Abstract
Infection of poultry with Salmonella enterica serovar Typhimurium poses a significant risk to public health through contamination of meat from infected animals. Vaccination has been proposed to control infections in chickens. However, the vaccines are currently largely empirical, and our understanding of the mechanisms that underpin immune clearance and protection in avian salmonellosis is not complete. In this study we describe the cytokine, chemokine, and antibody responses and cellular changes in primary and secondary infections of chickens with Salmonella serovar Typhimurium. Infection of 1-week-old chickens induced early expression of a macrophage inflammatory protein (MIP) family chemokine in the spleen and liver, followed by increased expression of gamma interferon accompanied by increased numbers of both CD4+ and CD8+ T cells and the formation of granuloma-like follicular lesions. This response correlated with a Th1-mediated clearance of the systemic infection. Primary infection also induced specific immunoglobulin M (IgM), IgG, and IgA antibody responses. In contrast to previously published studies performed with newly hatched chicks, the expression levels of proinflammatory cytokines in the gastrointestinal tract were not greatly increased following infection. However, significant expression of the anti-inflammatory cytokine transforming growth factor β4 was detected in the gut early in infection. Following secondary challenge, the birds were fully protected against systemic infection and showed a high level of protection against gastrointestinal colonization. Rapid expression of the MIP family chemokine and interleukin-6 was detected in the guts of these birds and was accompanied by an influx of lymphocytes. Increased levels of serum IgA-specific antibodies were also found following rechallenge. These findings suggest that cellular responses, particularly Th1 responses, play a crucial role in immune clearance in avian salmonellosis and that protection against rechallenge involves the rapid recruitment of cells to the gastrointestinal tract. Additionally, the high levels of inflammatory response found following Salmonella serovar Typhimurium infection of newly hatched chicks were not observed following infection of older birds (1 week old), in which the expression of regulatory cytokines appeared to limit inflammation.
Salmonella enterica remains a major cause of food-borne gastroenteritis throughout the world. Around 30,000 cases of human salmonellosis are reported per annum in the United Kingdom alone (32). The consumption of infected poultry meat and eggs is a major source of human cases, particularly infections caused by S. enterica serovars Typhimurium and Enteritidis (18). Therefore, the presence and control of Salmonella infections in chicken flocks remain important public health issues. Although Salmonella serovars Typhimurium and Enteritidis are both capable of causing severe systemic disease in newly hatched chicks, control in birds that are more than 3 or 4 days old is complicated by the fact that infection by these serovars leads to colonization of the gastrointestinal tract and shedding of Salmonella in feces for several weeks with no clinical disease (5, 19). A number of approaches have been used to control salmonellosis in flocks, including improved hygiene standards, improved animal husbandry, the use of prophylactic antibiotics, and vaccination (37), although the efficacy of such approaches has proved to be variable. The use of vaccination is perhaps the most straightforward of these strategies and largely avoids risks to public health and the difficulties associated with maintaining strict hygiene procedures on farms. Vaccination has proved to be successful in reducing levels of Salmonella serovar Enteritidis in flocks of egg-laying hens in the United Kingdom following its widespread introduction in 1998, and a decrease in human Salmonella serovar Enteritidis cases has been attributed to this approach (1). Thus, vaccination potentially is an effective approach for controlling salmonellosis in both egg and poultry meat (broiler) production.
Vaccination of chickens with live attenuated or killed vaccines has resulted in various degrees of protection in a range of experimental systems (4, 11-14, 16, 36). A number of killed and live attenuated vaccines produced from undefined mutants have been licensed in Europe for use in poultry to protect against both Salmonella serovar Enteritidis infection and Salmonella serovar Typhimurium infection. Despite the use of these vaccines, the immunology of protection in the chicken is not fully understood, and it is clear that infection with virulent Salmonella provides a significantly higher level of protection to rechallenge than the level of protection generated by infection with attenuated strains (4). Therefore, a better understanding of the immunological mechanisms that give rise to protection is required to allow a more rational approach to vaccination in the chicken. In many previous studies workers have investigated the serological responses and cellular changes associated with infection or vaccination, but in more recent studies workers have begun to investigate T-cell function and the expression of cytokines, along with serological changes associated with both primary and secondary infection by Salmonella serovar Typhimurium (6, 7). Infection with Salmonella leads to increased levels of immunoglobulin G (IgG; also called IgY), IgM, and IgA antibodies (2, 6, 7, 9, 15, 16) and to changes in the distribution of B and T lymphocytes (8). Clearance of Salmonella serovar Typhimurium from the spleen correlates with an increase in T-cell proliferation and expression of gamma interferon (IFN-γ) at this site, suggesting that a Th1-dominated T-cell response may clear the systemic stages of primary infection (6, 7). Clearance from the gastrointestinal tract occurred considerably later than clearance from the spleen and liver. While antigen-specific T-cell proliferation of splenic cells remained high following primary infection, a significant drop in the response was found at the time of gastrointestinal tract clearance, suggesting that trafficking of lymphocytes to the gut may play a key role in clearance of Salmonella (6, 7). Following rechallenge, previously infected birds demonstrated a high level of protection against systemic or gastrointestinal tract infection compared to age-matched birds that received a primary infection (6, 7). However, following secondary infection, there was little change in the systemic antibody levels or IFN-γ expression, and there was a decrease in T-cell proliferation, which again was consistent with trafficking of lymphocytes to intestinal sites.
In this study we investigated changes in cytokine and chemokine expression following primary and secondary infection with Salmonella serovar Typhimurium of the chicken, along with cellular and serological changes associated with the infection. The aims of these experiments were to characterize the immune response during a primary infection and to determine the changes associated with a protective response, both systemically and in the gastrointestinal tract, in previously infected chickens.
MATERIALS AND METHODS
Experimental animals.
One-week-old specific-pathogen-free (SPF) Rhode Island Red chickens were obtained from the Poultry Production Unit, Institute for Animal Health. The birds were reared in wire cages, initially at 30°C and then at 20°C from the time that they were 3 weeks old, and they were given ad libitum access to water and a vegetable protein-based diet (Special Diet Services, Witham, United Kingdom). SPF flocks are monitored on a regular basis for antibody to chicken anemia virus, and no virus has ever been detected in the Institute for Animal Health SPF flocks.
Bacterial strains.
Spontaneous nalidixic acid-resistant and spectinomycin-resistant mutants of Salmonella serovar Typhimurium phage type 14 strain F98 were used in the experimental infections. Salmonella serovar Typhimurium strain F98 is an invasive strain that is virulent in young chicks that are less than 3 days old and is capable of persistent colonization of the gastrointestinal tract of older birds (5, 29). Bacteria were maintained as glycerol stocks at −70°C and were grown in Luria-Bertani (LB) broth (Difco, West Molesey, United Kingdom) at 37°C in an orbital shaking incubator at 150 rpm.
Primary infection with Salmonella serovar Typhimurium.
One-week-old chickens were infected orally with 108 CFU of Salmonella serovar Typhimurium strain F98 in 0.1 ml of LB broth. The control group was mock infected with 0.1 ml of LB broth alone. Five birds from each group were killed by cervical dislocation at 1, 3, 7, 14, 21, and 28 days postinfection (dpi) for postmortem analysis. At each time, cardiac blood was collected for serology. Tissue samples of the liver, spleen, jejunum, ileum, and cecal tonsils were collected aseptically in liquid nitrogen for extraction of total RNA and for immunohistochemistry. Liver and cecal contents were used for enumeration of viable bacteria. Bacterial culturing and enumeration were performed as previously described (5, 29). Briefly, homogenized samples were plated onto selective brilliant green agar (Difco) containing 20 μg of sodium nalidixic acid/ml and 1 μg of novobiocin/ml. Plates were incubated at 37°C for 24 h before enumeration of the colonies. For histological examination, tissue samples were fixed in 4% buffered formalin, embedded in paraffin wax, and then cut into 4-μm sections. The sections were stained with hematoxylin and eosin as described previously (35).
Secondary (rechallenge) infection with Salmonella serovar Typhimurium.
Seventy-five 1-week-old chickens were divided into three groups. The first group (group 1) served as the control group and was mock infected with LB broth at both the primary and secondary infection times. For the primary infection birds in group 2 were initially inoculated with LB broth, whereas group 3 birds were orally infected with 108 CFU of a nalidixic acid-resistant mutant of Salmonella serovar Typhimurium strain F98 in 0.1 ml of LB broth. Infected chickens were monitored for the excretion of Salmonella by cloacal swabbing after the primary infection (3). Following clearance of Salmonella as determined by cloacal swabbing, at 9 weeks after the primary infection the birds in both group 2 and group 3 were orally infected with 108 CFU of the spectinomycin-resistant mutant of Salmonella serovar Typhimurium strain F98. Five birds from each group were killed at 1,3, 7, 14, and 21 dpi, and the tissues were collected as described above.
Immunohistochemistry.
Liver, spleen, jejunum, ileum, and cecal tonsil specimens were collected, snap frozen in liquid nitrogen, and stored at −80°C until use. Frozen sections were embedded in OCT medium, and serial 7-μm sections were cut using a cryostatic microtome (SME Cryotome; Shandon Scientific, Runcorn, United Kingdom). After air drying, the embedding medium was removed by dipping slides in 0.1 M phosphate-buffered saline (PBS) for 15 min at room temperature. The sections were incubated with 1% skim milk for 30 min at room temperature to minimize nonspecific binding, before incubation with specific mouse monoclonal antibodies against chicken CD3, CD4, CD8, IgA, IgG (IgY), and IgM (Southern Biotechnology Associates, Birmingham, AL). The antibody dilutions recommended by the manufacturer were used. A Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) was used for detection of T and B lymphocytes, and the specific color reaction was developed with 3,3′-diaminobenzidine in Tris buffer used according to the manufacturer's recommendations. All incubations were at room temperature for 1 h, and the sections were thoroughly washed between incubations with 0.1 M PBS. For negative controls, 3% bovine serum albumin was used instead of primary antibody. T and B cells were observed and photographed by light microscopy (model HFX-DX; Nikon, Tokyo, Japan). Quantification of cellular changes in organs was performed by counting cells in photographs taken at a magnification of ×200 as previously described (34). Five photographs per organ for each bird, taken randomly from serial sections, and four birds per group for each time were used to determine changes. Student's t test was used to compare groups.
Quantitative real-time RT-PCR for cytokine expression.
Total RNA was prepared from snap-frozen samples using an RNeasy mini kit (QIAGEN, Crawley, United Kingdom) following the manufacturer's instructions. Purified RNA was eluted in 50 μl RNase-free water and stored at −70°C until use. Expression of the cytokines interleukin-1β (IL-1β), IL-4, IL-6, IL-10, IFN-γ, and transforming growth factor β4 (TGF-β4), the chemokines K60, IL-8, and lymphotactin, and a CC macrophage inflammatory protein (MIP) family chemokine was determined by quantitative reverse transcription (RT)-PCR using the ABI PRISM 7700 sequence detection system (PE Applied Biosystems, Warrington, United Kingdom) as previously described (20, 27, 35). The MIP family chemokine has previously been described as the chicken orthologue of mammalian MIP-1β, including by us (36). However, our recent analysis of the cytokine and chemokine complement of the chicken genome sequence (P. Kaiser, unpublished data) suggests that this annotation may be incorrect. There is no doubt that this chemokine is a MIP family chemokine, but in the absence of any functional data or determination of ligand-receptor relationships for the chicken MIP family chemokine members, its previous annotation as chicken MIP-1β is at best optimistic and most likely incorrect.
Preparation of soluble Salmonella lysate antigen.
Overnight cultures of Salmonella serovar Typhimurium strain F98 (as described above) were used to inoculate 250-ml conical flasks containing 100 ml LB broth and were placed in an orbital incubator (150 rpm at 37°C) overnight. Bacterial cells were pelleted by centrifugation at 4,080 × g for 20 min and then washed twice and resuspended in 20 ml PBS. The bacterial suspension was freeze-thawed three times in liquid nitrogen and then sonicated (nine 20-s bursts on ice) in 10-ml portions at an amplitude of 15 μm using a Soniprep 150 sonicator (MSE Scientific Instruments, Crawley, United Kingdom). The suspension was clarified by centrifugation at 4,080 × g for 20 min, followed by ultracentrifugation of the supernatant at 30,000 × g for 20 min to remove the insoluble fraction. The concentration of protein in the soluble antigen preparation was determined using a Bradford protein determination kit (Merck, Poole, United Kingdom), and aliquots were frozen at −20°C until they were needed.
Enzyme-linked immunosorbent assay (ELISA).
Flat-bottom 96-well enzyme-linked immunosorbent assay (ELISA) plates (BD Biosciences, Oxford, United Kingdom) were coated with the soluble antigen preparation diluted to a concentration of 16.2 μg/ml in carbonate-bicarbonate buffer (pH 9.6) overnight at 4°C and then washed with PBS containing Tween 20 (0.05%) (PBS-T). Nonspecific binding sites were blocked using PBS-T containing 3% skim milk powder (Oxoid, Basingstoke, United Kingdom) (blocking buffer) for 1 h at 37°C. Serum samples were diluted 1:400 in blocking buffer (when testing for IgM and IgG) or were diluted 1:25 (when testing for IgA) and added to the plate, which was then incubated at 37°C for 1 h. Plates were washed in PBS-T, and bound immunoglobulins were detected by incubation at 37°C for 1 h with horseradish peroxidase conjugated to either goat anti-chicken IgM (1:1,000; Serotec, Oxford, United Kingdom), rabbit anti-chicken IgG (1:2,000; Sigma), or goat anti-chicken IgA (1:20,000; Serotec) diluted in blocking buffer. After the conjugation plates were washed with PBS-T, 50 μl per well of a 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS)-hydrogen peroxide solution (Sigma, Poole, United Kingdom) was added to each of the plates, which were incubated at room temperature in the dark for 30 to 60 min. The reaction was stopped by adding 1% sodium dodecyl sulfate, and the absorbance at 405 nm was determined with a Benchmark microplate reader (Bio-Rad, Hemel Hempstead, United Kingdom).
RESULTS
Primary infection with Salmonella serovar Typhimurium.
Salmonella was found in the livers, spleens, and cecal contents of all infected birds by 3 to 7 dpi (Table 1). The systemic counts of Salmonella peaked at 14 dpi, following which they declined, Salmonella was detected only following enrichment with selenite broth in the majority of liver and spleen samples by 28 dpi. The levels of Salmonella in the cecal contents peaked at 7 dpi and then gradually declined, although four of five birds still had detectable Salmonella in their cecal contents at 28 dpi. No Salmonella was detected in the control group throughout the experiment.
TABLE 1.
Tissue distribution of Salmonella bacteria following oral infection of 1-week-old Rhode Island Red chickens with 108 CFU of Salmonella serovar Typhimurium strain F98
| Days postinfection | Results for the following tissues:
|
|||||
|---|---|---|---|---|---|---|
| Liver
|
Spleen
|
Cecal contents
|
||||
| Mean log10 CFU/g (SEM) | No. positive/total no. | Mean log10 CFU/g (SEM) | No. positive/total no. | Mean log10 CFU/g (SEM) | No. positive/total no. | |
| 1 | <1 | 0/5 | <1 | 0/5 | 2.26 (0.31) | 5/5 |
| 3 | <1 | 0/5 | <1 | 0/5 | 3.28 (0.51) | 5/5 |
| 7 | 1.30 (0.31) | 4/5 | 2.38 (0.39) | 4/5 | 4.33 (0.33) | 5/5 |
| 14 | 2.10 (0.47) | 5/5 | 3.55 (0.18) | 5/5 | 2.49 (0.68) | 5/5 |
| 21 | 1.23 (0.40) | 4/5 | 2.86 (0.44) | 5/5 | 1.35 (0.71) | 4/5 |
| 28 | <1 | 2/5a | 1.21 (0.35) | 4/5 | 1.17 (0.37) | 4/5 |
Positive following enrichment with only selenite broth.
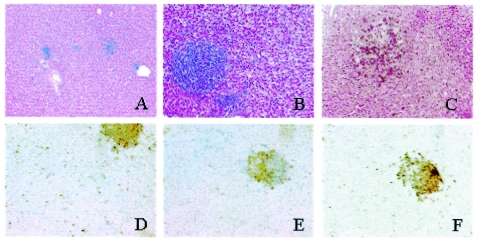
To determine any pathological changes, livers, spleens, jejuna, ilea, and cecal tonsils were examined macroscopically. Signs of mild inflammation, such as hyperaemia in the walls of jejuna and ilea, slight diarrhea, and mild hepatosplenomegaly, were seen in infected birds. On microscopic examination very small infiltrates consisting mainly of mononuclear cells were found in the liver 3 dpi (Fig. 1A). More organized follicular lesions were seen in the livers from 7 dpi onward (Fig. 1B) along with areas of necrosis and some loss of normal hepatic structure (Fig. 1C). Lesions were not distinguishable in the spleen, but because of the dense structure of this organ Salmonella-associated lesions are difficult to visualize even in animals with severe systemic salmonellosis (33). Examination of intestinal tissue revealed slight infiltration of heterophils, the functional equivalent of mammalian neutrophils (21, 25). The changes in the numbers of different cell subsets in the intestinal samples could not be quantified, but immunohistochemical examination revealed that both CD4+ and CD8+ cells were involved in the formation of the follicular lesions in the livers of the Salmonella-infected chickens (Fig. 1D to F). Quantification of the specifically stained cell subtypes showed that there was a significant (P < 0.05) increase in the number of T cells, but not B cells, 7 and 14 days after primary Salmonella infection in the livers (Fig. 2). The numbers of the T cells appeared to be lower in the spleens from infected birds at 14 dpi. The numbers of T cells seemed to be increased in both the ilea and cecal tonsils, but as discussed above, these cells could not be easily quantified.
FIG. 1.
Development of follicular lesions in the livers of Salmonella serovar Typhimurium-infected chickens. (A to C) Hematoxylin and eosin-stained sections at a magnification of ×200 show the development of lesions for 3 dpi (A), 7 dpi (B), and 14 dpi (C). (D to F) Sections taken at 14 dpi for immunohistological examination show the involvement of T lymphocytes in lesion formation. Staining for CD3+ cells (D) revealed large numbers of T lymphocytes within a lesion. Staining of serial sections indicated that both CD4+ (E) and CD8+ T lymphocytes are involved in the formation of lesions.
FIG. 2.
Numbers and distribution of B and T lymphocytes in the livers of chickens following Salmonella serovar Typhimurium infection determined by immunohistochemistry. Sections were stained with monoclonal antibodies to chicken immunoglobulin (B cells) or CD3 (T cells). (A) Uninfected liver stained for B cells. (B) Uninfected liver stained for T cells. (C) Liver from a bird at 7 dpi, showing little change in the number of B lymphocytes. (D) Increased number of scattered CD3+ T cells in the liver of an infected bird at 7 dpi. (E) Little change in the number of B lymphocytes in the liver at 14 dpi. (F) Increased numbers of scattered T cells remaining in the liver at 14 dpi.
Secondary (rechallenge) infection with Salmonella serovar Typhimurium.
Following primary infection with Salmonella serovar Typhimurium, fecal excretion was detected up to 8 weeks postinfection by cloacal swabbing (Fig. 3). Following clearance groups 2 and 3 were infected with the spectinomycin-resistant mutant of Salmonella serovar Typhimurium strain F98. As expected, birds that had previously received Salmonella serovar Typhimurium (group 3) showed a high level of protection to both systemic and gastrointestinal infection compared to previously uninfected birds (group 2) (Table 2). No Salmonella was detected in the spleens or livers of previously infected birds, while there was a reduction in the number of Salmonella cells in the cecal contents and clearance of Salmonella from the gastrointestinal tract within 14 days. In contrast, the birds that received a primary infection treatment (group 2) had Salmonella in their spleens and livers and in their cecal contents until the end of the experiment. No Salmonella was detected in the control group.
FIG. 3.
Percentage of Rhode Island Red chickens shedding Salmonella serovar Typhimurium following oral infection of 1-week-old birds. The numbers of birds shedding were determined by cloacal swabbing followed by direct plating onto brilliant green agar and plating following enrichment in selenite broth for 24 h.
TABLE 2.
Tissue distribution of Salmonella bacteria following primary or secondary rechallenge oral infection of 9-week-old Rhode Island Red chickens with 108 CFU of Salmonella serovar Typhimurium strain F98
| Days postinfection | Tissue | Group 1 (control)
|
Group 2 (primary infection)
|
Group 3 (rechallenge)
|
|||
|---|---|---|---|---|---|---|---|
| Mean log10 CFU/g | No. positive/total no. | Mean log10 CFU/g (SEM) | No. positive/total no. | Mean log10 CFU/g (SEM) | No. positive/total no. | ||
| Spleen | <1 | 0/5 | <1 | 0/5 | <1 | 0/5 | |
| 1 | Liver | <1 | 0/5 | <1 | 0/5 | <1 | 0/5 |
| Cecal contents | <1 | 0/5 | 2.95 (1.12) | 4/5 | 2.92 (0.21) | 5/5 | |
| Spleen | <1 | 0/5 | 1.23 (0.43) | 4/5 | <1 | 0/5 | |
| 3 | Liver | <1 | 0/5 | 2.02 (0.21) | 5/5 | <1 | 0/5 |
| Cecal contents | <1 | 0/5 | 4.95 (0.61) | 5/5 | 1.13 (0.91) | 3/5 | |
| Spleen | <1 | 0/5 | 2.73 (0.19) | 5/5 | <1 | 0/5 | |
| 7 | Liver | <1 | 0/5 | 2.54 (0.35) | 5/5 | <1 | 0/5 |
| Cecal contents | <1 | 0/5 | 4.08 (0.55) | 5/5 | 1.01 (0.89) | 2/5 | |
| Spleen | <1 | 0/5 | 1.36 (0.77) | 5/5 | <1 | 0/5 | |
| 14 | Liver | <1 | 0/5 | 2.17 (0.30) | 5/5 | <1 | 0/5 |
| Cecal contents | <1 | 0/5 | 3.69 (0.30) | 5/5 | <1 | 0/5 | |
| Spleen | <1 | 0/5 | 2.22 (1.15) | 4/5 | <1 | 0/5 | |
| 21 | Liver | <1 | 0/5 | <1 | 1/5a | <1 | 0/5 |
| Cecal contents | <1 | 0/5 | 1.11 (0.94) | 3/5 | <1 | 0/5 | |
Positive following enrichment in only selenite broth.
Little or no pathology was found in either the primary or secondary infected birds. Microscopic examination revealed cellular changes in the primary infected birds (group 2) that were similar to, although less pronounced than, those found after primary infection of younger birds in experiment 1. No distinct cellular changes were found in the rechallenged birds other than an early influx of mononuclear cells into the gastrointestinal tract (group 3).
Quantification of proinflammatory cytokine and chemokine mRNA expression.
A linear relationship between the amount of input RNA and the Ct values for the various reactions was seen as expected in a log10 dilution series of standard samples for each cytokine or chemokines that also acted as positive controls for RT and PCR. Regression analysis of the Ct values generated with the log10 dilution series of standards gave R2 values for all reactions that were greater than 0.97. To account for the variation in sampling and RNA preparations, the Ct values for cytokines and chemokines specific for each sample were standardized using the Ct value for 28S rRNA for the same sample from a reaction performed simultaneously. Using the slopes of the cytokine and chemokine and 28S rRNA log10 dilution series regression lines, the difference in input total RNA, as represented by the 28S rRNA, was then used to adjust cytokine- and chemokine-specific Ct values. Standardization did not dramatically alter the distribution of the results as a whole.
Cytokine and chemokine expression following primary infection.
Following infection, expression of IFN-γ mRNA was significantly up-regulated in infected birds (it was up to 200-fold greater than the control levels; P < 0.05) in the liver, ileum, and cecal tonsils at 3, 7, and 14 dpi (Fig. 4A, B, and D). In contrast, significant down-regulation of IFN-γ expression was found in the spleen at 21 and 28 dpi (Fig. 4D). In the jejunum and ileum, significantly increased expression of TGF-β4 mRNA was found 1 dpi (Fig. 5), whereas expression of proinflammatory cytokines and chemokines (IL-1β, IL-6, IL-8, K60) was not significantly changed in the gastrointestinal tract of the chickens infected with Salmonella at 1, 3, or 7 dpi (data not shown), although increased expression of IL-6 was found in the ileum and cecal tonsils from 14 and 21 dpi onward, respectively (Fig. 6); little or no change in expression was found in the spleen or liver (data not shown). Interestingly, up to about 30-fold-higher expression of TGF-β4 mRNA was seen in a range of tissues at 7 dpi (Fig. 5). Expression of IL-4 and IL-10 mRNA was not detected in this experiment. For the chemokines examined, the mRNA levels of the CC MIP family chemokine were significantly greater (12-fold; P < 0.05) in the livers of infected birds at 3 dpi and remained somewhat higher in infected birds at 7 and 14 dpi (data not shown), although no significant changes were found in other tissues during primary infection.
FIG. 4.
Expression of IFN-γ in the livers (A), cecal tonsils (B), spleens (C), and ilea (D) of chickens following infection with Salmonella serovar Typhimurium. The data are the fold changes in mRNA compared with age-matched mock-infected controls based on triplicate samples from five birds for each time determined by quantitative RT-PCR. The error bars indicate standard errors of the means.
FIG. 5.
Expression of TGF-β4 in livers (A), cecal tonsils (B), spleens (C), and ilea (D) of chickens following infection with Salmonella serovar Typhimurium. The data are the fold changes in mRNA compared with age-matched mock-infected controls based on triplicate samples from five birds for each time determined by quantitative RT-PCR. The error bars indicate standard errors of the means.
FIG. 6.
Expression of IL-6 in ilea (A) and cecal tonsils (B) of chickens following infection with Salmonella serovar Typhimurium. The data are fold changes in mRNA compared with age-matched mock-infected controls based on triplicate samples from five birds for each time determined by quantitative RT-PCR. The error bars indicate standard errors of the means.
Cytokine and chemokine expression following secondary infection.
Significant expression of the MIP family chemokine and IL-6 mRNAs was found in both the ileum and cecal tonsils from 1 day after the secondary infection compared to uninfected controls (P < 0.05) (Fig. 7). Expression of the MIP family chemokine was also seen in the cecal tonsils and ileum of the age-matched birds (group 2) that received a primary infection, although the levels of expression were significantly lower (P < 0.05) than those in the rechallenged birds (group 3) and expression was not detected in the cecal tonsils until 3 dpi. No pattern of expression in the spleen or liver for these or any other cytokines or chemokines could be determined following secondary infection, nor could any pattern of expression be found for other cytokines in the ileum or cecal tonsils (data not shown).
FIG. 7.
Expression of a MIP family chemokine in ilea (A) and cecal tonsils (B) and expression of IL-6 in ilea (C) and cecal tonsils (D) of chickens following primary and secondary rechallenge infection with Salmonella serovar Typhimurium in age-matched chickens. The data are fold changes in mRNA compared with age-matched mock-infected controls based on triplicate samples from five birds for each time determined by quantitative RT-PCR. The error bars indicate standard errors of the means.
Salmonella-specific antibody production following primary infection.
Specific antibodies to Salmonella were detected in infected birds by ELISA following infection, and the results showed a classical pattern; significant levels of IgM antibodies were detected by 14 dpi, and IgG (IgY) and IgA antibodies were detected at 21 dpi (Fig. 8). The levels remained higher than those in uninfected controls until the end of the experiment at 28 dpi.
FIG. 8.
Specific serum antibody response to Salmonella serovar Typhimurium following oral infection of chickens, determined by an ELISA with lysate antigen. (A) IgM anti-Salmonella antibody response (serum diluted 1:400). (B) IgG response (serum diluted 1:400). (C) IgA response (serum diluted 1:25). The data are the mean values for five birds per group for each time. The error bars indicate standard errors of the means. OD 405 nm, optical density at 405 nm.
Salmonella-specific antibody production following secondary infection.
The levels of circulating IgG antibodies were high in the previously infected birds (group 3) prior to the secondary infection and changed little following infection (Fig. 9). The levels of IgM antibodies also changed little during the secondary infection. The levels of IgA antibodies rose significantly during the secondary infection from 7 dpi onward. The age-matched primary infected birds again showed a classical pattern of antibody production, although the production was more rapid than that in the younger birds in the previous experiment and significant levels of IgG and IgA antibodies were found by 14 dpi, possibly reflecting the increased immunological maturity of 10-week-old birds compared to 1-week-old birds.
FIG. 9.
Specific serum antibody responses to Salmonella serovar Typhimurium following primary and secondary rechallenge infection in age-matched chickens, determined by an ELISA with lysate antigen. (A) IgM anti-Salmonella antibody response (serum diluted 1:400). (B) IgG response (serum diluted 1:400). (C) IgA response (serum diluted 1:25). The data are the mean values for five birds per group for each time. The error bars indicate standard errors of the means.
DISCUSSION
The results of this study revealed a number of key points in the development of the primary immune response to Salmonella infection in the chicken and suggest that chemokines and cytokines play a key role in the protective response to salmonellosis. Clearance of primary infection by Salmonella serovar Typhimurium in the chicken appeared to involve IFN-γ-mediated T-cell responses, confirming other recent findings (6, 7). This indicates that clearance is primarily Th1 mediated, particularly as Th2 cytokines were undetectable. The central role of IFN-γ in the first 2 weeks of infection is consistent with its role in Salmonella serovar Typhimurium infection in mice, including the formation of focal granulomas and activation of macrophages (24). The increases in IFN-γ expression (up to 100-fold) found in the liver at 7 to 14 dpi coincided with increased numbers of T cells and the appearance of follicular lesions in the liver. Both CD8+ and CD4+ T cells are involved in the formation of these lesions (Fig. 1), and in addition to the accumulation of mononuclear cells in the follicles, there are significantly higher numbers of scattered T cells throughout the liver (Fig. 2). It is likely that IFN-γ plays a key role in initiating these responses. Interestingly, T-cell numbers appeared to decline in the spleen but increased in the ileum at 14 dpi. This is consistent with the decrease in T-cell proliferation described by Beal et al. (6) following Salmonella serovar Typhimurium infection and is consistent with trafficking of T cells from the spleen to the intestines, where relatively high numbers of Salmonella bacteria persist. High levels of expression of the chemokine MIP-1β were found in the liver early in primary infection. In mammals, the CC MIP family chemokines are chemoattractants for a range of cell types, including lymphocytes, particularly Th1 CD4+ cells and macrophages or monocytes (23). The expression found in this study is consistent with the accumulation of mononuclear cells in the liver at 3 dpi onward observed by microscopy.
In contrast to studies with 1-day-old chicks (35), in Salmonella serovar Typhimurium infections in 1-week-old birds the levels of proinflammatory cytokine and chemokine expression are not dramatically increased in the intestines. In very young birds Salmonella serovar Typhimurium infection leads to high levels of pathology in the gut, whereas the 1-week-old birds in this experiment displayed inflammation and an influx of heterophils and there were not the hemorrhagic lesions or bloodied, impacted cecal contents described previously (35). Expression of the regulatory cytokine TGF-β4 was found in the intestines early in the infection, and this may have inhibited inflammatory responses. Interestingly, high levels of TGF-β expression have been associated with macrophage activation but not inflammation in the intestinal mucosa of piglets infected with a rough lipopolysaccharide mutant of Salmonella serovar Typhimurium (30). The later expression of IL-6 found in the ileum and cecal tonsils may be associated with lymphocyte or macrophage development, regulation, or activation rather than with initiation of an inflammatory response. High levels of TGF-β4 expression were also found in a wide range of tissues at 7 dpi, consistent with the role of this cytokine in down-regulating inflammatory responses.
In the second experimental infection, previously infected birds demonstrated high levels of protection against systemic and gastrointestinal infection when they were rechallenged. Such a high degree of protection is consistent with previous studies (4). Birds that received the secondary Salmonella infection showed few signs of inflammation or little change in cytokine or chemokine expression, with the notable exceptions of IL-6 and the MIP family chemokine in the cecal tonsils and ileum. Following the secondary infection there was a significant and rapid increase in IL-6 in intestinal tissue. Although IL-6 is primarily considered a proinflammatory cytokine, it has number of functions, including activation of T and B lymphocytes and the hematopoesis and development of macrophages (17, 22). The coincident expression of a MIP family chemokine suggests that these signals attract cells to the gut and are involved in their activation. Immunohistological examination of these tissues was inconclusive as to whether T or B lymphocytes or indeed macrophages are the main cell types associated with these increases in expression. However, previous studies have indicated that proliferation of splenic T cells occurs immediately following secondary infection with Salmonella serovar Typhimurium (6), and it is believed that this is due to trafficking of these cells to the intestines. It is tempting to speculate that the MIP family chemokine and IL-6 are involved in the recruitment of T cells to the intestines and that these cells play a major role in protective immunity to gastrointestinal tract Salmonella infection in the chicken.
The level of inflammatory signals in primary infections of 10-week-old birds (group 2) was only marginally higher than that in uninfected controls. This in stark contrast to the results obtained for Salmonella serovar Typhimurium infection of 1-day-old chicks (35) or even 1-week-old birds described here. This lack of expression of proinflammatory signals may reflect the immunological maturity of the birds at 10 weeks of age, as older birds are likely to have larger numbers of and more effective regulatory T cells. Although the role of such cells in the chicken is poorly understood at this time, the cells play a significant role in controlling inflammatory responses in mammals, including responses to infection, and in regulation of inflammation in the gastrointestinal tract (26, 31). The lack of inflammation and the absence of diarrhea in older chickens infected with Salmonella appear to support the hypothesis that T lymphocytes may regulate pathogen-induced inflammation found in younger birds.
The antibody response to Salmonella seen in this study is consistent with previous descriptions, following a classical pattern of IgM followed by IgG (IgY) and then IgA antibodies (6, 7). The levels of IgG antibody remained high throughout the primary infection and were still high during the secondary infection. Circulating antibodies may play a key role in protection against secondary systemic infection as killed vaccines generate high titers of circulating anti-Salmonella antibodies and lead to good protection against systemic challenge (10, 36), although they are less effective than live vaccines or natural infection in protecting against enteric infection (4). Secondary infection does not greatly change the levels of IgM or IgG antibodies, although a significant increase in the level of IgA antibodies was found. This is consistent with the absence of systemic infection and the presence of Salmonella in the intestines leading to a mucosa-associated IgA response. Although in this study we did not directly assess secreted IgA, previous studies have indicated that elevated levels of IgA in serum correspond well to elevated levels of IgA in the chicken gut lumen (9, 28)
The findings of this study indicate that IFN-γ-mediated T-cell responses play a key role in clearance of primary Salmonella serovar Typhimurium infections of chickens, particularly in the clearance of the systemic stages of infection. IFN-γ appears to be involved with T lymphocytes and macrophages in the development of follicular lesions, and it seems likely that the foci represent bacteria being cleared by the developing immune response. The clearance of primary infection is dominated by a Th1-type response, as it is in mice. It is clear that Salmonella infection of chickens gives rise to a high degree of protection against subsequent rechallenge. The protection appears to be associated with both high levels of circulating antibodies and cellular responses. In particular, protection against enteric infection, the aim of chicken Salmonella vaccines, appears to involve rapid induction of cellular responses in the intestines and cecal tonsils that may subsequently involve IgA antibody responses. IL-6 and the CC MIP family chemokine appear to play a role in the protective response. The data presented here characterize the immunological changes associated with primary infection and the protective response to secondary rechallenge with Salmonella serovar Typhimurium. These data indicate the importance of antibody and particularly cellular responses in both immune clearance and protection.
Acknowledgments
We thank Lisa Rothwell for helping G.S.K.W. with Taqman assays and providing standards for these assays.
We also thank the Biotechnology and Biological Sciences Research Council for funding this study (grant BFP 11365 to P.B. and grant 11367 to I.M.)
Editor: F. C. Fang
REFERENCES
- 1.Anonymous. 2003. Salmonella in livestock 2002. Veterinary Laboratory Agency/Department of Environment, Food and Rural Affairs, London, United Kingdom.
- 2.Barrow, P. 1991. Serological analysis for antibodies to S. enteritidis. Vet. Rec. 128:43-44. [DOI] [PubMed] [Google Scholar]
- 3.Barrow, P. A., N. Bumstead, K. Marston, M. A. Lovell, and P. Wigley. 2004. Faecal shedding and intestinal colonisation of Salmonella enterica in in-bred chickens; the effect of host-genetic background. Epidemiol. Infect. 132:117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrow, P. A., J. O. Hassan, M. A. Lovell, and A. Berchieri. 1990. Vaccination of chickens with aroA and other mutants of Salmonella typhimurium and S. enteritidis. Res. Microbiol. 141:851-853. [DOI] [PubMed] [Google Scholar]
- 5.Barrow, P. A., J. F. Tucker, and J. M. Simpson. 1987. Inhibition of colonization of the chicken alimentary tract with Salmonella typhimurium gram-negative facultatively anaerobic bacteria. Epidemiol. Infect. 98:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beal, R. K., C. Powers, P. Wigley, P. A. Barrow, and A. L. Smith. 2004. Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathol. 33:25-33. [DOI] [PubMed] [Google Scholar]
- 7.Beal, R. K., P. Wigley, C. Powers, S. D. Hulme, P. A. Barrow, and A. L. Smith. 2004. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet. Immunol. Immunopathol. 100:151-164. [DOI] [PubMed] [Google Scholar]
- 8.Berndt, A., and U. Methner. 2001. Gamma/delta T cell response of chickens after oral administration of attenuated and non-attenuated Salmonella typhimurium strains. Vet. Immunol. Immunopathol. 78:143-161. [DOI] [PubMed] [Google Scholar]
- 9.Brito, J. R., M. Hinton, C. R. Stokes, and G. R. Pearson. 1993. The humoral and cell mediated immune response of young chicks to Salmonella typhimurium and S. Kedougou. Br. Vet. J. 149:225-234. [DOI] [PubMed] [Google Scholar]
- 10.Clifton-Hadley, F. A., M. Breslin, L. M. Venables, K. A. Sprigings, S. W. Cooles, S. Houghton, and M. J. Woodward. 2002. A laboratory study of an inactivated bivalent iron restricted Salmonella enterica serovars Enteritidis and Typhimurium dual vaccine against Typhimurium challenge in chickens. Vet. Microbiol. 89:167-179. [DOI] [PubMed] [Google Scholar]
- 11.Cooper, G. L., L. M. Venables, R. A. Nicholas, G. A. Cullen, and C. E. Hormaeche. 1993. Further studies of the application of live Salmonella enteritidis aroA vaccines in chickens. Vet. Rec. 133:31-36. [DOI] [PubMed] [Google Scholar]
- 12.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants elicit protective immune responses to homologous and heterologous serovars in chickens. Infect Immun. 69:7950-7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2003. Salmonella DNA adenine methylase mutants prevent colonization of newly hatched chickens by homologous and heterologous serovars. Int. J. Food Microbiol. 80:153-159. [DOI] [PubMed] [Google Scholar]
- 14.Feberwee, A., E. G. Hartman, J. J. de Wit, and T. S. de Vries. 2001. The spread of Salmonella gallinarum 9R vaccine strain under field conditions. Avian Dis. 45:1024-1029. [PubMed] [Google Scholar]
- 15.Hassan, J. O., and R. Curtiss III. 1990. Control of colonization by virulent Salmonella typhimurium by oral immunization of chickens with avirulent delta cya delta crp S. typhimurium. Res. Microbiol. 141:839-850. [DOI] [PubMed] [Google Scholar]
- 16.Hassan, J. O., and R. Curtiss III. 1994. Development and evaluation of an experimental vaccination program using a live avirulent Salmonella typhimurium strain to protect immunized chickens against challenge with homologous and heterologous Salmonella serotypes. Infect. Immun 62:5519-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano, T. 1998. Interleukin-6, p. 145-168. In A. Thomson (ed.), The cytokine handbook, 3rd ed. Academic Press, San Diego, Calif.
- 18.Humphrey, T. J. 1999. Contamination of eggs and poultry meat with Salmonella enterica serovar Enteritidis, p. 183-192. In A. M. Saeed (ed.), Salmonella enterica serovar Enteritidis in humans and animals: epidemiology, pathogenesis and control. Iowa University State Press, Ames.
- 19.Humphrey, T. J., A. Baskerville, H. Chart, and B. Rowe. 1989. Infection of egg-laying hens with Salmonella enteritidis PT4 by oral inoculation. Vet. Rec. 125:531-532. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser, P., L. Rothwell, E. E. Galyov, P. A. Barrow, J. Burnside, and P. Wigley. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 146:3217-3226. [DOI] [PubMed] [Google Scholar]
- 21.Kogut, M. H., G. I. Tellez, E. D. McGruder, B. M. Hargis, J. D. Williams, D. E. Corrier, and J. R. DeLoach. 1994. Heterophils are decisive components in the early responses of chickens to Salmonella enteritidis infections. Microb. Pathog. 16:141-151. [DOI] [PubMed] [Google Scholar]
- 22.Lynagh, G. R., M. Bailey, and P. Kaiser. 2000. Interleukin-6 is produced during both murine and avian Eimeria infections. Vet. Immunol. Immunopathol. 76:89-102. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani, A., M. Locati, and S. Sozzani. 2003. CC chemokines, p. 1083-1100. In M. L. Thomson (ed.), The cytokine handbook, 4th ed., vol. 2. Academic Press, San Diego, Calif.
- 24.Mastroeni, P., and N. Menager. 2003. Development of acquired immunity to Salmonella. J. Med. Microbiol. 52:453-459. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell, M. H., and G. Robertson. 1998. The avian heterophil leucocyte: a review. World Poult. Sci. J. 54:155-178. [Google Scholar]
- 26.Mittrucker, H. W., and S. H. Kaufmann. 2004. Mini-review: regulatory T cells and infection: suppression revisited. Eur. J. Immunol. 34:306-312. [DOI] [PubMed] [Google Scholar]
- 27.Moody, A., S. Sellers, and N. Bumstead. 2000. Measuring infectious bursal disease virus RNA in blood by multiplex real-time quantitative RT-PCR. J. Virol. Methods 85:55-64. [DOI] [PubMed] [Google Scholar]
- 28.Rose, M. E., E. Orlans, A. W. Payne, and P. Hesketh. 1981. The origin of IgA in chicken bile: its rapid active transport from blood. Eur. J. Immunol. 11:561-564. [DOI] [PubMed] [Google Scholar]
- 29.Smith, H. W., and J. F. Tucker. 1975. The effect of antibiotic therapy on the faecal excretion of Salmonella typhimurium by experimentally infected chickens. J. Hyg. (London) 75:275-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trebichavsky, I., V. Dlabac, Z. Rehakova, M. Zahradnickova, and I. Splichal. 1997. Cellular changes and cytokine expression in the ilea of gnotobiotic piglets resulting from peroral Salmonella typhimurium challenge. Infect. Immun. 65:5244-5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker, L. S. 2004. CD4+ CD25+ Treg: divide and rule? Immunology 111:129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wall, P. G., and L. E. Ward. 1999. Epidemiology of Salmonella enterica Enteritidis phage type 4 in England and Wales, p. 19-26. In A. M. Saeed (ed.), Salmonella enterica serovar Enteritidis in humans and animals: epidemiology, pathogenesis and control. Iowa State University Press, Ames.
- 33.Wigley, P., S. D. Hulme, N. Bumstead, and P. A. Barrow. 2002. In vivo and in vitro studies of genetic resistance to systemic salmonellosis in the chicken encoded by the SAL1 locus. Microbes Infect. 4:1111-1120. [DOI] [PubMed] [Google Scholar]
- 34.Withanage, G. S., K. Sasai, T. Fukata, T. Miyamoto, E. Baba, and H. S. Lillehoj. 1998. T lymphocytes, B lymphocytes, and macrophages in the ovaries and oviducts of laying hens experimentally infected with Salmonella enteritidis. Vet. Immunol. Immunopathol. 66:173-184. [DOI] [PubMed] [Google Scholar]
- 35.Withanage, G. S. K., P. Kaiser, P. Wigley, C. Powers, P. Mastroeni, H. Brooks, P. Barrow, A. Smith, D. Maskell, and I. McConnell. 2004. Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar Typhimurium. Infect. Immun. 72:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodward, M. J., G. Gettinby, M. F. Breslin, J. D. Corkish, and S. Houghton. 2002. The efficacy of Salenvac, a Salmonella enterica subsp. enterica serotype Enteritidis iron-restricted bacterin vaccine, in laying chickens. Avian Pathol. 31:383-392. [DOI] [PubMed] [Google Scholar]
- 37.Zhang-Barber, L., A. K. Turner, and P. A. Barrow. 1999. Vaccination for control of Salmonella in poultry. Vaccine 17:2538-2545. [DOI] [PubMed] [Google Scholar]