Abstract
The four following commercially available enzyme immunoassays (EIAs) were assessed and compared for their performance in detecting Mycoplasma pneumoniae immunoglobulin G (IgG)- and IgM-specific antibodies Platelia EIA, ImmunoWELL M. pneumoniae ELISA IgG and IgM, ETI-MP-IgG and IgM EIAs and Biotest anti-M. pneumoniae IgG and IgM ELISA (referred to herein as EIA-Platelia, EIA-BMD, EIA-Sorin, and EIA-Biotest). Three groups of patients were investigated: 39 patients (27 children and 12 adults) with respiratory infections who tested positive by PCR for M. pneumoniae in respiratory specimens (group I; 52 serum samples), 61 healthy children and adults (group II; 61 serum samples), and 20 patients with rheumatoid factor or antinuclear antibodies, or who tested positive for antiviral IgM (group III; 20 serum samples). In group III, the IgM specificity for EIA-Platelia, EIA-BMD, EIA-Biotest, and EIA-Sorin was 100, 90, 65, and 25%, respectively. In the children from group I, the four EIAs had similar IgM sensitivities (89 to 92%); the sensitivity for IgG was greater with EIA-BMD and EIA-Biotest than with EIA-Platelia and EIA-Sorin (66 and 78% versus 55 and 52%, respectively). In adult patients from group I, 9 to 10 serum samples were positive for IgG with a concordant sensitivity of 75 to 83% between the four EIAs but a striking difference in IgM sensitivity: 16% by EIA-Platelia and EIA-BMD, 50% by EIA-Biotest, and 58% by EIA-Sorin. Discrepant and unexpected results were observed in IgM detection from control healthy patients using EIA-Sorin and EIA-Biotest, confirming the lack of specificity of these two EIAs and making them inaccurate for routine diagnosis. A good concordance of IgG seroprevalence in healthy adults was found between the four EIAs (66 to 70%), though this concordance was lower with EIA-Platelia (43%). In healthy children, EIA-BMD and EIA-Biotest gave a higher IgG seroprevalence than EIA-Sorin and EIA-Platelia (45% each for the former compared to 17 and 20%, respectively, for the latter). These results confirm that the IgM EIA serology test is a valuable tool for the early diagnosis of M. pneumoniae infections in children, as long as the EIA used is specific. In adults, the difficult interpretation of EIAs suggests that paired sera, combined with PCR detection on respiratory tract specimens collected on admission of patient, should be required for accurate diagnosis.
Mycoplasma pneumoniae is a common respiratory pathogen responsible for mild acute respiratory infections such as sore throats, pharyngitis, and tracheobronchitis in younger children. It is the most common cause of primary atypical pneumonia resistant to β-lactam antibiotics in older children and young adults (5, 10, 15). M. pneumoniae can also be associated with severe extrapulmonary complications (9, 21, 24). The standard laboratory methods for the specific diagnosis of M. pneumoniae infection have been isolation in culture and serological methods. Culture is time-consuming and relatively insensitive. The conventional complement fixation test (CFT) using a glycolipid antigen gives unspecific reactions and lacks sensitivity (16, 19). Alternative tests have recently been developed to obtain more-accurate and prompt diagnosis: indirect enzyme immunoassay (EIA) measuring separately immunoglobulin G (IgG) and IgM class antibodies (8, 14, 27, 33, 35) and PCR for rapid and sensitive detection of M. pneumoniae in respiratory tract specimens (1, 4, 6, 31, 32, 36). The objective of this retrospective study was to compare the performance of four commercially available EIAs for the detection of specific M. pneumoniae IgG and IgM antibodies in assessing the antibody response of suspected M. pneumoniae patients on the basis of PCR-positive results for M. pneumoniae in respiratory samples. Furthermore, we evaluated the ability of the serological tests to accurately establish an earlier serodiagnosis of M. pneumoniae infections.
MATERIALS AND METHODS
Patients and controls.
For a 4-year period, January 1997 through December 2000, 39 patients (group I: 27 children and 12 adults), admitted to Caen’s hospital with clinical features of upper or lower respiratory tract infection consistent with M. pneumoniae infection, were retrospectively selected on the basis of a specific M. pneumoniae-positive PCR in respiratory tract samples. Serum specimens and respiratory tract specimens were obtained on admission from all 39 patients. In addition, serum specimens were obtained 5 to 22 days later from 13 patients. Upon their receipt, the 52 serum samples were serologically investigated for M. pneumonia-specific IgM and IgG antibodies by the Platelia EIAs IgG and IgM (Diagnostica Pasteur Sanofi) (referred to herein as EIA-Platelia) and were subsequently stored frozen at −20°C until required for testing.
A control group consisting of 61 single serum samples from healthy individuals matched for age and sex (group II: 40 children and 21 adults) was included in the study. Furthermore, the specificity of the four EIAs was assessed by using 20 additional serum samples (group III): 2 serum samples positive for rheumatoid factor (RF), 2 serum samples positive for antinuclear antibodies (ANAs), and 16 serum samples from serologically proven cases of recent viral infections (5 heterophil-positive infectious mononucleosis-positive infections, 2 IgM cytomegalovirus infections, 2 IgM viral capsid antigen Epstein-Barr virus infections, 2 IgM hepatitis A virus infections, 2 IgM herpes simplex virus infections, 1 IgM varicella-zoster virus infection, 1 IgM measles virus infection, and 1 IgM mumps virus infection). Sera for all three groups were thawed and tested by the four EIAs; the acute- and convalescent-phase serum specimens were tested for IgG and IgM in the same run.
PCR for M. pneumoniae.
Respiratory tract specimens, five bronchoalveolar lavage (BAL) samples, 14 throat swabs, and 19 nasal aspirates were examined for M. pneumoniae by PCR on the admission of the patient. Nucleic acids were extracted by a Chelex procedure. A 250-μl specimen in transport medium (200 mM sucrose phosphate for throat specimens, Eagle minimal essential medium for nasal aspirates and BAL samples) was carefully mixed with 150 μl of a suspension containing 20% Chelex 100 (Bio-Rad, Marnes-la-Coquette, France) in 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, and 1% Tween 20 aqueous solution. The mixture was then heated to 100°C for 15 min and centrifuged at 12,000 × g for 10 min. The supernatant was directly used for PCR with P1 gene-specific primers MP-P11 and MP-P12 (6). The PCR amplification products were detected by hybridization with a biotinylated M. pneumoniae-specific probe, MP-I (6), in a DNA EIA (GEN-ETI-K DEIA; Sorin). An index value was defined as optical density (OD) sample value/OD cutoff value. A positive hybridization signal was considered positive if the index value was ≥2. To identify possible false-negative results caused by inhibitory factors in the specimens, control amplification of each DNA extract with human β-globin primers GH20 and PCO4 were performed in separate tubes (28). A positive signal was defined by a 268-bp fragment visualized on an ethidium bromide-stained agarose gel. A positive control and negative controls (sterile water, DNA-free extraction mixture, and DNA-free PCR mixture) were systematically treated identically to the samples throughout. The results of M. pneumoniae PCR were considered only when DNA was found to be amplified with β-globin-specific primers and when no hybridization signal was detected with negative controls. To minimize the risk of contamination, DNA extraction, PCR setup, and product analysis were each carried out in separate rooms.
Commercial EIAs.
The four commercially available EIAs contained all reagents to be used and were performed using the protocols supplied with each kit. Negative and positive control sera were included for each run to validate the test quality. The interpretation of the results was performed after assay validation defined for each test.
(i) EIA-Platelia.
IgG antibodies to M. pneumoniae were detected by the Platelia indirect EIA (Sanofi Diagnostica Pasteur). In this test, the antigen solution used to coat the microplate was a solubilized ultrasonicate of an M. pneumoniae culture containing a high proportion of membrane proteins. The specimen arbitrary unit (AU) values were determined from the calibration curve and were interpreted as follows. A value of <10 AU/ml for a single serum specimen was considered insignificant, a value of 10 to 19 AU/ml was considered low, a value of 20 to 39 AU/ml was considered moderate, and a value of >40 AU/ml was considered high.
IgM antibodies to M. pneumoniae were detected by the Platelia M. pneumonia IgM EIA. This test is a double-sandwich immunoenzymatic assay with capture of the serum IgM antibodies on the solid phase (microplate coated with human anti-μ chain antibodies).
(ii) EIA-BMD.
ImmunoWELL M. pneumoniae ELISA IgG and IgM (BMD s.a.) (referred to herein as EIA-BMD) was performed as in indirect EIAs, using a purified M. pneumoniae glycolipid (strain FH) as the antigen. For IgM titration, diluted controls and specimens were initially absorbed with a human anti-IgG solution in order to prevent interference due to RF or residual human IgG. The titers of IgG and IgM in the serum were calculated as the ratio of sample absorbance value to calibrator absorbance value that is then multiplied by the value assigned (in arbitrary units per milliliter) to the positive calibrator. The result was then interpreted as follows: titers for M. pneumoniae IgG were considered insignificant if they measured less than 200 AU/ml, low if they were between 200 and 320 AU/ml, and highly significant if they were higher than 320 AU/ml. The corresponding IgM cutoffs were ≤770, 770 to 950, and >950 AU/ml.
(iii) EIA-Sorin.
The ETI-M. pneumoniae IgG and IgM EIAs (Diasorin, Antony, France) (referred to herein as EIA-Sorin) use a microtiter plate coated with a preparation of purified membrane protein as the antigen, including the immunodominant cytoadhesin P1. For IgM antibody detection, the sera were diluted to 1/105 with a solution of human anti-IgG in order to rule out residual human IgG. The ODs of samples were transformed to arbitrary units determined from the calibration curve (10, 50, and 100 AU/ml), and the corresponding titers of the serum were interpreted, as indicated by the manufacturer, as follows: a titer of less than 10 AU/ml was considered insignificant, a value of 10 to 50 AU/ml was considered a significant titer of specific antibodies, and a value of ≥50 AU/ml was considered a high titer.
(iiii) EIA-Biotest.
The Biotest anti-M. pneumoniae IgG and IgM ELISA (Biotest AG, Buc, Germany) kit (referred to herein as EIA-Biotest) contains microtiter reaction wells that were precoated with an ultrasonicated purified homogeneous antigen. The cutoff value was calculated as the absorbance of the negative control (OD value < 0.100) plus mean value of all cutoff controls, and the cutoff range was defined (cutoff value ± 10%). The titer of the patient sera may be determined from a semiquantitative curve obtained with the calculated cutoff value and a titrated positive control.
RESULTS
The cutoff values established by the manufacturer were used for interpretation of results of the four IgM and IgG EIAs. Concerning the IgG and IgM EIA-BMD, IgG and IgM EIA-Biotest, and IgM EIA-Platelia, the uneasy definition of an EIA end point has been dealt with by the designation of an “equivocal” or “low-positive” range. In the interpretation of the results, when a single serum sample was tested, equivocal or low-positive results were considered to be negative, whereas in a paired serum sample, they were considered positive if they became positive when the second serum sample was tested.
Specificity of the assays.
The specificity of the assays was assessed on sera from group III. The different EIA results are presented in Table 1 as the number of individuals who were IgM positive. EIA-Platelia appears to be the most specific method for demonstrating M. pneumoniae IgM antibody; none of the RF, ANAs, or IgM antiviral positive sera were positive by IgM EIA-Platelia (specificity, 100%). Specificities of EIA-BMD, EIA-Biotest, and EIA-Sorin were, respectively, 90, 65, and 25%.
TABLE 1.
Specificities of four EIAs for 20 serum specimens from patients positive for RF, ANA, or anti-viral IgM (group III)
| Group III category | No. of serum specimens (n = 20) | No. of serum samples positive for IgM by:
|
|||
|---|---|---|---|---|---|
| EIA-Platelia | EIA-Sorin | EIA-BMD | EIA-Biotest | ||
| Infectious mononucleosis | 5 | 0 | 5 | 0 | 3 |
| IgM positive | |||||
| Cytomegalovirus | 2 | 0 | 1 | 0 | 0 |
| Epstein-Barr virus VCAa | 2 | 0 | 1 | 0 | 1 |
| Hepatitis A | 2 | 0 | 1 | 0 | 1 |
| Herpes simplex virus | 2 | 0 | 2 | 0 | 0 |
| Varicella-zoster virus | 1 | 0 | 1 | 0 | 0 |
| Measles virus | 1 | 0 | 1 | 1 | 0 |
| Mumps virus | 1 | 0 | 1 | 1 | 1 |
| RF positive | 2 | 0 | 1 | 0 | 0 |
| ANA positive | 2 | 0 | 1 | 0 | 1 |
| % Specificity | 100 | 25 | 90 | 65 | |
VCA, viral capsid antigen.
Specific IgG and IgM determination in sera from 39 patients with M. pneumoniae infection.
From 39 patients with a positive M. pneumoniae PCR (group I), acute- and available convalescent-phase sera (from 13 out of the 39 patients) were examined for specific IgG and IgM detection by the four EIA assays. The diagnostic performance of the four EIAs are shown in Table 2. In children, the four EIAs detected IgM antibodies from 24 to 25 patients, giving a concordant sensitivity of 89 to 92%, but the sensitivity of the EIA-BMD and the EIA-Biotest IgG test were greater (66 and 78%, respectively) than those of the EIA-Platelia and the EIA-Sorin IgG test (55 and 52%, respectively). In adult patients, we observed a concordant IgG sensitivity of 75 to 83% but a striking difference in IgM sensitivity: 16% by EIA-Platelia and EIA-BMD, 50% by EIA-Biotest, and 58% by EIA-Sorin.
TABLE 2.
Sensitivities of EIAs for detection of M. pneumoniae IgG and IgM antibodies in sera from 39 patients with positive M. pneumoniae PCR results (group I)
| Patient group | No. of serum samples | Immunoglobulin | No (%) of serum samples positive bya:
|
|||
|---|---|---|---|---|---|---|
| EIA-Platelia | EIA-Sorin | EIA-BMD | EIA-Biotest | |||
| Children | 27 | IgG | 15 (55) | 14 (52) | 18 (66) | 21 (78) |
| IgM | 24 (89) | 25 (92) | 25 (92) | 24 (89) | ||
| Adults | 12 | IgG | 10 (83) | 9 (75) | 9 (75) | 9 (75) |
| IgM | 2 (16) | 7 (58) | 2 (16) | 6 (50) | ||
| Total | 39 | IgG | 25 (64) | 23 (59) | 27 (69) | 30 (77) |
| IgM | 26 (66) | 32 (82) | 27 (69) | 30 (77) | ||
Convalescent- and/or acute-phase serum samples.
Particular attention was paid to the four EIA serological results from the 13 patients with paired sera from patients with proved M. pneumoniae infection (Table 3). Both in adults and children, the overall IgG sensitivities were 61% for the EIA-BMD and the EIA-Biotest, 31% for the EIA-Sorin, and 15% for the EIA-Platelia with the acute-phase serum specimens, whereas a similar IgG sensitivity was observed by the four EIAs with the convalescent-phase serum specimens. In IgM detection in acute-phase serum samples, EIA-Sorin and EIA-Biotest were globally more sensitive than EIA-Platelia and EIA-BMD (46 and 61% versus 15 and 31%), but the results were slightly different between adults and children. There was a good concordance between IgM detection in acute- and convalescent-phase sera in children but, in adult patients, the sensitivities were very high for EIA-Sorin (29 and 57%) and EIA-Biotest (57 and 71%) compared to EIA-Platelia and EIA-BMD (0 and 14% for both).
TABLE 3.
Sensitivities of four M. pneumoniae EIAs in 13 pairs of acute- and convalescent-phase serum samples from patients with positive M. pneumoniae PCR results (group I)
| Patient group | No. of serum samples | Immunoglobulin | No. (%) of serum samples positive by:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EIA-Platelia
|
EIA-Sorin
|
EIA-BMD
|
EIA-Biotest
|
|||||||
| Acute phase | Convalescent phase | Acute phase | Convalescent phase | Acute phase | Convalescent phase | Acute phase | Convalescent phase | |||
| Children | 6 | IgG | 0 | 4 (66) | 2 (33) | 5 (83) | 3 (50) | 5 (83) | 3 (50) | 5 (83) |
| IgM | 2 (33) | 4 (66) | 4 (66) | 5 (83) | 4 (66) | 5 (83) | 4 (66) | 5 (83) | ||
| Adults | 7 | IgG | 2 (29) | 6 (86) | 2 (29) | 6 (86) | 5 (71) | 5 (71) | 5 (71) | 6 (86) |
| IgM | 0 | 1 (14) | 2 (29) | 4 (57) | 0 (0) | 1 (14) | 4 (57) | 5 (71) | ||
| Total | 13 | IgG | 2 (15) | 10 (77) | 4 (31) | 11 (84) | 8 (61) | 10 (77) | 8 (61) | 11 (85) |
| IgM | 2 (15) | 5 (38) | 6 (46) | 9 (69) | 4 (31) | 6 (46) | 8 (61) | 10 (77) | ||
Although a good correlation was found between PCR results and serology, negative serological tests with a positive PCR result were obtained in five adults and one child (Table 4). None of the sera from the four patients with paired sera (patients 1, 2, 3, and 4) showed a seroconversion or a significant increase in the IgG titer. Equivocal IgM titer was found by EIA-BMD in single serum sample from adult patient 5, and equivocal IgG titer and low IgG titer were, respectively, found by EIA-Biotest and EIA-BMD in a single serum sample from patient 6; these serological results were uninterpretable without convalescent-phase serum samples collected 8 to 15 days later.
TABLE 4.
Results obtained in six patients with positive M. pneumoniae PCR results without positive serological criteria in the four EIAs
| Patient no. | Sex | Age | Diagnosis(es) | Days after onset | Immunoglobulin | Titer (AU/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EIA-Plateliaa
|
EIA-Sorinb
|
EIA-BMDc
|
EIA-Biotestd
|
||||||||||
| Acute phase | Convalescent phase | Acute phase | Convalescent phase | Acute phase | Convalescent phase | Acute phase | Convalescent phase | ||||||
| 1 | F | 5 mo | Asthmatic bronchitis | 8 | |||||||||
| IgG | <10 | <10 | <10 | <10 | <200 | <200 | <75 | <75 | |||||
| IgM | Neg | Neg | <10 | <10 | <770 | <770 | <65 | <65 | |||||
| 2 | M | 56 yr | Pneumonia, AIDS | 5 | |||||||||
| IgG | 51 | 48 | 39 | 33 | 657 | 475 | 774 | 491 | |||||
| IgM | Neg | Neg | 32 | 30 | <770 | <770 | 205 | 150 | |||||
| 3 | F | 67 yr | Pneumonia, respiratory distress | 17 | |||||||||
| IgG | <10 | <10 | <10 | <10 | <200 | <200 | <75 | <75 | |||||
| IgM | Neg | Neg | <10 | 20 | <770 | <770 | <65 | 115e | |||||
| 4 | M | 21 yr | Pneumonia, renal transplant | 9 | |||||||||
| IgG | 28 | 21 | 17 | 19 | 1,129 | 1,343 | 898 | 801 | |||||
| IgM | Neg | Neg | <10 | <10 | 920e | 908e | <65 | <65 | |||||
| 5 | F | 47 yr | Fever | ||||||||||
| IgG | 17 | ND | <10 | ND | <200 | ND | <75 | ND | |||||
| IgM | Neg | ND | <10 | ND | 903e | ND | <65 | ND | |||||
| 6 | M | 71 yr | Pneumonia, wasting syndrome | ||||||||||
| IgG | <10 | ND | 13 | ND | 342 | ND | 91e | ND | |||||
| IgM | Neg | ND | <10 | ND | <770 | ND | <65 | ND | |||||
Negative IgG, <10 AU/ml; negative IgM, OD sample < 0.8 × OD cutoff-serum.
Negative IgG and negative IgM, <10 AU/ml.
IgG: negative, <200 AU/ml; equivocal, 200 ≤ X < 320; positive, ≥320; IgM: negative, <770 AU/ml; equivocal, 770 ≤ X < 950; positive, ≥950.
IgG: negative, <75 U/ml; equivocal, 75 < X < 125; positive, >125; IgM: negative, <65 U/ml; equivocal, 65 < titer < 135; positive, >65.
Equivocal result or low titer.
Control group seroprevalence.
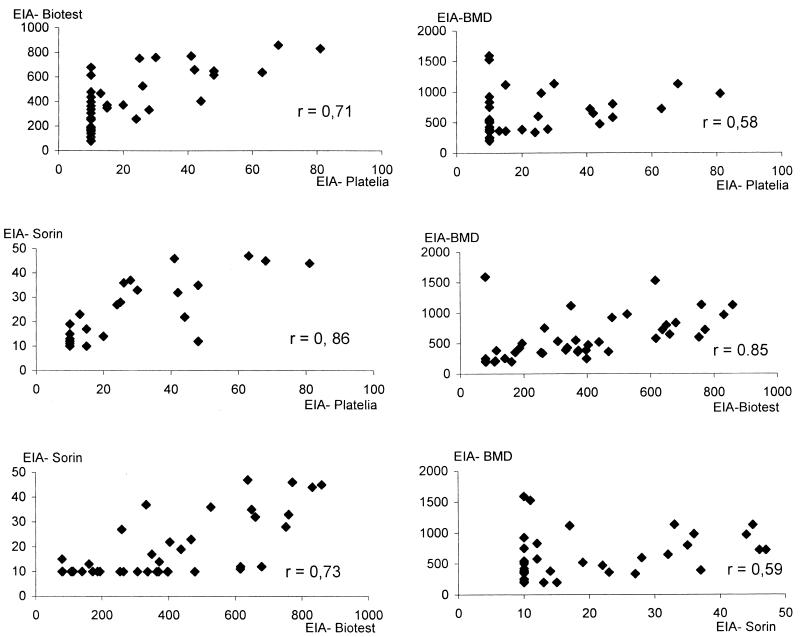
Out of the 61 healthy patients (group II), depending on the EIA used, 17 to 33 (28 to 54%) were IgG positive and 1 to 45 (1.6 to 74%) were IgM positive (Table 5). There was a good concordance in adult IgG seroprevalence (66 to 71%), though it was lower by EIA-Platelia (43%). In children, EIA-BMD and EIA-Biotest gave a higher seroprevalence than EIA-Sorin and EIA-Platelia (45% for both versus 17 and 20%). A comparative assessment of the amount of specific IgG antibodies in the 61 control serum specimens was undertaken with the four EIAs, using the Spearman correlation (Fig. 1). All six correlations were highly significant (P < 0.001). Most correlation coefficients were as high as 0.71 to 0.86, but weaker coefficients (0.58 and 0.59) were found between EIA-BMD titers and those of either EIA-Platelia or EIA-Sorin. Several sera contained high levels of IgG in the EIA-BMD but low levels of IgG when tested by EIA-Platelia or EIA-Sorin. This is of particular interest in the specific definition of the EIA cutoff of each EIA.
TABLE 5.
Anti-M. pneumoniae IgG and IgM antibodies in sera of 61 clinically healthy persons (group II)
| Patient group | No. of serum samples | Immunoglobulin | No (%) of serum samples positive by:
|
|||
|---|---|---|---|---|---|---|
| EIA-Platelia | EIA-Sorin | EIA-BMD | EIA-Biotest | |||
| Children | 40 | IgG | 8 (20) | 7 (17) | 18 (45) | 18 (45) |
| IgM | 0 (0) | 33 (82) | 3 (7.5) | 6 (15) | ||
| Adults | 21 | IgG | 9 (43) | 14 (66) | 14 (66) | 15 (71) |
| IgM | 1 (4.7) | 12 (57) | 1 (4.7) | 3 (14) | ||
| Total | 61 | IgG | 17 (28) | 21 (34) | 32 (52) | 33 (54) |
| IgM | 1 (1.6) | 45 (74) | 4 (6.5) | 9 (15) | ||
FIG. 1.
Spearman correlation between IgG titers obtained by using the four EIAs for M. pneumoniae IgG antibody in 61 serum samples from the patients in control group II. All correlations were significant at a P of <0.001.
Discrepant and unexpected results were observed in IgM detection. One (1.6%) to 45 (74%) subjects were IgM positive with an incredibly high IgM EIA-Sorin seroprevalence in adults and children (57 and 82%, respectively) (Table 5).
DISCUSSION
In M. pneumoniae infection, it is difficult to set up criteria for the “gold standard” to detect acute or remote infections. Cultural isolation is 100% specific but lacks sensitivity and is too slow to be of timely diagnostic value. There is no universally agreed upon gold standard serological assay for detection of antibodies to M. pneumoniae. CFT measures IgM and IgG antibodies together and has been shown to be both nonspecific and insensitive. Several EIAs have been developed in recent years; they include assays for the determination of both specific IgG and IgM or for IgG only and assays for IgM only (8, 14, 33, 34, 35). The μ-capture ELISA has previously been found to have a high sensitivity and specificity. It has been validated for early diagnosis of M. pneumoniae infection (13, 23, 37, 39). Although IgM EIAs are more sensitive and specific than CFT, the IgM response may be nonspecific (3, 19, 26, 29) or absent, particularly in the elderly with reinfection (2, 14, 22, 23, 30, 33, 37). It has also been shown that healthy people often have a relatively high background of specific M. pneumoniae IgG antibodies in their sera, probably because of past M. pneumoniae infections (2, 8, 14, 22, 25, 34, 35). Therefore, paired sera obtained with a time interval of 1 to 3 weeks are used in adults to confirm reinfection by M. pneumoniae, which is demonstrated by a significant rise in titer in IgG antibodies. More recently, a sensitive and specific PCR diagnostic method has proved its usefulness for accurate and early diagnosis (1, 4, 31, 32, 36), but its use as the reference method is complicated by the fact that a small number of healthy persons may carry the organism in the respiratory tract and it may be shed only weeks or months after resolution of an acute infection. Combined use of PCR and IgM serology tests should thus allow the maximal number of diagnoses at a very early phase of M. pneumoniae pneumonia in children (38), a practice that we encourage physicians to adopt in our laboratory.
The present data raise several important issues in the interpretation of M. pneumoniae EIAs.
The first important data were the results for sera from patients with other infections (group III), in which an excellent IgM specificity of the μ-capture EIA-Platelia (100%), a good specificity of EIA-BMD (90%), and a very bad IgM specificity of EIA-Biotest and EIA-Sorin (65 and 25%, respectively) were found. Because of this very low IgM specificity, the IgM EIA-Biotest and -Sorin should not be used as the single assay to diagnose Mycoplasma infection. The μ-capture EIA seems, thus, the most specific technique for M. pneumoniae IgM detection.
The second important data come from the 39 patients with positive M. pneumoniae PCR results (group I). There was a good agreement (85%) between the serological results obtained by the four EIAs and the direct PCR. The four IgM EIAs diagnosed a current M. pneumoniae infection in a large number of children (89 to 92%), with a sensitivity of 81 to 89% in the acute-phase serum specimens. In the present study, these young patients were hospitalized, which underlies the importance of IgM serology test to diagnose M. pneumoniae at a very early phase of infection. In adults, discrepant results were observed in the detection of IgM. Several sera had high levels of IgM by the indirect EIA and low levels of IgM when tested by μ-capture EIA, in agreement with a previous report (39). The stronger IgM reaction observed by EIA-Sorin and EIA-Biotest, in our study, is probably due to either nonspecific binding of macroglobulins or low levels of cross-reacting antibodies binding to nonprotein constituents of the EIA antigen preparation (3, 19, 26). Because it has been previously demonstrated that the main antigenic components reacting are proteins, better-defined specific protein antigen preparations should give more-accurate results and should be more specific than the use of a glycolipid or whole-cell antigen (8, 14, 27). However, other work proposed viability for a glycolipid antigen-based EIA with chloroform-methanol extraction (17). So, IgM specificity of EIA-BMD is better than EIA-Biotest and EIA-Sorin in which whole-cell lysates are used. Nevertheless, in processing the test, the potential interference of RF in indirect EIA has been eliminated by including an IgG absorbent. Concerning the IgG antibodies in group I (Table 2), they are best detected with EIA-BMD and EIA-Biotest (69 and 77%, respectively). EIA-Platelia and EIA-Sorin are less sensitive at identifying subjects infected with M. pneumoniae (64 and 59% sensitivity, respectively), which is confirmed by the results obtained with the acute-phase specimen of the 13 paired serum samples that show 15 and 31% sensitivity, respectively, versus 61% with both EIA-BMD and EIA-Biotest (Table 3).
Third, our study confirms that some control healthy subjects did have specific IgG (Table 5). The relatively high seroprevalence observed in EIA-BMD and EIA-Biotest is in agreement with the greatest IgG sensitivity found in infected patients. Because PCR was not performed, we cannot rule out an asymptomatic M. pneumoniae infection. However, since 1 of the 61 controls positive for IgG had IgM antibody detectable by EIA-Platelia and 4 of the 61 controls positive for IgG had IgM antibody detectable by EIA-BMD, it is more likely that the difference in sensitivity is to be explained by the different antigens used in coating plates. It is well-known that the serum immunoglobulins produced during an M. pneumoniae infection are very heterogeneous and that their kinetics is related to the type of antigen (14). Furthermore, given the high frequency with which M. pneumoniae infects the general population and the great sensitivity of IgG EIAs, it is not surprising to find a high endemic IgG seroprevalence; it has been shown previously that healthy persons often have elevated levels of specific M. pneumoniae IgG antibodies in their sera, probably because of past M. pneumoniae infections (2, 8, 14, 22). These data support the idea that the degree of accuracy of IgG detection is dependent on the definition of a particular cutoff value for discriminating between groups of infected patients and healthy subjects, and this cutoff should be high enough to avoid false-positive results but not too high for a good sensitivity. As for the BMD-EIA and Biotest-EIA, this interpretive dilemma may be dealt with by the designation of an equivocal or low-positive range. More surprising is the high IgM seroprevalence observed with EIA-Sorin and EIA-Biotest, supporting the argument that the specificity of IgM detection is essential.
Finally, discordant serological and PCR results were observed. A negative serological test with a positive PCR result was found for samples from six patients of group I. Probably because of its high sensitivity, it is possible that PCR is able to detect M. pneumoniae in circumstances other than acute infection; different studies have shown that M. pneumoniae can be detected in healthy persons. Persistence of mycoplasmas after a recent infection or possible carriage of M. pneumoniae in healthy people has been suggested by several authors to explain the absence of immune response (11, 12, 18, 20, 31, 32). This seems, however, very unlikely in the present cases because sera were obtained from patients with clinical features: one patient with pneumonia had clinical and radiographic improvement under macrolide treatment, two patients were immunocompromised (AIDS, renal transplant), a 71-year-old patient presented with pneumonia, and a neonate of 5 months had an asthmatic bronchitis. Our findings seem due rather to an impaired immune response (patients 1, 2, 3, and 4) and/or to a lack of antibody responses in samples taken too early in the disease (patients 1, 2, 5, and 6) (Table 4). Numerous M. pneumoniae PCR-positive samples have already been reported for patients less than 12 months old with negative serological responses, in immunocompromised patients, or in patients with serum samples taken too early in the disease (7, 31, 38). Thanks to strict guidelines for the general handling of the PCR procedure and high hybridization index of each respiratory sample, we can exclude the possibility of false-positive PCR results related to contamination by carryover of PCR products.
In conclusion, a positive specific IgM result allows the diagnosis of a recent M. pneumoniae infection with high reliability in children on a single serum sample, but only if a specific test is used. Because of their lack of specificity, the EIA-Sorin and EIA-Biotest are inaccurate for the routine detection of M. pneumoniae IgM. However, more washing in the EIA-Sorin procedure assay improves specificity (data not shown). Our results show that the μ-capture EIA-Platelia is the most specific technique for detecting M. pneumoniae-specific IgM and that the EIA-BMD has an acceptable specificity with a greater sensitivity in IgG detection. Both tests are suitable for routine diagnosis, and the choice depends on what processing and supplying facilities are available. In adults, when a single serum sample is used, and in the absence of IgM antibodies, the presence of specific IgG antibodies does not discriminate between a current and previous infection. A correct interpretation of EIAs requires paired sera with a rise in antibody titer between both tests, which consequently delays the results. Therefore, PCR should be combined with IgG and IgM antibody detection to allow fast and reliable diagnosis of M. pneumoniae infection. In the case of a negative PCR or negative IgM result with the acute-phase serum sample, IgG and IgM detection using paired sera is necessary to either confirm or reject the diagnosis of M. pneumoniae infection.
REFERENCES
- 1.Abele-Horn, M., U. Busch, H. Nitschko, E. Jacobs, R. Bax, F. Pfaff, B. Schaffer, and J. Heeseman. 1998. Molecular approaches to diagnosis of pulmonary diseases due to Mycoplasma pneumoniae. J. Clin. Microbiol. 36:548–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biberfeld, G. 1971. Antibody responses in Mycoplasma pneumoniae infection in relation to serum immunoglobulins, especially IgM. Acta Pathol. Microbiol. Scand. B 79:620–634. [PubMed] [Google Scholar]
- 3.Biberfeld, G. 1971. Antibodies to brain and other tissues in cases of Mycoplasma pneumoniae infection. Clin. Exp. Immunol. 8:319–333. [PMC free article] [PubMed] [Google Scholar]
- 4.Blackmore, T. K., M. Reznikov, and D. L. Gordon. 1995. Clinical utility of the polymerase chain reaction to diagnose Mycoplasma pneumoniae infection. Pathology 27:177–181. [DOI] [PubMed] [Google Scholar]
- 5.Clyde, W. A., Jr. 1993. Clinical overview of typical Mycoplasma pneumoniae infections. Clin. Infect. Dis. 17(Suppl. 1):S32–S36. [PubMed] [Google Scholar]
- 6.De Barbeyrac, B., C. Bernet-Poggi, F. Febrer, H. Renaudin, M. Dupon, and C. Bebear. 1993. Detection of Mycoplasma pneumoniae and Mycoplasma genitalium in clinical samples by polymerase chain reaction. Clin. Infect. Dis. 17(Suppl. 1):S83–S89. [DOI] [PubMed] [Google Scholar]
- 7.Dorigo-Zetsma, J. W., S. A. J. Zaat, P. M. E. Wertheim-van Dillen, L. Spanjaard, J. Rijntjes, G. van Waveren, J. S. Jensen, A. F. Angulo, and J. Dankert. 1999. Comparison of PCR, culture, and serological tests for diagnosis of Mycoplasma pneumoniae respiratory tract infection in children. J. Clin. Microbiol. 37:14–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dussaix, E., A. Slim, and P. Tournier. 1983. Comparison of enzyme-linked immunosorbent assay (ELISA) and complement fixation test for detection of Mycoplasma pneumoniae antibodies. J. Clin. Pathol. 36:228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez, C. V., R. Bortolussi, K. Gordon, S. H. Lee, J. G. Gatien, and M. S. Shahdrabadi. 1993. Mycoplasma pneumoniae infection associated with central nervous system complications. J. Child Neurol. 8:27–31. [DOI] [PubMed] [Google Scholar]
- 10.Foy, H. M., G. E. Kenny, M. K. Cooney, and I. D. Allan. 1979. Long term epidemiology of infections with Mycoplasma pneumoniae. J. Infect. Dis. 139:681–687. [DOI] [PubMed] [Google Scholar]
- 11.Foy, H. M. 1993. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin. Infect. Dis. 17(Suppl. 1):S37–S46. [DOI] [PubMed] [Google Scholar]
- 12.Gnarpe, J., A. Lundbäck, B. Sundelöf, and H. Gnarpe. 1992. Prevalence of Mycoplasma pneumoniae in subjectively healthy individuals. Scand. J. Infect. Dis. 24:161–164. [DOI] [PubMed] [Google Scholar]
- 13.Hirschberg, L., A. Krook, C. A. Pettersson, and T. Vikerfors. 1988. Diagnosis of Mycoplasma pneumoniae infection by the detection of specific IgM with a μ-capture ELISA. Eur. J. Clin. Microbiol. 7:420–423. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs, E., A. Bennewitz, and W. Bredt. 1986. Reaction pattern of human anti-Mycoplasma pneumoniae antibodies in enzyme-linked immunosorbent assays and immunoblotting. J. Clin. Microbiol. 23:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, E. 1997. Mycoplasma infections of the human respiratory tract. Wien. Klin. Wochenschr. 109:574–577. [PubMed] [Google Scholar]
- 16.Jacobs, E. 1993. Serological diagnosis of Mycoplasma pneumoniae infections: a critical review of current procedures. Clin. Infect. Dis. 17(Suppl. 1):S79–S82. [DOI] [PubMed] [Google Scholar]
- 17.Jensen, J. S., J. Sondergard-Andersen, N. Stranberg Pedersen, and K. Lind. 1990. Enzyme linked immunosorbent assay for the detection of antibodies to a lipid extract of Mycoplasma pneumoniae. Zentbl. Bakteriol. 20(Suppl.):784–788. [Google Scholar]
- 18.Kleemola, S. R. M., J. E. Karjalainen, and R. K. H. Raty. 1990. Rapid diagnosis of Mycoplasma pneumoniae infection: clinical evaluation of a commercial probe test. J. Infect. Dis. 162:70–75. [DOI] [PubMed] [Google Scholar]
- 19.Leinikki, P. O., P. Pekka, and H. Tykka. 1978. Immunoglobulin M antibody response against Mycoplasma pneumoniae lipid antigen in patients with acute pancreatitis. J. Clin. Microbiol. 8:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leng, Z., G. E. Kenny, and M. C. Roberts. 1994. Evaluation of the detection limits of PCR for identification of Mycoplasma pneumoniae in clinical samples. Mol. Cell. Probes 8:125–130. [DOI] [PubMed] [Google Scholar]
- 21.Levy, M., and N. H. Shear. 1991. Mycoplasma pneumoniae infections and Stevens-Johnson syndrome. Report of eight cases and review of the literature. Clin. Pediatr. 30:42–49. [DOI] [PubMed] [Google Scholar]
- 22.Mitzutani, H., and H. Mitzutani. 1983. Immunologic responses in patients with Mycoplasma pneumoniae infections. Am. Rev. Respir. Dis. 127:175–179. [DOI] [PubMed] [Google Scholar]
- 23.Moule, J. H., E. O. Caul, and T. G. Wreghitt. 1987. The specific IgM response to Mycoplasma pneumoniae infection: interpretation and application to early diagnosis. Epidemiol. Infect. 99:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, H. W., H. Masur, L. B. Senterfit, and R. B. Roberts. 1975. The protean manifestations of Mycoplasma pneumoniae infections in adults. Am. J. Med. 58:229–242. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura, S., I. Ebisawa, O. Kitamoto, and T. Sato. 1970. Persistence of serum antibody following Mycoplasma pneumoniae infection. Am. Rev. Respir. Dis. 101:620–622. [DOI] [PubMed] [Google Scholar]
- 26.Pönkä, A., T. Pönkä, S. Sarna, and K. Penttinen. 1981. Questionable specificity of lipid antigen in the Mycoplasma pneumoniae complement fixation test in patients with extrapulmonary manifestations. J. Infect. 3:332–338. [DOI] [PubMed] [Google Scholar]
- 27.Raisanen, S. M., J. I. Suni, and P. Leinikki. 1980. Serological diagnosis of Mycoplasma pneumoniae infection by enzyme immunoassay. J. Clin. Pathol. 33:836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saiki, R. K., D. H. Gelfland, S. Stoffel, S. J. Scharf, R. Higuchi, G. T. Horn, K. B. Mullis, and H. A. Erlich. 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491. [DOI] [PubMed] [Google Scholar]
- 29.Sillis, M. 1993. Modern methods for diagnosis of Mycoplasma pneumoniae pneumonia. Rev. Med. Microbiol. 4:24–31. [Google Scholar]
- 30.Sillis, M. 1990. The limitations of IgM assays in the serological diagnosis of Mycoplasma pneumoniae infections. J. Med. Microbiol. 33:253–258. [DOI] [PubMed] [Google Scholar]
- 31.Skakni, L., A. Sardet, J. Just, J. Landman-Parker, J. Costil, N. Moniot-Vill, F. Bricout, and A. Garbarg-Chenon. 1992. Detection of Mycoplasma pneumoniae in clinical samples from pediatric patients by polymerase chain reaction. J. Clin. Microbiol. 30:2638–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tjhie, J. H. T., F. J. M. van Kuppeveld, R. Roosendall, W. J. G. Melchers, R. Gordijn, D. M. Mac Laren, J. M. M. Walboomers, C. J. L. M. Meijer, and A. J. C. van den Brule. 1994. Direct PCR enables detection of Mycoplasma pneumoniae in patients with respiratory tract infections. J. Clin. Microbiol. 32:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uldum, S. A., J. S. Jensen, J. Sondergard-Andersen, and K. Lind. 1992. Enzyme immunoassay for detection of immunoglobulin M (IgM) and IgG antibodies to Mycoplasma pneumoniae. J. Clin. Microbiol. 30:1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uldum, S. A., J. Sondergard-Andersen, J. S. Jensen, and K. Lind. 1990. Evaluation of a commercial enzyme immunoassay for detection of Mycoplasma pneumoniae specific immunoglobulin G antibodies. Eur. J. Clin. Microbiol. Infect. Dis. 9:221–223. [DOI] [PubMed] [Google Scholar]
- 35.Van Griethuysen, A. J. A., R. de Graaf, J. A. M. van Druten, F. W. A. Heessen, J. T. M. van der Logt, and A. M. van Loon. 1984. Use of the enzyme linked immunosorbent assay for the early diagnosis of Mycoplasma pneumoniae infection. Eur. J. Clin. Microbiol. 3:116–121. [DOI] [PubMed] [Google Scholar]
- 36.Van Kuppeveld, F. J., K. E. Johansson, J. M. Galama, J. Kissing, G. Bolske, E. Hjelm, J. T. van der Logt, and W. J. Melchers. 1994. 16sRNA based polymerase chain reaction compared with culture and serological methods for diagnosis of Mycoplasma pneumoniae infection. Eur. J. Clin. Microbiol. Infect. Dis. 13:401–405. [DOI] [PubMed] [Google Scholar]
- 37.Vikerfors, T., G. Brodin, M. Grandien, L. Hirschberg, A. Krook, and C.-A. Pettersson. 1988. Detection of specific IgM antibodies for the diagnosis of Mycoplasma pneumoniae infections: a clinical evaluation. Scand. J. Infect. Dis. 20:601–610. [DOI] [PubMed] [Google Scholar]
- 38.Waris, M. E., P. Toikka, T. Saarinen, S. Nikkari, O. Meurman, R. Vainionpää, J. Mertsola, and O. Ruuskanen. 1998. Diagnosis of Mycoplasma pneumoniae pneumonia in children. J. Clin. Microbiol. 36:3155–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wreghitt, T. G., and M. Sillis. 1985. A micro-capture ELISA for detecting Mycoplasma pneumoniae IgM: comparison with indirect immunofluorescence and indirect ELISA. J. Hyg. 94:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]