Abstract
Vibrio cholerae causes diarrhea by colonizing the human small bowel and intoxicating epithelial cells. Colonization is a required step in pathogenesis, and strains defective for colonization are significantly attenuated. The best-characterized V. cholerae colonization factor is the toxin-coregulated pilus (TCP). It has been demonstrated that TCP is required for V. cholerae colonization in both humans and mice. TCP enhances bacterial interactions that allow microcolony formation and thereby promotes survival in the intestine. We have recently discovered that the TCP biogenesis apparatus also serves as a secretion system, mediating the terminal step in the extracellular secretion pathway of TcpF. TcpF was identified in classical isolates of V. cholerae O1 as a soluble factor essential for colonization in the infant mouse cholera model. In the present study, we expanded our analysis of TcpF to include the O1 El Tor and O139 serogroups and investigated how TCP and TcpF act together to mediate colonization. Additionally, we demonstrated that antibodies generated against TcpF are protective against experimental V. cholerae infection in the infant mouse cholera model. This observation, coupled with the fact that TcpF is a potent mediator of colonization, suggests that TcpF should be considered as a component of a polyvalent cholera vaccine formulation.
Vibrio cholerae is a gram-negative bacillus that causes the acute diarrheal disease cholera (for a review see reference 22). Although there are over 200 serogroups of V. cholerae based on the surface polysaccharide O antigen and several of these serogroups may cause sporadic, minor cases of cholera, epidemic V. cholerae isolates are represented by only two serogroups, serogroups O1 and O139. The O1 serogroup is further separated into two biotypes, classical and El Tor, based on physiologic variability. The easily demonstrable physiological differences between El Tor and classical isolates include hemagglutination of chicken erythrocytes, polymyxin B resistance, and hemolysis of sheep erythrocytes; all of these properties are characteristic of the El Tor biotype (22).
Cholera is transmitted via the oral-fecal route, and ingestion of a significant V. cholerae inoculum is required to produce the clinical syndrome (5). After a short incubation period, patients with cholera present with voluminous, watery diarrhea. In the absence of rehydration therapy, hypovolemic shock and death can ensue (4). These clinical manifestations are the direct result of intoxication of intestinal epithelial cells by cholera toxin (CT). CT is delivered to epithelial cells by V. cholerae that has successfully colonized the upper small intestine; colonization is therefore a required step in V. cholerae pathogenesis.
The molecular mechanism by which CT causes diarrhea is well understood. CT enters the endocytic pathway of intestinal epithelial cells and through a cascade of intermediates constitutively alters the permeability of these cells to ions and water (6, 20, 21, 47). Increased fluid and electrolyte secretion coupled with decreased absorption leads to abnormal luminal accumulation of fluid. Much less is known about how the proteins and other factors involved in intestinal colonization mediate interactions with intestinal epithelial cells and among bacteria to promote a productive infection. One possible way to conceptualize intestinal colonization is by comparison with a potentially similar bacterial process, biofilm formation. This is a general mechanism by which bacteria colonize surfaces and can be thought of as a stepwise process composed of at least three distinct events: (i) surface attachment, (ii) microcolony formation, and (iii) assembly of higher-order structures (macrocolonies or biofilms) (10, 50). Based on this model, it would be expected that mutations in genes encoding proteins involved in each of these steps would cause deficiencies that prevent progression of the biofilm formation process. This model is supported by the fact that mutations resulting in deficiencies in most of these steps have been described in Pseudomonas aeruginosa, for which colonization of plastic surfaces was used as a screening tool to identify such mutants (32-34). Additionally, more recent work has established that V. cholerae biofilm formation on plastic surfaces is a process that requires particular gene products to accomplish various steps, all of which are required for the formation and maintenance of biofilms (3, 50, 51).
Extending this concept to include intestinal colonization by V. cholerae, proteins believed to be involved in mediating the first two steps have been identified and characterized to varying degrees. For example, it has been suggested that OmpU, a major V. cholerae outer membrane protein, binds to fibronectin in the cellular matrix of eukaryotic cells, placing it among the mediators of the first step. Antibodies against OmpU were shown to block colonization in passive immunization experiments (37). In addition, we recently identified an outer membrane protein (GbpA) that appears to mediate direct attachment to epithelial cells by binding to surface-exposed sugars (Kirn et al., submitted for publication). Deletion of the gene encoding this protein results in a significant in vivo colonization defect.
While analysis of the proteins involved in the first step of colonization has been limited, the best-characterized V. cholerae colonization factor is the toxin-coregulated pilus (TCP), which is a representative factor involved in mediating the second step of colonization (bacterium-bacterium interactions leading to microcolony formation). TCP is a type 4 pilus that has long been recognized as a protein that is structurally related to the bundle-forming pilus of enteropathogenic Escherichia coli (11). More recently, it has become clear that based on the arrangement of the genes encoding the TCP biogenesis apparatus, TCP is also closely related to the type 4 pili elaborated by enterotoxigenic E. coli (ETEC) and Citrobacter rodentium (CFA/III and CFC, respectively) (31, 42). The elaboration of TCP confers several important properties upon V. cholerae in vitro and in vivo. Since TCP is the high-affinity receptor for the CTX phage, TCP+ bacteria are efficiently transduced by the CTX derivative CTX-Knφ, while TCP− strains are poorly transduced (49). Furthermore, TCP+ V. cholerae strains are protected from complement-mediated cytolysis, while TCP− strains are sensitive (8, 24). Perhaps most importantly, it has been shown that TCP is required for colonization in human volunteers and in the infant mouse cholera model for all epidemic serogroups and biotypes of V. cholerae (18, 41, 44, 45). While many type 4 pili function by mediating a direct interaction between bacteria and epithelial cells, TCP appears to enhance bacterial interactions, thereby promoting the assembly of intestinal microcolonies (24). Presumably, the formation of intestinal microcolonies protects the bacteria from biological, chemical, or physical threats, allowing them to persist and cause disease. As might be expected, blocking the function of the pilin subunit (TcpA) with antibodies significantly reduces the ability of V. cholerae to colonize and is protective (38, 52).
V. cholerae mutants defective for the third intestinal colonization step have not been described yet, suggesting that this step is not required for colonization or that it is impossible to evaluate this phenotype in vitro. However, we recently demonstrated that TcpF, a soluble protein secreted via the TCP biogenesis apparatus, plays an important role in colonization (23). Mutants lacking the ability to produce TcpF are severely defective for colonization in the infant mouse cholera model, while they essentially retain all of the in vitro properties associated with the elaboration of a functional TCP. Based on these observations, it is possible that TcpF may be the first representative of a class of proteins required for the third step of colonization. As mentioned above, operons encoding the proteins involved in the biogenesis of the ETEC CFA/III and C. rodentium CFC pili have recently been characterized. Interestingly, alignment of the genes encoding pilus biogenesis functions in these two organisms with the tcp operon revealed that in each case a nonhomologous gene occupies the same position within the operon as tcpF. In both cases, the predicted proteins have no known function and exhibit limited homology to well-characterized proteins. It is intriguing to consider that these proteins may play roles in ETEC and Citrobacter pathogenesis analogous to the role of TcpF in V. cholerae.
In the present study, we compared the phenotypes of mutants defective for TCP and mutants defective for TcpF with respect to intestinal clearance, in vitro microcolony formation, and epithelial cell attachment. Additionally, since all previously reported experiments designed to evaluate the secretion of TcpF were conducted with the classical O1 strain of V. cholerae, we expanded our analysis of TcpF to include El Tor O1 and O139 serotypes. Finally, we demonstrated that antibodies directed against TcpF are protective against experimental cholera infection in the infant mouse cholera model.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacteria were grown either in Luria-Bertani (LB) liquid broth at 37°C for 16 h (standard conditions), in LB broth adjusted to a starting pH of 6.5 at 30°C for 16 h (classical O1 TCP-inducing conditions), or in AKI broth (O139 and El Tor O1 TCP-inducing conditions) (19). V. cholerae strains O395, 569B, and N16961 were obtained from laboratory stocks, and strain CVD112 was obtained from the Center for Vaccine Development at the University of Maryland (40).
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
Each strain was grown under the appropriate TCP-inducing conditions, and culture supernatants were harvested by centrifugation for 5 min at 10,000 × g. Each pellet was resuspended in phosphate-buffered saline (PBS) and mixed with an equal volume of 2× loading buffer. Supernatants were then filtered through a 0.2-μm syringe barrel filter and mixed with an equal volume of 2× protein loading buffer. Proteins from culture supernatants and whole-cell extracts were separated on 12.5% polyacrylamide gels containing SDS. Total-protein determinations were made using the bicinchoninic acid protein assay (Pierce), and culture supernatant or pellet fractions were diluted appropriately. For immunodetection, proteins were transferred to nitrocellulose at 4°C in transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol; pH 8.3) using a wet transfer apparatus (48). TcpF was detected using polyclonal anti-TcpF antiserum generated as previously described (23).
CTX-Knφ transduction assay.
CTX-Knφ-containing supernatants were generated by growing strain CL101 under standard conditions and filtering the culture supernatant through a 0.2-μm syringe filter (26). Transductions were performed as previously described (49). Briefly, each strain to be tested was grown under TCP-inducing conditions and mixed with an equal volume of supernatant containing CTX-Knφ. After 30 min of incubation at room temperature, appropriate dilutions were prepared, and the bacteria were plated on solid medium containing kanamycin. The transduction frequency was calculated by dividing the number of Kanr colonies recovered by the number of input CFU. All transductions were performed on the same day with a single phage stock.
LD50 and competitive index analysis.
The 50% lethal dose (LD50) of each strain was assessed by orally inoculating groups of four 5- to 7-day-old mice with dilutions of bacteria grown under TCP-inducing conditions. The inocula tested were 10-fold dilutions containing from 5 × 108 to 5 × 105 bacteria per dose. When antibodies were evaluated for the ability to provide protection, an equal volume of antiserum was mixed with a bacterial suspension just before the mice were inoculated. The LD50 was based on the extrapolated dose that resulted in a mean survival rate of 50% after 48 h.
For the in vivo competitive index determinations, strains to be tested were grown under TCP-inducing conditions and then mixed with equal numbers of the isogenic ΔlacZ O395 derivative CG842. Five- to 7-day-old CD-1 mice from mixed litters were orally inoculated with 50 μl of a 1 × 10−2 dilution of the mixture and incubated at 30°C for 24 h. The bacteria were then recovered by homogenizing harvested intestines with a Tissue Tearor in 4 ml of 10% glycerol in LB broth. The homogenate was appropriately diluted and plated on solid medium containing streptomycin and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). The competitive index was calculated by comparing the ratio of test strain bacteria recovered from the intestine to the test strain input with the input/output ratio of CG842. The values plotted were the competitive index for each mouse along with the average.
In vitro competitive index values were obtained by inoculating 2 ml of LB liquid medium with equal numbers of the test strain and CG842, growing the organisms overnight at 37°C, and plating dilutions on solid medium containing streptomycin and X-Gal. The change in the ratio of the test strain to CG842 was then determined.
Serum resistance assay.
The serum resistance assay was performed as previously described (8). Briefly, each strain was grown for 12 h under TCP-inducing conditions, pelleted in a microcentrifuge, and resuspended in PBS. The cells were then diluted 1:100 in PBS, and reconstituted lyophilized guinea pig complement was added to a final concentration of 20%. This mixture was divided into multiple 500-μl aliquots, and either anti-O395 antibody at a final concentration of 1:2,500 or PBS was added. The samples were incubated at 37°C with agitation for 45 min and then diluted appropriately and plated on solid medium. Serum resistance was expressed as the number of bacteria recovered after treatment with antiserum divided by the input number of CFU.
Mixed microcolony assay.
Briefly, unmarked wild-type strains together with mutants bearing pGreenTIR were inoculated into liquid culture media and grown overnight under TCP-inducing conditions. The presence of fluorescent bacteria in the resulting bacterial aggregates was scored by confocal fluorescence microscopy using an overlay of the output from the fluorescein isothiocyanate channel with the differential interference contrast image.
Cell culture and bacterial attachment assay.
HT-29 cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and 1× Pen-Strep (Gibco) under 5% CO2 (1). Confluent monolayers were infected with 1 ml of a bacterial suspension containing 106 bacteria/ml and incubated at 37°C. Bacteria and epithelial cells were imaged by confocal microscopy, and differential interference contrast images were overlaid with images obtained on the green fluorescent protein (GFP) channel after 4 h. For quantitation of attached bacteria, the monolayers and attached bacteria were washed three times with PBS and then incubated for 30 min in a 1% Triton X-100 solution. The resulting bacterial suspensions were appropriately diluted and plated on LB medium plates.
In an effort to ensure that each bacterial colony truly represented a single bacterium and not a cluster of bacteria (microcolony), a simple control experiment was performed. Briefly, the LacZ+ wild-type V. cholerae isolate O395 was mixed at a 1:1 ratio with a LacZ− derivative of O395, grown overnight, and then incubated with epithelial cells as described above. Following lysis of the epithelial cells, appropriate dilutions of the resulting bacterial suspension were plated on LB medium plates containing X-Gal. After overnight incubation, an equal mixture of blue and white colonies was observed on the plates, and no mixed colonies were observed. Ten blue colonies and 10 white colonies were then restreaked on LB medium plates containing X-Gal and again incubated overnight. Confirming the hypothesis that each colony represented a single bacterium, each blue colony gave rise to only blue colonies and each white colony gave rise to only white colonies.
RESULTS
TcpF is secreted by all epidemic types of V. cholerae.
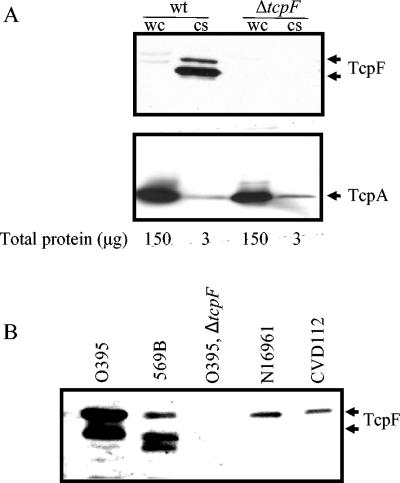
We previously demonstrated that TcpF is secreted at a level similar to the level of CT secreted by the classical O1 V. cholerae strain O395 (23). In an effort to determine the ultimate localization of this secreted protein in vitro, proteins from whole-cell extracts and culture supernatants were separated by SDS-PAGE, transferred to nitrocellulose, and then probed with antibodies directed against TcpF. As shown in Fig. 1, although the total amount of whole-cell protein loaded on the polyacrylamide gel was 50 times greater than the amount of culture supernatant protein loaded, TcpF was barely detectable in the whole-cell fraction, while the culture supernatant fraction yielded a pair of very robust bands. This pattern of hybridization (i.e., two distinct bands corresponding to TcpF) is consistent with previously reported results (see below). While this experiment was not precisely quantitative, it clearly demonstrated that a large majority of TcpF was secreted into the culture supernatant and remained there in a soluble form during in vitro growth. Immunoblotting these same fractions for TcpA revealed that the large majority of the TcpA was in the whole-cell fraction, which served as a control for a bacterial associated protein. A band for TcpF was not detected in the whole-cell or culture supernatant fractions from the O395 ΔtcpF mutant, demonstrating the specificity of the anti-TcpF antiserum for the two species of TcpF secreted by the wild-type strain.
FIG. 1.
Secretion of TcpF from V. cholerae. (A) Determination of TcpF and TcpA levels in whole-cell extracts (wc) and culture supernatants (cs) from V. cholerae O395 (wt) and O395 ΔtcpF (ΔtcpF). The numbers below the lanes indicate the total amounts of protein loaded as determined by the bicinchoninic acid protein assay (Pierce). (B) Immunoblot analysis of culture supernatants from strains representing each epidemic serogroup (O1 and O139), the two biotypes of O1, and the two serotypes of classical O1 V. cholerae. The primary antiserum was generated using a purified TcpF-GST fusion protein.
In addition to the more rigorous assessment of the localization of TcpF in classical O1 isolates of V. cholerae, we expanded our analysis of TcpF secretion to include additional strains representing all epidemic serotypes. As shown in Fig. 1, TcpF was detectable in culture supernatants isolated from O395 (O1, classical, Ogawa), 569B (O1, classical, Inaba), N16961 (O1, El Tor), and CVD112 (O139) grown under TCP-inducing conditions (see Materials and Methods). As previously described, TcpF was represented by two distinct bands (33 and 36 kDa) in culture supernatants isolated from O395 (23). Interestingly, 569B produced three bands (30, 32, and 36 kDa) that were cross-reactive with the anti-TcpF sera. These multiple bands most likely represented degradation products of TcpF or otherwise modified forms of TcpF. This hypothesis is supported by the fact that culture supernatants prepared from the O395 tcpF mutant were devoid of any proteins that cross-reacted with this antibody. In the case of N16961 and CVD112, TcpF was represented by a single band on the Western immunoblot. This may have been a result of the fact that, overall, less TcpF was present and the levels of less abundant bands at lower molecular weights were below the limits of detection. A lower level of TcpF expression in these strains is consistent with what is known about TCP expression (25, 46). Alternatively, it is possible that, unlike classical biotype strains, O139 and O1 El Tor strains do not process or modify TcpF.
TcpF is required for colonization of the infant mouse intestine.
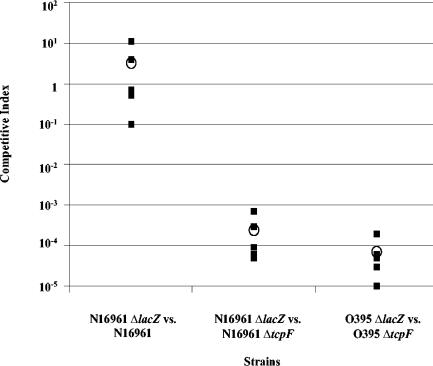
We previously reported that in the case of classical V. cholerae O1, TcpF is required for colonization in the infant mouse cholera model (23). We have also demonstrated that the colonization defect of a tcpF mutant could be rescued by expression of a TcpF-His fusion protein in trans (S. J. Krebs, T. J. Kirn, and R. K. Taylor, Abstr. 103rd Gen. Meet. Am Soc. Microbiol., abstr. B-432, 2003). Because few current clinical cases of cholera are caused by classical V. cholerae isolates, we expanded this study to include a more clinically relevant V. cholerae O1 El Tor isolate (N16961). This strain is also of interest because it was the strain used in the recently completed V. cholerae genome sequencing project (17). Additionally, since epidemic O139 isolates are physiologically similar to El Tor O1 isolates and may have even evolved from such strains, it is likely that results obtained with El Tor strains can be used as a reliable indicator of the behavior of O139 strains (2, 30). We generated an in-frame tcpF deletion in the N16961 background to use for in vivo colonization assays. As shown in Fig. 2, while the wild-type strain N16961 was able to colonize as well as an isogenic N16961 ΔlacZ mutant, a ΔtcpF mutant was significantly defective for colonization compared to the wild-type strain. This was reflected by a competitive index that was found to be more than 1,000-fold less than that of the parental strain. As also shown in Fig. 2, this defect was similar to what was observed for the ΔtcpF mutation in the classical O1 background (O395) when it competed against the isogenic parental strain. As expected, the mutant and wild-type strains competed with a ratio of one in vitro (data not shown). This result demonstrated that TcpF is required in colonization of the infant mouse intestine by both biotypes of V. cholerae O1, and this result may be inferred to extend to the O139 serogroup.
FIG. 2.
In vivo competition of wild-type and tcpF mutants in the infant mouse cholera model. ΔtcpF mutations were generated in O1 El Tor (N16961) and classical (O395) strains and used in competition assays with the corresponding wild-type parental strains. The competitive index for each mouse (▪) and the average for each group (○) are shown.
TcpF and TCP are both required for efficient colonization.
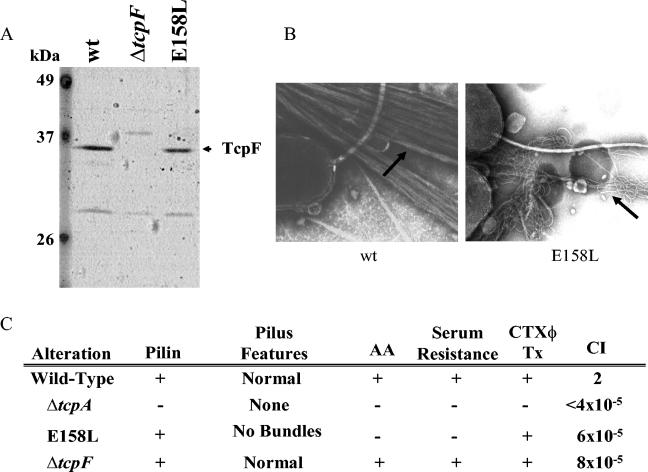
We showed previously that TcpF secretion is dependent upon assembly of the TCP biogenesis apparatus (23). Furthermore, it has been reported that TCP− strains are defective for colonization in infant mice and in humans (18, 44, 45). Since TCP− strains are necessarily deficient for TcpF secretion, it has been impossible to discern if the phenotype of these mutants is a result of the loss of the ability to secrete TcpF or the absence of TCP. To directly determine if TCP and TcpF are both required for colonization, we assessed the ability of a unique tcpA point mutant to compete against the parental wild-type strain in the infant mouse cholera model. As we described previously, E158L is a single amino acid change in the C terminus of TcpA that results in elaboration of a morphologically aberrant pilus (24). While wild-type TCP are bundled, straight structures, the E158L pilus is wavy, unbundled, and deficient for some TCP-associated properties, including colonization (Fig. 3). However, the E158L mutant retains the ability to secrete TcpF (Fig. 3A), making it an excellent strain with which to separate elaboration of a functional TCP from TcpF secretion. As shown in Fig. 3C, the E158L mutant was nearly as defective for colonization as the ΔtcpA mutant (which was TCP− and did not secrete TcpF). This result demonstrates that TCP and TcpF must both be expressed by the same bacterium for efficient colonization of the infant mouse intestine. Interestingly, loss of either of these factors reduced colonization by several logs (in most cases no mutant bacteria were recovered from the mice), suggesting a cooperative or synergistic function for TCP and TcpF in colonization.
FIG. 3.
TcpF secretion and TCP biogenesis in the E158L tcpA point mutant. (A) Culture supernatants isolated from wild-type, ΔtcpF, and E158L strains were analyzed for TcpF secretion by SDS-PAGE and immunoblotting with anti-TcpF antiserum. (B) Transmission electron micrograph showing TCP elaborated by wild-type V. cholerae (wt) and the nonfunctional, morphologically aberrant pili elaborated by the E158L mutant. (C) Relevant in vitro and in vivo features of wild-type, ΔtcpA, E158L, and ΔtcpF strains. The presence of stable pilin, pilus features, autoagglutination (AA), serum resistance, CTX phage transduction efficiency (CTXφ Tx), and competitive indices (CI) were determined as previously described (24).
TCP−, TcpF−, and TCP− TcpF− mutants are all cleared from the infant mouse intestine with similar kinetics.
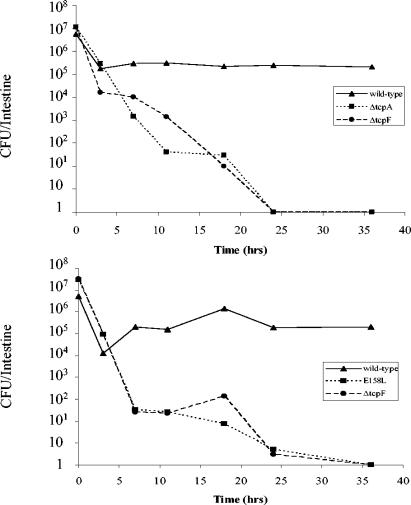
Since TCP and TcpF were found to be required for colonization to similar extents, we wanted to determine if we could discern a difference in the kinetics of clearance of TCP−, TcpF−, and TCP− TcpF− mutants from the mouse intestine. As shown in Fig. 4, the number of wild-type V. cholerae cells in the intestine initially declined and then remained nearly constant for the next 35 h (Fig. 4). In contrast, mutants lacking functional TCP (E158L), TcpF (ΔtcpF), or both factors (ΔtcpA) were continuously cleared from the intestine over the course of the experiment, and the level approached or reached zero at 35 h. Importantly, no obvious difference between kinetics was observed for any of the mutants. This result may be interpreted in two ways. It is possible that TCP and TcpF act in a cooperative manner during infection and that they both are required at the same step (the second step) during colonization. Alternatively, the presence of both of these factors may be required to progress past a certain step (the third step) in the colonization process that determines whether a stable population of bacteria remains in the bowel.
FIG. 4.
Kinetics of bacterial clearance from the infant mouse intestine. (Top panel) Wild-type, ΔtcpA, or ΔtcpF bacteria were individually inoculated into infant mice, and the number of bacteria colonizing the small bowel was determined at various times. Each point represents the average of two mice. (Bottom panel) Wild-type, E158L, and ΔtcpF samples were each mixed with an equal volume of wild-type bacteria (which were ΔlacZ mutants) prior to inoculation, and the numbers of LacZ+ colonies remaining in the intestine at various times were determined. Each point represents the average of two mice.
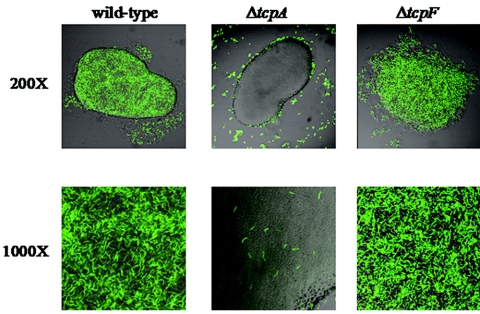
ΔtcpF mutants are able to form microcolonies in vitro.
Our previous experiments demonstrated that in vitro microcolony formation correlates with in vivo colonization (24). Specifically, tcpA mutants incapable of forming mixed microcolonies with wild-type bacteria were uniformly defective for colonization. Based on this finding, we tested the ΔtcpF mutant for the ability to agglutinate together with the wild-type parental strain. As shown in Fig. 5, when O395/pGreenTIR was grown together with unlabeled O395, large microcolonies formed that appeared to be approximately equally composed of the two strains. In contrast, the ΔtcpA pGreenTIR mutant failed to enter the wild-type microcolonies, and just a few individual fluorescent bacteria were visible at high magnification. The ΔtcpF mutant had a somewhat intermediate phenotype. Although the microcolonies included nearly equal numbers of wild-type and ΔtcpF pGreenTIR bacteria, they were consistently not as tightly packed as the wild-type microcolonies, as indicated most notably by the decreased adherence of bacteria around the edges of the microcolonies. Although an intermediate phenotype was evident, large microcolonies were formed. If TcpF enhances colonization by mediating increased bacterial interactions, as TCP does, then the result of a loss of TcpF in vitro is quite dampened compared to the tcpF phenotype in vivo. Perhaps a small defect in vitro translates to a large defect in bacterial interactions in vivo. An alternative explanation is that TcpF mediates its effects through an entirely different pathway in vivo.
FIG. 5.
Wild-type in vitro microcolony formation compared to microcolony formation by the ΔtcpA and ΔtcpF mutants. Wild-type V. cholerae was grown under TCP-inducing conditions along with wild-type, ΔtcpA, and ΔtcpF V. cholerae strains bearing a plasmid encoding GFP. The images of the mixed microcolonies include fluorescein isothiocyanate channel signals (green) overlaying differential interference contrast signals (grey).
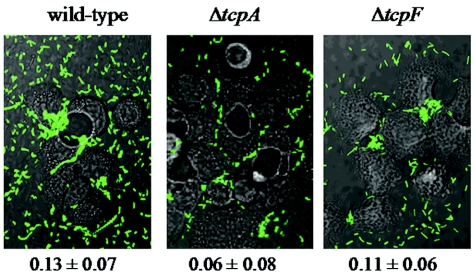
In an effort to support or refute the role of TcpF in microcolony formation in vivo, we assessed the ability of the ΔtcpF mutant to form microcolonies in an in vitro colonization assay. Human colonic epithelial cells (HT-29) were infected with the wild-type, ΔtcpF, or ΔtcpA strain and incubated for 4 h. After 4 h, adherent bacteria were observed by fluorescence microscopy. As shown in Fig. 6, the wild-type and ΔtcpF strains formed large, multicellular microcolonies after 4 h, while the ΔtcpA mutant was capable of attaching but displayed a reduced ability to form microcolonies. In order to determine if TcpF plays a role in epithelial cell attachment, the number of attached bacteria was also determined (Fig. 6). Although the results of this experiment suggested a reduction in attachment for the ΔtcpA mutant, the standard deviations overlapped and no significant difference (P > 0.05) in attachment was noted for either of the mutants when they were compared to each other or the wild type. These results support our previous observation that TCP is chiefly involved in mediating interbacterial interactions and are consistent with other data showing that TCP increases attachment to HT-29 cells by only a small amount (1). Furthermore, these results separate the functions of TCP and TcpF, suggesting that they act at different steps in the pathway in order to have a considerable effect on colonization. Specifically, while neither of these factors appeared to be required for initial attachment in our in vitro assay, TCP was required for microcolony formation, while TcpF seemed to play a minor role in this step. Overall, these results support a multistep model for colonization, in which TcpF functions after TCP to enhance microcolony formation or mediate other effects which are ultimately required for intestinal colonization.
FIG. 6.
Attachment of V. cholerae mutants to HT-29 epithelial cells. Wild-type, ΔtcpA, and ΔtcpF strains bearing a plasmid encoding GFP were inoculated into tissue culture wells containing a confluent layer of HT-29 human colonic epithelial cells. After growth for 4 h, representative photographs were taken, and the number of attached bacteria was determined by appropriately diluting and plating bacteria. The numbers below the micrographs are the ratios of the number of bacteria attached to epithelial cells to the input numbers (means ± standard deviations).
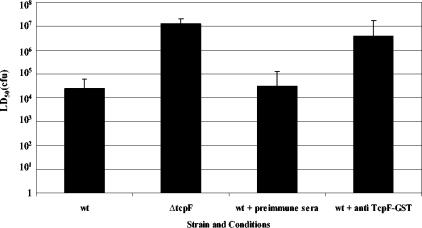
Antibodies directed against TcpF are protective in the infant mouse cholera model.
Since TcpF plays such a critical role in colonization by all epidemic strains, we wanted to determine if it was a reasonable target for incorporation into a multivalent vaccine formulation. We therefore investigated if blocking the TcpF function could protect infant mice inoculated with wild-type bacteria from disease. As shown in Fig. 7, coinoculation of wild-type bacteria with sera collected from rabbits immunized with a TcpF-glutathione S-transferase (GST) fusion protein increased the LD50 approximately 100-fold compared to preimmune serum. This reduction in virulence was intermediate between complete abrogation of the TcpF function (ΔtcpF mutant) and the wild-type phenotype. This level of protection is similar to that seen for antibodies directed against TcpA (38). Since TcpF is a secreted protein, it is likely that the mechanism by which the hyperimmune sera provide protection involves blocking of colonization by direct binding to TcpF in the intestinal lumen. This hypothesis is supported by the fact that the TcpF antisera failed to agglutinate wild-type bacteria in vitro, and, despite numerous attempts, we have been unable to demonstrate binding of the TcpF antisera to the bacterial surface or to TCP using immunoelectron microscopy (data not shown).
FIG. 7.
Protection of infant mice from experimental cholera infection by passive immunization with anti-TcpF antiserum. Dilutions of overnight cultures of wild-type V. cholerae (wt) were mixed with either preimmune or hyperimmune sera from rabbits immunized with purified TcpF-GST. The bars indicate the LD50 at 48 h.
DISCUSSION
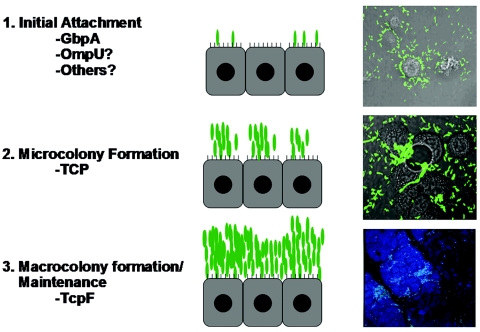
Colonization of the small bowel by V. cholerae may be conceptualized as a series of discrete steps, much like biofilm formation on abiotic or biotic surfaces (10, 50). We propose that colonization likely begins with surface attachment, proceeds to microcolony formation, and ultimately concludes with the production of the large, multicellular bacterial communities characteristic of biofilms (Fig. 8). Such aggregations of V. cholerae have previously been demonstrated to form in the intestines of experimentally infected mice and in resected specimens of human small bowel (24, 53). V. cholerae undoubtedly employs the functions of a variety of proteins to efficiently colonize the human small intestine, and it is likely that each of the steps requires a number of proteins or protein complexes. In the current investigation we extended our studies of one protein, TcpF, which has a potent effect on this process. tcpF mutants are capable of adhering to intestinal epithelial cells and producing microcolonies (Fig. 5 and 6), yet they are severely attenuated for colonization of the infant mouse intestine (Fig. 2). While we previously showed that V. cholerae mutants lacking the ability to form microcolonies in vitro were uniformly defective for colonization, the work presented here separated these two phenotypes (24).
FIG. 8.
Proposed model for V. cholerae intestinal colonization. We propose the following three-step model for colonization: (i) initial attachment (step 1), (ii) microcolony formation (step 2), and (iii) macrocolony formation and maintenance (step 3). Factors that may be involved in each of these steps are listed. Relevant micrographs show GFP-expressing V. cholerae (green) colonizing HT-29 cells (grey) in vitro after 1 and 4 h of incubation; for step 1 the magnification is ×400, and for step 2 the magnification is ×1,000. The micrograph for step 3 shows an infant mouse intestine (blue) colonized by GFP-expressing V. cholerae (green) 16 h after oral inoculation (magnification, ×200).
The phenotype of the ΔtcpF mutant suggests that the TcpF protein may exert its effect at the level of formation of higher-order bacterial complexes. Alternatively, it is possible that our in vitro microcolony or attachment assays were not sensitive enough to detect small defects in bacterial interactions that translate into severe defects in vivo. This is suggested by the results shown in Fig. 5, which revealed a slight defect in agglutination when mutants were mixed with wild-type bacteria. Additionally, previously we showed that the ΔtcpF mutant was slightly defective for in vitro autoagglutination by employing a quantitative assay for this phenotype and that expression of TcpF in trans complemented this defect (23). Since the in vitro colonization assay that we employed to investigate the first two steps of the colonization process is not suitable for analysis of the formation of macrocolonies, we had to rely on the results of the in vivo experiments to investigate these later events. As shown in Fig. 4, the TcpF− strain was cleared from the infant mouse intestine with the same kinetics as a TCP− strain, regardless of whether the mutant was mixed with a wild-type strain. This is notable because it suggests that no real “competition” occurred in these experiments but that the mutant which was unable to colonize simply transited the infant mouse intestine quite rapidly. Our in vitro experiments predicted that the TcpF− strain is capable of forming microcolonies in vivo, while the TCP− mutant is not. Taken together, these results may be interpreted to demonstrate that both of these mutants are blocked at the same step (microcolony formation) or that the TCP− mutant is defective for microcolony formation and the TcpF mutant is defective at a later step, yet both of these deficiencies result in an inability to establish a productive infection, leading to rapid intestinal clearance. In other words, the ability to form microcolonies in vivo does not protect the bacteria from rapid clearance, while the formation of higher-order structures does. Furthermore, it is possible that these two steps (namely, microcolony formation and the formation of higher-order structures) are mechanistically separate events (the former mediated by TCP and the latter mediated by TcpF), yet temporally very closely related.
Interestingly, when wild-type V. cholerae was mixed with the tcpF mutant and both strains were orally fed to infant mice, the wild-type strain was unable to rescue the tcpF phenotype in trans. This result was unexpected because our previous experiments and the data shown in Fig. 1 demonstrated that TcpF is a soluble factor and therefore would be expected to function in trans. Several explanations for this observation are equally plausible. It is possible that TcpF interacts with TCP or the bacterial membrane in vivo, yet is recovered as a soluble protein in vitro. A second possibility is that TcpF may exert its effect immediately upon secretion, in close proximity to the bacteria that secrete it. This is consistent with a model in which TCP-mediated interactions bring bacteria into close proximity with one another and then TcpF, secreted by the bacteria in a given microcolony, strengthens or stabilizes the interbacterial interaction.
Regardless of the exact mechanism by which TcpF enhances colonization, blocking its function could certainly be a reasonable approach to preventing disease in humans. Recent approaches to cholera vaccine design have largely focused on the production of live attenuated oral formulations. Several live vaccines have been developed, including CVD103-HgR (classical, Inaba), CVD 111 (El Tor, Ogawa), combined CVD 103-HgR, CVD 111, Peru-15 (El Tor, Inaba), Bengal-15 (O139), and V. cholerae 638 (El Tor, Ogawa) (9, 27, 43). The results of field trials with some of these formulations were quite favorable, although protection was often highly variable and required more than one dose (35, 36). These results may have been in part related to differential expression of antigens that engender protective immunity. Several studies have documented the efficacy of antibodies directed against V. cholerae subunits (TcpA and lipopolysaccharide) for protection from disease in the infant mouse cholera model (7, 28, 38, 39, 52). Subunit vaccines may prove to be superior to whole-cell live vaccines since the appropriate antigens can reliably be introduced into each individual at the appropriate dose. In addition, enhanced immunologic responses can be induced by incorporating appropriate biological response mediators into the vaccine formulation (29). Based on the results presented here, we propose that TcpF may be a reasonable antigen to include in a polyvalent subunit vaccine formulation. Combined with anti-lipopolysaccharide and anti-TCP responses, antibodies directed against TcpF may potently inhibit colonization in the human intestine, thereby preventing disease (7, 52). Furthermore, if antigens representing proteins involved in each step of the colonization process are combined into a vaccine formulation, a higher degree of protection may be provided than that obtained with a single subunit or with multiple subunits, each of which is a portion of proteins involved in the same step of colonization. This concept is well illustrated by the evolution of the pertussis vaccine in the United States. Pertussis is a severe, acute respiratory disease caused by the gram-negative bacterium Bordetella pertussis. Much like cholera, pertussis is primarily a toxin-mediated disease. Following colonization of the respiratory epithelium, B. pertussis produces toxins that paralyze the cilia and cause inflammation in the respiratory tract, potentially causing pneumonia. A whole-cell pertussis vaccine was developed in the mid-1930s and was combined into the diphtheria-tetanus-pertussis combination vaccine in the mid-1940s. This combination vaccine was reasonably efficacious (70% to 90%), with protection lasting 5 to 10 years. Local adverse reactions were common with this vaccine, and severe systemic events occurred less frequently yet were common enough to prompt interest in the development of a safer vaccine. Based on advances in our understanding of the molecular mechanisms of B. pertussis pathogenesis, several acellular, multisubunit vaccines were developed. The components present in these vaccines include adhesive proteins involved in colonization (pertactin, filamentous hemagglutinin, fimbria types 2 and 3), and pertussis toxoid. These acellular vaccines were introduced into clinical practice in the United States in 1991 as the fourth and fifth doses of a five-dose series and were approved for use in the primary series in 1996 as part of the diphtheria-tetanus-acellular pertussis combination vaccine. In addition to reducing the number of adverse events associated with pertussis vaccination, these formulations also proved to be at least as protective as their whole-cell predecessor (7, 12-15, 28). Recent studies have demonstrated that humans are capable of mounting an immune response to TcpF during V. cholerae infection, further validating the hypothesis that TcpF is a viable option for inclusion in a multisubunit cholera vaccine (16).
Obviously, further work is required to more completely understand TcpF function in vivo and to identify specific regions within TcpF that elicit protective immunity. We are currently using genetic and immunological approaches to further define the function of TcpF in the colonization process and to identify discrete regions of this protein that are important for protection.
Acknowledgments
This work was supported by grant AI25096 from the NIAID to R.K.T. T.J.K. was supported by NIH training grant AI007519 and by a Rosalind Borison Memorial fellowship.
Editor: V. J. DiRita
REFERENCES
- 1.Benitez, J. A., R. G. Spelbrink, A. Silva, T. E. Phillips, C. M. Stanley, M. Boesman-Finkelstein, and R. A. Finkelstein. 1997. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect. Immun. 65:3474-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bik, E. M., A. E. Bunschoten, R. D. Gouw, and F. R. Mooi. 1995. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 14:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bomchil, N., P. Watnick, and R. Kolter. 2003. Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J. Bacteriol. 185:1384-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunwald, E. 2001. Harrison's principles of internal medicine, 15th ed. McGraw-Hill, New York, N.Y.
- 5.Cash, R. A., S. I. Music, J. P. Libonati, M. J. Snyder, R. P. Wenzel, and R. B. Hornick. 1974. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J. Infect. Dis. 129:45-52. [DOI] [PubMed] [Google Scholar]
- 6.Cassel, D., and Z. Selinger. 1977. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. USA 74:3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernyak, A., S. Kondo, T. K. Wade, M. D. Meeks, P. M. Alzari, J. M. Fournier, R. K. Taylor, P. Kovac, and W. F. Wade. 2002. Induction of protective immunity by synthetic Vibrio cholerae hexasaccharide derived from V. cholerae O1 Ogawa lipopolysaccharide bound to a protein carrier. J. Infect. Dis. 185:950-962. [DOI] [PubMed] [Google Scholar]
- 8.Chiang, S. L., R. K. Taylor, M. Koomey, and J. J. Mekalanos. 1995. Single amino acid substitutions in the N-terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance. Mol. Microbiol. 17:1133-1142. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, M. B., R. A. Giannella, J. Bean, D. N. Taylor, S. Parker, A. Hoeper, S. Wowk, J. Hawkins, S. K. Kochi, G. Schiff, and K. P. Killeen. 2002. Randomized, controlled human challenge study of the safety, immunogenicity, and protective efficacy of a single dose of Peru-15, a live attenuated oral cholera vaccine. Infect. Immun. 70:1965-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davey, M. E., and A. O'Toole, G. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giron, J. A., A. S. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 12.Greco, D., S. Salmaso, P. Mastrantonio, M. Giuliano, A. E. Tozzi, A. Anemona, M. L. Ciofi degli Atti, A. Giammanco, P. Panei, W. C. Blackwelder, D. L. Klein, and S. G. Wassilak. 1996. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. N. Engl. J. Med. 334:341-348. [DOI] [PubMed] [Google Scholar]
- 13.Guris, D., P. M. Strebel, R. Tachdjian, B. Bardenheier, M. Wharton, and S. C. Hadler. 1997. Effectiveness of the pertussis vaccination program as determined by use of the screening method: United States, 1992-1994. J. Infect. Dis. 176:456-463. [DOI] [PubMed] [Google Scholar]
- 14.Halperin, S. A., B. Smith, M. Russell, P. Hasselback, R. Guasparini, D. Skowronski, W. Meekison, R. Parker, P. Lavigne, and L. Barreto. 2000. An adult formulation of a five-component acellular pertussis vaccine combined with diphtheria and tetanus toxoids is safe and immunogenic in adolescents and adults. Vaccine 18:1312-1319. [DOI] [PubMed] [Google Scholar]
- 15.Halperin, S. A., B. Smith, M. Russell, D. Scheifele, E. Mills, P. Hasselback, C. Pim, W. Meekison, R. Parker, P. Lavigne, and L. Barreto. 2000. Adult formulation of a five component acellular pertussis vaccine combined with diphtheria and tetanus toxoids and inactivated poliovirus vaccine is safe and immunogenic in adolescents and adults. Pediatr. Infect. Dis. J. 19:276-283. [DOI] [PubMed] [Google Scholar]
- 16.Hang, L., M. John, M. Asaduzzaman, E. A. Bridges, C. Vanderspurt, T. J. Kirn, R. K. Taylor, J. D. Hillman, A. Progulske-Fox, M. Handfield, E. T. Ryan, and S. B. Calderwood. 2003. Use of in vivo-induced antigen technology (IVIAT) to identify genes uniquely expressed during human infection with Vibrio cholerae. Proc. Natl. Acad. Sci. USA 100:8508-8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrington, D. A., R. H. Hall, G. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwanaga, M., K. Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 30:1075-1083. [DOI] [PubMed] [Google Scholar]
- 20.Kahn, R. A., and A. G. Gilman. 1984. ADP-ribosylation of Gs promotes the dissociation of its alpha and beta subunits. J. Biol. Chem. 259:6235-6240. [PubMed] [Google Scholar]
- 21.Kahn, R. A., and A. G. Gilman. 1986. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J. Biol. Chem. 261:7906-7911. [PubMed] [Google Scholar]
- 22.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirn, T. J., N. Bose, and R. K. Taylor. 2003. Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae. Mol. Microbiol. 49:81-92. [DOI] [PubMed] [Google Scholar]
- 24.Kirn, T. J., M. J. Lafferty, C. M. Sandoe, and R. K. Taylor. 2000. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae. Mol. Microbiol. 35:896-910. [DOI] [PubMed] [Google Scholar]
- 25.Kovacikova, G., and K. Skorupski. 2000. Differential activation of the tcpPH promoter by AphB determines biotype specificity of virulence gene expression in Vibrio cholerae. J. Bacteriol. 182:3228-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaPointe, C. F., and R. K. Taylor. 2000. The type 4 prepilin peptidases comprise a novel family of aspartic acid proteases. J. Biol. Chem. 275:1502-1510. [DOI] [PubMed] [Google Scholar]
- 27.Levine, M. M., J. B. Kaper, D. Herrington, J. Ketley, G. Losonsky, C. O. Tacket, B. Tall, and S. Cryz. 1988. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet ii:467-470. [DOI] [PubMed] [Google Scholar]
- 28.Meeks, M. D., R. Saksena, X. Ma, T. K. Wade, R. K. Taylor, P. Kovac, and W. F. Wade. 2004. Synthetic fragments of Vibrio cholerae O1 Inaba O-specific polysaccharide bound to a protein carrier are immunogenic in mice but do not induce protective antibodies. Infect. Immun. 72:4090-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meeks, M. D., T. K. Wade, R. K. Taylor, and W. F. Wade. 2001. Immune response genes modulate serologic responses to Vibrio cholerae TcpA pilin peptides. Infect. Immun. 69:7687-7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mooi, F. R., and E. M. Bik. 1997. The evolution of epidemic Vibrio cholerae strains. Trends Microbiol. 5:161-165. [DOI] [PubMed] [Google Scholar]
- 31.Mundy, R., D. Pickard, R. K. Wilson, C. P. Simmons, G. Dougan, and G. Frankel. 2003. Identification of a novel type IV pilus gene cluster required for gastrointestinal colonization of Citrobacter rodentium. Mol. Microbiol. 48:795-809. [DOI] [PubMed] [Google Scholar]
- 32.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 33.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 34.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 35.Richie, E. E., N. H. Punjabi, Y. Y. Sidharta, K. K. Peetosutan, M. M. Sukandar, S. S. Wasserman, M. M. Lesmana, F. F. Wangsasaputra, S. S. Pandam, M. M. Levine, P. P. O'Hanley, S. J. Cryz, and C. H. Simanjuntak. 2000. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 18:2399-2410. [DOI] [PubMed] [Google Scholar]
- 36.Ryan, E. T., and S. B. Calderwood. 2001. Cholera vaccines. J. Travel Med. 8:82-91. [DOI] [PubMed] [Google Scholar]
- 37.Sperandio, V., J. A. Giron, W. D. Silveira, and J. B. Kaper. 1995. The OmpU outer membrane protein, a potential adherence factor of Vibrio cholerae. Infect. Immun. 63:4433-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, D., M. J. Lafferty, J. A. Peek, and R. K. Taylor. 1997. Domains within the Vibrio cholerae toxin coregulated pilin subunit that mediate bacterial colonization. Gene 192:79-85. [DOI] [PubMed] [Google Scholar]
- 39.Sun, D. X., J. J. Mekalanos, and R. K. Taylor. 1990. Antibodies directed against the toxin-coregulated pilus isolated from Vibrio cholerae provide protection in the infant mouse experimental cholera model. J. Infect. Dis. 161:1231-1236. [DOI] [PubMed] [Google Scholar]
- 40.Tacket, C. O., G. Losonsky, J. P. Nataro, L. Comstock, J. Michalski, R. Edelman, J. B. Kaper, and M. M. Levine. 1995. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J. Infect. Dis. 172:883-886. [DOI] [PubMed] [Google Scholar]
- 41.Tacket, C. O., R. K. Taylor, G. Losonsky, Y. Lim, J. P. Nataro, J. B. Kaper, and M. M. Levine. 1998. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect. Immun. 66:692-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taniguchi, T., Y. Akeda, A. Haba, Y. Yasuda, K. Yamamoto, T. Honda, and K. Tochikubo. 2001. Gene cluster for assembly of pilus colonization factor antigen III of enterotoxigenic Escherichia coli. Infect. Immun. 69:5864-5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor, D. N., C. O. Tacket, G. Losonsky, O. Castro, J. Gutierrez, R. Meza, J. P. Nataro, J. B. Kaper, S. S. Wasserman, R. Edelman, M. M. Levine, and S. J. Cryz. 1997. Evaluation of a bivalent (CVD 103-HgR/CVD 111) live oral cholera vaccine in adult volunteers from the United States and Peru. Infect. Immun. 65:3852-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thelin, K. H., and R. K. Taylor. 1996. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect. Immun. 64:2853-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas, S., S. G. Williams, and P. A. Manning. 1995. Regulation of tcp genes in classical and El Tor strains of Vibrio cholerae O1. Gene 166:43-48. [DOI] [PubMed] [Google Scholar]
- 47.Torgersen, M. L., G. Skretting, B. van Deurs, and K. Sandvig. 2001. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 114:3737-3747. [DOI] [PubMed] [Google Scholar]
- 48.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 50.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu, J. Y., W. F. Wade, and R. K. Taylor. 2001. Evaluation of cholera vaccines formulated with toxin-coregulated pilin peptide plus polymer adjuvant in mice. Infect. Immun. 69:7695-7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto, T., and T. Yokota. 1988. Electron microscopic study of Vibrio cholerae O1 adherence to the mucus coat and villus surface in the human small intestine. Infect. Immun. 56:2753-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]