Abstract
We report the humanization and characterization of monoclonal antibody (MAb) T1-2 or tefibazumab, a monoclonal antibody that recognizes clumping factor A expressed on the surface of Staphylococcus aureus. We demonstrate that the binding kinetics of MAb T1-2 is indistinguishable compared to that of its murine parent. Furthermore, MAb T1-2 is shown to enhance the opsonophagocytic uptake of ClfA-coated latex beads, protect against an intravenous challenge in a prophylactic model of rabbit infective endocarditis, and enhance the efficacy of vancomycin therapy in a therapeutic model of established infective endocarditis.
Staphylococcus aureus is a major pathogen in a significant number of serious nosocomial and community-acquired infections. It has been estimated that approximately 56% of all S. aureus strains causing infections in the intensive care unit are methicillin resistant (17). More recently, reports documenting an increase in community-associated infections caused by methicillin-resistant Staphylococcus aureus (MRSA) (13) and vancomycin-resistant S. aureus strains in patients from Michigan (3), Pennsylvania (1), and New York (2) have served to further highlight the increasing dilemma clinicians face in treating serious S. aureus infections. With a limited number of approved therapeutic options available to address such infections, the development of novel therapies is clearly warranted.
Over the past decade, a family of S. aureus cell surface adhesins, known as MSCRAMMs (for microbial surface components recognizing adhesive matrix molecules), have been shown to interact with high affinity and specificity to host tissues or implanted biomaterials (5, 15), and this step is considered to be the first in the infection process. Clumping factor A (ClfA) is a fibrinogen-binding adhesin thought to be a primary factor contributing to the colonization of implanted biomaterials or damaged endothelial surfaces at the site of endovascular infections (18). The biological role of ClfA in vivo in such infections has been demonstrated in numerous studies (12, 16, 19) and suggests that ClfA is a major virulence factor of S. aureus. The clfA gene has been shown to be expressed in vivo and is present in nearly all clinical S. aureus strains examined to date (4, 21). Furthermore, the biological impact of targeting ClfA was demonstrated by Josefsson and colleagues, who found that active immunization with recombinant ClfA protein and passive immunization with human polyclonal anti-ClfA antibodies protected mice against S. aureus septic arthritis and sepsis-induced death (7).
We recently described the generation of a murine monoclonal antibody (MAb 12-9) that is specific for ClfA, binds with high affinity, inhibits the adherence of S. aureus to immobilized fibrinogen, and protects mice against sepsis-induced death by MRSA (6). We now report the production and characterization of a MAb, T1-2, or tefibazumab, a humanized version of murine MAb 12-9.
Humanization and selection of the NSO T1-2 cell line.
12-9 variable region mRNA was isolated from hybridoma cells using standard molecular biology techniques and sequenced. Humanization was carried out using a process described by Padlan (14). Using this technique, targeted residues were mutated to mimic the most homologous human germ line subgroup using mutagenic oligonucleotides via PCR (PCR Reagent System; Life Technologies, Gaithersburg, Maryland). In the VL sequence, only 7 amino acids (6.25% of total) were changed: VL 1 (N→D), 3 (M→V), 9 (S→D), 15 (A→L), 18 (K→R), 22 (S→N), and 63 (T→S). In the VH region, a total of 9 amino acids (7.4% of total) were changed: VH 13 (A→K), 17 (S→T), 23 (A→T), 76 (S→N), 83 (Q→T), 84 (Y→A), 85 (D→A), 89 (M→V), and 113 (A→S). Appropriate humanized V-regions, human constant regions (immunoglobulin G1 [IgG1]), and mammalian leader sequences were subcloned into glutamine synthetase (GS) selection vectors licensed from Lonza Biologics (Berkshire, United Kingdom).
The NS0 (GS) cell line (Lonza Biologics, Berkshire, United Kingdom) was transfected (FuGene 6; Roche Diagnostics, Indianapolis, IN) with the linearized plasmid containing DNA encoding the humanized 12-9 variable heavy and light chains as directed by Lonza protocols (Lonza Biologics, Berkshire, United Kingdom). Immunoglobulin present in the supernatant from growth positive clones was assayed for reactivity to ClfA by surface plasmon resonance (SPR) by a two-step method as previously described (6), i.e., a goat anti-human-F(ab′)2 antibody (GAH-F(ab′)2; Jackson ImmunoResearch, West Grove, PA)-labeled CM5 chip to facilitate antibody capture (step 1) and passing recombinant ClfA protein over the captured MAb (step 2). ClfA-positive clones were expanded, and the most stable clone with the highest production, MAb T1-2, was chosen as the lead candidate for characterization and production.
In vitro analysis of MAb T1-2.
MAb T1-2 bound to both recombinant and native ClfA, and provided the same level of inhibition of ClfA/fibrinogen binding in vitro as its murine predecessor (data not shown). SPR analysis revealed that the binding kinetics of MAb12-9 and MAb T1-2 were indistinguishable (MAb 12-9, equilibrium dissociation constant [KD] = 2.10 × 10−10; T1-2, KD = 2.54 × 10−10), indicating that the site-specific mutations made did not alter the critical antigen-binding regions of the MAb.
Conversion of the murine Fc region to human IgG1 was designed to increase the opsonic potential of MAb T1-2. This functional activity was evaluated by using a latex bead-based flow cytometric assay with human polymorphonuclear cells (PMNs), thus eliminating the interference of protein A on the surface of intact S. aureus cells. The phagocytic product (PP = mean beads per cell × percent fluorescent PMN) was calculated for each reaction as described in detail previously (9-11). The data in Fig. 1 clearly show that MAb T1-2 facilitates enhanced PMN uptake of the ClfA-coated beads (P values versus MAb T1-2: beads only, <0.001; complement alone, <0.001; and human IgG1, <0.001). Complement alone and nonspecific human IgG1 resulted in minimal levels of phagocytosis by the human PMNs (Fig. 1).
FIG. 1.
Opsonization of ClfA-coated fluorescent beads. PMNs were incubated with unopsonized beads, complement-opsonized beads, T1-2 plus complement-opsonized beads, and nonspecific human IgG1 complement-opsonized beads. The PP represents the mean beads per cell multiplied by the percent fluorescent PMNs, as determined via flow cytometric analysis. C′, complement.
In vivo efficacy of MAb T1-2.
To assess the in vivo therapeutic potential of MAb T1-2 against invasive MRSA infections, a rabbit infective endocarditis (IE) model was utilized. Female outbred New Zealand White rabbits (Irish Farms, Corona, CA) weighing approximately 2.5 kg underwent carotid artery-to-left ventricle catheterization as previously described (8). All rabbits were maintained according to National Institutes of Health animal husbandry standards. For prophylactic studies, approximately 18 h after catheterization animals received a single dose of MAb T1-2 intravenously (i.v.) (10 mg/kg or 30 mg/kg) or normal human i.v. Ig (IVIG; 300 mg/kg) (Panglobulin; ZLB, Bern, Switzerland). Twenty-four hours later, the passively immunized rabbits were challenged i.v. with 2.7 × 106 CFU of MRSA strain 67-0 (courtesy of Henry Chambers, University of California—San Francisco and San Francisco General Hospital, San Francisco). Limited pharmacokinetic analysis revealed specific anti-ClfA titers increased following MAb administration and then dissipated gradually over the 72-h postinfection period (Fig. 2A). None of the animals receiving MAb T1-2 were blood culture positive at sacrifice. In contrast, 4/6 animals (60%) given human IVIG were bacteremic (Fig. 2B). Moreover, only one animal receiving the lower MAb T1-2 (10 mg/kg) dose and none of the animals receiving 30 mg/kg of MAb T1-2 experienced seeding of vegetations or kidneys. All of the animals that were treated with normal IVIG developed bacterial seeding in both the vegetation and kidney (Fig. 2C and 2D). The levels of bacteria recovered from the target organs of rabbits receiving MAb T1-2, compared with the normal IVIG-treated animals, were significantly lower (Fig. 2B, C, and D; P < 0.02).
FIG. 2.
Serum levels of MAbs and blood or target tissue densities in the prophylactic rabbit model of IE (harvested 72 h postinfection). (A) Blood levels of T1-2 (at 10 [n = 7] and 30 [n = 9] mg/kg) were assessed at 48, 72, and 96 h post-antibody treatment. (B) Number of MRSA recovered from the blood at 96 h in prophylactically treated rabbits with MAb T1-2 (10 or 30 mg/kg) versus that in animals receiving IVIG. Vegetation bacterial densities (C), as well as infection in other target organs, such as the kidney (D), were also quantified 96 h after MAb treatment. In panels B, C, and D, data are represented as the bacterial density in each animal, with the horizontal line indicating the median. Detection limits: blood, 1.0 CFU/ml; vegetations, 9.0 CFU/g average; kidneys, 4.0 CFU/g average. Analysis of the infection rates in each rabbit treatment group was carried out using the Fisher exact test analysis with GraphPad Prism for Windows (version 3.02). The staphylococcal densities in target tissues (log10 CFU/g tissue) for each group were also compared using Kruskal-Wallis analysis of variance, with Dunn's multiple comparison post-test analysis, and P values of <0.05 were considered statistically significant.
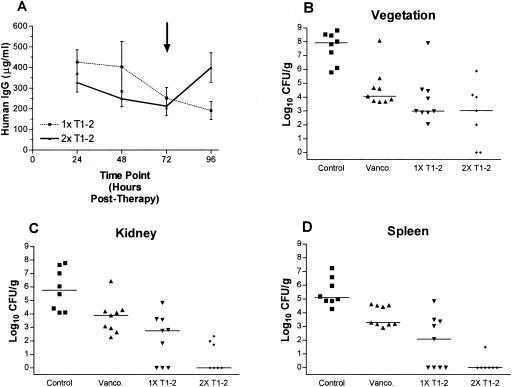
In the therapeutic IE model, MAb T1-2 was used as adjunctive therapy with vancomycin and was administered 24 h after infection with MRSA 67-0. We used both a single-dose or two-dose regimen (successive doses administered 72 h apart). Blood levels of MAb T1-2 indicated that anti-ClfA antibody titers diminished over time in the single-dose group, and in the two-dose group MAb T1-2 levels rose after the second dose to levels similar to that achieved after the primary dose (Fig. 3A). Within 24 h of administration, in the cohort MAb T1-2 plus vancomycin treatment, nearly all blood cultures were rendered culture negative (8/9) (data not shown), and all but one animal remained blood culture negative for the next 24 h. At 96 h posttherapy, two additional animals in this group developed recrudescent bacteremia (data not shown). These cases of recrudescent bacteremia supported the rationale for a treatment group that received a second dose of MAb T1-2 at 72 h following the initiation of therapy (Fig. 3; 2× MAb T1-2). Of note, all animals in the two-dose antibody group remained blood culture negative during the entire postinfection period (data not shown), while all control animals remained blood culture positive. Vancomycin alone exerted a slow impact on bacteremia clearance over the treatment period, with 33%, 67%, and 67% of blood cultures being culture negative at 24, 48, and 72 h posttherapy, respectively (data not shown). In the aortic vegetations, vancomycin alone reduced MRSA densities compared to untreated controls, although these differences did not reach statistical significance (Fig. 3B). Coadministration of one or two doses of MAb T1-2 with vancomycin significantly reduced vegetation MRSA densities (Fig. 3B) as compared to untreated controls. In the kidneys (Fig. 3C) and spleen (Fig. 3D), vancomycin alone reduced MRSA densities as compared to those of untreated controls, although this did not reach statistical significance. Similar to the vegetation data above, single-dose and two-dose MAb T1-2 administrations significantly reduced MRSA densities in kidneys (Fig. 3C) and spleen (Fig. 3D) as compared to untreated controls.
FIG. 3.
Serum levels of MAbs and target tissue densities associated with MRSA challenge in the therapeutic rabbit model of IE. Bacterial challenge was at 0 h, 1× MAb therapy was at 24 h, 2× MAb therapy was at 96 h, and harvest of tissues was at 120 h postinfection. Control, no treatment (n = 8); vancomycin (Vanco.), (twice daily) alone at 7.5 mg/kg (n = 9); and vancomycin (twice daily) at 7.5 mg/kg plus MAb T1-2 at 30 mg/kg (n = 9). Animals given a second dose of MAb T1-2 received it 72 h after the initial dose (96 h postchallenge, designated by the arrow in panel A). (A) Blood levels of MAb T1-2 post-i.v. infusion. (B) Vegetation bacterial densities. (C) Kidney bacterial densities. (D) Spleen bacterial densities. All values were from sacrifices performed at 96 h after vancomycin ± MAb T1-2 (1× or 2×) treatment (120 h postinfection). In panels B, C, and D, data are represented as the bacterial density observed in tissues of individual animals with the horizontal line indicating the median. Detection limits: vegetations, 54 CFU/g average; kidneys, 9.5 CFU/g average; spleen, 7.3 CFU/g average. Statistical analysis was performed as described for Fig. 2.
Previous data from our laboratories have indicated that polyclonal antibodies to recombinant ClfA are effective in preventing bacteremia (20). In this report we show that a single prophylactic administration of the MAb T1-2 is extremely effective in reducing target organ seeding and/or an increase in clearance, indicating that MAb T1-2, by itself, is capable of reducing the severity of IE MRSA infection. We also demonstrate the benefit of combining antibody therapy with conventional antibiotic treatment, as therapeutic administration of the MAb T1-2 with vancomycin significantly enhanced the clearance and/or reduced seeding of MRSA to target tissues compared to vancomycin alone. This is an important observation, given that in the clinical setting, MAb T1-2 would most likely be coadministered with an antibiotic, representing the current standard of care. Currently, the exact mechanism by which T1-2 reduces the level of bacteremia, vegetation, and organ screening is not precisely defined; however, the observed biological activities are consistent with an affinity for ClfA, inhibition of adherence of S. aureus to fibrinogen, and opsonophagocytosis. In conclusion, these findings strongly support further evaluation of such humanized MAbs directed against key adhesins in well-designed clinical trials to either prevent or mitigate serious S. aureus infections.
Acknowledgments
We thank Jin Wang, Jeffery Robbins, and Laurie Donald for their expert technical assistance and Yin Lee for assistance with the animal model.
Editor: F. C. Fang
REFERENCES
- 1.Anonymous. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 2.Anonymous. 2004. Vancomycin-resistant Staphylococcus aureus—New York, 2004. Morb. Mortal. Wkly. Rep. 53:322-323. [PubMed] [Google Scholar]
- 3.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 4.Colque-Navarro, P., M. Palma, B. Söderquist, J.-I. Flock, and R. Möllby. 2000. Antibody responses in patients with staphylococcal septicemia against two Staphylococcus aureus fibrinogen binding proteins: clumping factor and an extracellular fibrinogen binding protein. Clin. Diagn Lab. Immunol. 7:14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster, T. J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 6.Hall, A. E., P. J. Domanski, P. R. Patel, J. H. Vernachio, P. J. Syribeys, E. L. Gorovits, M. A. Johnson, J. M. Ross, J. T. Hutchins, and J. M. Patti. 2003. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect. Immun. 71:6864-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josefsson, E., O. Hartford, L. O'Brien, J. M. Patti, and T. Foster. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 184:1572-1580. [DOI] [PubMed] [Google Scholar]
- 8.Kupferwasser, L. I., M. R. Yeaman, S. M. Shapiro, C. C. Nast, P. M. Sullam, S. G. Filler, and A. S. Bayer. 1999. Acetylsalicylic acid reduces vegetation bacterial density, hematogenous bacterial dissemination, and frequency of embolic events in experimental Staphylococcus aureus endocarditis through antiplatelet and antibacterial effects. Circulation 99:2791-2797. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann, A. K., A. Halstensen, I. S. Aaberge, J. Holst, T. E. Michaelsen, S. Sornes, L. M. Wetzler, and H.-K. Guttormsen. 1999. Human opsonins induced during meningococcal disease recognize outer membrane proteins PorA and PorB. Infect. Immun. 67:2552-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehmann, A. K., A. Halstensen, and C. F. Bassoe. 1998. Flow cytometric quantitation of human opsonin-dependent phagocytosis and oxidative burst responses to meningococcal antigens. Cytometry 33:406-413. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann, A. K., A. Halstensen, J. Holst, and C. F. Bassoe. 1997. Functional assays for evaluation of serogroup B meningococcal structures as mediators of human opsonophagocytosis. J. Immunol. Methods 200:55-68. [DOI] [PubMed] [Google Scholar]
- 12.Moreillon, P., J. M. Entenza, P. Francioli, D. McDevitt, T. J. Foster, P. Francois, and P. Vaudaux. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63:4738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morin, C. A., and J. L. Hadler. 2001. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J. Infect. Dis. 184:1029-1034. [DOI] [PubMed] [Google Scholar]
- 14.Padlan, E. A. 1991. A possible procedure for reducing the immunogenicity of antibody variable domains while preserving their ligand-binding properties. Mol. Immunol. 28:489-498. [DOI] [PubMed] [Google Scholar]
- 15.Patti, J. M., B. L. Allen, M. J. Mcgavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 16.Que, Y.-A., J.-A. Haefliger, P. Francioli, and P. Moreillon. 2000. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect. Immun. 68:3516-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes et al. 1999. Nosocomial infections in medical intensive care units in the United States. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 18.Siboo, I. R., A. L. Cheung, A. S. Bayer, and P. M. Sullam. 2001. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect. Immun. 69:3120-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stutzmann Meier, P., J. M. Entenza, P. Vaudaux, P. Francioli, M. P. Glauser, and P. Moreillon. 2001. Study of Staphylococcus aureus pathogenic genes by transfer and expression in the less virulent organism Streptococcus gordonii. Infect. Immun. 69:657-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernachio, J., A. S. Bayer, T. Le, Y.-L. Chai, B. Prater, A. Schneider, B. Ames, P. Syribeys, J. Robbins, and J. M. Patti. 2003. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob. Agents Chemother. 47:3400-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolz, C., C. Goerke, R. Landmann, W. Zimmerli, and U. Fluckiger. 2002. Transcription of clumping factor A in attached and unattached Staphylococcus aureus in vitro and during device-related infection. Infect. Immun. 70:2758-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]