Abstract
Clinical tuberculosis (TB), whether noncavitary or cavitary, is the late stage of a chronic disease process, since Mycobacterium tuberculosis is a slowly growing organism. Our studies have shown that the profiles of antigenic proteins expressed by the in vivo bacteria that elicit antibodies differ in cavitary and noncavitary TB (K. Samanich, J. T. Belisle, and S. Laal, Infect. Immun. 69:4600-4609, 2001). To gain insight into antigenic proteins expressed during incipient, subclinical TB, an expression library of M. tuberculosis genomic DNA was screened with sera obtained during subclinical TB from guinea pigs infected with aerosols of M. tuberculosis H37Rv. One of the proteins recognized by antibodies elicited during subclinical TB infection of guinea pigs is the 309-kDa PPE55 (Rv3347c) protein. Genomic hybridization studies suggest that the PPE55 gene is specific to the M. tuberculosis complex and is present in a majority of clinical isolates tested. Antibodies to the C-terminal, ∼100-kDa fragment of PPE55 (PPE-C) were detectable in sera from 29/30 (97%) human immunodeficiency virus-negative/TB-positive (HIV− TB+) patients and 17/24 (71%) HIV+ TB+ patients tested but not in sera from purified-protein derivative-positive healthy controls, suggesting that the in vivo expression of PPE55 protein correlates with active M. tuberculosis infection. Anti-PPE-C antibodies were also detected in retrospective sera obtained months prior to manifestation of clinical TB from 17/21 (81%) HIV+ TB+ individuals tested, providing evidence that the protein is expressed during incipient, subclinical TB in HIV-infected humans. Thus, PPE55 is a highly immunogenic protein that may be useful for differentiating between latent TB and incipient, subclinical TB.
Approximately 5 to 10% of individuals who get infected with Mycobacterium tuberculosis progress to clinical tuberculosis (TB), whereas the remaining individuals develop a latent infection with the organism. Another 5 to 10% of these latently infected individuals reactivate their infection and progress to clinical TB during subsequent years/decades. In either case, active infection with M. tuberculosis is identified only when progression to bacteriologically detectable disease occurs. Thus, clinical TB, whether noncavitary paucibacillary or cavitary multibacillary disease, represents the late stages of a chronic disease process.
Our studies of humoral immune responses elicited by M. tuberculosis at different stages of infection and disease progression have shown that the profile of antigenic proteins expressed by the in vivo bacteria that elicit antibodies correlates with the stage of the infection (21-23, 35-37, 45). Thus, purified-protein derivative-positive (PPD+) healthy individuals have antibodies to only a small subset (4-6) of the >100 culture filtrate proteins (CFP) of M. tuberculosis. In contrast, patients with noncavitary paucibacillary TB have antibodies directed against ∼10 to 12 additional CFP antigens (35). As the disease progresses to the development of cavitary lesions, besides the presence of antibodies to the above-mentioned antigens, antibodies to an additional ∼10 to 12 antigens appear. These results provide evidence that as M. tuberculosis adapts to different in vivo environments, the profile of antigenic proteins that are expressed changes. Evidence for adaptation by M. tuberculosis to different environmental conditions by altering gene/protein expression has also come from several other labs (3, 11, 12, 29, 32, 41, 42, 47, 49).
M. tuberculosis is a slowly growing organism, and it takes weeks to months for the infection to progress to primary clinical TB. The time that elapses between the initiation of reactivation of latent infection and the manifestation of clinical TB is not known. The goal of the current studies was to identify antigenic proteins that are expressed during the asymptomatic, subclinical stages of infection when the in vivo M. tuberculosis bacilli replicate actively but the infection has not progressed to clinically identifiable disease (incipient, subclinical TB). Insight into these antigenic proteins will aid understanding of the host-pathogen interactions that lead to the progression of infection to clinical disease, and modulation of these host-pathogen interactions could potentially alter the outcome of infection. Moreover, antigenic proteins expressed during subclinical stages of active infection would also be useful for devising diagnostic markers that can differentiate between truly latent TB that is unlikely to progress to clinical disease and incipient, subclinical TB.
Humans with incipient, subclinical TB are asymptomatic and cannot be identified, since neither PPD reactivity nor any other markers of infection (ESAT-6, CFP10) can differentiate between individuals who have latent TB and those who have incipient, subclinical TB (21, 24). To determine the profile of antigenic proteins expressed by M. tuberculosis during incipient, subclinical TB, we used sera obtained from guinea pigs that were infected with low-dose aerosols of M. tuberculosis H37Rv and bled prior to progression of the infection to clinical TB. These subclinical-TB sera were used for immunoscreening of an expression library of genomic DNA of M. tuberculosis. Of the several clones that were identified, two clones expressed different portions of the same PPE55 (Rv3347c) protein. The PPE protein family was first identified when the genome of M. tuberculosis was sequenced, and the role of these proteins in the pathogenesis of TB is a subject of intense investigation (7). Some PPE proteins have been reported to elicit strong humoral and cellular responses in M. tuberculosis-infected animals and humans (5, 8, 9, 31, 46). In the current studies, we provide evidence that the PPE55 gene is specific to the M. tuberculosis complex and is present in a majority of the clinical isolates tested. The C-terminal region of the PPE55 protein was expressed in Escherichia coli and used to evaluate immune recognition by antibodies in sera from TB patients at different stages of the disease. Results provide evidence that PPE55 is a strongly immunogenic protein whose in vivo expression correlates with active infection with M. tuberculosis in humans with both incipient, subclinical TB and active clinical TB. Thus, the presence of anti-PPE55 antibodies can serve to distinguish between latent and active M. tuberculosis infection.
MATERIALS AND METHODS
Serum samples from guinea pigs.
Twenty-nine randomly bred, Hartley strain guinea pigs (350 to 500 g) were infected with 4 to 10 CFU airborne, virulent M. tuberculosis H37Rv in an aerosol infection chamber (27). Guinea pigs infected in this manner develop clinical signs of TB (weight loss and respiratory distress) around 8 to 10 weeks postinfection (p.i.) and die of TB by 14 to 18 weeks p.i. (27). To obtain sera representing the subclinical stage of active infection, groups of infected animals were euthanized and bled at 1 to 6 weeks p.i. Sera were also obtained from infected animals bled at 10 and 15 weeks p.i. to obtain specimens from animals with clinical TB. Since guinea pigs are exquisitely sensitive to infection with M. tuberculosis and do not develop latent infection, sera from three uninfected guinea pigs were included as specimens from animals who do not harbor actively replicating M. tuberculosis.
Serum samples from humans.
Serum samples obtained with informed consent from 132 individuals were included in these studies. These individuals can be categorized into the following groups.
(i) PPD+ healthy individuals.
Twenty-two of the 29 PPD+ healthy individuals were recent immigrants from areas where TB is endemic (India, China, Cameroon, and eastern Europe) who were working at the Manhattan Veterans Affairs Medical Center (VAMC) in New York, N.Y. A vast majority of these individuals were vaccinated with Mycobacterium bovis BCG, and it is likely that a high proportion would also have been exposed to M. tuberculosis. Seven U.S. citizens who were PPD+ were most likely latently infected with M. tuberculosis.
(ii) PPD− individuals.
The 10 PPD− individuals were healthy individuals working at the VAMC. These individuals were unlikely to be infected with M. tuberculosis.
(iii) HIV+ TB− individuals.
The 20 human immunodeficiency virus-positive/TB-negative (HIV+ TB−) individuals were HIV-infected individuals from the VAMC with no history or clinical symptoms of TB. Their PPD statuses were not known. All individuals except one were on antiretroviral therapy.
(iv) HIV+ TB+ patients.
The 24 HIV+ TB+ patients were acid-fast-bacillus (AFB) sputum smear-positive or smear-negative, culture-positive patients from the VAMC. These patients were bled at the time of clinical diagnosis of TB. These sera are considered equivalent to sera obtained from animals that were bled at 10 and 15 weeks p.i.
(v) HIV+ TB+ patients with subclinical TB.
Twenty-one of the 24 HIV+ TB+ patients described above were HIV+ patients who were routinely monitored for their T-cell profiles during the late 1980s and early 1990s (prior to highly active antiretroviral therapy) and who developed TB during the course of progression of HIV infection. Thus, sera that were obtained from these patients prior to progression to clinical TB (HIV+ subclinical TB) were available. These retrospective sera are considered the equivalent of sera obtained from guinea pigs bled during weeks 1 to 6 p.i. (22).
(vi) HIV− TB+ patients.
The 30 HIV− TB+ patients were AFB smear-positive TB patients with cavitary lesions, and sera were obtained from the Lala Ram Sarup Institute of Tuberculosis and Respiratory Diseases (LRSITRD), New Delhi, India. These sera are considered to be equivalent to sera obtained at weeks 10 and 15 p.i. from guinea pigs.
(vii) Household contacts of smear-positive TB patients.
The 19 household contacts of smear-positive TB patients (HIV− TB− HH contacts) were clinically asymptomatic household contacts of infectious, untreated, smear-positive TB patients, and these sera were also obtained from the LRSITRD, New Delhi. The PPD statuses of these individuals were not known. However, despite being asymptomatic, because of their frequent exposure to smear-positive family members, a small proportion of these individuals (estimated to be from 6 to 29% in different studies) are expected to have an active infection (6, 10, 14, 51).
M. tuberculosis H37Rv antigen preparations.
The M. tuberculosis H37Rv antigen preparations used were obtained from John T. Belisle under the NIH/NIAID TB Research Material and Vaccine Testing contract (Colorado State University, Fort Collins) and included (i) lipoarabinomannan-free CFP (LFCFP), (ii) sodium dodecyl sulfate-derived cell wall proteins (SDS-CW), (iii) cell wall fractions (CW), (iv) whole M. tuberculosis bacterial lysates, (v) purified native 45-kDa (MPT32) proteins, and (vi) purified native 38-kDa (PstS) proteins. The preparation of these proteins has been described earlier (23, 37).
Immunoscreening of the M. tuberculosis λgt11 library and identification of recombinant clones.
The λgt11 expression library of M. tuberculosis H37Rv DNA (World Health Organization) was screened with a serum pool prepared from guinea pigs bled at 1 to 6 weeks p.i. (38, 52). Serum from one animal was randomly selected from each time point for inclusion into the pool. DNA sequencing of the M. tuberculosis inserts, analysis of sequences, and identification of the M. tuberculosis proteins expressed in the positive recombinant λgt11 clones were performed as described earlier (45).
Mycobacterial-DNA isolation and Southern blot analysis.
Genomic DNA from M. tuberculosis H37Rv, M. tuberculosis H37Ra, M. tuberculosis Erdman, and clinical isolates CSU 11, CSU 17, CSU 19, CSU 22, CSU 25, CSU 26, and CSU 27 were obtained from John T. Belisle. Stock cultures of M. bovis, M. bovis BCG, M. africanum, M. microti, M. smegmatis, M. vaccae, M. phlei, M. chelonae, and M. xenopi were obtained from the ATCC (American Type Culture Collection, Manassas, VA) and grown in Middlebrook 7H9 broth (Difco) supplemented with 0.2% glycerol, 0.05% Tween 80, and 1× ADS (0.5% bovine serum albumin [BSA], fraction V [Sigma, St. Louis, MO]; 0.2% dextrose; and 0.85% NaCl). The genomic-tip system (QIAGEN Inc., Valencia, CA) was used to extract genomic DNA from mid-log-phase bacterial cultures per the manufacturer's protocols. One microgram of each genomic DNA was digested with SacI, the fragments were separated on a 0.8% agarose gel, and the Southern blots that were prepared were probed with a 480-bp digoxigenin (DIG)-labeled probe amplified with primers PPE1 (5′-GTGTTGTACACGCCCGAGATGCCC-3′) and PPE2 (5′-GAACCCGACGACACTGCACTTCCC-3′) from M. tuberculosis H37Rv genomic DNA (DIG probe synthesis kit; Roche Diagnostic Corporation, IN). These primers allow the amplification of a 480-bp fragment only from the PPE55 gene. Hybridization and detection were performed by using a DIG standard hybridization buffer and a chemiluminescence detection system according to the manufacturer's protocols (Roche).
Expression and purification of recombinant M. tuberculosis PPE55 protein.
A 3,072-bp fragment encoding the C-terminal 978 amino acids (aa) of PPE55 (designated PPE-C) was amplified from M. tuberculosis H37Rv genomic DNA with primers 5′-CTTCCATATGGGCAGCATCAACACC-3′ (forward primer) and 5′-TGCCCTCGAGGACTTCTTATTTCATAC-3′ (reverse primer) containing NdeI and XhoI sites (underlined), respectively. The amplified PPE-C fragment was cloned into a pCR BluntII TOPO vector (Invitrogen Corporation, Carlsbad, CA) and verified by sequencing. Subsequently, the PPE-C fragment was cloned at NdeI-XhoI sites into expression vector pET-14b (Novagen Inc., Madison, WI) to generate pET14b-PPE-C. After verification of the reading frame, the recombinant plasmid pET14b-PPE-C was transformed into E. coli expression host Rosetta (DE3), and a single colony containing the recombinant plasmid was used as the source of the recombinant PPE-C protein. Uninduced E. coli harboring the recombinant plasmid and induced cultures of E. coli containing the pET14b vector alone were used as controls. The recombinant protein was purified from lysates of induced E. coli cells by using Ni-nitrilotriacetic acid agarose affinity chromatography according to the manufacturer's protocols (QIAGEN).
Synthetic peptides.
The amino acid sequence of the PPE-C protein was subjected to analysis for regions of high antigenicity by computer algorithms that predict antigenic regions based on probability of surface exposure, local hydrophilicity, beta-turns, etc. (PCGENE [Intelligenetics, Inc., Mountain View, CA]; EMBOSS Antigenic [EMBO software]; Epitope Informatics [Epitope Informatics Ltd., Durham, United Kingdom]; Peptool [Life Sciences Software Resources, Inc.]) (16, 17, 20, 33). The prediction of antigenic regions and synthesis of corresponding peptides tagged with an N-terminal biotin residue were performed by Global Peptide Services, Fort Collins, CO.
Western blot analysis.
Reactivity of guinea pig or human sera with LFCFP or lysates of E. coli expressing His-tagged PPE-C protein or purified His-PPE-C protein was tested by Western blotting as described previously (44). Briefly, LFCFP, lysates of E. coli expressing the PPE-C protein, appropriate control lysates, or purified PPE-C protein was fractionated on 10% SDS-polyacrylamide gels under reducing conditions, and the Western blots were probed with anti-His monoclonal antibody (MAb) or E. coli lysate-absorbed sera from guinea pigs or humans (1:100) (23). The blots of purified PPE-C protein were probed with unabsorbed sera from PPD+ healthy individuals or smear-positive TB patients (1:100). The detection system was comprised of alkaline phosphatase-conjugated anti-mouse immunoglobulin G (IgG), anti-guinea pig IgG, or anti-human IgG (Zymed Laboratories, Inc., CA) and BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium (KPL, Inc., Gaithersburg, MD) as the substrate.
ELISA with M. tuberculosis antigens and peptides.
Enzyme-linked immunosorbent assays (ELISA) were optimized to detect the reactivities of sera from infected guinea pigs with M. tuberculosis antigens (37). LFCFP, SDS-CW, CW, and M. tuberculosis cell lysates were coated at 5 μg/ml, and purified MPT32 and 38-kDa antigens were coated at 2 μg/ml (50 μl/well). The guinea pig sera were absorbed against E. coli lysates to remove cross-reactive antibodies and tested for reactivity with these antigens at a 1:100 dilution (23, 37).
For assessment of the reactivity of the PPE-C protein with sera from TB patients and control individuals, purified recombinant PPE-C was coated at 10 μg/ml (50 μl/well) overnight at 4°C. The plates were washed three times with phosphate-buffered saline (PBS) containing 0.1% Tween 20, and the wells were blocked with 1% BSA-PBS containing 0.1% Tween 20 (blocking buffer) for 2 h at 37°C. After three washes, the antigen-coated wells were exposed to sera (1:50 in 0.1× blocking buffer) for 30 min at 37°C, followed by exposure to a mixture of diluted alkaline phosphatase-conjugated protein A (1:2,000; Sigma) and goat anti-human IgA (1:1,000; Sigma) for 30 min at 37°C. The wells were washed eight times with Tris-buffered saline (50 mM Tris, 150 mM NaCl), and the color was developed using an Invitrogen amplification system (Invitrogen). The mean optical density at 490 nm (OD490) obtained with sera from PPD+ and PPD− individuals, plus 3 standard deviations (SD), was used as the cutoff to determine positive responses in the patients.
For assessing the reactivities of biotinylated peptides with sera from TB patients and controls, a mixture of 50 μl of peptide (1 μg/ml) diluted in blocking buffer (7.5% fetal bovine serum [HyClone, Utah] and 2.5% BSA in PBS) and 50 μl of sera (1:10 in 0.1× blocking buffer) was added to wells of streptavidin-coated ELISA plates (Roche) and incubated for 1 h at 37°C. The peptide-antibody complexes bound to the wells were detected as described above.
Statistical analysis.
The Fisher test was used to determine the correlation between the presence of antibodies to peptides PPE-C1 and PPE-C2 in the HIV− TB+, HIV+ TB+, and subclinical-TB patient groups.
Bioinformatic analysis.
DNA and protein sequence similarities were performed by using the BLAST program on the National Center for Biotechnology Information (NCBI) website. BLAST searches with genome databases of M. tuberculosis H37Rv, M. tuberculosis CDC1551, M. tuberculosis 210, M. bovis, M. leprae, M. avium 104, M. avium subsp. paratuberculosis, M. marinum, and M. smegmatis were also performed using the genomic BLAST program on the NCBI website. The BLAST search with the M. ulcerans genome database was done on the BuruList World Wide Web server. Prediction of theoretical molecular weight and amino acid composition by ProtParam, determination of transmembrane helices by TMpred, identification of repetitive sequences by statistical analyses of protein sequences, signal peptide assessment by SignalP (version 2.0) trained on gram-positive bacteria, determination of glycosylation sites by NetOGlyc 2.0, and Kyte & Doolittle hydropathicity plotting by ProtScale were carried out with the respective softwares available on the ExPASy Proteomics server.
RESULTS
Anti-M. tuberculosis humoral responses in aerosol-infected guinea pigs.
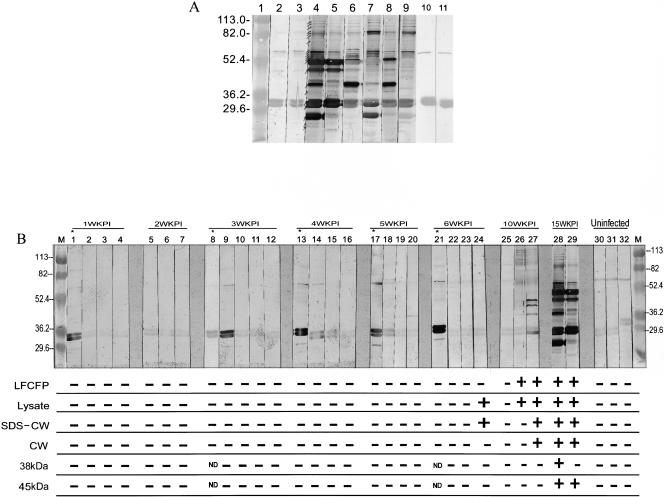
Previous studies from our lab have shown that sera from PPD+ healthy individuals have antibodies to a doublet of antigens at ∼32 to 33 kDa and another protein band at ∼65 to 67 kDa in the LFCFP (35). The same three antigens are also recognized by sera from PPD− healthy individuals (35). Mapping of these antigens identified the doublet to be the antigen 85 complex (35). Sera from cavitary-TB and noncavitary-TB patients have antibodies to these three cross-reactive antigens and several additional antigens in the LFCFP ranging from ∼26 to 115 kDa (35). To validate the use of guinea pigs for studies of humoral immune responses to M. tuberculosis antigens, reactivities of LFCFP with sera from two guinea pigs with TB (15 weeks p.i.) and from four randomly chosen TB patients were evaluated by Western blotting. The sera from uninfected guinea pigs and PPD+ healthy individuals, neither of whom would have had replicating M. tuberculosis, were included as controls. The sera from the uninfected animals and PPD+ humans showed reactivity with the doublet of M. tuberculosis antigens at ∼32 to 33 kDa and a single band at ∼68 kDa (Fig. 1A, lanes 2, 3, 10, and 11). In contrast, sera from guinea pigs with TB showed reactivity with several additional bands ranging from ∼26 kDa to ∼115 kDa (Fig. 1A, lanes 4 and 5). The guinea pigs with TB and the human TB patients had antibodies to similar profiles of proteins in the LFCFP (Fig. 1A, lanes 6 to 9, and additional patients in reference 35). As reported earlier with sera from different TB patients, some variation in the intensities of reactivity with individual proteins between the sera from the two animals and the four human patients was observed (35).
FIG. 1.
(A and B) Reactivities of sera from guinea pigs and humans with antigens of M. tuberculosis. (A) Western blots of LFCFP (10 μg/ml) were probed with sera from guinea pigs and humans. Lanes 2 and 3 were probed with sera from two uninfected guinea pigs, lanes 4 and 5 with sera from two M. tuberculosis H37Rv-infected guinea pigs bled at 15 weeks p.i., lanes 6 to 9 with sera from four HIV− TB+ patients, and lanes 10 and 11 with sera from two PPD+ healthy individuals. Lane 1 contains molecular weight markers in thousands. The major protein bands recognized by sera from tuberculous guinea pigs and HIV− TB+ patients are indicated by arrows in lane 4. (B) Western blots of LFCFP (10 μg/ml) were probed with sera from guinea pigs. Lanes 1 to 4 were probed with sera from four M. tuberculosis-infected guinea pigs obtained at 1 week p.i. (1WKPI), lanes 5 to 7 with sera from three animals obtained at 2 weeks p.i., lanes 8 to 12 with sera from five animals obtained at 3 weeks p.i., lanes 13 to 16 with sera from four animals obtained at 4 weeks p.i., lanes 17 to 20 with sera from four animals obtained at 5 weeks p.i., lanes 21 to 24 with sera from four animals obtained at 6 weeks p.i., lanes 25 to 27 with sera from three animals obtained at 10 weeks p.i., lanes 28 and 29 with sera from two animals obtained at 15 weeks p.i., and lanes 30 to 32 with sera from three uninfected guinea pigs. Lanes M contain molecular weight markers (in thousands). The positive and negative signs below each lane indicate the reactivities of the respective serum specimens with M. tuberculosis LFCFP, lysate, SDS-CW, CW, and purified 38-kDa and 45-kDa antigens, as determined by ELISA. The mean OD490 obtained with sera from uninfected guinea pigs plus 3 SD was used as the cutoff. ND, not done.
M. tuberculosis antigens recognized during subclinical TB in infected guinea pigs.
After confirmation that PPD+/PPD− healthy individuals and uninfected guinea pigs (lacking actively replicating bacteria) recognize similar sets of LFCFP antigens and that TB patients and tuberculous guinea pigs recognize similar profiles of antigens, the antigen profile recognized by antibodies obtained from the guinea pigs during subclinical TB (1 to 6 weeks p.i.) was investigated. Sera from animals with TB (10 and 15 weeks p.i.) were included as positive controls, and sera from uninfected animals were included as negative controls (Fig. 1B). Sera obtained 1 to 6 weeks p.i. reacted primarily with the doublet at ∼32 to 33 kDa, and some sera also recognized proteins of ∼68 kDa or, weakly, proteins of 29 kDa (Fig. 1B, lanes 1 to 24). In contrast, sera from two out of three animals bled at 10 weeks p.i. and from both animals bled at 15 weeks p.i. recognized multiple antigens in the LFCFP (Fig. 1B, lanes 26 to 29). The reactivities of all of these sera with cell lysate and cell wall antigens of M. tuberculosis and with the purified 38-kDa (PstS) and 45-kDa (MPT32) proteins, which are known to be associated with advanced clinical TB in humans, were also tested by ELISA (4, 35). Using the mean OD plus 3 SD obtained with the sera from the three uninfected guinea pigs as the cutoff, except for one animal bled at 6 weeks p.i., reactivity with the mycobacterial antigens was observed only with sera from animals bled at 10 or 15 weeks p.i. (Fig. 1B). These results suggested that strong antibody responses to antigens present in these mycobacterial fractions and to the purified 38- and 45-kDa antigens are elicited only during the advanced stages of active infection, when clinical TB manifests. During subclinical TB, either the antibody titers are too low or the target antigens are absent or quantitatively underrepresented in the antigen preparations being used.
Screening of the M. tuberculosis λgt11 library and characterization of recombinant clones.
To explore the possibility that antibodies are present in subclinical-TB sera but that the antigens that elicit these antibodies are poorly represented in our antigen preparations, a subclinical-TB serum pool containing one serum from each time point (1 to 6 weeks p.i.) was used for immunoscreening of the λgt11 expression library of M. tuberculosis H37Rv. Nine strongly reactive recombinant clones were obtained. This study describes the results obtained with two of the nine clones (clones 1 and 2).
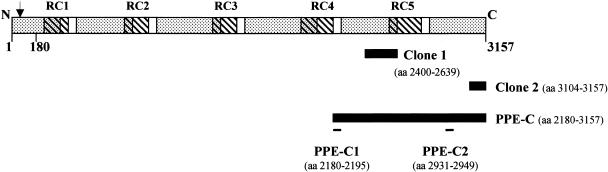
DNA sequence and restriction analyses revealed that the peptides expressed in clones 1 and 2 represent aa 2400 to 2639 and 3104 to 3157, respectively, in the C-terminal half of the same PPE55 (Rv3347c) gene product (Fig. 2). PPE55 is a 3,157-aa (309-kDa) protein which belongs to the MPTR (major polymorphic tandem repeat) subfamily of the PPE family of M. tuberculosis proteins (7). PPE55 contains 47 tandem copies of the motif Asn-X-Gly-X-Gly-Asn-X-Gly (perfect repeats) and 49 tandem copies of the same motif with alterations at positions 5 and 6 (imperfect repeats; total of 96 repeats) in five clusters (repetitive clusters [RC]) that show 76 to 81% identity with each other. The protein has six large hydrophobic regions alternating with five hydrophilic regions (20), and each of the five RC overlaps with portions of both the hydrophobic and the hydrophilic regions (Fig. 2). PPE55 has a signal peptide, a putative signal peptidase cleavage site between aa 29 and 30, 29 putative transmembrane helices, and 41 putative O-glycosylation sites. The contents of Gly (19.9%), Asn (11.1%), and hydrophobic amino acids (29.6%, including Leu, Val, Ile, Phe, and Met) are relatively high.
FIG. 2.
Schematic representation of the PPE55 (Rv3347c) protein of M. tuberculosis H37Rv. The dotted areas show hydrophobic regions. The striped areas indicate the positions of the repetitive clusters (RC1 to RC5). The black bars denote the portions of the PPE55 protein expressed in λgt11 clones 1 and 2 and in the PPE-C protein. The positions of the peptides are indicated by black lines. The arrow indicates the signal peptidase cleavage site. The conserved N-terminal 180-aa region is marked.
The N-terminal 180 aa of PPE55 showed 60 to 90% homology with the N-terminal amino acids of other PPE proteins in the M. tuberculosis database. In contrast, the C-terminal region of PPE55 (2,977 aa) is strongly homologous (67%) to PPE56, but only small regions of homology are observed with the other PPE proteins. In the M. bovis isolate that is sequenced, the homologous gene is split into two parts as a result of a single base deletion, and the existence of two proteins, PPE55a (2,096 aa) and PPE55b (1,061 aa), is predicted. A BLAST analysis of the PPE55 N-terminal 180-aa region against the M. leprae and M. avium subsp. paratuberculosis databases showed 30 to 66% homology with multiple proteins, while the C-terminal region showed poor homology in small regions. Similar results were obtained when a BLAST analysis against the unfinished databases of M. avium 104, M. marinum, M. ulcerans, and M. smegmatis was performed.
Distribution of the PPE55 gene in mycobacteria.
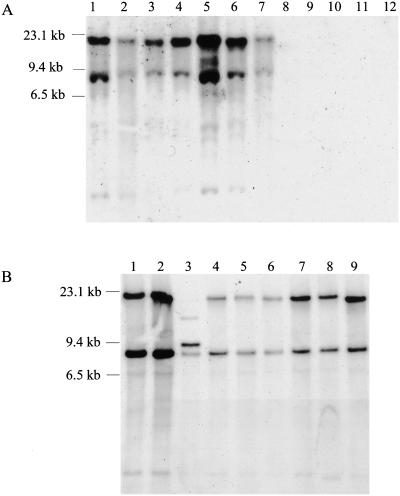
A BLAST analysis suggested that no genes that were strongly homologous to PPE55 were present in the non-TB mycobacterial species that have been sequenced. To further determine if the PPE55 gene is specific to M. tuberculosis and to determine if the gene is conserved between clinical isolates, the distribution of the PPE55 gene in the M. tuberculosis complex, M. tuberculosis clinical isolates, and nontuberculous mycobacterial species was determined by Southern blot analysis. The DIG-labeled, 480-bp PCR product of primers PPE1 and PPE2, which was used to probe the Southern blots, hybridized with two fragments of ∼23 kb and ∼8 kb in genomic DNA from all M. tuberculosis complex species but not with any of the nontuberculous mycobacterial species tested (Fig. 3A). When the same blot was reprobed with a PCR-generated 23S gene probe, all mycobacterial species showed a hybridization signal (data not shown). Although primers PPE1 and PPE2 were designed to amplify a 480-bp fragment only from the PPE55 gene, the nucleotide sequence of this fragment showed 83% identity with a 462-bp fragment from PPE56 and 89% identity with a 194-bp fragment from PPE54. Restriction analysis of the PPE55 gene and flanking regions showed that the ∼23-kb fragment contains the portion of the PPE55 gene encompassing the probe sequence and the complete PPE54 gene. The ∼8-kb fragment contains a portion of the PPE56 gene encompassing the region which is homologous with the probe sequence. Thus, hybridization with the ∼23-kb fragment would be due to hybridization with the PPE55 gene and cross-hybridization with the PPE54 gene. The hybridization with the ∼8-kb fragment would be due to hybridization with the PPE56 gene. The same pattern of hybridization with two bands at ∼23 kb and ∼8 kb was also observed with the genomic DNA from 6 out of 7 clinical isolates tested (Fig. 3B). The different hybridization pattern obtained with one clinical isolate (CSU 17) could be due to changes in the nucleotide sequence of either the PPE55 gene or the flanking regions and was not investigated further. These results indicate that the PPE55 gene is specific to M. tuberculosis complex species and is present in a majority of the clinical isolates tested.
FIG. 3.
(A and B) Presence of the PPE55 (Rv3347c) gene in different mycobacterial species and M. tuberculosis clinical isolates. Southern blots of SacI-digested genomic DNA were probed with a 480-bp, DIG-labeled, PCR-generated PPE55 gene fragment. (A) DNA from M. tuberculosis H37Rv (lane 1), M. tuberculosis H37Ra (lane 2), M. bovis BCG (lane 3), M. bovis (lane 4), M. africanum (lane 5), M. microti (lane 6), M. tuberculosis Erdman (lane 7), M. smegmatis (lane 8), M. vaccae (lane 9), M. phlei (lane 10), M. chelonae (lane 11), and M. xenopi (lane 12). (B) DNA from M. tuberculosis H37Rv (lane 1); M. tuberculosis clinical isolates CSU 11, CSU 17, CSU 19, CSU 22, CSU 25, CSU 26, and CSU 27 (lanes 2 through 8, respectively); and M. tuberculosis Erdman (lane 9).
Expression of recombinant PPE55 protein and its reactivity with sera from M. tuberculosis-infected guinea pigs and TB patients.
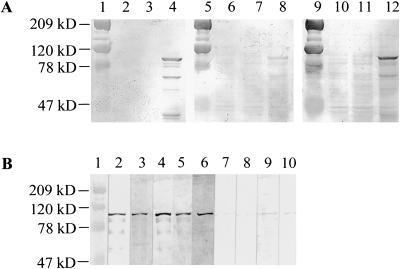
Since the PPE55 protein was immunogenic in M. tuberculosis-infected guinea pigs, its immunogenicity in humans with TB was investigated. Both of the clones (1 and 2) identified by the guinea pig sera expressed fragments located in the C-terminal region of the PPE55 protein, suggesting that this portion of the protein is immunogenic. Since the PPE55 protein is too large to be expressed as one unit, the C-terminal 978 amino acids (PPE-C; ∼99 kDa) were cloned into pET14b in frame with the N-terminal His tag (Fig. 2). The PPE-C protein encompasses the regions that were expressed in clones 1 and 2 and contains the RC5 region of PPE55 (Fig. 2). Lysates of induced E. coli cells expressing the His-tagged PPE-C protein contained an ∼99-kDa protein recognized by anti-His MAb (Fig. 4A, lane 4). Two other bands (∼66 kDa and ∼36 kDa) reactive with anti-His antibody were also present, suggesting that the PPE-C protein may undergo processing/degradation in E. coli. No protein bands that react with anti-His antibody were detectable in either of the control lysates. The serum pool used for immunoscreening of the library, but not the pool from the uninfected guinea pigs, showed strong reactivities with the ∼99-kDa and ∼66-kDa bands but not with the ∼36-kDa band (Fig. 4A, lane 12). These data suggest that immunogenic regions are present only in the ∼99-kDa and ∼66-kDa proteins and also confirm that PPE55 is expressed during subclinical TB in guinea pigs and that the PPE-C fragment of the PPE55 protein is immunogenic.
FIG. 4.
(A and B) Expression of a recombinant His-tagged, C-terminal fragment of PPE55 (PPE-C) and its reactivities with guinea pig and human sera. (A) Western blots of E. coli lysates were probed with anti-His MAb (lanes 2 to 4), a serum pool from uninfected guinea pigs (lanes 6 to 8), and a serum pool from M. tuberculosis H37Rv-infected guinea pigs bled 1 to 6 weeks p.i. (lanes 10 to 12). Lanes 1, 5, and 9 contain molecular mass markers; lanes 2, 6, and 10 contain lysates of induced E. coli containing vector alone; lanes 3, 7, and 11 contain lysates of uninduced E. coli containing recombinant plasmid; and lanes 4, 8, and 12 contain lysates of induced E. coli containing recombinant plasmid. (B) Western blots of purified PPE-C protein were probed with anti-His MAb (lane 2), sera from four HIV− TB+ patients (lanes 3 to 6), or sera from four PPD+ healthy individuals (lanes 7 to 10). Lane 1 contains molecular mass markers.
To confirm that anti-PPE-C antibodies are detectable in TB patients, the recombinant protein was purified by Ni-nitrilotriacetic acid agarose affinity chromatography. A Western blot of purified PPE-C protein probed with anti-His MAb confirmed the presence of the ∼99-kDa band in the purified protein preparation (Fig. 4B, lane 2). As was observed with lysates of E. coli expressing PPE-C protein, some additional protein bands, probably degradation products of the recombinant protein, were also present. Western blots of purified PPE-C protein were probed with sera from four HIV− TB+ patients and four PPD+ healthy individuals. Antibodies that recognized the ∼99-kDa PPE-C protein were present only in the sera from TB patients and not in the sera from the PPD+ healthy individuals tested (Fig. 4B, lanes 3 to 6).
Reactivity of purified PPE-C protein with sera from various groups of TB patients.
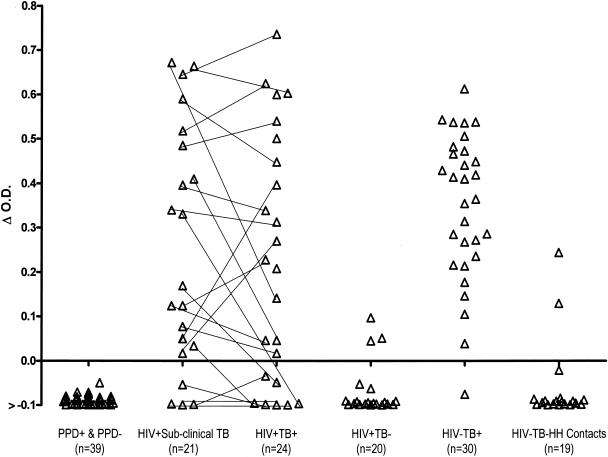
After confirmation that anti-PPE-C antibodies are present in TB patients but not in PPD+ healthy individuals, the ability of the protein to distinguish between patients with active infection and latent or no infection with M. tuberculosis was determined. The reactivity of the purified PPE-C protein was evaluated with sera from PPD− and PPD+ healthy individuals, as well as from patients with subclinical TB. Sera from patients with clinical TB were also included. There was no difference in the reactivities of sera obtained from PPD+ or PPD− healthy individuals, suggesting that antibodies to this protein are absent in healthy individuals with latent, inactive M. tuberculosis infection or in BCG-vaccinated individuals (Fig. 5). With the mean OD obtained with the PPD+ and PPD− healthy individuals plus 3 SD as the cutoff, none of the sera from these individuals contained anti-PPE-C antibodies. In contrast, subclinical-TB sera (obtained from 3 months to 2 years prior to manifestation of clinical TB) from 17/21 (81%) HIV+ TB+ patients had anti-PPE-C antibodies (Fig. 5). Sera from 29/30 (97%) HIV− TB+ patients and 17/24 (71%) HIV+ TB+ patients also had detectable anti-PPE-C antibodies (Fig. 5), confirming the correlation of active M. tuberculosis infection and anti-PPE-C antibodies. Each serum specimen was tested in three separate ELISA, and only specimens which tested positive three of three or two of three times were considered positive. No correlation between the presence or absence of anti-PPE-C antibodies and the AFB smear status, radiological features, or lymphocyte profile was observed for the HIV+ TB+ patients (Table 1). Anti-PPE-C antibodies were detected in sera from 10 to 15% of the individuals considered to be at high risk for TB; 3/20 (15%) HIV+ TB− individuals and 2/19 (11%) household contacts of smear-positive patients possessed anti-PPE-C antibodies (Fig. 5).
FIG. 5.
Reactivities of purified PPE-C protein with sera from humans. Serum samples from PPD+ and PPD− healthy individuals; HIV+ subclinical-TB, HIV+ TB+, HIV+ TB−, and HIV− TB+ patients; and HIV− TB− HH contacts were tested by ELISA for reactivity with purified PPE-C. The mean OD490 obtained with PPD+ and PPD− healthy individuals, plus 3 SD, was used as the cutoff. The OD obtained with any serum specimen minus the cutoff (Δ O.D.) for each specimen is plotted. Each line connects the reactivity of serum obtained during subclinical-TB infection with that of serum obtained at the time of diagnosis of TB from the same HIV+ TB+ patient.
TABLE 1.
Correlation between AFB smear status, radiological features, lymphocyte profile, and antibodies to PPE-Ce
| Disease status (no. of patients) | AFB smear status (no. of patients) | Radiological appearance (no. of patients) | Lymphocyte profile
|
No. (%) of patients screened for antibodies to PPE-C
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute cell count
|
% Cells
|
||||||||
| T | CD4 | CD4 | CD8 | B | Positive | Negative | |||
| HIV+ subclinical TB (21) | ND | ND | 225-3,350 | 9-525 | 1-23 | 31-82 | 1-25 | 17 (81) | 4 (19) |
| HIV+ TB+ (24)a | Positive (16) | Cav. (2)d; non-cav. (14)b | 155-3,962 | 7-552 | 1-29 | 24-71 | 3-29 | 13 (81)d | 3 (19) |
| Negative (7) | Cav. (0); non-cav. (7)c | 467-1,309 | 71-332 | 9-22 | 46-70 | 2-19 | 3 (43) | 4 (57) | |
| HIV+ TB− (20) | ND | ND | 641-2,698 | 108-862 | 8-54 | 19-72 | 3-27 | 3 (15) | 17 (85) |
| HIV− TB+ (30) | Positive (30) | Cav. (30); non-cav. (0) | ND | ND | ND | ND | ND | 29 (97) | 1 (3) |
| HIV− TB− HH contact (19) | ND | ND | ND | ND | ND | ND | ND | 2 (11) | 17 (89) |
For one patient, AFB smear and radiographic results were not available.
Eight patients had infiltration, one had patchy areas, one had pleural effusion, and four showed no pulmonary changes.
One patient had infiltration, one had patchy areas, and five showed no pulmonary changes.
Both cavitary-TB patients were positive for antibodies to PPE-C.
ND, not done; cav., cavitary TB; non-cav., non-cavitary TB.
Reactivities of antigenic peptides of PPE-C protein with sera from TB patients.
Since PPE55 is a member of a protein family, to determine if the anti-PPE-C antibodies are elicited by this protein or by other PPE proteins with cross-reactive regions, efforts to identify PPE55-specific immunogenic regions were made. The analysis by computer algorithms of the PPE-C amino acid sequence for regions with high antigenicity identified two immunogenic regions (Fig. 2). Peptides corresponding to both regions (PPE-C1 and PPE-C2) were synthesized commercially. The sequence of PPE-C1 (aa 2180 to 2195; GSINTGWFNTGNANTG) showed homology with the majority of PPE proteins from the M. tuberculosis H37Rv, M. tuberculosis CDC1551, and M. bovis genome databases. This peptide also showed homology with multiple fragments from the M. tuberculosis 210 and M. marinum unfinished-genome databases. No match was obtained when analyses of the M. leprae and M. avium subsp. paratuberculosis genomes and of the unfinished genomes of M. ulcerans, M. smegmatis, and M. avium 104 were performed. In contrast, PPE-C2 (aa 2931 to 2949; GFKVRPSFSFFAVGPDGMP) was specific to PPE55 and showed no homology with any other PPE protein or any protein/region from the M. tuberculosis H37Rv, M. tuberculosis CDC1551, M. bovis, M. tuberculosis 210, and M. ulcerans databases. Despite the 67% homology of the C-terminal regions of PPE55 and PPE56, no region homologous to the PPE-C2 peptide was found with the latter. The PPE-C2 sequence showed weak homology (∼40%) with two proteins (not PPE proteins) from the M. leprae genome database. One protein from each of the M. avium subsp. paratuberculosis, M. marinum, M. avium 104, and M. smegmatis databases also showed ∼40% homology. Thus, while PPE-C1 encompasses a cross-reactive immunogenic region, PPE-C2 appears to be specific to PPE55.
Antibodies to the PPE-C1 peptide were detected in 1/39 (3%) control individuals (97% specificity). In contrast, 6/21 (29%), 11/24 (46%), and 17/30 (57%) serum samples from HIV+ subclinical-TB, HIV+ TB+, and HIV− TB+ patients, respectively, contained anti-PPE-C1 antibodies. Similarly, anti-PPE-C2 antibodies were detected in only 1/39 (3%) healthy individuals but in 12/21 (57%), 12/24 (50%), and 16/30 (53%) serum samples from HIV+ subclinical-TB, HIV+ TB+, and HIV− TB+ patients, respectively (Table 2). Even when additive reactivities with both peptides were considered, the sensitivities of antibody detection (57%, 63%, and 67% in HIV+ subclinical-TB, HIV+ TB+, and HIV− TB+ patients, respectively) were lower than the sensitivity obtained with the PPE-C protein in each group of patients, indicating that additional immunogenic regions must exist on the protein (Table 2).
TABLE 2.
Reactivities of antigenic peptides of PPE-C with sera from TB patientsa
| Disease status (no. of patients) | No. (%) of patients with antibodies to:
|
No. (%) of PPE-C antibody-positive patients with antibodies to:
|
P valueb | |||||
|---|---|---|---|---|---|---|---|---|
| PPE-C protein | PPE-C1 peptide | PPE-C2 peptide | PPE-C1 and/or PPE-C2 peptides | PPE-C1 and/or PPE-C2 peptides | PPE-C1 peptide | PPE-C2 peptide | ||
| HIV+ subclinical TB (21) | 17 (81) | 6 (29) | 12 (57) | 12 (57) | 11 (65) | 5 (45) | 11 (100) | 0.0351 |
| HIV+ TB+(24) | 17 (71) | 11 (46) | 12 (50) | 15 (63) | 12 (71) | 9 (75) | 10 (83) | 0.0104 |
| HIV− TB+ (30) | 29 (97) | 17 (57) | 16 (53) | 20 (67) | 20 (69) | 17 (85) | 16 (80) | 0.0086 |
The presence of antibodies to PPE-C and peptides PPE-C1 and PPE-C2 in sera from TB patients and PPD+/PPD− healthy controls was determined by ELISA. The mean OD plus 3 SD obtained with healthy controls was used as the cutoff to determine positive responses in patients.
Correlation between the presence of antibodies to PPE-C1 and PPE-C2, as determined by the Fisher test.
To determine if the anti-PPE-C antibodies are elicited by the PPE55 protein or another PPE protein(s), the proportion of patients with anti-PPE-C1 (cross-reactive epitope) or anti-PPE-C2 (specific epitope) antibodies among patients with PPE-C antibodies was determined. Of the patients who had antibodies to the PPE-C protein, anti-PPE-C1 antibodies were present in 5/11 (45%), 9/12 (75%), and 17/20 (85%) patients, and anti-PPE-C2 antibodies were present in 11/11 (100%), 10/12 (83%), and 16/20 (80%) patients from the HIV+ subclinical-TB, HIV+ TB+, and HIV− TB+ patient groups, respectively (Table 2). There was a statistically significant correlation between the presence of antibodies to the two peptides in the three patient groups (P values were 0.0351, 0.0104, and 0.0086 for patients from the HIV+ subclinical-TB, HIV+ TB+, and HIV− TB+ groups, respectively), indicating that the anti-PPE-C antibodies detected in the patients are elicited by the in vivo expression of PPE55.
DISCUSSION
The goal of these studies was to identify antigens expressed by actively replicating in vivo M. tuberculosis during subclinical stages of TB but not by the latent bacteria. Since humans with incipient, subclinical TB cannot be recognized, antibodies in sera from M. tuberculosis aerosol-infected guinea pigs were used as markers of in vivo-expressed proteins. Several studies have shown that M. tuberculosis infection in guinea pigs resembles M. tuberculosis infection in humans in the development of typical mononuclear cell granulomas in the lungs, caseation, extrapulmonary dissemination, hematogenous reseeding of apical lobes, and development of the delayed-type-hypersensitivity reaction (26). The use of the guinea pig model for studies of anti-M. tuberculosis humoral responses was validated in the current studies by the recognition of similar profiles of antigens in the LFCFP by antibodies from tuberculous humans and guinea pigs. Earlier studies have shown that in guinea pigs infected with a low dose (4 to 10 CFU) of M. tuberculosis, the inhaled mycobacteria replicate exponentially in the lungs during the first 3 to 4 weeks p.i. Dissemination via the lymphatics to the lymph nodes draining the lungs occurs at about 8 to 10 days p.i., with organisms reaching the spleen via the bloodstream between 14 and 20 days. Within 4 weeks following initiation of pulmonary infection, there is seeding of mycobacteria into secondary foci throughout the lungs via hematogenous dissemination (15, 28). The clinical signs of TB, such as weight loss and respiratory distress, usually occur around 8 to 10 weeks p.i., and mortality is generally observed around 14 to 18 weeks (27). Based on these well-defined kinetics of infection, the period of 1 to 6 weeks p.i. for these animals corresponds to the stage when they have incipient, subclinical TB but have not yet progressed to clinically evident TB.
The antibodies in the guinea pig subclinical-TB serum pool identified PPE55 as one of the proteins expressed during incipient M. tuberculosis infection. The characteristics of PPE55 (signal sequence and transmembrane regions) indicate that PPE55 should be a cell surface-associated or -secreted protein. However, no protein of this size was detectable by Western blot analysis of the LFCFP (Fig. 1B) or the SDS-CW preparations (data not shown). These results indirectly suggest that PPE55 is either poorly expressed during growth in bacteriological media or degraded during preparation of the mycobacterial-antigen fractions. Two other PPE proteins, PPE34 (Rv1917c) and PPE68 (Rv3873), have also been shown to be cell surface associated, and the latter protein has been reported to be quantitatively a minor component in preparations derived from in vitro-grown bacteria (8, 31, 39). Moreover, proteomic studies of M. tuberculosis have rarely identified any PPE proteins as dominant components in preparations made from M. tuberculosis grown in bacteriological media, suggesting poor expression in this environment (2, 19, 34, 40).
The recognition of the PPE-C protein by sera obtained during subclinical-TB infection from HIV+ TB+ patients provides evidence that it is also expressed at this stage of infection in humans. Moreover, the absence of anti-PPE-C antibodies in PPD+ individuals shows that anti-PPE-C antibodies may be useful as markers that can discriminate between latent and incipient infection with M. tuberculosis, especially since PPE55 appears to be restricted to the M. tuberculosis complex. This is important since no clinical, bacteriological, or radiological markers for distinguishing between these patients, who are clinically asymptomatic, are currently available. Earlier studies of the reactivities of culture filtrate proteins of M. tuberculosis with multiple serial subclinical-TB sera from the same HIV+ TB+ patients provided evidence for the sustained detection of antimycobacterial antibodies in specimens obtained over several months prior to the clinical presentation of TB (22). Future studies of the kinetics of appearance of anti-PPE-C antibodies and their titers will provide additional information on the practical utility of these antibodies as markers for subclinical TB.
Antibodies to the PPE-C protein were detectable in 81% of the smear-positive HIV+ TB+ patients and 97% of the smear-positive HIV− TB+ patients but in only 40% of the smear-negative HIV+ TB+ patients. Although this suggests that antibodies are elicited when the disease is advanced and the bacterial load is high, the presence of antibodies in 81% of the subclinical-TB sera contradicts this assertion (Table 1). The smear-negative patient group comprised only seven individuals; additional patients need to be evaluated before the actual sensitivity for this group can be determined. The presence of anti-PPE-C antibodies in patients with subclinical and clinical TB suggests that the protein should be useful for identifying all stages of an active infection. Recognition by both HIV+ TB+ and HIV− TB+ patients is an added advantage, since other antigens, like the 38-kDa (PstS) protein and antigen 85B, are poorly immunogenic in HIV+ patients (25, 48). The presence of anti-PPE-C antibodies in both cavitary-TB and noncavitary-TB patients provides evidence that, unlike some other proteins (e.g., the 38-kDa protein) which are expressed primarily during replication in cavitary lesions, PPE55 is expressed by in vivo bacteria during growth in cavitary as well as noncavitary environments (Table 1) (4, 35). This is further emphasized by the finding that anti-PPE-C antibodies are present in the retrospective, subclinical-TB sera obtained months before clinical TB was identified. Also, recognition of PPE-C by a majority of the TB patients from India and the United States confirms its expression by clinical isolates from different geographical locations. The ability of the PPE-C protein, which encompasses only about one-third of the PPE55 protein, to identify ∼70 to 95% of HIV− TB+ or HIV+ TB+ patients reflects the strong immunogenicity of this protein. Whether inclusion of the remaining portions of the PPE55 protein will further enhance the sensitivity of detection of antibodies in TB patients is being investigated.
It has been speculated that the PPE protein family contributes to antigenic variation in M. tuberculosis. It is not known if different clinical isolates of M. tuberculosis express the same PPE protein(s) in vivo and if the same PPE protein(s) is expressed at different stages of infection. While the entire C-terminal region of PPE55 (2,977 aa) shows strong homology only to PPE56, the PPE-C protein (978 aa) shows 42 to 69% homology with three PPE proteins (PPE34, -54, and -56) and <30% homology in small regions with large gaps with some other PPE proteins. Thus, it is possible that the anti-PPE-C antibodies were in fact elicited by other PPE proteins with cross-reactive epitopes. The prediction of two antigenic regions in the PPE-C protein, one of which is conserved between several PPE proteins and the other of which is specific to PPE55, provided the opportunity to determine if the anti-PPE-C antibodies were in fact elicited by the PPE55 protein. The strong correlation between the presence of anti-PPE-C1 and anti-PPE-C2 antibodies and the recognition of PPE-C2 by 80 to 100% of the patients who had detectable antipeptide antibodies indicates that PPE55 is a dominant PPE protein which is expressed by a large proportion of the M. tuberculosis isolates only during active infection in humans (Table 2). The delayed appearance of anti-PPE-C1 antibodies suggests that the specific epitope may be recognized earlier during the course of active infection.
Other PPE proteins have been reported to be strongly immunogenic (5, 8, 9, 31, 46). Antibodies against PPE41 (Rv2430c) are present in TB patients and not in healthy individuals (5); PPE68 (Rv3873) induces gamma interferon production from splenocytes of M. tuberculosis-infected mice and from peripheral blood mononuclear cells of TB patients and PPD+ healthy individuals (8, 31). Immune responses elicited by PPE18 (Rv1196) and PPE14 (Rv0915c) have been shown to provide some protection in mice infected with M. tuberculosis (9, 46). Together, these studies suggest that several PPE proteins are expressed in vivo.
Immune responses to ESAT-6 and CFP10 can distinguish between infection with M. tuberculosis and BCG vaccination; this is useful in countries where TB control programs are able to provide preventive therapy to M. tuberculosis-infected individuals (1, 50). In contrast, the ability of PPE-C (and its peptides) to distinguish between latent TB and incipient, subclinical TB would be of value in countries of TB endemicity where TB control is focused on detection and treatment only of patients with active TB. Preventive therapy with isoniazid has been shown to be beneficial in reducing the incidence of TB in both HIV− and HIV+ individuals and is especially recommended for HIV+ individuals (13, 18). However, monotherapy in individuals with active TB runs the risk of fostering drug resistance (30), and no reliable tests that can differentiate between a truly latent infection and incipient, subclinical TB are available. The presence of anti-PPE-C antibodies in the retrospective sera from HIV+ TB+ individuals and their absence in a vast majority of the HIV+ TB− patients on antiretroviral therapy indicate the potential of PPE-C to act as a marker for identification of HIV+ individuals with incipient, subclinical TB.
Anti-PPE-C antibodies were also detected in 11% of the household contacts of untreated, infectious TB patients. Interestingly, 6 to 29% of the household contacts of TB patients were estimated to be at risk of developing TB within 6 months to 2 years of infection (6, 10, 14, 51). Longitudinal studies with cohorts comprised of high-risk individuals like the household contacts of infectious smear-positive TB patients are planned to determine if the presence of anti-PPE-C antibodies in these individuals can identify M. tuberculosis-infected HIV− individuals who have active but subclinical infection with M. tuberculosis and are likely to progress to clinical TB.
Recent studies have suggested that high gamma interferon production induced by ESAT-6 in household contacts of infectious TB patients may correlate with a high risk of progression to clinical TB (10). Our earlier studies have shown that antibodies to the malate synthase (Rv1837c) and MPT51 (Rv3803c) proteins of M. tuberculosis are detectable in the retrospective sera from a majority of HIV+ TB+ patients (43). Antibodies against some M. tuberculosis proteins have also been shown to be present in sera obtained from M. tuberculosis H37Rv aerosol-infected rabbits with subclinical TB (45). Together, these results provide evidence that a selected subset of M. tuberculosis proteins is expressed in vivo and elicits immune responses months before the infection progresses to symptomatic, bacteriologically detectable TB. Further studies with these antigens will enable a definition of their role in the pathogenesis of active infection with M. tuberculosis.
Acknowledgments
This work was supported by a VA Merit Review grant, by the Research Enhancement Award Program from the Department of Veterans Affairs, and by grant AI-0562570 from the National Institutes of Health.
Editor: A. D. O'Brien
REFERENCES
- 1.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 2.Betts, J. C., P. Dodson, S. Quan, A. P. Lewis, P. J. Thomas, K. Duncan, and R. A. McAdam. 2000. Comparison of the proteome of Mycobacterium tuberculosis strain H37Rv with clinical isolate CDC 1551. Microbiology 146:3205-3216. [DOI] [PubMed] [Google Scholar]
- 3.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. [DOI] [PubMed] [Google Scholar]
- 4.Bothamley, G. H., R. Rudd, F. Festenstein, and J. Ivanyi. 1992. Clinical value of the measurement of Mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax 47:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhary, R. K., S. Mukhopadhyay, P. Chakhaiyar, N. Sharma, K. J. R. Murthy, V. M. Katoch, and S. E. Hasnain. 2003. PPE antigen Rv2430c of Mycobacterium tuberculosis induces a strong B-cell response. Infect. Immun. 71:6338-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claessens, N. J., F. F. Gausi, S. Meijnen, M. M. Weismuller, F. M. Salaniponi, and A. D. Harries. 2002. High frequency of tuberculosis in households of index TB patients. Int. J. Tuberc. Lung Dis. 6:266-269. [PubMed] [Google Scholar]
- 7.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seegar, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 8.Demangel, C., P. Brodin, P. J. Cockle, R. Brosch, L. Majlessi, C. Leclerc, and S. T. Cole. 2004. Cell envelope protein PPE68 contributes to Mycobacterium tuberculosis RD1 immunogenicity independently of a 10-kilodalton culture filtrate protein and ESAT-6. Infect. Immun. 72:2170-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillon, D. C., M. R. Alderson, C. H. Day, D. M. Lewinsohn, R. Coler, T. Bement, A. Campos-Neto, Y. A. W. Skeiky, I. M. Orme, A. Roberts, S. Steen, W. Dalemans, R. Badaro, and S. G. Reed. 1999. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect. Immun. 67:2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty, T. M., A. Demissie, J. Olobo, D. Wolday, S. Britton, T. Eguale, P. Ravn, and P. Andersen. 2002. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J. Clin. Microbiol. 40:704-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubnau, E., P. Fontán, R. Manganelli, S. Soares-Appel, and I. Smith. 2002. Mycobacterium tuberculosis genes induced during infection of human macrophages. Infect. Immun. 70:2787-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 96:11554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant, A. D., J. E. Kaplan, and K. M. De Cock. 2001. Preventing opportunistic infections among human immunodeficiency virus-infected adults in African countries. Am. J. Trop. Med. Hyg. 65:810-821. [DOI] [PubMed] [Google Scholar]
- 14.Grzybowski, S., G. D. Barnett, and K. Styblo. 1975. Contacts of cases of active pulmonary tuberculosis. Bull. Int. Union Tuberc. 50:90-106. [PubMed] [Google Scholar]
- 15.Ho, R. S., J. S. Fok, G. E. Harding, and D. W. Smith. 1978. Host-parasite relationships in experimental airborne tuberculosis. VII. Fate of Mycobacterium tuberculosis in primary lung lesions and in primary lesion-free lung tissue infected as a result of bacillemia. J. Infect. Dis. 138:237-241. [DOI] [PubMed] [Google Scholar]
- 16.Hopp, T. P., and K. R. Woods. 1981. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. USA 78:3824-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janin, J. 1979. Surface and inside volumes in globular proteins. Nature 277:491-492. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, J. L., A. Okwera, D. L. Hom, H. Mayanja, C. Mutuluuza Kityo, P. Nsubuga, J. G. Nakibali, A. M. Loughlin, H. Yun, P. N. Mugyenyi, A. Vernon, R. D. Mugerwa, J. J. Ellner, and C. C. Whalen. 2001. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS 15:2137-2147. [DOI] [PubMed] [Google Scholar]
- 19.Jungblut, P. R., U. E. Schaible, H. J. Mollenkopf, U. Zimny-Arndt, B. Raupach, J. Mattow, P. Halada, S. Lamer, K. Hagens, and S. H. Kaufmann. 1999. Comparative proteome analysis of Mycobacterium tuberculosis and Mycobacterium bovis BCG strains: towards functional genomics of microbial pathogens. Mol. Microbiol. 33:1103-1117. [DOI] [PubMed] [Google Scholar]
- 20.Kyte, J., and R. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 21.Laal, S. 2003. Immunodiagnosis, p. 185-192. In W. N. Rom and S. M. Garay (ed.), Tuberculosis, 2nd ed. Lippincott Williams & Wilkins, New York, N.Y.
- 22.Laal, S., K. M. Samanich, M. G. Sonnenberg, J. T. Belisle, J. O'Leary, M. S. Simberkoff, and S. Zolla-Pazner. 1997. Surrogate marker of preclinical tuberculosis in human immunodeficiency virus infection: antibodies to an 88-kDa secreted antigen of Mycobacterium tuberculosis. J. Infect. Dis. 176:133-143. [DOI] [PubMed] [Google Scholar]
- 23.Laal, S., K. M. Samanich, M. G. Sonnenberg, S. Zolla-Pazner, J. M. Phadtare, and J. T. Belisle. 1997. Human humoral responses to antigens of Mycobacterium tuberculosis: immunodominance of high-molecular-mass antigens. Clin. Diag. Lab. Imunnol. 4:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laal, S., and Y. A. W. Skeiky. 2004. Immune-based methods, p. 71-83. In S. T. Cole, K. D. Eisenach, D. N. McMurray, and W. R. Jacobs, Jr. (ed.), Tuberculosis and the tubercle bacillus. ASM Press, Washington, D.C.
- 25.McDonough, J. A., E. D. Sada, A. A. Sippola, L. E. Ferguson, and T. M. Daniel. 1992. Microplate and dot immunoassays for the serodiagnosis of tuberculosis. J. Lab. Clin. Med. 120:318-322. [PubMed] [Google Scholar]
- 26.McMurray, D. N. 2001. Disease model: pulmonary tuberculosis. Trends Mol. Med. 7:135-137. [DOI] [PubMed] [Google Scholar]
- 27.McMurray, D. N. 1994. Guinea pig model of tuberculosis, p. 135-147. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, D.C.
- 28.McMurray, D. N. 2003. Hematogenous reseeding of the lung in low-dose, aerosol-infected guinea pigs: unique features of the host-pathogen interface in secondary tubercles. Tuberculosis (Edinburgh) 83:131-134. [DOI] [PubMed] [Google Scholar]
- 29.Monahan, I. M., J. Betts, D. K. Banerjee, and P. D. Butcher. 2001. Differential expression of mycobacterial proteins following phagocytosis by macrophages. Microbiology 147:459-471. [DOI] [PubMed] [Google Scholar]
- 30.Mosimaneotsile, B., E. A. Talbot, T. L. Moeti, N. M. Hone, G. Moalosi, H. J. Moffat, E. J. Lee, and T. A. Kenyon. 2003. Value of chest radiography in a tuberculosis prevention programme for HIV-infected people, Botswana. Lancet 362:1551-1552. [DOI] [PubMed] [Google Scholar]
- 31.Okkels, L. M., I. Brock, F. Follmann, E. M. Agger, S. M. Arend, T. H. M. Ottenhoff, F. Oftung, I. Rosenkrands, and P. Andersen. 2003. PPE protein (Rv3873) from DNA segment RD1 of Mycobacterium tuberculosis: strong recognition of both specific T-cell epitopes and epitopes conserved within the PPE family. Infect. Immun. 71:6116-6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288:1436-1439. [DOI] [PubMed] [Google Scholar]
- 33.Rose, G. D., A. R. Geselowitz, G. J. Lesser, R. H. Lee, and M. H. Zehfus. 1985. Hydrophobicity of amino acid residues in globular proteins. Science 229:834-838. [DOI] [PubMed] [Google Scholar]
- 34.Rosenkrands, I., K. Weldingh, S. Jacobsen, C. V. Hansen, W. Florio, I. Gianetri, and P. Andersen. 2000. Mapping and identification of Mycobacterium tuberculosis proteins by two-dimensional gel electrophoresis, microsequencing and immunodetection. Electrophoresis 21:935-948. [DOI] [PubMed] [Google Scholar]
- 35.Samanich, K., J. T. Belisle, and S. Laal. 2001. Homogeneity of antibody responses in tuberculosis patients. Infect. Immun. 69:4600-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samanich, K. M., J. T. Belisle, M. G. Sonnenberg, M. A. Keen, S. Zolla-Pazner, and S. Laal. 1998. Delineation of human antibody responses to culture filtrate antigens of Mycobacterium tuberculosis. J. Infect. Dis. 178:1534-1538. [DOI] [PubMed] [Google Scholar]
- 37.Samanich, K. M., M. A. Keen, V. D. Vissa, J. D. Harder, J. S. Spencer, J. T. Belisle, S. Zolla-Pazner, and S. Laal. 2000. Serodiagnostic potential of culture filtrate antigens of Mycobacterium tuberculosis. Clin. Diag. Lab. Immunol. 7:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Sampson, S. L., P. Lukey, R. M. Warren, P. D. van Helden, M. Richardson, and M. J. Everett. 2001. Expression, characterization and subcellular localization of the Mycobacterium tuberculosis PPE gene Rv1917c. Tuberculosis (Edinburgh) 81:305-317. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt, F., S. Donahoe, K. Hagens, J. Mattow, U. E. Schaible, S. H. Kaufmann, R. Aebersold, and P. R. Jungblut. 2004. Complementary analysis of the Mycobacterium tuberculosis proteome by two-dimensional electrophoresis and isotope-coded affinity tag technology. Mol. Cell. Proteomics 3:24-42. [DOI] [PubMed] [Google Scholar]
- 41.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. USA 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh, K. K., Y. Dong, J. T. Belisle, J. Harder, V. K. Arora, and S. Laal. 2005. Antigens of Mycobacterium tuberculosis recognized by antibodies during incipient, subclinical tuberculosis. Clin. Diagn. Lab. Immunol. 12:354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh, K. K., Y. Dong, L. Hinds, M. A. Keen, J. T. Belisle, S. Zolla-Pazner, J. M. Achkar, A. J. Nadas, V. K. Arora, and S. Laal. 2003. Combined use of serum and urinary antibody for diagnosis of tuberculosis. J. Infect. Dis. 188:371-377. [DOI] [PubMed] [Google Scholar]
- 45.Singh, K. K., X. Zhang, A. S. Patibandla, P. Chien, Jr., and S. Laal. 2001. Antigens of Mycobacterium tuberculosis expressed during preclinical tuberculosis: serological immunodominance of proteins with repetitive amino acid sequences. Infect. Immun. 69:4185-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skeiky, Y. A., P. J. Ovendale, S. Jen, M. R. Alderson, D. C. Dillon, S. Smith, C. B. Wilson, I. M. Orme, S. G. Reed, and A. Campos-Neto. 2000. T cell expression cloning of a Mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection. J. Immunol. 165:7140-7149. [DOI] [PubMed] [Google Scholar]
- 47.Talaat, A. M., R. Lyons, S. T. Howard, and S. A. Johnston. 2004. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 101:4602-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thybo, S., C. Richter, H. Wachmann, S. Y. Maselle, D. H. Mwakyusa, I. Mtoni, and A. B. Andersen. 1995. Humoral response to Mycobacterium tuberculosis-specific antigens in African tuberculosis patients with high prevalence of human immunodeficiency virus infection. Tuber. Lung Dis. 76:149-155. [DOI] [PubMed] [Google Scholar]
- 49.Triccas, J. A., and B. Gicquel. 2000. Life on the inside: probing Mycobacterium tuberculosis gene expression during infection. Immunol. Cell Biol. 78:311-317. [DOI] [PubMed] [Google Scholar]
- 50.van Pinxteren, L. A. H., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verver, S., R. M. Warren, Z. Munch, M. Richardson, G. D. van der Spuy, M. W. Borgdorff, M. A. Behr, N. Beyers, and P. D. van Helden. 2004. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet 363:212-214. [DOI] [PubMed] [Google Scholar]
- 52.Young, R. A., B. R. Bloom, C. M. Grosskinsky, J. Ivannyi, D. Thomas, and R. W. Davis. 1985. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc. Natl. Acad. Sci. USA 82:2583-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]