Abstract
To determine the role of human β-defensin 2 (HBD-2) in human tuberculosis, we studied the in vitro induction of HBD-2 gene expression by Mycobacterium tuberculosis H37Rv infection in the human lung epithelial cell line A549, in alveolar macrophages (AM), and in blood monocytes (MN) by reverse transcription-PCR. We also studied the induction of HBD-2 gene expression by mannose lipoarabinomannan (manLAM) from M. tuberculosis. Intracellular production of HBD-2 peptide was detected by immunocytochemistry and electron microscopy. Our results demonstrated that there was induction of HBD-2 mRNA in A549 cells after infection with M. tuberculosis at various multiplicities of infection (MOI) and that there was stimulation with manLAM. AM expressed the HBD-2 gene only at a high MOI with M. tuberculosis. MN did not express HBD-2 at any of the experimental M. tuberculosis MOI. Immunostaining revealed the presence of intracellular HBD-2 peptide in A549 cells following infection with M. tuberculosis, and the staining was more intense in areas where there were M. tuberculosis clusters. By using electron microscopy we also demonstrated production of HBD-2 after M. tuberculosis infection and adherence of HBD-2 to the membranes of M. tuberculosis. Alveolar epithelial cells are among the first cells to encounter M. tuberculosis following aerogenic infection. As HBD-2 has been shown to control growth of M. tuberculosis and has chemotactic activity, our results suggest that HBD-2 induction by M. tuberculosis may have a role in the pathogenesis of human tuberculosis.
The high incidence and prevalence of tuberculosis (TB) are a continuous global public health problem of enormous proportions. AIDS and the development of multidrug-resistant Mycobacterium tuberculosis have aggravated this problem. Our understanding of the components of protective M. tuberculosis immunity specifically in the human host is still fragmented and contradictory. A better understanding of protective human immune responses, however, is needed for the development of new antituberculous treatment and vaccination approaches for use against infection with M. tuberculosis and human TB (13, 14, 44). The role of innate immune mechanisms in the protective immune response against M. tuberculosis is particularly poorly understood (43). Epidemiological data provide evidence that human protective immunity against M. tuberculosis exists (14). Individuals who are exposed to infectious patients with TB but remain tuberculin skin test negative and do not show evidence of adaptive immune responses may be models of innate protective immune mechanisms. While systemic immunity appears to play a dominant role in preventing dissemination of M. tuberculosis, local pulmonary host defenses probably determine the outcome of the primary infection.
In the murine model, acquired T-cell immunity protects against disseminated disease but does not prevent initial aerogenic pulmonary infection (7, 33). In humans, natural acquired M. tuberculosis-specific T-cell immunity similarly does not prevent exogenous reinfection in the lung (44).
We previously demonstrated compartmentalization of activated alveolar lymphocytes and increased M. tuberculosis antigen-specific production of gamma interferon (IFN-γ) in the bronchoalveolar spaces of patients with active TB (37). As bronchoalveolar cells (BAC) were obtained by bronchoalveolar lavage (BAL) from the sites of active TB disease, activation of alveolar lymphocytes and increased antigen-specific production of IFN-γ alone appeared to be insufficient to control M. tuberculosis replication and disease. Recent studies with bacillus Calmette-Guérin (Mycobacterium bovis BCG)-vaccinated subjects also showed that IFN-γ production did not correlate with protection as determined by functional in vitro killing assays (8). Additional immune mechanisms that confer protection in the lung and additional correlates of protection thus remain to be defined. Because it is conceivable that local T-cell-independent innate host immune mechanisms are involved in protection against primary pulmonary M. tuberculosis infection, we have begun to assess induction of human β-defensin 2 (HBD-2) in alveolar epithelial cell line A549 upon infection with M. tuberculosis.
HBD-2 is a cationic antimicrobial peptide that has a low molecular weight (4,000) (29) and exhibits a wide range of antimicrobial activity against viruses, bacteria, and fungi (9, 26, 29, 40a). Defensins can be divided into the alpha, beta, and theta groups, depending on the position of cysteines and disulfide bridges (3, 25, 29). Four different human β-defensin peptides have been described to date (9, 17, 19, 21), although numerous homologous genes have been observed in the human genome (27, 34, 36). While expression of HBD-1 is generally constitutive, levels of HBD-2 and HBD-3 mRNA can be induced by interleukin-1, tumor necrosis factor (TNF), bacteria, and fungi (26, 29, 40a). HBD-2 gene expression has been identified in various human epithelia, such as epithelia from skin, lungs, trachea, and the urogenital system (2, 26, 29, 31, 39, 40a).
HBD-2 has also been detected in bronchoalveolar lavage fluid from patients with Mycobacterium avium-intracellulare infection (1). Kisich et al. demonstrated that HBD-2-transfected blood monocytes (MN) control M. tuberculosis growth better than native MN, suggesting that HBD-2 plays a role in immunity against M. tuberculosis (28).
Because of its chemotactic effects on immature dendritic cells and memory lymphocytes (46), HBD-2 may constitute a link between innate and acquired immune responses.
M. tuberculosis is transmitted primarily by the respiratory route, and in the majority of cases human TB is a pulmonary disease (13, 43, 45). Thus, alveolar macrophages (AM) and alveolar epithelial cells are among the first host cells to encounter M. tuberculosis. Our finding that HBD-2 is expressed and produced in the human alveolar epithelial cell line A549 upon infection with M. tuberculosis is novel and provides evidence that HBD-2 plays a role in early immune responses to M. tuberculosis.
(Part of this research was presented at the Thirty-Eighth Tuberculosis and Leprosy Research Conference, Newark, N.J., 21 and 22 July 2003, and at the 12th International Congress of Immunology and 4th Annual Conference of FOCIS, Montreal, Quebec, Canada, 18 to 23 July 2004.)
MATERIALS AND METHODS
Study subjects.
Seven healthy subjects (five men and two women; ages, 24 to 40 years) were recruited at the National Institute for Respiratory Diseases (INER) in Mexico City. All subjects had a normal chest X ray, and none had a upper respiratory tract infection; all had negative serology for human immunodeficiency virus. Following written informed consent, the study subjects underwent venipuncture and fiberoptic bronchoscopy with BAL. Consent to perform these studies was obtained from the study subjects by a Spanish-speaking study nurse and physician and was obtained according to the guidelines of the U.S. Department of Health and Human Services. Approval to perform BAL and venipuncture was given by the Institutional Review Boards at INER and the University of Medicine and Dentistry of New Jersey.
Bacteria and recombinant cytokines.
M. tuberculosis strain H37Rv was obtained from the American Type Culture Collection (ATCC 25618). The Pseudomonas aeruginosa mucoid strain used was a clinical isolate obtained from a patient suffering from cystic fibrosis at INER in Mexico City. P. aeruginosa was identified by conventional biochemical tests. Recombinant human tumor necrosis factor (rhTNF) (Endogen, Woburn, MA) and lipopolysaccharide (LPS) (Sigma, St. Louis, MO) were obtained commercially. Mannosylated lipoarabinomannan (manLAM) was provided by J. Belisle (TB Research Material Vaccine Testing contract NIH NIAID NO1 AI-75320).
Alveolar epithelial cells.
Human type II alveolar pneumocytes (A549; American Type Culture Collection reference number CCL185) were cultured in 75-cm2 culture flasks (Costar, Ontario, Canada) with antibiotic-free Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Grand Island, NY) supplemented with 10% fetal calf serum (HyClone laboratories, Logan, Utah). A549 cultures were incubated at 37°C with 5% CO2, and cells were grown to semiconfluence. After a washing step with DMEM, A549 cells were seeded into 24-well plates at a concentration of 106 cells per ml of culture medium and rested for 24 h in the presence of 5% CO2 at 37°C until infection with M. tuberculosis or stimulation with manLAM.
Blood monocytes.
Heparinized whole blood was obtained from seven healthy donors by venipuncture. Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque (Nycomed Pharma AS, Oslo, Norway) density gradient centrifugation. Peripheral blood mononuclear cells were then cultured in 962-mm2 polystyrene dishes (Costar, Ontario, Canada) containing RPMI medium supplemented with 10% human AB serum (Gemini, Woodland, CA). After 2 h of incubation in the presence of 5% CO2 at 37°C, nonadherent cells were removed by washing with warm RPMI medium. The remaining adherent cells (referred to as blood monocytes) were incubated at 4°C for 1 h with Hanks salt solution (BioWhittaker, Walkersville, MD) and then removed with a cell scraper, counted, and readhered in 24-well plates (Costar, Corning, N.Y.) at a concentration of 106 cells per well for 2 h prior to M. tuberculosis infection. Cytospin preparations were prepared from adherent uninfected cells to allow evaluation of the nuclear and cellular morphology by Wright's staining.
Alveolar macrophages.
To obtain BAC, BAL was performed with seven healthy donors as previously described (38). Briefly, 150 ml of a 0.9% sterile sodium chloride solution was instilled into each of two segments of the right lung. A total of 200 to 250 ml of the saline was retrieved from each subject. BAC were obtained by centrifugation (1,200 rpm, 15 min, 4°C) of the BAL fluid. AM were then enriched from the BAC by removal of alveolar lymphocytes with sheep red blood cell (SRBC) rosetting. To do this, 500 μl of SRBC was mixed with 500 μl of neuraminidase (Sigma Chemical Co., St. Louis, MO), incubated for 1 h at 37°C, washed with 1× Hanks balance salt solution, and then resuspended in RPMI medium. Two milliliters of neuraminidase-treated SRBC and 2 ml of fetal calf serum were added to each preparation containing 1 × 107 to 2 × 107 BAC/ml, and the cell mixtures were incubated for 10 min at 37°C. SRBC-treated BAC were then centrifuged at 400 × g for 10 min at 4°C. The pelleted cells were incubated for 1 h on ice and centrifuged over a Ficoll density gradient. Ninety-five percent of the cells from the interface had morphological characteristics of AM as determined by Wright's staining of cytospins.
In vitro infection and cell stimulation. (i) AM and MN.
AM and MN were plated separately in 24-well dishes at a concentration of 105 cells per well with RPMI 1640 containing gentamicin and l-glutamine and adhered for 2 h in the presence of 5% CO2 at 37°C. The culture medium was then carefully removed, and cells were infected with M. tuberculosis in RPMI medium supplemented with 30% non-heat-inactivated pooled human AB serum. AM and MN were not treated (medium alone), were infected at multiplicities of infection (MOI) (bacterium/cell ratios) of 0.1:1, 1:1, 5:1, and 10:1 for M. tuberculosis and 200:1 for P. aeruginosa, or were stimulated with LPS (10 μg/ml). Cells were then incubated at 37°C with 5% CO2 for 1, 18, 24, and 48 h and lysed in 200 μl TRIzol reagent (Gibco BRL, Grand Island, NY) per 106 cells, and the lysates were stored at −80°C.
(ii) Human alveolar epithelial cell line A549.
A549 cells were plated in 24-well dishes at a concentration of 106 cells/well and were preincubated for 24 h prior to M. tuberculosis infection in DMEM with 2% fetal calf serum. A549 cells were infected separately with M. tuberculosis at MOI of 0.1:1, 1:1, 5:1, and 10:1 and with P. aeruginosa at an MOI of 200:1. Infection of A549 cells was confirmed using Ziehl-Neelsen stain. The following stimuli were used to induce HBD-2 expression and production: LPS (10 μg/ml), rhTNF (1 ng/ml), and manLAM (10 μg/ml). Following 1, 6, 12, 18, 24, and 48 h of incubation, A549 cells were lysed in TRIzol reagent, and the lysates were stored at −20°C.
RNA extraction.
RNA was extracted from the cell lysates by adding 20 μl of chloroform-isoamyl alcohol (49:1) and vortexing the mixture, followed by centrifugation at 14,000 rpm for 15 min at 4°C. The aqueous phase was then mixed with cold isopropanol, chilled on ice for 15 min, and centrifuged, and the RNA pellet was washed once with cold 75% ethanol. The pelleted RNA was then dried at 65°C and resuspended in 13 μl of diethyl pyrocarbonate-treated water.
Reverse transcription and cDNA synthesis.
HBD-2 mRNA was reverse transcribed using oligo(dT)12-18 primers (Invitrogen, Carlsbad, CA) and a commercial kit (Superscript II; Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Briefly, RNA was mixed with a reaction mixture that contained 10× PCR buffer, 25 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 10 mM, and the reverse transcriptase enzyme. The final mixture was synthesized in a thermocycler (iCycler; Bio-Rad, Hercules, CA). cDNA concentrations were then measured with a spectrophotometer at a ratio of UV absorbance at 260 nm to UV absorbance at 280 nm.
HBD-2 PCR.
The primers used for a 102-bp HBD-2 gene fragment were 5′-CAT CAG CCA TGA GGG TCT-3′ (forward) and 5′-AGG CAG GTA ACA GGA TCG-3′ (reverse) (10). The primers used for a 110-bp fragment of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (constitutive gene expression control) were 5′-CAT CAC CAT CTT CCA GGA GC-3′ (forward) and 5′-GGA TGA TGT TCT GGA GA-3′ (reverse) (21).
One microgram of cDNA was added to a reaction mixture containing PCR buffer, MgCl2 (10 mM), deoxynucleoside triphosphates (25 mM), 1 U of Taq polymerase (Invitrogen, Carlsbad, CA), and the forward and reverse primers for HBD-2 or GAPDH (20 ng). The HBD-2 and GAPDH cDNA were amplified with the following settings: 30 s of denaturation at 95°C, 30 s of annealing at 56°C (HBD-2) or 63°C (GAPDH), and 1 min of extension at 72°C for 35 cycles and a 10-min extension step at 72°C after the last cycle. The resulting HBD-2 and GAPDH cDNA amplicons were kept at −20°C until visualization by electrophoresis on 3% agarose gels stained with ethidium bromide.
cDNA samples obtained from A549 cells that had been stimulated either with 500 pg of rhTNF or with the P. aeruginosa mucoid strain at an MOI of 200:1 were used to generate positive control bands for HBD-2 (20). cDNA samples from AM, MN, and A549 cells that had been incubated in culture medium without stimuli were used as negative controls.
HBD-2 immunocytochemistry and fluorescent M. tuberculosis staining.
A549 cells were grown to confluence (95%) on four-well chamber slides (Costar, Ontario, Canada). After M. tuberculosis infection, cells were fixed with formaldehyde (10%) for 24 h and stored at 4°C in phosphate-buffered saline (PBS). An immunocytochemistry analysis of HBD-2 and H37Rv was done as previously described (19). Briefly, slides were blocked with 5% goat serum for 20 min and then incubated either with a 1:5,000 dilution of HBD-2 antibody or with preimmune serum (both gifts from Tomas Ganz) in 5% goat serum at 4°C for 18 h. The slides were then developed with biotinylated goat anti-rabbit immunoglobulin G (IgG) using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Following the immunostaining of HBD-2, the slides were acid-fast stained with auramine-O to detect M. tuberculosis using the BBL TB fluorescent stain (Becton Dickinson, Franklin Lakes, NJ) as described by the manufacturer. HBD-2 and M. tuberculosis were then visualized by fluorescent microscopy.
Subcellular detection of HBD-2 by immunoelectron microscopy.
A549 cells were infected with M. tuberculosis as described above. After 24 h, infected and uninfected (control) A549 cells were washed three times with PBS, fixed in 4% (vol/vol) paraformaldehyde, and dissolved in 0.2 M Sörensen buffer (1 volume of NaH2PO4 · H2O, 2 volumes of NaHPO4 · 7H2O) at pH 7.3 for 2 h at 4°C. Following washing of the cells with Sörensen buffer, free aldehyde groups were blocked with 0.5 M ammonium chloride in PBS for 1 h. Fixed cells were then dehydrated with a graded ethyl alcohol series and embedded in LR-White hydrosoluble resin (London Resin Co., Hampshire, United Kingdom). Thin sections that were 70 to 90 nm thick were placed on nickel grids. The grids were incubated overnight at 4°C with specific polyclonal rabbit anti-HBD-2 antibody (Peptide International, Osaka, Japan) diluted 1/200 in PBS with 1% bovine serum albumin and 0.5% Tween 20. After rinsing with PBS, the grids were incubated for 1 h at room temperature with goat anti-rabbit IgG (Sigma Chemical Co., St. Louis, MO) conjugated to 10-nm gold particles (Sigma) diluted 1/20 in PBS. The grids were stained with uranium salts (Electron Microscopy Sciences, Fort Washington, PA) and examined with an M-10 Zeiss electron microscope (Karl Zeiss, Jena Germany). For negative controls, the primary HBD-2 antibody was replaced by normal rabbit serum.
RESULTS
Expression of HBD-2 in M. tuberculosis-infected A549 cells.
Infection of A549 cells with M. tuberculosis H37Rv resulted in HBD-2 gene expression as early as 6 h postinfection for all the MOI used. Maximal HBD-2 expression was detected at 18 and 24 h postinfection (Fig. 1) for all MOI, including 0.1:1,1:1,5:1, and 10:1. After 48 h of infection, expression of HBD-2 was detected only at an MOI of 10:1. P. aeruginosa induced the expression of HBD-2 in A549 cells at an MOI of 200:1 at 6, 12, 18, 24, and 48 h. All experiments were performed in triplicate.
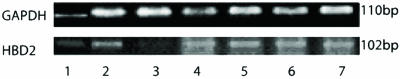
FIG. 1.
Expression of the HBD-2 gene in A549 cells infected with M. tuberculosis at different MOI for 24 h. Lane 1, molecular weight marker; lane 2, positive control; lane 3, negative control; lane 4, MOI of 0.1:1; lane 5, MOI of 1:1; lane 6, MOI of 5:1; lane 7, MOI of 10:1.
Expression of HBD-2 in M. tuberculosis-infected MN.
Infection of MN with M. tuberculosis at MOI of 0.1:1, 1:1, 5:1, and 10:1 did not induce HBD-2 gene expression at any of the times studied (18, 24, or 48 h). There was also no HBD-2 gene expression detectable at any infection time in P. aeruginosa-infected MN (MOI, 200:1). LPS stimulation of MN (10 μg/ml) induced HBD-2 expression in two of five healthy donors.
Expression of HBD-2 in M. tuberculosis-infected AM.
To assess expression of HBD-2 and its potential role in the mycobactericidal activity of AM, AM were stimulated with LPS (10 μg/ml) or infected with P. aeruginosa at an MOI of 200:1. No HBD-2 expression was detected with either of the stimuli at any of the times studied (1, 18, 24, or 48 h).
AM were then infected with M. tuberculosis at MOI of 0.1:1, 1:1, 5:1, 10:1, 70:1, and 350:1. Induction of HBD-2 was observed with M. tuberculosis MOI of 70:1 and 350:1 only at 18 and 24 h (Fig. 2).
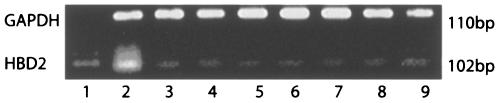
FIG. 2.
HBD-2 gene expression in alveolar macrophages. Lane 1, molecular weight marker; lane 2, positive control; lane 3, negative control; lanes 4 and 7, nonstimulated alveolar macrophages; lanes 5 and 8, HBD-2 expression in alveolar macrophages infected with M. tuberculosis at an MOI of 70:1 at 18 and 24 h postinfection, respectively; lanes 6 and 9, HBD-2 expression in alveolar macrophages infected at an MOI of 350:1 at 18 and 24 h postinfection, respectively.
Induction of HBD-2 expression by manLAM in A549 cells.
manLAM is an abundant immunogenic antigen of the cell wall (18) of M. tuberculosis. To assess its potential role in the induction of HBD-2 expression in A549 cells by M. tuberculosis, A549 cells were stimulated with manLAM (10 μg/ml) for 18, 24, and 48 h. Expression of the HBD-2 gene was observed after 18, 24, and 48 h of incubation (Fig. 3).
FIG. 3.
Expression of HBD-2 in A549 cells upon stimulation with manLAM. At 24 h after stimulation of A549 cells with manLAM at concentrations of 1 μg/ml (lane 8), 5 μg/ml (lane 9), 10 μg/ml (lane 10), and 20 μg/ml (lane 11) expression of the HBD-2 gene was detected by reverse transcription-PCR. Stimulation for 18 h with the same concentrations of manLAM (lanes 3 to 7) did not induce expression of HBD-2. A positive control band is shown in lane 2. Lane 12 contained a negative control.
A summary of the HBD-2 expression results for the different kinds of cells and stimuli is shown in Table 1.
TABLE 1.
HBD-2 expression at 24 in lung epithelial cells (A549), AM, and MNa
| Cell type | HBD-2 expression with:
|
||||
|---|---|---|---|---|---|
| P. aeruginosa (MOI, 200:1) | LPS (10 μg/ml) | M. tuberculosis (MOI, 0.1:1, 1:1, 5:1, and 10:1) | M. tuberculosis (MOI, 70:1 and 300:1) | manLAM (10 μg/ml) | |
| A549 | + | + | + | NDb | + |
| AM | − | − | − | + | ND |
| MN | − | + | − | − | ND |
Cells were stimulated with LPS, with manLAM, and with P. aeruginosa, and with M. tuberculosis at various MOI.
ND, not done.
Detection of HBD-2 by immunocytochemistry.
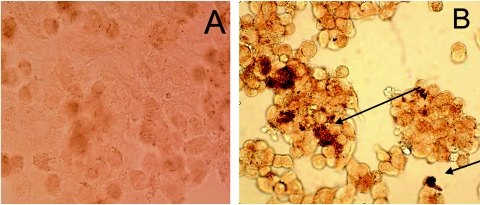
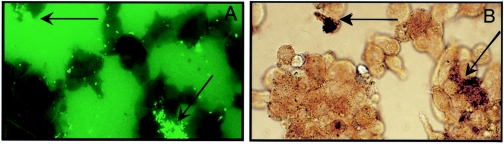
Figure 4 shows HBD-2 within A549 cells following infection with M. tuberculosis. Slides were developed with biotinylated goat anti-rabbit IgG using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA). In Fig. 5A, M. tuberculosis is visible due to auramine staining. Maximal HBD-2 expression was visible in the areas where M. tuberculosis was localized, and this suggests that there was an intracellular interaction between of HBD-2 and M. tuberculosis (Fig. 5B).
FIG. 4.
(A) Noninfected cells (negative control). (B) Immunodetection of HBD-2 in A549 cells 24 h after infection with M. tuberculosis at an MOI of 5:1.
FIG. 5.
(A) Immunofluorescence of M. tuberculosis 24 h after infection of A549 cells at an MOI of 5:1. (B) Colocalization and association of HBD-2 with M. tuberculosis in the same microscopic field (arrows).
Detection of HBD-2 by electron microscopy.
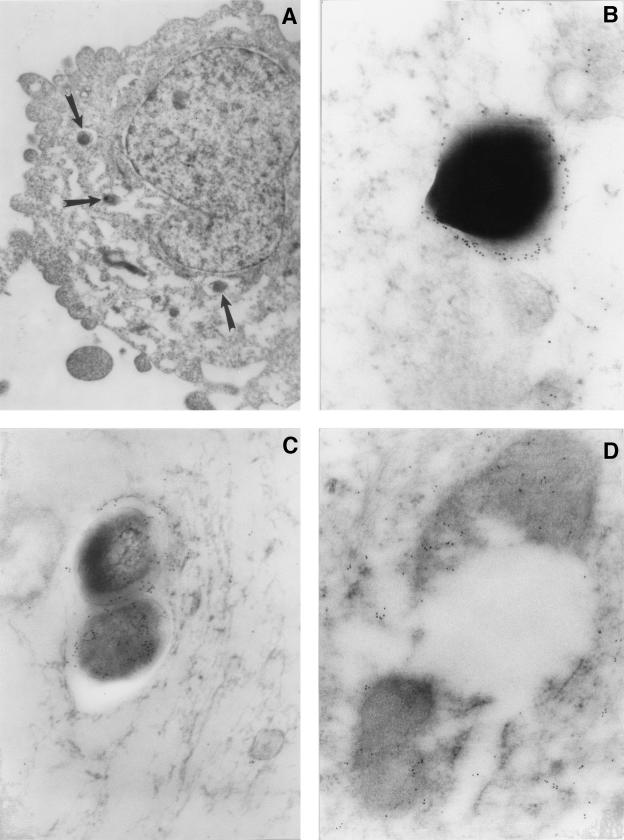
Electron microscopy showed that specific immunolabeling of HBD-2 was localized in the mycobacterial cell wall and the cytoplasm of extracellular bacteria (Fig. 6B and C), as well as in bacteria that were attached to the external surface of the cell membrane (Fig. 6B). The same pattern of subcellular HBD-2 distribution was found in intracellular mycobacteria (Fig. 6C and D).
FIG. 6.
Immunoelectron microcopy of HBD-2 in A549 cells following a 24-h in vitro infection with M. tuberculosis. (A) Low-power micrograph showing phagocytosed bacilli (arrows) in the cytoplasm of an A549 cell. Magnification, ×8,000. (B) Immunoelectron micrograph of a mycobacterium on the surface of the A549 cell membrane. There is HBD-2 immunoreactivity on the bacterial cell wall. Magnification, ×50,000. (C) Intracellular mycobacterium showing HBD-2 immunolabeling in the cell wall and cytoplasm. Magnification, ×50,000. (D) Endocytosed and disrupted mycobacterium showing HBD-2 immunoreactivity in its cytoplasm and cell wall. Magnification, ×40,000.
Interestingly, specific HBD-2 immunolabeling was also seen in disrupted intracellular bacilli (Fig. 6D). Noninfected control cells or M. tuberculosis-infected cells that were incubated without the primary anti-HBD-2 antibodies did not show immunolabeling for HBD-2 (not shown).
DISCUSSION
Innate immunity is the first line of defense against invading microorganisms in vertebrate organisms and triggers antigen-specific adaptive immune responses (43). Antimicrobial peptides are thought to be effectors of innate immunity through their antibiotic activity and direct killing of microorganisms (3)
HBD-2 is produced by epithelial cells in the respiratory tract and may be relevant in the pathogenesis of several diseases (1, 24, 40, 40a). High salt concentrations in the lung fluids of cystic fibrosis patients, for example, are associated with the functional impairment of HBD-2 that is associated with increased susceptibility to respiratory bacterial infections, such P. aeruginosa infections (40). Harder and coworkers (20) showed that human type II pneumocytes of the A549 cell line induced the HBD-2 gene and allowed isolation of the peptide from culture supernatants following in vitro infection with mucoid P. aeruginosa.
Whether HBD-2 is involved in the innate immunity against intracellular pathogens such as M. tuberculosis has not been determined. This is the first report showing induction of gene expression and production of HBD-2 upon infection of A549 cells by M. tuberculosis. Our data demonstrate that M. tuberculosis infection of A549 cells induces HBD-2 expression at MOI as low as 0.1:1. The earliest time of HBD-2 gene induction was 6 h following an M. tuberculosis infection at an MOI of 10:1. HBD-2 gene induction was maintained until 48 h, with peaks at 18 and 24 h postinfection. HBD-2 gene expression at a late time, 48 h, raised the question whether HBD-2 gene expression was due to direct M. tuberculosis infection alone and/or due to secondary events, such as interleukin-1β release from surrounding M. tuberculosis-infected or uninfected bystander cells as well.
Interestingly, LPS induction of HBD-2 was poor in A549 cells, whereas mucoid P. aeruginosa strongly induced HBD-2. The nonmucoid strain of P. aeruginosa resulted in only marginal HBD-2 induction (data not shown). This finding suggests that alginate, a prevalent antigen of a mucoid strain, may be an inducer of HBD-2.
As previously shown, TNF (20) induces the expression of HBD-2 in A549 cells. In the present study, TNF was used as a positive control for HBD-2 induction in A549 cells. Interestingly, TNF is produced by AM upon infection with M. tuberculosis (22, 33) and has a key role in the formation and maintenance of immune granulomas (22). Because TNF is an inducer of HBD-2 expression in pulmonary epithelial cells, modulated by NFκB (42), we hypothesize that in vivo expression of HBD-2 can be modulated both by a direct effect of M. tuberculosis on the alveolar epithelium and by TNF that is released from M. tuberculosis-infected AM.
We were interested in assessing if manLam could be a component of M. tuberculosis that induces the induction of HBD-2 in A549 cells. manLam is one of the most important lipid components of the cell wall of M. tuberculosis (6, 18) and plays an important role in the adherence and phagocytosis of M. tuberculosis in macrophages (35). Our finding that there was HBD-2 gene expression following stimulation of A549 cells with manLAM suggests that manLAM may be one of the components of M. tuberculosis that is responsible for the induction of HBD-2 gene expression.
Previous reports demonstrated that induction of HBD-2 expression requires Toll-like receptor 2 (TLR2)-mediated intracellular signaling (5, 41). Interestingly, A549 cells express TLR2, which activates NFκB to induce production of HBD-2 (5). We therefore hypothesize that M. tuberculosis induction of HBD-2 may depend on the interaction of manLAM with TLR2.
In vitro studies suggest that HBD-2 is involved in M. tuberculosis growth control. Kisich et al. showed that transfection of human MN-derived macrophages with the HBD-2 gene (28) increased the capacity of HBD-2 transfected MN to control M. tuberculosis growth compared with non-HBD-2-transfected MN-derived macrophages.
M. tuberculosis has been shown to be capable of invading alveolar epithelial cells (4), and type II pneumocytes are among the first cells in the alveolar spaces to come in contact with M. tuberculosis during a primary aerogenic infection (4, 43). In autopsy material from TB patients, M. tuberculosis DNA was found in type II pneumocytes, suggesting that these cells play a role in the pathogenesis of TB in vivo (23). Type II pneumocytes produce chemokines (30) and collectins, such as SP-A and SP-B (32), molecules that are important in the control of microbial invasion and defense against M. tuberculosis. However, so far there is no direct evidence that defensins are produced in vivo by type II pneumocytes during M. tuberculosis infection
Our PCR and immunocytochemistry data demonstrate that mycobacterial infection of A549 cells induces not only HBD-2 gene expression but also production of intracellular HBD-2. At the light microscopy level, the pattern of HBD-2 immunostaining suggests that HBD-2 production is induced by intracellular M. tuberculosis. This observation was corroborated by the immunoelectron microscopy findings that showed HBD-2 labeling in the mycobacterial cytoplasm and cell wall. Interestingly, extracellular bacilli that were in direct contact with the A549 cells also showed HBD-2 labeling, suggesting that not only intracellular bacteria but also extracellular bacilli can be targeted by HBD-2.
HBD-2 labeling of intracellular disrupted M. tuberculosis bacilli may allow speculation concerning participation of HBD-2 in intracellular mycobacterial killing. Bactericidal effects of HBD-2 from A549 cells may contribute to M. tuberculosis control. However, HBD-2 has also been shown to elicit chemokine-like activities (12). Alveolar epithelial cells thus may have an important function in the initiation of a primary immune response to M. tuberculosis by attracting MN, immature dendritic cells, and lymphocytes that constitute an efficient microenvironment for a secondary immune response.
AM and MN are important phagocytes of M. tuberculosis, migrate to sites where bacteria are present, participate in the formation of granulomas, and regulate immune responses during M. tuberculosis infection. The mechanisms by which human MN and AM kill M. tuberculosis have not been well described yet, and whether they produce HBD-2 is controversial. Our results show that infection of AM with M. tuberculosis at MOI of 0.1:1, 1:1, 5:1, and 10:1 cannot induce HBD-2 and that only artificially high doses of M. tuberculosis (MOI, 70:1 and 300:1) allow detection of HBD-2 gene expression in AM. These findings indicate that HBD-2 induction by AM may not have a relevant physiological function in a natural M. tuberculosis infection. Duits et al. demonstrated that there was induction of HBD-2 in MN after stimulation with LPS and IFN-γ (11); other authors failed to demonstrate HBD-2 production by MN (28). We have shown that HBD-2 gene expression is induced by LPS in MN. However, this was the case in only two of seven of our study subjects, a finding that corroborates earlier observations that not all subjects express HBD-2 under the same conditions (16). HBD-2 expression thus may depend on alterations of TLR4 expression or function or on genetic HBD-2 polymorphisms. We did not observe HBD-2 gene expression in M. tuberculosis-infected MN or MN-derived macrophages from the subjects studied (data not shown). However, due to the small number of subjects that were studied, induction of HBD-2 production by MN or macrophages by M. tuberculosis cannot be firmly excluded. Future studies may address in vivo HBD-2 expression in individuals with various levels of resistance to M. tuberculosis exposure.
In summary, our results show that A549 cells efficiently produce HBD-2 upon in vitro infection with M. tuberculosis and suggest that HBD-2 from lung epithelial cells may be a component of the innate immune response against M. tuberculosis both in the primary infection and in the immunopathogenesis of human TB.
Acknowledgments
This work was supported by NIH grant 2 RO1 HL51630-06, by Conacyt (Mexico) grant 3479-M, by grant ROI HL67871, and by a US EPA STAR fellowship.
We are thankful to Juan C. Contreras for his professional work in electronic microscopy.
Editor: J. L. Flynn
REFERENCES
- 1.Ashitani, J., H. Mukae, T. Hiratsuka, M. Nakazato, K. Kumamoto, and S. Matsukura 2001. Plasma and BAL fluid concentrations of antimicrobial peptides in patients with Mycobacterium avium-intracellulare infection. Chest 119:1131-1137. [DOI] [PubMed] [Google Scholar]
- 2.Bals, R., X. Wang, Wu, Z., T. Freeman, V. Banfa, M. Zasloff, and J. Wilson 1998. HBD-2 is a salt sensitivity peptide antibiotic expressed in human lung. Clin. Investig. 102:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals, R. 2000. Epithelial peptides in host defense against infection. Respir. Res. 1:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez, L. E., and J. Goodman. 1996. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immun. 64:1400-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birchler, T., R. Seibl, K. Buchner, S. Loeliger, R. Seger, J. P. Hossle, A. Aguzzi, and R. P. Lauener 2001. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur. J. Immunol. 31:3131-3137. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee, D., S. W. Hunter, M. McNeil, I. Jardine, and P. J. Brennan 1992. Structure and function of mycobacterial glycolipids and glycopeptidolipids. Acta Leprol. 7(Suppl. 1):81-84. [PubMed] [Google Scholar]
- 7.Cooper, A. M., J. E Callahan,. M. Keen, J. T. Belisle, and I. M. Orme 1997. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuber. Lung Dis. 78:67-73. [DOI] [PubMed] [Google Scholar]
- 8.Cowley, S. C., and K. L. Elkins 2003. CD4+ T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J. Immunol. 9:4689-4699. [DOI] [PubMed] [Google Scholar]
- 9.Diamond, G,. M. Zasloff, H. Eck, M. Brasseur, W. L. Maloy, and C. L. Bevins 1991. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc. Natl. Acad. Sci. USA 88:3952-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duits, L., M. Rademaker, and B. Ravensbergen 2001. Inhibition of HBD-3 but not HBD-1 and HBD-2, mRNA expression by corticosteroids. Biochem. Biophys. Res. 280:522-525. [DOI] [PubMed] [Google Scholar]
- 11.Duits, L., B. Ravensbergen, M. Rademaker, P. Hiemstra, and P. Nibbering 2002. Expression of β-defensin 1 and 2 mRNA by human monocytes, macrophages and dendric cells. Immunology 106:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durr, M., and A. Peschel 2002. Chemokine meet defensin: the emerging concepts of chemoattractants and antimicrobial peptides in host defense. Infect. Immun. 70:6515-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dye, C., S. Scheele, V. Dolin, T. Pathania, and M. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. W.H.O. global surveillance and monitoring project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 14.Ellner, J., C. Hirsch, and C. Whalen 2000. Correlates of protective immunity to Mycobacterium tuberculosis in humans. Clin. Infect. Dis. 30(Suppl. 3):S279-S282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Fang, X. M., Q. Shu, Q. X. Chen, M. Book, H. G. Sahl, A. Hoeft, and F. Stuber 2003. Differential expression of alpha- and beta-defensins in human peripheral blood. Eur. J. Clin. Investig. 1:82-87. [DOI] [PubMed] [Google Scholar]
- 17.Garcia, J. R., A. Krause, S. Schulz, F. J. Rodriguez-Jimenez, E. Kluver, K. Adermann, U. Forssmann, A. Frimpong-Boateng, R. Bals, and W. G. Forssmann 2001. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 10:1819-1821. [PubMed] [Google Scholar]
- 18.Gilleron, M,. L. Bala, T. Brando, A. Vercellone, and G. Puzo 2000. Mycobacterium tuberculosis H37Rv parietal and cellular lipoarabinomannans. Characterization of the acyl- and glyco-forms. Biol. Chem. 1:677-684. [DOI] [PubMed] [Google Scholar]
- 19.Goldman, M. J., G. M. Anderson, E. D. Stolzenberg, U. P. Kari, and M. Zassloff 1997. Human β-defensin-1 is a salt-sensitive antibiotic in the lung that is inactivated in cystic fibrosis. Cell 88:553-560. [DOI] [PubMed] [Google Scholar]
- 20.Harder, J., U. Meyer-Hoffert, and L. M. Teran 2000. Mucoid Pseudomonas aeruginosa, TNFα and IL1β, but not IL-6 induce HBD-2 in respiratory epithelia. Am. J. Respir. Cell Mol. Biol. 26:714-721. [DOI] [PubMed] [Google Scholar]
- 21.Harder, J., J. Bartels, and E. Christophers 2001. Isolation and characterization of human beta defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Pando, R., D. Aguilar, M. L. Hernandez, H. Orozco, and G. Rook 2004. Pulmonary tuberculosis in BALB/c mice with non-functional IL-4 genes: changes in the inflammatory effects of TNF-alpha and in the regulation of fibrosis. Eur. J. Immunol. 1:174-183. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Pando, R., M. Jeyanathan, G. Mengistu, D. Aguilar, H. Orozco, M. Harboe, G. A. Rook, and G. Bjune 2000. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 356:2133-2138. [DOI] [PubMed] [Google Scholar]
- 24.Hiratsuka, T., M. Nakazato, Y. Date, J. Ashitani, T. Minematsu, N. Chino, and S. Matsukara 1998. Identification of β-defensin-2 in respiratory tract and plasma and its increase in bacterial pneumonia. Biochem. Biophys. Res. Commun. 249:943-947. [DOI] [PubMed] [Google Scholar]
- 25.Hoover, D. M., K. R. Rajashankar, R. Blumenthal, A. Puri, J. J. Oppenheim, O. Chertov, and J. Lubkowski 2000. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J. Biol. Chem. 42:32911-32918. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser, V., and G. Diamond 2000. Expression of mammalian defensin genes. J. Leukoc. Biol. 68:779-784. [PubMed] [Google Scholar]
- 27.Kao, C. Y., Y. Chen, Y. H. Zhao, and R. Wu. 2003. ORFeome-based search of airway epithelial cell-specific novel human [beta]-defensin genes. Am. J. Respir. Cell Mol. Biol. 29:71-80. [DOI] [PubMed] [Google Scholar]
- 28.Kisich, K., L. Heifets, M. Higgins, and G. Diamond 2001. Antimycobacterial agent based on mRNA encoding human β-defensin 2 enables primary macrophages to restrict growth of Mycobacterium tuberculosis. Infect. Immun. 69:2692-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehrer, R., A. Lichtenstein, and T. Ganz 1993. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 11:105-128. [DOI] [PubMed] [Google Scholar]
- 30.Lin, Y., M. Zhang, and P. F. Barnes 1998. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect. Immun. 66:1121-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linzmeier, R., Ho, and C. H., T. Ganz 1999. A 450-kb contig of defensin genes on human chromosome 8p23. Gene 233:205-211. [DOI] [PubMed] [Google Scholar]
- 32.Madan, T., S. Saxena, K. J. Murthy, K. Muralidhar, and P. U. Sarma.2002. Association of polymorphisms in the collagen region of human SP-A1 and SP-A2 genes with pulmonary tuberculosis in Indian population. Clin. Chem. Lab. Med. 10:1002-1008. [DOI] [PubMed] [Google Scholar]
- 33.North, R. J., R. Lacourse, and L. Ryan 1999. Vaccinated mice remain more susceptible to Mycobacterium tuberculosis infection initiated via the respiratory route than via the intravenous route. Infect Immun. 67:2010-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Jimenez, F. J., A. Krause, S. Schulz, W. G. Forssmann, J. R. Conejo-Garcia, R. Schreeb, and D. Motzkus. 2003. Distribution of new human beta-defensin genes clustered on chromosome 20 in functionally different segments of epididymis. Genomics 81:175-183. [DOI] [PubMed] [Google Scholar]
- 35.Schlesinger, L. S., S. R Hull, and T. M. Kaufman. 1994. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J. Immunol. 152:4070-4079. [PubMed] [Google Scholar]
- 36.Schutte, B. C., J. P. Mitros, J. A. Bartlett, J. D. Walters, H. P. Jia, M. J. Welsh, T. L. Casavant, and P. B. McCray, Jr. 2002. Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc. Natl. Acad. Sci. USA 99:2129-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwander, S. K., M. Torres, E. Sada, C. Carranza, E. Ramos, M. Tary-Lehmann, R. S. Wallis, J. Sierra, and E. A. Rich 1998. Enhanced responses to Mycobacterium tuberculosis antigens by alveolar lymphocytes during active pulmonary tuberculosis. J. Infect. Dis. 178:1434-1445. [DOI] [PubMed] [Google Scholar]
- 38.Schwander, S. K., M. Torres, C. C. Carranza, D. Escobedo, M. Tary-Lehmann, P. Anderson, Z. Toossi, J. J. Ellner, E. A. Rich, and E. Sada. 2000. Pulmonary mononuclear cell responses to antigens of Mycobacterium tuberculosis in healthy household contacts of patients with active tuberculosis and healthy controls from the community. J. Immunol. 165:1479-1485. [DOI] [PubMed] [Google Scholar]
- 39.Singh, P., H. Jia, K. Wiles, J. Hesselberth, L. Liu, B. Conway, E. Greenberg, E. Valore, M. Welsh, T. Ganz, B. Tack, and P. J. McCray 1998. Production of β-defensin by human airway epithelia. Proc. Natl. Sci. USA 95:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, J., S. Travis, E. Greenberg, and M. Welsh 1996. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 2:229-236. [DOI] [PubMed] [Google Scholar]
- 40a.Stolzenberg, E. D., G. M. Anderson, M. R. Ackermann, R. H. Whitlock, and M. Zasloff. 1997. Epithelial antibiotic induced in states of disease. Proc. Natl. Acad. Sci. USA 94:8686-8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tapping, R. I., and P. S. Tobias 2003. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J. Endotoxin Res. 4:264-268. [DOI] [PubMed] [Google Scholar]
- 42.Tsutsumi-Ishii, Y., and I. Nagaoka. 2003. Modulation of human β-defensin-2 transcription in pulmonary epithelial cells by lipopolysaccharide-stimulated mononuclear phagocytes via proinflammatory cytokine production. J. Immunol. 170:4226-4236. [DOI] [PubMed] [Google Scholar]
- 43.van Crevel, R., T. Ottenhof, and J. van der Meer 2003. Innate immunity to Mycobacterium tuberculosis. Adv. Exp. Med. Biol. 531:294-309. [DOI] [PubMed] [Google Scholar]
- 44.van Rie, A., R. Warren, M. Richardson, T. C. Victor, R. P. Gie, A. Enarson, N. Beyers, and P. D. van Heiden 1999. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N. Engl. J. Med. 341:1174-1179. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. 2004. Global tuberculosis control: surveillance, planning, financing. W.H.O. report 18-38. World Health Organization, Geneva Switzerland.
- 46.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, O. M. Howard, and J. J. Oppenheim 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525-528. [DOI] [PubMed] [Google Scholar]