Abstract
The human monoclonal antibody to serotype 8 pneumococcal capsular polysaccharide D11 [immunoglobulin M(κ)] protects wild-type and complement component 4 knockout (C4 KO) mice against lethal intratracheal challenge with serotype 8 pneumococcus, but it does not promote polymorphonuclear leukocyte (PMN)-mediated pneumococcal killing in vitro. In this study, we investigated the effect of D11 on the blood and lung bacterial burdens and the serum and lung expression of inflammatory chemokines and cytokines in an intratracheal challenge model with serotype 8 pneumococcus in C4 KO mice. Pneumococcus was not detected in the blood of D11-treated mice, whereas control mice had high-grade bacteremia with >107 CFU. Control mice had a >5-log increase in lung CFU and D11-treated mice manifested a nearly 3-log increase in lung CFU compared to the original inoculum 24 h after infection. Serum and lung levels of soluble macrophage inflammatory protein 2 (MIP-2) and interleulin-6 (IL-6) as measured by an enzyme-linked immunosorbent assay were lower in D11-treated mice than in control mice 24 h after infection. Real-time PCR was performed to examine lung mRNA chemokine and cytokine expression. The results showed that D11-treated mice had significantly less gamma interferon, MIP-2, IL-12, monocyte chemoattractant protein 1/JE, and tumor necrosis factor alpha expression than control mice 24 h after infection. Histopathology and immunohistochemical staining of lung tissues revealed that D11-treated mice had less inflammation, fewer PMNs, and less myeloperoxidase staining than control mice 24 h after infection. These findings suggest that the efficacy of certain serotype-specific antibodies against pneumococcal pneumonia could be associated with modulation of the lung inflammatory response and a reduction in host damage.
The ability of serotype-specific antibodies to confer protection against invasive pneumococcal disease and pneumonia was established with therapeutic antisera during the serum therapy era (9). Subsequently, the discovery of antibiotics together with the toxicity of the antibody reagents available in the 1940s led to the discontinuation of antibody-based therapy for pneumococcal disease (9). However, rising antibiotic resistance and an increased number of persons who are at high risk for pneumococcal disease have led to renewed interest in active and passive antibody-based strategies for pneumococcal disease. The currently recommended adult pneumococcal vaccine prevents invasive (bacteremic) pneumococcal disease in certain populations, but it has had unpredictable efficacy against pneumonia (4, 23, 27, 40, 43, 64). This probably reflects variability in the study designs and clinical endpoints and the inability to definitively diagnose nonbacteremic pneumococcal pneumonia. Nonetheless, the functional mediators of antibody-mediated protection against pneumonia have not been defined, and it is not known whether they are the same in bacteremic and nonbacteremic disease.
The ability of serotype-specific immunoglobulin G (IgG) to promote polymorphonuclear leukocyte (PMN)-mediated pneumococcal killing in vitro is considered a surrogate for pneumococcal vaccine-elicited immunity (2, 21, 29, 49, 50, 63). However, the efficacy of the other antibody isotypes and the nature of antibodies that protect against pneumonia have not been as extensively investigated. The serotype 8-specific human monoclonal antibody (MAb) D11 (IgM) protected normal and complement component 4 knockout (C4 KO) mice against intraperitoneal (69) and intratracheal (i.t.) challenge with serotype 8 pneumococcus (10). Surprisingly, D11 promoted little or no human PMN-mediated killing of the same organism with or without complement, but it was found to downregulate PMN interleukin-8 (IL-8) secretion in vitro (10). Although macrophage-mediated phagocytosis (1a, 17) could be responsible for bacterial killing in vivo, this observation called into question the paradigm that antibody-mediated immunity requires opsonic serotype-specific IgG (29, 49) and led us to question whether D11-mediated protection was associated with modulation of the host inflammatory response in vivo. In this study, we sought to determine whether D11 administration affects the pulmonary inflammatory response to serotype 8 pneumococcus in an intratracheal model of infection.
MATERIALS AND METHODS
Antibody reagents.
Human MAb D11 [IgM(κ)] was previously shown to bind to the capsular polysaccharide of serotype 8 cells, to activate both the alternative and classical complement pathways, and to protect mice from death due to serotype 8 pneumococcus (10, 69). D11 was purified by affinity chromatography with anti-human IgM-coated agarose beads (Sigma-Aldrich, St. Louis, MO). A human myeloma IgM MAb (Calbiochem, San Diego, CA) was used as an isotype control. The IgM did not react with serotype 8 pneumococcal capsular polysaccharide as determined by an enzyme-linked immunosorbent assay (ELISA) and did not bind to whole cells in ultrastructural studies (69).
Bacteria.
Streptococcus pneumoniae serotype 8 strain ATCC 6308 (American Type Culture Collection, Rockville, Md.) was grown in tryptic soy broth (TSB) (Difco, Sparks, MD) to the mid-log phase at 37°C in 5% CO2, frozen in TSB in 10% glycerol, and stored at −80°C until it was used, as described previously (10). This strain is the same strain that was used to establish D11 efficacy in intraperitoneal and i.t. infection models in different strains of mice (10, 69) and to investigate its biological activity in vitro (10).
Mice.
C4 KO mice were used. D11 was previously shown to protect normal mice and this strain from death due to serotype 8 pneumococcus (10, 69). The innate immune response to pneumococcus depends on naturally occurring IgM and an intact classical complement pathway (7), whereas the alternative complement pathway is required for the efficacy of certain specific antibodies that mediate acquired immunity (13, 54, 69). Therefore, we used C4 KO mice, which lack a functional classical complement pathway, to evaluate antibody-mediated effects that are independent from innate, classical complement-dependent mechanisms. C4 KO mice (10, 13, 67, 69) were bred by the Institute for Animal Studies at the Albert Einstein College of Medicine in accordance with the rules and regulations of animal welfare at the Albert Einstein College of Medicine. Male and female mice that were 6 to 8 weeks old were used.
Intratracheal infection model.
D11 was previously shown to protect normal and C4 KO mice against death from intraperitoneal and i.t. infection with serotype 8 pneumococcus (10, 69). In the i.t. model, 1 μg of D11, an IgM control, or phosphate-buffered saline (PBS) was mixed with 20 CFU of pneumococcus and administered immediately i.t. In this model, 100% of the mice receiving 1 μg of D11 survived, compared to 20% of the PBS controls and none of the IgM controls (10). The same i.t. model was used in this study. Mice were anesthetized with sodium pentobarbital (65 mg/kg; Abbott Laboratories, North Chicago, IL) intraperitoneally, after which a tracheal incision was made and 20 CFU of serotype 8 pneumococcus ATCC 6308 (4 50% lethal doses) in 50 μl that had been mixed immediately before administration with PBS, 1 μg of D11, or the control IgM was injected i.t. Each mouse in each group was injected with the relevant inoculum before we proceeded to the next group of mice. After injection, the incision was sutured, and the mice were observed until recovery. The doses of D11 and IgM used were identical to those that were previously shown to protect against i.t. challenge with the same organism in the same strain of mice (10). Immediate replicate plating of the inocula was not possible, because the surgical procedure performed on the mice and the recovery period did not permit immediate plating. However, the number of CFU administered to the mice was estimated by mixing a final amount of serotype 8 S. pneumoniae (20 CFU) with either PBS or 1 μg of control IgM or D11 in 50 μl and plating the preparation on TSB containing 5% sheep blood (Becton Dickenson, Franklin Lakes, NJ). The number of CFU was determined after incubation overnight at 37°C in 5% CO2. The numbers of CFU for the PBS, IgM, and D11 mixtures were 11 ± 2, 15 ± 2, and 10 ± 2 (means ± standard errors of the means), respectively, and were not significantly different (P = 0.57 and P = 0.07 for comparisons of D11 to PBS and IgM, respectively, as determined by unpaired t tests). The mice were monitored daily to determine their clinical status and survival. Here we report the results of three independent experiments with C4 KO mice and one experiment with C57BL/6 × Sv129 and C4 KO mice (see below). The number of mice used in each experiment is indicated in the figure legends.
Determinations of blood and lung bacterial burdens.
Mice were bled from the retroorbital sinus 6 and 24 h after infection. At each time, mice were killed via cervical dislocation, and their lungs were removed aseptically, rinsed in sterile water, and homogenized in endotoxin-free Hanks balance salt solution without calcium, magnesium, or phenol red (Mediatech, Herndon, VA). The blood samples and lung lysates were serially diluted in TSB and then plated onto TSB with 5% sheep blood (Becton Dickenson) and incubated for 18 h at 37°C in the presence of 5% CO2, and the number of CFU was determined after 18 h of incubation.
Endotoxin avoidance precautions.
To avoid endotoxin contamination, surgical tools were washed in 70% ethanol after dissection of each mouse, and the probe of the homogenizer was rinsed in 70% ethanol between samples. Tests performed with a Limulus amebocyte lysate kit (Cambrex, Walkerville, M.D) revealed that there was no endotoxin in the human MAb or any other reagent used.
Determinations of serum and lung cytokine levels.
Serum and lung macrophage inflammatory protein 2 (MIP-2) and IL-6 levels were determined by and ELISA using samples from D11-, IgM-, and PBS-treated mice 6 and 24 h after infection. Blood was allowed to clot for 30 min on ice, after which the serum was separated by centrifugation for 10 min at 2,000 × g and stored at −80°C until use. The lungs were homogenized as described above and centrifuged at 1,000 × g for 10 min, and the supernatant was removed and stored at −80°C until use. ELISA kits (R&D Systems, Minneapolis, MN) were used according to the manufacturer's protocol to determine serum and lung MIP-2 and IL-6 levels. The positive controls were recombinant MIP-2 and IL-6 (R&D Systems), and the negative control was wells that contained only 1% bovine serum albumin in PBS.
Quantitative real-time PCR.
Quantitative real-time PCR was performed to determine the levels of lung gamma interferon (IFN-γ), IL-6, MIP-2, IL-12, monocyte chemoattractant protein 1 (MCP-1), tumor necrosis factor alpha (TNF-α), and IL-10 mRNA expression using the Bio-Rad Laboratories iCycler IQ real-time PCR detection system (Bio-Rad, Hercules, CA). Lungs were removed from D11-, IgM-, and PBS-treated mice 6 and 24 h after infection and placed in 6 ml of TRIzol (Invitrogen, Carlsbad, CA) for homogenization; RNA was extracted, and 1 μg of RNA was reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's instructions. The chemokine and cytokine primers that were used are listed in Table 1. PCR amplification was performed with IQ SYBR Green Supermix (Bio-Rad) by using 95°C for 5 min and 45 cycles of 95°C for 15 s followed by 62°C for 45 s for all mRNAs. A standard curve was generated by plotting threshold cycle values versus the input mouse splenic cDNA concentrations (Ambion, Austin, TX). The concentrations of the experimental samples were determined by interpolating threshold cycle values into the standard curve, and expression levels were computed by normalizing each cytokine or chemokine concentration to the concentration of glyceraldehyde-3-phosphate dehydrogenase in the sample. The results of four independent experiments to determine lung chemokine and cytokine expression were combined and analyzed statistically, and they included 10 to 12 data points per time per treatment group.
TABLE 1.
Sequences of the primers used for quantitative real-time PCR
| Gene | Directiona | Primer (5′ → 3′) | Product size (bp) | Final concn (nM)b | Reference |
|---|---|---|---|---|---|
| IFN-γ | For | CCTGCGGCCTAGCTCTGA | 81 | 100 | 67a |
| Rev | CAGCCAGAAACAGCCATGAG | 100 | |||
| IL-6 | For | ACAACCACGGCCTTCCCTACTT | 129 | 100 | 31a |
| Rev | CACGATTTCACAACCACGGCCTTCCCTACTT | 100 | |||
| MIP-2 | For | ATCCAGAGCTTGAGTGTGACGC | 90 | 100 | 31a |
| Rev | AAGGCAAACTTTTTGACCGCC | 100 | |||
| IL-12 | For | AGCAGTAGCAGTTCCCCTGA | 88 | 200 | 50a |
| Rev | AGTCCCTTTGGTCCAGTGTG | 200 | |||
| MCP-1/JE | For | CCACTCACCTGCTGCTACTCAT | 76 | 100 | 31a |
| Rev | TGGTGATCCTCTTGTAGCTCTCC | 300 | |||
| IL-10 | For | CTTGCACTACCAAAGCCACA | 86 | 100 | 50a |
| Rev | TAAGAGCAGGCAGCATAGCA | 100 | |||
| TNF-α | For | GTGTGGGTGAGGAGCACGTA | 198 | 100 | 1 |
| Rev | TCATCCTGCTCTTCTTTCTCGAAT | 100 | |||
| GAPDHc | For | CATCGCCTTCCGTGTTCCTA | 54 | 100 | http://medgen31.rug.ac .be/primerdatabase |
| Rev | GCGGCACGTCAGATCCA | 100 |
For, forward; Rev, reverse.
Final concentration of primer in 25 μl (final volume).
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Cytokine and chemokine expression in background strain and C4 KO mice.
C4 KO and C57BL/6 × Sv129 mice were infected i.t. with 20, 50, or 100 CFU as described above. At 24 h postinfection, mice were sacrificed via cervical dislocation, lungs were removed and homogenized in TRIzol, and RNA was extracted for cytokine expression studies as described above.
Histopathology and immunohistochemistry.
C4 KO mice treated with D11, IgM, or PBS were killed 6 and 24 h after infection; their lungs were inflated with 4% formalin (Fisher Scientific, Fairlawn, NJ), fixed in situ for 48 h, and removed. The tissue was embedded in paraffin (Blue Ribbon; Fisher Scientific), cut into 5-μm sections, and stained with hematoxylin and eosin (H&E) (Fisher Scientific). To detect myeloperoxidase (MPO) activity, slides were rehydrated and boiled in 10 mM sodium citrate, pH 6.0, for 20 min, blocked with 5% goat serum for 1 h, and incubated with ready-to-use rabbit polyclonal antibody to MPO (Labvision, Freemont, CA) for 90 min at room temperature. Slides of a human tonsil (Labvision) were used as positive control slides, and normal rabbit IgG (Santa Cruz Biotechnologies, Santa Cruz, CA) and no primary antibody were used as negative controls. The slides were incubated with ready-to-use biotinylated goat antipolyvalent antibody (Labvision) for 30 min at room temperature, incubated with streptavidin-horseradish peroxidase (Zymed, South San Francisco, CA) for 20 min at room temperature, and developed using Sigma Fast 3′-diaminobenzidine tablets (Sigma Aldrich, Minneapolis, MN) for 5 min at room temperature. To detect lung macrophages, slides were rehydrated and boiled in 10 mM sodium citrate, pH 6.0, for 30 min and then incubated with primary antibody MAC-3 (BD Biosciences Pharmingen, San Diego, CA) overnight at 4°C and a biotinylated goat anti-rat IgG for 1 h at room temperature. The slides were developed as described above.
Statistical analysis.
The numbers of CFU in blood samples and lung lysates and the levels of chemokine and cytokine expression in D11-, IgM-, and PBS-treated mice obtained by ELISA and real-time PCR, respectively, were compared by performing unpaired t tests after Grubbs' test was used to detect outliers. Correlations between bacteremia and real-time PCR-determined cytokine expression were examined by using Spearman's correlation test. All statistical analyses were performed using Prism (v.4.02 for Windows; GraphPad Software, San Diego, CA). A P value of <0.05 was used for statistical significance.
RESULTS
Comparison of cytokine profiles of C57BL/6 × Sv129 and C4 KO mice.
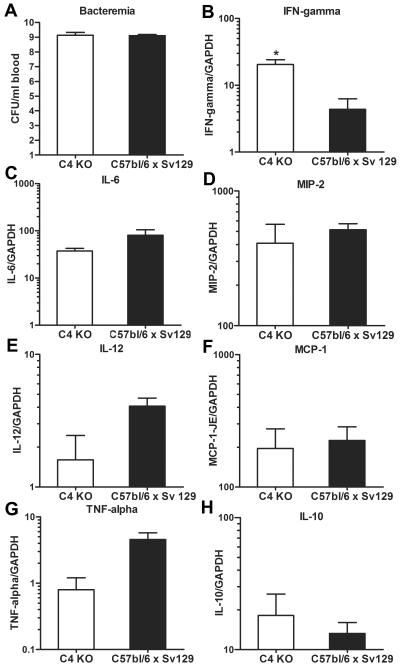
C4 KO and C57BL/6 × Sv129 mice had similar levels of bacteremia (Fig. 1A), and the levels of lung chemokine and cytokine mRNA expression (Fig. 1B to H) did not differ for the inocula studied (data not shown). Hence, the values for chemokine and cytokine expression in mice that received 20 and 100 CFU were combined. The levels of lung expression of IL-6 (Fig. 1C), MIP-2 (Fig. 1D), and MCP-1/JE mRNAs (Fig. 1F) were statistically similar for C4 KO and C57BL/6 × Sv129 mice; C4 KO mice exhibited significantly more IFN-γ (Fig. 1B) expression than C57BL/6 × Sv129 mice and more expression of IL-10 (Fig. 1H), but this difference was not significant. C57BL/6 × Sv129 mice exhibited more IL-12 (Fig. 1E) and TNF-α (Fig. 1G) expression than C4 KO mice, but the differences were not statistically significant.
FIG. 1.
Bacteremia and cytokine and chemokine expression in background strain and C4 KO mice: cytokine lung expression and number of CFU of type 8 pneumococcus in whole blood of C4 KO (open bars) and C57BL/6 × Sv129 (solid bars) mice infected i.t. with either 20 or 100 CFU of serotype 8 pneumococcus. (A) Bacteremia. (B) IFN-γ. (C) IL-6. (D) MIP-2. (E) IL-12. (F) MCP-1/JE. (G) TNF-α. (H) IL-10. The bars indicate the means, and the errors bars indicate the standard errors of the means. An asterisk indicates that the P value is 0.02, as determined by an unpaired t test (n = 3). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Pneumococcal burdens in C4 KO mice.
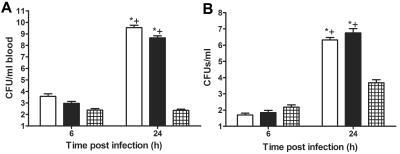
i.t. infection with 10 and 20 CFU of the serotype 8 strain used resulted in the death of 80% of C4 KO and C57BL/6 × Sv129 mice by 48 and 72 h after infection; infection with 5 CFU resulted in the death of 50% of the mice (data not shown). The number of CFU in blood samples from D11-treated mice was below the limit of detection (200 CFU) 6 and 24 h after infection. The levels of bacteremia were significantly higher in PBS- and IgM-treated mice than in D11-treated mice 24 h after infection (P = 0.001 and P < 0.0001, respectively). PBS- and IgM-treated mice had significantly higher levels of bacteremia at 24 after infection than at 6 h after infection (P = 0.002 and P < 0.0001) (Fig. 2A). The number of lung CFU in D11-treated mice was similar to the number in control mice at 6 h after infection but was significantly less than the number in PBS- and IgM-treated mice at 24 h after infection (P = 0.01 and P = 0.0002, respectively) (Fig. 2B). Control mice manifested a significant increase in the lung CFU burden at 24 h compared to the burden at 6 h after infection (P = 0.001 for PBS-tread mice and P < 0.0001 for IgM-treated mice) (Fig. 2B). The higher number of CFU in D11-treated mice at 24 h was not statistically significant. The numbers of lung CFU in D11-treated mice were also determined at later times after infection. There were 4 × 105, 1 × 103, and 6 × 102 CFU at 48, 72, and 96 h after infection, respectively (n = 3). The statistical comparisons were performed with unpaired t tests.
FIG. 2.
Bacteremia and lung CFU in C4 KO mice infected i.t. with serotype 8 pneumococcus: CFU in blood (A) and lungs (B) obtained from C4 KO mice inoculated with serotype 8 pneumococcus and PBS (open bars), 1 μg of IgM (solid bars), or 1 μg of D11 (cross-hatched bars) at 6 and 24 h after infection. An asterisk indicates that the P value is <0.05 for a comparison of control mice at 24 h to D11-treated mice, as determined by an unpaired t test; a plus sign indicates that the P value is < 0.05 for a comparison of the numbers of CFU at 6 h and 24 h after infection, as determined by an unpaired t test (n = 12 to 15 mice per time).
Serum and lung expression of soluble MIP-2 and IL-6.
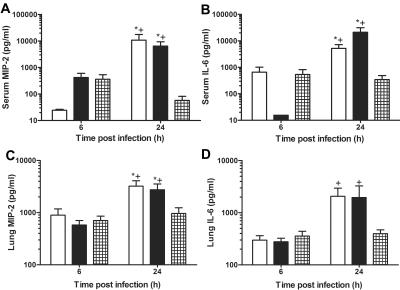
D11-treated mice had lower levels of serum MIP-2 and IL-6 than IgM-treated controls (P < 0.001) and PBS-treated controls (P < 0.02) at 24 h after infection (Fig. 3A and B). The MIP-2 serum level was higher (P > 0.06) and the IL-6 serum level was lower (P < 0.05) for IgM-treated mice than for PBS-treated mice at 6 h after infection. The MIP-2 and IL-6 serum levels were significantly increased at 24 h after infection compared to the levels at 6 h after infection in IgM-treated mice (P < 0.01) and PBS-treated mice (P < 0.01) (Fig. 3A and B). D11-treated mice had lower lung MIP-2 levels than IgM-treated mice (P < 0.03) and PBS-treated control mice (P < 0.01). Significant increases in lung MIP-2 and IL-6 levels were observed in PBS-treated mice (P < 0.01 and P < 0.02, respectively) and IgM-treated mice (P < 0.004 and P < 0.04, respectively) at 24 h after infection compared to the levels at 6 h after infection (Fig. 3C and D). The statistical comparisons were performed with unpaired t tests.
FIG. 3.
Serum and lung soluble MIP-2 and IL-6 levels in C4 KO mice infected i.t. with serotype 8 pneumococcus: serum MIP-2 and IL-6 (A and B) and lung MIP-2 and IL-6 (C and D) protein levels as determined by ELISA in PBS-treated mice (open bars), IgM-treated mice (solid bars), and D11-treated mice (cross-hatched bars) at 6 and 24 h after infection. The bars indicate the means of three independent experiments, and the error bars indicate the standard errors of the means. An asterisk indicates that the P value is <0.05 for a comparison of the control mice to D11-treated mice at 24 h after infection, as determined by an unpaired t test; a plus sign indicates that the P value is <0.05 for a comparison of the levels at 6 h and 24 h after infection, as determined by an unpaired t test (n = 12 to 15 mice per time).
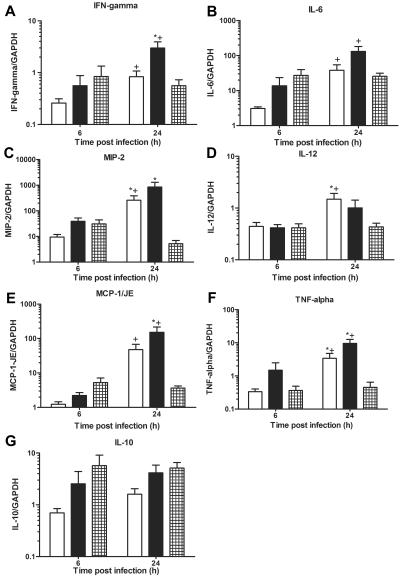
Lung expression of chemokine and cytokine mRNAs.
The results of the real-time PCR analysis of lung chemokine and cytokine RNA expression are shown in Fig. 4. The expression of IFN-γ (Fig. 4A), MIP-2 (Fig. 4C), and MCP-1/JE (Fig. 4E) compared to IgM-treated mice, the expression of IL-12 (Fig. 4D) and MIP-2 (Fig. 4C) compared to PBS-treated mice, and the expression of TNF-α (Fig. 4F) compared to IgM- and PBS-treated controls were significantly higher in D11-treated mice 24 h after infection (P < 0.05) (Fig. 4). IgM-treated mice also exhibited more IFN-γ expression than PBS-treated mice 24 h after infection, (P = 0.04), and IgM- and PBS-treated mice had significantly higher levels of IFN-γ (P < 0.04) (Fig. 4A), IL-6 (P < 0.02) (Fig. 4B), MCP-1/JE (P < 0.005) (Fig. 4E), TNF-α (P < 0.04) (Fig. 4F) 24 h than 6 h after infection. D11-treated mice did not manifest an increase in any chemokine or cytokine at 24 h compared to 6 h after infection; however, a significant decrease in MIP-2 expression was observed at 24 h after infection compared to that at 6 h postinfection, (P = 0.03) (Fig. 4C). No differences in expression of IL-10 (Fig. 4G) were observed for any of the groups either 6 or 24 h after infection. Expression of IL-6, MIP-2, MCP-1/JE, and IL-10 was positively correlated with the magnitude of bacteremia in IgM-treated mice (data not shown). Correlations between cytokine expression and bacteremia were not found for the other groups in this study. The statistical comparisons were performed with unpaired t tests.
FIG. 4.
Lung mRNA chemokine and cytokine expression in C4 KO mice infected i.t. with serotype 8 pneumococcus: lung mRNA chemokine and cytokine expression as determined by real-time PCR for mice inoculated i.t. with serotype 8 pneumococcus and PBS-treated mice (open bars), IgM-treated mice (solid bars), or D11-treated mice (cross-hatched bars) at 6 and 24 h after infection. (A) IFN-γ. (B) IL-6. (C) MIP-2. (D) IL-12. (E) MCP-1/JE. (F) TNF-α. (G) IL-10. The bars indicate the means of four independent experiments, and the error bars indicate the standard errors of the means. The y axis shows the mRNA concentrations of the mediators normalized to the concentration of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). An asterisk indicates that the P value is <0.05 for a comparison of control and D11-treated mice at 24 h after infection, as determined by an unpaired t test; a plus sign indicates that the P value is <0.05 for a comparison of the mRNA levels at 6 h and 24 h after infection, as determined by an unpaired t test (n = 10 to 12 mice per time).
Histology and immunohistochemistry.
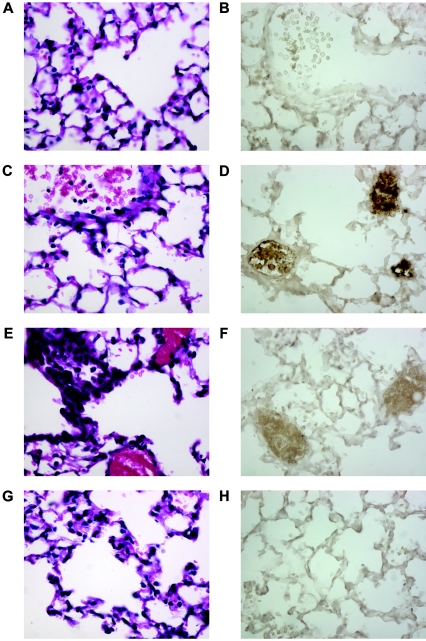
Hematoxylin- and eosin-stained lung sections from D11-treated mice contained more PMNs and myeloperoxidase-stained sections revealed more myeloperoxidase than sections from IgM-treated mice at 6 h after infection (Fig. 5A to D). In contrast, at 24 h after infection, more PMNs and more myeloperoxidase staining were observed in sections from IgM-treated mice than in sections from D11-treated mice (Fig. 5E to H). The sections from PBS-treated mice resembled those from IgM-treated mice (data not shown). MAC-3 staining did not reveal a difference in the number of macrophages between the control mice and the D11-treated mice (data not shown).
FIG. 5.
Histopathological appearance of C4 KO mice infected i.t. with type 8 pneumococcus: fixed tissue sections obtained 6 and 24 h after infection and stained with H&E and anti-myeloperoxidase antibody. (A) IgM, 6 h, H&E. (B) IgM, 6 h, MPO. (C) D11, 6 h, H&E. (D) D11, 6 h, MPO. (E) IgM, 24 h, H&E. (F) IgM, 24 h, MPO. (G) D11, 24 h, H&E. (H) D11, 24 h, MPO. Magnification, ×40. The images are representative of two sections per time.
DISCUSSION
The results of the experiments in this study showed that untreated or IgM-treated C4 KO mice develop high levels of bacteremia and lung bacterial burdens 24 h after i.t. infection with serotype 8 pneumococcus. In contrast, mice that received the protective human IgM MAb D11 did not develop detectable bacteremia and had lower lung burdens than the controls. Compared to control mice, D11-treated mice had lower levels of soluble serum and lung MIP-2 and IL-6, markedly less lung TNF-α, IFN-γ, IL-12, MIP-2, and MCP-1/JE mRNA expression, and fewer PMNs in lung tissues 24 h after infection. These findings revealed an association between antibody-mediated protection and modulation of the host inflammatory response to pneumococcal pneumonia that has not been described previously.
D11-treated mice in this study manifested an increase in lung CFU compared to the initial inoculum 24 h after infection, which was observed up to 96 h after infection. Similar findings were reported for surviving antibody-treated mice in a high-inoculum intranasal model of serotype 8 pneumonia (53). The mechanism by which lung CFU were reduced in the setting of D11 treatment in this study or in surviving mice with natural resistance (15) is uncertain. D11 did not promote killing of serotype 8 organisms by PMNs in vitro (10), although macrophages can promote killing (1a, 17) and additional opsonins and/or cytokines could affect the antibody opsonic potential in vivo (28). The reduction in lung CFU in D11-treated mice in our model or in surviving mice in other models (15, 53) could reflect growth inhibition and/or persistence without killing, as described previously for other gram-positive microbes (24, 57), but further studies are needed to identify the mechanism(s) which governs the CFU reduction in the setting of antibody therapy. Nonetheless, the fact that antibody-treated mice had persistent lung pneumococcal burdens without bacteremia establishes that sterilizing immunity is not required for antibody-mediated protection. In light of clinical evidence that vaccine-elicited immunity is more effective against invasive pneumococcal disease (bacteremia) than against pneumonia (16, 19, 22, 27, 56), our data suggest that protection against bacteremia and clearance from the lung could be mediated by distinct mechanisms.
D11-treated mice had lower levels of soluble serum MIP-2 and IL-6 and lung MIP-2 than control mice at 24 h after infection. In other models, the cytokine response in mice that survived intranasal infection with another serotype was associated with an early increase in IL-6, MIP-2, and PMN levels in lung tissue that declined, whereas dying mice had high levels of IL-6 and MIP-2 and increased numbers of PMNs in lung tissue at 24 h after infection (5, 15). IL-6 is a sepsis-associated cytokine (61), and MIP-2 is a cytokine that mediates inflammation and PMN recruitment in murine pneumococcal pneumonia (20, 47, 48). We found that lung sections from D11-treated mice had more PMNs and myeloperoxidase staining than control mice 6 h after infection, but this picture was reversed 24 h after infection, when sections from control mice had markedly more inflammation, PMNs, and myeloperoxidase staining. These findings paralleled a trend among the D11-treated mice toward higher levels of lung MIP-2 and MCP-1 mRNA expression at 6 h after infection than at 24 h after infection. Since MIP-2 is a PMN chemoattractant and MCP-1 recruits T cells and macrophages (5, 20, 32), we hypothesize that D11 administration could induce the kind of earlier cellular response that has been shown to be protective against pneumococcal pneumonia (15, 18, 41). This hypothesis is under investigation in our laboratory. Interestingly, there was also a trend toward lower serum IL-6 levels in control IgM-treated mice. This observation cannot be explained with the available knowledge; however, nonspecific antibodies can downregulate the expression of certain inflammatory mediators (37, 55). Perhaps a similar phenomenon could explain the failure to produce the early IL-6 response (15) that has been associated with protection in naïve mice. There is ample evidence that enhancement of the cellular immune response to a variety of pathogens is an important mechanism of antibody immunity, including the response to encapsulated pathogens (reviewed in reference 11).
The most intriguing finding in this study was that mice that received D11 had lower levels of lung TNF-α, IFN-γ, IL-12, MIP-2, and MCP-1/JE mRNA expression than control mice. The ability of the 1-μg dose of D11 used in this study to protect C4 KO mice against death was established previously in the same model (10) and reconfirmed in this study (data not shown). Since the antibody and pneumococcus were coadministered in this model, we considered the possibility that our results reflected a reduction in the inoculum that D11-treated mice received. Several factors make this unlikely. First, plating studies showed there were similar numbers of CFU in D11-, IgM-, and PBS-containing mixtures. Second, the experimental groups had similar numbers of lung CFU at 6 h after infection. Third, a 10-CFU inoculum (50% of the inoculum used) had the same lethality as a 20-CFU inoculum. Nonetheless, our results could reflect immune complex formation or agglutination in vivo, as described for protective IgMs to fungi (11, 25, 58). Although many mouse models of infectious diseases, including the one used in this study, have nonphysiologic features (12), mixed inocula were used to establish the efficacy and dose of therapeutic antisera in the serum therapy era (12) and MAb efficacy in a model of pulmonary tuberculosis (60). The use of C4 KO mice in this study allowed us to focus on acquired immune responses, since innate immunity to pneumococcal pneumonia depends on an intact classical complement pathway (7). Hence, the effect of D11 in this model could recapitulate the response of an immune and/or naturally resistant host who acquires pneumococcal pneumonia by aspiration, the most common route of infection.
The time at which inflammatory mediators are produced governs whether they are detrimental or beneficial (34, 41). Compared to control treatments, D11 administration was associated with a large (10-fold or more) decrease in lung MIP-2, MCP-1, and TNF-α mRNA expression 24 h after infection. In studies with other pneumococcal serotypes, resistance to experimental pneumonia was associated with increased production of soluble IFN-γ, IL-12, IL-6, and TNF-α soon after infection (15, 34, 46, 62, 68). In our study, lung expression of IFN-γ, IL-6, MIP-2, IL-12, and TNF-α mRNA was similar in D11-treated and control mice 6 h after infection. However, by 24 h after infection, the expression was markedly higher in controls but unchanged in D11-treated mice. This is consistent with reports from other groups which showed that secreted levels of MIP-2, IL-6, and MCP-1 were higher and increased with time in mice that succumbed with pneumococcal pneumonia than in mice that survived (15, 48, 65). We did not examine secreted IFN-γ, IL-12, MCP-1, or TNF-α levels, but we did find parallel increases in soluble and lung mRNA expression of MIP-2 and IL-6 at 6 and 24 h, which revealed a relationship between mRNA expression and secretion of these key mediators of the inflammatory response to pneumococcus. At present, the mechanism by which D11 administration results in modulation of the cytokine response is unknown. The increased bacterial burden and cytokine expression in control mice could reflect the activity of pneumolysin, which enhanced pneumococcal growth (51) and stimulated MIP-2 and IL-6 release (26, 39, 47) in other models. Currently, there is no information on the role of pneumolysin in the pathogenesis of serotype 8 pulmonary infections. Similarly, the effect of serotype-specific antibodies on pneumolysin activity is unknown. However, the ability of specific and nonspecific antibodies to mediate anti-inflammatory effects is well documented (reviewed in reference 11). Along these lines, intravenous immunoglobulin protected against death and limited bacteremia without inducing pneumococcal clearance from the lungs of serotype 8-infected mice (53), but inflammatory profiles were not examined.
There is increasing evidence that cellular immune mechanisms induce resistance to pneumococcal disease and promote pneumococcal clearance (15, 20, 30, 39) through a TH1-type cellular response (3, 33). We found that the levels of expression of IL-12 and IFN-γ were similar in controls and D11-treated mice 6 h after infection. Based on available studies (52, 68), examination of earlier times might have revealed earlier production of these TH1-type cytokines in D11-treated mice. The TH1-type response to one pneumococcal serotype was shown to be initiated by the early production of IL-12, which induced IFN-γ-mediated production of MIP-2 and TNF-α, and was associated with prolonged survival (68). Our data showed that lung mRNA expression of MCP-1 was numerically, but not statistically, higher in D11-treated mice than in control mice 6 h after infection. Upregulation of MCP-1, which is a monocyte chemoattractant, has been associated with macrophage recruitment in response to pneumococcal infection (20, 35, 44, 45). Although macrophages were not observed in lung sections from D11-treated mice obtained 24 h after infection, macrophage recruitment could occur later (36). Hence, examination of tissue from later times may have been necessary to identify macrophage recruitment, if it occurs in this model. We did not observe a change in lung IL-10 mRNA expression in any of the groups in this study. However, it may be necessary to examine earlier times to identify antibody-mediated modulation of IL-10, since IL-10 secretion increased 3 h after infection in other pneumococcal pneumonia models (38, 41).
The inflammatory response is a hallmark of clinical and experimental pneumococcal pneumonia (5, 8, 31, 42). The data presented here establish that administration of a serotype-specific, nonopsonic IgM was associated with modulation of the lung chemokine, cytokine, and lung inflammatory responses to i.t. infection with serotype 8 pneumococcus. These findings extend the in vitro observation that D11 downregulated IL-8 secretion by human PMNs (10). Control of IL-6 and TNF-α release in immunized mice challenged with another pneumococcal serotype was attributed to the murine inhibitory (FcgammaRIIb) Fc receptor (14). IgMs have also been shown to mediate anti-inflammatory effects (6, 10; reviewed in reference 11), but we could not assess the influence of isotype on our results, because a serotype-specific IgG was not available. The mechanism for D11-dependent downregulation of lung cytokine and chemokine mRNA expression is now under investigation in our laboratory. We wonder if D11 coating of the pneumococcal capsule could subvert the ability of pneumococcal virulence factors to trigger an inflammatory response by inhibiting or altering their usual receptor interactions (39, 48), as described for antibodies to other encapsulated pathogens (59, 66). To our knowledge, our findings are the first demonstration that antibody-mediated immunity is associated with modulation of the inflammatory response to pneumococcal pneumonia. This suggests that there are mechanisms of antibody immunity to pneumococcal pneumonia which depend on protection against pulmonary damage and disease without preventing infection. Validation of this hypothesis could explain the discordance between currently available pneumococcal vaccine efficacy against bacteremic disease and pneumonia, provide new approaches to vaccine design, and unravel the role of antibody in enhancing cellular immunity to the pneumococcus.
Acknowledgments
This work was supported by National Institutes of Health grants R01AI035370, R01AI045459, and R01AI044374. (L.-A.P.) and Ruth L. Kirschstein National Research Award 5F31GM20775 (T.B.).
We thank Jorge Bermudez for preparing the pathological slides and Arturo Casadevall for a critical review of the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Akilesh, S., D. J. Shaffer, and D. Roopenian. 2003. Customized molecular phenotyping by quantitative gene expression and pattern recognition analysis. Genome Res. 13:1719-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Ali, F., M. E. Lee, F. Iannelli, G. Pozzi, T. J. Mitchell, R. C. Read, and D. H. Dockrell. 2003. Streptococcus pneumoniae-associated human macrophage apoptosis after bacterial internalization via complement and Fcgamma receptors correlates with intracellular bacterial load. J. Infect. Dis. 188:1119-1131. [DOI] [PubMed] [Google Scholar]
- 2.Anttila, M., M. Voutilainen, V. Jantti, J. Eskola, and H. Kayhty. 1999. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin. Exp. Immunol. 118:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arva, E., and B. Andersson. 1999. Induction of phagocyte-stimulating and Th1-promoting cytokines by in vitro stimulation of human peripheral blood mononuclear cells with Streptococcus pneumoniae. Scan. J. Immunol. 49:417-423. [DOI] [PubMed] [Google Scholar]
- 4.Austrian, R., R. M. Douglas, G. Schiffman, A. M. Coetzee, H. J. Koornhof, S. Hayden-Smith, and R. D. Reid. 1976. Prevention of pneumococcal pneumonia by vaccination. Trans. Assoc. Am. Physicians 89:184-194. [PubMed] [Google Scholar]
- 5.Bergeron, Y., N. Ouellet, A. M. Deslauriers, M. Simard, M. Olivier, and M. G. Bergeron. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boes, M., A. P. Prodeus, T. Schmidt, M. C. Carroll, and J. Chen. 1999. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 99:16969-16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruunsgaard, H., P. Skinhoj, J. Qvist, and F. K. Pedersen. 1999. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J. Infect. Dis. 180:551-554. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald, U. K., and L. Pirofski. 2003. Immune therapy for infectious diseases at the dawn of the 21st century: the past, present and future of antibody therapy, therapeutic vaccination and biological response modifiers. Curr. Pharm. Des. 9:945-968. [DOI] [PubMed] [Google Scholar]
- 10.Burns, T., Z. Zhong, M. Steinitz, and L. A. Pirofski. 2003. Modulation of polymorphonuclear cell interleukin-8 secretion by human monoclonal antibodies to type 8 pneumococcal capsular polysaccharide. Infect. Immun. 71:6775-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadevall, A., and L. A. Pirofski. 2004. New concepts in antibody-mediated immunity. Infect. Immun. 72:6191-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadevall, A., and M. D. Scharff. 1994. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob. Agents Chemother. 38:1695-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, Q., Z. Zhong, A. Lees, M. Pekna, and L. Pirofski. 2002. Structure-function relationships for human antibodies to pneumococcal capsular polysaccharide from transgenic mice with human immunoglobulin loci. Infect. Immun. 70:4977-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clatworthy, M. R., and K. G. Smith. 2004. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J. Exp. Med. 199:717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dallaire, F., N. Ouellet, Y. Bergeron, V. Turmel, M. C. Gauthier, M. Simard, and M. G. Bergeron. 2001. Microbiological and inflammatory factors associated with the development of pneumococcal pneumonia. J. Infect. Dis. 184:292-300. [DOI] [PubMed] [Google Scholar]
- 16.Dear, K. B., R. R. Andrews, J. Holden, and D. P. Tatham. 2003. Vaccines for preventing pneumococcal infection in adults. Cochrane Database System Reviews 2003. [Online.] doi: 10.1002/14651858. CD004027. CD000422. [DOI] [PubMed]
- 17.Dockrell, D. H., M. Lee, D. H. Lynch, and R. C. Read. 2001. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J. Infect. Dis. 184:713-722. [DOI] [PubMed] [Google Scholar]
- 18.Dockrell, D. H., H. M. Marriott, L. R. Prince, V. C. Ridger, P. G. Ince, P. G. Hellewell, and M. K. Whyte. 2003. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol. 171:5380-5388. [DOI] [PubMed] [Google Scholar]
- 19.Fedson, D. S. 1999. The clinical effectiveness of pneumococcal vaccination: a brief review. Vaccine 17:S85-S90. [DOI] [PubMed] [Google Scholar]
- 20.Fillion, I., N. Ouellet, M. Simard, Y. Bergeron, S. Sato, and M. G. Bergeron. 2001. Role of chemokines and formyl peptides in pneumococcal pneumonia-induced monocyte/macrophage recruitment. J. Immunol. 166:7353-7361. [DOI] [PubMed] [Google Scholar]
- 21.Fine, D. P., J. L. Kirk, G. Schiffman, J. E. Schwinle, and J. C. Guckinan. 1988. Analysis of humoral and phagocytic defenses against Streptococcus pneumoniae serotypes 1 and 3. J. Lab. Clin. Med. 112:487-497. [PubMed] [Google Scholar]
- 22.Fine, M. J., M. A. Smith, C. A. Carson, F. Meffe, S. S. Sankey, L. A. Weissfeld, A. S. Detsky, and W. N. Capoor. 1994. Efficacy of pneumococcal vaccination in adults. A meta-analysis of randomized controlled trials. Arch. Int. Med. 154:2666-2677. [DOI] [PubMed] [Google Scholar]
- 23.French, N., J. Nakinyingi, L. M. Carpenter, E. Lugada, C. Watera, K. Moi, M. Moore, D. Antvelink, D. Mulder, E. N. Janoff, J. Whiworth, and C. F. Gilks. 2000. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 355:2106-2111. [DOI] [PubMed] [Google Scholar]
- 24.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 25.Han, Y., T. Kanbe, R. Cherniak, and J. E. Cutler. 1997. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect. Immun. 65:4100-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirst, R. A., A. Kadioglu, C. O'Callaghan, and P. W. Andrew. 2004. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin. Exp. Immunol. 138:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson, L. A., K. M. Neuzil, O. Yu, P. Benson, W. E. Barlow, A. L. Adams, C. A. Hanson, L. D. Mahoney, D. K. Shay, and W. W. Thompson. 2003. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N. Engl. J. Med. 348:1747-1755. [DOI] [PubMed] [Google Scholar]
- 28.Janoff, E. N., C. Fasching, J. M. Orenstein, J. B. Rubins, N. L. Opstad, and A. P. Dalmasso. 1999. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J. Clin. Investig. 104:1139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, S. E., L. Rubin, S. Romero-Steiner, J. K. Dykes, L. B. Pais, A. Rizvi, E. Ades, and G. M. Carlone. 1999. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J. Infect. Dis. 180:133-140. [DOI] [PubMed] [Google Scholar]
- 30.Kadioglu, A., and P. W. Andrew. 2004. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 25:143-149. [DOI] [PubMed] [Google Scholar]
- 31.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Kagari, T., H. Doi, and T. Shimozato. 2002. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J. Immunol. 169:1459-1466. [DOI] [PubMed] [Google Scholar]
- 32.Kasama, T., R. M. Strieter, N. W. Lukacs, M. D. Burdick, and S. L. Kunkel. 1994. Regulation of neutrophil-derived chemokine expression by IL-10. J. Immunol. 152:3559-3569. [PubMed] [Google Scholar]
- 33.Kemp, K., H. Bruunsgaard, P. Skinhoj, and B. K. Pedersen. 2002. Pneumococcal infections in humans are associated with increased apoptosis and trafficking of type-1 cytokine-producing T cells. Infect. Immun. 70:5019-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerr, A. R., J. J. Irvine, J. J. Search, N. A. Gingles, A. Kadioglu, P. W. Andrew, W. L. McPheat, C. G. Booth, and T. J. Mitchell. 2002. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect. Immun. 70:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knapp, S., L. Hareng, A. W. Rijneveld, P. Bresser, J. S. van der Zee, S. Florquin, T. Hartung, and T. van der Poll. 2004. Activation of neutrophils and inhibition of the proinflammatory cytokine response by endogenous granulocyte colony-stimulating factor in murine pneumococcal pneumonia. J. Infect. Dis. 189:1506-1515. [DOI] [PubMed] [Google Scholar]
- 36.Knapp, S., J. C. Leemans, S. Florquin, J. Branger, N. A. Maris, J. Pater, N. van Rooijen, and T. van der Poll. 2003. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167:171-179. [DOI] [PubMed] [Google Scholar]
- 37.Knoblach, S. M., and A. I. Faden. 2002. Administration of either anti-intercellular adhesion molecule-1 or a nonspecific control antibody improves recovery after traumatic brain injury in the rat. J. Neurotrauma 19:1039-1050. [DOI] [PubMed] [Google Scholar]
- 38.Ling, E., G. Feldman, R. Dagan, and Y. Mizrachi-Nebenzahl. 2003. Cytokine mRNA expression in pneumococcal carriage, pneumonia, and sepsis in young mice. J. Infect. Dis. 188:1752-1756. [DOI] [PubMed] [Google Scholar]
- 39.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 100:1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mangtani, P., F. Cutts, and A. J. Hall. 2003. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infect. Dis. 3:71-78. [DOI] [PubMed] [Google Scholar]
- 41.Mizrachi-Nebenzahl, Y., S. Lifshitz, R. Teitelbaum, S. Novick, A. Levi, D. Benharroch, E. Ling, and R. Dagan. 2003. Differential activation of the immune system by virulent Streptococcus pneumoniae strains determines recovery or death of the host. Clin. Exp. Immunol. 134:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenenesis, immunity, and treatment. Clin. Infect. Dis. 14:801-809. [DOI] [PubMed] [Google Scholar]
- 43.Ortqvist, A., J. Hedlund, L.-A. Burman, E. Elberl, M. Hofer, M. Leinonen, I. Lindblad, B. Sundelof, M. Kalin, and Swedish Pneumococcal Vaccination Study Group. 1998. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet 351:399-403. [DOI] [PubMed] [Google Scholar]
- 44.Perussia, B., M. Kobayashi, M. E. Rossi, I. Anegon, and G. Trinchieri. 1987. Immune interferon enhances functional properties of human granulocytes: role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J. Immunol. 138:765-774. [PubMed] [Google Scholar]
- 45.Petroni, K. C., L. Shen, and P. M. Guyre. 1988. Modulation of human polymorphonuclear leukocyte IgG Fc receptors and Fc receptor-mediated functions by IFN-gamma and glucocorticoids. J. Immunol. 140:3467-3472. [PubMed] [Google Scholar]
- 46.Rijneveld, A. W., F. N. Lauw, M. J. Schultz, S. Florquin, A. A. Te Velde, P. Speelman, S. J. Van Deventer, and T. van der Poll. 2002. The role of interferon-gamma in murine pneumococcal pneumonia. J. Infect. Dis. 185:91-97. [DOI] [PubMed] [Google Scholar]
- 47.Rijneveld, A. W., G. P. van den Dobbelsteen, S. Florquin, T. J. Standiford, P. Speelman, L. Van Alphen, and T. van der Poll. 2002. Roles of interleukin-6 and macrophage inflammatory protein-2 in pneumolysin-induced lung inflammation in mice. J. Infect. Dis. 185:123-126. [DOI] [PubMed] [Google Scholar]
- 48.Rijneveld, A. W., S. Weijer, S. Florquin, P. Speelman, T. Shimizu, S. Ishii, and T. van der Poll. 2004. Improved host defense against pneumococcal pneumonia in platelet-activating factor receptor-deficient mice. J. Infect. Dis. 189:711-716. [DOI] [PubMed] [Google Scholar]
- 49.Robbins, J. B., R. Schneerson, and S. C. Szu. 1995. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 171:1387-1398. [DOI] [PubMed] [Google Scholar]
- 50.Romero-Steiner, S., D. M. Musher, M. S. Cetron, L. B. Pais, J. E. Groover, A. E. Fiore, B. D. Plikaytis, and G. M. Carlone. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 29:281-288. [DOI] [PubMed] [Google Scholar]
- 50a.Rosen, S., and H. J. Skaletsky. 2000. Primer 3 on the WWW for general users and for biological programmers, p. 365-386. In S. Krawetz and S. Misener (ed.), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 51.Rubins, J. B., D. Charboneau, J. C. Paton, T. J. Mitchell, P. W. Andrew, and E. N. Janoff. 1995. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J. Clin. Investig. 95:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubins, J. B., and C. Pomeroy. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect. Immun. 65:2975-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saeland, E., G. Vidarsson, and I. Jonsdottir. 2000. Pneumococcal pneumonia and bacteremia model in mice for the analysis of protective antibodies. Microb. Pathog. 29:81-91. [DOI] [PubMed] [Google Scholar]
- 54.Saeland, E., G. Vidarsson, J. H. Leusen, E. Van Garderen, M. H. Nahm, H. Vile-Weekhout, V. Walraven, A. M. Stemerding, J. S. Verbeek, G. T. Rijkers, W. Kuis, E. A. Sanders, and J. G. Van de Winkel. 2003. Central role of complement in passive protection by human IgG1 and IgG2 anti-pneumococcal antibodies in mice. J. Immunol. 170:6158-6164. [DOI] [PubMed] [Google Scholar]
- 55.Samuelsson, A., T. L. Towers, and J. V. Ravetch. 2001. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291:484-486. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro, E. D., A. T. Berg, R. Austrian, D. Schroeder, V. Parcells, A. Margolis, R. K. Adair, and J. D. Clemens. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1425-1460. [DOI] [PubMed] [Google Scholar]
- 57.Staali, L., M. Morgelin, L. Bjorck, and H. Tapper. 2003. Streptococcus pyogenes expressing M and M-like surface proteins are phagocytosed but survive inside human neutrophils. Cell. Microbiol. 5:253-265. [DOI] [PubMed] [Google Scholar]
- 58.Taborda, C., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 59.Taborda, C. P., and A. Casadevall. 2002. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16:791-802. [DOI] [PubMed] [Google Scholar]
- 60.Teitelbaum, R., R. Glatman-Freedman, B. Chen, J. B. Robbins, E. Unanue, A. Casadevall, and B. R. Bloom. 1998. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA 95:15688-15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turnbull, I. R., P. Javadi, T. G. Buchman, R. S. Hotchkiss, I. E. Karl, and C. M. Coopersmith. 2004. Antibiotics improve survival in sepsis independent of injury severity but do not change mortality in mice with markedly elevated interleukin 6 levels. Shock 21:121-125. [DOI] [PubMed] [Google Scholar]
- 62.van der Poll, T., C. V. Keogh, X. Guirao, W. A. Buurman, M. Kopf, and S. F. Lowry. 1997. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis. 176:439-444. [DOI] [PubMed] [Google Scholar]
- 63.Vidarsson, G., S. T. Sigurdardottir, T. Gudnason, S. Kjartansson, K. G. Kristinsson, G. Ingolfsdottir, S. Jonsson, H. Valdimarsson, G. Schiffman, R. Schneerson, and I. Jonsdottir. 1998. Isotypes and opsonophagocytosis of pneumococcus type 6B antibodies elicited in infants and adults by an experimental pneumococcus type 6B-tetanus toxoid vaccine. Infect. Immun. 66:2866-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner, C., W. Popp, M. Posch, C. Vlasich, and A. Rosenberger-Spitzy. 2003. Impact of pneumococcal vaccination on morbidity and mortality of geriatric patients: a case-controlled study. Gerontology 49:246-250. [DOI] [PubMed] [Google Scholar]
- 65.Wang, E., M. Simard, N. Ouellet, Y. Bergeron, D. Beauchamp, and M. G. Bergeron. 2000. Modulation of cytokines and chemokines, limited pulmonary vascular bed permeability, and prevention of septicemia and death with ceftriaxone and interleukin-10 in pneumococcal pneumonia. J. Infect. Dis. 182:1255-1259. [DOI] [PubMed] [Google Scholar]
- 66.Weiser, J. N., N. Pan, K. L. McGowan, D. Musher, A. Martin, and J. Richards. 1998. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 187:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wessels, M. R., R. Butko, N. Ma, H. B. Warren, A. L. Lage, and M. C. Carroll. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 92:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67a.Wuthrich, M. H., I. Filutowicz, T. Warner, G. S. Deepe, Jr., and B. S. Klein. 2003. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J. Exp. Med. 197:1405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamamoto, N., K. Kawakami, Y. Kinjo, K. Miyagi, T. Kinjo, K. Uezu, C. Nakasone, M. Nakamatsu, and A. Saito. 2004. Essential role for the p40 subunit of interleukin-12 in neutrophil-mediated early host defense against pulmonary infection with Streptococcus pneumoniae: involvement of interferon-gamma. Microbes Infect. 6:1241-1249. [DOI] [PubMed] [Google Scholar]
- 69.Zhong, Z., T. Burns, Q. Chang, M. Carroll, and L. Pirofski. 1999. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect. Immun. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]