Abstract
Shiga toxins (Stxs) induce apoptosis in a variety of cell types. Here, we show that Stx1 induces apoptosis in the undifferentiated myelogenous leukemia cell line THP-1 in the absence of tumor necrosis factor alpha (TNF-α) or death receptor (TNF receptor or Fas) expression. Caspase-8 and -3 inhibitors blocked, and caspase-6 and -9 inhibitors partially blocked, Stx1-induced apoptosis. Stx1 induced the mitochondrial pathway of apoptosis, as activation of caspase-8 triggered the (i) cleavage of Bid, (ii) disruption of mitochondrial membrane potential, and (iii) release of cytochrome c into the cytoplasm. Caspase-8, -9, and -3 cleavage and functional activities began 4 h after toxin exposure and peaked after 8 h of treatment. Caspase-6 may also contribute to Stx1-induced apoptosis by directly acting on caspase-8. It appears that functional Stx1 holotoxins must be transported to the endoplasmic reticulum to initiate apoptotic signaling through the ribotoxic stress response. These data suggest that Stxs may activate monocyte apoptosis via a novel caspase-8-dependent, death receptor-independent mechanism.
Shiga toxins (Stxs) are a family of protein exotoxins expressed by the enteric pathogens Shigella dysenteriae serotype 1 and certain serotypes of Escherichia coli. Stxs share structural and functional properties: Stxs are holotoxins composed of a single A-subunit in noncovalent association with a pentameric ring of identical B-subunits, and the toxins are potent protein synthesis inhibitors in susceptible mammalian cells (11, 31). The B-subunits mediate toxin binding through interaction with the neutral membrane glycolipid globotriaosylceramide (Gb3) (24). Following internalization and retrograde transport of the toxins through the Golgi apparatus to the endoplasmic reticulum (ER), A-subunits are proteolytically nicked and reduced, and the resultant A1-fragments are translocated into the target cell cytoplasm (38). The A1-fragment acts as a highly specific N-glycosidase that cleaves a single adenine residue near the 3′ end of the 28S rRNA component of eukaryotic ribosomes (8, 39). Shigella dysenteriae serotype 1 produces Shiga toxin (Stx), while E. coli may express multiple toxins categorized as Shiga toxin type 1 (Stx1) or Shiga toxin type 2 (Stx2), based on their antigenic similarity to Stx (27).
Shigella dysenteriae serotype 1 and Shiga toxin-producing E. coli cause the bloody diarrheal diseases bacillary dysentery and hemorrhagic colitis, respectively. Patients infected with Stx-producing bacteria are at increased risk for developing life-threatening complications, including acute renal failure (hemolytic uremic syndrome) and central nervous system abnormalities such as disorientation, lethargy, seizures, paralysis, coma, and death (34, 40). Numerous studies in animals have shown that the extra-intestinal complications seen in humans are reproduced by the intravenous administration of purified Stxs (29). Affected organs in humans and experimental animals show evidence of profound vascular damage. These observations suggest that Stxs gain access to the bloodstream and target vascular endothelial cells in the kidneys and central nervous system for destruction. In addition to direct cytotoxic effects on endothelial cells, Stxs have also been shown to stimulate macrophages to produce the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1β in vitro (35, 48). These cytokines, in turn, up-regulate Gb3 biosynthesis and expression on endothelial cells (47) and sensitize target cells to the cytotoxic action of the toxins (25). Thus, the host innate immune response may exacerbate vascular damage initiated by Stxs.
Stxs have been demonstrated to induce apoptosis in many human cell types in vitro, including epithelial cell lines, primary renal epithelial cells, Burkitt's lymphoma cells, microvascular endothelial cells, and myelogenous leukemia cell lines (reviewed in reference 6). The mechanism(s) by which Stxs induce apoptosis in these cell types, and whether a common apoptosis pathway is triggered by Stxs in all cell types, remains to be fully characterized. Kojio et al. (21) showed that Stx-induced apoptosis in the human monocytic cell line THP-1 required transport of toxins through functional Golgi complexes and the activation of caspase-3. Fujii et al. (12) reported that Stx-induced HeLa cell apoptosis occurs via a pathway requiring caspase-8, -6, and -3, but not caspase-9. In contrast to studies using holotoxin molecules, binding toxin receptors with purified Stx B-subunits or anti-Gb3 antibodies has been reported to be sufficient to induce caspase-8 activation and apoptosis in Burkitt's lymphoma cells (20). Finally, it is unknown whether Stxs directly activate apoptosis in all cell types or if additional host factors are required in some instances. One potential host factor that may contribute to apoptosis induction is TNF-α (49). We previously showed that purified Stx1 induces TNF-α and interleukin-1β gene expression and apoptosis in the myelomonocytic cell line THP-1 in a cell maturation-dependent manner (14, 15). Differentiated (macrophage-like) THP-1 cells were relatively resistant to killing by Stxs and expressed cytokines, whereas undifferentiated (monocytic) THP-1 cells failed to secrete TNF-α and were sensitive to Stx cytotoxicity. Plastic-adherent human peripheral blood monocytes, like differentiated THP-1 cells, were relatively insensitive to apoptosis induction by Stx2 but responded by producing TNF-α and granulocyte-macrophage colony-stimulating factor (5). Thus, there appears to be a Stx-dependent, TNF-α-independent apoptotic signaling mechanism operative in monocytic THP-1 cells. Experiments reported here characterize Stx1-mediated apoptotic signaling in monocytic THP-1 cells.
MATERIALS AND METHODS
Cells.
The human myelogenous leukemia cell line THP-1 (46) was purchased from the American Type Culture Collection, Manassas, VA, and cultured in RPMI 1640 medium (Gibco-BRL, Grand Island, NY) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT). Cells were maintained at 37°C in 5% CO2 in a humidified incubator.
Toxins.
Stx1 used in this study was prepared as previously described (44). Briefly, Stx1 was purified from cell lysates prepared from E. coli DH5α(pCKS112) cells by sequential ion-exchange, chromatofocusing, and immunoaffinity chromatography. Purity of toxin preparations was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with silver staining and Western blot analysis using anti-Stx1 antibodies. Toxin preparations contained <0.1 ng endotoxin per ml as determined by Limulus amoebocyte lysate assay (Associates of Cape Cod, Falmouth, ME). Purified Stx1 pentameric B-subunits were the kind gift of Cheleste Thorpe, Tufts University School of Medicine, Boston, MA. Purified Stx1A− (E167Q-R170L), an enzymatic mutant in which glutamate at position 167 and arginine at position 170 were replaced by glutamine and leucine, respectively, by oligonucleotide-directed site-specific mutagenesis (32), was the kind gift of Yoshifumi Takeda, Jissen Women’s University, Tokyo, Japan.
Reagents.
General caspase inhibitor N-benzyl-oxycarbonyl-Val-Ala-Asp(OMe)-fluoromethyl-ketone (ZVAD-fmk), caspase-1 inhibitor Z-Tyr-Val-Ala-Asp-fmk (Z-YVAD-fmk), caspase-2 inhibitor Z-Val-Asp-Val-Ala-Asp-fmk (Z-VDVAD-fmk), caspase-3 inhibitor Z-Asp-Glu-Val-Asp-fmk (Z-DEVD-fmk), caspase-6 inhibitor Z-Val-Glu-Ile-Asp-fmk (Z-VEID-fmk), caspase-8 inhibitor Z-Ile-Glu-Thr-Asp-fmk (Z-IETD-fmk), and caspase-9 inhibitor Z-Leu-Glu-His-Asp-fmk (Z-LEHD-fmk) were purchased from Calbiochem, San Diego, CA. All caspase inhibitors were used at concentrations known to optimally inhibit specific caspase activity. Rabbit anti-human cytochrome c antibody was purchased from San Cruz Biotechnology, Inc., Santa Cruz, CA. Antibodies directed against human caspase-3, caspase-8, caspase-9, and Bid were obtained from Cell Signaling Technology, Beverly, MA. Cycloheximide and anisomycin were purchased from Sigma, St. Louis, MO. Fluorescein-conjugated and nonfluoresceinated monoclonal antibodies directed against human membrane-bound TNF-α, TNF receptor 1 (TNFR1), TNFR2, and Fas (APO/CD95) were purchased from R&D Systems, Inc., Minneapolis, MN. Human immunoglobulin G (IgG) and all other reagents were obtained from Sigma Chemical Co., St. Louis, MO.
Analysis of cell surface receptor expression.
THP-1 cells (2 × 106 cells/ml) were incubated in the presence or absence of Stx1 (400 ng/ml) for 0, 2, 4, and 6 h. We have previously shown that this toxin concentration produces optimal signaling for cytokine expression and apoptosis induction (14, 15). Cells were washed twice with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA). Before staining for cell surface receptor expression using antibodies, FcγRs were blocked by treatment of the cells with 1.0 μg of human IgG per 105 cells for 15 min at room temperature. FcγR-blocked cells were washed and suspended in PBS plus 0.5% BSA. Cells were then treated with fluorescein-conjugated monoclonal antibodies directed against human membrane-bound TNF-α (mTNF-α), TNFR1, TNFR2, or Fas for 30 to 45 min at 4°C. Following incubation, cells were washed and suspended in 0.5 ml PBS. Cell fluorescence was measured by flow cytometry using the FACSCalibur instrument (Becton Dickinson, Palo Alto, CA). Untreated cells and cells treated with fluorescein-labeled mouse IgG antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) served as control cells in the fluorescence-activated cell sorter (FACS) analysis.
Analysis of apoptosis by Annexin V and PI staining.
THP-1 cells were treated with Stx1 (400 ng/ml) for 12 h in RPMI 1640 plus 0.5% FBS in the presence or absence of anti-human TNF-α antibody (0.01 μg/ml), anti-human TNFR1 antibody (5.0 μg/ml), or recombinant human TNF-α (rhTNF-α; 40 ng/ml). In a separate set of experiments, cells were treated with Stx1 for 5 h in RPMI 1640 plus 0.5% FBS in the presence or absence of caspase-1, -2, -3, -6, -8, and -9 inhibitors or the general caspase inhibitor ZVAD-fmk (all at 40 μM). In some experiments, THP-1 cells were treated with cycloheximide (10 to 200 μM) or anisomycin (0.1 to 10 μg/ml) for 5 h. Following treatment, cells were centrifuged at 200 × g for 5 min, washed in ice-cold sterile PBS, and stained using the Annexin V-FLUOS staining kit (Roche Diagnostics Corp., Indianapolis, IN). Cells were incubated in the provided incubation buffer for 10 to 15 min at room temperature. Cells were then centrifuged, washed twice in incubation buffer, and suspended in 0.5 ml of incubation buffer. Apoptosis was measured by flow cytometry (Becton Dickinson, Palo Alto, CA). Fluorescence parameters were gated using unstained and single-stained untreated cells. Total apoptosis was expressed as the percentages of Annexin V-positive plus Annexin V and propidium iodide (PI) double-positive cells minus background fluorescence.
DNA fragmentation analysis.
DNA fragmentation was assayed using the Apoptotic DNA Ladder kit (Roche, Mannheim, Germany). THP-1 cells (2 × 106 cells/ml) were maintained in 12-well culture plates. Cells were treated with Stx1 (400 ng/ml) in RPMI 1640 and 0.5% FBS in the presence or absence of caspase-1, -2, -3, -6, -8, and -9 inhibitors or the general caspase inhibitor ZVAD-fmk (all at 40 μM) for 5 h. Cells were washed with ice-cold PBS and lysed with lysis buffer (6.0 M guanidine-HCl, 10 mM urea, 10 mM Tris-HCl, 20% Triton X-100 [vol/vol], pH 4.4). After 10 min of incubation at room temperature, lysates were centrifuged through DNA binding columns. DNA was eluted from the columns with 10 mM Tris (pH 8.5). Eluted DNA was treated with DNase-free RNase for 30 min. DNA concentrations were measured, and equal amounts of DNA (2.5 μg) were loaded on 1.2% agarose gels. Following electrophoresis, gels were stained with ethidium bromide and photographed on a UV transilluminator (Gel Imager; Bio-Rad, Hercules, CA).
Preparation of cellular lysates.
Prior to stimulation with Stx1, THP-1 cells (5 × 106 cells/ml) were washed once in cold Dulbecco's PBS and suspended in RPMI 1640 with 0.5% FBS for 2 h. Cells were then stimulated with Stx1 (400 ng/ml) for the various time periods indicated in the figures. For Western blot analyses of caspase and Bid activation, cells were harvested and lysed with modified radioimmunoprecipitation assay buffer (1.0% Nonidet P-40, 1.0% Na-deoxycholate, 150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 0.25 mM Na-pyrophosphate, 2.0 mM Na-vanadate, 2.0 mM Na-fluoride, 10 μg/ml aprotinin, 1.0 μg/ml leupeptin, 1.0 μg/ml pepstatin, and 200 mM phenylmethylsulfonyl fluoride) at 4°C. Extracts were collected and cleared by centrifugation at 15,000 × g for 10 min. Cleared extracts were stored at −80°C until used in Western blot analyses. For measurement of cytochrome c release from mitochondria, cytosolic fractions were prepared according to the method of Leist et al. (22). THP-1 cells (5 × 107 cells/ml) were stimulated with Stx1 as outlined above. Cells were harvested and suspended for 20 min at 4°C in permeabilization buffer (pH 7.2) containing 210 mM d-mannitol, 70 mM sucrose, 10 mM HEPES, 5.0 mM succinate, 0.2 mM EGTA, 0.15% BSA, and 80 μg/ml digitonin. Cells were centrifuged at 170 × g for 10 min at 4°C. Supernatants were collected and centrifuged at 13,000 × g for 10 min at 4°C. The resultant supernatants (cytosolic fraction) were used for cytochrome c analysis by Western blotting.
Western blot analysis of caspase activation, Bid cleavage, and cytochrome c release.
The protein content of cell extracts and cytosolic fractions was determined using the Micro BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of proteins (60 to 80 μg protein per gel lane) were separated by 12% Tris-glycine SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% milk prepared in TBST (20 mM Tris [pH 7.6], 137 mM NaCl, 0.1% Tween 20). Membranes were incubated with primary antibodies specific for human caspases, cytochrome c, or Bid in 5.0% BSA-TBST overnight at 4°C. The membranes were then incubated with the corresponding secondary antibodies (rabbit or mouse anti-IgG coupled to horseradish peroxidase) for 2 h at room temperature. Bands were visualized using the Western Lightning chemiluminescence system (NEN-Perkin-Elmer, Boston, MA). Data shown are from at least two independent experiments.
Analysis of mitochondrial membrane potential.
Alterations in mitochondrial membrane potential were measured using the mitochondrial membrane potential detection kit (Stratagene, La Jolla, CA). The mitochondrial membrane potential maintained in healthy cells allows the positively charged JC-1 reagent (5,5′,6,6′-tetraachloro-1,1′,3,3′-tetraethylbenz-imidazolocarbocyanine iodide) to accumulate in mitochondrial membranes, forming aggregates with red fluorescence. In apoptotic cells, as the mitochondrial membrane potential is disrupted, JC-1 does not accumulate in the membranes, and JC-1 in the cytoplasm has a green fluorescence. THP-1 cells (2 × 106 cells/ml) in 12-well culture plates were treated with Stx1 (400 ng/ml) in RPMI 1640 plus 0.5% FBS in the presence or absence of 40 μM caspase-1, -2, -3, -6, -8, and -9 inhibitors or ZVAD-fmk for 0, 2, 4, 6, and 8 h. Cells were harvested, washed in ice-cold PBS, and resuspended in 0.5 ml JC-1 assay buffer containing the JC-1 reagent. Following incubation for 15 min at 37°C in 5% CO2, the cells were centrifuged at 400 × g for 5 min and washed twice in assay buffer. Washed cells were resuspended in 0.5 ml of assay buffer, and maintenance of JC-1 in the mitochondrial membrane was assessed by flow cytometry.
Measurement of caspase activity.
Caspase activity was determined using caspase assay kits purchased from Chemicon, Temecula, CA, with colorimetric substrates for caspase-3 (Ac-DEVD-pNA), caspase-8 (Ac-IETD-pNA), and caspase-9 (Ac-LEHD-pNA). THP-1 cells (5 × 106 cells/ml) were treated with the caspase inhibitors (40 μM) for 1 h prior to the addition of Stx1 (400 ng/ml). At various time points after toxin stimulation, cells were washed in ice-cold PBS, treated with lysis buffer provided with the kits, and maintained on ice for 20 min. Cell lysates were centrifuged, and protein concentrations in the supernatants were determined using the Bio-Rad Dc protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein (60 μg per reaction mixture) were added to the caspase assay buffer and incubated for 2 h at 37°C. Caspase activation resulted in cleavage of the substrates, releasing free p-nitroaniline (pNA), which was detected spectrophotometrically (405 nm) in a microtiter plate reader. Caspase activity is expressed as pM pNA liberation per minute per μg protein. The data shown are the means ± standard errors of the means determined from three independent assays.
Statistics.
Statistical analyses of experiments were performed with Excel (Microsoft, Corp., Redmond, WA). All Annexin V/PI staining, Bid cleavage, and caspase activity data were analyzed by using Student's t test.
RESULTS
Roles of TNFR1, TNFR2, and Fas in Stx1-induced apoptosis.
Members of the TNFR family and Fas (APO/CD95) transduce apoptotic signals following engagement with their appropriate ligands. We investigated, therefore, whether TNFR1, TNFR2, or Fas is involved in Stx1-induced apoptosis of monocytic THP-1 cells. Following exposure to Stx1 for 6 h, membrane expression of TNFR1, TNFR2, mTNF-α (reported to specifically bind to TNFR2 [49]), and Fas was measured by flow cytometry (Fig. 1A). We failed to detect the expression of mTNF-α or death receptors on Stx1-treated cells. Control monocytic THP-1 cells cultured without Stx1 also failed to express death receptors. As a positive control, treatment of cells with phorbol-12-myristate-13-acetate induced the membrane expression of TNFR1, TNFR2, and mTNF-α (data not shown). Treatment of cells with Stx1 for 12 h resulted in 82.1 ± 4.9% apoptosis as measured by Annexin V and PI staining (Fig. 1B). In keeping with the lack of death receptor expression, purified rhTNF-α (40 ng/ml) failed to induce apoptosis in THP-1 cells. Pretreatment of cells with anti-TNFR1 or anti-TNF-α neutralizing antibodies did not prevent apoptosis induced by Stx1. Treatment of cells with the antibodies alone did not significantly affect cell viability. FasL has been reported to be constitutively produced and stored within THP-1 cells (4), and Tsan et al. (45) demonstrated that binding of a FasL agonist did not affect THP-1 cell growth. Collectively, these data suggest that Stx1-mediated cell death of monocytic THP-1 cells occurs through a TNFR/Fas-independent mechanism.
FIG. 1.
(A) Effect of Stx1 on death receptor expression by THP-1 cells. THP-1 cells were untreated (0 h) or treated with Stx1 (400 ng/ml) for 2, 4, and 6 h. After FcR blocking with human IgG (1.0 μg/105 cells), cells were incubated with anti-TNFR1, anti-TNFR2, anti-mTNF-α, anti-Fas, or control mouse IgG antibodies. Death receptor expression was assessed by flow cytometry. Representative results from three independent experiments are shown for untreated cells (shaded histogram) and 6-h Stx1 treatment (solid line). (B) Role of death receptors in Stx1-mediated apoptosis. THP-1 cells were untreated or treated with rhTNF-α for 12 h or pretreated with neutralizing anti-TNFR1 or anti-TNF-α antibodies for 1 h followed by treatment with Stx1 (400 ng/ml) for 12 h. Cells were stained with Annexin V and PI and analyzed by flow cytometry. Percentages of apoptotic (Annexin V-positive plus Annexin V/PI double-positive cells) were determined. Data shown are means ± standard errors of the means from three independent experiments. *, a significant difference (P < 0.01) relative to control (untreated) cells.
Requirement for caspase-8 and -3 activation for Stx1-induced apoptosis.
The sequential activation of a cascade of cysteine-dependent aspartate-specific proteases, called caspases, constitutes a major component of the programmed cell death machinery (43). In previous studies, we showed that Stx1-induced apoptosis of THP-1 cells was blocked by the general caspase inhibitor ZVAD-fmk (14). To further clarify the caspases required for Stx1-induced apoptosis, we measured apoptosis in the presence of specific caspase inhibitors using two different assays: DNA fragmentation and FACS analysis with Annexin V and PI double staining. In addition to ZVAD-fmk, the caspase-8-specific inhibitor and, to a lesser extent, the caspase-3-specific inhibitor were most effective in preventing Stx1-induced DNA laddering (Fig. 2A). Furthermore, inhibition of DNA fragmentation occurred in a caspase-8 inhibitor dose-dependent manner (Fig. 2B). Figure 3A shows representative scatter plots of FACS analyses of Annexin V/PI-stained cells treated with Stx1 with or without pretreatment with specific caspase inhibitors. Percentages of total apoptosis ([Annexin V-positive cells + Annexin V/PI double-positive cells] - background fluorescence) from three independent experiments are shown in Fig. 3B. After 5 h exposure to Stx1, 20.9 ± 3.3% of THP-1 cells were Annexin V or Annexin V/PI positive. Caspase-3 and -8 inhibitors almost completely blocked apoptosis (4.3 ± 2.5% and 3.55 ± 2.7%, respectively; P < 0.01). Caspase-6 and -9 inhibitors were partially effective in blocking Stx1-induced apoptosis, reducing Annexin V and Annexin V/PI positivity by approximately 50% (10.5 ± 2.1% and 10.1 ± 0.9%, respectively; P < 0.01). Caspase-1 and -2 inhibitors did not significantly decrease DNA laddering or Annexin V/PI staining. These data show that Stx1 triggers apoptosis in monocytic THP-1 cells primarily through activation of caspase-3 and -8, although caspase-6 and -9 also contribute to apoptosis induction.
FIG. 2.
(A) Effect of caspase inhibitors on Stx1-induced DNA fragmentation in THP-1 cells. Cells were incubated with specific caspase inhibitors (caspase-1 inhibitor [C1I], etc.) for 1 h and then treated with Stx1 (400 ng/ml) for 5 h. All caspase inhibitors were used at 40 μM. DNA was isolated and analyzed by ethidium bromide-agarose gel electrophoresis. ZVAD-fmk is a general caspase inhibitor. (B) A caspase-8 inhibitor (Z-IETD-fmk) blocks Stx1-induced DNA fragmentation in a dose-dependent manner. THP-1 cells were pretreated with the indicated concentrations of a caspase-8 inhibitor (C8I) for 1 h prior to the addition of Stx1 for 5 h. DNA was isolated and analyzed by ethidium bromide-agarose gel electrophoresis. “DNA ladder” is an apoptosis DNA ladder marker.
FIG. 3.
Effect of specific caspase inhibitors on Stx1-induced apoptosis of THP-1 cells. Cells (2 × 106 cells/ml) were incubated with various caspase inhibitors (all at 40 μM) for 1 h and then treated with Stx1 (400 ng/ml) for 5 h. Cells were stained with Annexin V and PI and analyzed by flow cytometry. (A) Representative scatter plots showing percentages of cells staining Annexin V positive (lower right quadrants) or Annexin V/PI double positive (upper right quadrants). (B) Percentages of total apoptosis of THP-1 cells treated with specific caspase inhibitors and Stx1. Data shown are the means ± standard errors of the means from three independent experiments. *, a significant difference (P < 0.01) relative to Stx1 treatment.
Activation of the mitochondrial pathway of apoptosis through Bid cleavage.
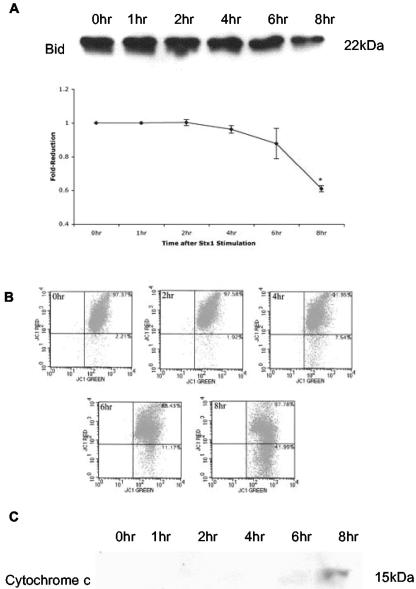
Cleavage of caspase-8 may directly activate caspase-3 or initiate apoptosis through the mitochondrial pathway (16). Caspase-8 converts the inactive 22-kDa form of Bid, a proapoptotic BH3 domain-only member of the Bcl2 family, to the active 15-kDa form called truncated Bid (tBid). tBid associates with the mitochondrial outer membrane, disrupts mitochondrial membrane potential (ΔΨm), and releases cytochrome c into the cytoplasm. Release of cytochrome c leads to the formation of a multimeric complex, the apoptosome, and the activation of caspase-9 (16, 53). To investigate possible activation of the mitochondrial pathway, THP-1 cells were incubated with Stx1 for 0 to 8 h, and Bid cleavage, ΔΨm, and cytochrome c release were measured (Fig. 4). As shown in Fig. 4A, levels of immunoreactive cytosolic Bid decreased beginning 4 h after Stx1 treatment. Densitometric scanning of the bands revealed a 50% reduction in the inactive form of Bid at 8 h of toxin treatment (P < 0.02). To measure ΔΨm, THP-1 cells stained with the dye JC-1 were analyzed by flow cytometry (Fig. 4B). At the start of the experiment and 2 h after toxin stimulation, JC-1 accumulated in the mitochondrial membrane as evidenced by >97% of the cells staining positive for red and green fluorescence. Beginning 4 h after Stx1 treatment, red fluorescence was reduced, and at 6 and 8 h of toxin treatment, ΔΨm was markedly reduced. Cytosolic fractions prepared from THP-1 cells treated with Stx1 for 0 to 8 h were analyzed by immunoblotting for the presence of cytochrome c (Fig. 4C). No cytochrome c was detected in the cytoplasm until 6 h after Stx1 treatment.
FIG. 4.
Kinetics of Bid cleavage, reduction in ΔΨm, and cytochrome c release in Stx1-treated THP-1 cells. Cells were harvested at 0, 1, 2, 4, 6, and 8 h after Stx1 treatment. (A) Cell lysates were prepared, and the uncleaved (22-kDa) form of Bid was detected in the lysates by immunoblotting (upper panel). Levels of Bid were quantitated by densitometric scanning and compared to control levels (lower panel). Data shown are the means ± standard errors of the means from two independent experiments. *, a significant difference (P < 0.02) compared to control cells. (B) Cells were collected after Stx1 treatment, and the JC-1 reagent was used to measure reductions in ΔΨm by flow cytometry. Percentages of cells staining for red and green fluorescence (upper right quadrants) and red fluorescence only (lower right quadrants) are shown in the upper right-hand corners of the quadrants. A decrease in ΔΨm is associated with a shift in reactive cells from the upper right quadrant to the lower right quadrant. Representative scatter plots from two independent experiments are shown. (C) Cytosolic fractions were prepared, and cytochrome c was detected in the fractions by immunoblotting. A representative blot from two independent experiments is shown.
To more directly examine the relationship between caspase-8 activation and ΔΨm, we treated THP-1 cells with the caspase-8 inhibitor or ZVAD-fmk and then treated with Stx1 for 8 h. Levels of JC-1 red fluorescence were then determined by flow cytometry (Fig. 5). Treatment with the caspase inhibitors almost completely blocked ΔΨm. These data suggest that Stx1 induces apoptosis, in part, through the activation of caspase-8, the cleavage of Bid, alterations in mitochondrial membrane potential, and the release of cytochrome c.
FIG. 5.
Effect of caspase inhibitors on ΔΨm after incubation of THP-1 cells with Stx1. THP-1 cells were incubated with the caspase-8 inhibitor (C8I) or the general caspase inhibitor (ZVAD-fmk) for 1 h before treatment with Stx1 (400 ng/ml). Caspase inhibitors were used at 40 μM. After 8 h of incubation with Stx1, cells were stained with JC-1 reagent and analyzed by flow cytometry. Control cells were not treated with Stx1. The percentages of cells staining for red and green fluorescence and green fluorescence only are shown in the scatter plots. The data shown are representative of at least two independent experiments.
Kinetics of Stx1-induced caspase activation.
Because Stx1-induced apoptosis of THP-1 cells required caspase-3, -8, and -9, we examined the kinetics of activation of these three caspases over a 24-h period of toxin stimulation. Caspase activation was monitored using caspase-specific colorimetric substrates coupled to pNA. Active caspases cleave the substrate, releasing free pNA. As shown in Fig. 6A, caspase-3, -8, and -9 showed similar kinetics of activation. Activities were significantly elevated (P < 0.05) 4 h after toxin treatment and peaked at 8 h. By 24 h, caspase activities appeared to return to basal levels. However, we have previously shown that approximately 85% of monocytic THP-1 cells treated with Stx1 for 24 h are not viable (14). The kinetics of caspase cleavage from the inactive procaspases were measured by Western blotting (Fig. 6B). The kinetics of caspase cleavage closely correlated with kinetics of functional activation.
FIG. 6.
Kinetics of caspase activation and cleavage in Stx1-treated THP-1 cells. (A) Cells were incubated with or without Stx1 (400 ng/ml) for 0 to 24 h. Caspase-3, -8, and -9 activities were then assayed as described in Materials and Methods. Control cells have not been treated with Stx1. Data shown are means ± standard errors of the means from three independent experiments (B) After Stx1 stimulation for 0, 2, 4, 6, and 8 h, cell lysates were subjected to SDS-PAGE and caspase-3, -8, and -9 cleavage was detected by immunoblotting with antibodies directed against the caspases. Data are representative of three independent experiments.
Role of caspase-6 in Stx1-induced apoptosis.
Although we showed that Stx1-induced apoptosis of THP-1 cells was associated with caspase-8 activation and the subsequent triggering of the mitochondrial pathway of cell death, we also noted that a caspase-6 inhibitor reduced Stx1-mediated apoptosis by approximately 50% (Fig. 3B). Caspase-3 has been reported to activate caspase-8 through a mitochondria-independent, caspase-6-dependent mechanism (13). Therefore, we investigated the role of caspase-6 in Stx1-induced apoptosis. THP-1 cells were treated with caspase-3, -6, and -8 inhibitors (40 μM) for 1 h prior to the addition of Stx1 (Fig. 7A). As expected, pretreatment of cells with the caspase-8 inhibitor dramatically reduced caspase-3, -8, and -9 activities, suggesting that caspase-8 activation may be an upstream event of caspase-3 and -9 activation. However, treatment of cells with caspase-3 and caspase-6 inhibitors also reduced caspase-8 and -9 activities. The abilities of caspase inhibitors to block Stx1-induced Bid cleavage (Fig. 7B, upper panel) and caspase-8 cleavage (Fig. 7B, lower panel) were also examined. Caspase-3, -6, and -8 inhibitors almost completely blocked Bid and caspase-8 cleavage in Stx1-treated THP-1 cells. These data suggest that in addition to triggering the mitochondrial pathway of apoptosis, Stx1 may also activate caspase-8 through a caspase-3- and caspase-6-dependent mechanism.
FIG. 7.
Role of caspase-6 in Stx1-induced apoptosis. THP-1 cells were pretreated with caspase-3, -6, or -8 inhibitor (40 μM) for 1 h and then treated with Stx1 (400 ng/ml) for 8 h. (A) Caspase activities were measured using specific colorimetric substrates as described in Materials and Methods. Control cells were not treated with Stx1. Data shown are means ± standard errors of the means from three independent experiments. *, **, and ***, significant differences (P < 0.01) within treatment groups relative to control (unstimulated) cells. (B) Cell lysates were separated by SDS-PAGE, and Bid (upper panel) and caspase-8 (lower panel) cleavage was detected using anti-Bid and anti-caspase-8 antibodies. Control cells were not treated with Stx1. A representative immunoblot from three independent experiments is shown.
Requirement for toxin enzymatic activity and ribotoxic stress response for Stx1-induced apoptosis.
To determine whether Stx1 binding to Gb3 and/or toxin enzymatic activity is required for the induction of THP-1 cell apoptosis, we treated cells with equal amounts of purified Stx1, Stx1 pentameric B-subunits, or Stx1A− (E167Q-R170L), a Stx1 holotoxin containing mutations in the A-subunit active site that reduce Vero cell cytotoxicity approximately 3 × 105-fold (32). As shown in Fig. 8A, after 6 h of treatment with Stx1, 35.8 ± 2.9% of THP-1 cells were apoptotic as detected by staining with Annexin V and PI. In contrast, Annexin V and PI staining of cells treated with B-subunits or Stx1A− (E167Q-R170L) were significantly decreased compared to Stx1-treated cells (3.75 ± 0.5% and 4.0 ± 2.2%, respectively; P < 0.01). Caspase-3, -8, and -9 activation were not detected in cells treated with B-subunits or Stx1A− (E167Q-R170L) (Fig. 8B). To investigate whether the activation of apoptosis via a caspase-8-dependent, death receptor-independent mechanism was a general response to protein synthesis inhibition or is unique to protein synthesis inhibitors acting on the ribosomal peptidyltransferase reaction center to initiate the ribotoxic stress response, we treated monocytic THP-1 cells with cycloheximide or anisomycin. As shown in Fig. 8C, even at high cycloheximide doses, apoptosis of THP-1 cells did not exceed 5.75 ± 0.8%. In contrast, anisomycin (10 μg/ml), a known inducer of the ribotoxic stress response (17), induced apoptosis in 28.6 ± 3.6% of the treated cells. Thus, binding of Stx1 to membrane Gb3 is not a sufficient signal for apoptosis in monocytic THP-1 cells. Furthermore, it would appear that active Stx1 must be transported to the ER, translocate into the cytoplasm, and trigger the ribotoxic stress response to initiate apoptotic signaling.
FIG. 8.
Requirement for Stx1 enzymatic activity and the ribotoxic stress response in Stx1-induced apoptosis of THP-1 cells. Cells were incubated with Stx1 or Stx1A− (E167Q-R170L) or Stx1 B-subunits (400 ng/ml and 800 ng/ml, respectively) for 6 h. (A) Percent apoptosis was analyzed by flow cytometry following Annexin V and PI staining. Data shown are means ± standard errors of the means from at least two independent experiments. (B) Caspase-3, -8 and -9 activities were measured using specific colorimetric substrates as described in Materials and Methods. Control cells were not treated with Stx1, Stx1A−, or Stx1 B-subunits. Data shown are means ± standard errors of the means from three independent experiments. *, **, and ***, significant differences (P < 0.01) within treatment groups relative to Stx1-treated cells. (C) Cells were treated with Stx1, cycloheximide (10 to 200 μM), or anisomycin (0.1 to 10 μg/ml) for 5 h, and apoptosis was measured by flow cytometry following Annexin V and PI staining. CHX, cycloheximide; Aniso, anisomycin.
DISCUSSION
It is clear that Stxs induce apoptosis in a wide variety of cell types, including cells of epithelial, endothelial, lymphocytic, and myelogenous origin (6). Apoptotic cell death is also evident in tissues of humans suffering from the hemolytic uremic syndrome (19). While cell death caused by Stxs may be critical in the pathogenesis of hemorrhagic colitis and systemic vascular complications, the mechanisms by which the toxins induce apoptosis remain to be fully characterized. We have shown that monocytic THP-1 cells are sensitive to the cytotoxic action of Stxs, with a 50% cytotoxic dose of approximately 14 pg/ml (35). Monocytic THP-1 cells undergo apoptosis via caspase-3 activation (21), which is blocked by the general caspase inhibitor ZVAD-fmk (14). The experiments performed in this study were designed to further clarify the apoptotic signaling pathways necessary for cell death in Stx1-stimulated monocytic THP-1 cells.
The role of death receptors in signaling for apoptosis has been well characterized. TNF-α/TNFR family or Fas/FasL ligation initiates the formation of the death-inducing signaling complex, an assembly of adaptor proteins, FADD and TRADD, and procaspase-8. Cleavage of procaspase-8 then initiates caspase cascades that ultimately trigger apoptosis (28). Recently, several research groups have shown that in the absence of death receptors of the TNFR family or Fas, caspase-8 may still be activated (12, 42). Our study and the studies of Fujii et al. (12) demonstrate that similar apoptotic signaling pathways may be triggered by Stxs in monocytic THP-1 cells and HeLa cells. In contrast, Stx1-induced apoptosis of Burkitt's lymphoma cells appears to involve Fas-mediated signaling (20). Bremner et al. (4) reported that THP-1 cells express FasL in the presence of lipopolysaccharide but that cell viability was not affected by treatment with anti-Fas antibodies. Our data show that in the presence or absence of Stx1, monocytic THP-1 cells fail to express membrane TNFR1, TNFR2, TNF-α, or Fas. However, Stx1 treatment triggers DNA fragmentation and exposure of membrane phosphatidylserine in the absence of these membrane-expressed death receptors. Thus, Stxs may induce apoptosis in different cell types through different signaling mechanisms. Exogenously added rhTNF-α did not induce THP-1 cell apoptosis, and anti-TNF-α or anti-TNFR1 neutralizing antibodies failed to inhibit Stx1-mediated apoptosis. As expected, treatment of cells with ZVAD-fmk and a caspase-3 specific inhibitor reduced Stx1-mediated DNA fragmentation and Annexin V staining, but we also noted that a caspase-8 inhibitor blocked these indicators of apoptosis. While we cannot rule out the possibility of poorly defined, novel death receptors participating in Stx1-mediated apoptosis induction, our data suggest that Stx1 induces THP-1 cell apoptosis in a TNFR/Fas-independent, caspase-8-dependent manner.
Activated caspase-8 may directly trigger the activation of executioner caspases, such as caspase-3 (1), or may trigger apoptosis through the mitochondrial pathway by cleavage of Bid (9). Once cleaved, tBid translocates from the cytosol to the mitochondrial outer membrane, mediating the homo-oligomerization of Bak and Bax proteins, the reduction in mitochondrial membrane potential, and the release of cytochrome c (51). Release of mitochondrial cytochrome c forms the apoptosome, a complex containing procaspase-9, Apaf-1, ATP, and cytochrome c. Procaspase-9 is cleaved, which then cleaves procaspase-3 (23, 53). We showed that Bid cleavage started 4 h after exposure of THP-1 cells to Stx1, with a 50% reduction in the uncleaved molecule by 8 h of treatment. Disruption of ΔΨm and cytochrome c release correlated with Bid cleavage. Furthermore, pretreatment of THP-1 cells with caspase-8 inhibitor almost completely prevented mitochondrial membrane disruption. These data indicate that caspase-8 is involved in Bid cleavage and cytochrome c release in Stx1-treated THP-1 cells. In addition, caspase-8 activation occurs upstream of caspase-9 and -3 activation.
It should be noted that we have observed Stx1-induced THP-1 cell death beginning at 4 h (14), but cytochrome c release was first evident 6 h after toxin treatment. These results suggest that caspase-8 may also directly activate caspase-3, while the mitochondrial pathway of apoptosis induction is triggered with slightly slower kinetics. Our data suggest that caspase-6 may be involved in the direct activation of caspase-8. Caspase-6 is a lamin protease, and activated lamin A induces nuclear disassembly and chromatin condensation (37). Furthermore, procaspase-6 is activated by caspase-3 and, in turn, activates procaspase-8 (7). Kojio et al. (21) showed a sixfold increase in caspase-6 activity in Stx-treated THP-1 cells, and we show here that a caspase-6 inhibitor reduces apoptosis by approximately 50%. To demonstrate a linkage among caspase-3, -6, and -8, we measured Stx1-induced caspase activities and procaspase cleavage in the presence or absence of specific inhibitors for each of the caspases. Although the percentages of inhibition differed among the different treatments, all the caspase-3, -6, and -8 inhibitors blocked caspase-3, -8, and -9 activities and procaspase-8 cleavage. Thus, blocking caspase-3 or -6 prevents caspase-8 activation and blocks Bid cleavage. These data suggest that Stx1 may trigger separate apoptosis signaling pathways in THP-1 cells. These signaling pathways are depicted in Fig. 9.
FIG. 9.
Proposed model of Stx1-induced apoptosis signaling pathways in monocytic THP-1 cells. See text for additional details.
Kojio et al. (21) reported caspase-3 activity peaking 5 h after exposure of THP-1 cells to Stx1. We measured caspase-3, -8, and -9 activities from 0 to 24 h after toxin treatment and found that all caspase activities reached the highest levels after 8 h. For the remaining 16 h of the experiments, caspase activities appeared to return to basal levels, although we have shown significant cell death occurring within this time period (14). Caspase-2 activity was reported to be increased 12-fold in Stx1-treated THP-1 cells (21), and we also found increased caspase-2 activity in Stx1-treated cells (data not shown). However, we found that inhibition of caspase-2 activity failed to decrease Stx1-mediated DNA laddering or Annexin V staining. The role of caspase-2 in apoptosis is controversial. Procaspase-2 is constitutively expressed in the nucleus and Golgi apparatus (26, 52). DNA damage by etoposide (an inhibitor of nuclear topoisomerase 2), the binding of TNF-α to TNFR1, and the activation of caspase-3 have all been shown to activate caspase-2 (33, 36). However, cleavage may not be required for caspase-2 activation. Procaspase-2 may oligomerize and become partially activated, although the role of procaspase-2 aggregates in apoptosis is unclear (2). Recently, Golgin-160, a Golgi apparatus-localized macromolecular complex, has been shown to be a substrate for caspase-2 involved in the disassembly of the Golgi complex (26). Whether treatment of THP-1 cells with Stx1 results in proteolysis of Golgin-160, and whether cleaved Golgin-160 is involved in apoptosis, will require additional experiments.
The treatment of Ramos Burkitt's lymphoma and astrocytoma cells with Stx B-subunits or anti-Gb3 monoclonal antibody has been reported to induce apoptosis and activate procaspase-8 (20). Thus, for these cells, binding of toxin receptors at the cell surface may generate sufficient signals to activate programmed cell death cascades. We show here that purified Stx1 B-subunits and an enzymatic (active site) mutant toxin are incapable of inducing apoptosis or caspase-3, -8, and -9 activities in monocytic THP-1 cells. Inhibition of the formation of functional Golgi complexes using brefeldin A has been shown to inhibit Stx1-induced caspase-3 activity and DNA fragmentation in THP-1 cells (21). Together, these data suggest that Stxs must be internalized and undergo retrograde transport through the Golgi apparatus to the ER, and the functional toxin A-subunit fragments must be translocated into the cytoplasm in order to trigger the signals necessary for apoptosis induction.
A key feature of the apoptotic pathways triggered by Stxs that remains to be characterized is the linkage between protein synthesis inhibition and the activation of caspases. A family of ribosome inactivating proteins, including Stxs, has been shown to induce the ribotoxic stress response (5, 10, 17, 41). As a result of the highly specific 28S rRNA depurination reaction mediated by Stxs, the stress-activated protein kinase cascades, JNK and p38, are activated. These mitogen-activated protein kinases (MAPKs) phosphorylate a number of downstream substrates, leading to activation of transcriptional factors such as NF-κB and AP-1. In response to Stxs, therefore, the cellular transcriptome may be rapidly altered by transcriptional and posttranscriptional mechanisms. The p38 MAPK cascade has also been reported to regulate the stability of some mRNA transcripts (50). Our data suggest that the activation of apoptotic signaling is not a general response to protein synthesis inhibition but that the ribotoxic stress response may be selectively involved in triggering caspase-8- and mitochondrion-dependent programmed cell death. Ribosome inactivation may not be the sole determinant in triggering apoptosis; rather, toxin effects directed to the ER membrane may contribute to signaling. Jimbo et al. (18) showed that ER stress initiated procaspase-8 activation by direct interaction of caspase-8 with ER membrane proteins. Bap31 is a polytopic integral membrane protein of the ER and a substrate for caspase-8 activity. Bap31 may interact with Bcl-2 to inhibit the interaction between procaspase-8 and Bap31. However, if Bap31 associates with procaspase-8 in the absence of Bcl-2, then procaspase-8 and Bap31 are cleaved and programmed cell death may be initiated (3, 30). Even though the precise role of Bap31 in apoptosis remains to be elucidated, it may explain how procaspase-8 is activated in the absence of death receptor expression by THP-1 cells.
Acknowledgments
These studies were supported by U.S. Public Health Service grant 2RO1-AI34530 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank Yoshifumi Takeda and Cheleste Thorpe for their gifts of toxins and Jane Miller for her excellent technical assistance with the FACS analyses. We thank David McMurray, Rajesh Miranda, and Jane Welsh for their careful reading of the manuscript.
Editor: A. D. O'Brien
REFERENCES
- 1.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 2.Baliga, B. C., S. H. Read, and S. Kumar. 2004. The biochemical mechanism of caspase-2 activation. Cell Death Diff. 11:1234-1241. [DOI] [PubMed] [Google Scholar]
- 3.Breckenridge, D. G., M. Nguyen, S. Kuppig, M. Reth, and G. C. Shore. 2002. The procaspase-8 isoform, procaspase-8L, is recruited to the BAP31 complex at the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 99:4331-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremner, T. A., D. Chatterjee, Z. Han, M.-F. Tsan, and J. H. Wyche. 1999. THP-1 monocytic leukemia cells express Fas ligand constitutively and kill Fas-positive Jurkat cells. Leukemia Res. 23:865-870. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, P., S. J. Smith, M. A. Giembycz, D. Rotondo, and R. Plevin. 2003. Verotoxin activates mitogen-activated protein kinase in human peripheral blood monocytes: role in apoptosis and proinflammatory cytokine release. Br. J. Pharmacol. 140:1320-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherla, R. P., S.-Y. Lee, and V. L. Tesh. 2003. Shiga toxins and apoptosis. FEMS Microbiol. Lett. 228:159-166. [DOI] [PubMed] [Google Scholar]
- 7.Cowling, V., and J. Downward. 2002. Caspase-6 is the direct activator of caspase-8 in the cytochrome c-induced apoptosis pathway: absolute requirement for removal of caspase-6 prodomain. Cell Death Diff. 9:1046-1056. [DOI] [PubMed] [Google Scholar]
- 8.Endo, Y., K. Tsurugi, T. Yutsudo, T., Y. Takeda, T. Ogasawara, and K. Igarashi. 1988. Site of action of a verotoxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171:45-50. [DOI] [PubMed] [Google Scholar]
- 9.Eskes, R., S. Desagher, B. Antonsson, and J.-C. Martinou. 2000. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20:929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, G. H., and V. L. Tesh. 2002. Shiga toxin 1-induced activation of c-Jun NH2-terminal kinase and p38 in the human monocytic cell line THP-1: possible involvement in the production of TNF-α. J. Leukoc. Biol. 71:107-114. [PubMed] [Google Scholar]
- 11.Fraser, M. E., M. M. Chernaia, Y. V. Kozlov, and M. N. G. James. 1994. Crystal structure of the holotoxin from Shigella dysenteriae at 2.5 Å resolution. Nat. Struct. Biol. 1:59-64. [DOI] [PubMed] [Google Scholar]
- 12.Fujii, J., T. Matsui, D. P. Heatherley, K. H. Schlegel, P. I. Lobo, T. Yutsudo, G. M. Ciraolo, R. E. Morris, and T. Obrig. 2003. Rapid apoptosis induced by Shiga toxin in HeLa cells. Infect. Immun. 71:2724-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fumarola, C., and G. G. Guidotti. 2004. Stress-induced apoptosis: toward a symmetry with receptor-mediated cell death. Apoptosis 9:77-82. [DOI] [PubMed] [Google Scholar]
- 14.Harrison, L. M., R. P. Cherla, C. van den Hoogen, W. C. E. van Haaften, S.-Y. Lee, and V. L. Tesh. 2005. Comparative evaluation of apoptosis induced by Shiga toxin 1 and/or lipopolysaccharides in human monocytic and macrophage-like cells. Microb. Pathog. 38:63-76. [DOI] [PubMed] [Google Scholar]
- 15.Harrison, L. M., W. C. E. van Haaften, and V. L. Tesh. 2004. Regulation of proinflammatory cytokine expression by Shiga toxin 1 and/or lipopolysaccharides in the human monocytic cell line THP-1. Infect. Immun. 72:2618-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 17.Iordanov, M. S., D. Pribnow, J. L. Magun, T.-H. Dinh, J. A. Pearson, S. L.-Y. Chen, and B. E. Magun. 1997. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of peptidyltransferase reaction and by sequence-specific RNA damage to the α-sarcin/ricin loop in the 28S rRNA. Mol. Cell. Biol. 17:3373-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimbo, A., E. Fujita, Y. Kouroku, J. Ohnishi, N. Inohara, K. Kuida, K. Sakamaki, S. Yonehara, and T. Momoi. 2003. ER stress induces caspase-8 activation, stimulating cytochrome c release and caspase-9 activation. Exp. Cell Res. 83:156-166. [DOI] [PubMed] [Google Scholar]
- 19.Karpman, D., A. Hakansson, M.-T. R. Perez, C. Isaksson, E. Carlemalm, A. Caprioli, and C. Svanborg. 1998. Apoptosis of renal cortical cells in the hemolytic uremic syndrome: in vivo and in vitro studies. Infect. Immun. 66:636-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiyokawa, N., T. Mori, T. Taguchi, M. Saito, K. Mimori, T. Suzuki, T. Sekino, N. Sato, H. Nakajima, Y. U. Katagiri, T. Takeda, and J. Fujimoto. 2001. Activation of the caspase cascade during Stx1-induced apoptosis in Burkitt's lymphoma cells. J. Cell. Biochem. 81:128-142. [DOI] [PubMed] [Google Scholar]
- 21.Kojio, S., H.-M. Zhang, M. Ohmura, F. Gondaira, N. Kobayashi, and T. Yamamoto. 2000. Caspase-3 activation and apoptosis induction coupled with the retrograde transport of Shiga toxin: inhibition by brefeldin A. FEMS Immunol. Med. Microbiol. 29:275-281. [DOI] [PubMed] [Google Scholar]
- 22.Leist, M., C. Volbracht, E. Fava, and P. Nicotera. 1998. 1-Methyl-4-phenyl-pyridinium induces autocrine excitoxicity, protease activation, and neuronal apoptosis. Mol. Pharmacol. 54:789-801. [DOI] [PubMed] [Google Scholar]
- 23.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 24.Lingwood, C. A. 2003. Shiga toxin receptor glycolipid binding. Pathology and utility. Methods Mol. Med. 73:165-186. [DOI] [PubMed] [Google Scholar]
- 25.Louise, C. B., and T. G. Obrig. 1991. Shiga toxin-associated hemolytic uremic syndrome: combined cytotoxic effects of Shiga toxin, interleukin-1β and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect. Immun. 59:4173-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancini, M., C. E. Machamer, S. Roy, D. Nicholson, N. A. Thornberry, L. Casciola-Rosen, and A. Rosen. 2000. Caspase-2 is localized at the Golgi complex and cleaves Golgin-160 during apoptosis. J. Cell Biol. 149:603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melton-Celsa, A. R., and A. D. O'Brien. 1998. Structure, biology and relative toxicity of Shiga toxin family members for cells and animals, p. 121-128. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 28.Micheau, O., and J. Tschopp. 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114:181-190. [DOI] [PubMed] [Google Scholar]
- 29.Moxley, R. A., and D. H. Francis. 1998. Overview of animal models, p. 249-260. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 30.Ng, F. W. H., M. Nguyen, T. Kawn, P. E. Branton, D. W. Nicholson, J. A. Cromlish, and G. C. Shore. 1997. p28 BAP31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum. J. Cell Biol. 139:327-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Brien, A. D., V. L. Tesh, A. Donohue-Rolfe, M. P. Jackson, S. Olsnes, K. Sandvig, A. A. Lindberg, and G. T. Keusch. 1992. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 180:65-94. [DOI] [PubMed] [Google Scholar]
- 32.Ohmura, M., S. Yamasaki, H. Kurazono, K. Kashiwagi, K. Igarashi, and Y. Takeda. 1993. Characterization of non-toxic mutants of verotoxin 1 that were constructed by replacing amino acids in the A subunit. Microb. Pathog. 15:169-176. [DOI] [PubMed] [Google Scholar]
- 33.Paroni, G., C. Henderson, C. Schneider, and C. Brancolini. 2001. Caspase-2-induced apoptosis is dependent on caspase-9, but its processing during UV- or tumor necrosis factor-dependent cell death requires caspase-3. J. Cell Biol. 276:21907-21915. [DOI] [PubMed] [Google Scholar]
- 34.Proulx, F., E. G. Seidman, and D. Karpman. 2001. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr. Res. 50:163-171. [DOI] [PubMed] [Google Scholar]
- 35.Ramegowda, B., and V. L. Tesh. 1996. Differentiation-associated toxin receptor modulation, cytokine production and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect. Immun. 64:1173-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson, J. D., V. Gogvadze, B. Zhitotovsky, and S. Orrenius. 2000. Distinct pathways for stimulation of cytochrome c release by etoposide. J. Biol. Chem. 275:32438-32443. [DOI] [PubMed] [Google Scholar]
- 37.Ruchaud, S., N. Korfali, P. Villa, T. J. Kottke, C. Dingwall, S. H. Kaufmann, and W. C. Earnshaw. 2002. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. EMBO J. 21:1967-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandvig, K., S. Grimmer, S. U. Lauvrak, M. L. Torgersen, G. Skretting, B. van Deurs, and T. G. Iversen. 2002. Pathways followed by ricin and Shiga toxin into cells. Histochem. Cell. Biol. 117:131-141. [DOI] [PubMed] [Google Scholar]
- 39.Saxena, S. K., A. D. O'Brien, and E. J. Ackerman. 1989. Shiga toxin, Shiga-like toxin II variant, and ricin are all single-site RNA N-glycosidases of 28S RNA when microinjected into Xenopus oocytes. J. Biol. Chem. 264:596-601. [PubMed] [Google Scholar]
- 40.Siegler, R. L. 1994. Spectrum of extrarenal involvement in postdiarrheal hemolytic uremic syndrome. J. Pediatr. 125:511-518. [DOI] [PubMed] [Google Scholar]
- 41.Smith, W. E., A. V. Kane, S. T. Campbell, D. W. K. Acheson, B. H. Cochran, and C. M. Thorpe. 2003. Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells. Infect. Immun. 71:1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Souza-Fagundes, E. M., G. Brumatti, O. A. Martins-Filho, R. Correa-Oliveira, C. L. Zani, and G. P. Amarante-Mendes. 2003. Myriadenolide, a labdane diterpene isolated from Alomia myriadenia (asteraceae) induces depolarization of mitochondrial membranes and apoptosis associated with activation of caspase-8, -9 and -3 in Jurkat and THP-1 cells. Exp. Cell Res. 290:420-426. [DOI] [PubMed] [Google Scholar]
- 43.Stennicke, H. R., and G. S. Salvesen. 2000. Caspases—controlling intracellular signals by protease zymogen activation. Biochim. Biophys. Acta 1477:299-306. [DOI] [PubMed] [Google Scholar]
- 44.Tesh, V. L., J. A. Burris, J. W. Owens, V. M. Gordon, E. A. Wadolkowski, A. D. O'Brien, and J. E. Samuel. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 61:3392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsan, M.-F., J. E. White, J. G. Maheshware, T. A. Bremner, and J. Sacco. 2000. Resveratrol induces Fas signaling-independent apoptosis in THP-1 human monocytic leukemia cells. Br. J. Haematol. 109:405-412. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada.1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 47.van de Kar, N. C. A. J., L. A. H. Monnens, M. A. Karmali, and V. W. M. van Hinsbergh. 1992. Tumor necrosis factor and interleukin 1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic uremic syndrome. Blood 80:2755-2764. [PubMed] [Google Scholar]
- 48.van Setten, P. A., L. A. H. Monnens, R. G. G. Verstraten, L. P. W. J. van den Heuvel, and V. W. M. van Hinsbergh. 1996. Effects of verocytotoxin-1 on nonadherent monocytes: binding characteristics, protein synthesis inhibition and induction of cytokine release. Blood 88:174-183. [PubMed] [Google Scholar]
- 49.Wajant, H., K. Pfizenmaier, and P. Scheurich. 2003. Tumor necrosis factor signaling. Cell Death Diff. 10:45-65. [DOI] [PubMed] [Google Scholar]
- 50.Wang, S. W., J. Pawlowski, S. T. Wathen, S. D. Kinney, H. S. Lichenstein, and C. L. Manthey. 1999. Cytokine mRNA decay is accelerated by an inhibitor of p38 mitogen-activated protein kinase. Inflamm. Res. 48:533-538. [DOI] [PubMed] [Google Scholar]
- 51.Wei, M. C., T. Lindsten, V. K. Mootha, S. Weiler, A. Gross, M. Ashiya, C. B. Thompson, and S. J. Korsmeyer. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14:2060-2071. [PMC free article] [PubMed] [Google Scholar]
- 52.Zhivotovsky, B., A. Samali, A. Gahm, and S. Orrenius. 1999. Caspases: their intracellular localization and translocation during apoptosis. Cell Death Diff. 6:644-651. [DOI] [PubMed] [Google Scholar]
- 53.Zou, H., W. J. Henzel, X. Liu, A. Lutschg, and X. Wang. 1997. Apaf-1, human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405-413. [DOI] [PubMed] [Google Scholar]