Abstract
The aim of this study was to determine if probiotics reduce epithelial injury following exposure to Escherichia coli O157:H7 and E. coli O127:H6. The pretreatment of intestinal (T84) cells with lactic acid-producing bacteria reduced the pathogen-induced drop in transepithelial electrical resistance. These findings demonstrate that probiotics prevent epithelial injury induced by attaching-effacing bacteria.
Escherichia coli serotype O157:H7 causes acute diarrhea, hemorrhagic colitis, and hemolytic uremic syndrome (18). The organism is the most common serotype identified in the group of enteric pathogens variously referred to as enterohemorrhagic E. coli, verotoxin-producing E. coli, and Shiga-like toxin-producing E. coli (15). Current therapy is limited to supportive treatment alone, because the use of antibiotics appears to increase the risk of systemic complications, such as acute renal failure occurring in hemolytic uremic syndrome, perhaps by promoting the release of toxin from the periplasm (34).
Enteropathogenic E. coli (EPEC) strains are non-toxin-producing bacteria associated with acute and protracted diarrhea in infants, particularly in the developing world. Both enterohemorrhagic E. coli and enteropathogenic E. coli bind to the surface epithelia, induce rearrangements of the cytoskeleton referred to as attaching and effacing lesions, and inject proteins (EspF) via a molecular syringe encoded by a type 3 secretion system that is targeted to intercellular tight junctions (16, 17).
Probiotics refer to a group of nonpathogenic organisms that are purported to have beneficial effects on health (26). A meta-analysis of randomized controlled trials provided evidence of the efficacy of lactic acid-producing bacteria for both the prevention and treatment of acute diarrhea in infants and young children (13). However, the precise mechanisms underlying these beneficial effects have not been clearly delineated. Therefore, the aims of the present study were to determine whether Lactobacillus species prevent injury to polarized intestinal T84 cell monolayers induced by infection with E. coli O157:H7 and E. coli O127:H6.
E. coli O157:H7 strain CL-56 was originally isolated from a stool sample obtained from a child with hemorrhagic colitis and hemolytic uremic syndrome (29). For comparative purposes, enteropathogenic E. coli strain E2348/69 (serotype O127:H6) was also employed. Lactobacillus strains, including Lactobacillus acidophilus strain R0052 and Lactobacillus rhamnosus strain R0011, were provided by Institut Rosell-Lallemand Inc. (Montreal, Quebec, Canada).
E. coli strains were grown overnight at 37°C in static, nonaerated Penassay broth (Difco, Detroit, MI), spun at 3,000 rpm for 5 min, washed with sterile phosphate-buffered saline (PBS, pH 7.4), and resuspended in PBS to a final concentration of 5 × 109 bacteria/ml. Lactobacillus strains were either used as concentrated industrial proprietary preparations or grown overnight at 37°C in static, nonaerated de Man, Rogosa, and Sharpe (MRS) broth (Difco), spun at 3,000 rpm for 5 min, and then washed and suspended in sterile PBS to a final concentration of 5 × 109 bacteria/ml. These two methods of preparation were used to determine whether the growth conditions of the Lactobacillus strains altered their probiotic effects. Tyndallized probiotics were prepared from industrially grown lactobacilli by heat treatment for 1 h at 70°C on three consecutive days and by gamma irradiation (71.3 krad for 1 h).
To determine if the effects of probiotics were solely due to the lower pH and lactic acid production, lactic acid (100 mmol; Sigma-Aldrich, St. Louis, MO) was added to the upper chamber of a 6.5-mm (0.4 μm pore size) 12-well Transwell (Corning Glass Works, Corning, NY), and E. coli strain CL-56 (O157:H7) or E2348/69 (O127:H6) (107 CFU in 1.0 ml of tissue culture medium) was added to the lower chamber and incubated overnight at 37°C in 5% CO2. There was no inhibition of E. coli growth by lactate. The pH levels of T84 culture medium during the 24-h period of incubation with the probiotic strains were not reduced below 6.0 (data not shown).
Microbial interactions with host epithelial cells were assessed using HEp-2 and T84 epithelial cells, which were purchased from the American Type Culture Collection (Manassas, VA) and grown in tissue culture flasks according to established methods. Briefly, HEp-2 cells were cultured in minimal essential medium (Gibco Laboratories, Grand Island, NY) supplemented with 15% heat-inactivated fetal bovine serum (Cansera International Inc., Rexdale, Ontario, Canada), 0.5% glutamine, 0.1% sodium bicarbonate, and 2% penicillin-streptomycin (all from Gibco). Cells were grown to confluence in 25-cm2 flasks (Corning) or in LabTek chamber slides (Miles Scientific, Naperville, IL) at 37°C in 5% CO2.
T84 cells were grown either in 25-cm2 flasks (Corning) until confluence, in LabTek chamber slides (Miles Scientific), or in 6.5-mm 12-well Transwells (Corning) at 37°C in 5% CO2 for 7 days. Cells grown in Transwells were cultured until the transepithelial electrical resistance (TER) reached a minimum of 1,000 Ω/cm2. T84 cells were then preincubated for 6 h at 37°C with 108 lactobacilli resuspended in 0.2 ml of tissue culture medium without antibiotics. Electrical resistance was measured with a Millicell probe (Millipore Corporation, Bedford, MA), and changes in response to exposure to bacteria were calculated as percentages of control values.
For infections of epithelial cells, the culture medium was replaced with antibiotic-free medium. Lactobacilli (106, 108, 109, or 1010 bacteria) were then either coinfected with E. coli (107 bacteria) or preincubated with the host epithelium for 3 h or 6 h prior to the addition of the pathogenic bacterium. Infected cells were then incubated for up to 18 h at 37°C in 5% CO2. The results are expressed as means ± standard errors of the means. Analysis of variance (ANOVA) was employed to determine statistical differences between multiple groups.
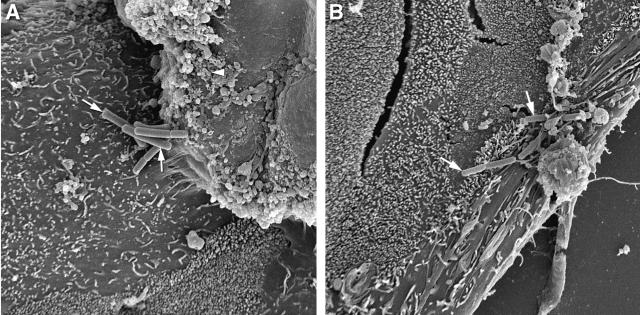
Scanning electron microscopy was used to detect the attachment of Lactobacillus strains to the surfaces of T84 cells. T84 cells were treated with lactobacilli for 3 h at 37°C and then collected and prepared for electron microscopy as described previously (28). Briefly, cells were fixed overnight in paraformaldehyde and glutaraldehyde (4% and 1%, respectively, at pH 7.0), incubated in osmium tetroxide for 1 h at room temperature, and dehydrated in a graded series of ethanol (from 50% to 100%). Cells were then critically point dried and sputter coated with gold before visualization with a scanning electron microscope (model JSM 820; Joel Ltd., Boston, MA). Both L. acidophilus and L. rhamnosus adhered to the surfaces of both HEp-2 cells (data not shown) and T84 cells (Fig. 1).
FIG. 1.
Lactobacilli adhere to the surfaces of T84 epithelial cells. The scanning electron photomicrographs show L. rhamnosus strain R0011 (A) and L. acidophilus strain R0052 (B) grown in MRS broth adhering to cell surfaces (arrows). Approximate original magnification, ×4,300.
Quantitation of the binding of Lactobacillus strains and E. coli to tissue culture cells was performed as described previously (31). Briefly, HEp-2 or T84 cells were grown overnight in antibiotic-free medium in 25-cm2 flasks (Corning) or in LabTek chamber slides (Miles Scientific). Cells were either pretreated with probiotics for 3 h or 6 h prior to E. coli infection or coincubated with probiotics for 3 h at 37°C in 5% CO2. Cells were then washed with sterile phosphate-buffered saline (Gibco) to remove nonadherent bacteria. Epithelial cells with adherent bacteria were trypsinized with 0.25% trypsin (Gibco) and centrifuged at 800 rpm for 5 min. The supernatant was discarded, and 1 ml of sterile distilled water plus 0.1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) was added to lyse the eukaryotic cells (5). To selectively culture lactobacilli, Columbia blood agar plates (PML Microbiologicals, Mississauga, Ontario, Canada) were employed, whereas MacConkey agar (PML Microbiologicals) was used to detect the CFU of E. coli O157:H7 and E2348/69. Plates were incubated in the absence of CO2 for 48 h at 37°C, and then individual colonies were enumerated. Staining with 10% Giemsa (Fisher Scientific, Pittsburgh, PA) was used as a complementary method to verify bacterial adherence to the tissue culture cells.
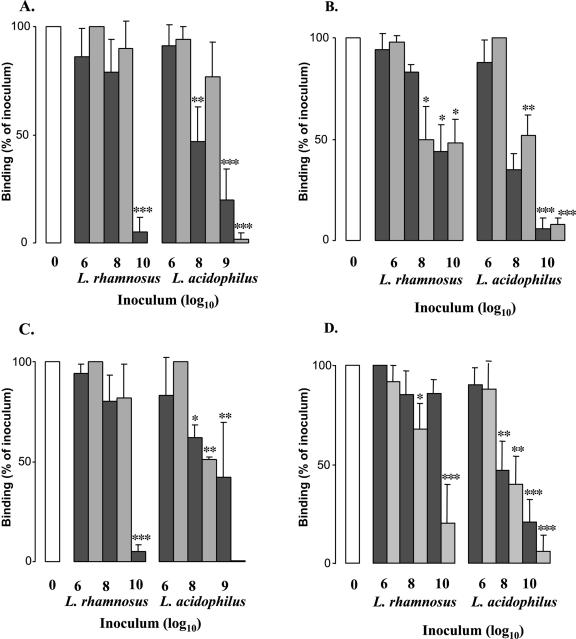
As shown in Fig. 2, pretreatment with L. acidophilus R0052 and L. rhamnosus R0011 (industrially rehydrated or grown in MRS broth) for 3 h resulted in a dose-dependent reduction in the adhesion of E. coli O157:H7 to HEp-2 (panel A) and T84 cells (panel B). Similarly, a reduced adherence of EPEC strain E2348/69 was observed when host epithelial cells were pretreated with lactobacilli prior to infection (Fig. 2C and D). The difference in the abilities of the Lactobacillus strains to prevent pathogenic adherence was both species and preparation dependent. In contrast to the case for preincubation with tissue culture cells, when lactic acid-producing bacteria were coincubated with the enteric pathogens there was no reduction in the adhesion of E. coli O157:H7 or EPEC strain E2348/69 to host epithelia (data not shown). In addition, neither preincubation nor coincubation with Tyndallized probiotics prevented E. coli adherence to HEp-2 and T84 cells (data not shown).
FIG. 2.
Lactobacilli inhibit adhesion of E. coli O157:H7 strain CL-56 and EPEC strain E2348/69 (O127:H7) to epithelial cells. Quantitative adhesion assays showed that both L. acidophilus R0052 and L. rhamnosus R0011, grown either in MRS broth (dark gray bars) or using industrial methods (light gray bars), reduced the binding of E. coli O157:H7 and EPEC to the surfaces of HEp-2 cells (A and C, respectively) and to T84 epithelia (B and D, respectively) in a dose-dependent manner. Open bars represent the adhesion of enteric pathogens to epithelial cells in the absence of probiotics. *, P < 0.05 by ANOVA; **, P < 0.01 by ANOVA; ***, P < 0.001 by ANOVA (compared to control values).
To determine the mechanism of action of probiotics, supernatants from spent MRS broth were removed and filtered twice through a Millipore filter (0.2-μm polysulfone membrane filter; Gelman Laboratories, Missisauga, Ontario, Canada). To ensure that viable organisms were not present, the medium was passed twice through a 0.2-μm filter (Millipore). Plating onto blood agar plates was employed to ensure that viable bacteria were not present in culture supernatants.
To prepare conditioned medium, T84 cells grown in T25 flasks were treated with either MRS broth-cultured or industrially rehydrated lactobacilli and then incubated at 37°C for 18 and 24 h. The conditioned medium was then removed, spun at 3,000 rpm for 5 min, and filtered twice through a Millipore filter (0.2-μm polysulfone membrane filter; Gelman Laboratories).
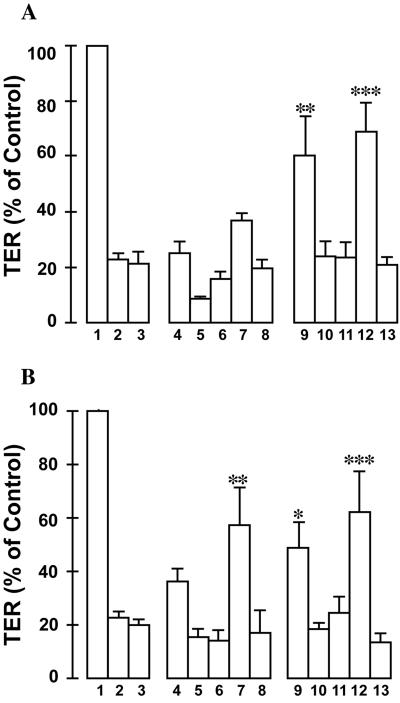
As shown in previous reports (24, 25), the incubation of E. coli O157:H7 (108 CFU) or an equal amount of EPEC strain E2348/69 for 18 h induced an 80% reduction in the TER of T84 cells. In contrast, an equal number of lactobacilli, whether industrially rehydrated or grown in MRS broth, had no effect on the TER of polarized intestinal epithelia (data not shown). The preincubation of viable probiotics for either 3 h (Fig. 3A) or 6 h (Fig. 3B) with T84 cells reduced the drop in TER induced by infection with E. coli O157:H7 or EPEC strain E2348/69. In contrast, the coincubation of L. acidophilus R0052 or L. rhamnosus R0011 with an equal number of pathogenic bacteria for 18 h did not prevent E. coli-induced reductions in the TER (data not shown). Viable lactic acid-producing bacteria were necessary to mediate the observed effects, since culture supernatants, conditioned medium, and Tyndallized preparations of L. acidophilus R0052 and L. rhamnosus R0011 did not prevent the drop in TER following infections of T84 cells with attaching-effacing E. coli enteropathogens.
FIG. 3.
Lactobacilli attenuate the drop in resistance induced by E. coli O157:H7 and EPEC infections. The pretreatment of T84 cells with Lactobacillus species (108 in 0.2 ml) for 3 h (A) or 6 h (B) prior to infection with E. coli O157:H7 or EPEC (107 in 0.2 ml) reduced the pathogen-induced drop in resistance. Lanes 1, uninfected T84 cells; lanes 2, cells infected with EPEC; lanes 3, Shiga-like toxin-producing E. coli-infected T84 cells; lanes 4 to 8, EPEC-infected cells preincubated with L. rhamnosus R0011 grown in MRS medium (lanes 4), L. acidophilus R0052 grown in MRS medium (lanes 5), L. rhamnosus R0011 grown under industrial conditions (lanes 6), L. acidophilus R0052 grown industrially (lanes 7), and Tyndallized L. acidophilus R0052 (lanes 8); lanes 9 to 13, E. coli O157:H7-infected T84 cells preincubated with L. rhamnosus R0011 grown in MRS medium (lanes 9), L. acidophilus R0052 grown in MRS medium (lanes 10), L. rhamnosus R0011 grown under industrial conditions (lanes 11), L. acidophilus R0052 grown industrially (lanes 12), and Tyndallized L. acidophilus R0052 (lanes 13). *, P < 0.05 by ANOVA; **, P < 0.01 by ANOVA; ***, P < 0.001 by ANOVA (compared to control values).
To detect attaching-effacing lesions, indirect immunofluorescence using a murine monoclonal antibody against the F-actin bridging protein alpha-actinin was employed as described previously (14). Briefly, HEp-2 cells grown overnight on slides in 5% CO2 at 37°C were washed with sterile PBS. Industrially rehydrated or MRS broth-cultured L. acidophilus or L. rhamnosus was added to a concentration of 108 CFU prior to or in conjunction with E. coli (107 CFU) infection. Cells were infected for 3 h and then fixed in 100% cold methanol (Caledon Laboratories, Georgetown, Ontario, Canada). A 1:100 dilution of anti-alpha-actinin (Sigma) was added to the cells and incubated at 37°C for 1 h prior to incubation with fluorescein isothiocyanate-conjugated AffiniPure goat anti-mouse alpha-chain-specific immunoglobulin M (Jackson Immunoresearch Laboratories, Missisauga, Ontario, Canada). Cells were then mounted in slow-fade component A-glycerol-PBS (Molecular Probes, Eugene, OR), and slides were examined by alternating phase-contrast and immunofluorescence microscopy at a magnification of ×40 (Leitz Dialuz 22; Leica Canada Inc., Willowdale, Ontario, Canada).
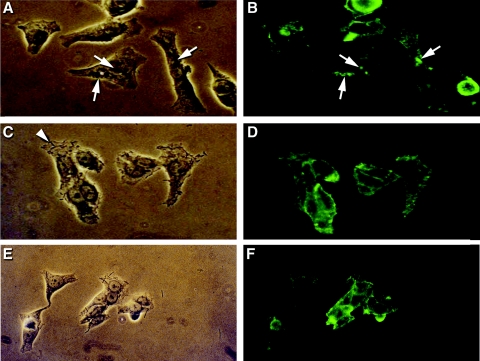
As shown in Fig. 4, indirect immunofluorescence microscopy demonstrated that viable probiotics reduced the number of foci of rearrangements of alpha-actinin, which was indicative of a reduced number of attaching and effacing lesions formed in response to E. coli O157:H7 and EPEC strain E2348/69 infections (14).
FIG. 4.
Lactobacilli inhibit attaching and effacing E. coli-induced rearrangements of alpha-actinin in HEp-2 cells. Phase-contrast microscopy showed the binding of EPEC strain E2348/69 (arrows) to epithelial cells following infection for 3 h at 37°C (A). Corresponding fluorescence micrographs demonstrated the aggregation of foci of alpha-actinin underneath adherent EPEC strain E2348/69 (B). Phase-contrast microscopy demonstrated adherent L. acidophilus R0052 (C, arrowhead). Corresponding fluorescence microscopy (D) showed a negative alpha-actinin response. Phase-contrast microscopy showed reduced E. coli O157:H7 adhesion (E) and alpha-actinin reorganization (F) in HEp-2 cells coincubated with L. acidophilus R0052. Approximate original magnification, ×40.
Using two strains of probiotic bacteria grown under two defined culture conditions, our findings indicate that the organisms did not adversely affect the functional integrity of intercellular tight junctions. In contrast to enterohemorrhagic E. coli serotype O157:H7 and enteropathogenic E. coli strain E2348/69, probiotics had no effect on the transepithelial electrical resistance of a polarized intestinal (T84 cell) monolayer. TER was employed as a marker of the integrity of intercellular tight junctions because it provides an electrical measurement that is inversely related to the permeability of the polarized epithelium to macromolecules such as mannitol and Cr-labeled EDTA (2). Moreover, preincubation, but not coincubation, of the monolayer with L. acidophilus and L. rhamnosus prevented the drop in TER induced by pathogenic E. coli infection. The findings of the present study are supported by previous observations reported using other probiotics, including nonpathogenic yeast. For example, Saccharomyces boulardii preserves the barrier function in T84 cells infected with either EPEC (7) or E. coli O157:H7 (8). However, in contrast to the results of the present study using lactic acid-producing bacteria, the results with S. boulardii did not correlate with a reduced binding of E. coli O157:H7 to T84 monolayers (8).
In the present study, viable probiotics provided a barrier which reduced the response of the host epithelium to pathogenic infections. Culture supernatants and Tyndallized bacteria did not provide a comparable beneficial effect. Previous studies support the need for viable probiotics. For example, Gotteland et al. (10) showed that heat-killed Lactobacillus GG does not mediate the protective effect on gastric damage induced by a nonsteroidal anti-inflammatory agent. Resta-Lenert and Barrett (27) reported that live bacteria are required for probiotics to protect intestinal epithelial cells in tissue culture from adverse effects induced by enteroinvasive E. coli.
The adhesion of lactobacilli to receptors on surface epithelial cells could compete for binding sites with enteric pathogens. It is also possible that lactic acid-producing bacteria reduce both the viability and the virulence properties of E. coli O157:H7 (6) and other diarrheagenic E. coli (19). In this study, we have demonstrated that these probiotics affect the virulence of E. coli O157:H7 and E. coli E2348/69 by factors other than their ability to reduce the pH or produce lactic acid. The ability of these probiotic strains to attenuate the pathogen-induced drop in TER at neutral pH values strongly supports this contention. Several previous reports indicated that factors other than lactic acid produced by probiotics, including bacteriocins, proteinases, peroxides, and exopolysaccharides, could exert antibacterial effects (3, 32). However, nondigestible oligosaccharides have not proven to be effective alternatives to viable probiotics in experimental animal models of bacterium-induced diarrheal disease (22, 30).
Previous studies indicated that Lactobacillus species are able to adhere to the surfaces of intestinal epithelial cells in tissue culture (11). Previous work has shown, for example, that E. coli O157:H7 binds to the surfaces of S. boulardii cells (9). On the other hand, Hirano and coworkers (12) reported that L. rhamnosus blocks the internalization, but not the adherence, of E. coli O157:H7 in Caco-2 cells. Other lactic acid-producing bacteria, including Lactobacillus gasseri, Lactobacillus casei, and Lactobacillus plantarum, have no effect on either the binding or internalization of E. coli O157:H7 in Caco-2 cell monolayers (12). This study shows that L. rhamnosus and L. acidophilus have the ability to adhere to host epithelial cells and reduce the binding of both E. coli O157:H7 and E. coli E2348/69 to host epithelial cells.
Probiotics have also been employed with success in vivo. For example, in an infant rabbit model of E. coli O157:H7 infection, L. casei promotes immune responses against the E. coli cytotoxin and enhances the elimination of O157:H7 from the intestinal tract (23). Using a model of streptomycin-treated mice, Asahara et al. (1) showed that Bifidobacterium breve inhibits the consequences of E. coli O157:H7 infection in parallel with a drop in the luminal pH due to the production of high levels of acetic acid. Probiotics are also effective at reducing O157:H7 gut colonization in ruminants (36), which serve as the environmental reservoir for enterohemorrhagic E. coli. For humans, randomized trials have provided evidence of a beneficial effect of probiotics (20), including both the prevention and treatment of acute diarrhea in children (13, 33).
Boudeau et al. (4) reported that a probiotic reduces both the binding and internalization of adherent, invasive E. coli strains originally isolated from subjects with Crohn's disease. Similar to the findings reported in the present study, the preincubation of host cells with the probiotic was better than coincubation at reducing binding of the E. coli strains (4). In summary, probiotic strains play an important role in attenuating host epithelial responses to pathogenic E. coli infections. Their role in modulating signal transduction responses in host epithelia infected with pathogenic bacteria, including enterohemorrhagic E. coli O157:H7 and E. coli O127:H6, is an important field warranting further investigation (21, 35).
Acknowledgments
This study was supported by operating grants from Institut Rosell-Lallemand Inc. (Montreal, Quebec, Canada) and the Canadian Institutes of Health Research. H.Y. was the recipient of a Summer Student Research Program Award from the Society for Pediatric Research. P.M.S. is the recipient of a Canada Research Chair in Gastrointestinal Disease.
Editor: V. J. DiRita
REFERENCES
- 1.Asahara, T., K. Shimizu, K. Nomoto, T. Hamabata, A. Ozawa, and Y. Takeda. 2004. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 72:2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkes, J., V. K. Viswanathan, S. D. Savkovic, and G. Hecht. 2003. Intestinal epithelial responses to enteric pathogens: effects on tight junction barrier, ion transport, and inflammation. Gut 52:439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernet-Camard, M.-F., V. Lievin, D. Brassart, J.-R. Neeser, A. L. Servin, and S. Hudault. 1997. The human Lactobacillus acidophilus strain LA1 secretes a non-bacteriocin antibacterial substance(s) active in vitro and in vivo. Appl. Environ. Microbiol. 63:2747-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boudeau, J., A.-L. Glasser, S. Julien, J.-F. Colombel, and A. Darfeuille-Michaud. 2003. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E. coli strains isolated from patients with Crohn's disease. Aliment. Pharmacol. Ther. 18:45-56. [DOI] [PubMed] [Google Scholar]
- 5.Boudeau, J., A.-L. Glasser, E. Masseret, B. Joly, and A. Darfeuille-Michaud. 1999. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect. Immun. 67:4499-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brashears, M. M., S. S. Reilly, and S. E. Gilliland. 1998. Antagonistic action of cells of Lactobacillus lactis toward Escherichia coli O157:H7 on refrigerated raw chicken meat. J. Food Prot. 61:166-170. [DOI] [PubMed] [Google Scholar]
- 7.Czerucka, D., S. Dahan, B. Mograbi, B. Rossi, and P. Ramphal. 2000. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect. Immun. 68:5998-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahan, S., G. Dalmasso, V. Imbert, J.-F. Peyron, P. Rampal, and D. Czerucka. 2003. Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli-induced signaling pathways in T84 cells. Infect. Immun. 71:766-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gedek, B. R. 1999. Adherence of Escherichia coli serogroup O157 and the Salmonella typhimurium mutant DT 104 to the surface of Saccharomyces boulardii. Mycoses 42:261-264. [DOI] [PubMed] [Google Scholar]
- 10.Gotteland, M., S. Cruchet, and S. Verbeke. 2001. Effect of Lactobacillus ingestion on the gastrointestinal mucosal barrier alterations induced by indomethacin in humans. Aliment. Pharmacol. Ther. 15:11-17. [DOI] [PubMed] [Google Scholar]
- 11.Greene, J. D., and T. R. Klaenhammer. 1994. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl. Environ. Microbiol. 60:4487-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano, J., T. Yoshida, T. Sugiyama, N. Koide, I. Mori, and T. Yokochi. 2003. The effect of Lactobacillus rhamnosus on enterohemorrhagic Escherichia coli infection of human intestinal cells in vitro. Microbiol. Immunol. 47:405-409. [DOI] [PubMed] [Google Scholar]
- 13.Huang, J. S., A. Bousvaros, J. W. Lee, A. Diaz, and E. J. Davidson. 2002. Efficacy of probiotic use in acute diarrhea in children. A meta-analysis. Dig. Dis. Sci. 47:2625-2634. [DOI] [PubMed] [Google Scholar]
- 14.Ismaili, A., D. J. Philpott, M. T. Dytoc, R. Soni, S. Ratnam, and P. M. Sherman. 1995. Alpha-actinin accumulation in epithelial cells infected with attaching and effacing gastrointestinal pathogens. J. Infect. Dis. 172:1393-1396. [DOI] [PubMed] [Google Scholar]
- 15.Ismaili, A., D. J. Philpott, D. McKay, M. Perdue, and P. M. Sherman. 1998. Epithelial cell responses to Shiga toxin-producing Escherichia coli infection, p. 245-256. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli. ASM Press, Washington, D.C.
- 16.Kaper, J. B., J. P. Nataro, and H. L. T. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 17.Knodler, L. A., J. Celli, and B. B. Finlay. 2001. Pathogenic trickery: deception of host cell processes. Nat. Rev. Mol. Cell. Biol. 2:578-588. [DOI] [PubMed] [Google Scholar]
- 18.LeBlanc, J. J. 2003. Implication of virulence factors in Escherichia coli O157:H7 pathogenesis. Crit. Rev. Microbiol. 29:277-296. [DOI] [PubMed] [Google Scholar]
- 19.Lievin-Le Moal, V., R. Amsellem, A. L. Servin, and M.-H. Coconnier. 2002. Lactobacillus acidophilus (strain LB) from the resident adult gastrointestinal microflora exerts activity against brush border damage promoted by a diarrhoeagenic Escherichia coli in human enterocyte-like cells. Gut 50:803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macfarlane, G. T., and J. H. Cummings. 1999. Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? Br. Med. J. 318:999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mimura, T., F. Rizzello, U. Helwig, G. Poggioli, S. Schreiber, I. C. Talbot, R. J. Nicholls, P. Gionchetti, M. Campieri, and M. A. Kamm. 2004. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 53:108-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naughton, P. J., L. L. Mikkelsen, and B. B. Jensen. 2001. Effects of nondigestible oligosaccharides on Salmonella enterica serovar Typhimurium and nonpathogenic Escherichia coli in the pig small intestine in vitro. Appl. Environ. Microbiol. 67:3391-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa, M., K. Shimizu, K. Nomoto, M. Takahashi, M. Watanuki, R. Tanaka, T. Tanaka, T. Hamabata, S. Yamasaki, and Y. Takeda. 2001. Protective effect of Lactobacillus casei strain Shirota on Shiga toxin-producing Escherichia coli O157:H7 infection in infant rabbits. Infect. Immun. 69:1101-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philpott, D. J., D. McKay, P. M. Sherman, and M. Perdue. 1996. Infection of T84 intestinal epithelial cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am. J. Physiol. 270:G634-G645. [DOI] [PubMed] [Google Scholar]
- 25.Philpott, D. J., D. McKay, W. Mak, M. Perdue, and P. M. Sherman. 1998. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect. Immun. 66:1680-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid, G., J. Jass, M. T. Sebulsky, and J. K. McCormick. 2003. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 16:658-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resta-Lenert, S., and K. E. Barrett. 2003. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52:988-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman, P. M., N. Fleming, J. Forstner, N. Roomi, and G. G. Forstner. 1987. Bacteria and the mucus blanket in experimental small bowel bacterial overgrowth. Am. J. Pathol. 126:527-534. [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman, P. M., R. Soni, M. Petric, and M. A. Karmali. 1987. Surface properties of the verocytotoxin-producing Escherichia coli serotype O157:H7. Infect. Immun. 55:1824-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman, P. M., R. Soni, S. Del Vedovo, and J.-R. Neeser. 1989. Effect of oligomannoside-type glycopeptides in diarrheal disease of rabbits induced by Escherichia coli strain RDEC-1. FEMS Microbiol. Lett. 61:121-126. [DOI] [PubMed] [Google Scholar]
- 31.Sherman, P. M., F. Cockerill III, R. Soni, and J. B. Brunton. 1991. Outer membranes are competitive inhibitors of Escherichia coli O157:H7 adherence to epithelial cells. Infect. Immun. 59:890-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van de Guchte, M., S. D. Ehrlich, and E. Maguin. 2001. Production of growth-inhibiting factors by Lactobacillus delbruekii. J. Appl. Microbiol. 91:147-153. [DOI] [PubMed] [Google Scholar]
- 33.Vanderhoof, J. 2000. Probiotics and intestinal inflammatory disorders in infants and children. J. Pediatr. Gastroenterol. Nutr. 30:S34-S38. [PubMed] [Google Scholar]
- 34.Wong, C., S. Lelacic, R. L. Habeeb, S. L. Watkins, and P. I. Tarr. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infection. N. Engl. J. Med. 342:1930-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan, F., and D. B. Polk. 2002. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 27:50959-50965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao, T., M. P. Doyle, B. G. Harmon, C. A. Brown, P. O. E. Mueller, and A. H. Parks. 1998. Reduction of carriage of enterohemorrhagic Escherichia coli O157:H7 in cattle by inoculation with probiotic bacteria. J. Clin. Microbiol. 36:641-647. [DOI] [PMC free article] [PubMed] [Google Scholar]