Abstract
The virulence of yersiniae is promoted in part by shared ≈70-kb plasmids (pCD in Yersinia pestis and pYV in enteropathogenic Yersinia pseudotuberculosis and Yersinia enterocolitica) that mediate a low-calcium response. This phenotype is characterized at 37°C by either bacteriostasis in Ca2+-deficient medium with expression of pCD/pYV-encoded virulence effectors (Yops and LcrV) or vegetative growth and repression of Yops and LcrV with ≥2.5 mM Ca2+ (Lcr+). Regulation of Yops and LcrV is well defined but little is known about bacteriostasis other than that Na+ plus l-glutamate promotes prompt restriction of Y. pestis. As shown here, l-aspartate substituted for l-glutamate in this context but only Na+ exacerbated the nutritional requirement for Ca2+. Bacteriostasis of Y. pestis (but not enteropathogenic yersiniae) was abrupt in Ca2+-deficient medium at neutral to slightly alkaline pH (7.0 to 8.0), although increasing the pH to 8.5 or 9.0, especially with added Na+ (but not l-glutamate), facilitated full-scale growth. Added l-glutamate (but not Na+) favored Ca2+-independent growth at acidic pH (5.0 to 6.5). Yops and LcrV were produced in Ca2+-deficient media at pH 6.5 to 9.0 regardless of the presence of added Na+ or l-glutamate, although their expression at alkaline pH was minimal. Resting Ca2+-starved Lcr+ cells of Y. pestis supplied with l-glutamate first excreted and then destroyed l-aspartate. These findings indicate that expression of Yops and LcrV is necessary but not sufficient for bacteriostasis of Ca2+-starved yersiniae and suggest that abrupt restriction of Y. pestis requires Na+ and the known absence of aspartate ammonia-lyase in this species.
The ≈70-kb plasmid termed pCD in Yersinia pestis, the causative agent of bubonic plague, and pYV in enteropathogenic Yersinia pseudotuberculosis and Yersinia enterocolitica encodes a type III secretion system (TTSS) capable of injecting cytotoxic yersinial outer proteins (Yops) directly into the host cell cytoplasm and secreting the immunomodulator LcrV directly into supernatant fluids (5, 17). Expression of the TTSS is mediated at 37°C by melting (54) a structural gene present on pCD/pYV encoding a transcriptional activator designated VirF in Y. enterocolitica (40) and LcrF in Y. pseudotuberculosis (58) and Y. pestis (34). Carriage of pCD/pYV confers a low-calcium response (LCR) in vitro at 37°C, characterized as the ability to undergo either vegetative growth without expression of the TTSS upon addition of Ca2+ or bacteriostasis in its absence coupled with synthesis of VirF/LcrF-activated functions (Lcr+) (10, 29, 53, 61). However, Ca2+ does not downregulate Yops in tissue culture, where their expression occurs following attachment of yersiniae to both professional and nonprofessional phagocytes (24, 27, 52). The mechanism accounting for the ability of host cells to upregulate the TTSS of docked yersiniae is not fully defined but may involve competition between Ca2+ and a host cell receptor for a bacterial outer membrane sensor (17).
Although Y. pestis and the enteropathogenic yersiniae share the pCD/pYV-encoded TTSS, the typically chronic diseases caused by Y. pseudotuberculosis and Y. enterocolitica are radically different from plague. This distinction is due in part to carriage by Y. pestis of a unique ≈10-kb plasmid termed pPCP (22) encoding a plasminogen activator (Pla) that facilitates bacterial metastasis (39), thereby accounting for prompt colonization of visceral organs following subcutaneous infection (8, 60). A second ≈100-kb plasmid termed pMT, encoding capsular antigen fraction 1 and murine toxin (38), is also specific to Y. pestis (22). However, these activities are not essential for virulence (12, 33, 64) and thus the primary role of pMT is to facilitate colonization of the flea (32). Transformation of pPCP into Y. pseudotuberculosis did not significantly enhance its dissemination in mice (37), suggesting that this enteropathogen possesses factors that curtail acute disease.
Despite the very recent evolution of Y. pestis from Y. pseudotuberculosis (1), comparison of the two species by DNA microarray analysis revealed the existence of 11 loci containing numerous Y. pestis-specific chromosomal genes (31). However, subsequent direct annotation of the two genomes using additional isolates of Y. pseudotuberculosis decreased the number of such plague-specific genes to 25, the bulk of which are unidentified open reading frames (16). Analysis of more strains of Y. pseudotuberculosis (especially the immediate progenitor of the plague bacillus) could further reduce this number, perhaps to zero. In this case, the remarkable lethality of Y. pestis would obviously entail an explanation other than the action of unique chromosomal gene products.
Since its emergence from Y. pseudotuberculosis, the chromosome of Y. pestis has undergone physical rearrangements, numerous small additions and deletions, and amplification of insertion sequence elements that eliminate about 10% of total genome function (16, 20, 50). This observation is consistent with the hypothesis that enhanced virulence of Y. pestis is a function of chromosomal gene loss in addition to lateral transfer of pPCP. Mutations that prevent utilization of nutrients found only in natural environments or those accounting for specific biochemical requirements would not be expected to favor acute disease. However, plague bacilli lack the cell invasins and adhesins required by the enteropathogenic yersiniae to initiate chronic gastrointestinal disease (16, 20, 50), as would be expected if these structures hinder dissemination in tissue (5, 7). The LCR of Y. pestis is also unique in that the organisms undergo especially abrupt restriction of growth (15) and Pla is utilized to degrade undelivered Yops (37, 55, 59).
At 37°C, Ca2+-starved yersiniae cease vegetative growth but remain viable and exhibit reduced adenylate energy charge, suggesting a lesion in energy metabolism (66). Bacteriostasis is enhanced by Mg2+ (10, 30, 65), Na+ (4, 26), and l-glutamate (26) and relieved by exogenous nucleotides (65). Furthermore, upregulation of VirF/LcrF-activated functions is increased by Mg2+ (10, 41), l-glutamate (42), and fermentable carbohydrates (10). Plague bacilli, as opposed to the enteropathogenic yersiniae, lack detectable aspartate ammonia-lyase (aspartase) (21) and glucose 6-phosphate dehydrogenase (45, 46) activities and therefore possess potentially serious lesions in bioenergetics and efficient flow of metabolic carbon.
The purpose of this report is to integrate these variables by demonstrating that both of the naturally occurring dicarboxylic amino acids (l-glutamate and l-aspartate) as well as their porter (Na+) favor bacteriostasis but not expression of VirF/LcrF-activated functions, the LCR is significantly modified by initial culture pH, and the abrupt onset of bacteriostasis in Y. pestis involves uptake, conversion, and excretion of l-glutamate in the form of l-aspartate. These observations provide important insights into the LCR and may relate directly to expression of acute disease.
MATERIALS AND METHODS
Bacteria.
The Y. pestis isolate used throughout this study was an Lcr+, nonpigmented mutant (35) of strain KIM-10 (25) known to have undergone high-frequency ≈100-kb chromosomal deletion (6, 23, 43) resulting in loss of the Hms locus and high-pathogenicity island encoding the ability to synthesize the siderophore yersiniabactin (11, 51). An isogenic Lcr− mutant lacking pCD was isolated by selection on magnesium oxalate agar (30). Enteropathogenic Y. pseudotuberculosis strain PB1/+ (13), Y. enterocolitica WA (14), and their similarly selected Lcr− mutants were used in comparative experiments. Y. pseudotuberculosis PB1/+ is of serotype O:1b and thus on the direct evolutionary line leading to Y. pestis (57).
Chemically defined medium.
The basic chemically defined medium was modified from that reported previously (26) by inclusion of 40 mM potassium d-gluconate and omission, unless stated otherwise, of l-glutamate, l-aspartate, and Na+. First and second transfers prepared to inoculate third cultures for use in experiments always contained N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) at a final concentration of 25 mM and pH 7.0. HEPES was replaced in the third culture by an equimolar concentration of another Good buffer depending upon the desired initial pH. The buffers used were morpholinethanesulfonic acid (MES) for a final pH of 5.0, 5.5, and 6.0, morpholinopropanesulfonic acid (MOPS) for a final pH of 6.5, 7.0, and 7.5, Tris for a final pH of 8.0 and 8.5, and 2-(cyclohexylamino)ethane-2-sulfonic acid (CHES) for a final pH of 9.0. Solid NaCl (100 mM) and l-glutamic acid (25 mM) were added when required, and when dissolved, the pH was adjusted precisely to the desired initial value by dropwise addition of 5 M HCl or 5 M KOH. After addition of all stock solutions (26) the fully constituted medium was sterilized by filtration and dispensed aseptically.
Cultivation.
Storage of bacteria at −20°C in buffered glycerol and cultivation on solid medium for use in experiments were previously described (26). Rates of growth in chemically defined medium were determined in stoppered Erlenmeyer flasks (25 ml of medium per 250-ml flask) aerated at 200 rpm in a temperature-controlled model G76 water bath gyrotory shaker (New Brunswick Scientific Co., Inc., New Brunswick, N.J.). Multiplication was compared in cultures containing Na+ plus l-glutamate, Na+ alone, l-glutamate alone, or neither nutrient. In these determinations, the organisms underwent two subcultures at 26°C in medium identical to that used for the third culture (with respect to Na+ and l-glutamate) except, as noted above, a buffered-salt stock solution containing HEPES was used and the initial pH was always adjusted to 7.0.
The first and second cultures were inoculated at an optical density (OD) of about 0.1 and transferred during late phase at an OD of between 5 and 6. Third cultures composed of the Good buffers noted above (initial pH of 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, or 9.0) were then inoculated at an OD of about 0.125 and incubated at 37°C. Bacterial growth was determined at 620 nm with a Beckman DU spectrophotometer using 1-ml cuvettes and cultures suitably diluted in 0.033 M potassium phosphate buffer (pH 7.0) to yield an OD of between 0.05 and 0.7. The results of separate control experiments indicated that the chosen Good buffers controlled pH without significantly interacting with other ingredients of the media. The pH of individual cultures remained remarkably stable for at least 9 h of incubation.
Excretion of l-aspartate.
Lcr+ or Lcr− cells of Y. pestis were grown for two transfers at 26°C in chemically defined medium (initial pH of 7.0 using HEPES with 100 mM Na+ plus 25 mM l-glutamate) and then aerated as described above in the same medium (initial pH of 7.5 using MOPS) with or without added 4.0 mM Ca2+ (200 ml of medium per stoppered 2-liter Erlenmeyer flask). After 7 h of cultivation, the organisms were harvested by centrifugation (10,000 × g for 30 min), washed twice in excess cold potassium phosphate buffer (pH 7.0), and suspended to a concentration equivalent to 20 mg of protein per ml of distilled water. A portion (1.0 ml) of this suspension was immediately added to a 50-ml Erlenmeyer flask containing 2.0 μmol of MgCl2 and 20 μmol of sodium l-glutamate in 1.0 ml of 0.066 M potassium buffer (pH 7.0). The flask was aerated at 37°C as described above, and samples of 0.1 ml were removed at intervals, sedimented for 1 min in a model 11 Microfuge (Beckman Coulter, Inc., Fullerton, Calif.), passed through a 4-mm Acrodisc HT Tuffryn low-protein-binding filter disk (Pall Life Sciences, Ann Arbor, Mich.), and frozen until analysis. After thawing, the samples were appropriately diluted in distilled water, applied (10 μl) to Whatman no. 1 filter paper, and chromatographed with phenol-water (4:1, wt/vol, in an HCN atmosphere). The sheets were then dried, sprayed with 0.25% (wt/vol) ninhydrin in water-saturated n-butanol and, after full development of color in a water-saturated environment at 26°C, scanned and evaluated against a standard curve using the SigmaGel software program (Systat Software, Inc., Point Richmond, Calif.).
Miscellaneous.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), silver staining, and immunoblotting were performed as previously described (9, 48). Positions on stained SDS-PAGE gels corresponding to individual Yops and LcrV were characterized previously (37).
RESULTS
Comparison of the LCR.
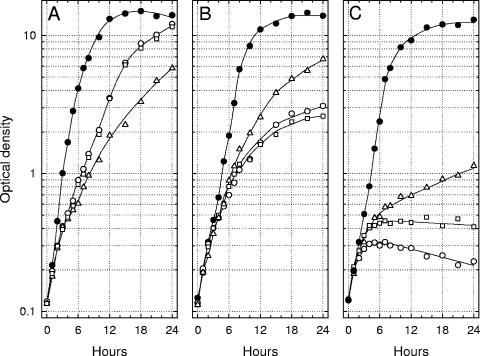
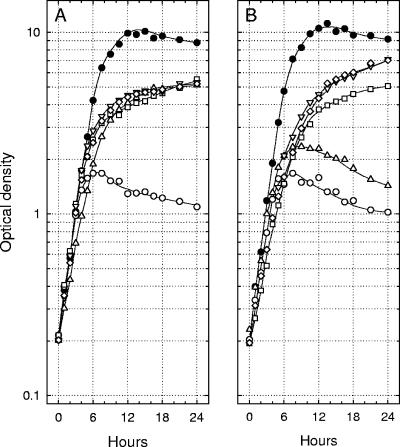
Lcr+ cells of Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis were cultured twice at 26°C in chemically defined medium containing 100 mM Na+ but lacking both added Ca2+ and l-glutamate, transferred to third cultures of the same medium either with or without Ca2+ (4.0 mM) and l-glutamate (25 mM), and aerated at 37°C. Increases in optical density were compared to those obtained in parallel third transfers of yersiniae previously grown with both Na+ and l-glutamate (Fig. 1). l-Glutamate did not influence doubling times at 37°C in the presence of Ca2+ although the terminal population of Y. pestis was slightly reduced in its absence (data not shown). The nutritional requirement of Lcr+ Y. enterocolitica for Ca2+ was minimal, and l-glutamate stimulated growth whether or not the organisms were previously adapted to this amino acid. In contrast, l-glutamate enhanced bacteriostasis of Ca2+-starved Y. pseudotuberculosis; this effect was not dependent on prior adaptation. Cells of Y. pestis exhibited an even more abrupt pattern of restriction that was also favored by l-glutamate, especially if the latter was present during prior growth at 26°C.
FIG. 1.
Growth of Yersinia enterocolitica strain WA (A), Yersinia pseudotuberculosis strain PB1/+ (B), and Yersinia pestis strain KIM (C) at 37°C in chemically defined medium containing added l-glutamate (25 mM) either with (•) or without (○) added 4.0 mM Ca2+ after two prior subcultures at 26°C in the same medium lacking added Ca2+. Parallel growth at 37°C of a third culture either lacking (▵) or containing (□) l-glutamate (25 mM) is shown after prior subculture at 26°C for two transfers without added l-glutamate or Ca2+. All cultures contained 100 mM Na+ and possessed an initial pH of 7.0.
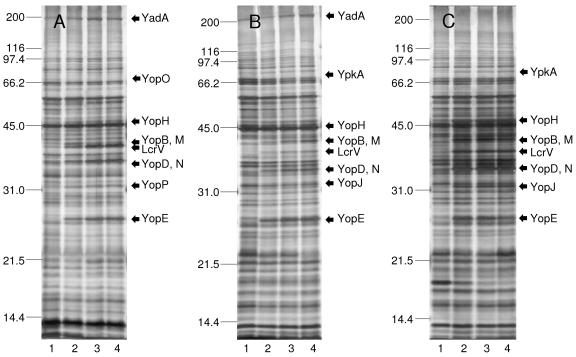
Growth with Ca2+ always prevented expression of VirF/LcrF-activated functions except for YadA regardless of the presence or absence of Na+ or l-glutamate (Fig. 1 and data not shown). Samples of the cultures illustrated in Fig. 1 were subjected to SDS-PAGE and silver staining after 7 h of incubation; full complements of Yops and LcrV were expressed during Ca2+ privation regardless of the presence or absence of added l-glutamate (Fig. 2). This finding indicates that l-glutamate enhances the nutritional requirement for Ca2+ as expressed by Y. pseudotuberculosis and Y. pestis but is not necessary for expression of LcrV and Yops, as reported for Y. enterocolitica (42).
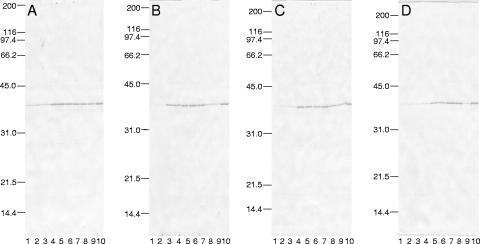
FIG. 2.
Silver-stained preparations following SDS-PAGE of whole cultures of Yersinia enterocolitica strain WA (A), Yersinia pseudotuberculosis strain PB1/+ (B), and Yersinia pestis strain KIM (C) after subculture at 26°C in chemically defined medium containing added 25 mM l-glutamate without Ca2+ followed by cultivation at 37°C in the same medium either without (lane 4) or with (lane 1) 4.0 mM Ca2+; comparable samples of cells previously subcultured twice at 26°C without Ca2+ or l-glutamate and then transferred to third Ca2+-deficient cultures at 37°C either lacking (lane 2) or containing (lane 3) l-glutamate are shown. All cultures contained 100 mM Na+ and exhibited an initial pH of 7.0. Expression of YopO/YpkA was too faint for reproduction.
Contribution of Na+ and l-glutamate to the LCR.
The results of previous studies showed that Na+ is essential for abrupt bacteriostasis of Y. pestis (26). To determine the specificity of this process, the ability of 100 mM Na+, K+, Li+, and Tris+ to inhibit growth of Lcr+ cells of Y. pestis in Ca2+-deficient media was compared in the constant presence of 25 mM l-glutamate. As shown in Fig. 3A, only Na+ promoted prompt bacteriostasis. These monovalent cations were not toxic to Lcr+ cells cultivated with Ca2+ nor were they inhibitory to Lcr− mutants grown with or without Ca2+ (data not shown). A similar experiment was undertaken to determine if l-amino acids metabolically related to l-glutamate could also promote abrupt bacteriostasis in Ca2+-deficient medium containing constant 100 mM Na+. Both l-glutamate and l-aspartate caused abrupt restriction, whereas the bacteriostasis observed with l-glutamine or l-asparagine was less pronounced (Fig. 3B). These amino acids were not inhibitory to either Lcr+ cells grown with Ca2+ or Lcr− mutants grown with or without this cation; a full component of VirF/LcrF-activated functions was observed in all media lacking added Ca2+ (data not shown).
FIG. 3.
Growth of Yersinia pestis strain KIM at 37°C in Ca2+-deficient chemically defined medium containing (A) constant l-glutamate (25 mM) and 100 mM Na+ (○), Li+ (▵), K+ (□), or Tris+ (▿); a parallel control culture containing Na+ and Ca2+ (4.0 mM) is included (•). Also shown (B) is growth of Y pestis strain KIM at 37°C in Ca2+-deficient chemically defined medium containing constant Na+ (100 mM) and 25 mM l-glutamate (○), l-aspartate (▵), l-glutamine (□), or l-asparagine (▿) or no added amino acid (⋄); a parallel control culture containing Na+ and Ca2+ (4.0 mM) is included (•). The initial pH of all cultures was 6.0.
Effect of pH on the LCR.
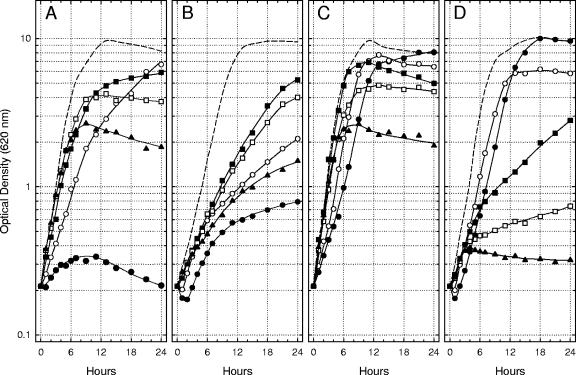
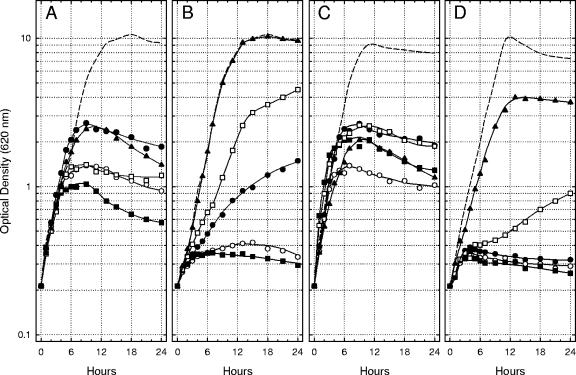
The initial culture pH dramatically influenced the ability of Na+ and l-glutamate to affect bacteriostasis after a shift of Lcr+ cells of Y. pestis from 26°C to 37°C in Ca2+-deficient medium. The significance of acidic pH was defined by measuring the growth of Lcr+ yersiniae at 37°C with 100 mM Na+ plus 25 mM l-glutamate, Na+ alone, l-glutamate alone, or neither nutrient at initial pHs ranging from 5.0 to 7.0. Mild to moderate acidity (pH 5.5 to 6.5) favored multiplication in all four Ca2+-deficient environments, although the extreme of pH 5.0 was often initially toxic (Fig. 4A). The favorable effect of moderate acidity was especially evident in the medium containing l-glutamate alone, where, at pH 6.0, the doubling time (≈70 min) approached that observed at pH 7.0 with added 4.0 mM Ca2+ (Fig. 4C). Addition of Na+, either alone (Fig. 4B) or with l-glutamate (Fig. 4D), depressed growth except at the lowest pHs tested. The toxic effect of Na+ was pronounced at pH 7.0 in the presence of l-glutamate (Fig. 4D), where the onset of bacteriostasis was especially abrupt.
FIG. 4.
Growth of Lcr+ cells of Yersinia pestis strain KIM at 37°C in Ca2+-deficient chemically defined medium lacking added Na+ and l-glutamate (A), containing only 100 mM Na+ (B), containing only 25 mM l-glutamate (C), and containing both Na+ and l-glutamate (D) at an initial pH of 5.0 (•), 5.5 (○), 6.0 (▪), 6.5 (□), and 7.0 (▴). Dashed lines indicate the extent of growth in control cultures containing 4.0 mM Ca2+ at an initial pH of 7.0.
Mild alkalinity (pH 7.5 and 8.0) inhibited multiplication in all four environments, although a higher initial pH of 8.5 and especially 9.0 promoted excellent growth provided Na+ was present (Fig. 5). Indeed, the multiplication observed at pH 9.0 in Ca2+-deficient medium containing Na+ alone (Fig. 5B) was indistinguishable from that detected in a parallel control culture containing added Ca2+ (doubling time, ≈70 min). Addition of l-glutamate to alkaline media containing Na+ significantly inhibited growth (Fig. 5D), as was observed in Fig. 4 for mildly acidic and neutral media. These results demonstrate that the temperature-dependent nutritional requirement of Lcr+ cells of Y. pestis for physiological levels of Ca2+ is essentially eliminated in the chemically defined medium by incubation at pH 6.0 with added l-glutamate alone or at pH 9.0 with the sole addition of Na+. The determinations further emphasize that the combination of Na+ plus l-glutamate at neutral to mildly alkaline pH in Ca2+-deficient medium promotes especially abrupt bacteriostasis of Lcr+ Y. pestis.
FIG. 5.
Same as Fig. 4 except that the cultures possessed an initial pH of 7.0 (•), 7.5 (○), 8.0 (▪), 8.5 (□), and 9.0 (▴).
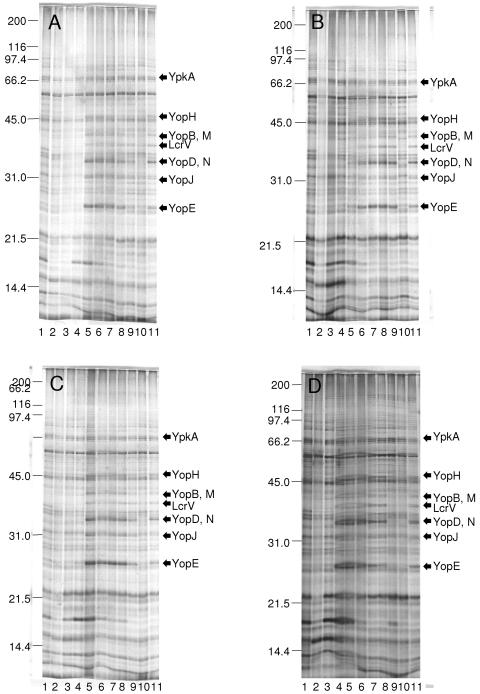
Samples of the cultures illustrated in Fig. 4 and 5 were subjected to immunoblotting against mouse monoclonal anti-LcrV (Fig. 6) and SDS-PAGE and silver staining (Fig. 7) after 7 h of incubation. Full components of Yops and LcrV were expressed regardless of the presence or absence of added Na+ or l-glutamate provided that the initial pH was 6.5 or above (Fig. 6); significant expression occurred even at pH 6.0 if only l-glutamate was added. This finding effectively uncouples activation of pCD/pYV-encoded genes by VirF/LcrF from the phenomenon of bacteriostasis as considered in the definition of the LCR provided above.
FIG. 6.
Immunoblot using mouse monoclonal anti-LcrV following SDS-PAGE of whole cultures of Yersinia pestis strain KIM cultured in Ca2+-deficient chemically defined medium lacking both Na+ and l-glutamate (A), containing only 100 mM Na+ (B), containing only 25 mM l-glutamate (C), and containing both Na+ and l-glutamate (D) at an initial pH of 5.0 (lane 1), 5.5 (lane 2), 6.0 (lane 3), 6.5 (lane 4), 7.0 (lane 5), 7.5 (lane 6), 8.0 (lane 7), 8.5 (lane 8), and 9.0 (lane 9); a comparable positive control of a Ca2+-deficient culture containing both Na+ and l-glutamate is shown in lane 10.
FIG. 7.
Silver-stained preparations following SDS-PAGE of whole cultures of Yersinia pestis strain KIM cultured in Ca2+-deficient chemically defined medium lacking both Na+ and l-glutamate (A), containing only 100 mM Na+ (B), containing only 25 mM l-glutamate (C), and containing both Na+ and l-glutamate (D) at an initial pH of 5.0 (lane 2), 5.5 (lane 3), 6.0 (lane 4), 6.5 (lane 5), 7.0 (lane 6), 7.5 (lane 7), 8.0 (lane 8), 8.5 (lane 9), and 9.0 (lane 10); comparable negative (Lcr− cells) and positive (Lcr+ cells) controls of Ca2+-deficient cultures containing both Na+ and l-glutamate are shown in lanes 1 and 11, respectively.
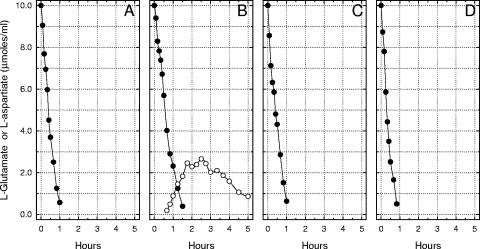
LCR-mediated excretion of l-aspartate.
Lcr+ cells of Y. pestis were cultivated under conditions that optimized abrupt bacteriostasis (no added Ca2+, 100 mM Na+, and 25 mM l-glutamate at pH 7.0 and 37°C) and prepared for incubation in buffer containing exogenous l-glutamate. Growth in this environment permitted catabolism of l-glutamate with net accumulation of l-aspartate followed by destruction of the latter after l-glutamate had disappeared (Fig. 8B). Lcr+ cells previously grown in the same medium with 4.0 mM Ca2+ (Fig. 8A) or Lcr− cells grown either with (Fig. 8C) or without (Fig. 8D) Ca2+ did not release detectable l-aspartate. Similar export of l-aspartate at 26°C or by Ca2+-starved cells of Y. enterocolitica or Y. pseudotuberculosis was not detected (data not shown).
FIG. 8.
Catabolic destruction of l-glutamate (•) with the appearance of metabolic l-aspartate (○) by resting cells of Yersinia pestis strain KIM previously grown at 37°C in synthetic medium containing 25 mM l-glutamate and 100 mM Na+. (A) Lcr+ cells grown with 4.0 mM Ca2+, (B) Lcr+ cells grown without added Ca2+, (C) Lcr− cells grown with 4.0 mM Ca2+, and (D) Lcr− cells grown without added Ca2+.
DISCUSSION
Y. pestis KIM, Y. pseudotuberculosis PB1, and Y. enterocolitica WA are well-defined type species of highly pathogenic yersiniae known to exhibit distinct patterns of growth at 37°C in Ca2+-deficient media (15). Fowler and Brubaker (26) used a modification of this environment to show that Na+ and l-glutamate were necessary for abrupt bacteriostasis of Lcr+ cells of Y. pestis and that, for full effectiveness, both metabolites had to be present in prior subcultures incubated at 26°C. In the present study, growth of Lcr+ Y. enterocolitica in Ca+-deficient medium containing Na+ was enhanced by l-glutamate whether or not the cells were adapted to this amino acid. In contrast, l-glutamate was inhibitory in parallel cultures of Lcr+ Y. pseudotuberculosis regardless of prior adaptation at 26°C and toxic to Lcr+ Y. pestis, especially after previous adaptation. This difference suggests that Y. pseudotuberculosis and Y. pestis possess a common sensitivity to l-glutamate not shared by the distantly related Y. enterocolitica and that an additional metabolic lesion involving Na+ that accounts for abrupt restriction of growth is superimposed on Y. pestis.
Lee et al. (42) reported that l-glutamate or metabolically related amino acids were necessary for expression of the TTSS by Y. enterocolitica in Ca2+-deficient tissue culture medium. l-Glutamate was unnecessary in the Ca2+-deficient chemically defined medium used here for production of LcrV and Yops by all three species of yersiniae. A likely explanation for this discrepancy is that the level of Mg2+ in the present study was increased to 20 mM. As noted previously, this concentration favors bacteriostasis in the absence of Ca2 + (30, 64) and ensures expression of LcrV (41). It is also equivalent to that present in mammalian cytoplasm (36) known to be released by invading yersiniae during rapid terminal growth within necrotic lesions of visceral organs (49, 63).
The observation that both Na+ and l-glutamate are necessary for abrupt bacteriostasis (26) was refined in this report by demonstrating that the contribution made by Na+ was specific (not due to increase of ionic strength or some allied effect), whereas the requirement for l-glutamate could also be met by l-aspartate (but not l-glutamine or l-asparagine). The literature regarding the role of Na+ in prokaryote physiology and the metabolism of dicarboxylic amino acids is voluminous although the overlapping set is limited to the observation that Na+ serves as a porter for the high-energy transport of both l-glutamic acid and l-aspartic acid via the action of GltS (18, 44, 56). This translocase exists in yersiniae (16, 20, 50) as do alternative Na+-independent l-glutamate transport systems mediated by GltJKL and GltP (2, 19, 62). As noted below, the possibility exists that GltS may modulate the LCR of Y. pestis, as judged by the release of l-aspartate by Ca2+-starved organisms.
Duplication of the modest acidic pH of mammalian cytoplasm (pH 6.0) permitted essentially full-scale growth of Lcr+ cells at 37°C in chemically defined medium lacking both added Ca2+ and Na+, provided that l-glutamate was present. This environment also facilitated full synthesis of LcrV and Yops, demonstrating that the correlation in vitro between bacteriostasis and expression of VirF/LcrF-activated genes is not obligatory (as implied in the present definition of the LCR). Medium adjusted to pH 9.0 and containing added Na+ but not l-glutamate also facilitated full-scale multiplication in the absence of added Ca2+. In this case, synthesis of LcrV and Yops was less pronounced than at neutral to mildly acidic pH but still detectable. One possible explanation for sustained Na+-dependent growth in this alkaline environment is the presence in Y. pestis of NADH:ubiquinone oxidoreductase (20, 50), an enzyme capable of serving as a primary electrogenic sodium pump at alkaline pH (68). Further study will be necessary to define the mechanism that excludes Na+ from the cytoplasm of yersiniae cultivated at neutral and acidic pHs and to determine if this process is inactivated during expression of LcrV and Yops. If so, then one or more VirF/LcrF-activated gene products may be involved in modulating bacteriostasis of Ca2+-starved yersiniae by enabling Na+ to access the cytoplasm.
Cells of Escherichia coli grown in enriched medium rapidly transaminate internalized l-glutamate (via aspartate aminotransferase) with a catalytic level of oxalacetate to yield 2-oxoglutarate and l-aspartate. 2-Oxoglutarate is then catabolized via enzymes of the tricarboxylic acid cycle to regenerate oxalacetate and accumulated l-aspartate is converted to fumarate via aspartase, thereby reentering the tricarboxylic acid cycle (28). Although the same process can function in the enteropathogenic yersiniae, the absence of aspartase activity in Y. pestis precludes use of this mechanism, limiting catabolism of l-aspartate to the formation of oxalacetate by transamination with some convenient amino group acceptor, especially 2-oxoglutarate (thereby generating l-glutamate). As a consequence, l-glutamate undergoes stoichiometric conversion to l-aspartate in resting plague bacilli (21). The observation provided here showing that secretion of l-aspartate occurs only in previously restricted Lcr+ cells is new information and was unsuspected. Since both bacteriostasis and secretion are dependent upon prior cultivation with Na+, the latter probably directly or indirectly promotes the export of l-aspartate. A likely candidate for this exit reaction would be GltS, where the normal flow of l-aspartate would become reversed by internalized Na+.
Considered together, these findings suggest that upregulation of VirF/LcrF-activated functions by cultivation at 37°C in Ca2+-deficient medium results in loss of the normal ability to exclude Na+ from the cytoplasm. One generally recognized consequence of this lesion would be interference with normal bioenergetic processes involving the proton motive force at acidic pH. A second effect unique to Y. pestis would involve Na+-facilitated export of l-aspartate, resulting in loss of metabolic carbon and consequent abrupt bacteriostasis; neither of these events could occur at alkaline pH due to the activity of NADH:ubiquinone oxidoreductase. This hypothesis can readily be subjected to experimental verification. Further study will also be necessary to determine what effect, if any, the ability to convert l-glutamate to l-aspartate has on expression of acute disease.
The results obtained by microarray analysis using essentially the same chemically defined medium described here showed that a shift of Y. pestis from 26°C to 37°C causes marked upregulation of oxidative metabolism with attendant profligate catabolism of nutrients readily converted to tricarboxylic cycle intermediates (47). This extravagant process, coupled with the potential of radically altering host l-glutamate and l-aspartate pools, may directly contribute to the acute symptoms of plague. An additional observation favoring this notion is the observation that the aspartase sequenced in attenuated Y. pestis strain 91001 (biovar microtus) (67) is similar to the active form found in Y. pseudotuberculosis (16). It may also be significant that l-glutamate undergoes stoichiometric conversion to l-aspartate in typhus rickettsiae (3) in a manner similar to that found for Y. pestis.
Acknowledgments
This work was sponsored by the NIH/NIAID Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (RCE) Program. I acknowledge membership within and support from the Region V ‘Great Lakes’ RCE (NIH award 1-U54-AI-057153).
The technical assistance of Janet Fowler was indispensable.
Editor: V. J. DiRita
REFERENCES
- 1.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 96:14043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blattner, F. R., G. Plunkett, III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, J. Rose, B. Mau, and Y. Shau. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 3.Bovarnick, M. R., and J. C. Miller. 1950. Oxidation and transamination of glutamate by typhus rickettsiae. J. Biol. Chem. 184:661-676. [PubMed] [Google Scholar]
- 4.Brubaker, R. R. 1967. Growth of Pasteurella pseudotuberculosis in simulated intracellular and extracellular environments. J. Infect. Dis. 117:403-417. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker, R. R. 2003. Interleukin-10 and the inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brubaker, R. R. 1970. Mutation rate to nonpigmentation in Pasteurella pestis. J. Bacteriol. 98:1404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brubaker, R. R. 8. September 2000, posting date. Yersinia pestis and bubonic plague. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackelbrandt (ed.), The prokaryotes: an evolving electronic resource for the microbiological community. [Online] http://et.springer-ny.com:8080/prokPUB/index.htm.
- 8.Brubaker, R. R., E. D. Beesley, and M. J. Surgalla. 1965. Pasteurella pestis: role of pesticin I and iron in experimental plague. Science 149:422-424. [DOI] [PubMed] [Google Scholar]
- 9.Brubaker, R. R., A. K. Sample, D.-Z. Yu, R. J. Zahorchak, P. C. Hu, and J. M. Fowler. 1987. Proteolysis of V antigen from Yersinia pestis. Microb. Pathogen. 2:49-62. [DOI] [PubMed] [Google Scholar]
- 10.Brubaker, R. R., and M. J. Surgalla. 1964. The effect of Ca++ and Mg++ on lysis, growth, and production of virulence antigens by Pasteurella pestis. J. Infect. Dis. 114:13-25. [DOI] [PubMed] [Google Scholar]
- 11.Buchrieser, C., M. Prentice, and E. Carniel. 1998. The 102-kilobase unstable region of Yersinia pestis comprises a high-pathogenicity island linked to a pigmentation segment which undergoes internal rearrangement. J. Bacteriol. 180:2321-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrows, T. W. 1957. Virulence of Pasteurella pestis. Nature 179:1246-1247. [DOI] [PubMed] [Google Scholar]
- 13.Burrows, T. W., and G. W. Bacon. 1960. V and W antigens in strains of Pasteurella pseudotuberculosis. Br. J. Exp. Pathol. 41:38-44. [PMC free article] [PubMed] [Google Scholar]
- 14.Carter, P. B., and F. M. Collins. 1974. Experimental Yersinia enterocolitica infection in mice: kinetics of growth. Infect. Immun. 9:851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter, P. B., R. J. Zahorchak, and R. R. Brubaker. 1980. Plague virulence antigens from Yersinia enterocolitica. Infect. Immun. 28:638-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chain, P., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, I. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 101:13826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deguchi, Y., I. Yamato, and U. Anraku. 1990. Nucleotide sequence of gltS, the Na+/glutamate symport carrier gene of Escherichia coli. J. Biol. Chem. 265:21704-21708. [PubMed] [Google Scholar]
- 19.Deguchi, Y., I. Yamato, and Y. Anraku. 1989. Molecular cloning of gltS and gltP, which encode glutamate carriers of Escherichia coli B. J. Bacteriol. 171:1314-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng, W., V. Burland, G. I. Plunkett, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, D. C. Schwartz, S. Zhou, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. L. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 104:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreyfus, L. A., and R. R. Brubaker. 1978. Consequences of aspartase deficiency in Yersinia pestis. J. Bacteriol. 136:757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferber, D. M., and R. R. Brubaker. 1981. Plasmids in Yersinia pestis. Infect. Immun. 31:839-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fetherston, J. D., and R. D. Perry. 1994. The pigmentation locus of Yersinia pestis KIM6+ is flanked by an insertion sequence and includes the structural genes for pesticin sensitivity and HMWP2. Mol. Microbiol. 13:697-708. [DOI] [PubMed] [Google Scholar]
- 24.Fields, K. A., M. L. Nilles, C. Cowan, and S. C. Straley. 1999. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect. Immun. 67:5395-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finegold, M. J., R. F. Petery, R. F. Berendt, and H. R. Adams. 1968. Studies on the pathogenesis of plague. Blood coagulation and tissue responses of Macaca mulatta following exposure to aerosols of Pasteurella pestis. Am. J. Pathol. 53:99-114. [PMC free article] [PubMed] [Google Scholar]
- 26.Fowler, J. M., and R. R. Brubaker. 1994. Physiological basis of the low calcium response in Yersinia pestis. Infect. Immun. 62:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grosdent, N., I. Maridonneau-Parini, M. P. Sory, and G. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpern, Y. S., and H. E. Umbarger. 1960. Conversion of ammonia to amino groups in Escherichia coli K-12 mutants. J. Bacteriol. 80:285-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higuchi, K., L. L. Kupferberg, and J. L. Smith. 1959. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J. Bacteriol. 77:317-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higuchi, K., and J. L. Smith. 1961. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J. Bacteriol. 81:605-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinchliffe, S. T., K. E. Isherwood, R. A. Stabler, M. P. Prentice, A. Rakin, R. A. Nichols, P. C. F. Oyston, J. Hinds, R. W. Titball, and B. W. Wren. 2003. Application of DNA microarrays to study the evolutionary genomics of Yersinia pestis and Yersinia pseudotuberculosis. Genome Res. 13:2018-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinnebusch, B. J., A. E. Rudolph, P. Cherepanov, J. D. Dixon, T. G. Schwan, and A. Forsberg. 2002. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science 296:733-735. [DOI] [PubMed] [Google Scholar]
- 33.Hinnebusch, J., C. P., Y. Du, A. Rudolph, J. D. Dixon, T. Schwan, and Å. Forsberg. 1999. Murine toxin of Yersinia pestis shows phospholipase D activity but is not required for virulence in mice. Int. J. Med. Microbiol. 290:483-487. [DOI] [PubMed] [Google Scholar]
- 34.Hoe, N. P., and J. D. Goguen. 1993. Temperature sensing in Yersinia pestis: translation of the LcrF activator protein is thermally regulated. J. Bacteriol. 175:7901-7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson, S., and T. W. Burrows. 1956. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br. J. Exp. Pathol. 37:570-576. [PMC free article] [PubMed] [Google Scholar]
- 36.Kugelmass, N. L. 1959. Biochemistry of blood in health and disease. Charles C. Thomas, Inc., Springfield, Ill.
- 37.Kutyrev, V., R. J. Mehigh, V. L. Motin, M. S. Pokrovskaya, G. B. Smirnov, and R. R. Brubaker. 1999. Expression of the plague plasminogen activator in Yersinia pseudotuberculosis and Escherichia coli. Infect. Immun. 67:1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutyrev, V. V., Y. A. Popov, and O. A. Protsenko. 1986. Pathogenicity plasmids of the plague microbe (Yersinia pestis). Mol. Gen. Mikrobiol. Virusol. 6:3-11. [PubMed] [Google Scholar]
- 39.Lähteenmäki, K., R. Virkola, A. Sarén, E. Emödy, and T. K. Korhonen. 1998. Expression of the plasminogen activator Pla of Yersinia pestis enhances bacterial attachment to the mammalian extracellular matrix. Infect. Immun. 66:5755-5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambert de Rouvroit, C., C. Sluiters, and G. R. Cornelis. 1992. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol. Microbiol. 6:395-409. [PubMed] [Google Scholar]
- 41.Lawton, W. D., R. L. Erdman, and M. J. Surgalla. 1963. Biosynthesis and purification of V and W antigen in Pasteurella pestis. J. Immunol. 91:179-184. [DOI] [PubMed] [Google Scholar]
- 42.Lee, V., S. Mazmanian, and O. Schneewind. 2001. A program of Yersinia enterocolitica type III secretion reactions is activated by specific signals. J. Bacteriol. 183:4970-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucier, T. S., and R. R. Brubaker. 1992. Determination of genome size, macrorestriction pattern polymorphism, and non-pigmentation-specific deletion in Yersinia pestis by pulsed-field gel electrophoresis. J. Bacteriol. 174:2078-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacDonald, R. E., J. K. Lanyi, and R. V. Greene. 1977. Sodium-stimulated glutamate uptake in membrane vesicles of Escherichia coli: the role of ion gradients. Proc. Natl. Acad. Sci. USA 74:3167-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mortlock, R. P. 1962. Gluconate metabolism of Pasteurella pestis. J. Bacteriol. 84:53-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortlock, R. P., and R. R. Brubaker. 1962. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase activities of Pasteurella pestis and Pasteurella pseudotuberculosis. J. Bacteriol. 84:1122-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Motin, V. L., A. M. Georgescu, J. P. Fitch, P. P. Gu, D. O. Nelson, S. L. Mabery, J. B. Garnham, B. A. Sokhansanj, L. L. Ott, M. A. Coleman, J. M. Elliott, L. M. Kegelmeyer, A. J. Wyrobeck, T. R. Slezak, R. R. Brubaker, and E. Garcia. 2004. Temporal global changes in gene expression during temperature transition in Yersinia pestis. J. Bacteriol. 186:6298-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. G. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Chercher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeño-Tárraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. F. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 50.Perry, R. D., M. L. Pendrak, and P. Schuetze. 1990. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J. Bacteriol. 172:5929-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pettersson, J., R. Nordfeith, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 52.Portnoy, D. A., S. L. Moseley, and S. Falkow. 1981. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect. Immun. 31:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohde, J. R., X.-S. Luan, H. Rohde, J. M. Fox, and S. A. Minnich. 1999. The Yersinia enterocolitica pYV virulence plasmid contains multiple intrinsic DNA bends which melt at 37°C. J. Bacteriol. 181:4198-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sample, A. K., and R. R. Brubaker. 1987. Post-translational regulation of Lcr plasmid-mediated peptides in pesticinogenic Yersinia pestis. Microb. Pathog. 3:239-248. [DOI] [PubMed] [Google Scholar]
- 55.Skulachev, V. P. 1987. Bacterial sodium transport: bioenergetic factors of sodium ions, p. 131-164. In B. P. Rosen and S. Silver (ed.), Ion transport in prokaryotes. Academic Press, New York, NY.
- 56.Skurnik, M., A. Peippo, and E. Ervelä. 2000. Characterization of the O-antigen gene clusters of Yersinia pseudotuberculosis and the cryptic O-antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b. Mol. Microbiol. 37:316-330. [DOI] [PubMed] [Google Scholar]
- 57.Skurnik, M., and P. Toivanen. 1992. LcrF is the temperature-regulated activator of the yadA gene of Yersinia enterocolitica and Yersinia pseudotuberculosis. J. Bacteriol. 174:2047-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sodeinde, O. A., A. K. Sample, R. R. Brubaker, and J. D. Goguen. 1988. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect. Immun. 56:2749-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sodeinde, O. A., Y. V. B. K. Subrahmanyam, K. Stark, T. Quan, Y. Bao, and J. D. Goguen. 1992. A surface protease and the invasive character of plague. Science 258:1004-1007. [DOI] [PubMed] [Google Scholar]
- 60.Straley, S. C., and R. R. Brubaker. 1981. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc. Natl. Acad. Sci. USA 78:1224-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tolner, B., B. Poolman, B. Wallace, and W. N. Konings. 1992. Revised nucleotide sequence of the gltP gene, which encodes the proton-glutamate-aspartate transport protein of Escherichia coli K-12. J. Bacteriol. 174:2391-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Une, T., R. Nakajima, and R. R. Brubaker. 1986. Roles of V antigen in promoting virulence in Yersinia. Contrib. Microbiol. Immunol. 9:179-185. [PubMed] [Google Scholar]
- 63.Winter, C. C., W. B. Cherry, and M. D. Moody. 1960. An unusual strain of Pasteurella pestis isolated from a fatal case of human plague. Bull. W.H.O. 23:408-409. [PMC free article] [PubMed] [Google Scholar]
- 64.Zahorchak, R. J., and R. R. Brubaker. 1982. Effect of exogenous nucleotides on Ca2+ dependence and V antigen synthesis in Yersinia pestis. Infect. Immun. 38:935-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zahorchak, R. J., W. T. Charnetzky, R. V. Little, and R. R. Brubaker. 1979. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J. Bacteriol. 39:792-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou, D., Z. Tong, Y. Song, Y. Han, D. Pei, X. Pang, J. Zhai, M. Li, B. Cui, Z. Qi, L. Jin, R. Dai, Z. Du, J. Wang, Z. Guo, J. Wang, P. Huang, and R. Yang. 2004. Genetics of metabolic variations between Yersinia pestis biovars and the proposal of a new biovar, microtus. J. Bacteriol. 186:5147-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou, W., Y. V. Bertsova, B. Feng, P. Tsatsos, M. I. Verkhovskaya, R. B. Gennis, A. V. Bogachev, and B. Barquera. 1999. Sequencing and preliminary characterization of the Na+-translocating NADH:ubiquinone oxidoreductase from Vibrio harveyi. Biochemistry 38:16246-16252. [DOI] [PubMed] [Google Scholar]