Abstract
Neisseria gonorrhoeae has well-characterized oxidative stress defense systems that protect against oxidative killing in in vitro assays. In contrast, mutant strains of N. gonorrhoeae lacking oxidative stress defenses are identical to the wild type when tested in an ex vivo survival assay using human polymorphonuclear leukocytes.
Neisseria gonorrhoeae, the causative agent of the sexually transmitted infection gonorrhea, is a host-adapted pathogen that poses serious health implications. Gonococcal infection is typically characterized by a symptomatic inflammatory response of the urogenital tract, which involves accumulation of activated polymorphonuclear leukocytes (PMNs) (4). The PMN-mediated inflammatory response involves migration of PMNs towards sites of infection, phagocytosis of microorganisms, and elimination of these organisms by oxygen-dependent and oxygen-independent mechanisms (reviewed in references 11 and 23).
Stimulation of PMNs results in a rapid increase in oxygen consumption referred to as the “oxidative burst.” Assembly and activation of the NADPH oxidase in the plasma membrane results in the generation of superoxide (5), which rapidly dismutates to hydrogen peroxide (27) and is then consumed by myeloperoxidase, generating hypochlorous acid (52). Nitric oxide is also produced by constitutive and inducible nitric oxide synthases of PMNs (12, 25, 28). Various secondary oxidants are generated from these reactive species, including chloramines, hydroxyl radicals, singlet oxygen, and peroxynitrite (12, 23). Concurrent with the oxidative burst, intracellular granules fuse with the plasma membrane or phagosomal membrane to release a broad array of biologically active molecules, including proteases and antimicrobial proteins (reviewed in reference 18).
Without antibiotic treatment, gonococcal infections are persistent and resolve slowly (33), indicating that the PMN response is relatively ineffective in eradicating infection. However, interaction of N. gonorrhoeae with PMNs has been a controversial topic; some studies have reported that N. gonorrhoeae cells survive and grow within PMNs, while other studies have reported that N. gonorrhoeae is rapidly killed within PMNs (reviewed in references 37 and 44). The majority of these studies were performed using a tumbling tube assay with nonadherent PMNs. The use of adherent PMNs mimics the natural disease process in which PMNs have migrated from the bloodstream and are attached to cells and extracellular matrix proteins present at the site of infection. PMNs adhered to plates coated with serum or extracellular matrix proteins produce a large respiratory burst in response to stimuli, whereas suspension PMNs are relatively unresponsive (32). A recent study investigating interactions between N. gonorrhoeae and adherent human PMNs found the PMNs generated a substantial respiratory burst in response to gonococci (45). Despite this, a significant proportion of phagocytosed N. gonorrhoeae cells survived PMN killing and replicated over time (45). Viable counts and microscopic analysis indicated that some level of killing occurs after ingestion, but a subpopulation of N. gonorrhoeae cells survive and replicate (75.0% ± 18.31% at 1 h and 80.21% ± 15.34% at 2 h), in contrast to efficient killing of Escherichia coli (1.83% ± 0.36% at 1 h and 1.37% ± 0.08% at 2 h) (45).
It seemed probable that the ability of N. gonorrhoeae cells to survive in the hostile environment of the PMN would be due to the diverse array of oxidative stress defenses that this organism possesses. These defenses have typically been characterized based on the sensitivity of mutant strains to in vitro oxidative killing (see Table 1 for a summary). N. gonorrhoeae possesses one superoxide dismutase (Sod), which is an inactive or weakly active cytoplasmic SodB (4, 24, 53) that does not protect against reactive oxygen species in vitro (49). However, accumulation of manganese by the MntABC transport system confers protection against oxidative stress (49). N. gonorrhoeae also has high catalase and peroxidase activities (4, 24, 53), afforded by the cytoplasmic catalase (KatA) (4) and the periplasmic cytochrome c peroxidase (Ccp), both of which protect against hydrogen peroxide in vitro (42, 50). Other oxidative defenses described for N. gonorrhoeae include the following: a potential thiol-disulfide oxidoreductase, Sco (41); methionine sulfoxide reductase (MsrA/B) (46); and the iron storage protein bacterioferritin (Bfr) (14). Regulators of these defenses include the peroxide-responsive repressors of KatA and MntC, OxyR (48) and PerR (H.-J. Wu, K. L. Seib, Y. N. Srikhanta, J. L. Edwards, S. P. Kidd, M. A. Apicella, A. G. McEwan, and M. P. Jennings, submitted for publication), respectively.
TABLE 1.
Strains used in the PMN phagocytosis assay and a summary of results
| Strain | Description of strain | In vitro sensitivitya
|
PMN sensitivityc | |
|---|---|---|---|---|
| Sensitivity (substance) | Reference | |||
| N. gonorrhoeae 1291 | Wild type | − | ||
| sodB | Sod deficient | X (PQ, X/XO) | 49 | − |
| mntC | Lacks periplasmic Mn-binding protein of MntABC transporter (decreased [Mn] in cells) | S (PQ) | 49 | − |
| katA | Catalase deficient | S (H2O2) | 42 | − |
| ccp | Cytochrome c peroxidase deficient | S (H2O2 [slight]) | 42 | − |
| ccp katA | Ccp and catalase double mutant | S (H2O2) | 42 | − |
| sco | Sco deficient (potential thiol-disulfide oxidoreductase) | S (PQ) | 41 | − |
| sco katA | Sco and catalase double mutant | S (PQ, H2O2) | 41 | − |
| oxyR | Overexpresses KatA | R (X/XO, H2O2) | 48 | − |
| perR | Overexpresses MntC (increased [Mn] in cells) | R (H2O2) | —b | − |
| E. coli DH5α | Wild type | + | ||
The strains used in the PMN phagocytosis assay are shown along with a summary of the results from this assay and results from in vitro assays from previous publications. Sensitivities of mutant strains to in vitro oxidative stress killing assays are shown relative to N. gonorrhoeae strain 1291 (wild type): X, same phenotype as wild type; S, sensitive to killing; R, resistant to killing (increased survival relative to wild type). These assays involved exposure of a suspension of 104 to 106 cells to either paraquat (PQ; 10 mM), xanthine (4.3 mM)/xanthine oxidase (300 mU/ml) (X/XO), or hydrogen peroxide (H2O2; 10 or 40 mM). For further details, see the references cited in the table.
—, H.-J. Wu, K. L Seib, Y. N. Srikhanta, J. L. Edwards, S. P. Kidd, M. A. Apicella, A. G. McEwan, and M. P. Jennings, submitted for publication.
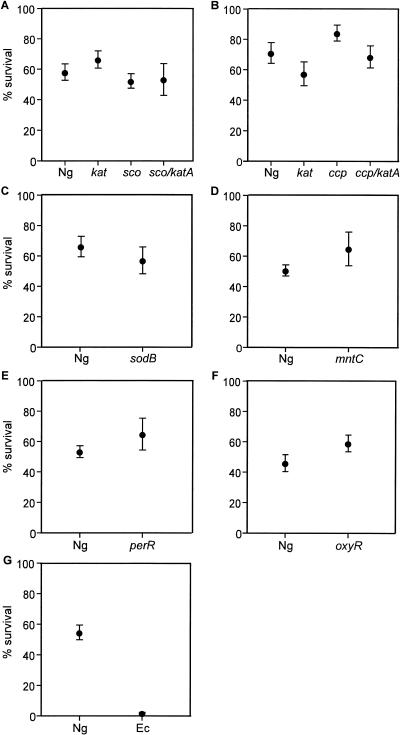
PMN sensitivity (+) is defined as a statistically significant difference in the mean percent survival of the strain relative to N. gonorrhoeae strain 1291 (wild type) in the PMN phagocytosis assay. P values that are <0.05 are considered statistically significant. Differences between N. gonorrhoeae 1291 and its mutant derivatives were not statistically significant (−) (Fig. 1) (P values ≥ 0.14).
The oxidative stress that N. gonorrhoeae encounters within the host environment is significantly more complex than that generated by in vitro assays. Indeed, the expression of numerous genes involved in defense against oxidative stress is upregulated in E. coli upon exposure to PMNs (47). Many of these defenses (i.e., catalase, Sod, and OxyR) have been shown to protect enteric bacteria against killing by activated PMNs (19, 29, 39, 47). The role of oxidative defenses in protecting N. gonorrhoeae from the PMN oxidative burst was determined using the phagocytosis assay described by Simons et al. (45). Briefly, approximately 2 × 106 PMNs isolated from peripheral blood were adhered to glass coverslips pretreated with collagen and autologous human serum. N. gonorrhoeae strain 1291 (wild type) and a set of mutant derivatives (Table 1) expressing opacity-associated protein (Opa+) and pili (P+) (as determined by colony morphology) were grown to log phase. Bacteria were added to PMNs at a multiplicity of infection of 1:1 and centrifuged, and the supernatant was removed (time A). Synchronized phagocytosis was stimulated by the addition of warm medium for 2 minutes (time B). Nonphagocytosed bacteria were removed by three wash steps (time 0 h). PMNs and bacteria were incubated at 37°C for 1 hour (time 1 h). Saponin was used to lyse PMNs, and viable CFU were enumerated after overnight culture of plated serial dilutions. Each assay was done with triplicate cultures of each mutant strain along with wild-type controls and was performed on at least three occasions. Laser scanning confocal microscopy, scanning electron microscopy, and transmission electron microscopy performed by Simons et al. (45) illustrated that PMN-associated N. gonorrhoeae cells were internalized and contained within defined phagosomes within the perimeter of the PMN membrane and that there were very few bacteria residing in the extracellular spaces.
This study is based on a set of previously constructed and defined mutant strains of N. gonorrhoeae that are deficient in various oxidative stress defense mechanisms or regulatory systems (see Table 1 and references cited therein for further detail), with the exception of the sco katA mutant strain, which was constructed by transforming the sco::kan plasmid (41) into the katA::tet strain (42). All mutant strains were confirmed by Southern hybridization, PCR, and/or sequence analysis. The set of mutant strains was compared to wild-type strain 1291 to determine relative sensitivity to killing by PMNs (Fig. 1). A summary of results is shown in Table 1, along with results of in vitro assays from previously published work. Surprisingly, none of the mutant strains tested had a phenotype distinct from the wild type in the PMN phagocytosis assay. Wild-type and mutant strains had similar levels of PMN association (time A) and phagocytosis (time B), indicating the mutations had no detectable effect on phagocytosis by PMNs.
FIG. 1.
PMN phagocytosis assay. PMNs were incubated with N. gonorrhoeae wild-type strain 1291 and mutant derivatives deficient in various oxidative stress defense mechanisms or regulatory systems to determine relative sensitivity to killing by PMNs (as described by Simons et al. [45]). Briefly, bacteria were added to a monolayer of PMNs (approximately 2 × 106) at a multiplicity of infection of 1:1 and incubated at 37°C for 1 h. PMN were then lysed to quantify CFU. Each panel (A to G) shows a set of experiments in which wild-type strain 1291 (Ng) was compared to a mutant strain, a set of mutant strains, or Escherichia coli strain DH5α (Ec), as shown on the x axis. Results shown are the percent survival of bacteria at time 1 h (expressed as a percentage of viable bacteria at time 0 h). Each data point is the mean of several individual experiments ± one standard deviation of the mean (number of repeat experiments: panel A, 3; B, 4; C, 2; D, 4; E, 2; F, 3; and G, 3) in which each experiment was conducted with three replicates of each strain. To determine whether the percent survival of mutant strains was statistically significant compared to the percent survival of the wild-type strain, P values were computed using unpaired two-sided Student's t test. P values that were less than 0.05 were considered statistically significant. Differences between N. gonorrhoeae wild-type strain 1291 and its mutant derivatives were not statistically significant (panels A to F, P ≥ 0.14). The only statistically significant difference was between wild-type N. gonorrhoeae and E. coli (panel G; P < 0.0001).
N. gonorrhoeae is a host-adapted pathogen which is able to survive killing by PMNs. Based on the broad range of oxidative stress defenses that N. gonorrhoeae cells possess, it was proposed that the ability to survive within PMNs was dependent, at least in part, on protection against the oxidative burst. This hypothesis is not supported by the similar phenotypes that were observed for the wild-type strain and the oxidative stress defense mutant strains in the PMN assay.
Several species of bacteria are able to survive PMN killing by inhibiting or subverting the oxidative burst of PMNs (reviewed in reference 1). As seen with N. gonorrhoeae in this study, Helicobacter pylori is able to evade PMN killing despite the activation of an oxidative response. H. pylori alters the targeting of the PMN NADPH oxidase so that it locates to the plasma membrane rather than the phagosomal membrane, thus releasing reactive oxygen species into the extracellular environment (2).
The features that govern N. gonorrhoeae-PMN interactions have not yet been defined, and while interactions do involve the gonococcal surface proteins porin (8, 21, 22, 26, 31) and Opa (7, 20, 31, 51), their roles in stimulating versus inhibiting the oxidative burst of PMNs have not been resolved. Further investigation using the adherent PMN phagocytosis assay (45) should provide a better understanding of the N. gonorrhoeae-dependent oxidative burst of PMNs. Despite the stimulation of an oxidative burst by N. gonorrhoeae (7, 31, 45, 51), the results described herein support previous studies which suggested that oxygen-independent mechanisms may be of greater significance than oxygen-dependent mechanisms during PMN killing of N. gonorrhoeae (9, 10, 13, 35, 36, 43; reviewed in reference 44).
The resistance of the specific mutant strains may also be due to the presence of redundant defenses. For example, in E. coli, a sodA or a sodB mutant strain is no more sensitive to PMN killing than the wild-type strain, but a sodA sodB double mutant is more susceptible to killing (29, 34). It is also important to note that the defenses investigated do not protect against all of the types of oxidants generated during the PMN oxidative burst (e.g., nitric oxide), some of which may be of greater importance in the killing of N. gonorrhoeae.
In light of our results, the presence of a wide range of defenses in N. gonorrhoeae suggests that this organism encounters significant oxidative stress from other sources in vivo. The primary sites of gonococcal infection are the ecto- and endocervical epithelium in women (17) and the urethral epithelium in men (3, 15). N. gonorrhoeae cells are able to survive and replicate within epithelial cells at these sites of infection (reviewed in reference 30). Intestinal and airway epithelial cells are able to kill bacteria by oxidative mechanisms (6, 16, 38, 40), and cervical epithelial cells also appear to have such oxidative defense capacity. We have recently observed that both MntC and PerR of N. gonorrhoeae are required for survival within primary human cervical epithelial cells (H.-J. Wu, K. L. Seib, Y. N. Srikhanta, J. L. Edwards, S. P. Kidd, M. A. Apicella, A. G. McEwan, and M. P. Jennings, submitted for publication).
Acknowledgments
This work was supported by Program Grant 284214 from the National Health and Medical Research Council of Australia and by U.S. Public Health service grants AI45728, AI43924, and AI38515 from the National Institute of Allergy and Infectious Diseases. K.L.S. acknowledges The University of Queensland Graduate School for providing a Graduate School Research Travel Award.
Editor: J. N. Weiser
REFERENCES
- 1.Allen, L. A. 2003. Mechanisms of pathogenesis: evasion of killing by polymorphonuclear leukocytes. Microbes Infect. 5:1329-1335. [DOI] [PubMed] [Google Scholar]
- 2.Allen, L. A. 2000. Modulating phagocyte activation: the pros and cons of Helicobacter pylori virulence factors. J. Exp. Med. 191:1451-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apicella, M. A., M. Ketterer, F. K. Lee, D. Zhou, P. A. Rice, and M. S. Blake. 1996. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J. Infect. Dis. 173:636-646. [DOI] [PubMed] [Google Scholar]
- 4.Archibald, F. S., and M. N. Duong. 1986. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect. Immun. 51:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babior, B. M., J. D. Lambeth, and W. Nauseef. 2002. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 397:342-344. [DOI] [PubMed] [Google Scholar]
- 6.Battistoni, A., F. Pacello, S. Folcarelli, M. Ajello, G. Donnarumma, R. Greco, M. G. Ammendolia, D. Touati, G. Rotilio, and P. Valenti. 2000. Increased expression of periplasmic Cu,Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells. Infect. Immun. 68:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belland, R. J., T. Chen, J. Swanson, and S. H. Fischer. 1992. Human neutrophil response to recombinant neisserial Opa proteins. Mol. Microbiol. 6:1729-1737. [DOI] [PubMed] [Google Scholar]
- 8.Bjerknes, R., H. K. Guttormsen, C. O. Solberg, and L. M. Wetzler. 1995. Neisserial porins inhibit human neutrophil actin polymerization, degranulation, opsonin receptor expression, and phagocytosis but prime the neutrophils to increase their oxidative burst. Infect. Immun. 63:160-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britigan, B. E., and M. S. Cohen. 1986. Effects of human serum on bacterial competition with neutrophils for molecular oxygen. Infect. Immun. 52:657-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britigan, B. E., D. Klapper, T. Svendsen, and M. S. Cohen. 1988. Phagocyte-derived lactate stimulates oxygen consumption by Neisseria gonorrhoeae. An unrecognized aspect of the oxygen metabolism of phagocytosis. J. Clin. Investig. 81:318-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burg, N. D., and M. H. Pillinger. 2001. The neutrophil: function and regulation in innate and humoral immunity. Clin. Immunol. 99:7-17. [DOI] [PubMed] [Google Scholar]
- 12.Carreras, M. C., G. A. Pargament, S. D. Catz, J. J. Poderoso, and A. Boveris. 1994. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett. 341:65-68. [DOI] [PubMed] [Google Scholar]
- 13.Casey, S. G., W. M. Shafer, and J. K. Spitznagel. 1986. Neisseria gonorrhoeae survive intraleukocytic oxygen-independent antimicrobial capacities of anaerobic and aerobic granulocytes in the presence of pyocin lethal for extracellular gonococci. Infect. Immun. 52:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, C. Y., and S. A. Morse. 1999. Neisseria gonorrhoeae bacterioferritin: structural heterogeneity, involvement in iron storage and protection against oxidative stress. Microbiology 145:2967-2975. [DOI] [PubMed] [Google Scholar]
- 15.Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. [DOI] [PubMed] [Google Scholar]
- 16.Deitch, E. A., Y. Haskel, N. Cruz, D. Xu, and P. R. Kvietys. 1995. Caco-2 and IEC-18 intestinal epithelial cells exert bactericidal activity through an oxidant-dependent pathway. Shock 4:345-350. [DOI] [PubMed] [Google Scholar]
- 17.Edwards, J. L., J. Q. Shao, K. A. Ault, and M. A. Apicella. 2000. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect. Immun. 68:5354-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faurschou, M., and N. Borregaard. 2003. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 5:1317-1327. [DOI] [PubMed] [Google Scholar]
- 19.Franzon, V. L., J. Arondel, and P. J. Sansonetti. 1990. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect. Immun. 58:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray-Owen, S. D., D. R. Lorenzen, A. Haude, T. F. Meyer, and C. Dehio. 1997. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol. Microbiol. 26:971-980. [DOI] [PubMed] [Google Scholar]
- 21.Haines, K. A., J. Reibman, X. Y. Tang, M. Blake, and G. Weissmann. 1991. Effects of protein I of Neisseria gonorrhoeae on neutrophil activation: generation of diacylglycerol from phosphatidylcholine via a specific phospholipase C is associated with exocytosis. J. Cell Biol. 114:433-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haines, K. A., L. Yeh, M. S. Blake, P. Cristello, H. Korchak, and G. Weissmann. 1988. Protein I, a translocatable ion channel from Neisseria gonorrhoeae, selectively inhibits exocytosis from human neutrophils without inhibiting O2− generation. J. Biol. Chem. 263:945-951. [PubMed] [Google Scholar]
- 23.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007-3017. [PubMed] [Google Scholar]
- 24.Johnson, S. R., B. M. Steiner, and G. H. Perkins. 1996. Cloning and characterization of the catalase gene of Neisseria gonorrhoeae: use of the gonococcus as a host organism for recombinant DNA. Infect. Immun. 64:2627-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kudoh, S., K. Suzuki, M. Yamada, Q. Liu, S. Nakaji, and K. Sugawara. 1999. Contribution of nitric oxide synthase to human neutrophil chemiluminescence. Luminescence 14:335-339. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzen, D. R., D. Gunther, J. Pandit, T. Rudel, E. Brandt, and T. F. Meyer. 2000. Neisseria gonorrhoeae porin modifies the oxidative burst of human professional phagocytes. Infect. Immun. 68:6215-6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makino, R., T. Tanaka, T. Iizuka, Y. Ishimura, and S. Kanegasaki. 1986. Stoichiometric conversion of oxygen to superoxide anion during the respiratory burst in neutrophils. Direct evidence by a new method for measurement of superoxide anion with diacetyldeuteroheme-substituted horseradish peroxidase. J. Biol. Chem. 261:11444-11447. [PubMed] [Google Scholar]
- 28.McCall, T. B., N. K. Boughton-Smith, R. M. Palmer, B. J. Whittle, and S. Moncada. 1989. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem. J. 261:293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McManus, D. C., and P. D. Josephy. 1995. Superoxide dismutase protects Escherichia coli against killing by human serum. Arch. Biochem. Biophys. 317:57-61. [DOI] [PubMed] [Google Scholar]
- 30.Merz, A. J., and M. So. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423-457. [DOI] [PubMed] [Google Scholar]
- 31.Naids, F. L., and R. F. Rest. 1991. Stimulation of human neutrophil oxidative metabolism by nonopsonized Neisseria gonorrhoeae. Infect. Immun. 59:4383-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathan, C. F. 1987. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J. Clin. Investig. 80:1550-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ober, W. B. 1970. Boswell's clap. JAMA 212:91-95. [PubMed] [Google Scholar]
- 34.Papp-Szabo, E., C. L. Sutherland, and P. D. Josephy. 1993. Superoxide dismutase and the resistance of Escherichia coli to phagocytic killing by human neutrophils. Infect. Immun. 61:1442-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rest, R. F. 1979. Killing of Neisseria gonorrhoeae by human polymorphonuclear neutrophil granule extracts. Infect. Immun. 25:574-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rest, R. F., S. H. Fischer, Z. Z. Ingham, and J. F. Jones. 1982. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect. Immun. 36:737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rest, R. F., and W. M. Shafer. 1989. Interactions of Neisseria gonorrhoeae with human neutrophils. Clin. Microbiol. Rev. 2(Suppl.):S83-S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rochelle, L. G., B. M. Fischer, and K. B. Adler. 1998. Concurrent production of reactive oxygen and nitrogen species by airway epithelial cells in vitro. Free Radic. Biol. Med. 24:863-868. [DOI] [PubMed] [Google Scholar]
- 39.Rosen, H., P. J. Lewis, and C. M. Nitzel. 2004. Neutrophil microbicidal activity: screening bacterial mutants for survival after phagocytosis using quantitative PCR. Jpn. J. Infect. Dis. 57:S19-S21. [PubMed] [Google Scholar]
- 40.Schmidt, H. H., and U. Walter. 1994. NO at work. Cell 78:919-925. [DOI] [PubMed] [Google Scholar]
- 41.Seib, K. L., M. P. Jennings, and A. G. McEwan. 2003. A Sco homologue plays a role in defence against oxidative stress in pathogenic Neisseria. FEBS Lett. 546:411-415. [DOI] [PubMed] [Google Scholar]
- 42.Seib, K. L., H. J. Tseng, A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2004. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J. Infect. Dis. 190:136-147. [DOI] [PubMed] [Google Scholar]
- 43.Shafer, W. M., V. C. Onunka, and L. E. Martin. 1986. Antigonococcal activity of human neutrophil cathepsin G. Infect. Immun. 54:184-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shafer, W. M., and R. F. Rest. 1989. Interactions of gonococci with phagocytic cells. Annu. Rev. Microbiol. 43:121-145. [DOI] [PubMed] [Google Scholar]
- 45.Simons, M. P., W. M. Nauseef, and M. A. Apicella. 2005. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect. Immun. 73:1971-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skaar, E. P., D. M. Tobiason, J. Quick, R. C. Judd, H. Weissbach, F. Etienne, N. Brot, and H. S. Seifert. 2002. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc. Natl. Acad. Sci. USA 99:10108-10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staudinger, B. J., M. A. Oberdoerster, P. J. Lewis, and H. Rosen. 2002. mRNA expression profiles for Escherichia coli ingested by normal and phagocyte oxidase-deficient human neutrophils. J. Clin. Investig. 110:1151-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tseng, H. J., A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2003. OxyR acts as a repressor of catalase expression in Neisseria gonorrhoeae. Infect. Immun. 71:550-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tseng, H. J., Y. Srikhanta, A. G. McEwan, and M. P. Jennings. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40:1175-1186. [DOI] [PubMed] [Google Scholar]
- 50.Turner, S. M., E. G. Reid, H. Smith, and J. A. Cole. 2003. A novel cytochrome c peroxidase from Neisseria gonorrhoeae, a lipoprotein from a Gram-negative bacterium. Biochem. J. 373:865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virji, M., and J. E. Heckels. 1986. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J. Gen. Microbiol. 132:503-512. [DOI] [PubMed] [Google Scholar]
- 52.Winterbourn, C. C., M. C. Vissers, and A. J. Kettle. 2000. Myeloperoxidase. Curr. Opin. Hematol. 7:53-58. [DOI] [PubMed] [Google Scholar]
- 53.Zheng, H. Y., D. J. Hassett, K. Bean, and M. S. Cohen. 1992. Regulation of catalase in Neisseria gonorrhoeae. Effects of oxidant stress and exposure to human neutrophils. J. Clin. Investig. 90:1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]