PPVs, and NPVs, of Dade Behring Enzygnost Anti-Measles-Virus/IgM immunoassay for DVB samples relative to the results for serum samples
| Sample storage time (mo) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Kappa value (% agreement) |
|---|---|---|---|---|---|
| All samples | 90.2 | 98.8 | 95.8 | 97.0 | 0.91 (96.7) |
| <6 (n = 88) | 100 | 97.1 | 90.0 | 100 | 0.93 (97.7) |
| 6–11 (n = 47) | 80.0 | 100 | 100 | 94.8 | 0.86 (95.7) |
| 12–17 (n = 42) | 100 | 100 | 100 | 100 | 1.00 (100) |
| 18–23 (n = 39) | 81.3 | 100 | 100 | 88.5 | 0.84 (92.3) |
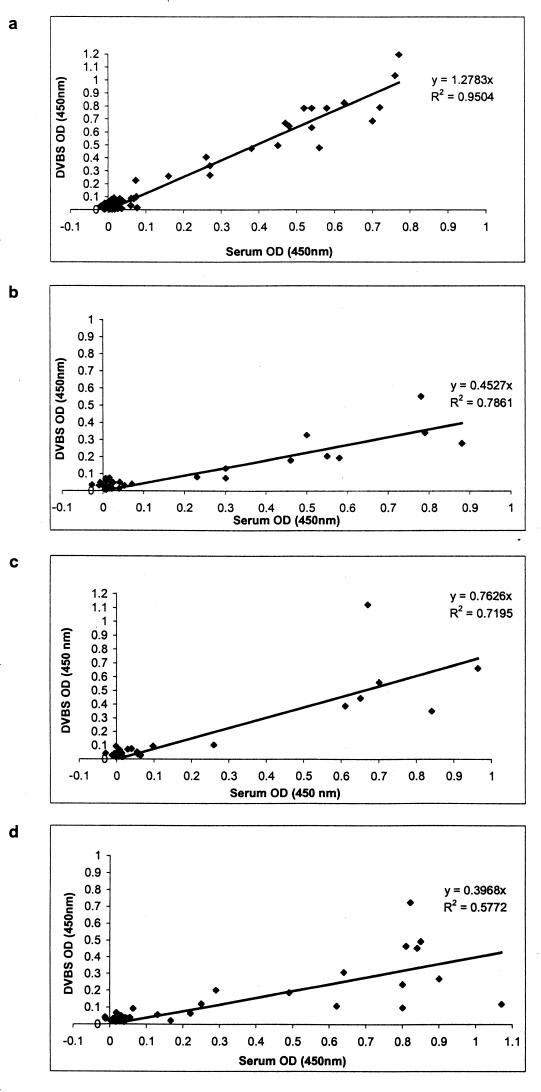
Figure 1 demonstrates the diminishing correlation between the ODs for serum and DVB samples over time. Analysis of covariance indicated a significant difference in the slopes of the regression lines (1.28 versus 0.50) when the results for samples tested within 6 months of collection were compared with those for samples tested after longer storage times (95% confidence interval for difference, −0.87, −0.69; P < 0.001).
FIG. 1.
ODs at 450 nm for serum samples and corresponding DVB samples (DVBS) according to time stored at 4°C before testing by the Dade Behring Enzygnost Anti-Measles-Virus/IgM EIA. Results are for DVB samples stored for <6 months (a), 6 to 11 months (b), 12 to 17 months (c), and 18 to 24 months (d). Serum samples were generally tested within 48 h of collection. Assay cutoff and plate validity requirements were as specified by the manufacturer for both serum samples and DVBS.
The slope of the regression line for samples stored for less than 6 months indicates that the ODs for the DVB samples were higher than those for the corresponding serum samples. However, this did not result in an increase in the number of samples with false-positive reactivities (Table 1).
DISCUSSION
Confirmation of all cases of suspected measles by a measles virus-specific IgM assay is recommended, especially where the incidence of measles is low (7, 13). It has previously been demonstrated that dried blood spots are a feasible alternative to venous blood as a specimen for use in investigations for measles virus antibodies (2, 3, 5, 10). The present study demonstrates for the first time the feasibility of laboratory diagnosis of measles by use of dried blood specimens with a commercial assay.
The sensitivity of the Dade Behring Enzygnost Anti-Measles-Virus/IgM EIA when it was used to test DVB samples within 6 months of collection was the same as that reported by the manufacturer when it is used to test serum samples. From the PPV and NPV of the assay for the DVB samples tested in the present study, it is apparent that DVB samples could serve as acceptable alternatives to venous blood samples for measles virus IgM serology. Testing of DVB samples stored at 4°C for periods longer than 6 months is likely to be less reliable, and testing of such samples so long after collection would be inappropriate in clinical practice. Other studies have demonstrated the stability of both antigen and antibodies in whole blood collected onto filter paper for up to 2 weeks or longer (1, 3).
In the present study, DVB samples were produced from venous blood for the comfort and convenience of patients, but in clinical practice it is likely that dried blood spots would be capillary samples. Several groups have demonstrated a good correlation between serum and peripheral (finger or heel) blood for determination of measles virus antibody titers (2, 3, 10, 15). Our results should therefore be equally applicable to capillary blood specimens.
The use of DVB samples for the diagnosis of measles virus infections has the potential to overcome the incomplete investigation of suspected cases of measles (4). This is especially an issue in the developing world and will become more problematic as the transmission of measles is interrupted in these areas and laboratory confirmation of all potential cases of measles is attempted. DVB samples can be collected in a clinic or in the field, and the samples can easily be transported to the laboratory. The use of a commercial assay allows testing of DVB samples to be performed at the local laboratory level, without the need for referral to a specialized laboratory that uses in-house assays. Further development of DVB samples for use in the investigation of other febrile rash diseases, such as rubella virus and parvovirus type B19 infections, will augment the laboratory surveillance of measles virus infection.
Acknowledgments
We thank David Anderson and Stephen Wesselingh for critical reading of the manuscript, Graham Byrnes for statistical advice, the Serology Laboratory staff at VIDRL for testing of serum specimens, and Debbie Gercovich and the Victorian Department of Human Services for collection of dried blood spot specimens.
Dade Behring provided IgM kits to VIDRL for a minimal handling fee. M.A.R. received a National Health and Medical Research Council Ph.D. Public Health Research Scholarship.
REFERENCES
- 1.Behets, F., M. Kashamuka, M. Pappaioanou, T. A. Green, R. W. Ryder, V. Batter, J. R. George, W. H. Hannon, and T. C. Quinn. 1992. Stability of human immunodeficiency virus type 1 antibodies in whole blood dried on filter paper and stored under various tropical conditions in Kinshasa, Zaire. J. Clin. Microbiol. 30:1179–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Condorelli, F., G. Scalia, A. Stivala, R. Gallo, A. Marino, C. M. Battaglini, and A. Castro. 1994. Detection of immunoglobulin G to measles virus, rubella virus, and mumps virus in serum samples and in microquantities of whole blood dried on filter paper. J. Virol. Methods 49:25–36 [DOI] [PubMed] [Google Scholar]
- 3.De Swart, R. L., Y. Nur, A. Abdallah, H. Kruining, H. S. El Mubarak, S. A. Ibrahim, B. Van Den Hoogen, J. Groen, and A. D. Osterhaus. 2001. Combination of reverse transcriptase PCR analysis and immunoglobulin M detection on filter paper blood samples allows diagnostic and epidemiological studies of measles. J. Clin. Microbiol. 39:270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Expanded Programme on Immunization. 1996. Meeting on advances in measles elimination: conclusions and recommendations. Wkly. Epidemiol. Rec. 71:305–309 [PubMed] [Google Scholar]
- 5.Grandolfo, M. E., E. Medda, F. Novello, and B. Ridolfi. 1998. Seroepidemiological evaluation of 1989–91 mass vaccination campaigns against measles, in Italy. Epidemiol. Infect. 121:645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly, H. A., M. A. Riddell, S. B. Lambert, J. A. Leydon, and M. G. Catton. 2001. Measles immunity among young adults in Victoria. Commun. Dis. Intell. 25:129–132 [PubMed] [Google Scholar]
- 7.Lambert, S. B., H. A. Kelly, R. M. Andrews, M. C. Catton, P. A. Lynch, J. A. Leydon, D. K. Gercovich, G. G. Hogg, M. L. Morgan, and R. A. Lester. 2000. Enhanced measles surveillance during an interepidemic period in Victoria. Med. J. Aust. 172:114–118 [DOI] [PubMed] [Google Scholar]
- 8.Meredith, N., and W. Hannon. 1993. Preparation of dried blood spot materials for quality assurance of assays for antibodies to human immunodeficiency virus, p.225–241. In B. J. Therrell (ed.), Laboratory methods for neonatal screening. American Public Health Association, Washington, D.C.
- 9.Nakano, J. H., D. L. Miller, S. O. Foster, and E. W. Brink. 1983. Microtiter determination of measles hemagglutination inhibition antibody with filter papers. J. Clin. Microbiol. 17:860–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novello, F., B. Ridolfi, L. Fiore, G. Buttinelli, E. Medda, A. Favero, D. Marchetti, and F. Gaglioppa. 1996. Comparison of capillary blood versus venous blood samples in the assessment of immunity to measles. J. Virol. Methods 61:73–77 [DOI] [PubMed] [Google Scholar]
- 11.Ratnam, S., V. Gadag, R. West, J. Burris, E. Oates, F. Stead, and N. Bouilianne. 1995. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J. Clin. Microbiol. 33:811–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratnam, S., G. Tipples, C. Head, M. Fauvel, M. Fearon, and B. J. Ward. 2000. Performance of indirect immunoglobulin M (IgM) serology tests and IgM capture assays for laboratory diagnosis of measles. J. Clin. Microbiol. 38:99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryall, L., C. Goodbourn, A. Taylor, and L. Teare. 1996. Laboratories should use serum IgM tests to confirm measles. BMJ 312:975–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steger, K. A., D. E. Craven, B. F. Shea, B. R. Fitzgerald, M. Schwerzler, and G. R. D. H. R. Seage. 1990. Use of paper-absorbed fingerstick blood samples for studies of antibody to human immunodeficiency virus type 1 in intravenous drug users. J. Infect. Dis. 162:964–967 [DOI] [PubMed] [Google Scholar]
- 15.Wassilak, S. G., R. H. Bernier, K. L. Herrmann, W. A. Orenstein, K. J. Bart, and R. Amler. 1984. Measles seroconfirmation using dried capillary blood specimens in filter paper. Pediatr. Infect. Dis. 3:117–121. [DOI] [PubMed] [Google Scholar]