Abstract
In recognition of the need for immunological memory-inducing components for future Neisseria meningitidis group B vaccines, we previously searched the proteome of N. meningitidis and identified T-cell-stimulating protein A (TspA). This study was designed to confirm the immunogencity of TspA and to examine the subset of T-helper cell responses to the protein in patients and nasopharyngeal carriers. The tspA gene was reconstructed, cloned, and expressed in Escherichia coli, and the recombinant TspA (rTspA) protein was affinity purified. T-cell proliferative responses to rTspA were detected in the peripheral blood mononuclear cells (PBMCs) of convalescent patients and carriers, confirming that TspA-specific T-cell responses were stimulated by invasive disease and nasopharyngeal colonization. Following stimulation of PBMCs with meningococcal lysate, increased frequencies of both Th1 and Th2 cells were observed, indicating that, as during carriage, invasive meningococcal disease induced an unbiased T-helper subset response. A similar unbiased T-helper response was also detected against rTspA in the PBMCs of convalescent patients. The response of PBMCs from the carriers to TspA stimulation, however, was very weak, and the frequencies of cytokine-positive CD4 cells were not significantly greater than the frequencies in unstimulated control cultures. All of the patients and carriers responded with serum antimeningococcal immunoglobulin G (IgG) antibodies, while four of six samples from patients and 5 of 14 samples from carriers contained detectable anti-rTspA IgG antibodies. Taken together, the results of this study confirmed the immunogenicity of TspA in humans during natural meningococcal infection, and therefore, TspA is worthy of further investigation as a possible T-cell stimulating component of future vaccines.
Neisseria meningitidis is exclusively a human pathogen which colonizes the nasopharynx and may invade underlying tissues, causing a number of severe, often fatal, clinical syndromes. It is a common cause of pyogenic meningitis and causes outbreaks of invasive disease. Meningococcal serogroups A, B, C, and Y are the most common causes of invasive disease throughout the world. Capsular polysaccharide vaccines are available against serogroups A, C, Y, and W135 but not against serogroup B since the structure of the polysaccharide is identical to the structure of a polysialic acid antigen present on neural tissues (13). This homology with self-antigens makes it a poor immunogen, a problem compounded by the risk of inducing autoimmune responses if it were included in a vaccine (25). Since the introduction of serogroup C conjugate vaccine (MenC) in the United Kingdom in 1999, laboratory reports of serogroup disease C have fallen by over 80% in the targeted groups (10), although immunity may be short lived in vaccinated infants (40). By contrast, serogroup B remains the most prevalent cause of meningococcal disease in England and Wales (10), emphasizing the need for a vaccine effective against this serogroup.
Despite extensive efforts no licensed vaccines are available for serogroup B meningococci, which are responsible for 32% of all meningococcal disease in the United States, for 45 to 80% of the cases in Europe, and for the majority of cases in the rest of the world, with the exception of sub-Saharan Africa, where serogroup A is responsible for 90% of the cases (14, 21). The approaches used for development of new meningococcal vaccines have focused on noncapsular immunogenic components, including pili (14), detoxified lipooligosaccharide (30, 41), outer membrane vesicles (5, 6), and purified outer membrane proteins (2, 15, 16, 23, 43). Several outer membrane proteins are known to have the capacity to induce protective immunity against serogroup B meningococcal disease, but major problems have arisen from antigenic variation between strains (25).
Previous clinical trials in Norway, Brazil, and Chile using vaccines based on outer membrane vesicles have failed to provide the required cross-protective immunity in children under 2 years old, the most vulnerable age group (5, 6, 11). The reasons for the disappointing results are not clear; however, it is likely that the vaccine preparations did not contain all the relevant T-cell and B-cell immunogenic antigens and that their presentation to the immune system was not optimal. These vaccine preparations consisted of crude mixtures of proteins, the identities and ratios of which were not known, although they were enriched with some proteins, including PorA and PorB, which are known to be antigenically hypervariable (12). It is now widely accepted that future preparations should consist of highly characterized antigens that are essential for the survival and pathogenesis of the organism. Moreover, greater understanding of the bacterial structure, physiology, and interaction with the host is required before better vaccine strategies are designed.
The most desirable properties for meningococcal vaccine candidates are that the molecules should be expressed in vivo and immunogenic to T cells and B cells and should induce immunological memory (18, 36). Immunity to N. meningitidis infection is believed to correlate with the presence of bactericidal immunoglobulin G (IgG) (42); however, help by CD4+ T cells is required for an efficient humoral immune response generating lytic IgG and memory B cells. CD4+ T cells recognize antigen peptides associated with major histocompatibility complex class II on antigen-presenting cells. Thus, proteins from N. meningitidis may enhance the effectiveness of meningococcal vaccines by acting as more appropriate carriers for the immunogenic capsular polysaccharides or as protective immunogens in their own right.
T-cell stimulating protein A (TspA) was identified previously from a screening analysis of the meningococcal proteome for components which stimulated the proliferation of peripheral blood mononuclear cells (PBMCs) taken from patients who had recovered from invasive meningococcal disease and also from healthy donors (19). The aim of this study was to characterize the TspA-specific T-cell response elicited during carriage and meningococcal disease. Studying the immune responses of patients who recovered from meningococcal disease, since they are usually immunologically protected from further episodes of disease, could reveal the characteristics required for protective immunity. This important information could be utilized for future vaccine design and delivery, using TspA as a T-cell stimulating carrier protein in combination with other protective immunogens.
MATERIALS AND METHODS
Cloning of TspA.
Genomic DNA was isolated from meningococcal cells using the method described by Chen and Kuo (7). Plasmid DNA was isolated using a Qiaprep spin miniprep kit (QIAGEN). Genes were amplified by PCR using genomic DNA of strain SD. tspA was amplified using primers 5′-GGCGGTCGGATCCTTCACATATTAAATG-3′ and 5′-ACGCGTCGACTCAAATACCCAATTCCTGCG-3′. Restriction enzymes and T4 DNA ligase were purchased from Fermentas. The Taq polymerase used in PCR was purchased form Roche Diagnostics Ltd., Lewes, United Kingdom. Restriction endonuclease digestions were carried out according to the manufacturer's instructions or at room temperature overnight to cut sites close to the ends of PCR products. Ligations and PCRs were carried out according to the recommendations of the manufacturers. Expression plasmid pKG107 was constructed by ligating BamHI- and SalI-digested PCR products to the expression vector pQE30 (QIAGEN), which was cut with the same enzymes. The resulting ligation preparations were used to transform Escherichia coli strain M15 harboring plasmid pREP4 (QIAGEN) by electroporation using standard protocols (37). The pQE30-derived constructs drove expression of recombinant proteins with an N-terminal six-histidine tag. The histidine tag facilitated purification of the recombinant protein (see below). Expression of the protein was under control of the E. coli lac promoter, which, in the absence of an inducer, was repressed by the Lac repressor protein encoded by plasmid pREP4. Cloned DNA segments were sequenced at the Protein and Nucleic Acid Chemistry Laboratory (University of Leicester) using an ABI 377 automated DNA sequencer.
Expression of recombinant TspA.
E. coli M15 containing plasmids pKG107 and pREP4 was grown overnight in 10 ml of LB medium containing 50 μg/ml ampicillin and 25 μg/ml kanamycin. One milliliter of the culture was added to 50 ml of fresh LB broth with antibiotics as described above and allowed to grow to an optical density at 600 nm (OD600) of 0.6 before addition of 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After 2 h, cells were harvested, and the recombinant proteins were purified under denaturing or native conditions using a nickel-resin column (Ni-NTA spin kit; QIAGEN) according to the manufacturer's instructions. To provide appropriate negative controls for experiments, eluates from an equivalent culture of E. coli transformed with pREP4 and pQE30 (with no meningococcal insert) were used. In order to obtain protein of higher purity for immunological assays, recombinant TspA (rTspA) was also separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and eluted from the gel as previously described (1).
Bioinformatic analysis.
Public databases containing previously published protein and DNA sequences were searched using the BLAST and PSI-BLAST programs available at http://www.ncbi.nlm.nih.gov/BLAST/index.html. The genome database of strain MC58 was interrogated at http://www.tigr.org/tdb/CMR/gnm/htmls/SeqSearch.html, while the strain Z2491 sequence and partial data for the group C FAM18 strain were obtained from http://www.sanger.ac.uk/Projects/N_meningitidis/blast_server.shtml. Sequence homology data were obtained using the CLUSTAL X software (http://www-igbmc.u-strasbg.fr/BioInfo/ClustalX/). The Protein Families database of alignments and hidden Markav models (www.sanger.ac.uk/Software/Pfam/) were used to view protein domain architectures. Other DNA and protein sequence analyses were carried out using the DNAMAN package of programs (Lynnon BioSoft).
Subjects for immunological study.
Thirty-one first-year medical students were recruited at the start of the academic year, and a blood sample and a posterior pharyngeal swab were taken from each student. Eight weeks later the students were swabbed again, and additional samples of blood were collected. In week 23, students volunteered to provide final swabs and to donate 50 ml of blood. A total of 26 students provided samples at all three times, and 14 of these students were confirmed to be carriers of N. meningitidis by culture of their pharyngeal swabs (Table 1). Seven patients with invasive meningococcal disease kindly donated blood samples at the acute and postconvalescent phases of infection (Table 2).
TABLE 1.
Confirmed meningococcal carriers from whom blood samples were obtained
| Carriera | Sexb | Isolates recovered
|
||
|---|---|---|---|---|
| Week 0 | Week 8 | Week 23 | ||
| 1 | F | —c | — | B:NT:P1.13 |
| 2 | F | — | — | B:4:P1.4 |
| 3 | F | — | — | B:4:P1.4 |
| 4 | F | — | — | NG:4:NT |
| 5 | F | — | — | NG:1:P1.14 |
| 6 | F | — | — | B:NT:1.9 |
| 7 | F | — | B:21:P1.14 | B:21:P1.14 |
| 8 | F | B:14:P1.14 | B:14:P1.14 | B:14:P1.14 |
| 9 | F | 29E:14:P1.16 | 29E:14:P1.16 | 29E:14:P1.16 |
| 10 | F | B:4:P1.4 | NG:4:P1.4 | B:4:P1.4 |
| 11 | F | — | B:NT:P1.9 | — |
| 12 | M | NG:14:NT | NG:14:NT | — |
| 13 | F | NG:NT:P1.1 | — | — |
| 14 | F | W135:NT:NT | — | — |
Sera and PBMCs were obtained from carriers 1 to 8, and sera (no PBMCs) were obtained from carriers 9 to 14.
F, female; M, male.
—, No meningococcal isolate was recovered.
TABLE 2.
Convalescent patients from whom blood samples were obtained
| Patient | Isolate | Age (yr) | Sexa | Clinical syndrome |
|---|---|---|---|---|
| 1 | Cb | 16 | F | Meningitis |
| 2 | B:4:P1.4 | 18 | M | Meningitis |
| 3 | Bb | 18 | M | Meningitis |
| 4 | B:4:P1.4 | 20 | M | Meningitis |
| 5 | B:NT:NT | 19 | M | Meningitis |
| 6 | C:2a:NT | 15 | M | Septicemia |
| 7 | B:NT:P1.4 | 20 | M | Meningitis |
F, female; M, male.
Diagnosed by PCR. Serotype and subtype data were not available.
Isolation of meningococci from pharyngeal swabs.
As described previously (34), pharyngeal swabs were directly plated onto selective VCNT agar plates (selective supplement SR91) (Oxoid Ltd., Basingstoke, United Kingdom) at the time of sampling. The plates were incubated at 37°C with 5% CO2 for 24 to 48 h. Isolates that produced colonies morphologically similar to N. meningitidis colonies were subcultured and Gram stained. Gram-negative diplococci were tested further using a Gonochek kit (EY Laboratories Inc., San Mateo, CA), and the bacteria thought to be N. meningitidis were serologically characterized at the HPA Meningococcal Reference Unit, Manchester, United Kingdom.
Clinical materials.
Blood samples (50 ml) were collected in 10-ml Vacutainer tubes containing EDTA anticoagulant. Samples of serum were collected, frozen, and stored at −20°C. PBMCs were purified by density gradient centrifugation using Histopaque 1077 (Sigma-Aldrich Company Ltd., Poole, United Kingdom). Washed PBMCs were resuspended in fetal calf serum (FCS) (Sigma) containing 10% dimethyl sulfoxide, split into three aliquots, and frozen at −80°C overnight before they were stored in liquid nitrogen vapor.
Meningococcal antigen preparation.
Meningococcal antigens were prepared as described previously (34). Briefly, overnight cultures of the H44/76 meningococcal strain in Mueller-Hinton broth (Oxoid Ltd.) were centrifuged at 1,600 × g for 15 min. The bacteria were harvested, washed, and resuspended in sterile phosphate-buffered saline (PBS) before heat inactivation at 56°C for 30 min. OD600 of the suspensions were determined. An OD600 of 0.30 was determined to be the optimum coating concentration for an enzyme-linked immunosorbent assay (ELISA).
Recombinant TspA and a control antigen preparation were purified from equivalent cultures of E. coli strains M15(pKG107, pREP4) and M15(pQE30, pREP4), respectively, by elution from SDS-PAGE gels as described previously (16).
Cell proliferation assays.
Cell proliferation assays were performed as described previously (19, 34). Briefly, frozen aliquots of PBMCs were resuscitated, washed, and resuspended at a concentration of 1 × 106 cells per ml in complete medium (RPMI 1640 supplemented with 10% human AB serum, 10 mM HEPES, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin [Sigma]). Aliquots (200 μl) of cells were placed into the wells of a sterile 96-well flat-bottom plate in quadruplicate together with 20 μl of antigen, mitogen, or medium. Final concentrations of the H44/76 meningococcal lysate ranging from 1 to 10 μg protein/ml, 0.1 to 5 μg/ml rTspA, and the equivalent dilutions of TspA control antigen were used in the assays, together with 0.5 to 5 flocculating units/ml tetanus toxoid (NIBSC) as a positive control antigen and phytohemagglutinin (PHA) (Sigma) at a concentration of 10 μg/ml as a mitogen-positive control.
The cells were incubated at 37°C in 5% CO2 for 7 days. For the final 18 h of incubation, 1 μCi of [3H]thymidine (Amersham Pharmacia Biotech, United Kingdom) was added to each well. The cells were harvested, and proliferation was detected by measurement of cellular [3H]thymidine incorporation. Stimulation indices (SIs) were calculated by determining the ratio of the mean counts per minute from stimulated cells to the mean counts per minute from unstimulated cells. Pilot experiments showed that fresh and frozen PBMCs gave reproducible results in these assays.
Assay of cytokine responses by intracellular staining and flow cytometry.
Using a previously described method (34), 5 × 105 PBMCs in 1 ml of complete medium were aliquoted into sterile culture test tubes (12 by 75 mm; Elkay Laboratory Products Ltd., Basingstoke, United Kingdom). Anti-CD28 antibody (Beckman Coulter, High Wycombe, United Kingdom) was added to each tube to a concentration of 1 μg/ml. The lysate from meningococcal strain H44/76 was added to a final concentration of 20 μg/ml. As a positive control, phorbol myristate acetate (PMA) and ionomycin were added to final concentrations of 20 ng/ml and 1 μM, respectively. Negative control tubes received anti-CD28 but no antigen or mitogen. Anti-CD28 antibody is commonly used to improve the sensitivity of ex vivo assays of T-cell cytokine expression, but there is no evidence from other studies that it causes skewing of the response profile (22).
The cultures were incubated for 2 h at 37°C in 5% CO2 before brefeldin A (Sigma) was added at a concentration of 10 μg/ml, and then the cultures were placed back in the incubator for a further 8 h. The positive control tubes containing PMA and ionomycin were incubated for only 4 h after brefeldin A was added.
The cells were pelleted by centrifugation at 200 × g for 5 min before incubation on ice for 30 min while they were stained with anti-CD4-phycoerythrin (PE)-Texas red x (ECD), anti-CD8-ECD, or anti-CD56-PE antibody conjugate (Beckman Coulter). The cells were washed three times with 1 ml PBA buffer (PBS containing 0.1% bovine serum albumin [BSA] and 0.1% sodium azide) supplemented with 2% FCS before they were fixed in 1 ml 0.5% formaldehyde in borate-buffered saline at 4°C overnight. The cells were then washed with 1 ml PBA buffer, permeabilized with 1 ml PBA buffer containing 0.1% saponin (Sigma), and washed with 1 ml PBA buffer-0.1% saponin-10% FCS before they were stained with anti-CD69-PC5, anti-gamma interferon (IFN-γ)-fluorescein isothiocyanate (Beckman Coulter), and anti-interleukin-5 (IL-5)-PE (Pharmingen, San Diego, CA) antibody conjugates at 4°C for at least 2 h. The cells were washed three times with PBA buffer-0.1% saponin before they were fixed in 0.5% formaldehyde. The data for fluorescently labeled cells were acquired using a Coulter EPICS Altra flow cytometer and were analyzed using WinMDI, version 2.8 (http://facs.scripps.edu/).
Serum antibody ELISA.
Serum antibody was detected by an ELISA as described previously (34). Briefly, 96-well ELISA plates (NUNC Maxisorp; Life Technologies, Paisley, Scotland) were coated overnight at 4°C with 50 μl/well of meningococcal strain whole-cell lysate (diluted to an optical density of 0.30), 5 μg/ml rTspA, or the equivalent dilution of TspA control antigen in 0.05 M carbonate/bicarbonate buffer (pH 9.6). So that a standard curve could be included, a portion of each plate was also coated with an optimal concentration of monoclonal anti-human IgG Fab specific antibody (Sigma). Plates were washed with PBS-0.05% Tween 20 (PBS-Tween) before they were blocked for 1 h at room temperature with 100 μl/well of 3% BSA in PBS-Tween. Sera were diluted in PBS-Tween before 50 μl/well was applied to the washed plates in duplicate. The plates were incubated at room temperature for 90 min before washing and addition of 50 μl/well of alkaline phosphatase-conjugated anti-human IgG diluted in PBS-Tween. After a further 90 min of incubation and rigorous washing, 100 μl/well of the substrate solution was added. This solution was composed of Sigma 104 phosphatase tablets dissolved in diethanolamine buffer (pH 9.8) (Sigma). The plates were incubated for 30 min, the OD405 was determined using an Emax Precision microplate reader (Molecular Devices), and standard curves were plotted. The limit of sensitivity of each assay plate was calculated by using the mean OD405 plus three times the standard deviation for six control wells that received no primary serum. The relative concentration of specific antibody in each sample was calculated by direct reference to the standard curve. ELISA plates coated with the control antigen preparation produced negligible signals compared to plates coated with the equivalent dilution of TspA.
SDS-PAGE and immunoblotting.
rTspA was dissolved in sample buffer (50 mM Tris-Cl, pH 6.8, 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, 10% glycerol) and incubated at 100°C for 5 min. Aliquots (10 μl) were loaded into the wells of 10% polyacrylamide minigels (Mini-Protean II; Bio-Rad), and proteins were electrophoretically separated as previously described (19). Proteins were transferred to nitrocellulose membranes (Schleicher & Schuell) using a semidry blotting system (Bio-Rad) and a buffer containing 39 mM glycine, 48 mM Tris base, 0.037% SDS, and 20% methanol.
Membranes were blocked with 3% BSA (fraction V; Sigma) in PBS-Tween for 90 min at room temperature before incubation overnight with serum from patients and carriers at a dilution of 1:100 in the blocking solution. As a positive control, polyclonal anti-TspA rabbit serum was used at a dilution of 1:500, and preimmune rabbit serum was used as the negative control. After washing with PBS-Tween, blots were incubated for 90 min with anti-human IgG- and anti-rabbit IgG-horseradish peroxidase conjugates (Bio-Rad) diluted in blocking solution as appropriate. After washing with PBS-Tween, the blots were developed using 1-chloro-4-naphthol as a chromogenic substrate solution (Sigma).
Statistical analysis.
Statistical tests of paired sets of data were carried out using the Wilcoxon signed-rank test. For unpaired data, the Mann-Whitney U test was used. Spearman's rank correlation coefficient was calculated to detect significant correlations in the data. In all cases, a significant difference or correlation was defined at a P value of ≤0.05.
RESULTS
Identification and reconstruction of TspA.
In the previously published search for T-cell immunogenic outer membrane vaccine candidates, the proteome of N. meningitidis strain SD was fractionated and screened using CD4+ T helper (Th) cells derived from patients and healthy donors. Potent T-cell stimulating antigens were identified, and their corresponding genes were detected in a genomic expression library using polyclonal rabbit antibodies raised against an oligomixture of partially purified proteins (19). One of the most prominent T-cell stimulating proteins identified in this search was designated TspA. Repeated screening of the genomic library led to detection of at least a dozen overlapping recombinant DNA fragments which contained only the terminal two-thirds of the gene, starting from a Sau3AI restriction site cut during library construction. No clones containing upstream DNA fragments were obtained despite repeated attempts. Other attempts to design minilibraries based on partially digested and purified genomic DNA, containing the tspA gene, also failed to yield any clones containing the upstream fragment of tspA. This failure was attributed to toxicity of the latter fragment to the E. coli host cells.
The partial recombinant tspA gene was sequenced and analyzed; reconstruction of the complete gene became possible after the subsequent release of the genome sequence data for meningococcal strains MC58 and Z2491 (27, 29). The full gene was subsequently amplified from strains SD and Z2491, and a series of attempts were made to clone the DNA into different plasmid vectors, including pUC18, pBluescript, and pQE30. These attempts, as well as attempts to clone the promoter region or the 5′ 0.5-kb region of the gene, were all unsuccessful, supporting our previous hypothesis that the upstream one-third of the molecule was toxic to the host E. coli cells. Cloning of the complete gene was achieved using the repressible expression vector pQE30 in the presence of the lacI suppressor gene carried on the multicopy plasmid pREP4. The cloned fragment was then fully sequenced for further analysis.
Molecular features of TspA.
Sequence analysis of tspA (EMBL accession number AJ010113) revealed that the 2,625-bp gene encodes a protein consisting of 875 amino acids (aa) with a predicted molecular mass of 92,443 Da, a pI of 4.13, and a net charge at pH 7.0 of −85.99. In the published genome sequence of strain MC58 (serogroup B; GenBank accession number AAF40784 [39]) the gene was flanked by the lactoylglutathione lyase gene (gloA; accession number AAF40783.1 [20]) and a homolog of the intracellular septation protein gene (ispA; accession number AAF40785.1). Both flanking genes are in the opposite orientation with respect to tspA, and a promoter is predicted upstream of tspA, indicating that it is monocistronic. A similar arrangement of genes occurs in the genomes of the serogroup A strain Z2491 and the serogroup C strain FAM18 (both available from the Sanger Centre).
Bioinformatic analysis of the TspA sequence (strain SD) predicted a 58-aa N-terminal signal peptide and a 22-aa hydrophobic transmembrane region between aa 372 and 394. The latter sequence separates a highly basic N-terminal domain from a strongly acidic C-terminal domain. Five 19-aa repeats (ADDLSALLQPXXXPXVEEN) occur between aa 635 and 766. The significance of these repeats, which are separated by 5 or 14 residues, is not known, although they may be associated with protein-protein interactions and/or dimerization (3, 8). A strong match was found in the Pfam database at aa 202 to 253 with the LysM motif (PF01476), which is present in a number of peptidoglycan hydrolases and is thought to form a peptidoglycan-binding domain (4, 38). A tricopeptide repeat region domain (PF00515) was also predicted toward the C terminus. Such motifs have been implicated in protein-protein interactions in many proteins (8). No runs of oligonucleotide repeats (often indicative of phase variation in Neisseria spp.) were present in the gene or its promoter region. Secondary structure predictions indicated that there is a lack of beta sheet regions, which are often found in integral outer membrane proteins. The only cystine residues are within the predicted signal peptide, indicating that the mature protein is unable to form disulfide bonds.
Cloning, expression, and purification of TspA.
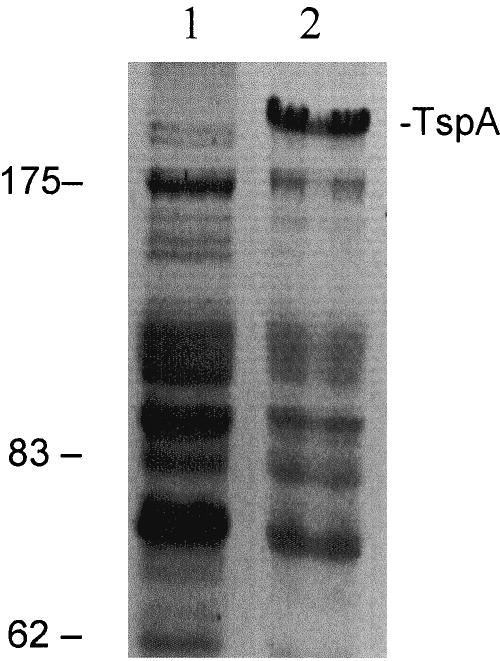
Full-length recombinant TspA, with a six-histidine tag at the N terminus, was expressed in E. coli and affinity purified. The apparent molecular mass of rTspA, based on its mobility in reducing SDS-PAGE gels, was approximately double the molecular mass predicted from its sequence (Fig. 1). Treatment of the rTspA preparations with alternative denaturing reagents, including 6 M urea and 8 M guanidine hydrochloride, did not affect the mobility of rTspA in SDS-PAGE gels (not shown). The possibility that the protein is capable of dimerizing to form the high-molecular-weight form observed on SDS-PAGE gels was considered, especially in light of the tricopeptide repeat region and five 19-aa repeat regions. Such motifs are found in a wide range of proteins and are implicated in protein-protein interactions and assembly of multiprotein complexes (3, 8). It would be surprising, however, if such a complex were stable enough to resist the denaturing conditions described above. The reason for the aberrant mobility on SDS-PAGE gels, therefore, remains unknown.
FIG. 1.
Gel electrophoresis protein profiles following expression of recombinant TspA: SDS-PAGE gel showing protein profiles of the control E. coli expression strain lysate (lane 1) and E. coli containing the TspA expression vector following induction to express TspA (lane 2). The positions of TspA and molecular weight markers (in kDa) are indicated on the right and left, respectively.
TspA-specific T-cell responses induced by natural infections with N. meningitidis.
It was important to characterize the TspA-specific human immune response elicited by meningococcal carriage and invasive disease. PBMCs collected at week 23 from eight confirmed carriers (carriers 1 to 8 [Table 1]) and from seven convalescent patients (Table 2) were tested for their proliferative responses to a range of concentrations of a whole-cell lysate of the H44/76 meningococcal strain (B:15:P1.7,16 belonging to the ET-5 complex), purified rTspA, tetanus toxoid (a positive control antigen), and PHA (a positive control mitogen). An SI of more than 2.0 was taken to indicate that proliferation had occurred (33).
Following stimulation with the meningococcal whole-cell lysate, PBMCs from all of the patients (median SI, 3.23) and all of the carriers (median SI, 3.67) responded with SIs of more than 2.0. PBMCs from seven of eight of the carriers responded to stimulation with rTspA (median SI, 3.57), and cells from six of seven patients also responded (median SI, 3.73) (Fig. 2). None of the cell samples responded to incubation with a negative control antigen (median SI for patients, 0.78; median SI for carriers, 1.09), which was prepared in manner equivalent to the manner used for rTspA but from the E. coli expression strain transformed with the vector lacking a tspA gene insert. The responses of PBMCs from both the carriers and patients to this control antigen were significantly lower than the responses obtained with rTspA (P < 0.01). There was a significant correlation in the responses of PBMCs from patients to H44/76 meningococcal lysate and rTspA (r = 0.905; P = 0.005), but there was no such correlation in the responses to H44/76 and tetanus toxoid. No correlations were found in the meningococcal antigen-specific responses of PBMCs from the carriers.
FIG. 2.
Proliferative responses of PBMCs to optimum stimulatory concentrations of a heat-killed whole-cell lysate of the H44/76 meningococcal strain, recombinant TspA, a negative control TspA preparation, or tetanus toxoid. Replicate wells of PBMCs were incubated with 1 to 10 μg/ml of the H44/76 strain lysate, 0.5 to 5 LfU/ml of tetanus toxoid (TT), 0.1 to 5 μg/ml of TspA, and equivalent volumes of a negative control preparation from the E. coli host strain used for TspA expression. PHA (10 μg/ml) was added as a positive control, while other wells received no antigen or mitogen. Cells were cultured for 7 days, and [3H]thymidine was added for the final 18 h. SIs were calculated by determining the ratio of mean counts per minute from antigen-stimulated cells to mean counts per minute from unstimulated cells. The data show the SIs obtained from the PBMCs of individual convalescent patients (a) and confirmed carriers (b). The bars indicate the geometric mean SIs for the groups, while the error bars indicate the standard deviations. The stimulation indices obtained from induction with PMA were greater than 139.2.
High SIs were obtained following positive control mitogenic PHA stimulation of all of the samples of PBMCs, indicating that there were highly proliferative responses and that cellular viability was good. The median SIs of cells from the carriers and patients were 579.5 and 816.1, respectively. The median SIs resulting from tetanus toxoid antigenic stimulation of PBMCs from the carriers and patients were 4.62 and 6.39, respectively, indicating that there were recall T-cell proliferative responses to a control antigen. The range of SIs obtained with tetanus toxoid was extremely wide (0.99 to 41.9), but six of eight carriers and all seven patients responded with proliferation.
TspA-specific T-helper subset responses induced by natural infections with N. meningitidis.
In order to investigate the T-helper subset response induced by meningococcal carriage and disease, the PBMCs from patients and carriers tested in proliferation assays (see above) were incubated with a lysate of the H44/76 meningococcal strain or rTspA in the presence of anti-CD28 monoclonal antibody for the optimal time (10 h), as described previously (34). Brefeldin A was added after the first 2 h in order to block cytokine secretion. The cells were stained for the surface marker CD4 and then fixed and permeabilized in order to detect the intracellular cytokines IFN-γ and IL-5 and also the activation marker CD69.
Compared with unstimulated controls, significant increases in the percentage of CD69-positive events were detected in all cultures stimulated with the mitogens PMA and ionomycin (for patients, P = 0.01; for carriers, P = 0.005) or with the meningococcal lysate (for patients, P = 0.01; for carriers, P = 0.005). Similar increases were obtained when PBMCs from the seven convalescent patients were stimulated with rTspA (P = 0.01). However, only small, nonsignificant increases in CD69+ events were observed in six of eight of the PBMC cultures from carriers. The unstimulated cells from patients exhibited higher percentages of CD69+ events than the cells collected from carriers exhibited (median percentage for patients, 35.56%; median percentage for carriers, 19.66%; P = 0.014), indicating that more of the mononuclear cells were already activated in the peripheral blood of infected patients (data not shown).
Stimulation with PMA and ionomycin reduces cellular CD4 expression (28), and therefore it was not possible to distinguish a CD4-positive population following immunostaining. Following PMA stimulation of PBMCs from patients, however, the median frequency of CD69+ IFN-γ+ events was significantly increased by 34-fold, from 2,097 events per 106 unstimulated cells to 71,200 events per 106 unstimulated cells (P = 0.01). The median frequency of CD69+ IL-5+ events also increased by threefold, from 894 to 2,798 events per 106 cells, confirming that the cells were capable of producing a cytokine response to mitogenic stimulation. Similar results were obtained with PMA-stimulated PBMCs from carriers (not shown).
T-helper subset responses of patients.
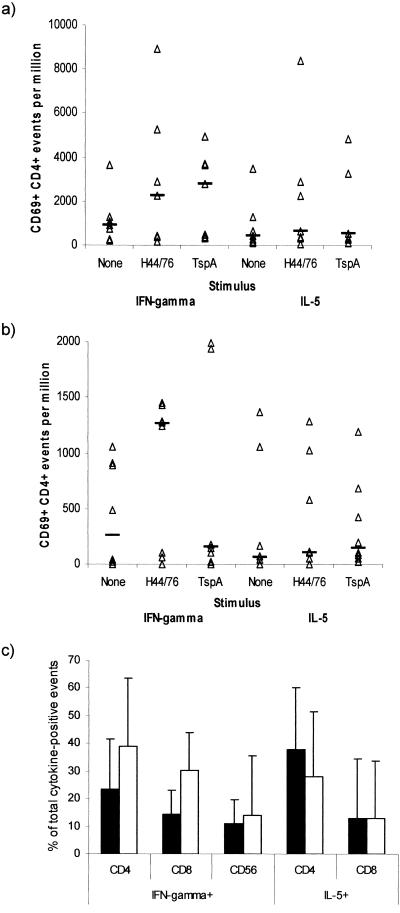
The unstimulated PBMCs of patients had a median of 912 CD69+ CD4+ IFN-γ+ events per 106 cells (Fig. 3a), and this was significantly increased by 2.5-fold to 2,275 events per 106 cells when preparations were stimulated with meningococcal lysate (P = 0.043) or by 3-fold to 2,820 events per 106 cells when preparations were stimulated with rTspA (P = 0.023). The unstimulated PBMCs also had a median of 428 CD69+ CD4+ IL-5+ events per 106 cells, which increased to 644 events per 106 cells (1.5-fold) and 521 events per 106 cells (1.2-fold) following stimulation with meningococcal lysate and TspA, respectively (the differences were not significant). Although only the frequency of IFN-γ+ events was significantly increased following stimulation with meningococcal antigens, there was no significant bias in the number of events positive for IFN-γ or IL-5. The mean ratios of the frequencies of IFN-γ+ events to the frequencies of IL-5+ events among CD69+ CD4+ cells in unstimulated cultures, cells incubated with meningococcal lysate, and cells incubated with TspA were 1.43, 1.00, and 1.17, respectively, and a positive correlation of IFN-γ and IL-5 events was detected with all treatments (unstimulated rs, 0.966 [P < 0.005]; TspA rs, 0.751 [P < 0.05]; meningococcal lysate rs, 0.838 [P < 0.02]). These data indicated that meningococcus- and TspA-specific Th1 and Th2 cells were present in the peripheral blood following infection.
FIG. 3.
Frequencies of activated CD4 cells staining positive for IFN-γ or IL-5 following incubation of PBMCs from patients (a) and carriers (b) for 10 h with a lysate of the H44/76 meningococcal strain or recombinant TspA. The data points indicate the individual frequencies of cytokine-positive CD69+ CD4+ events. The lines indicate the median frequencies for groups. (c) Proportion of IFN-γ- or IL-5-producing cells which expressed CD4, CD8, or CD56 for PBMCs from patients. The solid bars indicate the geometric mean response to the meningococcal lysate, and the open bars indicate the response to rTspA; the error bars indicate the standard deviations.
In order to assess the relative magnitude of the T-cell response, the cell types responsible for producing IFN-γ and IL-5 in response to antigenic stimulation were also investigated. These cells included CD4-, CD8-, and CD56-positive cells. In the PBMC samples from patients, the proportion of IFN-γ-producing cells that were CD4+ was significantly greater than the proportion of IFN-γ-producing cells expressing CD56 (P = 0.03), and the median percentage of CD4+ events was 30.4%, compared with 20.5% CD8+ events and 14.2% CD56+ events (Fig. 3c); 37.2% of IL-5+ cells were CD4+, and 16.2% (median) of IL-5+ cells were CD8+. A similar result was obtained when the cells were stimulated with rTspA (Fig. 3c); in this analysis a significantly higher percentage of CD4+ cells than of CD56+ cells was detected among IFN-γ-producing cells (P < 0.01) using paired statistical tests. The median percentage of IFN-γ+ events that were CD4+ was 42.8%, compared with 35.5% CD8+ events and 38.4% CD56+ events; 43.9% of IL-5+ cells were CD4+,and 18.2% (median) of IL-5+ cells were CD8+.
T-helper subset responses of carriers.
The antigen-stimulated cytokine response of the cells from the carriers was much weaker than the response of the cells from convalescent patients. The unstimulated PBMCs of carriers had a median of only 262 CD69+ CD4+ IFN-γ+ events per 106 cells (Fig. 3b), but the value was significantly increased by 4.8-fold to 1,260 events per 106 cells when preparations were stimulated with meningococcal lysate (P = 0.013). Incubation with rTspA did not induce a significant IFN-γ response (median, 158 events per 106 cells). The unstimulated PBMCs had a median of 59 CD69+ CD4+ IL-5+ events per 106 cells, which increased to 109 events per 106 cells (1.8-fold) and 148 events per 106 cells (2.5-fold) following stimulation with meningococcal lysate and rTspA, respectively, but the differences were also not significant.
Meningococcal TspA induces antibodies in patients and carriers.
In order to determine whether carriers, whose T cells did not respond well to TspA stimulation in vitro, did indeed produce an immune response to TspA, sera were tested by ELISA and immunoblotting for levels of antibody with specificity to rTspA and also for the H44/76 meningococcal strain (B:15:P1.7,16), which belongs to the ET-5 complex. Serum samples from six convalescent patients were also tested for comparison.
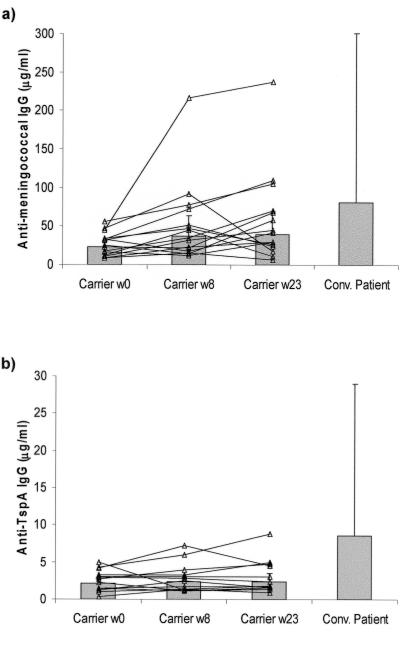
Sera from 14 carriers who provided blood samples at all of the times during the 23-week carriage study and from seven convalescent patients were assayed. The levels of antimeningococcal IgG increased twofold during the study, from 27 μg/ml at time zero (the point of entry into the university) to 52 μg/ml at week 8 (P < 0.02) and 59 μg/ml at week 23. An increased serum IgG concentration was detected in all individuals during the study, but in some individuals (carriers 7, 8, 9, and 11 [Table 1]) the increase occurred around week 8 and the responses declined by week 23 (Fig. 4a). The concentrations of antimeningococcal IgG in serum samples from the convalescent phase of infection were higher (median, 107.5 μg/ml) than those detected in serum from the carriers, but the differences were not significant.
FIG. 4.
Levels of antimeningococcal (a) and anti-TspA (b) IgG in serum samples from 14 carriers and six patients recovering from meningococcal disease (Conv. Patient). The bars indicate the geometric mean concentrations; the error bars indicate the standard deviations.
The levels of anti-TspA IgG in serum samples from the carriers were detectable but very low (medians in week 23, 1.75 μg/ml anti-TspA IgG and 30.0 μg/ml antimeningococcal IgG) and did not increase during the study (Fig. 4b). In comparison, anti-TspA IgG was found at significantly higher concentrations in serum samples from the convalescent patients (median, 6.75 μg/ml; P < 0.05).
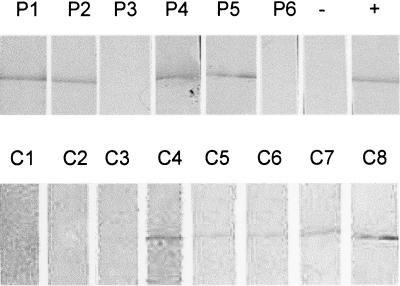
Immunoblots of rTspA incubated overnight with 1/100 dilutions of serum from six of the patients revealed that a signal could be detected only when the ELISA concentration was greater than or equal to 4.5 μg/ml specific IgG (Fig. 5 shows representative samples). From these experiments it was possible to confirm the ELISA results and to determine a limit of serum positivity. Four of the six patients (66.7%), but only 5 of the 14 carriers (35.7%), had serum antibodies against rTspA.
FIG. 5.
Detection of TspA-specific antibody in serum from convalescent patients (P1 to P6) and confirmed meningococcal carriers (C1 to C8). Recombinant TspA was loaded onto a 10% SDS-PAGE gel before electrophoretic transfer to nitrocellulose. The membrane was cut into strips and probed in immunoblotting experiments with human sera, followed by anti-human IgG-alkaline phosphatase conjugate. Positive controls (+) were probed with rabbit anti-TspA serum and an anti-rabbit IgG conjugate. Negative controls (−) were incubated only with the anti-human IgG conjugate antibody.
DISCUSSION
Currently, there is a great need for a vaccine that can provide long-lasting protection in all age groups against serogroup B N. meningitidis, which remains the most prevalent cause of meningococcal disease in many countries, including England and Wales, where the conjugated serogroup C vaccine was first implemented (32). In recognition of the need for T-cell, as well as B-cell, memory-inducing components for future group B vaccines, we previously searched the proteome of N. meningitidis and identified TspA as a potent T-cell stimulating protein (19). Here, we describe detailed immunological investigations and provide evidence for the T-cell and B-cell immunogenicity of TspA following natural infections.
In order to confirm the T-cell stimulatory characteristics of TspA after invasive disease or natural colonization, PBMCs from seven patients and eight carriers were stimulated with purified rTspA in vitro. Ideally, the responses of such subjects should be compared with those of unexposed negative controls. Unfortunately, since almost all individuals are colonized by meningococci or commensal Neisseria at some point during their lives, it is extremely difficult to identify suitable subjects and obtain samples. In lieu of these controls, the assays were carried out by comparing unstimulated and antigen-stimulated PBMCs. Positive responses were obtained from all of the test samples when they were incubated with a whole meningococcal cell lysate, confirming that natural meningococcal infection (carriage and disease) elicited a T-cell-mediated immune response. T-cell proliferative responses to rTspA were detected in the majority of PBMC samples from convalescent patients (six of seven patients) and carriers (seven of eight carriers), confirming that TspA-specific T-cell responses were stimulated by invasive disease and nasopharyngeal colonization. TspA-specific T-cell responses could not be detected for some individuals; this could have been due to a frequency of circulating memory T-cell clones that was below the detection threshold rather than a complete lack of responsiveness.
Cellular immunity to meningococcal disease has been poorly studied, possibly because protection is thought to be due to the presence of specific bactericidal antibodies (42). T cells and the cytokines that they secrete, however, play a pivotal role in regulating the immunoglobulin isotype response and inducing immunological memory (35). Our previous work on the cellular response to meningococcal infection focused upon carriage, and we determined that an unbiased T-helper subset response was elicited (34). In the present study we were able to confirm our previous results and to investigate the cellular response induced by invasive disease. Very few published studies on the acquired cellular response to meningococcal disease can be found. One such study, a study performed by Pollard et al. (31), showed that a T-helper 2 (Th2) subset response (defined by a high ratio of IL-10 concentration to IFN-γ concentration) was obtained upon restimulation of PBMCs from convalescent children. IL-10 is known to be produced by several cell types as the result of innate or acquired immunity (24), and IFN-γ can also be produced by B cells, CD8 T cells, or NK cells (26); therefore, measuring the concentrations of these factors in supernatants may not provide a good indication of the T-helper response. In this study, by using flow cytometry, it was possible to more accurately investigate T-helper subsets by determining the frequency of IFN-γ+ CD4+ Th1 and IL-5+ CD4+ Th2 cells. Following stimulation with meningococcal lysate, increased frequencies of both Th1 and Th2 cells were observed, indicating that, as during carriage, invasive meningococcal disease induced an unbiased T-helper subset response.
The T-helper responses of PBMCs from patients and carriers to antigenic stimulation with rTspA were also investigated, as this may aid future vaccine design to generate the appropriate response phenotype. An unbiased T-helper response, similar to responses obtained with meningococcal stimulation, was detected in the PBMCs of convalescent patients, and there was a significant correlation in the frequencies of IFN-γ- and IL-5-positive CD69+ CD4 cells. In response to rTspA, the majority of cytokine-producing cells were CD4 or CD8 T cells, and there was a significant predominance of IFN-γ+ CD4 T cells over CD56+ NK cells. This demonstrated that TspA is indeed a potent T-cell stimulating antigen. The response of PBMCs from the carriers to TspA stimulation was very weak compared to the response of PBMCs from the patients, and the frequencies of cytokine-positive CD4 cells were not significantly greater than those observed for unstimulated control cultures. This lower-level response may be explained in terms of the manner of exposure to meningococcal antigens and immune response priming. The low-level cytokine response to TspA might be due to the flow cytometry assay detecting only activated or effector memory T cells that can be induced to produce cytokines within a few hours, whereas the proliferation assay detects central memory T cells that are activated from a resting state over a period of days. During carriage, the interaction of immune cells and bacteria is probably limited to the nasopharyngeal mucosa, which makes detection (with insensitive methods) at the cellular level extremely difficult. Bacteria would provoke a much weaker mucosal immune response than systemic invasive disease, which involves much greater numbers of inflammatory and immune cells. These possibilities are supported by the detection of low levels of TspA-specific IgG antibodies in some of the carriers when the more sensitive detection methods were used. It should also be expected that a whole bacterial lysate (containing a large number of immunostimulatory molecules) is a more potent stimulator of cytokines than rTspA, a single T-cell stimulating antigen.
Furthermore, the molecular features of TspA suggest that it is a large protein which is likely to be in contact with the inner membrane (suggested by the predicted transmembrane region in the middle of the protein), the periplasm and peptidoglycan (it contains a putative peptidoglycan-binding LysM homologue motif), and the outer membrane (unpublished data). During invasive disease, followed by patient recovery, the entire protein is most likely to be processed by the immune system; hence, a potent T-cell and B-cell response is detected. However, during carriage, bacterial cell death and antigen processing by mucosal lymphoid tissue are unlikely to be as extensive, and the mucosal immune responses might even be suppressive (promoting tolerance). Finally, the lack of high (or detectable) levels of T-cell and antibody responses in some of the carriers may also have been due to the fact that most of the carriers (carriers 1 to 6) may have acquired N. meningitidis shortly before samples were obtained. Unfortunately, this remains a matter for speculation at this stage because PBMCs from carriers 9 to 14 were not available for testing.
A prerequisite for human vaccine antigens is that they should be demonstrated to be immunogenic in humans irrespective of the results obtained with animal models. It was previously reported that serum from four patients who recovered from meningococcal disease reacted with purified meningococcal TspA (19). In the present study, rTspA was used in an ELISA to determine the level of TspA-specific IgG present in six serum samples from convalescent patients and also in samples from 14 known meningococcal carriers. This could have provided confirmatory evidence that TspA is recognized by the immune system during infection. All of the carriers responded with serum antimeningococcal IgG antibodies during the course of the study, and the sera from patients also contained similar high levels of antibody. Not all of the sera contained significant levels of anti-TspA IgG, however. Four of the six samples from patients were positive, but only 5 of the 14 samples from carriers contained anti-TspA IgG, and the concentrations were significantly lower. Immunity to bacterial antigens at mucosal surfaces may be dominated by mucosal IgA antibodies rather than high-level systemic IgG responses. For example, an intranasally administered outer membrane vesicle vaccine was found to elicit persistent mucosal IgA antibodies in human volunteers but induced weak serum IgG responses (17). Other workers have shown recently that carriage of meningococci elicits a mucosal T-cell response, but serum bactericidal antibody responses may not always be produced (9). In the current study, the serum IgG responses measured during meningococcal carriage may therefore have been lower-level responses compared to the responses elicited by invasive disease. In order to check this, future experiments should quantify TspA-specific IgA and IgG in saliva and serum from colonized individuals.
The primary goal of this work was to confirm the T-cell stimulating activity of TspA. Demonstration of TspA-specific T-cell and antibody responses following invasive disease, in which the immune system was exposed to a complex mixture of antigens in the context of the whole meningococcus, supports our contention that TspA is an immunogenic antigen. Although fewer of the carriers had detectable serum antibodies, it was nevertheless encouraging to be able to demonstrate TspA-specific B- and T-cell responses following asymptomatic colonization of the nasopharynx. Together, these results demonstrate that TspA is indeed a potent T-cell stimulating immunogen in humans. Future vaccine studies should determine whether TspA is capable of stimulating a T-cell-dependent response to other meningococcal antigens (e.g., when it is used as a carrier protein with capsular polysaccharide) and if it is effective in eliciting protection against experimental meningococcal infections.
Acknowledgments
We acknowledge the practical help and advice of Kathy Wilks and Goksel Kizil. Meningococcal serotyping was carried out at the Health Protection Agency Meningococcal Reference Unit, Manchester Royal Infirmary, Manchester, United Kingdom. We acknowledge Ed Kaczmarski and Steve Gray for their assistance with this part of the study.
This work was supported in part by the Meningitis Research Foundation.
Editor: J. N. Weiser
REFERENCES
- 1.Abdel Hadi, H., K. G. Wooldridge, K. Robinson, and D. A. A. Ala'Aldeen. 2001. Identification and characterization of App: an immunogenic autotransporter protein of Neisseria meningitidis. Mol. Microbiol. 41:611-624. [DOI] [PubMed] [Google Scholar]
- 2.Ala'Aldeen, D. A., and S. P. Borriello. 1996. The meningococcal transferrin-binding proteins 1 and 2 are both surface exposed and generate bactericidal antibodies capable of killing homologous and heterologous strains. Vaccine 14:49-53. [DOI] [PubMed] [Google Scholar]
- 3.Andrade, M. A., C. Perez-Iratxeta, and C. P. Ponting. 2001. Protein repeats: structures, functions, and evolution. J. Struct. Biol. 134:117-131. [DOI] [PubMed] [Google Scholar]
- 4.Bateman, A., and M. Bycroft. 2000. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299:1113-1119. [DOI] [PubMed] [Google Scholar]
- 5.Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nokleby, E. Rosenqvist, et al. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 6.Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, W. Silva, et al. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Chilean National Committee for Meningococcal Disease. Vaccine 13:821-829. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W. P., and T. T. Kuo. 1993. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 21:2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Andrea, L. D., and L. Regan. 2003. TPR proteins: the versatile helix. Trends Biochem. Sci. 28:655-662. [DOI] [PubMed] [Google Scholar]
- 9.Davenport, V., T. Guthrie, J. Findlow, R. Borrow, N. A. Williams, and R. S. Heyderman. 2003. Evidence for naturally acquired T cell-mediated mucosal immunity to Neisseria meningitidis. J. Immunol. 171:4263-4270. [DOI] [PubMed] [Google Scholar]
- 10.Davison, K. L., and M. E. Ramsay. 2003. The epidemiology of acute meningitis in children in England and Wales. Arch. Dis. Child. 88:662-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Moraes, J. C., B. A. Perkins, M. C. Camargo, N. T. Hidalgo, H. A. Barbosa, C. T. Sacchi, I. M. Landgraf, V. L. Gattas, G. Vasconcelos Hde, I. M. Gral, et al. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 340:1074-1078. [DOI] [PubMed] [Google Scholar]
- 12.Feavers, I. M., A. J. Fox, S. Gray, D. M. Jones, and M. C. Maiden. 1996. Antigenic diversity of meningococcal outer membrane protein PorA has implications for epidemiological analysis and vaccine design. Clin. Diagn. Lab. Immunol. 3:444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finne, J., D. Bitter-Suermann, C. Goridis, and U. Finne. 1987. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J. Immunol. 138:4402-4407. [PubMed] [Google Scholar]
- 14.Frasch, C. E. 1989. Vaccines for prevention of meningococcal disease. Clin. Microbiol. Rev. 2(Suppl.):S134-S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granoff, D. M., G. R. Moe, M. M. Giuliani, J. Adu-Bobie, L. Santini, B. Brunelli, F. Piccinetti, P. Zuno-Mitchell, S. S. Lee, P. Neri, L. Bracci, L. Lozzi, and R. Rappuoli. 2001. A novel mimetic antigen eliciting protective antibody to Neisseria meningitidis. J. Immunol. 167:6487-6496. [DOI] [PubMed] [Google Scholar]
- 16.Hadi, H. A., K. G. Wooldridge, K. Robinson, and D. A. Ala'Aldeen. 2001. Identification and characterization of App: an immunogenic autotransporter protein of Neisseria meningitidis. Mol. Microbiol. 41:611-623. [DOI] [PubMed] [Google Scholar]
- 17.Haneberg, B., R. Dalseg, E. Wedege, E. A. Hoiby, I. L. Haugen, F. Oftung, S. R. Andersen, L. M. Naess, A. Aase, T. E. Michaelsen, and J. Holst. 1998. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect. Immun. 66:1334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holst, J., B. Feiring, J. E. Fuglesang, E. A. Hoiby, H. Nokleby, I. S. Aaberge, and E. Rosenqvist. 2003. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine 21:734-737. [DOI] [PubMed] [Google Scholar]
- 19.Kizil, G., I. Todd, M. Atta, S. P. Borriello, K. Ait-Tahar, and D. A. Ala'Aldeen. 1999. Identification and characterization of TspA, a major CD4+ T-cell- and B-cell-stimulating Neisseria-specific antigen. Infect. Immun 67:3533-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kizil, G., K. Wilks, D. Wells, and D. A. Ala'Aldeen. 2000. Detection and characterisation of the genes encoding glyoxalase I and II from Neisseria meningitidis. J. Med. Microbiol. 49:669-673. [DOI] [PubMed] [Google Scholar]
- 21.Kvalsvig, A. J., and D. J. Unsworth. 2003. The immunopathogenesis of meningococcal disease. J. Clin. Pathol. 56:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maino, V. C., and L. J. Picker. 1998. Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry 34:207-215. [DOI] [PubMed] [Google Scholar]
- 23.Martin, D., B. R. Brodeur, J. Hamel, F. Couture, U. de Alwis, Z. Lian, S. Martin, D. Andrews, and R. W. Ellis. 2000. Candidate Neisseria meningitidis NspA vaccine. J. Biotechnol. 83:27-31. [DOI] [PubMed] [Google Scholar]
- 24.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol 19:683-765. [DOI] [PubMed] [Google Scholar]
- 25.Morley, S. L., and A. J. Pollard. 2001. Vaccine prevention of meningococcal disease, coming soon? Vaccine 20:666-687. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann, T. 2000. Complexity or coherence? Cytokine secretion by B cells. Nat. Immunol. 1:465-466. [DOI] [PubMed] [Google Scholar]
- 27.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M. A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 28.Pelchen-Matthews, A., I. J. Parsons, and M. Marsh. 1993. Phorbol ester-induced downregulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J. Exp. Med. 178:1209-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 30.Plested, J. S., K. Makepeace, M. P. Jennings, M. A. Gidney, S. Lacelle, J. Brisson, A. D. Cox, A. Martin, A. G. Bird, C. M. Tang, F. M. Mackinnon, J. C. Richards, and E. R. Moxon. 1999. Conservation and accessibility of an inner core lipopolysaccharide epitope of Neisseria meningitidis. Infect. Immun. 67:5417-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollard, A. J., R. Galassini, E. M. Rouppe van der Voort, M. Hibberd, R. Booy, P. Langford, S. Nadel, C. Ison, J. S. Kroll, J. Poolman, and M. Levin. 1999. Cellular immune responses to Neisseria meningitidis in children. Infect. Immun. 67:2452-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsay, M. E., N. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 33.Robinson, K., T. Bellaby, and D. Wakelin. 1994. Vaccination against the nematode Trichinella spiralis in high- and low-responder mice. Effects of different adjuvants upon protective immunity and immune responsiveness. Immunology 82:261-267. [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson, K., K. R. Neal, C. Howard, J. Stockton, K. Atkinson, E. Scarth, J. Moran, A. Robins, I. Todd, E. Kaczmarski, S. Gray, I. Muscat, R. Slack, and D. A. Ala'Aldeen. 2002. Characterization of humoral and cellular immune responses elicited by meningococcal carriage. Infect. Immun 70:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romagnani, P., F. Annunziato, M. P. Piccinni, E. Maggi, and S. Romagnani. 2000. Th1/Th2 cells, their associated molecules and role in pathophysiology. Eur. Cytokine Netw. 11:510-511. [PubMed] [Google Scholar]
- 36.Rosenstein, N. E., M. Fischer, and J. W. Tappero. 2001. Meningococcal vaccines. Infect. Dis. Clin. N. Am. 15:155-169. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Steen, A., G. Buist, K. J. Leenhouts, M. El Khattabi, F. Grijpstra, A. L. Zomer, G. Venema, O. P. Kuipers, and J. Kok. 2003. Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J. Biol. Chem. 278:23874-23881. [DOI] [PubMed] [Google Scholar]
- 39.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 40.Trotter, C. L., N. J. Andrews, E. B. Kaczmarski, E. Miller, and M. E. Ramsay. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364:365-367. [DOI] [PubMed] [Google Scholar]
- 41.Verheul, A. F., H. Snippe, and J. T. Poolman. 1993. Meningococcal lipopolysaccharides: virulence factor and potential vaccine component. Microbiol. Rev. 57:34-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vermont, C., and G. van den Dobbelsteen. 2002. Neisseria meningitidis serogroup B: laboratory correlates of protection. FEMS Immunol. Med. Microbiol. 34:89-96. [DOI] [PubMed] [Google Scholar]
- 43.West, D., K. Reddin, M. Matheson, R. Heath, S. Funnell, M. Hudson, A. Robinson, and A. Gorringe. 2001. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect. Immun. 69:1561-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]