Abstract
In immunocompromised patients with disseminated infection, human cytomegalovirus (HCMV) is widespread in the microvascular endothelium of multiple organs. Human umbilical vein endothelial cells (HUVEC) were used in parallel to human embryonic lung fibroblasts (HELF) to recover HCMV from blood samples of immunocompromised patients. Using the shell vial technique, comparable median numbers of p72-positive HUVEC and HELF cells were found with the 26 HCMV-positive buffy coat samples out of 150 examined. Analysis of other clinical samples inoculated as controls revealed, in the presence of highly infected HELF monolayers, either the presence of very few infected HUVEC with urine specimens (n = 10 samples) or the lack of infected HUVEC with throat washes (n = 3) or amniotic fluid samples (n = 2). Thus, peripheral blood leukocytes (PBL) appear essential for primary isolation of HCMV in HUVEC. In this respect, HCMV strains, recovered from clinical samples other than buffy coats in HELF only, could be readily adapted to growth in HUVEC by coculturing PBL from healthy blood donors with infected HELF and then inoculating infected PBL onto HUVEC. Recently elucidated mechanisms of interaction of leukocytes and HUVEC with bidirectional transfer of virus seem to provide the basis for the restriction of HCMV primary isolation in HUVEC to blood samples. However, virus strains recovered from only HELF could be adapted to growth in HUVEC when inoculated with HELF-derived (either cell-associated or cell-free) HCMV strains upon primary isolation. In conclusion, due to the in vitro selection of virus variants provided with both PBL tropism and HUVEC tropism, HCMV recovery in HUVEC is PBL mediated and substantially restricted to blood samples. Lack of HCMV recovery in HUVEC from clinical samples other than blood leads to the assumption that epithelial cells, such as urinary, amniotic, or pharyngeal cells, do not possess adequate adhesion molecules to establish close contacts with HUVEC.
In vivo, human cytomegalovirus (HCMV) can infect a number of cell types of different origins, namely, fibroblasts, epithelial and endothelial cells, and smooth muscle cells (9). In particular, HCMV has been shown to infect and fully replicate in endothelial cells of the vascular tree in immunocompromised patients. In disseminated infection, cytomegalic endothelial cells may also circulate in peripheral blood (3, 5), and virus dissemination is mediated by peripheral blood leukocytes (PBL) carrying infectious virus acquired from infected endothelium and transmitting the infection to uninfected endothelial cells (4, 7, 10).
Recently, an in vitro model was developed in our laboratory showing that PBL from healthy blood donors may be infected following coculture with human umbilical vein endothelial cells (HUVEC) or human embryonic lung fibroblasts (HELF) infected with clinical isolates and may disseminate the infection to both uninfected cell types (1, 7). The mechanism underlying transfer of infectious virus and viral products from infected cells to PBL and from infected PBL to uninfected cells has been partly clarified, while the viral gene(s) involved in this process is now under investigation.
In the present study, the differential recovery of HCMV from PBL in HUVEC and HELF was investigated in parallel with clinical samples from other sources, documenting the fact that PBL are usually required for HCMV primary isolation in HUVEC. However, HCMV strains recovered only from HELF can be readily adapted to growth in HUVEC following inoculation with either PBL infected in vitro or HELF-derived (either cell-associated or cell-free) HCMV strains upon primary isolation, thus documenting the fact that growth in HUVEC is likely to depend on in vitro selection of PBL-tropic and HUVEC-tropic HCMV variants.
MATERIALS AND METHODS
Cell cultures.
HUVEC were obtained by trypsin treatment of umbilical cord veins according to a previously reported procedure (1, 7). The cells were grown in M199 medium supplemented with 20% fetal calf serum, heparin (5 U/μl), 1% endothelial growth factor (Sigma Chemical Co., St. Louis, Mo.), and antibiotics. The growing surface for HUVEC was pretreated with 0.2% gelatin. Cells were propagated weekly at a 1:2 ratio and used within five passages for primary HCMV isolation from clinical samples. All primary HUVEC cultures were tested for the presence of HCMV DNA by nested PCR (8). In parallel, HELF cultures, originally developed in the laboratory in 1980 and cultured according to standard procedures, were used at passage 20 to 30 for primary HCMV isolation from the same clinical samples.
Clinical samples.
Altogether, 180 clinical samples were inoculated in parallel onto HUVEC and HELF monolayers. The specimens examined included 150 buffy coat samples from immunocompromised patients (AIDS patients and heart, lung, and bone marrow transplant recipients); 21 urine samples, 11 of which were from five congenitally HCMV-infected newborns; six throat washes, three of which were from subjects excreting virus with saliva; and three amniotic fluid samples from pregnant women, two of which were from women transmitting virus to the fetus during primary HCMV infection.
HCMV isolation in HUVEC and HELF.
Buffy coat samples were obtained by sedimentation of 5.0 ml of heparinized blood at 37°C for 30 min. The supernatant was collected and centrifuged at 700 × g. The cell pellet was collected, and contaminating erythrocytes were removed by hypotonic lysis with 0.8% ammonium chloride. Following centrifugation at 700 × g and washings with phosphate-buffered saline, the final pellet was resuspended in Earle minimum essential medium supplemented with 2% fetal calf serum and the PBL were counted. HUVEC and HELF monolayers grown in shell vials were inoculated with 2 × 105 PBL or 100 μl of urine, throat washes, or amniotic fluid samples and centrifuged at room temperature for 45 min at 600 × g.
Early and late identification of HCMV isolates in HUVEC.
Early identification of HCMV isolates was achieved by staining shell vial cell cultures 24 to 48 h postinfection (p.i.) with a monoclonal antibody reactive to the major immediate-early (IE) protein p72 (nuclear staining) by the indirect immunofluorescence technique (2). In addition, some shell vial cultures were incubated for 4 to 7 days and then stained with either a monoclonal antibody to the late viral glycoprotein B (gB), which was kindly provided by L. Pereira (University of California—San Francisco) and gave a cytoplasmic staining mostly localized to the Golgi area, or a combination of both p72- and gB-specific monoclonal antibodies. HCMV p72- or gB-positive HUVEC and HELF were counted, and the numbers were recorded.
Adaptation to growth in HUVEC of HCMV strains recovered in HELF.
Three approaches were used to adapt the HCMV strains recovered in HELF from clinical specimens other than buffy coat to growth in HUVEC. The first was to mix infected HELF with uninfected HUVEC at a ratio of 1:2 and then propagate the infected cell mixture onto uninfected HUVEC at a ratio of 1:2 once weekly for 5 weeks. The infected cell cultures were then sonicated to remove infected HELF, and cell-free virus was inoculated onto fresh HUVEC. Finally, the virus was propagated as cell-associated preparations till passage 10. As reported previously, if more than 50% of HUVEC were infected at passage 10, the virus strain was considered HUVEC adapted (8). The second approach was to sonicate HCMV-infected HELF one or a few passages after virus recovery and directly infect HUVEC with cell-free virus following centrifugation at 600 × g for 45 min. Subsequent passages involved cocultivation of infected and uninfected HUVEC at a 1:2 ratio. With this approach, adaptation to HUVEC was often readily achieved within 5 passages. Finally, the third approach consisted of coculturing PBL from healthy blood donors and HELF infected with a primary HCMV isolate from a clinical sample other than buffy coat and then infecting HUVEC (and HELF, for comparison) with PBL infected in vitro. Thus, with the last approach, virus recovery in HUVEC from PBL was artificially reproduced in vitro.
RESULTS
Comparative efficiencies of HUVEC and HELF for HCMV recovery from clinical samples.
Overall, 42 of the 180 clinical samples inoculated simultaneously onto HUVEC and HELF shell vial monolayers were positive for HCMV rapid isolation (Table 1). Of the 26 buffy coat samples positive for HCMV isolation, 13 (50%) showed the presence of HCMV on both HUVEC and HELF, while 7 (27%) were positive on HELF only and 6 (23%) were positive on HUVEC only. Thus, the two cell substrates appeared to be comparably sensitive for HCMV recovery from clinical samples yet complementary to one another.
TABLE 1.
Efficiency of HUVEC and HELF cultures for HCMV rapid recovery from 180 clinical samples
| Clinical sample | No. of samples tested | Total no. of positive samples | No. (%) of samples positive on both HELF and HUVEC | No. (%) of samples positive on HELF only | No. (%) of samples positive on HUVEC only |
|---|---|---|---|---|---|
| Buffy coat | 150 | 26 | 13 (50) | 7 (27) | 6 (23) |
| Urine | 21 | 11 | 7 (64) | 4 (36) | 0 |
| Throat wash | 6 | 3 | 0 | 3 (100) | 0 |
| Amniotic fluid | 3 | 2 | 0 | 2 (100) | 0 |
| Total | 180 | 42 | 20 (48) | 16 (38) | 6 (14) |
Of the 11 urine samples positive for HCMV isolation on HELF, 7 were also positive on HUVEC but none was positive on HUVEC only. In addition, HCMV was not recovered on HUVEC following inoculation of three HCMV-positive throat washes and two HCMV-positive amniotic fluid samples, which revealed the presence of a large amount of virus in HELF.
In more detail, when the differential efficiency in HCMV recovery was considered not only qualitatively but quantitatively, a striking difference was observed between the two cell substrates (Table 2). The median numbers of p72-positive cells of the 19 buffy coat samples positive for HCMV recovery on HUVEC and of the 20 buffy coat samples positive on HELF were comparable (2 [range, 1 to 58] on HUVEC and 4 [range, 1 to 175] on HELF). Buffy coat samples positive on either cell type contained only a very low number of infectious virus particles, and the restriction of virus isolation to a single cell substrate was likely due to a stochastic effect (Table 2).
TABLE 2.
Median number of HCMV p72-positive cells following inoculation of the same clinical samples onto the two cell substrates
| HCMV-positive sample | Median no. of p72-positive cells (range)
|
No. of samples | |
|---|---|---|---|
| HUVEC | HELF | ||
| Buffy coat | |||
| Positive in both cell types | 2 (1–58) | 4 (1–175) | 13 |
| Positive in only one cell type | 1 (1–2) | 3 (1–7) | 13 |
| Urine | |||
| Positive in both cell types | 2 (1–39) | 8 × 104 (4.5 × 103−1 × 105) | 7 |
| Positive in HELF only | 0 | 5 × 103 (1.9 × 102−1 × 104) | 4 |
| Throat wash | 0 | 1 × 103 (67–3 × 103) | 3 |
| Amniotic fluid | 0 | 1 × 105 (8 × 104−1.2 × 105) | 2 |
| Urine fractions | |||
| Sediment cell pellet | 2 | 1 × 104 | 1 |
| Supernatant | 7 | 1 × 105 | 1 |
On the other hand, of the 11 HCMV-positive urine samples tested, the 7 positive on both cell substrates showed a median number of HCMV-positive cells much higher (80,000; range, 4,500 to 100,000) on HELF (Fig. 1A) than on HUVEC (2; range, 1 to 39) (Fig. 1B), while the 4 samples positive for HCMV on HELF only showed a median number of p72-positive HELF cells more than 1 log10 unit lower than the former samples (5,000; range, 190 to 10,000). Finally, no HCMV-positive HUVEC were detected after inoculation of three HCMV-positive throat washes and two HCMV-positive amniotic fluid samples, whereas the same samples gave a median number of 1,000 p72-positive HELF cells (range, 67 to 3,000) for throat washes and 100,000 for the two amniotic fluid samples.
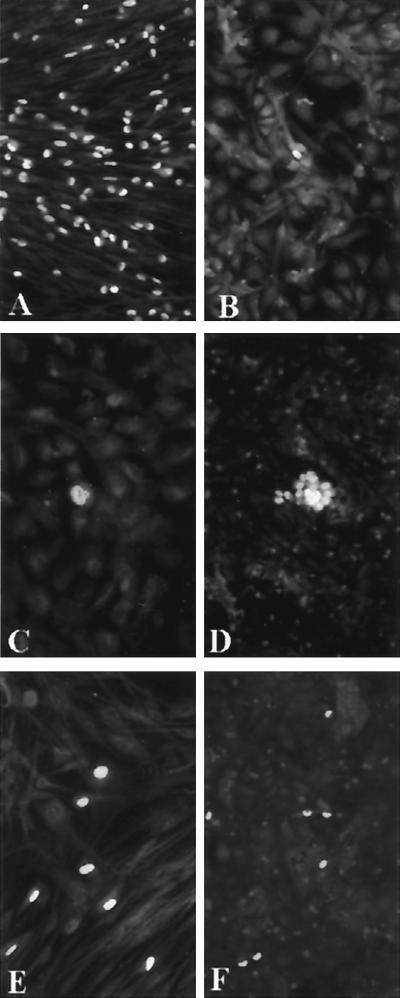
FIG. 1.
Inoculation of urine sample (100 μl) from a congenitally HCMV-infected newborn in parallel onto HELF (A) and HUVEC (B to D). In contrast to an exceedingly high number of p72-positive HELF in panel A (nuclear staining), a single positive cell is seen in panel B. However, these very rare cells also progress to late phases of virus replication, as shown by the presence of gB (cytoplasmic staining) in panel C 96 h p.i. and a small plaque in panel D 7 days p.i. In panels E (HELF) and F (HUVEC), comparable numbers of p72-positive cells (n = 7) are shown, following inoculation of PBL cocultured with HELF infected with the same virus strain upon primary isolation. When infection is mediated by cocultured PBL, the numbers of infected cells on the two cell systems are comparable. Immunofluorescence staining with p72-specific monoclonal antibodies (A, B, E, and F) and with both p72- and gB-specific monoclonal antibodies (C and D) was used to detect both IE and late viral antigens.
Differential recovery of cell-free and cell-associated HCMV from clinical samples on HELF and HUVEC.
To verify whether the differential recovery of HCMV from clinical samples was dependent on the amount of virus present in the cell-free or cell-associated fraction, two clinical samples (urine and amniotic fluid) containing large amounts of virus (106 and 107 PFU/ml, respectively) were centrifuged and fractionated into cell pellets and supernatants. Then, equal volumes (100 μl) were inoculated in parallel onto HELF and HUVEC cultures. The results showed that, while both fractions were found to contain large amounts of infectious virus on HELF (similar to those found on HELF for whole samples within 1 log10 unit difference), consistently less than 10 p72-positive cells were found in HUVEC cultures infected with the relevant fractions. Table 2 reports only data obtained for the fractionated urine sample.
Progression from IE to late phases of replication of HCMV primary isolates on HUVEC.
Five buffy coat samples positive for HCMV isolation on HUVEC at 24 h p.i. were stained for gB at 96 and 168 h p.i. As a rule, nearly 100% of IE-protein-positive HUVEC progressed to gB-positive cells (96 h) and to plaques (168 h). Thus, HUVEC appeared to be highly permissive to HCMV on primary isolation when virus was transmitted by PBL. The same finding, i.e., progression to late phases of virus replication and to plaques, was observed for each of the very few p72-positive HUVEC following inoculation of clinical samples other than buffy coat and, specifically, urine samples containing very large amounts of infectious virus as detected on HELF (Fig. 1C and D).
Adaptation to growth on HUVEC of non-endothelial-cell-tropic HCMV strains following transfer to PBL.
All non-endothelial-cell-tropic HCMV strains recovered on HELF from clinical samples other than buffy coats could be promptly adapted to growth on HUVEC following coculture of PBL from healthy donors with HELF infected with the relevant strain and then inoculation of infected PBL onto uninfected HUVEC (Fig. 2, option 3). When the same number of PBL carrying infectious virus was inoculated in parallel onto HUVEC and HELF, comparable numbers of infected cells or plaques were observed in the two cell systems (Fig. 1E and F).
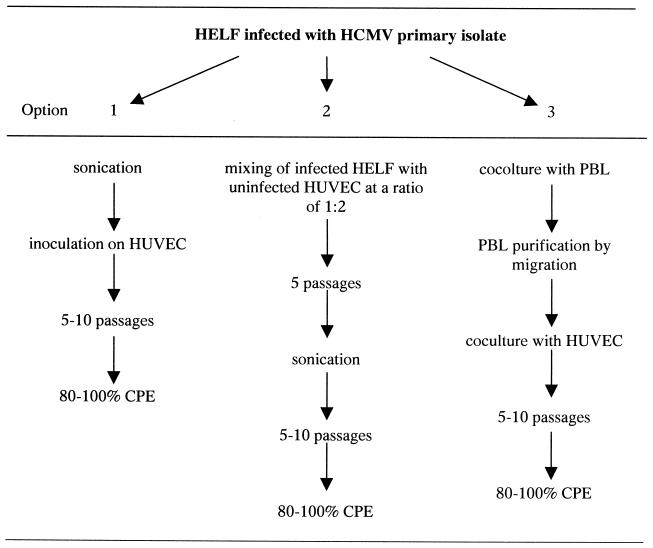
FIG. 2.
Diagram of three methods of adaptation to growth in HUVEC of HCMV strains recovered in HELF from clinical samples other than buffy coat. Passages indicate propagation of cell-associated virus. CPE, cytopathic effect.
Comparative growth on HUVEC and HELF of HCMV strains from buffy coat upon primary isolation and following different stages of propagation on HUVEC.
Inoculation of HCMV-positive buffy coats onto HUVEC and HELF yielded comparable numbers of infected cells 24 h p.i. (Fig. 3A and B). However, following propagation on HUVEC, inoculation of cell-free virus onto HUVEC and HELF evidenced small plaques on HUVEC at either passage 10 or 20 (Fig. 3C and E) and much larger plaques on HELF (Fig. 3D, passage 10), where a trend towards plaque confluency was observed at passage 20 (Fig. 3F).
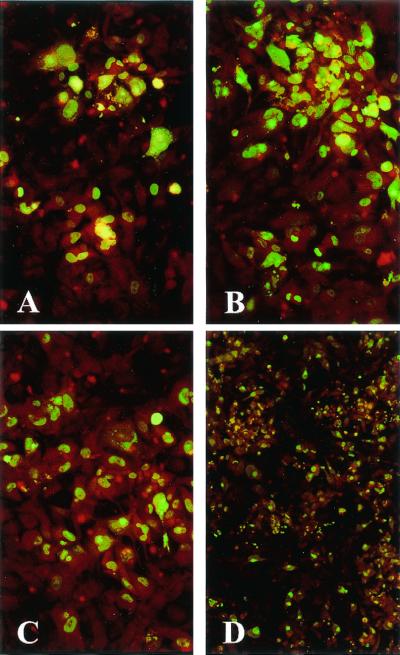
FIG. 3.
Comparative growth on HUVEC (A, C, and E) and HELF (B, D, and F) of an HCMV strain recovered on HUVEC (A) and HELF (B) from buffy coat and inoculated as cell-free virus onto both cell systems after 10 (C and D) and 20 (E and F) passages on HUVEC. Immunofluorescence staining with p72-specific monoclonal antibody (A and B) and with both p72-specific (nuclear staining) and gB-specific (cytoplasmic staining) monoclonal antibodies (C to F) was performed. Virus spread to contiguous cells becomes more efficient on HELF than on HUVEC between passages 10 and 20, whereas on HUVEC the efficiency of spreading appears unchanged.
Adaptation to growth on HUVEC of HCMV strains recovered on HELF from clinical samples other than buffy coat.
Adaptation to growth on HUVEC was achieved for all four of the HCMV strains recovered from urine samples only on HELF according to the following two protocols (Fig. 2, options 1 and 2). The first protocol consisted of mixing HELF infected with a primary HCMV isolate with uninfected HUVEC (Fig. 2, option 2) and then propagating the infected cell mixture once weekly for 5 weeks (Fig. 4A to C). Following cell sonication to remove HELF and subsequent propagation of infected HUVEC onto uninfected HUVEC, extensive (80 to 100%) infection of the HUVEC monolayer was generally achieved within passage 10 (Fig. 4D). The second protocol required sonication of infected HELF at passages 1 to 3 after virus recovery followed by adsorption onto HUVEC of HELF-derived cell-free virus during low-speed centrifugation (Fig. 2, option 1). Infected cells were used for subsequent propagation. With this protocol, the number of infected HUVEC increased with passages till the great majority of the cell monolayer was involved within passage 10.
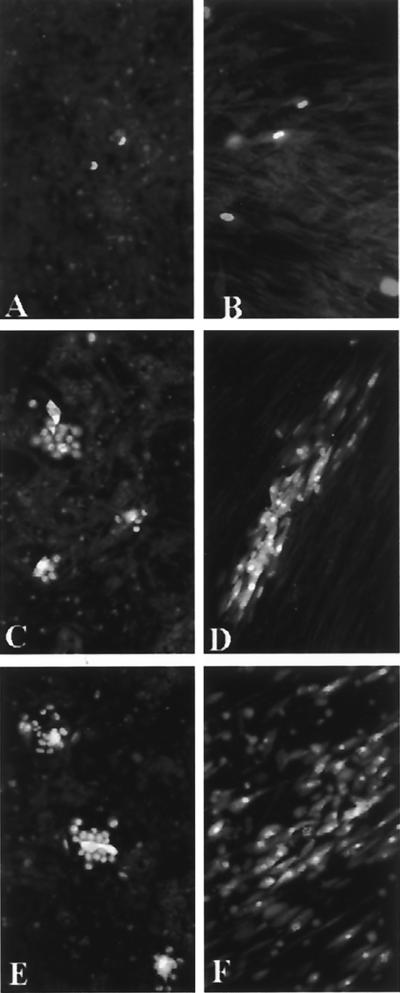
FIG. 4.
Progressive adaptation to growth on HUVEC of a cell-associated HCMV strain originally recovered on HELF. (A) Passage 2 (two small plaques); (B) passage 4 and (C) passage 6 (large plaques); (D) passage 10, following sonication at passage 6 (lower magnification to show that the great majority of the cell monolayer is infected). Immunofluorescence staining with a combination of p72-specific (nuclear staining) and gB-specific (cytoplasmic staining) monoclonal antibodies was performed.
DISCUSSION
Human fibroblasts, and specifically embryonic or neonatal fibroblasts, have represented the conventional cell substrate for recovery of HCMV from clinical samples since the beginning of medical virology. Due to the increasing need for recovery of HCMV from the blood of immunocompromised patients, we investigated whether use of HCMV-free HUVEC could increase the recovery rate of HCMV from blood (buffy coat) samples in comparison to HELF. Clinical samples other than blood were inoculated in parallel onto the two cell substrates as controls. Surprisingly, while the isolation rate from blood samples did not increase on HUVEC compared to HELF, no or very little virus could be recovered on primary isolation on HUVEC from clinical samples other than blood, even when virus was present in exceedingly large amounts. Thus, HELF were confirmed as the cell substrate of choice for isolation of HCMV from the broadest series of clinical samples, whereas HCMV recovery on HUVEC was substantially restricted to blood samples.
In light of the present knowledge of the interaction of PBL, whether polymorphonuclear (PMN) leukocytes (4, 7) or monocytes (10), and HUVEC, HCMV can be transmitted to HUVEC only by leukocytes. In fact, adhesion of PMN leukocytes to HUVEC occurs through interaction of CD18 molecules (integrins) on the surfaces of leukocytes and ICAM-1 molecules on the surfaces of HUVEC. When PMN leukocytes carry infectious virus, adhesion is followed by a series of microfusion events between the two adhering cells, with transfer of infectious virus from PMN leukocytes to HUVEC (1). Since HCMV circulates in peripheral blood of immunocompromised patients and immunocompetent subjects during primary infection inside leukocytes, and specifically PMN leukocytes, HCMV recovery on HUVEC must be mediated by leukocytes. This appears to be indirectly confirmed by the lack of HCMV isolation on HUVEC from clinical samples other than blood. Since the clinical samples other than blood tested in this study all contained epithelial cells, the lack of HCMV recovery on HUVEC from these samples leads to the assumption that epithelial cells, such as urinary, amniotic, or pharyngeal cells, do not possess the adhesion molecules required to establish close contacts with HUVEC. Thus, epithelial cells would be unable to adhere to HUVEC and to transfer virus to them.
In addition, the cell-free virus present in clinical samples other than blood was not able to infect HUVEC, whereas the infection was successful when virus was propagated from HELF (using either cell-free or cell-associated virus) to HUVEC. This implies that the cell-free virus in these samples is somehow complexed with antibody or nonantibody viral inhibitors, hindering its adsorption to HUVEC but not to HELF. In addition, all these HCMV strains could be readily transmitted to and propagated on HUVEC if they were first transferred to leukocytes from infected HELF and then from infected leukocytes to HUVEC.
Thus far, PMN tropism and endothelial cell tropism have been found to be consistently associated (8). However, both properties have been shown to be peculiar to clinical HCMV isolates and missing in reference HCMV strains, such as AD169, Towne, Davis, and even Toledo (1, 7), or in HCMV variants selected during propagation of clinical isolates on HELF (8). These variants, not detected upon primary isolation, were identified between passages 10 and 50 on HELF based on loss of both PMN and endothelial cell tropism. In this study, the finding that the numbers of infectious foci were comparable on HUVEC and HELF with blood samples and enormously lower on HUVEC than on HELF for samples from other sources suggests that endothelial-cell-tropic variants were selected on HUVEC. According to this hypothesis, non-endothelial-cell-tropic variants were unable to grow on HUVEC. This conclusion appears to be confirmed by the adaptation to growth on HUVEC of apparently non-endothelial-cell-tropic HCMV strains following transfer to PBL.
In conclusion, we believe that primary recovery of HCMV on HUVEC from blood may be considered as a marker of both endothelial cell tropism and HCMV dissemination. This appears to be indirectly confirmed by the consistent lack of recovery of the Towne vaccine strain from the blood of vaccinated individuals (6).
Acknowledgments
We thank Linda D’Arrigo for revision of the English. We are also indebted to Gabriella Garbagnoli and Viviana Landini for technical assistance.
This work was partially supported by Ministero della Sanità, Istituto Superiore di Sanità, III Progetto Nazionale AIDS, grant no. 50C.12; Ricerca Finalizzata grant no. 820RFM99/01 and 820RFM95/01; and Ricerca Corrente IRCCS Policlinico San Matteo, grant no. 80206.
REFERENCES
- 1.Gerna, G., E. Percivalle, F. Baldanti, S. Sozzani, P. Lanzarini, E. Genini, D. Lilleri, and M. G. Revello. 2000. Human cytomegalovirus replicates abortively in polymorphonuclear leukocytes after transfer from infected endothelial cells via transient microfusion events. J. Virol. 74:5629–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerna, G., M. G. Revello, E. Percivalle, M. Zavattoni, M. Parea, and M. Battaglia. 1990. Quantification of human cytomegalovirus viremia by using monoclonal antibodies to different viral proteins. J. Clin. Microbiol. 28:2681–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grefte, A., M. van der Giessen, W. van Son, and T. H. The. 1993. Circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J. Infect. Dis. 167:270–277. [DOI] [PubMed] [Google Scholar]
- 4.Grundy, J. E., K. M. Lawson, L. P. MacCormac, J. M. Fletcher, and K. L. Yong. 1998. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J. Infect. Dis. 177:1465–1474. [DOI] [PubMed] [Google Scholar]
- 5.Percivalle, E., M. G. Revello, L. Vago, F. Morini, and G. Gerna. 1993. Circulating endothelial giant cells permissive for human cytomegalovirus (HCMV) are detected in disseminated HCMV infections with organ involvement. J. Clin. Investig. 92:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plotkin, S. A., S. E. Starr, H. M. Friedman, E. Gonczol, and R. E. Weibel. 1989. Protective effect of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J. Infect. Dis. 159:860–865. [DOI] [PubMed] [Google Scholar]
- 7.Revello, M. G., E. Percivalle, E. Arbustini, R. Pardi, S. Sozzani, and G. Gerna. 1998. In vitro generation of human cytomegalovirus pp65 antigenemia, viremia, and leukoDNAemia. J. Clin. Investig. 101:2686–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Revello, M. G., F. Baldanti, E. Percivalle, A. Sarasini, L. De-Giuli, E. Genini, D. Lilleri, N. Labò, and G. Gerna. 2001. In vitro selection of human cytomegalovirus variants unable to transfer virus and virus products from infected cells to polymorphonuclear leukocytes and to grow in endothelial cells. J. Gen. Virol. 82:1429–1438. [DOI] [PubMed] [Google Scholar]
- 9.Sinzger, C., A. Grefte, B. Plachter, A. S. H. Gouw, T. H. The, and G. Jahn. 1995. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 76:741–750. [DOI] [PubMed] [Google Scholar]
- 10.Waldman, W. J., D. A. Knight, E. H. Huang, and D. D. Sedmak. 1995. Bidirectional transmission of infectious cytomegalovirus between monocytes and vascular endothelial cells: an in vitro model. J. Infect. Dis. 171:263–272. [DOI] [PubMed] [Google Scholar]