Abstract
Polymorphisms in exon 1 of the MBL-2 gene, resulting in reduced plasma levels of mannose binding lectin, were significantly overrepresented in 23 patients with primary antibody deficiency and culture-proven mycoplasma infections (P = 0.0038). This association persisted with the inclusion of a further nine suspected (doxycycline-responsive) cases (P = 0.0087). The lectin was shown to bind to three strains of mycoplasma.
Patients with primary antibody deficiency (PAD) are susceptible to mycoplasma infection (2), and the most common presenting feature is arthritis, often involving large joints (1). Although antibody deficiency is the major susceptibility factor, there must be cofactors that determine significant infection.
Mannose binding lectin (MBL) is an oligomeric protein able to bind to repeating sugar arrays present on a variety of microorganisms (11). It has similarities in structure to C1q and can activate complement in the absence of antibody and the C1 complex. Three allelic variants in the coding region of the MBL gene have been described, and they are associated with low plasma levels of oligomeric MBL. These mutations in codons 52 (D variant), 54 (B variant), and 57 (C variant) cause disruption of the collagenous triple helix, which is critical for oligomerization. Polymorphisms in the promoter region also influence the serum MBL level (7).
Against this background, using a large cohort of PAD patients having either common variable immunodeficiency (CVID) or X-linked agammaglobulinemia (XLA), we have investigated a possible role for MBL in protecting patients from overt mycoplasma infection.
Whole-blood samples for DNA extraction were obtained from 32 patients with either proven or probable mycoplasma infections. Eleven of these patients had X-linked agammaglobulinemia (XLA confirmed by mutation screening of the Btk gene) and were attending clinics at The Royal Free Hospital, London, United Kingdom; Great Ormond Street Hospital for Children, London, United Kingdom; and Huddinge Hospital, Sweden. The remaining 21 samples were from patients with CVID who were attending clinics at the Royal Free Hospital. The diagnosis of CVID was made in accordance with International Union of Immunological Societies criteria (3), and all patients were established on regular immunoglobulin therapy.
Mycoplasma infection was confirmed by positive culture from synovial fluid, synovial biopsy, urine, urethral swab, or lung biopsy (1, 2). For one patient, the diagnosis was confirmed by PCR for ureaplasmas. Mycoplasma infection was defined as probable when the clinical course was typical, with negative routine bacterial cultures and a good response to doxycycline. Culture for mycoplasmas either was unavailable for these patients or was negative, possibly because of sampling difficulties, prolonged transport, or limited laboratory culture techniques.
The Avon Longitudinal Study of Parents and Children was set up as a multifaceted study on approximately 14,000 children (http:/www.alspac.bris.ac.uk), and a subset of individuals has been genotyped for all known structural and promoter polymorphisms of the MBL gene and was used as the “normal” control group in this study.
DNA was extracted by using standard techniques from heparinized or EDTA-anticoagulated venous blood. Genotyping was performed as described by Jack et al. (4) and Turner et al. (12).
Mycoplasma A39 (NCTC 11740) and Mycoplasma pneumoniae strain 5167 were provided by Mycoplasma Experience Ltd., Reigate, United Kingdom. These strains and Mycoplasma hominis (NCTC 10111) and Mycoplasma orale (a clinical isolate) were cultured at 36°C in 50-ml volumes of Mycoplasma liquid medium (ML5A) supplied by Mycoplasma Experience or modified SP4 broth (made in house). Cultures were harvested when the density of mycoplasma organisms was sufficient, as indicated by a color change (10).
The binding of MBL to bacteria was determined by modification of a procedure described previously (5). Briefly, a suspension (50 μl) of the mycoplasma organisms was centrifuged at 12,000 × g for 2 min in a microcentrifuge, and the pellet was resuspended in Hanks balanced salt solution (HBSS) without phenol red containing MBL purified according to the method of Jack et al. (6) (5 μg/ml) for 30 min at 37°C. Following further centrifugation at 12,000 × g for 2 min, the cell pellet was washed with 200 μl of HBSS before resuspension in HBSS containing fluorescein isothiocyanate-anti-MBL. After incubation at 37°C for 30 min, the mycoplasma preparations were pelleted (12,000 × g, 2 min) and washed. Samples were prepared for flow cytometry by resuspension in 200 μl of phosphate-buffered saline and fixed by the addition of 200 μl of Cell-Fix (in the proportion of 1:10) (catalog no. 340181; Becton Dickinson). An aliquot of 10 μl of propidium iodide was added to each sample before analysis. Organisms were identified by forward and side scatter to select particles of between 0.4 and 0.6 μm. Further gating to include only propidium iodide-positive events was used to exclude nonbacterial particles. The specificity of binding was examined by preincubating the MBL (5 μg/ml) at 37°C for 10 min with EDTA (5 mM), galactose (25 mM; Sigma), or mannose (25 mM; Sigma).
A total of 32 patients with either a confirmed or probable diagnosis of mycoplasma infection were identified. A variety of mycoplasmas, including M. hominis, M. pneumoniae, Mycoplasma salivarium, Ureaplasma urealyticum, and Mycoplasma maculosum, were isolated from either joints or urogenital or respiratory tracts. Patients were genotyped for all known exon 1 and promoter polymorphisms. There was a statistically significant increase in MBL exon 1 mutations in patients with microbiologically proven mycoplasma infections (P = 0.0038) and also in such patients when a mycoplasma infection was either proven or probable (P = 0.0087) (Table 1). Indeed, more than two-thirds of patients with mycoplasma infections had genotypic MBL deficiency (compared with one-third in the general population). These significant differences were not enhanced when promoter polymorphisms were also included in the analysis, probably reflecting the small size of the cohort.
TABLE 1.
Frequency of MBL structural gene polymorphisms in patients with proven or probable mycoplasma infection
| Cohort | No. of individuals | No. of A/Aa individuals | No. of O/Oa and O/A individuals | P valueb (disease group vs. control group) |
|---|---|---|---|---|
| Population controlc | 1,051 | 653 | 398 | |
| Cohort with proven mycoplasma infection | 23 | 7 | 16 | 0.0038 |
| Cohort with proven and probable mycoplasma infection | 32 | 12 | 20 | 0.0087 |
A indicates MBL wild type, and O indicates the presence of an exon 1 variant (B, C, or D).
Fisher's exact test (two-tailed).
The Avon Longitudinal Study of Parents and Children cohort (see the text) incorporating previously published data on 302 individuals (8).
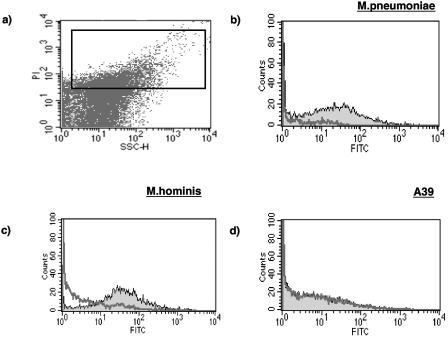
Flow cytometry was used to investigate the interaction of MBL with various mycoplasma strains (Fig. 1). In our previous studies using other bacteria (9), extensive washing was required to remove nonspecific binding of the fluorescein isothiocyanate-conjugated-MBL-specific antibody. Such treatment of mycoplasma preparations resulted in considerable disruption of the microorganisms, and as such, there was always some binding of the MBL detector antibody in the absence of MBL. However, when M. pneumoniae organisms were incubated with MBL, there was evidence of a log shift in median fluorescence intensity (Fig. 1b). Similarly, with M. hominis (Fig. 1c) and M. orale (not shown) organisms, there was a clear increase in the median fluorescence intensity. However, in the case of the A39 NCTC11740 strain (Fig. 1d), no such shift was seen.
FIG. 1.
Panel a shows a dot plot of a preparation of M. hominis with propidium iodide staining depicted on the y axis and side scatter along the x axis (expressed in arbitrary units). The rectangular acquisition gate is shown. Panels b, c, and d show histograms of three mycoplasma preparations before (thick line) and after (thin line) incubation with MBL. A clear distinction can be seen between organisms that bind MBL (b and c) and the A39 strain (d), which does not. FITC, fluorescein isothiocyanate.
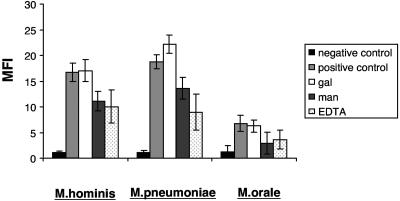
The specificity of the MBL binding observed with M. hominis, M. orale, and M. pneumoniae organisms was investigated by using various inhibitors (Fig. 2). For each of the three strains, 5 mM EDTA and 25 mM mannose inhibited binding of the lectin, whereas 25 mM galactose had no effect. These results are characteristic of a C-type lectin.
FIG. 2.
Inhibition of MBL binding to three strains of mycoplasmas M. hominis, M. pneumoniae, and M. orale. Preparations of each organism were either incubated in the absence of MBL (negative control) or incubated with MBL (5 μg/ml) at 37°C for 30 min (positive control). Inhibition of MBL binding was attempted using 25 mM galactose (gal), 25 mM mannose (man), and 5 mM EDTA, each of which was added to the MBL at 37°C 10 min before the incubation with organisms. Three separate experiments were performed, and the data are plotted as means ± standard errors of the means.
The mycoplasma strains most commonly isolated from joints and abscesses are M. hominis, M. pneumoniae, Ureaplasma urealyticum, M. salivarium, and M. orale strains. Apart from M. pneumoniae, most of the other organisms are considered to be commensals or semicommensals with low pathogenicity. Little is known of the sugar repertoire of mycoplasmas, but we detected MBL binding to three of four strains tested, and inhibition studies were compatible with the presence of mannose or N-acetylglucosamine residues on the surface of mycoplasmas.
How might MBL protect against mycoplasma infection? All of the mycoplasmas tested, except for mycoplasma A39, can invade mucosal surfaces and spread systemically in susceptible PAD patients. MBL may help prevent invasive disease by inhibiting growth, similar to the way in which specific antibody can inhibit growth in vitro. The failure of MBL to bind to mycoplasma A39, a novel organism recently found in the sputa of many PAD patients, is interesting because this mycoplasma appears to have low pathogenicity and does not spread systemically.
This is the first study to show a role for MBL in mycoplasma infection, which can be very difficult to treat once the infection has spread systemically. In the future, it may be possible to increase the plasma level of MBL by regular infusion of either recombinant or plasma-derived material (13) in order to protect and treat those PAD patients with recurrent mycoplasma infections.
Acknowledgments
We thank I. Vorechovsky, C. Heilmann, and T. Lester for providing samples of DNA from L. Hammarstrom’s patients; P. Furr and D. Taylor-Robinson for culturing some of the mycoplasmas; and D. Jack and C. Chatterton for advice and assistance with the genotyping.
Research at the Institute of Child Health and Great Ormond Street Hospital for Children National Health Service Trust benefits from Research & Development funding received from the National Health Service Executive. We also gratefully acknowledge support from the European Union (contract no. QLRT-CT-2001-01039).
Informed consent was obtained for this study from all of the patients or from their parents or guardians. The official guidelines of each participating institution were followed in the conduct of the clinical research.
M.W.T. and N.J.K. both act as scientific consultants for NatImmune, a Danish company exploring the therapeutic potential of MBL.
Editor: J. N. Weiser
REFERENCES
- 1.Franz, A., A. D. Webster, P. M. Furr, and D. Taylor-Robinson. 1997. Mycoplasmal arthritis in patients with primary immunoglobulin deficiency: clinical features and outcome in 18 patients. Br. J. Rheumatol. 36:661-668. [DOI] [PubMed] [Google Scholar]
- 2.Furr, P. M., D. Taylor-Robinson, and A. D. Webster. 1994. Mycoplasmas and ureaplasmas in patients with hypogammaglobulinaemia and their role in arthritis: microbiological observations over twenty years. Ann. Rheum. Dis. 53:183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Union of Immunological Societies.1999. Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. Clin. Exp. Immunol. 118(Suppl. 1):1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack, D., J. Bidwell, M. Turner, and N. Wood. 1997. Simultaneous genotyping for all three known structural mutations in the human mannose-binding lectin gene. Hum. Mutat. 9:41-46. [DOI] [PubMed] [Google Scholar]
- 5.Jack, D. L., A. W. Dodds, N. Anwar, C. A. Ison, A. Law, M. Frosch, M. W. Turner, and N. J. Klein. 1998. Activation of complement by mannose-binding lectin on isogenic mutants of Neisseria meningitidis serogroup B. J. Immunol. 160:1346-1353. [PubMed] [Google Scholar]
- 6.Jack, D. L., R. C. Read, A. J. Tenner, M. Frosch, M. W. Turner, and N. J. Klein. 2001. Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J. Infect. Dis. 184:1152-1162. [DOI] [PubMed] [Google Scholar]
- 7.Madsen, H. O., P. Garred, S. Thiel, J. A. Kurtzhals, L. U. Lamm, L. P. Ryder, and A. Svejgaard. 1995. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J. Immunol. 155:3013-3020. [PubMed] [Google Scholar]
- 8.Mead, R., D. Jack, M. Pembrey, L. Tyfield, M. Turner, et al. 1997. Mannose-binding lectin alleles in a prospectively recruited UK population. Lancet 349:1669-1670. [DOI] [PubMed] [Google Scholar]
- 9.Neth, O., D. L. Jack, A. W. Dodds, H. Holzel, N. J. Klein, and M. W. Turner. 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poveda, J. B., and R. Nicholas. 1998. Serological identification of mycoplasmas by growth and metabolic inhibition tests. Methods Mol. Biol. 104:105-111. [DOI] [PubMed] [Google Scholar]
- 11.Turner, M. W. 1996. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol. Today 17:532-540. [DOI] [PubMed] [Google Scholar]
- 12.Turner, M. W., L. Dinan, S. Heatley, D. L. Jack, B. Boettcher, S. Lester, J. McCluskey, and D. Roberton. 2000. Restricted polymorphism of the mannose-binding lectin gene of indigenous Australians. Hum. Mol. Genet. 9:1481-1486. [DOI] [PubMed] [Google Scholar]
- 13.Valdimarsson, H., M. Stefansson, T. Vikingsdottir, G. J. Arason, C. Koch, S. Thiel, and J. C. Jensenius. 1998. Reconstitution of opsonizing activity by infusion of mannan-binding lectin (MBL) to MBL-deficient humans. Scand. J. Immunol. 48:116-123. [DOI] [PubMed] [Google Scholar]