Abstract
Endocarditis is frequently attributable to oral streptococci, but mechanisms of pathogenesis are not well understood, although monocytes appear to be important. High titers of interleukin-12 (IL-12) are produced by peripheral blood mononuclear cells (PBMC) after engaging Streptococcus mutans, but monocytes in developing endocardial vegetations tend to disappear rather than become macrophages. These data prompted the hypothesis that streptococcus-infected monocytes differentiate into short-lived IL-12-producing dendritic cells (DCs) rather than macrophages. PBMC from healthy subjects were stimulated with six isolates of oral streptococci, three nonstreptococcal oral bacteria, or IL-4 plus granulocyte-macrophage colony-stimulating factor, and the appearance of cells with markers typical of mature DCs (CD83+, CD86+, CD11c+, and CD14−) was monitored. Supernatant fluids from the PBMC cultures were harvested and IL-12 p70 levels were determined. S. mutans-stimulated monocytes were analyzed for their ability to elicit allogeneic mixed-lymphocyte reactions. All streptococci examined, except one strain of Streptococcus oralis (35037), rapidly induced up-regulation of CD83 and CD86 and a loss of CD14 in the CD11c+ monocyte population within 20 h. Induction of IL-12 was CD14 dependent and correlated with streptococcal isolates that promoted the DC phenotype. Major histocompatibility complex (MHC) class II expression was up-regulated by S. mutans, and these cells were short-lived and elicited potent allogeneic mixed-lymphocyte reactions typical of DCs. In summary, monocytes stimulated with endocarditis-associated oral streptococci rapidly exhibited the DC phenotype and functions. These data suggest that the initiation of bacterial endocarditis by oral streptococci may involve monocyte-to-DC differentiation, and this may help explain the low levels of macrophages in the site.
Viridans group streptococci are the most common cause of native valve endocarditis, accounting for 45 to 80% of cases, and Streptococcus sanguis, Streptococcus mutans, Streptococcus gordonii, and Streptococcus oralis are frequently isolated from these lesions (35). Virulence factors such as exopolysaccharide (26), fibronectin binding protein (24), and platelet aggregation association protein (9, 17) have been implicated in the initial fibrin colonization on damaged cardiac valves. However, oral streptococci without these properties are also isolated from the endocarditis lesions.
Monocytes are prominent in early endocarditis lesions, and the number of monocytes correlates with infectivity and higher tissue factor activity in vegetations (3, 6). However, the actual role of the monocytes in the initiation and perpetuation of the vegetation remains controversial. The work of Durack and Beeson indicates that the majority of S. sanguis strains in the vegetations are phagocytosed, transported in the blood, and deposited on the vegetations in adherent monocytes (13). The monocyte population then disappears, and viable bacteria persist in the vegetations. Carrizosa et al., using scanning electron microscopy, confirmed their observations with Streptococcus mitis in a rabbit infective endocarditis model (7). However, studies of monocytopenic rabbits indicate that S. sanguis can directly interact with and infect vegetations and that streptococci do not have to be phagocytosed by monocytes before being deposited on the surface of vegetations (2, 33). The mechanism by which monocytes contribute to tissue factor activity during the progression of infective endocarditis is not well understood. Nevertheless, interactions between streptococci, monocytes, and platelets are necessary for the increased tissue factor activity in vegetations (1, 3).
The high levels of interleukin-12 (IL-12) induced by S. mutans in our previous study (14) together with the rapid disappearance of monocytes and the lack of numerous macrophages in vegetations prompted the hypothesis that streptococcus-infected monocytes are transformed to dendritic cells (DCs) rather than macrophages. Mature DCs are capable of producing high levels of IL-12 and tend to live only for a few days (18, 20), whereas macrophages can survive in sites of inflammation and kill microorganisms for long periods of time. We reasoned that adhesin-rich, streptococcus-infected monocytes would adhere to damaged heart valves where they could differentiate into DCs and die, leaving the site infected with viable streptococci. To begin testing this hypothesis, we examined the phenotype and functional characteristics of streptococcus-infected monocytes. The results indicate that oral streptococci can prompt monocytes to differentiate into mature short-lived DCs that produce high levels of IL-12 and that this transformation can be completed within a single day in vitro.
MATERIALS AND METHODS
Bacterial preparation.
S. mutans ATCC 25175, S. mutans v1311 (Centers for Disease Control and Prevention clinical isolate from an endocarditis patient generously provided by Frank Macrina, Philips Institute, Virginia Commonwealth University), S. oralis ATCC 35037, S. oralis ATCC 10557, S. mitis ATCC 903, S. sanguis ATCC 10556, and Actinomyces viscosus ATCC 15978 were cultured in brain heart infusion broth (Becton Dickinson, Sparks, MD) overnight in an anaerobic chamber. Lactobacillus casei ATCC 4646 was cultured in lactobacillus MRS broth (Difco, Detroit, MI), and Prevotella intermedia ATCC 25611 was cultured in brain heart infusion enriched with hemin (5 μg/ml) and menadione (1 μg/ml) overnight in an anaerobic chamber. Bacteria were harvested and washed three times with sterile phosphate-buffered saline. Individual bacterial concentrations were determined with a spectrophotometer at a wavelength of 650 nm and stored at −20°C.
PBMC preparation.
Medically healthy donors were recruited by the Clinical Research Center for Periodontal Disease, School of Dentistry, Virginia Commonwealth University. Venous blood was drawn after appropriate informed consent was received and approved by the University Institutional Review Board. Peripheral blood mononuclear cells (PBMC) were prepared by differential density centrifugation with lymphocyte separation medium (MP Biomedicals, Auropa, OH). Cells were then washed three times with RPMI 1640, and the number of viable cells was determined by trypan blue exclusion. PBMC preparations (106/ml) were challenged with bacteria (105, 106, or 107/ml), IL-4 (500 U/ml; R&D, Minneapolis, MN) plus granulocyte-macrophage colony-stimulating factor (GM-CSF) (800 U/ml; R&D), or macrophage colony-stimulating factor (M-CSF) (1,000 U/ml; R&D) in enriched RPMI 1640 (0.01 M HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin; Invitrogen, Carlsbad, CA) and 10% fetal calf serum (HyClone, Logan, UT) for 20 h before harvesting.
Antibodies and blockade of Toll-like receptors.
Some PBMC preparations were pretreated with neutralizing anti-TLR2 (20 μg/ml, clone TL2.1; eBioscience, San Diego, CA), anti-TLR4 (20 μg/ml, clone HAT125; eBioscience), and anti-CD14 (1 μg/ml, clone 61D3; eBioscience) antibodies or their respective isotypes (immunoglobulin G2a [IgG2a] for Toll-like receptors [TLRs] or IgG1 for CD14) for 1 h in 5% CO2 before the addition of bacteria.
Adherent cell preparation.
PBMC (5 × 106/ml) were inoculated into each well of a six-well plate in RPMI 1640 and incubated for 1 h at 37°C in a 5% CO2 incubator. Nonadherent cells were removed by three washes with RPMI 1640, and the adherent cells were stimulated with various bacteria in enriched RPMI 1640 for 1 day before harvesting. Positive controls for immature DCs were made using IL-4 (500 U/ml) plus GM-CSF (800 U/ml)-stimulated adherent cells in enriched RPMI 1640 for 5 to 7 days. Positive controls for macrophages were made using M-CSF (1,000 U/ml)-stimulated adherent cells for 5 to 7 days.
Flow cytometry.
Dendritic cells were identified using monoclonal antibodies (BD Pharmingen, San Diego, CA) reactive with CD14, CD11c, CD83, CD86, and major histocompatibility complex class II (MHC-II) using flow cytometry and analyzed with Cytomics software (Beckman Coulter, Miami, FL). The percentages of CD14− CD11c+ population, MHC-II expression, and CD86+ or CD83+ cells were calculated using the monocyte population gated using forward and side scatter. The mean fluorescent intensity (MFI) of CD86 expression was compared between PBMC controls and bacterially stimulated cultures at the dose containing the most mature DCs. DC maturity was indicated by the maximal up-regulation of CD83. The up-regulation of MHC-II by S. mutans ATCC 25175 was compared to that by A. viscosus. The percentage of live or dead CD83+ cells was obtained using propidium iodide (PI) and a CD83-specific monoclonal antibody, and the number of live or dead cells was calculated from the total cell count using a hemacytometer.
ELISA.
Concentrations of IL-12 p70 in culture supernatant fluids were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (BD Pharmingen). Comparisons were made between PBMC controls and bacterially stimulated cultures containing the most mature DCs, as determined by CD83 expression at the maximal stimulatory dose of bacteria.
Mixed-lymphocyte reaction.
T cells were prepared using CD14, CD19, and CD56 reactive microbeads (Miltenyi Biotec, Auburn, CA) to deplete PBMC of unwanted cells. Antigen-presenting cells (APC) were prepared from PBMC after overnight culture with S. mutans, A. viscosus, or medium control in enriched RPMI 1640. Cells were washed three times with sterile phosphate-buffered saline, and the unwanted cells (CD3+, CD19+, and CD56+) were depleted by a microbead depletion method according to the manufacturer's instructions (Miltenyi Biotec). APC were then irradiated with 3,000 rad using a 137Cs source. APC (103, 104, and 105 per well) were added to a 96-well plate and cocultured with T-cell preparations in triplicate wells (105 per well) in enriched RPMI 1640 for 5 days. The cultures were then pulsed with [3H]thymidine (1 μCi/well) for 18 h before harvesting. The radioactivity (in counts per minute) was measured in a beta counter (Top Count; Parker Instrument, Meriden, CT).
Statistical analysis.
The differences between PBMC controls and experimental groups were analyzed using repeated measures of analysis of variance. Significant differences were identified at P values of <0.05 with Tukey honestly significant difference corrected for multiple comparisons.
RESULTS
Rapid loss of CD14 on the CD11c+ monocyte population.
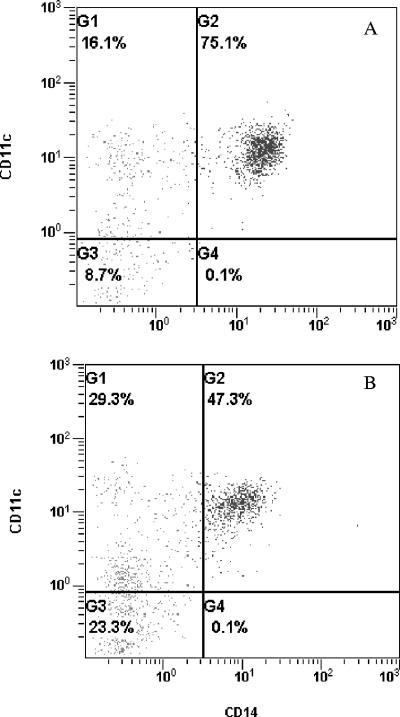
When PBMC cultures stimulated with S. mutans were compared with the untreated controls, a shift in the CD11c+ monocyte population from CD14+ to CD14− cells was apparent. Forward and side scatter were used to gate the monocyte population, and a typical shift under the influence of S. mutans is illustrated in Fig. 1. Note that the entire CD11c+ CD14+ population shifted to the left when stimulated with S. mutans and that double-positive cells dropped from about 75% to less than 50%, while the CD11c+ CD14− population nearly doubled in just 20 h. This effect of S. mutans was compared with other oral microorganisms and cytokines, and the results are shown in Table 1. Note that monocytes treated with IL-4 plus GM-CSF, L. casei, and S. mutans ATCC 25175 for 20 h all showed increased levels of the CD11c+ CD14− population (P < 0.05), while PBMC treated with M-CSF, P. intermedia, or A. viscosus did not differ from the untreated PBMC control (Table 1).
FIG. 1.
Change in CD14 expression by monocytes after exposure to S. mutans. (A) PBMC control; (B) PBMC treated with S. mutans ATCC 25175 for 20 h. Monocytes were stained with CD14 and CD11c monoclonal antibodies. Note the reduction in CD14 when incubated with S. mutans.
TABLE 1.
Rapid shift of the CD11c+ CD14+ monocyte population to CD14−a
| Treatment | % of CD11c+ CD14− cells
|
|
|---|---|---|
| (least square mean ± SE)b | P valuec | |
| S. mutans ATCC 25175 | 26.4 ± 5.0 | <0.05 |
| L. casei | 26.2 ± 5.2 | <0.05 |
| IL-4 + GM-CSF | 24.4 ± 5.2 | <0.05 |
| P. intermedia | 19.5 ± 5.2 | NSd |
| A. viscosus | 14.0 ± 5.0 | NS |
| M-CSF | 7.9 ± 5.2 | NS |
| None (PBMC control) | 13.2 ± 4.9 | |
Triplicate PBMC cultures (106/ml) were treated with bacteria (106/ml), IL-4 plus GM-CSF, or M-CSF for 20 h. Cells were then washed and stained with monoclonal antibodies for flow cytometry analysis.
The percentage of CD11c+ CD14− cells inside the monocyte gate was calculated and reported as the least square mean of the results from four individual experiments.
The statistical comparison was made with the PBMC control. P values from Tukey honestly significant difference were corrected for multiple comparisons.
NS, not significant.
Up-regulation of CD83, CD86, and MHC class II.
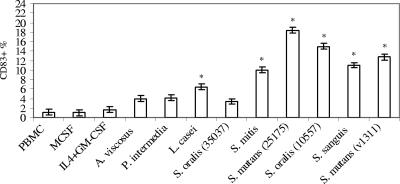
A hallmark for DC maturation is the expression of CD83, and the effect of bacterial or cytokine challenge on the CD83+ population is illustrated in Fig. 2. M-CSF or IL-4 plus GM-CSF treatment did not promote the production of mature DCs, as indicated by minimal up-regulation of CD83 within 20 h in culture. A. viscosus and P. intermedia tended to increase CD83 expression, but it was not significantly higher than that of the PBMC control. L. casei induced a modest elevation in CD83 expression when compared with the PBMC control (P < 0.05). In marked contrast, all isolates of oral streptococci, except S. oralis ATCC 35037, stimulated significantly elevated expression of CD83+ cells by 10 to 20% of cells in culture (Fig. 2).
FIG. 2.
Increase in CD83 expression upon stimulation with oral streptococci. PBMC (106/ml) were stimulated with M-CSF, IL-4 plus GM-CSF, or bacteria (105, 106, or 107/ml) for 20 h in triplicate. Cells were stained with CD83-fluorescein isothiocyanate monoclonal antibody and analyzed by flow cytometry. The maximal stimulatory responses from each bacterial isolate were compared to the PBMC control. The vertical bars represent standard errors, and asterisks represent significant elevations of CD83 expression of cells inside the monocyte gate. This experiment is representative of the results from three experiments with three different individuals.
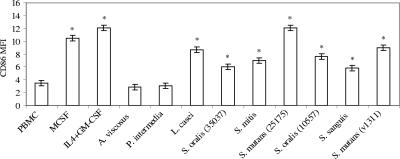
CD86 is an important monocyte costimulatory molecule that is up-regulated early in the differentiation process leading to mature DCs. The ability of 6 streptococcal isolates, other oral bacteria, and cytokines to up-regulate CD86 expression is illustrated in Fig. 3. The MFI of CD86 clearly increased when PBMC were stimulated with M-CSF, IL-4 plus GM-CSF, L. casei, S, oralis ATCC 35037, S. mitis, S. mutans ATCC 2517), S. oralis ATCC 10557, S. sanguis, and S. mutans v1311 (P < 0.05).
FIG. 3.
Effect of various bacteria on the MFI of CD86 expression by PBMC inside the monocyte gate. PBMC (106/ml) were stimulated with M-CSF, IL-4 plus GM-CSF, or bacteria for 20 h in triplicate. Cells were stained with a CD86-phycoerythrin antibody and analyzed by flow cytometry. The vertical bars represent standard errors, and asterisks represent significant elevations of CD86 expression of cells inside the monocyte gate. This experiment is representative of the results from three experiments with three different individuals.
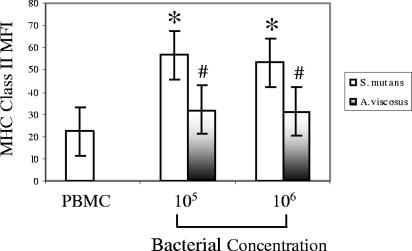
Increased expression of MHC class II is also a characteristic of mature DCs, and this was apparent when PBMC were stimulated with S. mutans ATCC 25175 (Fig. 4). A. viscosus tended to increase MHC class II expression, but statistically, it was not significantly higher than that of the PBMC control. However, S. mutans was more potent in up-regulating MHC class II expression than A. viscosus at both 105 and 106 concentrations (P < 0.05).
FIG. 4.
Up-regulation of MHC class II expression by S. mutans-stimulated monocytes. PBMC from 3 individuals were cultured with two concentrations (105 and 106/ml) of S. mutans ATCC 25175 or A. viscosus for 20 h. Cells were then washed and stained with anti-human MHC class II monoclonal antibody. The vertical bars represent standard errors, and asterisks represent significant increases of the MFI of MHC class II labeling compared with the PBMC control. #, significant difference in MFI between S. mutans- and A. viscosus-stimulated cultures.
Short-lived CD83+ cells.
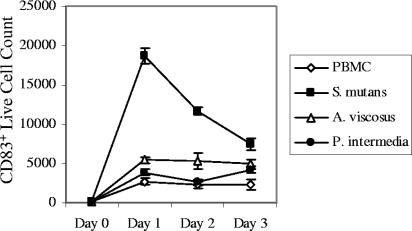
The life span of mature DCs is on the order of 2 to 3 days (18, 20) and is short relative to macrophages, prompting us to study the life span of the CD83+ cells induced by oral streptococci (Fig. 5). PBMC were challenged with S. mutans, and numerous live CD83+ cells appeared quickly on day 1 and declined on days 2 and 3. In addition, we found many PI+ CD83+ cells (∼2,000 on day 1) along with the ∼19,000 live CD83+ cells illustrated in Fig. 5. In contrast, macrophages (M-CSF derived) treated with S. mutans survived, and the number of viable macrophages was not different from the untreated macrophage control for up to 3 days (data not shown). A. viscosus and P. intermedia, which did not significantly up-regulate CD83 expression (Fig. 2), were similar to the PBMC control (Fig. 5).
FIG. 5.
CD83+ cells induced by S. mutans had a short life span. PBMC (0.5 × 106/0.5 ml) were stimulated with S. mutans ATCC 25175, A. viscosus, or P. intermedia (0.5 × 106/0.5 ml of enriched RPMI media that contains 1% penicillin and streptomycin). Cells were harvested in triplicate on days 1, 2, and 3 and labeled with CD83 and PI. The number of live CD83+ cells was calculated using the percentage of PI− CD83+ cells and the total cell count obtained using a hemacytometer. The vertical bars represent standard errors. The S. mutans data are representative of results from five different individuals, although in two cases, the peak number of viable DCs was found on day 2.
IL-12 production.
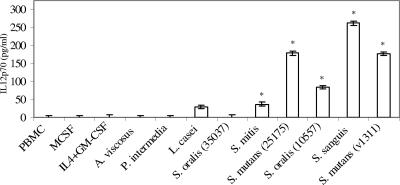
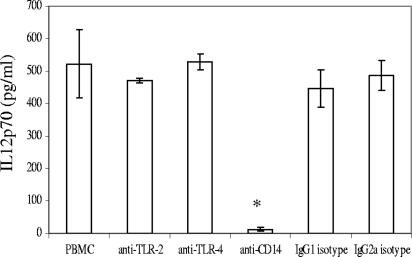
PBMC cultures stimulated with all of the oral streptococcal isolates for 20 h produced elevated titers of IL-12 p70, except S. oralis ATCC 35037 (Fig. 6). A statistically insignificant trend toward IL-12 production by L. casei was also apparent but IL-4 plus GM-CSF, M-CSF, A. viscosus, or P. intermedia did not result in an increase of IL-12 production in the 20-h period. The roles of pattern recognition receptors (TLR2 and TLR4) and CD14 in IL-12 induction were also investigated using specific receptor-blocking antibodies. Only anti-CD14 blocked IL-12 induction by S. mutans ATCC 25175, suggesting involvement of a receptor distinct from TLR2 or TLR4 (Fig. 7).
FIG. 6.
Induction of IL-12 p70 responses by PBMC cultures stimulated with oral streptococci. PBMC (106/ml) were stimulated with M-CSF, IL-4 plus GM-CSF, or bacteria in triplicate for 20 h. Supernatants were harvested and assayed for IL-12 concentration by ELISA. The vertical bars represent standard errors, and asterisks represent significant increases of IL-12 titers compared with the PBMC control. This experiment is representative of the results from three experiments with three different individuals.
FIG. 7.
IL-12 p70 production by PBMC was CD14 dependent. PBMC (106/ml) were stimulated with S. mutans ATCC 25175 (106/ml) in the presence of neutralizing antibodies for TLR2, TLR4, or CD14 for 20 h in triplicate. IgG2a and IgG1 isotype controls were included for TLRs and CD14 antibodies, respectively. The vertical bars represent standard deviations. Asterisks represent significant decreases in IL-12 induction. This experiment is representative of the results from three experiments with three different individuals. Controls indicated that the anti-TLR2 and anti-TLR4 antibodies were effective under the conditions of these experiments. While anti-TLR2 failed to inhibit IL-12 production, it did inhibit the S. mutans (106/ml)-induced IL-10 response (from 731.3 ± 101.9 pg/ml to 272.4 ± 71.11 pg/ml; P < 0.01). Furthermore, anti-TLR4 inhibited IL-12 production induced in PBMC by LPS (E. coli, 100 ng/ml) from 491.8 ± 54.03 pg/ml to 77.26 ± 5.519 pg/ml (P < 0.01).
Allogeneic mixed-lymphocyte reaction.
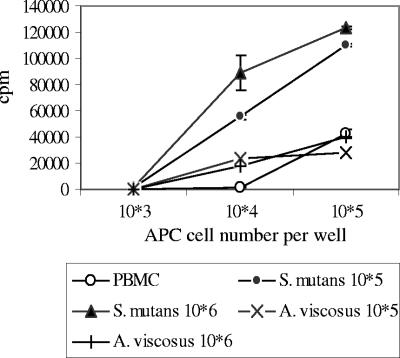
Mature DCs are potent stimulators of allogeneic T-cell proliferation, prompting us to reason that the mature DCs induced by streptococci should yield enhanced mixed-lymphocyte reactions. APC prepared by stimulating PBMC overnight with S. mutans were potent stimulators of allogeneic T cells compared with monocytes stimulated with A. viscosus or PBMC medium controls (Fig. 8) (P < 0.05 at 104 and 105 APC concentrations).
FIG. 8.
Enhanced allogeneic mixed-lymphocyte reaction by S. mutans-stimulated monocytes. APC preparations were derived from overnight stimulation of PBMC with S. mutans ATCC 25175 (105 and 106/ml) or A. viscosus (105 and 106/ml). T-cell preparations from different individual PBMC were obtained using microbeads. Three concentrations of APC (103, 104, and 105/well) were cocultured with T cells (105/well) for 5 days. The cultures were then pulsed with [3H]thymidine (1 μCi/well) for 18 h, harvested, and counted for radioactivity (cpm) using Top Count. The T-cell control had 1,422 ± 1,284 cpm. The vertical bars represent standard errors. This experiment is representative of the results from two experiments with four different individuals.
DISCUSSION
Monocytes are capable of differentiating into macrophages or dendritic cells, depending on the microenvironment. GM-CSF with IL-4 is known to induce DC differentiation, while M-CSF drives macrophage differentiation in vitro (10). As monocytes differentiate into immature DCs, CD14 expression is down-regulated (38). In the present study, exposure to gram-positive bacteria such as S. mutans and L. casei resulted in a rapid loss of CD14 expression on monocytes (<24 h), but this was not seen with A. viscosus or P. intermedia. A loss of CD14 by monocytes upon encountering bacteria has been reported by Karlsson et al. (21), and they demonstrated a greater down-regulation of CD14 on monocytes stimulated with Escherichia coli than with Lactobacillus plantarum. They suggested that the down-regulation of CD14 could be due to the fact that pattern recognition receptors are internalized together with the microbes or that activation induced negative feedback needed to control the inflammation.
The increases in CD83, MHC class II, and costimulatory molecules (CD86) observed in the present study support the concept that oral streptococci induce monocytes to differentiate into mature DCs. The dendritic cell activity of the streptococcus-stimulated monocytes was further confirmed by demonstration of high levels of IL-12 production and enhanced allogeneic mixed-lymphocyte reactions. The pattern of IL-12 induction correlated with the ability of the various streptococci to express CD83 and up-regulate CD86. The clinical isolate of S. mutans (v1311) from an endocarditis patient induced a level of costimulatory molecule up-regulation and IL-12 titer similar to those of ATCC type strain 25175. This finding indicates that a laboratory strain and a clinical strain of S. mutans behave similarly. ATCC strains were used in the present study to make it easy for others to confirm and extend our work. Two ATCC strains (S. sanguis ATCC 10556 and S. oralis ATCC 10557) were isolated from endocarditis patients, and both strains were potent DC inducers. Our data suggest that the ability to promote differentiation of monocytes to DCs that produce proinflammatory cytokines is likely widely associated with oral streptococci.
Compared to other streptococci, S. oralis strain ATCC 35037 induced low titers of IL-12 and was not able to up-regulate CD83 above background control levels. These data suggest that this strain differs in some important way from other oral streptococci, including another S. oralis strain (ATCC 10557). At this point, we do not know what is different about this strain. It would also follow that if DCs are important in the initiation of endocarditis, this strain would be less frequently involved in endocarditis than the other five streptococci. L. casei induced a pattern of phenotypic changes on monocytes similar to those of the five oral streptococcal isolates examined in this study. Interestingly, L. casei has also been identified in infected endocardial vegetation where it can serve as a pathogen (39, 40).
Adherent monocytes could also be stimulated to express CD83 when incubated with S. mutans but not with A. viscosus or P. intermedia (data not shown). However, DC differentiation and CD83 expression in the adherent cell cultures were modest compared to those of PBMC cultures (data not shown). These results suggest that interaction with other cell types in PBMC cultures, such as NK cells that are known to be able to rapidly produce gamma interferon and other cytokines, may contribute to DC maturation (23, 29).
Many microbial ligands and synthetic compounds that act on distinct TLRs are known to control DC maturation (e.g., mycobacterial extracts for TLR2 and TLR4, lipopolysaccharide for TLR4, and bacterial DNA and CpG deoxynucleotides for TLR9). Inflammatory cells express different TLRs that facilitate responses to different microbes. Monocytes preferentially express TLR1, 2, 4, 5, and 8, which may be found both intracellularly and on cell surfaces (34). TLR2 has been implicated mainly as the signaling receptor for gram-positive cell wall components (25), while TLR4 is associated with gram-negative bacteria. Activation of these two receptors results in IL-12 p70 production (28, 32). The finding that IL-12 induction by S. mutans was CD14 dependent suggests the involvement of a TLR, but anti-TLR2 and anti-TLR4 had no inhibitory effect. Henneke et al. reported that activation of macrophages by group B streptococcus in vitro is also independent of TLR2 and TLR4 but is dependent on CD14 and myeloid differentiation factor 88 (16). It is also known that heat-killed Streptococcus pyogenes can stimulate immature DCs to express CD83 and produce IL-12, but S. pyogenes did not bind to TLR2 or TLR4 but to β2 integrins, such as CD11c and CD18 (27). Thus, it is possible that multiple TLRs or other receptors are activated by S. mutans, and therefore, the blockade with anti-TLR2 and/or anti-TLR4 does not prevent the activation of other TLRs. Immature DCs lack membrane CD14 but are able to respond to low doses of LPS and produce IL-12 via use of soluble CD14 (37). Soluble CD14 released by monocytes in our PBMC cultures is likely important in stimulating DC differentiation and maturation by oral streptococci. Adding DNase to the PBMC cultures with S. mutans reduced the IL-12 titer by about 40% (data not shown), prompting the speculation that TLR9 might play a role in IL-12 production. Mature DCs can also be generated from immature DCs through pathways distinct from TLRs, including Fcγ receptors for immune complexes (30) and CD40L (8), suggesting a TLR-independent pathway might be used by streptococci. Streptococci such as S. gordonii and S. pyogenes are potent inducers of DC maturation from immature DCs in vitro, but the molecules involved have not been defined (11, 19).
Viridans group streptococci are commensal oral bacteria and are present in saliva, dental caries, and periodontal plaques. They are extracellular bacteria and are typically thought of as having low virulence. However, when they are out of their normal habitat and in the bloodstream as a consequence of chewing, brushing, or dental procedures, they can adhere to damaged endothelial cells or fibrin clots (vegetations) and become the main pathogens in infective endocarditis (12, 22). Bacteremias are usually cleared by the reticuloendothelial system within minutes (12). Certain bacteria such as enteric gram-negative rods frequently cause bacteremias but rarely cause endocarditis. In contrast, the various oral streptococci are isolated from only one-third of dental trauma-induced bacteremias (31), but they account for 45 to 80% of cases of native valve endocarditis in humans (4, 36). It appears that oral streptococci can uniquely avoid the clearance by the reticuloendothelial system and are capable of adhering to and proliferating in endocardial vegetations. Data presented here indicate that mature DCs can be rapidly generated from monocytes upon encountering oral streptococci. Thus, blood monocytes could serve as the protective vehicles that deliver viable streptococci to infection-susceptible sites and, as a consequence, promote endocarditis.
Mature DCs exhibit large numbers of adhesion molecules such as ICAM-1, ICAM-2, and LFA-1, which could contribute to their ability to adhere to injured endothelium or fibrinogen in blood clots (15). Mature DCs also exhibit significantly higher titers of tissue factor than macrophages in vitro (5) and could thus facilitate intravascular coagulation and vegetation formation. Furthermore, mature DCs have a short half-life, as demonstrated by our data and those of others (18, 20), which would explain why the numerous monocytes initially associated with endocarditis disappear rather than becoming long-lived macrophages. Studies of pathogenesis of streptococcal endocarditis have mainly focused on bacterial virulence factors, which are important in bacterial adherence to platelets or fibrin clots in vegetation. Our results suggest that alterations in the immune response of the host could also contribute to the infectious process. Studies of the fate of internalized oral streptococci in short-lived DCs may be helpful in further elucidating the possible role of streptococcus-infected monocytes/DCs in the initiation and progression of streptococcal endocarditis.
Acknowledgments
We gratefully acknowledge Kimberly Lake and Gail Smith for clinical management of the subjects participating in this study.
This work was supported by grant DE13102 from the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Editor: J. D. Clements
REFERENCES
- 1.Bancsi, M. J., J. Thompson, and R. M. Bertina. 1994. Stimulation of monocyte tissue factor expression in an in vitro model of bacterial endocarditis. Infect. Immun. 62:5669-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancsi, M. J., M. H. Veltrop, R. M. Bertina, and J. Thompson. 1996. Influence of monocytes and antibiotic treatment on tissue factor activity of endocardial vegetations in rabbits infected with Streptococcus sanguis. Infect. Immun. 64:448-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bancsi, M. J., M. H. Veltrop, R. M. Bertina, and J. Thompson. 1996. Role of phagocytosis in activation of the coagulation system in Streptococcus sanguis endocarditis. Infect. Immun. 64:5166-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayliss, R., C. Clarke, C. M. Oakley, W. Somerville, A. G. Whitfield, and S. E. Young. 1983. The microbiology and pathogenesis of infective endocarditis. Br. Heart J. 50:513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broussas, M., P. Cornillet-Lefebvre, J. Bernard, J. C. Adjizian, G. Potron, and P. Nguyen. 2000. Separation of dendritic cells from highly purified human monocytes by counterflow centrifugation induces tissue factor expression. Transfusion (Paris) 40:1088-1094. [DOI] [PubMed] [Google Scholar]
- 6.Buiting, A. G., J. Thompson, D. van der Keur, W. C. Schmal-Bauer, and R. M. Bertina. 1989. Procoagulant activity of endocardial vegetations and blood monocytes in rabbits with Streptococcus sanguis endocarditis. Thromb. Haemost. 62:1029-1033. [PubMed] [Google Scholar]
- 7.Carrizosa, J., K. Kaye, and W. Kobasa. 1978. Experimental streptococcal endocarditis. The early vegetation. Arch. Pathol. Lab. Med. 102:518-521. [PubMed] [Google Scholar]
- 8.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chia, J. S., Y. L. Lin, H. T. Lien, and J. Y. Chen. 2004. Platelet aggregation induced by serotype polysaccharides from Streptococcus mutans. Infect. Immun. 72:2605-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomarat, P., J. Banchereau, J. Davoust, and A. K. Palucka. 2000. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 1:510-514. [DOI] [PubMed] [Google Scholar]
- 11.Corinti, S., D. Medaglini, A. Cavani, M. Rescigno, G. Pozzi, P. Ricciardi-Castagnoli, and G. Girolomoni. 1999. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 163:3029-3036. [PubMed] [Google Scholar]
- 12.Debelian, G. J., I. Olsen, and L. Tronstad. 1994. Systemic diseases caused by oral microorganisms. Endod. Dent. Traumatol. 10:57-65. [DOI] [PubMed] [Google Scholar]
- 13.Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis. II. Survival of a bacteria in endocardial vegetations. Br. J. Exp. Pathol. 53:50-53. [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn, C. L., A. M. Best, and J. G. Tew. 2000. Cytokine induction by Streptococcus mutans and pulpal pathogenesis. Infect. Immun. 68:6785-6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harley, S. L., J. Sturge, and J. T. Powell. 2000. Regulation by fibrinogen and its products of intercellular adhesion molecule-1 expression in human saphenous vein endothelial cells. Arterioscler. Thromb. Vasc. Biol. 20:652-658. [DOI] [PubMed] [Google Scholar]
- 16.Henneke, P., O. Takeuchi, R. Malley, E. Lien, R. R. Ingalls, M. W. Freeman, T. Mayadas, V. Nizet, S. Akira, D. L. Kasper, and D. T. Golenbock. 2002. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and -independent signaling pathways. J. Immunol. 169:3970-3977. [DOI] [PubMed] [Google Scholar]
- 17.Herzberg, M. C. 1996. Platelet-streptococcal interactions in endocarditis. Crit. Rev. Oral Biol. Med. 7:222-236. [DOI] [PubMed] [Google Scholar]
- 18.Holt, P. G., S. Haining, D. J. Nelson, and J. D. Sedgwick. 1994. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J. Immunol. 153:256-261. [PubMed] [Google Scholar]
- 19.Itoh, T., Y. Ueda, K. Okugawa, H. Fujiwara, N. Fuji, T. Yamashita, H. Fujiki, S. Harada, T. Yoshimura, and H. Yamagishi. 2003. Streptococcal preparation OK432 promotes functional maturation of human monocyte-derived dendritic cells. Cancer Immunol. Immunother. 52:207-214. (First published 25 February 2003; doi: 10.1007/s00262-002-0337-8.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamath, A. T., S. Henri, F. Battye, D. F. Tough, and K. Shortman. 2002. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 100:1734-1741. [PubMed] [Google Scholar]
- 21.Karlsson, H., P. Larsson, A. E. Wold, and A. Rudin. 2004. Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect. Immun. 72:2671-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy, H. F., D. Morrison, D. Tomlinson, B. E. Gibson, J. Bagg, and C. G. Gemmell. 2003. Gingivitis and toothbrushes: potential roles in viridans streptococcal bacteraemia. J. Infect. 46:67-70. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi, T., C. L. Hahn, S. Tanaka, S. E. Barbour, H. A. Schenkein, and J. G. Tew. 2004. Dendritic cells stimulated with Actinobacillus actinomycetemcomitans elicit rapid gamma interferon responses by natural killer cells. Infect. Immun. 72:5089-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitten, T., C. L. Munro, S. M. Michalek, and F. L. Macrina. 2000. Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect. Immun. 68:4441-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 26.Munro, C. L., S. M. Michalek, and F. L. Macrina. 1995. Sucrose-derived exopolymers have site-dependent roles in Streptococcus mutans-promoted dental decay. FEMS Microbiol. Lett. 128:327-332. [DOI] [PubMed] [Google Scholar]
- 27.Nakahara, S., T. Tsunoda, T. Baba, S. Asabe, and H. Tahara. 2003. Dendritic cells stimulated with a bacterial product, OK-432, efficiently induce cytotoxic T lymphocytes specific to tumor rejection peptide. Cancer Res. 63:4112-4118. [PubMed] [Google Scholar]
- 28.Nau, G. J., A. Schlesinger, J. F. Richmond, and R. A. Young. 2003. Cumulative Toll-like receptor activation in human macrophages treated with whole bacteria. J. Immunol. 170:5203-5209. [DOI] [PubMed] [Google Scholar]
- 29.Pan, J., M. Zhang, J. Wang, Q. Wang, D. Xia, W. Sun, L. Zhang, H. Yu, Y. Liu, and X. Cao. 2004. Interferon-gamma is an autocrine mediator for dendritic cell maturation. Immunol. Lett. 94:141-151. [DOI] [PubMed] [Google Scholar]
- 30.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogosa, M., E. G. Hampp, T. A. Nevin, H. N. Wagner, E. J. Driscoll, and P. N. Baer. 1960. Blood sampling and cultural studies in the detection of postoperative bacteremias. J. Am. Dent. Assoc. 60:171-180. [DOI] [PubMed] [Google Scholar]
- 32.Thoma-Uszynski, S., S. M. Kiertscher, M. T. Ochoa, D. A. Bouis, M. V. Norgard, K. Miyake, P. J. Godowski, M. D. Roth, and R. L. Modlin. 2000. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J. Immunol. 165:3804-3810. [DOI] [PubMed] [Google Scholar]
- 33.Thorig, L., J. Thompson, F. Eulderink, J. J. Emeis, and R. Van Furth. 1980. Effects of monocytopenia and anticoagulation in experimental Streptococcus sanguis endocarditis. Br. J. Exp. Pathol. 61:108-116. [PMC free article] [PubMed] [Google Scholar]
- 34.Uronen-Hansson, H., J. Allen, M. Osman, G. Squires, N. Klein, and R. E. Callard. 2004. Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: integrity of microtubules is required for interleukin-12 production in response to internalized bacteria. Immunology 111:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Rijn, I., M. George, A. Bouvet, and R. B. Roberts. 1986. Enzyme-linked immunosorbent assay for the detection of antibodies to nutritionally variant streptococci in patients with endocarditis. J. Infect. Dis. 153:116-121. [DOI] [PubMed] [Google Scholar]
- 36.van der Meer, J. T., W. van Vianen, E. Hu, W. B. van Leeuwen, H. A. Valkenburg, J. Thompson, and M. F. Michel. 1991. Distribution, antibiotic susceptibility and tolerance of bacterial isolates in culture-positive cases of endocarditis in The Netherlands. Eur. J. Clin. Microbiol. Infect. Dis. 10:728-734. [DOI] [PubMed] [Google Scholar]
- 37.Verhasselt, V., C. Buelens, F. Willems, D. De Groote, N. Haeffner-Cavaillon, and M. Goldman. 1997. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J. Immunol. 158:2919-2925. [PubMed] [Google Scholar]
- 38.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249-255. [DOI] [PubMed] [Google Scholar]
- 39.Wallet, F., R. Dessein, S. Armand, and R. J. Courcol. 2002. Molecular diagnosis of endocarditis due to Lactobacillus casei subsp. rhamnosus. Clin. Infect. Dis. 35:e117-119. (First published 28 October 2002; doi: 10.1086/344181). [DOI] [PubMed] [Google Scholar]
- 40.Ze-Ze, L., R. Tenreiro, A. Duarte, M. J. Salgado, J. Melo-Cristino, L. Lito, M. M. Carmo, S. Felisberto, and G. Carmo. 2004. Case of aortic endocarditis caused by Lactobacillus casei. J. Med. Microbiol. 53:451-453. [DOI] [PubMed] [Google Scholar]