Abstract
Virulence characteristics of diarrheal outbreak-associated Escherichia coli O55:NM, O126:NM, and O111:NM were examined. The E. coli O55:NM strains were atypical enteropathogenic E. coli (EPEC), while the E. coli O126:NM and O111:NM strains should be classified as enteroaggregative E. coli (EAggEC). The contributions of EPEC and EAggEC to the human disease burden in Japan might be significantly greater than is currently appreciated.
There are six categories of Escherichia coli that cause diarrhea: enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli, enteroaggregative E. coli (EAggEC), enteroinvasive E. coli, and diffusely adherent E. coli (21). EPEC causes characteristic attaching-and-effacing lesions (A/E), which can be observed by intestinal biopsy in both human patient (19) and animal (29) models. A/E is characterized by loss of microvilli, intimate adherence of bacteria between epithelial cell membranes (27, 30), and cytoskeletal changes such as actin polymerization directly beneath the adherent bacteria (15). Generally, EPEC causes infantile diarrhea in developing countries and sporadic diarrhea in developed countries (21). EAggEC, on the other hand, is an enteric pathogen defined by its distinctive aggregative or “stacked-brick” pattern of adherence to cultured human epithelial cells (22). EAggEC associates mainly with persistent diarrhea in developing countries (21). Only two reports in Japan have described diarrheal outbreaks caused by EAggEC or EPEC. Itoh et al. (11) reported the isolation of EAggEC from the stools of patients with severe diarrhea in elementary and junior high schools. Makino et al. (18) reported the isolation of EPEC from a mass outbreak. In this paper, we describe three cases of diarrheal outbreaks in Japan caused by E. coli belonging to the traditional EPEC serotype.
Chromosomal DNA-embedded agarose plugs for pulsed-field gel electrophoresis (PFGE) analysis were prepared by using the CHEF Bacterial DNA Plug Kit (Bio-Rad, Hercules, Calif.) and were digested with XbaI (Nippon gene; Osaka, Japan) at a concentration of 30 U/plug for 4 h at 37°C. The plugs were applied to a 1% PFC Grade Agarose (Bio-Rad) gel. Electrophoresis was performed in 0.5× Tris-Borate EDTA buffer at 14°C using a CHEF DR-II PFGE apparatus (Bio-Rad) under the following conditions: voltage, 6 V/cm; block 1, 11 h, with initial switching time of 4 s to final switching time of 8 s; block 2, 9 h, with initial switching time of 8 s to final switching time of 50 s. The HEp-2 cell assay was performed following the method described by Craviotto et al. (4), with modifications involving 3 or 6 h of incubation (15). The E. coli isolates were examined for the presence of the following virulence genes by PCR: stx1 (Shiga toxin) and stx2 (16), eaeA (E. coli attaching and effacing) (12), bfpA (bundle-forming pilus) (9), perA (EPEC plasmid-encoded regulatory region) (8), astA (EAggEC heat-stable enterotoxin) (28), aggR (transcriptional activator for EAggEC aggregative adherence fimbria I expression) (20), and pet (EAggEC plasmid-encoded heat-labile toxin) (6) using the primers listed in Table 1. EPEC E2348/69 and EAggEC 17-2 were kindly provided by James B. Kaper, and EAggEC 042 was kindly provided by James P. Nataro, University of Maryland School of Medicine, Baltimore, Md.
TABLE 1.
PCR primers used in this study
| Designation | Location | Sequence (5′ to 3′) | Target gene | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|
| V1 | 213–230 | AGT-TAA-TGT-GGT-GGC-GAA | |||
| V5 | 1013–1029 | GAC-TCT-TCC-ATC-TGC-CG | stx1 | 817 | 16 |
| V3 | 289–306 | TTC-GGT-ATC-CTA-TTC-CCG | |||
| V4 | 745–762 | TCT-CTG-GTC-ATT-GTA-TTA | stx2 | 474 | 16 |
| EA-1 | 1846–1865 | AAA-CAG-GTG-AAA-CTG-TTG-CC | |||
| EA-2 | 2280–2299 | CTC-TGC-AGA-TTA-ACC-TCT-GC | eaeA | 454 | This study |
| EP-1 | 2773–2793 | AAT-GGT-GCT-TGC-GCT-TGC-TGC | |||
| EP-2 | 3076–3096 | GCC-GCT-TTA-TCC-AAC-CTG-GTA | bfpA | 324 | 9 |
| PerAS | 522–541 | TGT-CAT-CCT-TAG-TGC-TTC-AT | |||
| PerAAS | 856–875 | GGC-AAT-GTT-CCT-TGT-GTA-AT | perA | 354 | This study |
| EAST-1S | 63–82 | GCC-ATC-AAC-ACA-GTA-TAT-CC | |||
| EAST-1AS | 149–168 | GAG-TGA-CGG-CTT-TGT-AGT-CC | astA | 106 | This study |
| AggRks1 | 100–120 | GTA-TAC-ACA-AAA-GAA-GGA-AGC | |||
| AggRkas2 | 353–334 | ACA-GAA-TCG-TCA-GCA-TCA-GC | aggR | 254 | 26 |
| PetS | 557–576 | TCA-TTT-CCA-GCA-CTT-CCT-GT | |||
| PetAS | 979–998 | CTC-CGA-CAG-TAT-TTG-CTC-GT | pet | 442 | This study |
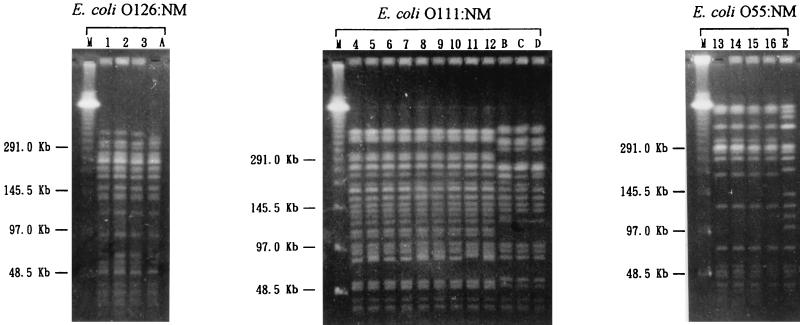
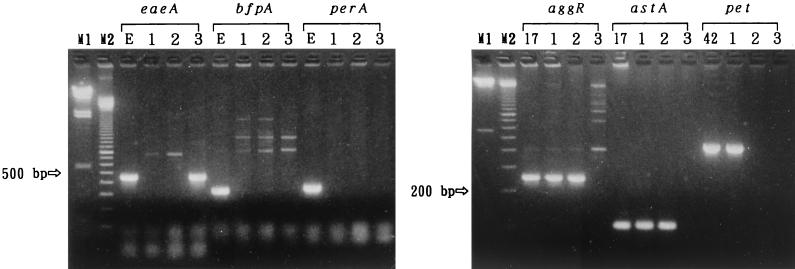
The diarrheal patients were junior high school students in case 1, adults who attended a party in case 2, and infants of a day care center in case 3. The only diarrheagenic bacterial pathogens isolated from the patients were three E. coli O126:NM isolates from four of nine patients in case 1, nine E. coli O111:NM isolates from 9 of 21 patients in case 2, and four E. coli O55:NM isolates from four of four patients in case 3. As shown in Fig. 1, E. coli strains isolated within the same case showed identical PFGE patterns, suggesting that the strains originated from the common infectious sources in the respective cases. These results indicated that these E. coli strains were the causative agents of the diarrheal outbreak cases. As shown in Fig. 2, E. coli O55:NM possessed eaeA and showed a localized HEp-2 cell adherence pattern only in the 6-h assays but was negative for bfpA and perA, indicating that E. coli O55:NM is an atypical EPEC. The E. coli O55:NM isolate was negative for aggR, astA, and pet. On the other hand, both E. coli O126:NM and O111:NM strains were negative for the eaeA, bfpA, and perA genes but positive for the aggR and astA genes and showed an aggregative HEp-2 cell adherence pattern, indicating that they should be classified as EAggEC, displaying features of the traditional EPEC serotypes. The E. coli O126:NM was pet positive, while E. coli O111:NM was negative for this gene. All three E. coli isolates were negative for both stx1 and stx2 genes.
FIG. 1.
PFGE patterns of the diarrheal isolates. Lanes: M, lambda molecular weight ladder; 1 to 3 (case 1, E. coli O126:NM); 4 to 12 (case 2, O111:NM isolates); 13 to 16 (case 3, O55:NM isolates); A to E (sporadic isolates of respective organisms).
FIG. 2.
Agarose gel electrophoresis of the PCR products of E. coli virulence genes showing virulence traits of the E. coli isolates from three outbreak cases. Lanes: M1, lambda/HindIII molecular weight marker; M2, 100-bp-ladder molecular weight marker; 1, E. coli O126:NM EC-152 from case 1; 2, E. coli O111:NM EC-560 from case 2; 3, E. coli O55:NM EC-1045 from case 3; E, EPEC E2348/69; 17, EAggEC 17-2; 42, EAggEC 042.
At the Second International Symposium on EPEC (13), a consensus on the basic characteristics of EPEC infection was reached, identifying them as the presence of A/E histopathology and the absence of Shiga toxin. The A/E phenotype is closely related to the localized adherence phenomenon displayed by EPEC (14). DNA probes and PCR primers have been developed and used for the evaluation of the three major characteristics of EPEC: A/E (12), the presence of a ca. 60-MDa plasmid designated EPEC adherence factor plasmid (EAF) (23), and lack of Shiga toxin (16). Some EPEC strains possess EAF-encoding bundle-forming pilus (BFP) (5). Typical EPEC strains possess the eaeA for A/E and the EAF or bfpA, while atypical EPEC strains possess the eaeA gene only, and there is some controversy over whether atypical EPEC strains are true diarrheagenic pathogens (13). On the other hand, EAggEC infection is diagnosed definitively by isolation from the stools of patients of E. coli showing the aggregative HEp-2 cell adherence pattern (21). EAggEC strains possess the aggA gene that encodes the aggregative adherent fimbria I (AAF/I) protein (24), the aggR gene for transcriptional activation of AAF/I expression (20), and the astA gene that encodes the enteroaggregative E. coli heat-stable enterotoxin I protein (28). In this study, we examined E. coli isolated from patients with diarrhea from three outbreak cases. Serotyping of E. coli isolates showed the pathogenic strains to be O55:NM, O111:NM, and O126:NM, representing traditional EPEC serotypes. Based on phenotypic and genotypic tests, the O55:NM strain was identified as an atypical EPEC; it showed localized adherence and possessed the eaeA without the EAF. However, E. coli isolates from the other two cases were identified as EAggEC, because they showed aggregative adherence and possessed astA and aggR but not eaeA or EAF. In general, EPEC infection is primarily a disease of infants younger than two years old (17), and EAggEC is associated with persistent diarrhea (1). Several outbreaks of diarrhea due to EPEC have been reported in the United States, the United Kingdom, Finland, and other developed countries. These outbreaks frequently occur in day care centers (2, 25) and occasionally occur in pediatric wards (10). However, reports of outbreaks due to atypical EPEC are infrequent. Recently, Hedberg et al. (10) reported an outbreak caused by an atypical EPEC among adults who ate at a gourmet buffet in the United States. This atypical EPEC strain was unique, because its serotype was O39:NM, which did not belong to any of the traditional EPEC serotypes, and it was positive for astA along with eaeA but negative for the EAF. Our present data, along with Hedberg’s observation, suggest that atypical EPEC is a diarrheic pathogen. Furthermore, the number of reports describing outbreaks due to EAggEC is increasing (3, 7). In Japan, there are only two reports describing outbreaks of diarrhea involving EPEC and EAggEC (11, 18). Based on our cases and the two reports just mentioned, the contribution of EPEC and EAggEC to the human disease burden in Japan might be significantly greater than is currently appreciated.
REFERENCES
- 1.Bahn, M. K., P. Raj, M. M. Levine, J. B. Kaper, N. Bhandari, R. Srivastava, R. Kumar, and S. Sazawal. 1989. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J. Infect. Dis. 159:1061–1064. [DOI] [PubMed] [Google Scholar]
- 2.Bower, J. R., B. L. Congeni, T. G. Cleary, R. T. Stone, A. Wanger, B. E. Murray, J. J. Mathewson, and L. K. Pickering. 1989. Escherichia coli O114:nonmotile as a pathogen in an outbreak of severe diarrhea associated with a day care center. J. Infect. Dis. 160:243–247. [DOI] [PubMed] [Google Scholar]
- 3.Cobeljic, M., B. Miljkovic-Selimovic, D. Paunovic-Todosijevic, Z. Velickovic, Z. Lepsanovic, D. Savic, R. Ilic, S. Konstantinovic, B. Jovanovic, and V. Kostic. 1996. Enteroaggregative Escherichia coli associated with an outbreak of diarrhoea in a neonatal nursery ward. Epidemiol. Infect. 117:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craviotto, A., R. J. Gross, S. M. Scotland, and B. Rowe. 1979. An adhesive factor found in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Curr. Microbiol. 3:95–99. [Google Scholar]
- 5.Donnenberg, M. S., J. A. Girron, J. P. Nataro, and J. B. Kaper. 1992. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 6:3427–3437. [DOI] [PubMed] [Google Scholar]
- 6.Eslava, C., F. Navarro-Garcia, J. R. Czeczulin, I. R. Henderson, A. Craviotto, and J. P. Nataro. 1998. Pet, as autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect. Immun. 66:3155–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giron, J. A., F. Qadri, K. J. Jarvis, J. B. Kaper, and M. J. Albert. 1995. Monoclonal antibodies specific for the bundle-forming pilus of enteropathogenic Escherichia coli. Infect. Immun. 63:4949–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunzberg, S. T., N. G. Tornieporth, and L. W. Riley. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedberg, C. W., S. J. Savarino, J. M. Besser, C. J. Paulus, V. M. Thelen, L. J. Myers, D. N. Cameron, T. J. Barrett, J. B. Kaper, and M. T. Osterholm. 1997. An outbreak of foodborne illness caused by Escherichia coli O39:NM: an agent that does not fit into the existing scheme for classifying diarrheagenic E. coli. J. Infect. Dis. 176:1625–1628. [DOI] [PubMed] [Google Scholar]
- 11.Itoh, Y., I. Nagano, M. Kunishima, and T. Ezaki. 1997. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J. Clin. Microbiol. 35:2546–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaper, J. B. 1996. Defining EPEC. Rev. Microbiol. Sao Paulo 27:130–133. [Google Scholar]
- 14.Knutton, S., A. D. Phillips, H. R. Smith, R. J. Gross, R. Shaw, P. Watson, and E. Price. 1991. Screening for enteropathogenic Escherichia coli in infants with diarrhea by the fluorescent-actin staining test. Infect. Immun. 59:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knutton, S., P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic Escherichia coli. Infect. Immun. 57:1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi, K. 1991. Detection of enterohemorrhagic Escherichia coli using PCR. Rinsyo to Biseibutsu. 18:507–513. (In Japanese.) [Google Scholar]
- 17.Levine, M. M., and R. Edelman. 1984. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol. Rev. 6:31–51. [DOI] [PubMed] [Google Scholar]
- 18.Makino, S., H. Asakura, T. Shirahata, T. Ikeda, K. Takeshi, K. Arai, M. Nagasawa, T. Abe, and T. Sadamoto. 1999. Molecular epidemiological study of a mass outbreak caused by enteropathogenic Escherichia coli O157:H45. Microbiol. Immunol. 43:381–384. [DOI] [PubMed] [Google Scholar]
- 19.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nataro, J. P., D. Yikang, D. Yingkang, and K. Walker. 1994. aggR, transcriptional activator of aggregative adherence fimbria I expression in enteroaggregative Escherichia coli. J. Bacteriol. 176:4691–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nataro, J. P., J. B. Kaper, R. Robins-Browne, V. Prado, P. Vial, and M. M. Levine. 1987. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr. Infect. Dis. J. 6:829–831. [DOI] [PubMed] [Google Scholar]
- 23.Nataro, J. P., M. M. Baldini, J. B. Kaper, R. E. Black, N. Bravo, and M. M. Levine. 1985. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J. Infect. Dis. 152:560–565. [DOI] [PubMed] [Google Scholar]
- 24.Nataro, J. P., Y. Deng, D. R. Maneval, A. L. German, W. C. Martin, and M. M. Levine. 1992. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect. Immun. 60:2297–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulozzi, L. J., K. E. Johnson, L. M. Kamahere, C. R. Clausen, L. W. Riley, and S. D. Helgerson. 1986. Diarrhea associated with adherent enteropathogenic Escherichia coli in an infant and toddler center, Seattle, Washington. Pediatrics 77:296–300. [PubMed] [Google Scholar]
- 26.Ratchtrachenchai, O. A., S. Subpasu, and K. Ito. 1997. Investigation on enteroaggregative Escherichia coli infection by multiplex PCR. Bull. Dept. Med. Sci. 39:211–220. [Google Scholar]
- 27.Rothbaum, R., A. J. McAdams, R. Giannella, and J. C. Partin. 1982. A clinicopathological study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology 83:441–454. [PubMed] [Google Scholar]
- 28.Savarino, S. J., A. Fasano, J. Watson, B. M. Martin, M. M. Levine, S. Guandalini, and P. Guerry. 1993. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc. Natl. Acad. Sci. USA 90:3093–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor, C. J., A. Hart, R. M. Batt, C. McDougall, and L. McLean. 1986. Ultrastructural and biochemical changes in human jejunal mucosa associated with enteropathogenic Escherichia coli (O111) infection. J. Pediatr. Gastroenterol. Nutr. 5:70–73. [DOI] [PubMed] [Google Scholar]
- 30.Ulshen, M. H., and J. L. Rallo. 1980. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N. Engl. J. Med. 302:99–101. [DOI] [PubMed] [Google Scholar]