Abstract
We report the cloning of a novel antimicrobial peptide gene, termed rtCATH_1, found in the rainbow trout, Oncorhynchus mykiss. The predicted 216-residue rtCATH_1 prepropeptide consists of three domains: a 22-residue signal peptide, a 128-residue cathelin-like region containing two identifiable cathelicidin family signatures, and a predicted 66-residue C-terminal cationic antimicrobial peptide. This predicted mature peptide was unique in possessing features of different known (mammalian) cathelicidin subgroups, such as the cysteine-bridged family and the specific amino-acid-rich family. The rtCATH_1 gene comprises four exons, as seen in all known mammalian cathelicidin genes, and several transcription factor binding sites known to be of relevance to host defenses were identified in the 5′ flanking region. By Northern blot analysis, the expression of rtCATH_1 was detected in gill, head kidney, and spleen of bacterially challenged fish. Primary cultures of head kidney leukocytes from rainbow trout stimulated with lipopolysaccharide or poly(I · C) also expressed rtCATH_1. A 36-residue peptide corresponding to the core part of the fish cathelicidin was chemically synthesized and shown to exhibit potent antimicrobial activity and a low hemolytic effect. Thus, rtCATH_1 represents a novel antimicrobial peptide gene belonging to the cathelicidin family and may play an important role in the innate immunity of rainbow trout.
Rapid and effective defense against pathogens is essential for the survival of all living organisms that coexist with microbes. Increasing evidence suggests that endogenous peptides with nonspecific antimicrobial properties play a critical role in the host first line of defense (1, 15, 19). More than 700 antimicrobial peptides have been isolated from diverse species. Most of them share characteristics, such as small size, cationic charge, and an amphipathic conformation. They tend to display broad-spectrum antimicrobial activity and have many potential roles in innate immunity (15). These peptides are found on epithelial surfaces (7, 16), where they act as a local defense mechanism, and in circulating myeloid-derived cells (25, 38), which gather at the site of microbial invasion. Based on their common features, these peptides can be divided into several families (5) that include well-known molecules, such as the cecropins from (primarily) insects, magainins from amphibians, and in mammals, defensins and cathelicidins.
The cathelicidin family was first recognized in the early 1990s (46). They are a numerous group of proteins that share a highly conserved preproregion that contains the cathelin-like domain, a 12-kDa protein from porcine leukocytes (36), at the N terminus and carry diverse antimicrobial peptides at the C terminus. In most species, cathelicidins are expressed in myeloid precursor cells (34) and stored in neutrophil granules as propeptides without antimicrobial activities. After neutrophil activation leading to elastase-mediated endoproteolytic cleavage, the highly variable C-terminal antimicrobial peptide is released (19). The mature cathelicidin peptides are relatively small, most <100 amino acids, with an amphipathic molecular structure and a net positive charge. These two characteristics are related to the interaction of such peptides with bacterial membranes as part of their bactericidal mechanism. Cathelicidins are typically found in neutrophils and at mucosal surfaces, suggesting they contribute to systemic and local defense (13). Thus far, the cathelicidins have been described only in mammals, including human, monkey, horse, cattle, sheep, goat, pig, rabbit, mouse, and guinea pig (34), except for two sequences from hagfish published recently and cited as representing an “ancient ancestor of the cathelicidin gene family” (43).
Relatively few studies of antimicrobial peptides from fish have been performed, despite the problem of infectious diseases and the limitation of using antibiotics in aquaculture worldwide. Attempts to find antimicrobial peptides have had a number of successes in fish but have so far focused mainly on the epithelial surfaces. For example, paradaxin, a 33-amino-acid toxic polypeptide with bactericidal activity, was discovered in the mucous glands of Moses sole fish (30); pleurocidin, a 25-residue linear peptide active against a broad spectrum of microorganisms, was found in skin mucous secretions of winter flounder (7) and immunolocalized to mucin granules of goblet cells in the intestine; misgurin, a 21-amino-acid pore-forming antimicrobial peptide without significant hemolytic activity, was isolated from the loach (31); piscidin, a 22-amino-acid peptide rich in histidine and phenylalanine, was first isolated from skin of hybrid striped bass and immunolocalized to eosinophilic granular cells in gill, skin, and gut and those lining blood vessels in the viscera (40); and moronecidin, an α-helical antimicrobial peptide with 22 residues, was found in the skin and gills of hybrid striped bass (23). Neither systemic antimicrobial peptides produced by myeloid cells nor members belonging to the cathelicidin family have yet been found in jawed fish.
Through routine screening of rainbow trout Oncorhynchus mykiss clones for immunologically important genes, a partial sequence was obtained with apparent homology to the mammalian cathelicidin gene family. This allowed the design of gene-specific primers to confirm and extend the sequence. In this paper, we report the cloning of this novel antimicrobial peptide gene, termed the rtCATH_1 gene (from rainbow trout cathelicidin_1). The gene organization and expression were studied, and a peptide based on the mature peptide sequence of the rtCATH_1 gene was synthesized chemically and found to have potent antimicrobial activities against fish-pathogenic bacteria.
MATERIALS AND METHODS
Fish, cell culture, and microorganisms.
Rainbow trout O. mykiss weighing 300 to 350 g, obtained from a local fish farm (Almond Bank, Perthshire, United Kingdom), were maintained and acclimatized in a recirculating freshwater system at 14°C with regular feeding.
The reference strain Escherichia coli NCIMB12210 (ATCC 25922) was obtained from NCIMB Ltd., Aberdeen, United Kingdom. Nine field isolates of fish pathogens were provided by FRS Marine Laboratory, Aberdeen, United Kingdom. Aeromonas salmonicida A-layer-negative avirulent strain MT004 and the A-layer-positive virulent strain MT423 (17) were isolated from rainbow trout. Photobacterium damselae subsp. piscicida MT1415 was a virulent capsulated strain isolated from sea bass Dicentrarchus labrax, and EPOY8803II was an avirulent noncapsulated strain isolated from red grouper Epinephelly akaara (26). Vibrio anguillarum serogroup O1 MT1741 (3) and serogroup O2 MT1742 (4) and Yersinia ruckeri MT252 were isolated from rainbow trout. Lactoccus garvieae noncapsulated strain MT2055 and capsulated strain MT2291 were originally isolated from yellowtail and rainbow trout, respectively (2). E. coli was maintained in Luria-Bertani (LB) medium at 37°C. The aquatic strains were maintained in tryptic soy broth medium at 22°C. All the strains were transferred to Mueller-Hinton broth (Sigma) and cultured to optimal concentrations before the antimicrobial assays.
Isolation of the full-length cDNA.
Wax-mediated hot-start PCR was performed using 0.5 μl of an enzyme mixture of Taq (5 U/μl; Bioline, United Kingdom) DNA polymerase and Pfu (3 U/μl; Promega, United Kingdom) DNA polymerase (50:1) in a 50-μl reaction volume containing the following components: 5 μl of 10× NH4 buffer, 2 μl of 10 mM deoxynucleoside triphosphate mixture, 10 μl of 10 mM MgCl2, 2 μl of 10 μM forward primer, 2 μl of 10 μM reverse primer, 2 μl of DNA template, and 26.5 μl of sterile H2O. The cycling protocol was 1 cycle of 94°C for 5 min and 28 to 32 cycles of 94°C for 45 s, 58 to 62°C for 45 s, and 72°C for 1 min, followed by 1 cycle of 72°C for 10 min. Initial PCR using rtCATH_1-F1 forward (Table 1) and rtCATH_1-R1 reverse primers was performed on a rainbow trout phytohemagglutinin-stimulated leukocyte cDNA library (inserted into the λ ZAP express vector using the ZAP Express cDNA synthesis kit [Stratagene]) (9) and gave a product of 613 bp, confirming the presence of the gene (Fig. 1). Anchored PCR using the gene-specific primer rtCATH_1-F1 with the vector-specific primer M13F, followed by a nested PCR using rtCATH_1-F2 with the vector-specific primer T7, was used to obtain the 3′ end of the gene and gave a product of 541 bp. However, attempts to obtain the 5′ end of the gene by a similar procedure failed. Therefore, the cloning strategy to obtain the 5′ end was switched to random amplification of cDNA ends PCR.
TABLE 1.
Primers used for cloning and sequencing of rainbow trout rtCATH_1
| Name | Sequence (5′ → 3′) | Position |
|---|---|---|
| Trout cathelicidin primers | ||
| rtCATH_1-F1 | CATCCTGCTCGCTGTGGCTGTCCT | +34 to +57 |
| rtCATH_1-F2 | GATTCGGACAAGAAGAAGCAAAG | +1056 to +1078 |
| rtCATH_1-F3 | GAAGATGAAGGTCCAGGTGAGA | +7 to +28 |
| rtCATH_1-F4 | CTGACCTGTCTACATTTGTTTG | +728 to +749 |
| rtCATH_1-R1 | GCGATTTCCATCACTGTGATCTCT | +1249 to +1272 |
| rtCATH_1-R2 | CACAATTTTTGCCTCTGGAGCATATTCT | +1081 to +1108 |
| rtCATH_1-R3 | CACAAACAAATGTAGACAGGTCAGTGTT | +724 to +751 |
| Other primers | ||
| RT-BT-F | ATGGAAGATGAAATCGCC | |
| RT-BT-R | TGCCAGATCTTCTCCATG | |
| M13F | CGTTGTAAAACGACGGCCAG | |
| T7 | TGTAATACGACTCACTATAGGG | |
| GR5P | CGACUGGAGCACGAGGACACUGA | |
| GR5NP | GGACACUGACAUGGACUGAAGGAGUAGAAA | |
| AP1 | GTAATACGACTCACTATAGGGC | |
| AP2 | ACTATAGGGCACGCGTGGT |
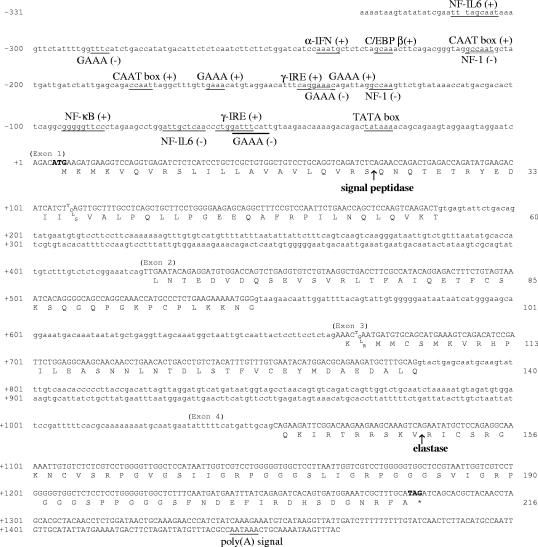
FIG. 1.
Nucleotide and deduced amino acid sequences of rainbow trout rtCATH_1. Numbering is on the left for nucleotides and on the right for amino acids. Exons are shown in uppercase; introns and 5′ flanking region are shown in lowercase. The predicted translation of exon-coding regions is given. The putative cleavage sites for the signal peptidase and elastase are indicated by arrows. Features in boldface represent the start/stop codons. Consensus binding motifs for transcription factors, the TATA box, and the polyadenylation site are underlined.
The transcription initiation start was determined using a GeneRACER kit (Invitrogen, United Kingdom) that allowed amplification of full-length capped mRNA. Total mRNA was extracted from stimulated rainbow trout head kidney using an OligotexmRNA Mini Kit (QIAGEN, United Kingdom). The full-length, ligase-mediated rapid amplification of 5′ end RNA was conducted according to the manufacturer's instructions. The treated RNA was reverse transcribed by treatment with Moloney murine leukemia virus reverse transcriptase (RNase H low; Bioline, United Kingdom) at 60°C for 20 min, followed by 65°C for 50 min. The full-length 5′ end of the transcript was then obtained by PCR with primers GR5P (supplied with the kit) and rtCATH_1-R2, followed by a nested PCR using primers GR5NP (supplied with the kit) and rtCATH_1-R3, and gave a product of 425 bp.
Isolation of genomic DNA.
Genomic DNA was isolated from rainbow trout liver using a genomic DNA isolation kit (QIAGEN, United Kingdom). The resultant DNA was dissolved in distilled H2O to a final concentration of 0.25 mg/ml. The genomic sequence was obtained by PCR using the primers rtCATH_1-F3 and rtCATH_1-R1 and gave a product of 1,266 bp. The 5′ flanking region was obtained using a gene-walking approach. Gene-walking libraries were constructed with a Universal GenomeWalker kit (Clontech) according to the manufacturer's instructions. Amplification of the 5′ end of the gene was performed using primers AP1 (supplied with the kit) and rtCATH_1-R2, which gave a product of 1,118 bp. Nested PCRs were carried out on the DraI-restricted DNA using primers AP2 (supplied with the kit) and rtCATH_1-R3. The cycling protocol for the PCR was 1 cycle of 94°C for 5 min, 5 cycles of 94°C for 45 s and 72°C for 2 min, 5 cycles of 94°C for 45 s and 70°C for 2 min, and 25 cycles of 94°C for 45 s, 64°C for 45 s, and 70°C for 2 min, followed by 1 cycle of 72°C for 10 min.
Cloning and sequencing.
All products obtained by PCR were directly cloned into the pGEM-T Easy vector (Promega, United Kingdom). Plasmid DNA was isolated from bacterial colonies that had an appropriately sized insert using a Qiaprep spin miniprep kit (QIAGEN, United Kingdom). Three randomly selected clones representing each product were sequenced on an ABI 377 Automated Sequencer (Applied Biosystems) using vector- or gene-specific primers. Comparisons of nucleotide and amino acid sequences with GenBank/EMBL databases were performed using the FASTA program (32). Direct comparisons between two sequences were performed using the GAP alignment program (29) within the Wisconsin Genetics Computer Group Sequence Analysis Software Package (version 9.1; 1997), and multiple sequence alignments were generated using CLUSTAL W (version 1.74) (42). Putative transcription factor binding sites were predicted by MatInspector version 2.2/TRANSFAC 4.0, GBF (http://www.genomatix.de/) (33).
Expression studies.
Northern blot analysis was used to study the sites of expression of the rtCATH_1 gene in normal and infection situations. For the in vivo studies, fish were injected intraperitoneally with A. salmonicida MT423 at 2 × 106 bacteria (suspended in 100 μl phosphate-buffered saline [PBS]) per fish, a dose and route shown in other studies to lead to mortalities in this species (18). Fish were killed separately at 8 and 24 h postinjection. Tissues were sampled from gill, head kidney, liver, intestine, skin, and spleen. Total RNA was extracted from all tissues using Trizol (Invitrogen, United Kingdom). Twenty micrograms of RNA was loaded per lane of a 1.1% agarose gel. Following electrophoresis at 100 V for 3 h in 1× MOPS (morpholinepropanesulfonic acid) buffer (Amersham, United Kingdom) and transfer to an N+ nylon filter (Amersham, United Kingdom), the membrane was baked at 80°C for 1 h. The 18S rRNA was visualized and photographed to assess the quality of the RNA and to ensure equal loading of RNA for each sample before transfer. The region of rtCATH_1 cDNA between the rtCATH_1 F3 and rtCATH_1 R3 primers was amplified by wax-mediated hot-start PCR, labeled with [32P] dCTP using the Megaprime radiolabeling kit (Amersham, United Kingdom), and hybridized to RNA. After stringent washing, the membrane was exposed to X-ray film (Kodak) at −70°C for 72 h. The relative levels of mRNA were quantified by densitometric scanning of the exposed film using a UVP gel-imaging system and UVP Gelworks ID advanced software.
Reverse transcription-PCR was used to perform the in vitro studies. The head kidney was collected under sterile conditions from freshly killed trout and gently pushed through a 100-μm nylon mesh (John Staniar, United Kingdom) with ice-cold Leibovitz medium (L15; Gibco). After being washed with L15 medium, the cells were resuspended in L15 and cultured at 18°C in the absence or presence of 0.1, 1, 10, and 100 μg/ml of lipopolysaccharide (LPS) (E. coli O127:B8; Sigma) or poly(I · C) (Pharmacia Biotech), respectively. Four hours after stimulation, cells were harvested and total RNA was extracted using Trizol (Invitrogen). cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (RNase H low; Bioline, United Kingdom) from 5 μg total RNA at 42°C for 50 min with the oligo(dT) primer (Invitrogen, United Kingdom). PCR was performed on the resulting cDNAs using the specific primers rtCATH_1-F4 and R1, which span intron 3 of the trout genomic sequence. β-Actin was amplified as a positive control for RT-PCR, using the RT-BT-F and RT-BT-R primers listed in Table 1. PCR reagents were as described above, and the cycling protocol was 1 cycle of 94°C for 4 min and 33 cycles of 94°C for 45 s, 62°C for 45 s, and 70°C for 1 min, with a final extension step of 10 min at 72°C. Selections of PCR products were sequenced to confirm correct amplification of the rtCATH_1 gene in each tissue/cell type with primers F4 and R1.
Construction of prtCATH_1 expression vector.
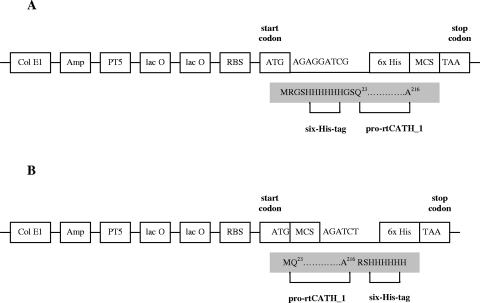
The cDNA of rtCATH_1 was amplified by PCR, sequenced, and cloned into pGEM-T easy vector (Promega). Using the sequenced plasmid DNA as a template, the cDNA fragment encoding the proregion of rtCATH_1 (prtCATH_1), from Q23 to the C terminus, was amplified again by PCR, and a translation start codon ATG was added. Two expression vectors named pQE30 and pQE70 (QIAGEN) containing a six-His tag at the N terminus and C terminus, respectively, were used to produce the recombinant proteins. To ligate into the pQE30 vector, restriction enzyme sites for BamHI and for HindIII were added by PCR at the 5′ and 3′ ends, respectively. To ligate into the pQE70 vector, restriction enzyme sites for SphI and BglII were added by PCR at the 5′ and 3′ ends, respectively. The resultant expression plasmids were named pQE30-rtCATH_1 and pQE70-rtCATH_1 (Fig. 2).
FIG. 2.
Diagram showing the prtCATH_1 expression vectors: pQE30-rtCATH_1 (A) and pQE70-rtCATH_1 (B). Col E1, Col E1 origin of replication; Amp, ampicillin resistance gene; PT5, T5 promoter; lac O, lac operator; RBS, ribosome-binding site; six-His, six-His tag sequence; MCS, multiple cloning site. The shaded areas represent the amino acid sequences translated from the vector.
Production and purification of prtCATH_1.
To express the recombinant prtCATH_1 protein, the plasmids pQE30-rtCATH_1 and pQE70-rtCATH_1 were transformed into E. coli JM109 cells (Promega). A single colony containing target plasmid was cultured in 5 ml LB medium (Sigma) containing ampicillin (100 μg/ml) and cultured at 37°C with shaking (300 rpm) overnight prior to culture in 250 ml LB medium containing ampicillin for 4 h and then incubated for 2 h with the addition of isopropyl β-d-1-thiogalactopyranoside (IPTG; 0.5 mM final concentration). The E. coli cells were then harvested by centrifugation at 4°C, lysed in phosphate buffer (100 mM NaH2PO4, 10 mM Tris-Cl, pH 8.0) containing 8 M urea, and loaded onto an Ni-nitrilotriacetic acid column (QIAGEN). Purification was conducted according to the QIAGEN protocols. Briefly, the column containing bacterial lysate was washed several times with the above-mentioned buffer at pH 6.3 to remove proteins without a six-His tag. The recombinant protein was then eluted using the phosphate buffer at pH 5.9 and 4.5, respectively. The protein size, purification, and concentrations of flowthrough fractions were determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and UVP image analysis. The resultant proteins generated from plasmids pQE30-rtCATH_1 and pQE70-rtCATH_1 were named 6His-prtCATH_1 and prtCATH_1-6His, respectively.
Enzymatic cleavage of prtCATH_1 and Western blot analysis.
Human neutrophil elastase (ELAStin Products Company, Inc., Owensville, Mo.) was applied to analyze the cleavage site of the prtCATH_1 protein. Fifty nanograms of elastase in Tris buffer (2 M Tris, 0.1 M NaOAc, 0.01% bovine serum albumin, pH 7.4) was incubated with 6His-prtCATH_1 or prtCATH_1-6His protein (5 μg) at 20°C for different times (see the legend to Fig. 4). The reaction was stopped by adding an equal volume of 5% acetic acid.
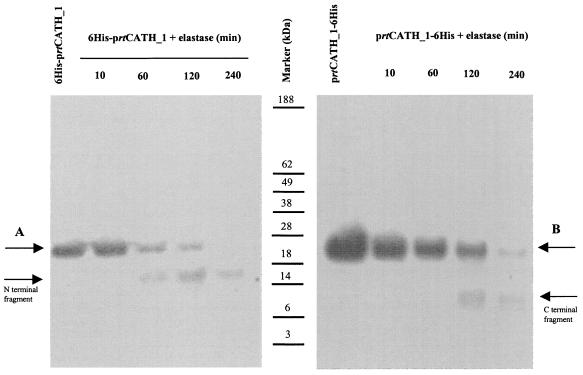
FIG. 4.
Time-dependent cleavage of prtCATH_1 by elastase. Shown is a Western blot of a 4 to 12% polyacrylamide gel after reaction with an anti-six-His tag antibody. Five micrograms of 6His-prtCATH_1 (A) and prtCATH_1-6His (B) was incubated with elastase (50 ng) separately for the times indicated, and the reactions were stopped by acidification with equal volumes of 5% acetic acid. Note the appearance of the N-terminal and C-terminal fragments of 15 kDa and 8 kDa, respectively, with time.
Western blotting was performed using the Westernbreeze Chemiluminescent Immunodetection System (Invitrogen) according to the manufacturer's instructions. Briefly, samples were loaded onto a 4 to 12% polyacrylamide gel and electroblotted to a nitrocellulose membrane. The membrane was blocked with the Westernbreeze blocking solution for 30 min and incubated with a monoclonal anti-six-His antibody (Invitrogen) to detect the six-His tag at the N terminus and C terminus of 6His-prtCATH_1 and prtCATH_1-6His, respectively, for 1 h. The membranes were rinsed with antibody washing solution and water, covered with detection buffer, and exposed to X-ray film.
Peptide synthesis.
Since the yield of recombinant protein cleaved by elastase was low and could be detected only by Western blotting, it was decided to test the antimicrobial activity using synthesized peptides. However, the full length of the predicted mature peptide (66 amino acids) was difficult to make chemically, and thus, a 36-amino-acid peptide, rtCATH_1(R151-V186), with a net charge of +6 and containing key features of the known cathelicidins, was synthesized commercially by the Proteome Facility at the University of Aberdeen (United Kingdom) using solid-phase 9-fluorenylmethoxy carbonyl chemistry and purified by reverse-phase high-performance liquid chromatography. The peptide mass of 3,464.49 Da was verified by mass spectroscopy using an ABI Voyager DE-STR matrix-assisted laser desorption ionization-COF (AME Bioscience, United Kingdom) and was not C-terminally amidated. To compare the rtCATH_1 peptide activity directly against that of a mammalian cathelicidin, the bovine cyclic dodecapeptide (35) was also synthesized, since it is similar to the N-terminal region of the rtCATH_1 mature peptide in molecular structure and charge.
Antimicrobial assays.
The antimicrobial activities were determined as MICs using the modified twofold microtiter broth dilution method (45). Briefly, serial twofold dilutions of peptides were prepared in 0.2% bovine serum albumin-0.01% acetic acid buffer in tubes. Eighty microliters of each concentration was added to each corresponding well of a 96-well microtiter plate. Bacteria were incubated to mid-logarithmic phase and diluted in Mueller-Hinton broth to give a final inoculum concentration of 105 CFU/ml. A suspension of 20 μl of bacteria was added to each well of a 96-well plate and incubated for 24 to 48 h at 22°C. The growth was determined by the optical density at 600 nm. Inhibition was defined as growth less than or equal to one-half of the growth observed in control wells, to which no peptide was added.
Kinetic analysis.
A total of 103 A. salmonicida CFU were incubated at 22°C for increasing times (1 min to 8 h) with one of three concentrations (0.5×, 1×, and 2× MIC) of rtCATH_1(R151-V186). The reactions were terminated by plating on Mueller-Hinton agar (Sigma), and plates were incubated at 22°C for 48 h. Viable colonies were counted and shown as percentage of control rtCATH_1(R151-V186), plated at time zero.
Hemolytic assay.
The hemolytic activity was assayed as described by Skerlavaj et al. (41) with a slight modification described by Park et al. (31). Freshly bled rainbow trout erythrocytes (3 ml), from blood collected in lithium-heparin-coated vacutainers, were washed with PBS, pH 7.4, until the supernatant was colorless. The washed erythrocytes were resuspended in 30 ml PBS supplemented with glucose (0.2% [wt/vol]). To 190 μl of the cell suspension in microcentrifuge tubes, peptide samples (10 μl, serially diluted in PBS) or mellitin (a hemolytic peptide) were added. Following gentle mixing, the samples were incubated for 1 h at 22°C and centrifuged for 10 min at 1,100 × g at room temperature. One hundred microliters of supernatants was transferred to a microtiter plate, and the optical density was determined at 405 nm. The percentage of hemolysis was defined relative to the hemolysis obtained with the erythrocyte suspension treated with 0.2% Triton X-100 (100% hemolysis).
Nucleotide sequence accession number.
The genomic and cDNA sequences reported in this paper have been submitted to the GenBank/EMBL Data Bank with accession numbers AY594646 and AY382478, respectively.
RESULTS
Cloning and characterization of rainbow trout antimicrobial peptide gene.
PCR with primers F1 and R1, using a rainbow trout phytohemagglutinin-stimulated leukocyte cDNA library as a template, generated a 613-bp product that corresponded to the core sequence of rtCATH_1. Anchored PCR was used to isolate the 3′ end of this transcript. Using a nested approach, PCR with primers F1 and M13F was followed by reamplification with primers F2 and T7 and resulted in a 541-bp product that contained the 3′ end of the gene through to the poly(A) tail. The 3′ untranslated region of the transcript contained 183 nucleotides, with a polyadenylation signal (AATAAA) 17 bp upstream from the poly(A) tail. The transcription initiation start and the 5′ end of the mature peptide were determined by a GeneRacer kit using RNA from A. salmonicida-stimulated rainbow trout head kidney. A 425-bp product was obtained by nested PCR with primers GR5P and R2, followed by reamplification with primers GR5NP and R3, and contained 4 nucleotides of 5′ untranslated region and the 5′ end of the mature peptide. Analysis of the three sequenced overlapping products enabled the contiguous open reading frame of 651 nucleotides to be compiled (Fig. 1), which translated into a 216-residue peptide representing the first bony-fish homolog of the cathelicidin gene family.
Primers designed from the rtCATH_1 cDNA sequence were used to isolate the full genomic sequence from rainbow trout. PCR with primers F3 and R1 gave a 1,266-bp product that was composed of four exons—the same as the gene structures of all mammalian cathelicidins—that were 180, 123, 117, and 231 bp, respectively. The three introns, 239, 117, and 270 bp, respectively, all possessed a classical “GT-AG” intron splicing motif (Fig. 1). By gene walking with primers AP1 and R2, followed by primers AP2 and R3, a 1,118-bp PCR product was obtained. An overlapping region of 700 bp allowed the assembly of a contiguous sequence. The genomic sequence contained 1,464 nucleotides from the start to the end of the transcript described above.
Scanning of the 5′ flanking region of the rtCATH_1 sequence for eukaryotic promoter elements indicated the presence of a typical TATA box (TATAAA) at −31 to −26 (Fig. 1). Two putative CCAAT boxes were found in a sense orientation at −209 and −180. Clusters of potential recognition sites for nuclear factors involved in the transcription of immune-related molecules were found in the region. Within 331 bp upstream of the transcriptional start site there was one nuclear factor kappa B (NF-κB) recognition site, one alpha interferon recognition site, one binding site for CCAAT/enhancer-binding protein β (C/EBPβ), one nuclear factor 1, and two nuclear factor interleukin 6 motifs, two gamma interferon response elements, and five GAAA motifs.
The coding region of trout rtCATH_1 was compared with the sequences of four different mammalian cathelicidins (LL-37 of human, BCT1 of cow, SC52 of sheep, and PR39 of pig), using the GAP program in the Genetics Computer Group package. Comparing the full cDNA sequence, rtCATH_1 was found to share 41 to 44% nucleotide identity with mammalian cathelicidins. The highest homology (44 to 48%) was seen in exon 2, which encoded the cathelin-like domain. The lowest homology (31 to 36%) was seen in exon 4, which encoded the last few residues of the proregion and all of the predicted mature antimicrobial peptide.
The full-length cDNA sequence of the novel cathelicidin had an open reading frame of 216 amino acid residues, corresponding to a 23.6-kDa polypeptide with a calculated pI of 9.4. The peptide showed the characteristic features of cathelicidins, including a conserved N-terminal preproregion and a unique C-terminal sequence. Multiple alignment using the CLUSTAL program indicated conservation of both the mammalian cathelicidin family signature I, Y-X-[ED]-X-V-X-[RQ]-A-[LIVMA]-[DQG]-X-[LIVMFY]-N-[EQ], and signature II, F-X-[LIVM]-K-E-T-X-C-X10-C-X-F-[KR]-[KE], in the rainbow trout sequence (12), with a few exceptions, e.g., V to I and N to L in signature I and K to Q and F to L in signature II (Fig. 3). Analysis of the N-terminal region predicted a cleavage site for signal peptidase at residues S22-Q23. The putative signal peptide is followed by a proregion that conserves the four cysteine residues present in all the congeners characterized thus far. This proregion is predicted to end at residues V150-R151, which is a common elastase cleavage site for many mammalian cathelicidins (13). Thus, the predicted mature peptide would be 66 residues in size, of which 14 are positively charged. The peptide as a whole is basic (calculated pI, 11.71), with four residues being acidic.
FIG. 3.
Multiple alignment of the predicted translation of rtCATH_1 propreprotein with cathelin and other known cathelicidins from human, bovine, sheep, pig, rabbit, and hagfish. Identical (*) and similar (. or :) residues identified by the CLUSTAL program are indicated. The cathelicidin family signature is boxed, and the critical residues conforming to the signature are in boldface. Cysteines involved in disulfide bond formation are in boldface text and indicated with a %. The mature peptides are in italics.
Elastase cleavage of prtCATH_1.
Enzymatic cleavage and Western blot analysis were performed to confirm that the prtCATH_1 molecule is indeed processed by elastase and to determine the size of the mature rtCATH_1 after processing. Elastase (50 ng) cleaved prtCATH_1 (5 μg) in a time-dependent manner into two fragments (Fig. 4). The molecular masses of the generated fragments detected after cleavage of the 6His-prtCATH_1 and prtCATH_1-6His proteins were 15 kDa and 8 kDa, respectively, and matched the sizes of the predicted precursor fragment (Fig. 4A) and mature (Fig. 4B) rtCATH_1 peptide. In both cases, the second fragment was not visualized, as it did not contain a six-His tag. The uncleaved precursor decreased as digestion time increased.
Antimicrobial and hemolytic assays.
The effects of synthesized peptides against a panel of bacteria were evaluated as the minimal concentration capable of inhibiting visible microbial growth. The rtCATH_1(R151-V186) peptide displayed various antibacterial activities against all 10 different microorganisms studied, including gram-negative and gram-positive bacteria, with MICs of 2 to 40 μM (Table 2). The mammalian cathelicidin, bovine cyclic dodecapeptide, also demonstrated antimicrobial activities against a broad spectrum of the fish bacterial pathogens tested but was noticeably less effective than the rtCATH_1(R151-V186) peptide against A. salmonicida MT423,P. damsela MT1415, and V. anguillarum MT1741.
TABLE 2.
Antimicrobial activities of rtCATH_1(R151-V186) and bovine cyclic dodecapeptide (Bovdode)
| Organism and strain | Relevant phenotype | MIC (μM)
|
|
|---|---|---|---|
| Bovdode | rtCATH_1 (R151-V186) | ||
| Gram negative | |||
| Escherichia coli NCIMB 12210 | ATCC25922 | 4 | 5 |
| Aeromonas salmonicida MT004 | A-layer negative | 16 | 8 |
| A. salmonicida MT423 | A-layer positive | >64 | 20 |
| Photobacterium damsela MT1415 | Capsulated | 40 | 10 |
| P. damsela EPOY 8803 II | Noncapsulated | 2 | 16 |
| Vibrio anguillarum MT1741 | Serogroup O1 | 10 | 2 |
| V. anguillarum MT1742 | Serogroup O2 | 1 | 2 |
| Yersinia ruckeri MT252 | 16 | 10 | |
| Gram positive | |||
| Lactococcus garvieae MT2055 | Noncapsulated | 2.5 | 40 |
| L. garvieae MT2291 | Capsulated | 1.25 | 2 |
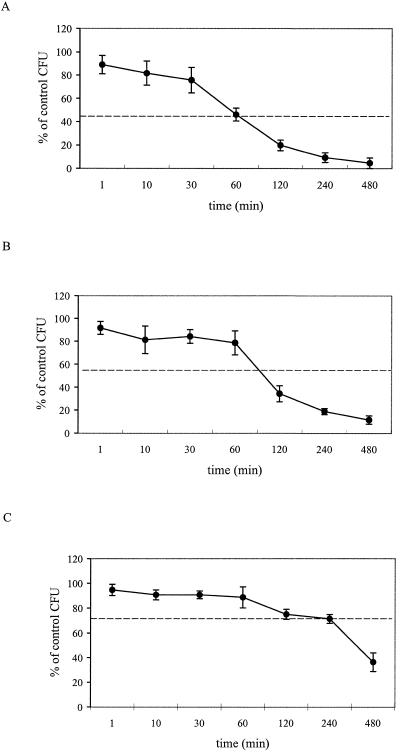
To determine the rate of bactericidal activity of rtCATH_1, a kinetic study was performed using A. salmonicida MT004 (Fig. 5). A time-dependent mechanism of action was demonstrated, with increased bacterium-rtCATH_1(R151-V186) incubation time leading to higher bactericidal activity (i.e., lower numbers of CFU). At 2× MIC (16.0 μM), antibacterial action increased dramatically after 30 min, and at 120 min, less than 20% of the CFU remained. At 1× MIC (8.0 μM), antibacterial action was noted after 60 min, with few CFU at 240 min. At 0.5× MIC (4.0 μM), antibacterial action started after 240 min, and more than 30% of the bacteria were still viable at 480 min. The overall mean activity, as represented by the average percentage of control CFU, increased with decreasing rtCATH_1(R151-V186) concentration.
FIG. 5.
Kinetics of rtCATH_1(R151-V186) bactericidal activity against A. salmonicida MT004. The results represent viable colonies (CFU) shown as percent of control (without rtCATH_1, plated at time zero). (A) 16.0 μM (2× MIC); (B) 8.0 μM (1× MIC); (C) 4.0 μM (0.5× MIC). The dotted horizontal lines indicate the overall mean bactericidal activity for each concentration. The error bars indicate standard deviations.
Sodium, calcium, and magnesium were added to determine their effects on the MICs of rtCATH_1(R151-V186). An increased calcium or magnesium concentration resulted in decreased antimicrobial activity against A. salmonicida MT004 (Table 3). NaCl, added at concentrations 10-fold greater than those of either CaCl2 or MgCl2, did not affect the antimicrobial activity of rtCATH_1(R151-V186).
TABLE 3.
Effects of increasing concentrations of common cations on MICs of rtCATH_1(R151-V186) against A. salmonicida MT004
| Salt | Concn (mM) | MIC (μM) |
|---|---|---|
| NaCl | 0 | 8 |
| 10 | 8 | |
| 50 | 8 | |
| 100 | 8 | |
| CaCl2 | 0 | 8 |
| 1 | 10 | |
| 5 | 32 | |
| 10 | >64 | |
| MgCl2 | 0 | 8 |
| 1 | 8 | |
| 5 | 16 | |
| 10 | >64 |
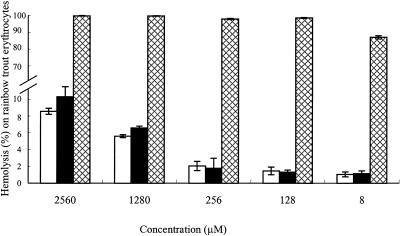
Very modest hemolytic activity (<12% hemolysis) was observed with the rtCATH_1(R151-V186) peptide and bovine cyclic dodecapeptide (up to 2.56 mM, 40 to 1,000 times the MIC levels) when tested against rainbow trout erythrocytes (Fig. 6). In contrast, melittin, a hemolytic peptide, induced complete lysis of trout erythrocytes, with the exception of the lowest concentration used, where >80% lysis still occurred.
FIG. 6.
Hemolysis assays of rtCATH_1(R151-V186) peptide (open bars), bovine cyclic dodecapeptide (solid bars), and melittin (hatched bars) on rainbow trout erythrocytes. The values are expressed relative to total hemolysis, obtained by adding 0.2% Triton X-100 to the cell suspension. The results are the mean of three independent experiments with standard error values ranging from ±0.1 to ±1.2.
Expression studies.
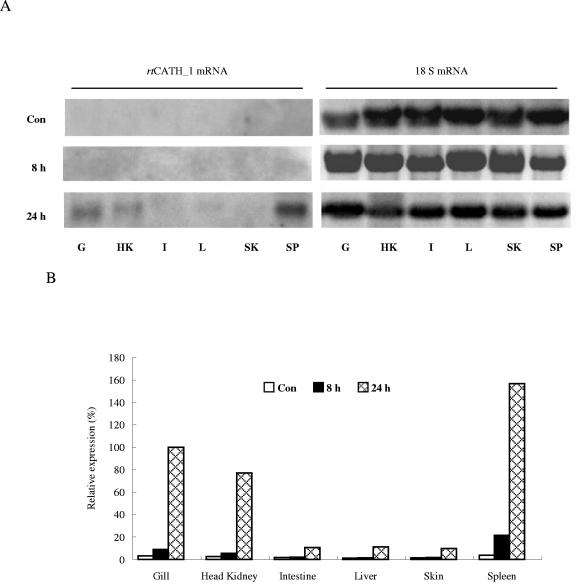
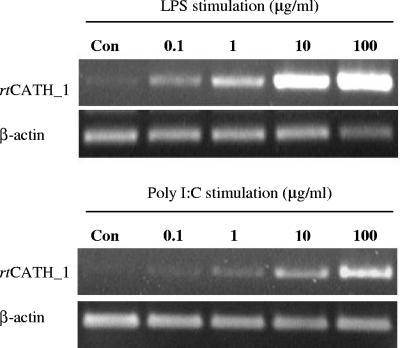
Northern blot analysis showed that no significant transcript level of rtCATH_1 was apparent in samples from normal fish. However, after stimulation with bacteria, the spleen samples showed a significant increase in expression level, with samples from gill and head kidney also showing detectable levels of the rtCATH_1 transcript (Fig. 7). By RT-PCR, clear dose-dependent effects of both LPS and poly(I · C) stimulation were apparent using the head kidney leukocytes. Stimulation of the head kidney cells with increasing concentrations of LPS or poly(I · C) resulted in an increased rtCATH_1 transcript level (Fig. 8).
FIG. 7.
Northern blot analysis of rtCATH_1 mRNA expression. Exposed film (A) and densitometric readings of the film (B) are shown. The mRNA levels were normalized to the 18S RNA level and expressed as percentages of the mRNA level in the gills of 24-h-challenged fish (100%). Con, control fish; 8 h, fish injected intraperitoneally (i.p.) with 2 × 106 CFU of Aeromonas salmonicida MT423 for 8 h; 24 h, fish injected i.p. with A. salmonicida for 24 h; G, gill; HK, head kidney; I, intestine, L, liver; SK, skin; SP, spleen.
FIG. 8.
RT-PCR analysis of rtCATH_1 and β-actin expression in head kidney cells after stimulation without (Con) or with various concentrations of LPS (top) and poly(I · C) (bottom) for 5 h.
DISCUSSION
Thus far, all cathelicidin family genes show similar structural organizations, with the coding sequence distributed over four exons. Exon 1 encodes the 29- to 30-residue signal peptide and some initial residues of the proregion, exons 2 and 3 encode 98 to 114 residues of the cathelicidin-like proregion, and exon 4 encodes several final residues of the proregion and the mature peptide, which varies in length from 12 to 100 residues (13). Rainbow trout cathelicidin has some interesting differences from known mammalian molecules. It has a shorter (22 residues) signal peptide and a longer (128 residues) cathelin-like domain. Homology analysis indicated that the percentage nucleotide identity in trout and mammalian cathelicidin genes drops in exon 4, which encodes the mature peptide and is the most diverse part of all the cathelicidin molecules. The next lowest homology was seen in exon 1, suggesting that the signal peptide of the fish cathelicidin was markedly different from those in mammals.
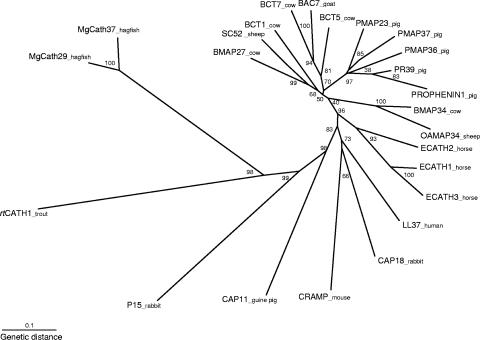
Phylogenetic analysis (Fig. 9) shows rainbow trout cathelicidin has branched away from the mammalian cathelicidins, especially the molecules belonging to species of the grandorder Ungulata. The hagfish cathelicidin family (MgCATH29 and MgCATH37) branches with rtCATH_1 but has a very distant evolutionary relationship.
FIG. 9.
Unrooted phylogenetic tree showing the relationship between rtCATH_1 amino acid sequence of the precursor molecule and other known cathelicidin precursor sequences. The tree was constructed by the neighbor-joining method using the CLUSTAL W and PHYLIP packages and was bootstrapped 1,000 times.
Four invariant cysteines are present in mammalian cathelicidins, within the motif C-X10-C-X5-G-X4-C-X16-C, clustered in the conserved region of the cathelin-like propiece. The two pairs of intramolecular disulfide bonds formed by the four cysteines may impose structural constraints on the molecule (34). In the rtCATH_1 sequence, four cysteines (C84, C95, C106, and C130) were also found to be coded for in exon 2 and exon 3. The disulfide bridge motif was C-X10-C-X5-G-X4-C-X23-C, which conformed very closely to the mammalian motif. In hagfish cathelicidin (43), the four cysteines were present but there was no glycine between cysteines 2 and 3, giving a motif of C-X10-C-X12-C-X17-C. The fact that two pairs of cysteines would be needed within the proregion of cathelicidins, together with an analysis of possible elastase cut sites and the likelihood that the mature peptide would be encoded entirely within the final exon (exon 4), allowed the putative antimicrobial peptide to be deduced in the C-terminal region of rtCATH_1. Probably the most interesting feature of the rtCATH_1 molecule is that the C-terminal 66-amino-acid sequence has features present in multiple cathelicidin subgroups, including the cysteine-bridged peptide family and the specific amino-acid-rich family (13), where, unusually, a high content of glycine is present in the fish sequence.
The rtCATH_1(R151-V186) peptide, tested for antimicrobial activity in this study, represented the first 36 amino acids of the predicted mature peptide. It is highly basic (net charge, +6), due to the presence of multiple arginine residues and a lysine residue, and had potent antimicrobial activity. Bovine cyclic dodecapeptide was also tested in these studies, and in some cases it was more active than the rtCATH_1(R151-V186) peptide, as seen with P. damsela EPOY 8803 II and the noncapsulated strain of L. garvieae. However, with A. salmonicida, which causes the well-known salmonid bacterial disease furunculosis, the rtCATH_1(R151-V186) peptide had more killing activity than bovine cyclic dodecapeptide. Our kinetic study (Fig. 5) indicates a direct relationship between the concentration of peptide, incubation time, and bactericidal activity.
Bacteria can evolve different strategies to resist bactericidal agents. For example, producing β-lactamase to destroy some antibiotics (6), secreting an outer capsule to prevent phagocytosis by host leukocytes (27), or producing an A layer to aggregate bacteria and decrease susceptibility to antimicrobial peptides (17). The results in this study showed that production of a capsule did not affect susceptibility to the rtCATH_1(R151-V186) peptide, since the MICs for the two capsulated strains of P. damsela MT1415 and L. garvieae MT2055 were lower than those for the noncapsulated strains. In the case of A-layer production, A. salmonicida strains with an A layer were shown previously to be less susceptible to cecropins B and P1 (17), and a similar conclusion was made regarding resistance to the rtCATH_1(R151-V186) peptide, where the MIC against the A-layer-positive strain MT423 was higher than that to the A-layer-negative strain MT004.
Divalent cations can increase bacterial membrane stabilization (8), thus affecting the microbicidal activities of some antimicrobial peptides. Calcium and magnesium have been reported to inhibit the activities of mammalian cathelicidins (34) and some fish antimicrobial peptides (7, 23), where increasing calcium or magnesium concentration resulted in decreased antimicrobial activity against the strains tested. A similar trend was seen with the rainbow trout antimicrobial peptide rtCATH_1(R151-V186) against A. salmonicida MT004. The divalent calcium and magnesium, but not monovalent sodium, affected the interaction between the antimicrobial peptide and the bacterial membrane. The functional relevance of such findings relates to the aquatic habitat in which fish reside. Although normally found in freshwater, rainbow trout can live in seawater, in which both the calcium and magnesium concentrations (52.3 and 10.0 mM, respectively) are much higher than the levels tested here and would thus presumably decrease the activity of rtCATH_1 at external surfaces. The reported concentrations of calcium and magnesium in trout body fluids, though, are on the order of 1 mM (22), which is within the active range, and thus, rtCATH_1 would be expected to be active within physiologic fluids under all environmental circumstances.
Expression studies revealed an inducible expression of rtCATH_1 in rainbow trout. The levels of rtCATH_1 expression in rainbow trout were markedly increased after bacterial challenge, when the rtCATH_1 mRNA was appreciable in gill, head kidney, and spleen. Induction at the systemic sites was correlated with the detection of bacteria internally, suggesting that pathways to upregulate rtCATH_1 expression exist, possibly within myeloid cells at these sites. In mammals, cathelicidins are also expressed at various mucosal and systemic sites. The human cathelicidin, FALL-39/LL-37, is present in testis, airway epithelia, thymus, and liver (34). The porcine cathelicidin, PR-39, appears in bone marrow and lymphoid organs, including the thymus, spleen, and mesenteric lymph nodes (44). A murine cathelicidin, CRAMP, was found in the testis, spleen, stomach, and intestine (11). In the present study, it is possible that the challenge route (injection), which was chosen because rainbow trout do not typically succumb to an immersion challenge with A. salmonicida, affected the sites of expression detected. Studies with other routes of exposure and other pathogens, which are now possible, will confirm if other mucosal tissues express rtCATH_1 in trout. Mammalian cathelicidins were originally isolated in neutrophils, as seen in humans, cattle, pigs, rabbits, horses, and mice (34). While this still remains to be determined for fish, the reverse transcription-PCR studies showed that rtCATH_1 can be expressed in head kidney cells after stimulation with LPS or poly(I · C) (Fig. 7) and is thus present in one or more of the cell types present, which are predominantly leukocytes. Additional experiments are needed to investigate further the cellular source(s) of trout cathelicidin.
Analysis of the 5′ flanking region of the rtCATH_1 gene revealed a number of consensus binding sequences present in the promoter regions of other immune-relevant genes, including those for C/EBPβ, nuclear factor 1, nuclear factor interleukin 6, and NF-κB. Such transcription factors are frequently present adjacent to others, and the factors interact to effect transcription of antimicrobial peptide genes in different species, including the inducible antimicrobial peptides in invertebrates (20), the bovine β-defensin produced by respiratory tract epithelial cells (10), and other mammalian cathelicidin gene family members (14, 37, 47). In fish, such regulatory elements are known to be present in the promoters of the antimicrobial peptide gene in flounder (7), as well as the promoters of the transferrin gene in salmon (21), the interferon-inducible Mx genes (24) and the insulin-like growth factor gene in trout (39), and the antifreeze protein gene in flounder (28). Thus, in agreement with expression results from the bacterially challenged fish presented above, rtCATH_1 gene expression is likely to be affected by transcription factors released in response to infection and inflammation, in a manner similar to the modulation of other immune-relevant genes in insects, fish, amphibians, and mammals.
Binding sites for various transcription factors known to be involved in viral defense were also found in the 5′ flanking region of the rtCATH_1 gene, including multiple GAAA motifs commonly found upstream of interferon-induced genes, two gamma interferon response elements, and one alpha interferon recognition site. Indeed, poly(I · C), a synthetic double-stranded RNA that is used as a viral mimic, enhanced the mRNA expression level of rtCATH_1 in head kidney cells (Fig. 8). Thus, it is possible that the rtCATH_1 gene has some role in host clearance of viral infections, and this requires further study.
Overall, the above-mentioned results confirmed that cathelicidin genes evolved at an early stage of vertebrate evolution and are present in bony fish. The rtCATH_1 molecule was shown to be novel, in terms of possessing combined features of known mammalian subgroups, and the rtCATH_1(R151-V186) peptide was shown to have potent antimicrobial activity against a broad spectrum of fish bacterial pathogens while showing low hemolysis to host red blood cells. Thus, it appears that cathelicidins represent an important first line of defense at mucosal sites in fish as in mammals and that rtCATH_1, as the first bony-fish cathelicidin discovered, may be a good candidate antibiotic for preventing disease outbreaks in aquaculture and a starting point for the development of novel synthetic antimicrobial agents for fish.
Acknowledgments
This work was supported by a Marie Curie Research Training grant from the EC to O.P. (FAIR-GT-1997-3355) and by EWOS Innovation. Thanks also go the Taiwanese government for financial assistance to C.-I.C.
We thank Tony Ellis (FRS Marine Laboratory, United Kingdom) for kindly providing the field isolates of fish pathogens and Ian Davidson (Proteome Facility, University of Aberdeen, Aberdeen, United Kingdom) for synthesizing the rtCATH_1 peptide.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Andreu, D., and L. Rivas. 1998. Animal antimicrobial peptides: an overview. Biopolymers 47:415-433. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, A. C., C. Guyot, B. G. Hansen, K. Mackenzie, M. T. Horne, and A. E. Ellis. 2002. Resistance to serum killing may contribute to differences in the abilities of capsulated and non-capsulated isolates of Lactococcus garvieae to cause disease in rainbow trout. Fish Shellfish Immunol. 12:155-168. [DOI] [PubMed] [Google Scholar]
- 3.Boesen, H. T., J. L. Larsen, and A. E. Ellis. 1999. Bactericidal activity by sub-agglutinating levels of rainbow trout (Oncorhynchus mykiss) antiserum to Vibrio anguillarum serogroup O1. Fish Shellfish Immunol. 9:633-636. [DOI] [PubMed] [Google Scholar]
- 4.Boesen, H. T., M. H. Larsen, J. L. Larsen, and A. E. Ellis. 2001. In vitro interactions between rainbow trout (Oncorhynchus mykiss) macrophages and Vibrio anguillarum serogroup O2a. Fish Shellfish Immunol. 11:415-431. [DOI] [PubMed] [Google Scholar]
- 5.Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 6.Coghlan, A. 1996. Peptides punch it out with superbugs. New Scientist 150:20. [Google Scholar]
- 7.Cole, A. M., R. O. Darouiche, D. Legarda, N. Connell, and G. Diamond. 2000. Characterization of a fish antimicrobial peptide: gene expression, subcellular localization, and spectrum of activity. Antimicrob. Agents Chemother. 44:2039-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coughlin, R. T., S. Tonsager, and E. J. McGroarty. 1983. Quantitation of metal cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry 22:2002-2007. [DOI] [PubMed] [Google Scholar]
- 9.Davidson, J., T. Smith, and S. A. M. Martin. 1999. Cloning and sequence analysis of trout LMP2 cDNA and differential expression of the mRNA. Fish Shellfish Immunol. 9:621-632. [Google Scholar]
- 10.Diamond, G., D. E. Jones, and C. L. Bevins. 1993. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc. Natl. Acad. Sci. USA 90:4596-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallo, R. L., K. J. Kim, M. Bernfield, C. A. Kozak, M. Zanetti, L. Merluzzi, and R. Gennaro. 1997. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 272:13088-13093. [DOI] [PubMed] [Google Scholar]
- 12.Gattiker, A., E. Gasteiger, and A. Bairoch. 2002. ScanProsite: a reference implementation of a PROSITE scanning tool. Appl. Bioinform. 1:107-108. [PubMed] [Google Scholar]
- 13.Gennaro, R., and M. Zanetti. 2000. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers 55:31-49. [DOI] [PubMed] [Google Scholar]
- 14.Gudmundsson, G. H., K. P. Magnusson, B. P. Chowdhary, M. Johansson, L. Andersson, and H. G. Boman. 1995. Structure of the gene for porcine peptide antibiotic PR-39, a cathelin gene family member: comparative mapping of the locus for the human peptide antibiotic FALL-39. Proc. Natl. Acad. Sci. USA 92:7085-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock, R. E. W., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 16.Harder, J., J. Bartels, E. Christophers, and J.-M. Schröder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 17.Henry, M. A., and C. J. Secombes. 2000. The A-layer influences the susceptibility of Aeromonas salmonicida to antibacterial peptides. Fish Shellfish Immunol. 10:637-642. [DOI] [PubMed] [Google Scholar]
- 18.Hong, S., S. Peddie, J. J. Campos-Pérez, J. Zou, and C. J. Secombes. 2003. The effect of intraperitoneally administered recombinant IL-1 on immune parameters and resistance to Aeromonas salmonicida in the rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 27:801-812. [DOI] [PubMed] [Google Scholar]
- 19.Huttner, K. M., and C. L. Bevins. 1999. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 45:785-794. [DOI] [PubMed] [Google Scholar]
- 20.Kim, Y. S., S. J. Han, J. H. Ryu, K. H. Choi, Y. S. Hong, Y. H. Chung, S. Perrot, A. Raibaud, P. T. Brey, and W. J. Lee. 2000. Lipopolysaccharide-activated kinase, an essential component for the induction of the antimicrobial peptide genes in Drosophila melanogaster cells. J. Biol. Chem. 275:2071-2079. [DOI] [PubMed] [Google Scholar]
- 21.Kvingedal, A. M. 1994. Characterization of the 5′ region of the Atlantic salmon (Salmo salar) transferrin-encoding gene. Gene 150:335-339. [DOI] [PubMed] [Google Scholar]
- 22.Laidley, C. W., and J. F. Leatherland. 1988. Cohort sampling, anaesthesia and stocking-density effects on plasma cortisol, thyroid hormone, metabolite and ion levels in rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 33:73-88. [Google Scholar]
- 23.Lauth, X., H. Shike, J. C. Burns, M. E. Westerman, V. E. Ostland, J. M. Carlberg, J. C. V. Olst, V. Nizet, S. W. Taylor, C. Shimizu, and P. Bulet. 2002. Discovery and characterization of two isoforms of moronecidin, a novel antimicrobial peptide from hybrid striped bass. J. Biol. Chem. 277:5030-5039. [DOI] [PubMed] [Google Scholar]
- 24.Leong, J. C., G. D. Trobridge, C. H. Kim, M. Johnson, and B. Simon. 1998. Interferon-inducible Mx proteins in fish. Immunol. Rev. 166:349-363. [DOI] [PubMed] [Google Scholar]
- 25.Levy, O. 1996. Antibiotic proteins of polymorphonuclear leukocytes. Eur. J. Haematol. 56:263-277. [DOI] [PubMed] [Google Scholar]
- 26.López-Dóriga, M. V., A. C. Barnes, N. M. S. Santos, and A. E. Ellis. 2000. Invasion of fish epithelial cells by Photobacterium damselae subsp. Piscicida: evidence for receptor specificity, and effect of capsule and serum. Microbiology 146:21-30. [DOI] [PubMed] [Google Scholar]
- 27.Madigan, M. T., J. M. Martinko, and J. Parker. 2003. Essentials of Immunology, p. 755-786. In M. T. Madigan, J. M. Martinko, and J. Parker (ed.), Biology of microorganisms, 10th ed. Pearson Education, Inc., N.J.
- 28.Miao, M., S.-L. Chan, C. L. Hew, and G. L. Fletcher. 1998. Identification of nuclear proteins interacting with the liver-specific enhancer B element of the antifreeze protein gene in winter flounder. Mol. Mar. Biol. Biotechnol. 7:197-203. [PubMed] [Google Scholar]
- 29.Needleman, S. B., and C. D. Wunsch. 1970. A general method applicable to search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 48:443-453. [DOI] [PubMed] [Google Scholar]
- 30.Oren, Z., and Y. Shai. 1996. A class of highly potent antibacterial peptides derived from paraxin, a pore-forming peptide isolated from Moses sole fish Pardachirus marmoratus. Eur. J. Biochem. 237:303-310. [DOI] [PubMed] [Google Scholar]
- 31.Park, C. B., J. H. Lee, I. Y. Park, M. S. Kim, and S. C. Kim. 1997. A novel antimicrobial peptide from the loach, Misgurnus anguillicandatus. FEBS Lett. 411:173-178. [DOI] [PubMed] [Google Scholar]
- 32.Pearson, W. R., and D. I. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quandt, K., K. Frech, H. Karas, E. Wingender, and T. Werner. 1995. MatInd and MatInspector—new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 23:4878-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramanathan, B., E. G. Davis, C. R. Ross, and F. Blecha. 2002. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microb. Infect. 4:361-372. [DOI] [PubMed] [Google Scholar]
- 35.Remeo, D., B. Skerlavaj, M. Bolognesi, and R. Gennaro. 1988. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J. Biol. Chem. 263:9573-9575. [PubMed] [Google Scholar]
- 36.Ritonja, A., M. Kopitar, R. Jerala, and V. Turk. 1989. Primary structure of a new cysteine proteinase inhibitor from pig leucocytes. FEBS Lett. 255:211-214. [DOI] [PubMed] [Google Scholar]
- 37.Scocchi, M., S. Wang, and M. Zanetti. 1997. Structural organization of the bovine cathelicidin gene family and identification of a novel member. FEBS Lett. 417:311-315. [DOI] [PubMed] [Google Scholar]
- 38.Selsted, M. E., M. J. Novotny, W. L. Morris, Y.-Q. Tang, W. Smith, and J. S. Cullor. 1992. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J. Biol. Chem. 267:4292-4295. [PubMed] [Google Scholar]
- 39.Shamblott, M. J., S.-M. Leung, M. W. Greene, and T. T. Chen. 1998. Characterization of a teleost insulin-like growth factor II (IGF-II) gene: evidence for promoter CCAAT/enhancer-binding protein (C/EBP) sites, and the presence of hepatic C/EBP. Mol. Mar. Biol. Biotechnol. 7:181-190. [PubMed] [Google Scholar]
- 40.Silphaduang, U., and E. J. Noga. 2001. Peptide antibiotics in mast cells of fish. Nature 414:268-269. [DOI] [PubMed] [Google Scholar]
- 41.Skerlavaj, B., R. Gennaro, L. Bagella, L. Merluzzi, A. Risso, and M. Zanetti. 1996. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J. Biol. Chem. 271:28375-28381. [DOI] [PubMed] [Google Scholar]
- 42.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uzzell, T., E. D. Stolzenberg, A. E. Shinnar, and M. Zasloff. 2003. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides 24:1655-1667. [DOI] [PubMed] [Google Scholar]
- 44.Wu, H., G. Zhang, C. R. Ross, and F. Blecha. 1999. Cathelicidn gene expression in porcine tissue: roles in ontogeny and tissue specificity. Infect. Immun. 67:439-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu, M., and R. E. W. Hancock. 1999. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J. Biol. Chem. 274:29-35. [DOI] [PubMed] [Google Scholar]
- 46.Zanetti, M., G. Del Sal, P. Storici, C. Schneider, and D. Romeo. 1993. The cDNA of the neutrophil antibiotic Bac5 predicts a pro-sequence homologous to a cysteine proteinase inhibitor that is common to other neutrophil antibiotics. J. Biol. Chem. 268:522-526. [PubMed] [Google Scholar]
- 47.Zhao, C., T. Ganz, and R. I. Lehrer. 1995. Structure of genes for two cathelin-associated antimicrobial peptides: prophenin-2 and PR-39. FEBS Lett. 376:130-134. [DOI] [PubMed] [Google Scholar]