Abstract
Although the essential role of tumor necrosis factor (TNF) in resistance to Listeria monocytogenes infection is well established, the roles of the related cytokines lymphotoxin alpha (LTα) and lymphotoxin beta (LTβ) are unknown. Using C57BL/6 mice in which the genes for these cytokines were disrupted, we examined the contributions of TNF, LTα, and LTβ in the host response to Listeria. To overcome the lack of peripheral lymph nodes in LTα−/− and LTβ−/− mice, bone marrow chimeras were constructed. TNF−/− and LTα−/− chimeras that lacked both secreted LTα3 and membrane-bound LTα1β2 and LTα2β1 were highly susceptible and succumbed 4.5 and 6 days, respectively, after a low-dose infection (200 CFU). LTβ−/− chimeras, which lacked only membrane-bound LT, controlled the infection in a manner comparable to wild-type (WT) chimeras. The Listeria-specific proliferative and gamma interferon T-cell responses were equivalent in all five groups of infected mice (LTα−/− and LTβ−/− chimeras, WT chimeras, and TNF−/− and WT mice). TNF−/− mice and LTα−/− chimeras, however, failed to generate the discrete foci of lymphocytes and macrophages that are essential for bacterial elimination. Rather, aberrant necrotic lesions comprised predominantly of neutrophils with relatively few lymphocytes and macrophages were observed in the livers and spleens of TNF−/− and LTα−/− chimeras. Therefore, in addition to TNF, soluble LTα3 plays a separate essential role in control of listerial infection through control of leukocyte accumulation and organization in infected organs.
Tumor necrosis factor (TNF) and lymphotoxin alpha (LTα) are two structurally related cytokines that mediate proinflammatory activities. The functions of TNF have been extensively characterized, and this factor is required for host control of both mycobacteria and other intracellular pathogens, including Listeria monocytogenes (2, 12, 29). The activities of LTα are less well understood, but recently an essential role for LTα in the host response to pulmonary tuberculosis has been demonstrated (28).
L. monocytogenes is a gram-positive facultative intracellular bacterium that infects macrophages, as well as epithelial cells and hepatocytes. Although initially contained in the phagosome, Listeria is able to penetrate the vacuolar membrane and enter the host cell cytosol through the action of the pore-forming toxin listeriolysin O (LLO) (24). As a result, L. monocytogenes is a potent inducer of CD8 T-cell responses (3, 5). Listeria infection also induces strong activation of antigen-specific CD4+ T cells. Although the role of CD4 T cells remains less well defined, it has recently been demonstrated that CD4 T-cell help is required for generation of functional CD8 T-cell memory (31, 32). While the effector mechanisms used by each cell type have not been elucidated fully, studies in which CD8+ gamma interferon (IFN-γ)-deficient T cells were transferred indicated that IFN-γ is not required to effect the protection provided by these cells (14). Further studies revealed that transfer of wild-type (WT) and TNF-deficient CD8+ T cells mediated antilisterial immunity in wild-type but not TNF-deficient host mice, demonstrating that TNF plays an essential role in the expression of antilisterial resistance (14, 34, 35). The function of lymphotoxin in protective immunity to Listeria has not been reported.
In this study we examined the roles of TNF, LTα, and lymphotoxin beta (LTβ) in the host response to L. monocytogenes infection by using mice with targeted disruption of the LTα, LTβ, and TNF genes. Gene knockout mice were generated directly in C57BL/6 animals (18), and LTα−/−, LTβ−/−, and WT donors were used to generate bone marrow radiation chimeras in syngeneic RAG−/− recipients. This strategy overcame problems related to the lack of peripheral lymphoid tissue in mice with an LTα or LTβ gene disruption (18, 23). TNF−/− and LTα−/− chimeric mice exhibited severe susceptibility to low-dose intravenous infection with L. monocytogenes, despite evidence of T-cell activation. LTβ−/− and WT chimeric mice both controlled and resolved the infection normally, indicating that LTα, but not LTβ, is essential in the host response to L. monocytogenes.
MATERIALS AND METHODS
Mice.
WT C57BL/6 and C57BL/6.RAG-1−/− mice (RAG is the recombinase activation gene) were obtained from the Animal Resources Centre (Perth, Australia). TNF, LTα, and LTβ gene knockout mice were generated in the C57BL/6 genetic background as previously described (22, 26). Adult (>6-week-old) mice were used in all experiments. The mice were housed under specific-pathogen-free conditions at the Centenary Institute animal facility until infection, when they were transferred and maintained in a level 2 physical containment facility. Experiments were conducted by using protocols approved by the University of Sydney Animal Care and Ethics Committee.
Generation of radiation bone marrow chimeras.
Bone marrow cells were harvested from the long bones of donor mice by flushing with phosphate-buffered saline, washed, and counted. Recipient RAG−/− mice were preconditioned with 5.5 Gy gamma radiation on day −2 and again on day 0. Bone marrow cells (1 × 107 cells/recipient) from WT, LTα−/−, and LTβ−/− mice were injected intravenously on day 0. The cage water was supplemented with trimethoprim (50 μg/ml) and sulfamethoxazole (0.25 mg/ml) (DBL, Sydney Australia) from weeks 2 to 4 after transplantation. Chimeras were used at least 3 months after preparation, when complete cellular reconstitution had occurred. Cellular reconstitution was monitored by analysis of peripheral blood by flow cytometry.
Bacteria and experimental infections.
L. monocytogenes was grown in tryptic soy broth (Difco, Detroit, Mich.) from a sample kindly provided by C. Cheers (University of Melbourne, Melbourne, Australia). The culture was initiated from a single colony and cultured for 16 h at 37°C. Bacterial stocks were frozen in 30% (vol/vol) glycerol and stored at −70°C. LTα, LTβ, and WT chimeric mice, as well as TNF−/− and WT mice, were infected with 200 CFU of L. monocytogenes intravenously via a lateral tail vein. The numbers of viable bacteria in target organs were monitored over time by plating serial dilutions of whole-organ homogenates on supplemented tryptic soy agar (Difco) and counting the bacterial colonies that had formed after 24 h of culture. Heat-killed L. monocytogenes (HKL) was prepared by incubating bacteria at 80°C for 1 h.
Cytokine production from purified T cells.
Single-cell suspensions from the spleens of L. monocytogenes-infected mice 7 days after infection were stained with either rat anti-CD4 (clone GK1.5) or anti-CD8 (clone 53.6-7) monoclonal antibody for 30 min at 4°C. Cells were then washed in 2% (vol/vol) fetal calf serum in phosphate-buffered saline and incubated with anti-rat immunoglobulin Dynabeads (Dynal, Australia) for 30 min at 4°C. CD4+ and CD8+ T cells were then isolated by two rounds of magnetic separation. The purities of the cell populations were confirmed by fluorescence-activated cell sorting staining and analysis of an aliquot of cells from each isolation. Purities of more than 95% were obtained following separation (data not shown). For analysis of expression of TNF and LTα mRNA transcripts, total RNA was prepared from cell homogenates using RNAzol B (Tel-Test, Friendswood, Tex.). cDNA was synthesized using SUPERSCRIPT II RNase H reverse transcriptase (GIBCO BRL) and was analyzed by reverse transcription-PCR using primers and methods described previously (26).
T-cell responses to Listeria antigens.
Spleens were removed, and single-cell suspensions were prepared. Erythrocytes were lysed in a hypotonic ammonium chloride lysis buffer, and the remaining cells were washed, counted, and suspended in complete RPMI medium (RPMI 1640 [Cytosystem, Sydney, Australia] with 10% fetal bovine serum [Trace, Sydney, Australia], 2 mM l-glutamine [Sigma], 10 mM HEPES [Sigma], 10 mM Na2CO3, 0.5 μM 2-mercaptoethanol [Sigma], 100 U/ml penicillin [Trace], and 100 μg/ml streptomycin [CSL, Melbourne, Australia]). To measure antigen-specific T-cell responses, splenocytes were cultured with HKL (2 ×107 CFU/ml) or medium (control) for 72 h. To assess proliferative responses, cells were pulsed with 1 μCi of [3H]thymidine (NEN Life Sciences, Boston, Mass.) for the final 6 h of culture and then harvested onto glass fiber filters. The amount of incorporated [3H]thymidine was determined by liquid scintillation spectroscopy (Pharmacia/Wallace Oy, Turku, Finland). Specific [3H]thymidine incorporation was calculated by subtracting the mean counts per minute in unstimulated wells from the mean counts per minute of test samples.
The concentration of IFN-γ in culture supernatants was determined using a capture enzyme-linked immunosorbent assay and a monoclonal antibody capture assay with the antibodies R4-6A2 and XMG1.2-biotin (Endogen, Woburn, Mass.) as previously described (17). No significant proliferation or IFN-γ release was detected in splenocytes from uninfected chimeric and unmanipulated mice. To determine the frequency of IFN-γ-producing cells, splenocytes were cultured for 16 h in Multiscreen 96-well filtration plates (Millipore, Bedford, Mass.) precoated with anti-IFN-γ antibody R4-6A2 in the presence of HKL, Listeria lysin O peptide296-304 (LLO296-304) (13), or medium alone. The enzyme-linked immunospot assay preparations were then developed with anti-IFN-γ antibody XMG1.2-biotin and avidin-alkaline phosphatase as previously described (17).
Histology.
Liver tissue samples were fixed in 10% neutral buffered formalin, processed in paraffin blocks, and sectioned (thickness, 5 μm). The sections were stained with hematoxylin and eosin and examined to assess the histopathological responses of infiltrating leukocytes in the livers of L. monocytogenes-infected mice.
Statistical analysis.
Statistical analyses of the results of immunological assays and log-transformed bacterial counts were conducted using analysis of variance (ANOVA). Fisher's protected least-significant difference ANOVA post hoc test was used for pairwise comparisons of multigroup data sets. P values of <0.05 were considered significant. Survival was calculated on a Kaplan-Meier nonparametric survival plot, and significance was assessed by the Mantel-Cox log rank test.
RESULTS
CD8+ and CD4+ T cells express LTα and TNF following infection with L. monocytogenes.
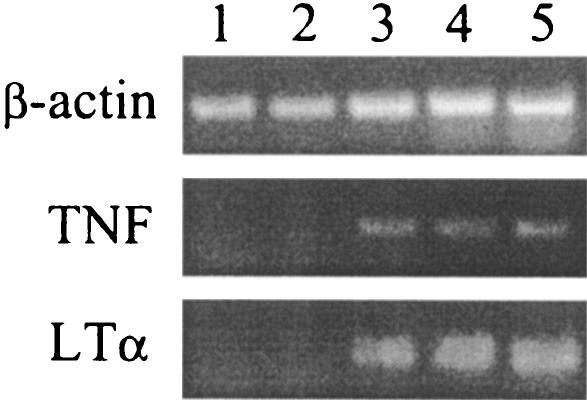
The expression of transcripts for LTα and/or TNF was examined in CD4+ and CD8+ T-cell populations to determine if both CD4+ and CD8+ T cells produce LTα and/or TNF during infection with L. monocytogenes. At 7 days postinfection CD4+ and CD8+ cells were isolated separately from the spleen using antibody-coated magnetic beads, total RNA was extracted, and cDNA was reverse transcribed. Analysis of cDNA by reverse transcription-PCR indicated that following infection both CD4+ and CD8+ T cells up-regulated the expression of LTα and TNF (Fig. 1).
FIG. 1.
Expression of LTα and TNF is up-regulated in both CD4+ and CD8+ T cells during infection with L. monocytogenes. WT mice were infected with 200 CFU of L. monocytogenes intravenously, and at 7 days postinfection highly purified (>95%) CD4+ and CD8+ T cells were isolated from pooled spleen samples. RNA was extracted and cDNA was synthesized as outlined in Materials and Methods. The expression of TNF and LTα was examined by reverse transcription-PCR. Lane 1, naive CD4+ spleen cells; lane 2, naive CD8+ spleen cells; lane 3, CD4+ spleen cells; lane 4, CD8+ spleen cells; lane 5, total spleen cells from infected mouse.
TNF−/− and LTα−/− chimeric mice, but not LTβ−/− chimeric mice, succumb to low-dose L. monocytogenes infection.
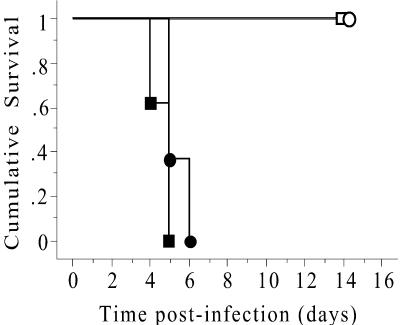
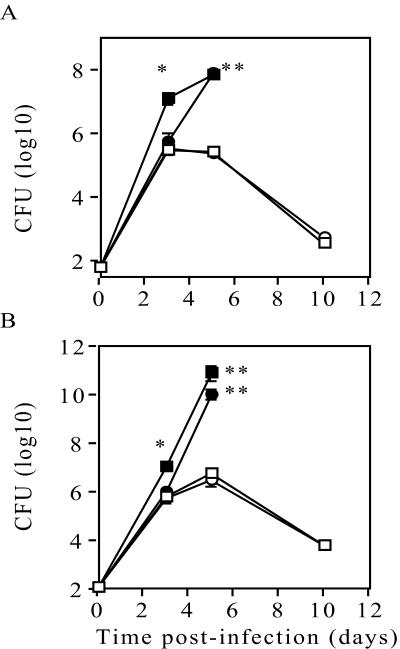
To study functions of LTα and LTβ independent of secondary lymphoid organogenesis, LTα−/−, LTβ−/−, and WT bone marrow chimeras were generated in irradiated RAG−/− recipients. Full reconstitution of peripheral lymphoid tissue with normal lymphoid architecture was observed 3 months after transplantation, and the numbers of peripheral blood leukocytes and percentages of CD4+ and CD8+ T lymphocytes were comparable (26, 28). To assess the contribution of secreted LTα and TNF in the host response to L. monocytogenes infection, LTα−/−, LTβ−/−, and WT chimeric mice, as well as TNF−/− and normal WT mice, were infected with 200 CFU of L. monocytogenes via a lateral tail vein. The survival of the infected mice and the bacterial load were monitored for 2 weeks. All of the normal WT mice and WT chimeric mice survived and had similar bacterial loads in their spleens and livers following infection with L. monocytogenes (data not shown). TNF−/− mice were extremely susceptible to infection and succumbed 4 to 5 days postinfection (Fig. 2). These mice had significantly increased bacterial loads in their spleens (Fig. 3A) and livers (Fig. 3B) as early as 3 days postinfection compared to WT chimeras (Fig. 3) and normal WT controls (data not shown). LTα−/− chimeric mice were highly susceptible to Listeria infection and succumbed 5 to 6 days postinfection (Fig. 2). Although similar numbers of bacteria were recovered from both the spleens (Fig. 3A) and livers (Fig. 3B) of LTα−/− and WT chimeras at 3 days postinfection, by day 5 there were significantly increased bacterial loads in LTα−/− chimeric mice. In contrast, the LTβ−/− chimeras controlled the infection in a manner comparable to WT chimeric mice (Fig. 3). Therefore, both TNF and secreted LTα independently mediate essential actions in the host response to L. monocytogenes infection.
FIG. 2.
Survival of TNF−/− mice and LTα−/−, LTβ−/−, or WT chimeric mice following infection with L. monocytogenes. WT chimeric (□), LTα chimeric (•), LTβ chimeric (○), and TNF−/− (▪) mice were infected intravenously with 200 CFU of L. monocytogenes. The infected mice were monitored daily and euthanized when they showed signs of ill health. The data are the times to euthanasia for eight mice/group and are representative of one of three separate experiments. The P value was <0.001 for TNF−/− versus WT chimeric mice and for LTα chimeric versus WT chimeric mice as determined by a Mantel-Cox log rank test.
FIG. 3.
TNF and LTα−/− chimeric mice fail to control infection with L. monocytogenes. WT chimeric (□), TNF−/− (▪), LTα chimeric (•), and LTβ chimeric (○) mice were infected intravenously with 200 CFU of L. monocytogenes, and the bacterial loads in the spleens (A) and livers (B) were monitored over time. The data are the means and standard errors for four mice per group and are representative of one of three experiments. The significance of differences between WT chimeric and TNF−/− mice, as well as between LTα−/− and WT chimeric mice, was determined by ANOVA (one asterisk, P < 0.01; two asterisks, P < 0.001).
Antigen-specific T-cell responses following L. monocytogenes infection.
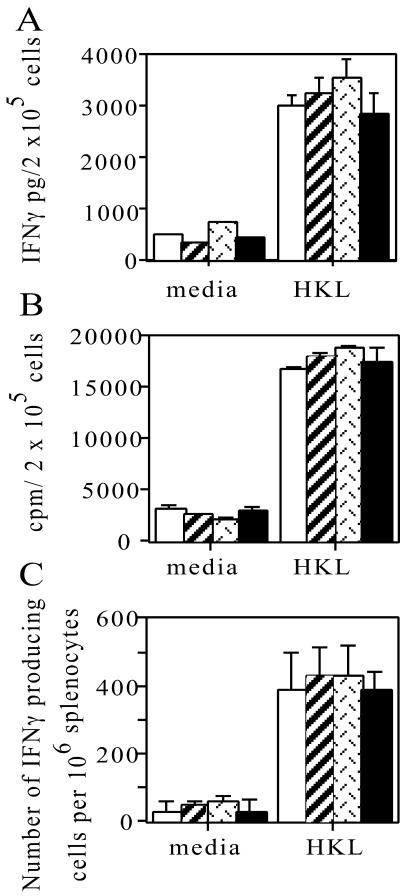
The T-cell response to Listeria infection peaked between days 7 and 10 in WT mice (data not shown), but an antigen-specific T-cell response could be detected after 5 days. The ability of the TNF−/− mice or LTα−/− chimeric mice to mount T-cell responses to L. monocytogenes was assessed to determine if the failure to control infection was due to a deficiency in the activation of T cells. Splenocytes from TNF−/− mice and LTα−/−, LTβ−/−, and WT chimeras infected for 5 days all produced comparable amounts of IFN-γ following 3 days of ex vivo culture in the presence of HKL (Fig. 4A). The proliferative potential of these cells was also assessed at this time by determining the incorporation of [3H]thymidine during the final 6 h of culture. Splenocytes from all groups displayed similar antigen-specific proliferative responses (Fig. 4B). There were also comparable numbers of Listeria-specific splenic IFN-γ-producing cells at 5 days postinfection in all groups in response to HKL stimulation (Fig. 4C). Further analysis to determine if the susceptibility of the LTα chimeric mice was due to a lack of a CD8 T-cell response was then undertaken. WT and LTα chimeric mice displayed a similar number of T cells producing IFN-γ in response to the CD8-specific LLO296-304 epitope (Table 1). Furthermore, the percentages of IFN-γ-producing T cells detected by intracellular staining were also similar in both CD4+ and CD8+ T cells from WT and LTα chimeric mice.
FIG. 4.
TNF−/− and LTα−/− chimeric mice have the potential to mount normal T-cell responses. WT chimeric (open bars), LTα chimeric (bars with diagonal lines), LTβ chimeric (bars with patterned lines), and TNF−/− (solid bars) mice were infected intravenously with 200 CFU of L. monocytogenes. At 5 days postinfection splenocytes were prepared and cultured in the presence of HKL or medium alone (media) for 3 days. Total IFN-γ release was measured by an enzyme-linked immunosorbent assay (A), and proliferation was determined by measuring the incorporation of [3H]thymidine during the final 6 h of culture (B). (C) Cells were cultured in the presence of HKL overnight, and the frequency of IFN-γ-producing cells was determined by the enzyme-linked immunospot assay. The data are the means ± standard errors of the means for four mice per group and are representative of one of two experiments.
TABLE 1.
Equivalent CD4+ and CD8+ T-cell responses in WT and LTα−/− chimeric mice at 5 days postinfectiona
| Parameter | WT chimeric mice | LTα chimeric mice | WT uninfected mice |
|---|---|---|---|
| % CD4+ IFN-γ-producing cells | 3.2 + 0.99 | 2.4 + 0.84 | 0.6 + 0.58 |
| % CD8+ IFN-γ-producing cells | 1.64 + 0.22 | 1.96 + 0.38 | 0.62 + 0.49 |
| No. of IFN-γ-producing cells (106 spleen cells) | 184 ± 107 | 200 ± 106 | 36 ± 43 |
Mice were infected with 200 CFU of L. monocytogenes. Five days later spleens were harvested and either incubated overnight on anti-CD3-coated plates and stained for CD4, CD8, and intracellular IFN-γ or incubated overnight with LLO296-304 and subjected to an enzyme-linked immunospot assay to determine the number of IFN-γ-producing cells. The data are the means and standard errors for three mice per group from one of two independent experiments.
LTα−/− chimeric mice do not form normal foci of leukocytes in response to infection with L. monocytogenes.
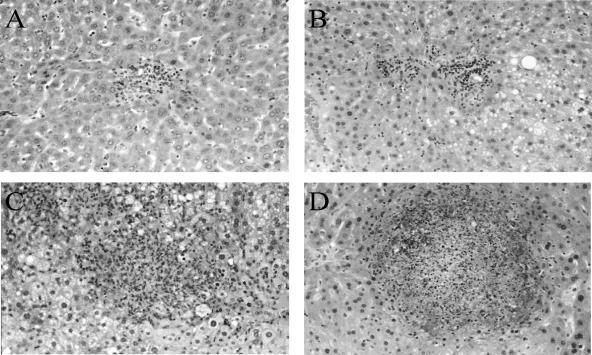
To determine if the increased susceptibility of TNF−/− and LTα−/− chimeric mice to infection was associated with different patterns of cellular recruitment, liver histology was assessed at days 3 and 5 postinfection. A few small discrete foci (10 or more cells) were seen in the WT and LTβ chimeric mice on day 3, with even fewer foci evident in the LTα chimeric mice (WT chimeric mice, 19 ± 3.4 foci/liver section; LTβ chimeric mice, 14 ± 3.2 foci/liver section; LTα chimeric mice, 6 ± 3.4 foci/liver section). At this time TNF−/− mice already displayed extensive inflammation and necrosis in the liver. By day 5, compact, discrete collections of macrophages and lymphocytes were seen in WT chimeras (Fig. 5A) and LTβ−/− chimeras (Fig. 5B), and these collections were similar to those observed in normal WT mice (data not shown). In contrast, the foci that formed in LTα−/− chimeric mice (Fig. 5C) and in TNF−/− mice (Fig. 5D) were grossly abnormal. These aberrant foci contained numerous neutrophils and relatively few lymphocytes and macrophages, and extensive areas of necrosis were observed in all lesions.
FIG. 5.
Histopathological response to L. monocytogenes infection is abnormal in the livers of TNF−/− and LTα−/− chimeric mice. Livers from infected WT, LTα−/−, and LTβ−/− chimeric mice and TNF−/− mice were removed at day 5 postinfection and processed for histology. WT (A) and LTβ−/− (B) chimeric mice showed infiltrating macrophages and lymphocytes in small, compact, discrete collections of cells, whereas LTα−/− chimeras (C) and TNF−/− (D) mice exhibited large necrotic lesions with numerous neutrophils. Original magnification, ×100. The sections show typical lesions from one of four livers per group from one of two representative experiments.
DISCUSSION
This study defined independent, essential, functions for TNF and LTα in the murine response to the intracellular pathogen L. monocytogenes. The requirement for TNF was confirmed here by progressive infection and profound susceptibility to L. monocytogenes in TNF−/− mice. The median survival time after infection was only 4.5 days, and a significant increase in the bacterial load in the spleen and liver was evident by 3 days postinfection. This most likely reflects the role of TNF in facilitating early recruitment of neutrophils, which are crucial for the containment of early Listeria infection (6, 9). This is in addition to the role of TNF during the adaptive phase of the response, in which TNF is essential for the recruitment of T cells and macrophages into lesions to effect bacterial clearance (2). These data are consistent with the profound susceptibility of mice treated with anti-TNF antisera and TNF receptor I-deficient mice to L. monocytogenes (23, 29) and with the observation that therapy with recombinant TNF protected mice from a lethal L. monocytogenes challenge (15).
The role of other TNF superfamily members in antilisterial immunity has been less clear. This study revealed a separate and independent role for the soluble form of LT, the homotrimer LTα3, but not for the membrane-bound heterotrimeric forms, LTβ2α1 and LTβ1α2, in host control of L. monocytogenes infections. The membrane-bound heterotrimers signal through the unique LTβ receptor, and the signal is essential for formation of peripheral lymphoid tissue during embryogenesis (25). Use of LTα−/− and LTβ−/− chimeras overcomes the problem of the lack of secondary lymphoid organs in LTβ−/−-deficient mice (26, 28), which limits the development of normal T-cell responses.
LTβ−/− chimeras lack membrane-bound LT but are sufficient in the LTα secreted homotrimer LTα3, whereas LTα−/− chimeras are deficient in both soluble and membrane-bound LT. The LTα−/− chimeras, but not the LTβ−/− chimeras, were highly susceptible and succumbed 6 days after low-dose infection. Similar susceptibility was observed in LTα-deficient chimeras and TNF−/− mice exposed to Mycobacterium tuberculosis by the aerosol route (2, 28). The LTα3 defect was not due to an indirect effect of a failure to produce TNF, as macrophages from LTα-deficient mice (and LTβ-deficient mice) exhibited normal TNF secretion in response to lipopolysaccharide and IFN-γ stimulation (28). LTα was also not required to control early bacterial replication. Unlike TNF−/− mice, LTα−/− chimeras contained bacillary growth as effectively as WT chimeric mice for the first 72 h. However, LTα was required for effective containment and elimination of Listeria, as after 5 days of infection both the spleens and livers of the LTα−/− chimeric mice displayed increased bacterial loads. This was not due to an inability to develop an antigen-specific T-cell response as both LTα−/− chimeras and TNF−/− mice developed normal Th-1-like T-cell responses.
The significant, overriding defect observed in both LTα−/− chimeras and TNF−/− mice was a profoundly inadequate inflammatory response. After 72 h of infection the number of inflammatory foci identified in the LTα−/− chimeric mice was lower than the number seen in both the WT and LTβ chimeric mice. These differences were most apparent by 5 days postinfection, when, rather than responding to L. monocytogenes by formation of tight foci of T cells and macrophages, as seen in the WT and LTβ−/−chimeras, the LTα−/− chimeras and TNF−/− mice responded with diffuse collections of cells, mainly neutrophils, that failed to contain the infection. Extensive tissue necrosis resulting from the secretion of oxidative products from activated neutrophils and increased protein exudation were observed. Similar aberrant lesions were observed in TNF- and LTα-deficient mice infected with M. tuberculosis (28). Recombinant murine LTα3 induces the leukocyte adhesion molecules vascular cell adhesion molecule 1, intracellular adhesion molecule 1, and E-selectin on endothelial cells in vitro and stimulates the T-cell- and monocyte-attracting chemokines RANTES, IP-10, and MCP-1 (7, 8). Dysregulated chemokine expression accompanied abnormal granuloma formation in TNF−/− chimeras infected with M. tuberculosis (2, 27, 28). Since TNF and LTα clearly display independent proinflammatory activities and LTα3 can signal through the same receptors, TNFRI and TNFRII, it is possible that LTα3 activity is also mediated via an additional signaling pathway, such as HVEM. Alternatively, LTα3 may signal through the same receptors as TNF, but the timing and site of production and therefore the effects of LTα3 signaling may be temporally distinct.
Independent effector functions for TNF and LTα have been described in several other murine models of infection. Control of Toxoplasma gondii required both TNF and LTα acting in synergy (30), and protection against Mycobacterium bovis BCG infection was more profoundly impaired in TNF−/− LTα−/− double-knockout mice than in their LTα−/− counterparts (4, 16). In a murine model of cerebral malaria, LTα was essential for the development of the immunopathology, whereas the central nervous system inflammation progressed independent of TNF (11). Interpretation of the outcome of infections in nonchimeric LT-deficient mice is complicated by their lack of peripheral lymphoid tissues. Impaired activation and expansion of virus-specific CD8+ T cells have been observed in LTα−/− mice infected with herpes simplex virus (19), lymphocytic choriomeningitis virus (33), Theiler’s murine encephalomyelitis virus (20), and influenza virus (21). Impaired T-cell responses to lymphocytic choriomeningitis virus (33) and also to Leishmania major (36) required intact peripheral lymphoid tissue; however, interestingly, LTβ-deficient chimeras with sufficient LTα3 resisted L. major infection (36).
Studies of M. tuberculosis and high-dose Listeria infections in LTβ receptor knockout mice revealed susceptibility to both infections which was not solely attributable to a lack of secondary lymphoid tissue and implicated membrane-bound LTαβ heterotrimer signaling in protective responses (10). This contrasts with our observations of protective responses to Listeria and M. tuberculosis in LTβ−/− chimeras (28; this study). These discrepancies may reflect indirect effects of LTβ receptor deficiency, such as a failure in maturation of antigen-presenting dendritic cells (1, 10).
In conclusion, this study revealed independent functions of TNF and LTα, but not LTβ, in the protective response to L. monocytogenes. TNF- and LTα-deficient T cells do not display intrinsic defects in activation or effector function. Rather, both of the cytokines are required for the early recruitment of macrophages and T cells to the site of infection and activating macrophages to eliminate the intracellular bacterial infection.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and the New South Wales Department of Health through its research infrastructure grant to the Centenary Institute of Cancer Medicine and Cell Biology. D.R. was supported by an Australian postgraduate award.
We thank Jenny Kingham and staff for excellent animal care.
Editor: J. L. Flynn
REFERENCES
- 1.Abe, K., F. O. Yarovinsky, T. Murakami, A. N. Shakhov, A. V. Tumanov, D. Ito, L. N. Drutskaya, K. Pfeffer, D. V. Kuprash, K. L. Komschlies, and S. A. Nedospasov. 2003. Distinct contributions of TNF and LT cytokines to the development of dendritic cells in vitro and their recruitment in vivo. Blood 101:1477-1483. [DOI] [PubMed] [Google Scholar]
- 2.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 3.Bhardwaj, V., O. Kanagawa, P. E. Swanson, and E. R. Unanue. 1998. Chronic Listeria infection in SCID mice: requirements for the carrier state and the dual role of T cells in transferring protection or suppression. J. Immunol. 160:376-384. [PubMed] [Google Scholar]
- 4.Bopst, M., I. Garcia, R. Guler, M. L. Olleros, T. Rulicke, M. Muller, S. Wyss, K. Frei, M. Le Hir, and H. P. Eugster. 2001. Differential effects of TNF and LTalpha in the host defense against M. bovis BCG. Eur. J. Immunol. 31:1935-1943. [DOI] [PubMed] [Google Scholar]
- 5.Brunt, L. M., D. A. Portnoy, and E. R. Unanue. 1990. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J. Immunol. 145:3540-3546. [PubMed] [Google Scholar]
- 6.Conlan, J. W., and R. J. North. 1991. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J. Exp. Med. 174:741-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuff, C. A., R. Sacca, and N. H. Ruddle. 1999. Differential induction of adhesion molecule and chemokine expression by LTalpha3 and LTalphabeta in inflammation elucidates potential mechanisms of mesenteric and peripheral lymph node development. J. Immunol. 162:5965-5972. [PubMed] [Google Scholar]
- 8.Cuff, C. A., J. Schwartz, C. M. Bergman, K. S. Russell, J. R. Bender, and N. H. Ruddle. 1998. Lymphotoxin alpha3 induces chemokines and adhesion molecules: insight into the role of LT alpha in inflammation and lymphoid organ development. J. Immunol. 161:6853-6860. [PubMed] [Google Scholar]
- 9.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836-1846. [PubMed] [Google Scholar]
- 10.Ehlers, S., C. Holscher, S. Scheu, C. Tertilt, T. Hehlgans, J. Suwinski, R. Endres, and K. Pfeffer. 2003. The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J. Immunol. 170:5210-5218. [DOI] [PubMed] [Google Scholar]
- 11.Engwerda, C. R., T. L. Mynott, S. Sawhney, J. B. De Souza, Q. D. Bickle, and P. M. Kaye. 2002. Locally up-regulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principle mediator of murine cerebral malaria. J. Exp. Med. 195:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 13.Geginat, G., S. Schenk, M. Skoberne, W. Goebel, and H. Hof. 2001. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J. Immunol. 166:1877-1884. [DOI] [PubMed] [Google Scholar]
- 14.Harty, J. T., R. D. Schreiber, and M. J. Bevan. 1992. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc. Natl. Acad. Sci. USA 89:11612-11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havell, E. A. 1989. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J. Immunol. 143:2894-2899. [PubMed] [Google Scholar]
- 16.Jacobs, M., N. Brown, N. Allie, and B. Ryffel. 2000. Fatal Mycobacterium bovis BCG infection in TNF-LT-alpha-deficient mice. Clin. Immunol. 94:192-199. [DOI] [PubMed] [Google Scholar]
- 17.Kamath, A. T., T. Hanke, H. Briscoe, and W. J. Britton. 1999. Co-immunization with DNA vaccines expressing granulocyte-macrophage colony-stimulating factor and mycobacterial secreted proteins enhances T-cell immunity, but not protective efficacy against Mycobacterium tuberculosis. Immunology 96:511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korner, H., M. Cook, D. S. Riminton, F. A. Lemckert, R. M. Hoek, B. Ledermann, F. Kontgen, B. Fazekas de St. Groth, and J. D. Sedgwick. 1997. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol 27:2600-2609. [DOI] [PubMed] [Google Scholar]
- 19.Kumaraguru, U., I. A. Davis, S. Deshpande, S. S. Tevethia, and B. T. Rouse. 2001. Lymphotoxin alpha−/− mice develop functionally impaired CD8+ T cell responses and fail to contain virus infection of the central nervous system. J. Immunol. 166:1066-1074. [DOI] [PubMed] [Google Scholar]
- 20.Lin, X., X. Ma, M. Rodriguez, X. Feng, L. Zoecklein, Y. X. Fu, and R. P. Roos. 2003. Membrane lymphotoxin is required for resistance to Theiler's virus infection. Int. Immunol. 15:955-962. [DOI] [PubMed] [Google Scholar]
- 21.Lund, F. E., S. Partida-Sanchez, B. O. Lee, K. L. Kusser, L. Hartson, R. J. Hogan, D. L. Woodland, and T. D. Randall. 2002. Lymphotoxin-alpha-deficient mice make delayed, but effective, T and B cell responses to influenza. J. Immunol. 169:5236-5243. [DOI] [PubMed] [Google Scholar]
- 22.Ngo, V. N., H. Korner, M. D. Gunn, K. N. Schmidt, D. S. Riminton, M. D. Cooper, J. L. Browning, J. D. Sedgwick, and J. G. Cyster. 1999. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189:403-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeffer, K. 2003. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 14:185-191. [DOI] [PubMed] [Google Scholar]
- 24.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rennert, P. D. 1998. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity 9:71-79. [DOI] [PubMed] [Google Scholar]
- 26.Riminton, S. D., H. Korner, D. H. Strickland, F. A. Lemckert, J. D. Pollard, and J. D. Sedgwick. 1998. Challenging cytokine redundancy: inflammatory cell movement and clinical course of experimental autoimmune encephalomyelitis are normal in lymphotoxin-deficient, but not tumor necrosis factor-deficient, mice. J. Exp. Med. 187:1517-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roach, D. R., A. G. Bean, C. Demangel, M. P. France, H. Briscoe, and W. J. Britton. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168:4620-4627. [DOI] [PubMed] [Google Scholar]
- 28.Roach, D. R., H. Briscoe, B. M. Saunders, M. P. France, S. Riminton, and W. J. Britton. 2001. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J. Exp. Med. 193:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364:798-802. [DOI] [PubMed] [Google Scholar]
- 30.Schluter, D., L. Y. Kwok, S. Lutjen, S. Soltek, S. Hoffmann, H. Korner, and M. Deckert. 2003. Both lymphotoxin-alpha and TNF are crucial for control of Toxoplasma gondii in the central nervous system. J. Immunol. 170:6172-6182. [DOI] [PubMed] [Google Scholar]
- 31.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 32.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suresh, M., G. Lanier, M. K. Large, J. K. Whitmire, J. D. Altman, N. H. Ruddle, and R. Ahmed. 2002. Role of lymphotoxin alpha in T-cell responses during an acute viral infection. J. Virol. 76:3943-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White, D. W., V. P. Badovinac, G. Kollias, and J. T. Harty. 2000. Cutting edge: antilisterial activity of CD8+ T cells derived from TNF-deficient and TNF/perforin double-deficient mice. J. Immunol. 165:5-9. [DOI] [PubMed] [Google Scholar]
- 35.White, D. W., and J. T. Harty. 1998. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J. Immunol. 160:898-905. [PubMed] [Google Scholar]
- 36.Wilhelm, P., D. S. Riminton, U. Ritter, F. A. Lemckert, C. Scheidig, R. Hoek, J. D. Sedgwick, and H. Korner. 2002. Membrane lymphotoxin contributes to anti-leishmanial immunity by controlling structural integrity of lymphoid organs. Eur. J. Immunol. 32:1993-2003. [DOI] [PubMed] [Google Scholar]