Abstract
Five recombinant antigens (Ags; 85A, 85B, 85C, superoxide dismutase [SOD], and 35-kDa protein) were purified from Mycobacterium avium subsp. paratuberculosis and evaluated for their ability to stimulate peripheral blood mononuclear cells (PMBCs) from fecal-culture-positive cows (low and medium shedders) and culture-negative healthy cows. Recombinant Ags 85A, 85B, and 85C induced significant lymphocyte proliferation as well as the production of gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-12, and tumor necrosis factor alpha (TNF-α), but not IL-4, from low and medium shedders. The 85 antigen complex did not stimulate PMBC proliferation from culture-negative healthy cows. The 35-kDa protein also induced significant lymphocyte proliferation as well as the production of IFN-γ and IL-4 from low and medium shedders. CD4+ T cells and CD25+ (IL-2R) T cells were stimulated the most by 85A and 85B, while the 35-kDa protein primarily stimulated CD21+ B cells involved in humoral immune responses. Interestingly, SOD was less immunostimulatory than other antigens but strongly induced γδ+ T cells, which are thought to be important in the early stages of infection, such as pathogen entry. These data provide important insight into how improved vaccines against mycobacterial infections might be constructed.
Mycobacterium avium subspecies paratuberculosis is the causative agent of Johne's disease (JD), which causes chronic granulomatous enteritis in ruminants. Clinically affected animals develop chronic diarrhea and progressive weight loss that eventually results in death, while subclinically infected animals mainly have decreased production of milk. JD is of tremendous economic importance to the worldwide dairy industry, causing major losses due to reduced production and early culling of animals with estimates of 20% of U.S. dairy herds affected and costs of $220 million per year to the dairy industry (56). Cattle are most susceptible to infection with this organism within the first 6 months of life, but disease typically does not become evident until 3 to 5 years of age. Infection occurs by ingestion of contaminated manure, colostrum, or milk from infected cows (50). Fetal infection also occurs, particularly in pregnant cows with advanced disease (52).
Although JD is an important infectious disease of ruminants, there is no effective vaccine against this disease. The only currently available vaccine in the United States consists of killed M. avium subsp. paratuberculosis in an oil adjuvant (26, 28, 39). Studies have demonstrated that vaccination with this bacterin does not prevent infection but diminishes the multiplication of bacteria in the intestinal wall and limits the progression of lesions associated with clinical signs (19). Also, there is a strong reaction at the injection sites after vaccination with this killed bacterin (26, 28). Another drawback of this vaccine is that the vaccinated animals become tuberculin skin test positive (26, 28). Clearly, there is a need for the development of more effective vaccines against JD that can be used as either a prophylactic and/or a therapeutic vaccine without hindering tuberculosis screening of the cattle industry.
M. avium subsp. paratuberculosis, like M. tuberculosis, is an intracellular pathogen, and cell-mediated immunity plays a major role in the control of bacterial propagation and protecting against JD (15, 16). Animal studies indicate that acquired protection against tuberculosis is mediated by sensitized T lymphocytes. In particular, gamma interferon (IFN-γ)-secreting CD4+ T lymphocytes are critical in mediating protection in mice (6, 10, 17, 46, 47). IFN-γ is a central cytokine in the control of M. tuberculosis infection, as demonstrated by the high susceptibility to mycobacterial infections in mice with a disrupted IFN-γ gene (11, 17) or in people with a mutated IFN-γ receptor (21, 22, 37). In addition, major histocompatibility complex class I-restricted CD8+ T cells that produce cytokines such as IFN-γ and tumor necrosis factor alpha (TNF-α) are also required for resistance to M. tuberculosis infection (41-43). Thus, the identification of mycobacterial antigens (Ags) that preferentially activate both CD4 and CD8 T cells that secrete IFN-γ is critical to the development of recombinant and/or DNA vaccines against JD.
In recent years, several novel antigens from M. tuberculosis have been identified that induce protection in a mouse model when used as either adjuvant proteins (single or fusion proteins), plasmid DNA, or live bacterial vectors (12, 18, 23, 33, 54). These include the 85 antigen complex (23, 53), Mtb8.4 (9, 10), Mtb32 (46), Mtb39 (46), ESAT-6 (4), rv3407 (32), phosphate transport receptors (55), heparin-binding hemagglutinin (38), and others that induce the production of IFN-γ in vaccinated animals. Since M. avium subsp. paratuberculosis and M. tuberculosis share many homologous antigens, these studies may provide important information for the development of effective DNA vaccines against JD.
The aim of this study was to compare various M. avium subsp. paratuberculosis antigens for their capacities to stimulate peripheral blood lymphocyte subsets from medium shedders, low shedders, and healthy control cattle, respectively. We analyzed the phenotypic changes and cytokine profiles of lymphocytes stimulated in vitro with several purified recombinant M. avium subsp. paratuberculosis antigens by flow cytometric analysis and real-time PCR.
MATERIALS AND METHODS
Animals.
A total of 38 Holstein cows, 2 to 3 years old, were divided into three groups. Healthy controls (n = 18) were negative for M. avium subsp. paratuberculosis infection, as determined by negative fecal culture and negative IS900 PCR testing. The healthy controls came from a farm that had been fecal culture and IS900 PCR negative for the past 10 years. Positive animals were subdivided into low shedders (n = 16; 1 to 30 CFU/g of feces) and medium shedders (n = 4; 31 to 300 CFU/gm of feces). In this study, heavy fecal shedders (>300 CFU/g of feces) were unavailable since they are culled immediately from farms once they are identified. Fecal culturing and IS900 PCR testing to determine M. avium subsp. paratuberculosis infection status were performed as previously described (44).
Recombinant antigen preparation.
M. avium subsp. paratuberculosis recombinant antigens 85A, 85B, 85C, 35-kDa antigen, and superoxide dismutase (SOD) were cloned and expressed (13, 45) and purified as previously described (46). The antigens used in this study had negligible (10 pg/ml) endotoxin in a Limulus amoebocyte assay.
Isolation and culture of bovine peripheral blood mononuclear cells (PBMCs).
Peripheral blood (20 ml) of all cows was collected from the tail vein with heparinized vacuum tubes. Isolation of lymphocytes from heparinized blood was performed by differential centrifugation using Histopaque 1.077 (Sigma). Twenty ml of heparinized whole blood was layered over 15 ml Histopaque in a 50-ml sterile polypropylene tube (Falcon) and then centrifuged at 200 × g for 30 min at room temperature. The plasma layer was discarded, and the mononuclear cell layer was carefully collected and washed three times with phosphate-buffered saline (PBS; pH 7.2). Contaminating red blood cells were lysed with 0.87% ammonium KCl buffer by inverting them for 2 min at room temperature and then immediately by adding 30 ml PBS.
The washed cell pellets were suspended in PBS and counted using a hemacytometer and trypan blue to determine percent viability. Differential cell counts consistently showed greater than 96% lymphocytes, 1% monocytes, and less than 3% granulocytes in the cell suspension.
The lymphocytes were resuspended at 2 × 106/ml in RPMI 1640 containing 10% endotoxin-free fetal calf serum (FCS, Cellect Gold; ICN Biomedicals, Inc., Costa Mesa, CA), 2 mM l-glutamine, 10 mM HEPES, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamicin (Sigma), and 250 μl was added to either 96-well round-bottom plates or flat-bottom plates, depending on the purposes of the experiment.
Lymphocyte proliferation assay.
To investigate lymphocyte proliferation in response to individual antigens, a blastogenesis assay was performed. PBMCs were initially incubated in a 96-well flat-bottom microplate for 3 days at 37°C in a humidified atmosphere with 5% CO2. Cultures were then stimulated with concanavalin A (ConA; 10 μg/ml), purified protein derivative (PPD; 10 μg/ml), or each purified recombinant protein (10 μg/ml), and 40 μl (1.0 μCi) of methyl-3H-thymidine (PerkinElmer Life Science Inc, MA) in culture medium was added to each well. The cells were incubated for an additional 18 h in the same conditions, and the cells were then harvested using a semiautomatic cell harvester (Skatronas, Lier, Norway). Blastogenic activity was recorded as counts per minute (cpm) of radioactivity based on liquid scintillation counting.
Results were expressed as stimulation indices (SI) calculated as follows:
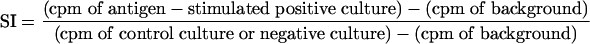 |
IFN-γ assay.
IFN-γ was measured in culture supernatants using a commercial kit specific for bovine IFN-γ following the manufacturer's instructions (Biosource Int., Camarillo, CA). The plates were read at 450 nm in a model 312E enzyme-linked immunosorbent assay (ELISA) reader (BioTEK Instruments, Inc., Winooski, VT), using any reference filter from 630 nm to 750 nm. The results were calculated based on a comparison of negative- and positive-control optical density (OD). Results were determined as either negative (<OD of positive control) or positive (>OD of positive control) relative to the cutoff value according to the manufacturer's instructions.
Comparison of antibody responses.
The ELISA was performed to evaluate the seroreactivity of the recombinant antigens following steps as previously described (45). An indirect ELISA was optimized using 2.5, 5, or 10 μg/ml of each antigen and 1:100 diluted serum by checkerboard titration. Flat-bottom 96-well plates (Maxisorp; Nunc, Roskilde, Denmark) were coated with 100 μl of each antigen in carbonate-bicarbonate buffer (14.2 mM Na2CO3, 34.9 mM NaHCO3, 3.1 mM NaN3, pH 9.5) at 4°C overnight, followed by washing three times with PBS containing 0.05% Tween 20 (PBST washing buffer) using an ELx405 microwell plate washer (BioTEK Instruments, Inc., Winooski, VT). Uncoated sites in the wells were blocked with 5% skim milk in PBST at 37°C for 1 h. The plates were washed twice with PBST, and 100 μl of optimally diluted (1:25,000) conjugated anti-bovine immunoglobulin G (IgG)-horseradish peroxidase (Sigma) was added to all wells and incubated at 37°C for 1 h. The plates were washed three times in PBST, and 200 μl of 2,2′-azinobis-thiazoline-6-sulfonic acid (Sigma) was added to each well. The plates were incubated at 37°C in the dark. After 30-min incubation, stop solution (1 M HCl) was added and the plates were read three times at 405 nm at 2-min intervals in the model 312 ELISA reader (BioTEK Instruments, Inc., Winooski, VT). Positive and negative sera and antigen and antibody controls were included in each plate.
Flow cytometric analysis.
A single-color flow cytometric analysis was performed with monoclonal antibodies (interleukin-A11 [IL-A11], IgG2a, CD4 [5]; CACT80C, IgG1, CD8α [5]; MM1a, IgG1, CD3 [5]; BAQ15A, IgM, CD21 [5]; CACT116A, IgG1, CD25 [IL-2Ra] [5]; and CACT63A, IgG1, γδ T [5] cells) against bovine lymphocyte markers. Briefly, cells were washed three times in fluorescence-activated cell sorter (FACS) buffer, incubated with the first antibody for 30 min at 4°C, washed three times, subsequently incubated with a fluorescein isothiocyanate-labeled horse anti-mouse immunoglobulin antibody (Vector) for 30 min at 4°C, washed twice, and collected in 200 μl of FACS fixed buffer prior to analysis. Analysis was done on a flow cytometer (FACSCalibur; Becton Dickinson). A forward-scatter-side-scatter live gate was used to measure 5,000 to 10,000 lymphocytes per sample. Based on the fluorescence data of the lymphocytes, the results were expressed as the percentage of cells with positive staining relative to that of a sample stained with an irrelevant isotype control antibody.
Preparation of RNA and DNase I treatment.
Cell pellets were washed twice in 50 ml PBS and pelleted, and 5 × 106 cells were lysed with 350 μl lysis buffer according to the manufacturer's recommendations (RNeasy mini kit; QIAGEN, Valencia, CA) and kept at −80°C until RNA extraction and cDNA synthesis. Total RNA was extracted from lysed cells or PMBCs using the RNeasy mini kit (QIAGEN). The extracted total RNA was treated with 10 U/μl of RNase-free DNase I at 37°C for 10 min followed by heat inactivation at 95°C for 5 min and then chilled on ice.
RT of total RNA for cDNA synthesis.
Reverse transcription (RT) was performed in a 20-μl final volume containing 1.6 μl total RNA, 200 U Superscript II reverse transcriptase (Gibco BRL), 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 0.01 M dithiothreitol, and 0.5 mM concentrations of deoxynucleoside triphosphates. The reaction mix was subjected to RT at 42°C for 50 min and inactivated at 70°C for 15 min. The cDNA was analyzed immediately or stored at −20°C until use.
Real-time quantitative RT-PCR.
Approximately 1 to 5 μg of total RNA from each treatment group was reverse transcribed by using Superscript reverse transcriptase, random hexamers, and reverse transcriptase reagents (Gibco BRL). Real-time primers and probes were designed with Primer Express software (Applied Biosystems) using bovine glyceraldehyde-3-phosphate dehydrogenase (GAPDH), cytokine, and growth factor gene DNA sequences derived from GenBank (Table 1). The internal probes were labeled with the fluorescent reporter dye 6-carboxyfluorescein (FAM) at the 5′ end and the quencher dye N′,N′,N′,N′,N′-6-carboxytetramethylrhodamine (TAMRA) at the 3′ end. The PCR mixture consisted of 400 nM concentrations of primers, 80 nM TaqMan probe, and commercially available Universal PCR Master Mix (Applied Biosytems) containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 5 mM MgCl2, 2.5 mM concentrations of deoxynucleotide triphosphates, 0.625 U AmpliTaq Gold DNA polymerase per reaction, 0.25 U AmpErase uracil-N-glycosylase per reaction, and 10 μl of the diluted cDNA sample in a final volume of 25 μl. The samples were placed in 96-well plates and amplified in an automated fluorometer (ABI Prism 7700 sequence detection system; Applied Biosystems). Amplicon conditions were 2 min at 50°C and 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Final quantitation was done using the comparative cycle threshold (CT) method and is reported as the relative transcription or the n-fold difference relative to a calibrator cDNA.
TABLE 1.
Sequences of PCR primers and TaqMan probes specific for bovine GAPDH and cytokines
| Cytokine and primers | Sequence (5′-3′) | Length | Probe | Probe sequence (5′-3′) | Accession no. |
|---|---|---|---|---|---|
| GAPDH | 67 | P-492 | CACTGTCCACGCCATCACTGCCA | AF077815 | |
| F-470 | GCATCGTGGAGGGACTTATGA | ||||
| R-536 | GGGCCATCCACAGTCTTCTG | ||||
| IL-12p40 | 101 | P-906 | TGCCAACGTCCGCGTGCAA | U11815 | |
| F-884 | CCACCGTCACATGCCACAAGG | ||||
| R-944 | CTGTAGTAGCGGTCCCGGG | ||||
| IL-4 | 66 | P-332 | TCCTGGGCGGACTTGACAGGAATC | M77120 | |
| F-311 | GCCACACGTGCTTGAACAAA | ||||
| R-376 | TCTTGCTTGCCAAGCTGTTG | ||||
| IL-2 | 81 | P-317 | CCCCAGAGATCAAGGATTCAATGGACA | M12791 | |
| F-293 | TTTAGCTCCAAGCAAAAACCTGA | ||||
| R-373 | TGTAGTTCCAAAACGATTCTCTTCA | ||||
| IFN-γ | 106 | P-564 | TCTCTTTCGAGGCCGGAGAGCATCA | M29867 | |
| F-504 | CAGAAAGCGGAAGAGAAGTCAGA | ||||
| R-609 | CAGGCAGGAGGACCATTACG | ||||
| TNF-α | 118 | P-3310 | AACCAGCCTCAGAGTTTCAGCGAGTTCC | Z14137 | |
| F-3261 | TCTCCGTGCAAAAGTTGGG | ||||
| R-3378 | CGAACTCCAGCACAGCAGG |
Statistical analysis.
Statistical analysis of the data was performed with Excel and GraphPad Prism software package version 2.0. Differences between individual groups and antigens and cytokine gene expression were analyzed with Student's t test. Differences were considered significant if probability values of <0.05 were obtained.
RESULTS
Lymphoproliferative responses to individual antigens.
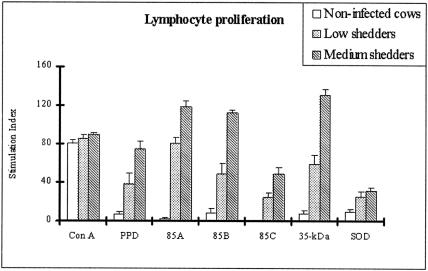
Five unique M. avium subsp. paratuberculosis recombinant proteins were analyzed for their ability to elicit proliferative responses in PBMCs obtained from cows with different M. avium subsp. paratuberculosis shedding levels. Proliferative activities of bovine PBMCs from medium shedder cows were higher than those in other groups in response to all recombinant proteins and two positive controls (P < 0.05), although there was variation among individual cows. PBMCs from medium shedder cows treated with 85A, 85B, and the 35-kDa protein antigens demonstrated an SI significantly higher (P < 0.05) than that of PBMCs treated with other recombinant antigens. In addition, proliferative responses to the 35-kDa protein in low shedders were even greater than those of medium shedders in response to SOD (P < 0.05) (Fig. 1).
FIG. 1.
Proliferative responses of peripheral blood mononuclear cells from infected and healthy control cows stimulated in vitro with five M. avium subsp. paratuberculosis recombinant proteins. The results are expressed as a stimulation index, and the error bars represent standard deviations from the means. No significant proliferation was noted to any antigen by PBMCs from noninfected cows (P > 0.05). 85A and the 35-kDa protein showed the most proliferative activity in low and medium shedders, respectively.
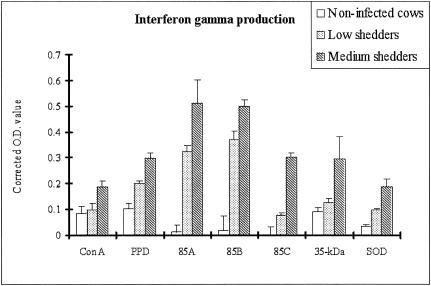
Production of IFN-γ by recombinant antigens.
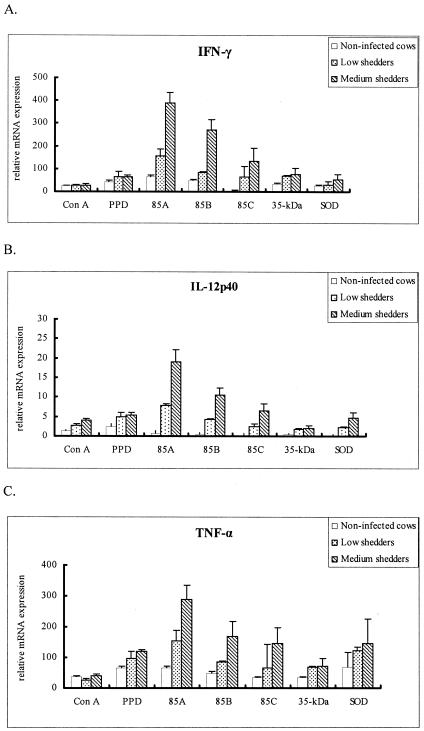
IFN-γ production after stimulation with five recombinant antigens or two positive controls was measured in PBMCs from infected and uninfected control cows. The results are given in corrected OD values (OD of antigen stimulated minus OD of control) representing the elevation of IFN-γ production by the various antigens. All recombinant antigens tested induced the significant release of IFN-γ in cultures of bovine PBMCs from infected cattle compared to uninfected controls (P < 0.05), and IFN-γ levels were consistently higher in medium shedders than in low shedders (P < 0.05) (Fig. 2). The recombinant antigens 85A and 85B induced significantly higher levels of IFN-γ in the low shedders than the other recombinant antigens tested and the two positive controls (P < 0.05).
FIG. 2.
IFN-γ production in response to individual antigens is related to M. avium subsp. paratuberculosis shedding levels. The results are given as OD values in stimulated wells minus OD values in control (naturally produced IFN-γ) wells. Error bars represent standard deviations from the means. 85A and 85B were the most inducible antigens to produce IFN-γ in bovine peripheral blood mononuclear cells from both shedders.
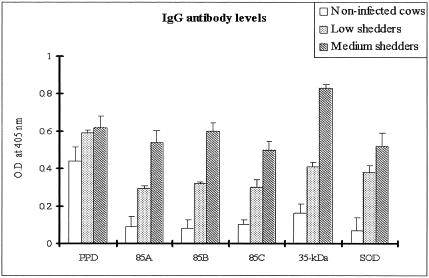
Comparison of antibody responses to recombinant antigens.
Levels of IgG antibodies to all recombinant antigens were measured in sera from both shedder groups and healthy controls. Although there was a wide variation in antibody content in sera from individual cows, the mean IgG antibody responses against all recombinant antigens increased significantly in both the low and medium shedder groups. No significant differences were observed among the mean levels of antibody of the low shedder group to any of the antigens tested (Fig. 3). Strikingly, antibody responses to the 35-kDa protein were significantly higher in the medium shedder group than those to the other antigens (P < 0.05).
FIG. 3.
Antibody responses to individual antigens in related to M. avium subsp. paratuberculosis shedding levels. Bars represent the means OD values at 405 nm. Error bars represent standard deviations from the means. All recombinant antigens showed increases of antibody responses according to shedding levels and antibody responses to the 35-kDa protein were positively separated between noninfected healthy cows and both shedders (P < 0.01).
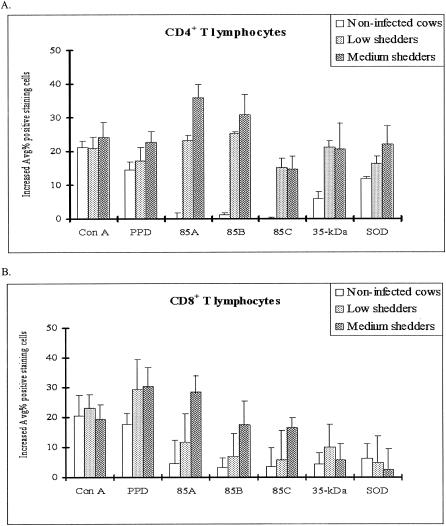
Changes in lymphocyte subset distribution in response to recombinant antigens.
Antigen-stimulated T-cell and/or B-cell subsets were examined by single-color flow cytometry for differences in the percentages of CD4+, CD8+, CD3+, CD21+, and CD25+ lymphocyte subsets, as well as of γδ+ T cells in PBMC cultures from both shedder groups and healthy controls after stimulation with each recombinant antigen (Fig. 4 and 5). All lymphocyte subsets investigated in this study increased, but depending on bacterial shedding levels, there were slight differences (P < 0.05) between noninfected cattle and low shedders according to recombinant antigens.
FIG. 4.
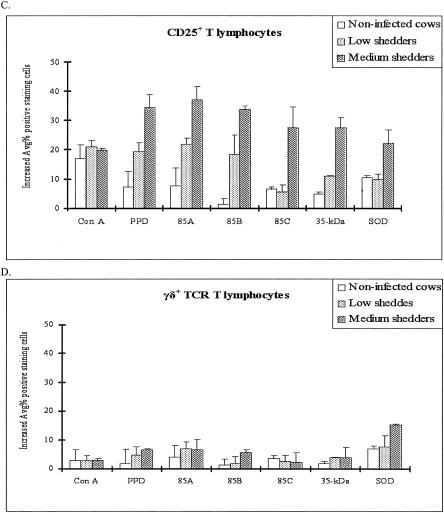
Changes in T-cell subset distribution in bovine peripheral blood lymphocytes after stimulation with recombinant antigens as determined by FACS analysis. (A) CD4. Ag 85A and Ag 85B induced a higher proportion of CD4+ T lymphocytes in medium shedders than in low shedders, while the percentage of CD4+ lymphocytes was unchanged in noninfected control cattle. (B) CD8. Ag 85A increased the proportion of CD8+ T lymphocytes in medium shedders, while the increased percentage of CD8+ lymphocytes was very low in noninfected cattle. (C) CD25. Ag 85A and Ag85B increased the proportion of CD25+ T cells in both shedder groups, while they had little effect in noninfected cattle. In contrast, Ag 85C and the 35-kDa protein significantly increased the proportion of CD25+ T cells in only the medium shedders (P < 0.05). (D) γδ+ T cells. All antigens resulted in significantly lower increases in all cell subsets in both the low and medium shedder groups except SOD for γδ+ T cells in medium shedders.
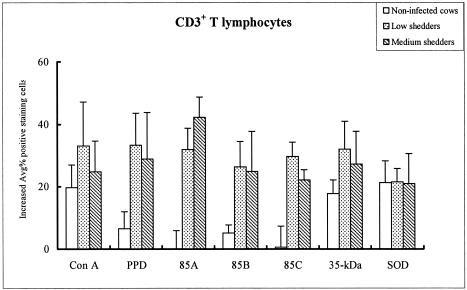
FIG. 5.
Differential changes of CD3+ T lymphocytes in response to stimulation with recombinant proteins and two controls (ConA and PPD). Data are expressed as the average of cells staining positive for CD3 (1 standard error of the mean) in response to each recombinant antigen relative to the shedding level.
CD3 is a pan T-cell marker that is expressed by CD4+ and CD8+ cells as well as γδ+ T cells. The proportion of CD25+ T cells increased in culture regardless of the recombinant antigen used (P < 0.05) (Fig. 4C). These results suggest that all antigens used in this study are able to stimulate sensitized T cells.
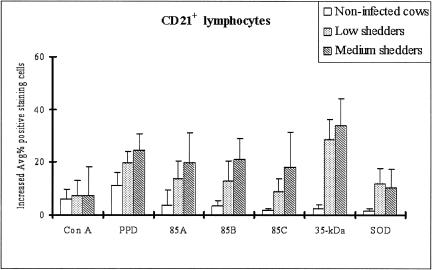
While all recombinant antigens tested increased the proportion of CD4+ cells in cultures of bovine PBMCs from infected cattle compared to uninfected controls (P < 0.05), 85A and 85B increased the proportion of CD4+ T cells to significantly higher levels than 85C, the 35-kDa protein, and SOD (P < 0.05). The proportion of CD4+ T cells was also greater in cultures treated with 85A and 85B among PBMCs from medium shedders than that from low shedders (P < 0.05) (Fig. 4A). In contrast, a significant increase in the proportion of CD8+ T cells was found only in cultures treated with 85A, 85B, and 85C and the proportion of CD8+ cells was also greater in cultures treated with 85A and 85B among PBMCs from medium shedders than that from low shedders (P < 0.05) (Fig. 4B). Only SOD was able to significantly increase the proportion of γδ+ T cells in the cultures of medium shedders (P < 0.05) (Fig. 4D). Lastly, all recombinant antigens tested significantly increased the proportion of CD21+ B cells in cultures of bovine PBMCs from both low and medium shedders compared to uninfected controls (P < 0.05) (Fig. 6). Interestingly, the proportion of CD21+ B cells was significantly higher in cultures of bovine PBMCs from medium shedders than that of the other recombinant antigens tested (P < 0.05).
FIG. 6.
Increased CD21+ B lymphocyte subsets in bovine peripheral blood lymphocytes after stimulation with recombinant antigens as determined by FACS analysis. The results are reported as the average percent increase in positive-staining cells, and the error bars represent 1 standard error of the mean. Recombinant 35-kDa protein induced the largest increase in CD21+ B lymphocytes in medium shedders. No significant increase in the proportion of B lymphocytes was observed in response to the other antigens regardless of bacterial shedding levels (P > 0.05).
Comparison of cytokine mRNA expression levels.
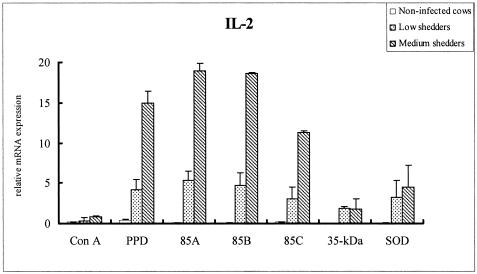
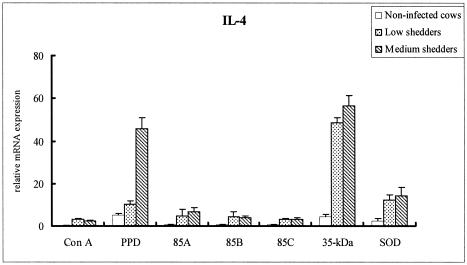
All recombinant antigens stimulated high levels of IL-2 mRNA from PBMCs of medium shedders, with the antigen 85 complex having a greater effect than either the 35-kDa protein or SOD (P < 0.05) (Fig. 7). 85A also induced a high level of IFN-γ, IL-12p40, and TNF-α mRNA (Fig. 8A, B, and C, respectively) in medium shedders (P < 0.05). Strikingly, PBMCs stimulated with the 35-kDa protein antigen highly expressed IL-4 mRNA in both low and medium shedders (P < 0.05) (Fig. 9).
FIG. 7.
IL-2 profiles of bovine PBMCs from noninfected cattle and low and medium shedders after stimulation with recombinant antigens for 24 h. Results represent the mean fold increases of IL-2 over unstimulated PBMCs, which served as calibrators. Ags 85A and 85B most strongly stimulated medium shedders, while the 35-kDa protein and SOD had lesser effects (P < 0.05).
FIG. 8.
Comparison of cytokine mRNA profiles for IFN-γ (A), IL-12p40 (B), and TNF-α (C) of bovine PBMCs from noninfected cattle and low and medium shedders after stimulation with recombinant antigens for 24 h. Results represent the mean fold increase over unstimulated PBMCs, which served as calibrators. The results are similar with Ags 85A and 85B most strongly stimulating medium shedders, while the 35-kDa protein and SOD had lesser effects.
FIG. 9.
IL-4 mRNA profiles of bovine PBMCs from noninfected cattle and low and medium shedders after stimulation with recombinant antigens for 24 h. Results represent the mean fold increases of IL-4 over unstimulated PBMCs, which served as calibrators. The 35-kDa protein strongly stimulated IL-4 mRNA expression in both low and medium shedders.
DISCUSSION
This is the first report to describe lymphoproliferation, cytokine responses, and changes in lymphocyte subset distribution from subclinically M. avium subsp. paratuberculosis-infected cows stimulated with five unique M. avium subsp. paratuberculosis antigens. The antigenic repertoire of an outbred cattle population is both diverse and heterogeneous. It is possible, nevertheless, that a few antigens could elicit protective immunity against M. avium subsp. paratuberculosis infection in ruminants, particularly if the component T-cell epitopes demonstrate indiscriminate reactivity and evoke responses with different host major histocompatibility complex. One of the requirements of a candidate vaccine antigen, therefore, is recognition by the immune system during the course of infection by the majority of animals of the target population. To test this requirement, we used PMBC from M. avium subsp. paratuberculosis-infected (low and medium shedders) and uninfected healthy cows to assess the five candidate M. avium subsp. paratuberculosis antigens. The parameters used as indicators of T-cell responses included Ag-specific proliferation, flow cytometric analysis of T/B-cell subsets, and measurement of cytokine production to identify Ags most likely to stimulate CD4+ lymphocytes of the Th1 phenotype that are considered to play pivotal roles in the response against M. avium subsp. paratuberculosis infection.
Although the nature of an effective immune response to Johne's disease in ruminants is not completely understood, it is generally agreed that the most effective vaccination strategies are those that stimulate T-cell responses, both CD4 and CD8, to produce Th1-associated cytokines (15-17). Therefore, a cocktail of several antigens that induces the production of enduring Th1 responses is desirable and is probably an essential element of a successful vaccine. Among the five chosen M. avium subsp. paratuberculosis Ags, 85A, 85B, and 85C are homologous to 85A, 85B, and 85C from M. tuberculosis, respectively (13). These Ags have been shown to contain T-cell epitopes and could induce protection against tuberculosis or leprae or Buruli ulcer infection in a mouse model by using either recombinant protein antigens or DNA vaccines (30, 35, 36, 54). SOD induces a Th1 response in a mouse model (34), and the 35-kDa protein is a potential virulence factor of M. avium subsp. paratuberculosis (2).
CD4+ T cells that secrete IFN-γ are probably most important in controlling the progression of M. avium subsp. paratuberculosis infection (17, 25). CD4+ T cells that responded to 85A and 85B were significantly higher in both shedder groups than other antigens. Further, 85A and 85B did not increase CD4+ T cells in noninfected cattle, indicating that 85A and 85B antigens induce specifically sensitized CD4+ T cells by M. avium subsp. paratuberculosis and may provide protective immunity against M. avium subsp. paratuberculosis infection through maintaining circulating CD4+-T-cell populations in the early infectious phase. Also, 85A and 85B increased the number of CD25+-T-cell lymphocytes, which are associated with IFN-γ production. 85C may also induce protective immunity since it is able to maintain circulating CD4+ T cells and induce CD25+ T cells in the early phase. However, 85C-antigen-induced CD4+-T-cell immune responses were less than those evoked by 85A and 85B. These results may reflect differences in the immunogenicity of 85C compared to 85A and 85B (20, 29).
T cells expressing the γδ form of the T-cell receptor are a major subpopulation of circulating lymphocytes in ruminants, particularly in young animals, which are most susceptible to paratuberculosis infection (27). Interestingly, SOD stimulated lymphocytes to a lesser degree than the other antigens tested, except for γδ+ T lymphocytes. The number of γδ T cells was significantly higher in PBMC cultures treated with SOD in noninfected cattle as well as in both shedder groups. Thus, SOD may preferentially stimulate γδ T lymphocytes compared to the other antigens tested in this study. γδ T cells are numerous in mucosal tissues, the point of entry for mycobacterial pathogens. It has also been postulated that γδ T cells provide a link between innate and acquired immune responses (24, 31). Other investigators propose a regulatory role for γδ T cells (14) by immunomodulation of α/β+ T cells in both tuberculosis and paratuberculosis in cattle (8, 40). These studies suggest that SOD antigen may be important in the earlier stages of infection by preferentially stimulating γδ T cells (7). Additionally, SOD induced less IFN-γ production than the other antigens used in this study, even in medium shedders.
Cytokine expression is generally lower in peripheral blood than at local sites of infection (3). Also, expression of cytokines is uniquely associated with the polarity of disease states in mycobacterial infections (49). In this study, cytokine profiles were significantly different depending on individual antigens and shedding levels. Induction of IL-4 mRNA by both PPD and the 35-kDa protein significantly increased in the medium shedding levels (P < 0.001). In contrast, no significant differences were observed among the other antigens (P > 0.05). Secretion of IL-4 is upregulated in cells from human tuberculosis patients compared to healthy skin-test-positive controls. These results suggest that a shift in Th1 to Th2 cell activation has occurred in severely affected patients compared to patients with early tuberculosis infection (49). Similar results have been noted for cattle infected with M. avium subsp. paratuberculosis (51). These studies are in agreement with our results indicating that immune responses to the 35-kDa protein are likely to be more important in the later stage of disease since flow cytometric analysis showed that the 35-kDa protein strongly induced proliferation of B lymphocytes, especially in medium shedders.
Other investigators have shown that IFN-γ and IL-2 production by Th1 cells is significantly higher in PBMCs isolated from naturally infected cows with subclinical paratuberculosis than that from control noninfected cows after stimulation of cells with either ConA or a whole-cell sonicate of M. avium subsp. paratuberculosis (48). Similar results were obtained in this study after stimulation with 85-complex antigens. In particular, IFN-γ and TNF-α gene expression induced by 85A was significantly higher depending on bacterial shedding levels (P < 0.001). However, Adams et al. did not find significant differences in IFN-γ mRNA expression from peripheral blood monocytes in subclinically infected cattle and suggested that IFN-γ production is a local phenomenon restricted to infected tissues (1). It is possible that some M. avium subsp. paratuberculosis antigens induce the expression of IFN-γ inhibitory cytokines, such as IL-10, in subclinical infections. In the present study, IL-10 was slightly increased but no significant differences were observed according to shedding levels and individual antigens (data not shown). Our results corroborate the pattern of cytokine expression observed in the different stages of paratuberculosis with a high degree of peripheral blood T-cell responsiveness noted for subclinically infected animals.
To identify the antigens most suitable for vaccine development against JD, we have tested the ability of recombinant M. avium subsp. paratuberculosis antigens to induce the expression of cytokines that have been shown to play a key role in either protection against or susceptibility to JD. IFN-γ, IL-12, and TNF-α are associated with protective responses, while IL-4 correlates with susceptibility to the disease (54). Therefore, an effective vaccine should include mycobacterial antigens that induce protective Th1 responses. In this study, we show that 85A, 85B, and 85C induces the secretion of protective cytokines IFN-γ, IL-12 and TNF-α while SOD induces TNF-α only. It has been suggested that the 85 complex of antigens has a strong ability to protect against disease progression in the early infectious stage by induction of proinflammatory cytokines such as TNF-α, IL-12, and IFN-γ, which are important cytokines in controlling mycobacterial growth (29). However, to effectively induce protective immunity in a large outbred ruminant population, a vaccine based on the 85 complex might need to be combined with other important molecules that could induce a biased protective Th1 immune response. Such a combination vaccine could provide various types of epitopes and then may induce effective protection against M. avium subsp. paratuberculosis infection, especially in the early stages of disease.
Acknowledgments
This work was partially supported by the Biotechnology Research and Development Corporation (BRDC) and by a contract through a cooperative agreement between the NYS Department of Agriculture and Markets and the USDA-APHIS. Sung Jae Shin was supported by the Korea Research Foundation Grant, funded by the Korean government (MOEHRD, Basic Research Promotion Fund, KRF-M01-2004-000-10072-0).
Editor: J. L. Flynn
REFERENCES
- 1.Adams, J. L., M. T. Collins, and C. J. Czuprynski. 1996. Polymerase chain reaction analysis of TNF-alpha and IL-6 mRNA levels in whole blood from cattle naturally or experimentally infected with Mycobacterium paratuberculosis. Can. J. Vet. Res. 60:257-262. [PMC free article] [PubMed] [Google Scholar]
- 2.Bannantine, J. P., J. F. Huntley, E. Miltner, J. R. Stabel, and L. E. Bermudez. 2003. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 149:2061-2069. [DOI] [PubMed] [Google Scholar]
- 3.Begara-McGorum, I., L. A. Wildblood, C. J. Clarke, K. M. Connor, K. Stevenson, C. J. McInnes, J. M. Sharp, and D. G. Jones. 1998. Early immunopathological events in experimental ovine paratuberculosis. Vet. Immunol. Immunopathol. 63:265-287. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodersen, R., F. Bijlsma, K. Gori, K. T. Jensen, W. Chen, J. Dominguez, K. Haverson, P. F. Moore, A. Saalmuller, D. Sachs, W. J. Slierendrecht, C. Stokes, O. Vainio, F. Zuckermann, and B. Aasted. 1998. Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species. Vet. Immunol. Immunopathol. 64:1-13. [DOI] [PubMed] [Google Scholar]
- 6.Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 7.Chan, J., and S. H. Kaufmann. 1994. Immune mechanisms of protection. American Society for Microbiology, Washington, D.C.
- 8.Chiodini, R. J., and W. C. Davis. 1992. The cellular immunology of bovine paratuberculosis: the predominant response is mediated by cytotoxic gamma/delta T lymphocytes which prevent CD4+ activity. Microb. Pathog. 13:447-463. [DOI] [PubMed] [Google Scholar]
- 9.Coler, R. N., A. Campos-Neto, P. Ovendale, F. H. Day, S. P. Fling, L. Zhu, N. Serbina, J. L. Flynn, S. G. Reed, and M. R. Alderson. 2001. Vaccination with the T cell antigen Mtb 8.4 protects against challenge with Mycobacterium tuberculosis. J. Immunol. 166:6227-6235. [DOI] [PubMed] [Google Scholar]
- 10.Coler, R. N., Y. A. Skeiky, T. Vedvick, T. Bement, P. Ovendale, A. Campos-Neto, M. R. Alderson, and S. G. Reed. 1998. Molecular cloning and immunologic reactivity of a novel low molecular mass antigen of Mycobacterium tuberculosis. J. Immunol. 161:2356-2364. [PubMed] [Google Scholar]
- 11.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denis, O., A. Tanghe, K. Palfliet, F. Jurion, T.-P. van den Berg, A. Vanonckelen, J. Ooms, E. Saman, J. B. Ulmer, J. Content, and K. Huygen. 1998. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 66:1527-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dheenadhayalan, V., K. S. Shin, C. F. Chang, C. D. Chang, S. J. Wang, S. McDonough, P. McDonough, S. Stehman, S. Shin, A. Torres, and Y. F. Chang. 2002. Cloning and characterization of the genes coding for antigen 85A, 85B and 85C of Mycobacterium avium subsp. paratuberculosis. DNA Seq. 13:287-294. [DOI] [PubMed] [Google Scholar]
- 14.D'Souza, S., O. Denis, T. Scorza, F. Nzabintwali, H. Verschueren, and K. Huygen. 2000. CD4+ T cells contain Mycobacterium tuberculosis infection in the absence of CD8+ T cells in mice vaccinated with DNA encoding Ag85A. Eur. J. Immunol. 30:2455-2459. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, J. L. 2004. Immunology of tuberculosis and implications in vaccine development. Tuberculosis 84:93-101. [DOI] [PubMed] [Google Scholar]
- 16.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 17.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geluk, A., K. E. van Meijgaarden, K. L. Franken, J. W. Drijfhout, S. D'Souza, A. Necker, K. Huygen, and T. H. Ottenhoff. 2000. Identification of major epitopes of Mycobacterium tuberculosis AG85B that are recognized by HLA-A*0201-restricted CD8(+) T cells in HLA-transgenic mice and humans. J. Immunol. 165:6463-6471. [DOI] [PubMed] [Google Scholar]
- 19.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harth, G., B.-Y. Lee, J. Wang, D. L. Clemens, and M. A. Horwitz. 1996. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect. Immun. 64:3038-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jouanguy, E., F. Altare, S. Lamhamedi, P. Revy, J. F. Emile, M. Newport, M. Levin, S. Blanche, E. Seboun, A. Fischer, and J. L. Casanova. 1996. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335:1956-1961. [DOI] [PubMed] [Google Scholar]
- 22.Jouanguy, E., S. Lamhamedi-Cherradi, D. Lammas, S. E. Dorman, M. C. Fondaneche, S. Dupuis, R. Doffinger, F. Altare, J. Girdlestone, J. F. Emile, H. Ducoulombier, D. Edgar, J. Clarke, V. A. Oxelius, M. Brai, V. Novelli, K. Heyne, A. Fischer, S. M. Holland, D. S. Kumararatne, R. D. Schreiber, and J. L. Casanova. 1999. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat. Genet. 21:370-378. [DOI] [PubMed] [Google Scholar]
- 23.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy, H. E., M. D. Welsh, D. G. Bryson, J. P. Cassidy, F. I. Forster, C. J. Howard, R. A. Collins, and J. M. Pollock. 2002. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1+ γδ T cells. Infect. Immun. 70:1488-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koets, A., V. Rutten, A. Hoek, F. van Mil, K. Müller, D. Bakker, E. Gruys, and W. van Eden. 2002. Progressive bovine paratuberculosis is associated with local loss of CD4+ T cells, increased frequency of γδ T cells, and related changes in T-cell function. Infect. Immun. 70:3856-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kormendy, B. 1992. Paratuberculosis vaccine in a large dairy herd. Acta Vet. Hung. 40:171-184. [PubMed] [Google Scholar]
- 27.Larsen, A. B., R. S. Merkal, and R. C. Cutlip. 1975. Age of cattle as related to resistance to infection with Mycobacterium paratuberculosis. Am. J. Vet. Res. 36:255-257. [PubMed] [Google Scholar]
- 28.Larsen, A. B., A. I. Moyle, and E. M. Himes. 1978. Experimental vaccination of cattle against paratuberculosis (Johne's disease) with killed bacterial vaccines: a controlled field study. Am. J. Vet. Res. 39:65-69. [PubMed] [Google Scholar]
- 29.Lim, J.-H., J.-K. Park, E.-K. Jo, C.-H. Song, D. Min, Y.-J. Song, and H.-J. Kim. 1999. Purification and immunoreactivity of three components from the 30/32-kilodalton antigen 85 complex in Mycobacterium tuberculosis. Infect. Immun. 67:6187-6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozes, E., K. Huygen, J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, P. Vandenbussche, J. P. Van Vooren, A. Drowart, J. B. Ulmer, and M. A. Liu. 1997. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine 15:830-833. [DOI] [PubMed] [Google Scholar]
- 31.Mak, T. W., and D. A. Ferrick. 1998. The gammadelta T-cell bridge: linking innate and acquired immunity. Nat. Med. 4:764-765. [DOI] [PubMed] [Google Scholar]
- 32.Mollenkopf, H. J., L. Grode, J. Mattow, M. Stein, P. Mann, B. Knapp, J. Ulmer, and S. H. Kaufmann. 2004. Application of mycobacterial proteomics to vaccine design: improved protection by Mycobacterium bovis BCG prime-Rv3407 DNA boost vaccination against tuberculosis. Infect. Immun. 72:6471-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mollenkopf, H. J., D. Groine-Triebkorn, P. Andersen, J. Hess, and S. H. Kaufmann. 2001. Protective efficacy against tuberculosis of ESAT-6 secreted by a live Salmonella typhimurium vaccine carrier strain and expressed by naked DNA. Vaccine 19:4028-4035. [DOI] [PubMed] [Google Scholar]
- 34.Mullerad, J., A. H. Hovav, Y. Fishman, R. G. Barletta, and H. Bercovier. 2002. Antigenicity of Mycobacterium paratuberculosis superoxide dismutase in mice. FEMS Immunol. Med. Microbiol. 34:81-88. [DOI] [PubMed] [Google Scholar]
- 35.Mullerad, J., I. Michal, Y. Fishman, A. H. Hovav, R. G. Barletta, and H. Bercovier. 2002. The immunogenicity of Mycobacterium paratuberculosis 85B antigen. Med. Microbiol. Immunol. (Berlin) 190:179-187. [DOI] [PubMed] [Google Scholar]
- 36.Naito, M., M. Matsuoka, N. Ohara, H. Nomaguchi, and T. Yamada. 1999. The antigen 85 complex vaccine against experimental Mycobacterium leprae infection in mice. Vaccine 18:795-798. [DOI] [PubMed] [Google Scholar]
- 37.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1949. [DOI] [PubMed] [Google Scholar]
- 38.Parra, M., T. Pickett, G. Delogu, V. Dheenadhayalan, A. S. Debrie, C. Locht, and M. J. Brennan. 2004. The mycobacterial heparin-binding hemagglutinin is a protective antigen in the mouse aerosol challenge model of tuberculosis. Infect. Immun. 72:6799-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson, C. J., M. LaVenture, S. S. Hurley, and J. P. Davis. 1988. Accidental self-inoculation with Mycobacterium paratuberculosis bacterin (Johne's bacterin) by veterinarians in Wisconsin. J. Am. Vet. Med. Assoc. 192:1197-1199. [PubMed] [Google Scholar]
- 40.Rhodes, S. G., R. G. Hewinson, and H. M. Vordermeier. 2001. Antigen recognition and immunomodulation by gamma delta T cells in bovine tuberculosis. J. Immunol. 166:5604-5610. [DOI] [PubMed] [Google Scholar]
- 41.Serbina, N. V., and J. L. Flynn. 2001. CD8+ T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect. Immun. 69:4320-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serbina, N. V., and J. L. Flynn. 1999. Early emergence of CD8+ T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect. Immun. 67:3980-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serbina, N. V., C. C. Liu, C. A. Scanga, and J. L. Flynn. 2000. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J. Immunol. 165:353-363. [DOI] [PubMed] [Google Scholar]
- 44.Shin, S. J., Y. F. Chang, C. Huang, J. Zhu, L. Huang, H. S. Yoo, K. S. Shin, S. Stehman, and A. Torres. 2004. Development of a polymerase chain reaction test to confirm Mycobacterium avium subsp. paratuberculosis in culture. J. Vet. Diagn. Investig. 16:116-120. [DOI] [PubMed] [Google Scholar]
- 45.Shin, S. J., H. S. Yoo, S. P. McDonough, and Y. F. Chang. 2004. Comparative antibody response of five recombinant antigens in related to bacterial shedding levels and development of serological diagnosis based on 35 kDa antigen for Mycobacterium avium subsp. paratuberculosis. J. Vet. Sci. 5:111-117. [PubMed] [Google Scholar]
- 46.Skeiky, Y. A., M. R. Alderson, P. J. Ovendale, J. A. Guderian, L. Brandt, D. C. Dillon, A. Campos-Neto, Y. Lobet, W. Dalemans, I. M. Orme, and S. G. Reed. 2004. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 172:7618-7628. [DOI] [PubMed] [Google Scholar]
- 47.Skeiky, Y. A., P. J. Ovendale, S. Jen, M. R. Alderson, D. C. Dillon, S. Smith, C. B. Wilson, I. M. Orme, S. G. Reed, and A. Campos-Neto. 2000. T cell expression cloning of a Mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection. J. Immunol. 165:7140-7149. [DOI] [PubMed] [Google Scholar]
- 48.Stabel, J. R. 1996. Production of gamma-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J. Vet. Diagn. Investig. 8:345-350. [DOI] [PubMed] [Google Scholar]
- 49.Stabel, J. R. 2000. Transitions in immune responses to Mycobacterium paratuberculosis. Vet. Microbiol. 77:465-473. [DOI] [PubMed] [Google Scholar]
- 50.Sweeney, R. W. 1996. Transmission of paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 12:305-312. [DOI] [PubMed] [Google Scholar]
- 51.Sweeney, R. W., D. E. Jones, P. Habecker, and P. Scott. 1998. Interferon-gamma and interleukin 4 gene expression in cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 59:842-847. [PubMed] [Google Scholar]
- 52.Sweeney, R. W., R. H. Whitlock, and A. E. Rosenberger. 1992. Mycobacterium paratuberculosis isolated from fetuses of infected cows not manifesting signs of the disease. Am. J. Vet. Res. 53:477-480. [PubMed] [Google Scholar]
- 53.Tanghe, A., J. Content, J.-P. Van Vooren, F. Portaels, and K. Huygen. 2001. Protective efficacy of a DNA vaccine encoding antigen 85A from Mycobacterium bovis BCG against Buruli ulcer. Infect. Immun. 69:5403-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanghe, A., O. Denis, B. Lambrecht, V. Motte, T. van den Berg, and K. Huygen. 2000. Tuberculosis DNA vaccine encoding Ag85A is immunogenic and protective when administered by intramuscular needle injection but not by epidermal gene gun bombardment. Infect. Immun. 68:3854-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanghe, A., P. Lefevre, O. Denis, S. D'Souza, M. Braibant, E. Lozes, M. Singh, D. Montgomery, J. Content, and K. Huygen. 1999. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J. Immunol. 162:1113-1119. [PubMed] [Google Scholar]
- 56.Wells, S. J., and B. A. Wagner. 2000. Herd-level risk factors for infection with Mycobacterium paratuberculosis in US dairies and association between familiarity of the herd manager with the disease or prior diagnosis of the disease in that herd and use of preventive measures. J. Am. Vet. Med. Assoc. 216:1450-1457. [DOI] [PubMed] [Google Scholar]