Abstract
Predominant T-helper 1 (Th1) responses with increased gamma interferon (IFN-γ) levels have been proposed to play an important role in Helicobacter pylori-induced gastritis and peptic ulceration. However, bacterial factors contributing to the initiation of Th1 polarization of H. pylori-specific immune responses have not been characterized in detail thus far. We report here on the identification of Helicobacter cysteine-rich protein A (HcpA) as a novel proinflammatory and Th1-promoting protein. The capacity of HcpA to induce immune activation was studied in splenocyte cultures of naive H. pylori-negative mice. HcpA stimulated the release of high concentrations of the proinflammatory and Th1-promoting cytokines interleukin-6 (IL-6) and IFN-γ, in addition to significant levels of IL-12, tumor necrosis factor alpha, and IL-10. The observed cytokine profile was comparable to that induced by lipopolysaccharide but differed in the kinetics and maximum levels of cytokine production. In addition, HcpA-induced cytokine release resembled that observed upon incubation with H. pylori except for IL-10, which was only moderately released upon HcpA stimulation. Both HcpA- and H. pylori-mediated IFN-γ production was drastically reduced by a neutralizing antibody against IL-12 but not by an anti-IL-2 antibody. Thus, HcpA seems to represent a novel bacterial virulence factor triggering the release of a concerted set of cytokines to instruct the adaptive immune system for the initiation of proinflammatory and Th1-biased immunity.
Helicobacter pylori is a spiral-shaped, gram-negative microorganism, which permanently colonizes the gastric lumen of humans. Colonization of the gastric mucosa is associated with chronic active gastritis (32) and increases the risk of developing peptic ulceration (5), gastric adenocarcinoma (38, 40, 49), and mucosa-associated lymphoid tissue (MALT) lymphoma (39, 53). However, despite intensive investigations for more than 2 decades, the molecular mechanisms underlying long-term persistence of H. pylori within the gastric epithelium, as well as initiation of inflammation and disease progression, remain only incompletely understood (6, 33). Dominant T-helper 1 (Th1)-polarized immune responses have been suggested to enable H. pylori to evade elimination by the immune system and to persist lifelong. CD4+ T cells from-infected ulcer patients show a typical Th1 phenotype (10, 21, 45), and excess local gamma interferon (IFN-γ) production induced in response to H. pylori infection is thought to be a key mediator in driving H. pylori-induced gastritis. Consistent with these observations, no gastric inflammation was observed in IFN-γ-deficient mice upon H. pylori infection (43). Although H. pylori has been reported to stimulate the secretion of IFN-γ from natural killer (NK) cells in vitro (51) as well as the production of interleukin-12 (IL-12) from human peripheral blood mononuclear cells (PBMC) (1) and dendritic cells (16, 19, 22, 28), the molecular mechanisms of H. pylori-induced Th1-polarization remain enigmatic.
There is accumulating evidence that an interaction of distinct bacterial components with cellular effectors of the host's innate immune system may essentially contribute to the induction of H. pylori-induced inflammation and Th1 polarization of immune responses (2, 9, 13, 44), thereby orchestrating the full virulence phenotype. A number of secreted proteins of H. pylori have been recently identified as important virulence factors that induce distinct pathogen-host interactions during the course of infection. The most prominent examples for secreted proteins with immunomodulatory activities are the vacuolating cytotoxin A (VacA) and urease (3, 15, 17, 23, 48, 50).
An actual systematic study of secreted proteins (the secretome) has identified additional putative proteins that might be involved in mediating the pathogenic virulence phenotype of H. pylori (7). In addition, Cao et al. (8) identified several immunogenic extracellular H. pylori proteins in the supernatant of broth cultures. One of these proteins was the Helicobacter cysteine-rich protein A (HcpA) encoded by the open reading frame (ORF) hp0211. The family of Helicobacter cysteine-rich proteins (Hcp) is unique to the Helicobacter and Campylobacter genera and comprises HP0160, HP0211 (HcpA), HP0235, HP0336, HP0628, HP1098, HP1117, JHP0318, JHP01437, and CJ0413 (35). Furthermore, H. hepaticus also encodes several cysteine-rich proteins with homologies to members of the Hcp family (HH0143/144, HH0718, HH0816, HH1222, and HH1827) (47). In addition, very recently, a novel putative member of the Hcp family has been identified by our group within the genome of Wolinella succinogenes, termed WcpA (NCBI accession number BX571656). In HcpA six pairs of cysteine residues, which are separated by exactly seven amino acid residues, form disulfide bridges (34). Although no in vivo function has been assigned to members of the Hcp family thus far, it could be demonstrated that HcpA (HP0211), HcpB (HP0336), and HcpD (HP0160) possess weak penicillin-binding activities (31, 34). In a comprehensive immunoproteomics study, increased HcpC antibody titers were detected in sera of patients with gastric adenocarcinoma (20). In addition, antibodies against HcpA, HcpB, HcpC, and HcpE were found in most H. pylori-infected individuals (35). These results clearly indicated that different Hcp proteins are expressed in the course of H. pylori infection and recognized by the immune system of infected individuals.
In our study we aimed to investigate suspected immunomodulatory properties of recombinant HcpA in cultures of murine splenocytes, which have been repeatedly used as a model system to determine innate immune activation by bacterial components. Using splenic cells of H. pylori-negative naive mice, we found that both recombinant HcpA and H. pylori are potent activators of innate immune responses, as demonstrated by stimulating the release of substantial levels of proinflammatory and Th1-promoting cytokines. We found here that production of IFN-γ in response to stimulation with recombinant HcpA and H. pylori was substantially inhibited by an anti-IL-12 antibody. The present data strongly suggest HcpA to be a novel secreted virulence factor, which may contribute to H. pylori-mediated pathogenicity by adjusting a nonprotective Th1-biased immunity.
MATERIALS AND METHODS
Antibodies and polyclonal sera.
Neutralizing goat anti-mouse IL-12 (I7642) and IL-2 immunoglobulin G (IgG; I8398) fractions of antisera were obtained from Sigma-Aldrich, Taufkirchen, Germany. A polyclonal HcpA-specific rabbit serum was raised by immunizing a Chinchilla White rabbit (Charles River, Kisslegg, Germany) at an age of 3 months with 500 μg of purified HcpA protein adjuvanted with Titermax (Sigma-Aldrich, Steinheim, Germany) and boosting the animal with the same immunogen at weeks 6 and 10 after the primary injection. The rabbit was bled 3 weeks after the third injection, and titers of HcpA-specific antibodies were quantified by an endpoint dilution enzyme-linked immunosorbent assay (ELISA) as described previously in detail (11).
The obtained serum reached HcpA-specific endpoint titers of greater than 1:1,000,000. The specificity of the produced rabbit serum for HcpA was demonstrated by analyzing cytosolic and periplasmic protein fractions from the parental H. pylori strains 60190 and 2802, as well as the isogenic hcpA-deficient strain 2802 hcpA::cat. Here a protein with an apparent molecular mass of 24 kDa, representing the processed form of HcpA lacking its N-terminal signal sequence was detected in both preparations from H. pylori strains 2802 and 60190 but was absent in the isogenic knockout strain 2802 hcpa::cat (see Fig. 6).
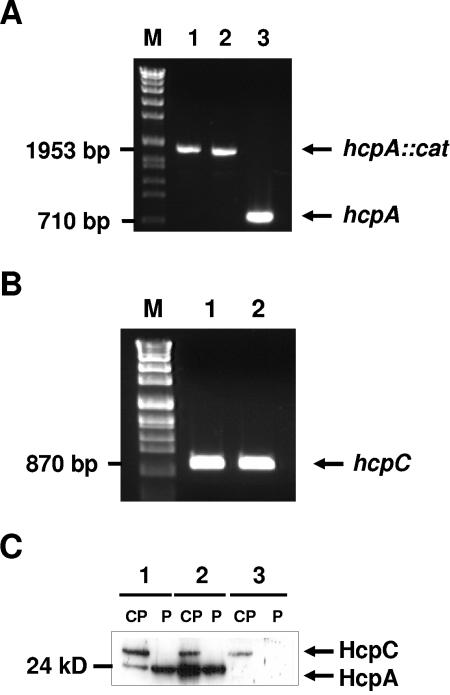
FIG. 6.
Generation and characterization of an isogenic HcpA-deficient H. pylori mutant strain. (A) PCR analysis of two transformants of HcpA mutagenesis (lanes 1 and 2) confirmed a larger amplicon in 2802 hcpA::cat due to the presence of cat resistance gene in the hcpA locus in comparison to the H. pylori parental strain 2802 (lane 3). (B) Amplification of hcpC gene in 2802 hcpA::cat (lane 1) and 2802 (lane 2) was performed to verify the specificity of the hcpA mutagenesis. (C) Comparative immunoblot analysis of HcpA expression in cytoplasmic (CP) and periplasmic (P) fractions of H. pylori 2802 (lane 2) and the isogenic 2802 hcpA::cat H. pylori strain (lane 3) with H. pylori 60190 (lane 1).
As can be also seen in Fig. 2B and C and in Fig. 6C, an additional protein was detected by the HcpA antiserum in cytosol and supernatant, respectively. We suggest that this protein might be HcpC because of the predicted molecular mass of ca. 30 kDa and the presence of a common B-cell epitope within HcpA and HcpC (M. Aigner et al., in preparation). These results were confirmed by immunoblotting experiments revealing a reactivity of the rabbit anti-HcpA serum against recombinant HcpA and HcpC (data not shown).
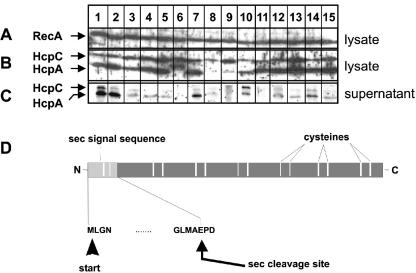
FIG. 2.
Expression and secretion of HcpA in different H. pylori strains. (A) Expression of the strictly cytoplasmic protein RecA in H. pylori lysates was determined as a loading control. (B and C) HcpA expression and secretion were investigated in H. pylori whole cellular lysates (B) or cell-free culture supernatants (C) by immunoblotting with a HcpA-specific rabbit serum. Lanes 1 to 4 and 7, CagA-positive H. pylori strains from ulcer patients; lane 5, CagA-positive isolate of a carcinoma patient; lanes 6 and 8, CagA-positive strains from MALT lymphoma patients; lanes 9, 10, and 15, CagA-negative strains from gastritis patients; lanes 11 to 14, CagA-negative isolates from carcinoma patients. The band beyond HcpA represents HcpC, which also belongs to the gene family 12 and shows the highest degree of similarity to HcpA among all Hcp members. (D) Schematic illustration of the hcpA ORF indicating the positions of the signal peptide and the putative internal cleavage site.
Cultivation of H. pylori.
H. pylori was grown on Wilkins-Chalgren agar plates (Oxoid, Basingstoke, United Kingdom) containing 10% lysed defibrinated horse blood (Elocin-Lab, Mühlheim an der Ruhr, Germany) and 25 mg of H. pylori selective supplement (DENT; Oxoid)/liter in a microaerophilic atmosphere (80% N2, 11% O2, 9% CO2) at 36°C. For in vitro cell stimulation experiments, H. pylori was harvested after 48 h with a sterile cotton swab, suspended, and washed three times in ice-cold phosphate-buffered saline (PBS). Then bacteria were dissolved in PBS at serial dilutions ranging from 103 to 108 bacterial cells/ml in ice-cold PBS. The solution's optical density at 600 nm (OD600) was measured and used to calculate the number of H. pylori per ml by using a factor determined earlier by serial dilutions (1 OD600 = 2.28 × 108 H. pylori/ml). The bacterial strain used in the stimulation experiments was H. pylori 2802, a clinical isolate from a patient suffering from ulcer disease. H. pylori 2802 has been previously reported to induce substantial IL-8 secretion from gastric epithelial cells (19). The presence of the cagA gene was determined by PCR as described previously (19).
Generation of an isogenic HcpA deletion in H. pylori 2802.
HcpA gene disruption was generated by sequence-specific insertion of a chloramphenicol resistance (cat) cassette into the hcpA ORF of H. pylori 2802. The hp0211 gene was amplified from genomic DNA of H. pylori 2802 by PCR by using forward primer hp211A (5′-GACACAGGCATATGCTAGGAAACGTTAAAAAAACCC-3′) and reverse primer hp211B (5′-GGACTCGAGAAGTTCTATTTTTAATTCCTTG-3′). The obtained PCR product was blunt end cloned into a SmaI-digested pUC18 plasmid according to standard procedures. The resulting plasmid pUChcpA was used as a template for a linearizing PCR with primers hp211Cfor (5′-TAAATCTTGTGAATTGAACCATGC-3′) and hp211Drev (5′-GAGTAATATTGTGAGGCTTTTTTGG-3′). Both oligonucleotides bind within the center of the hcpA gene and are oriented outward. After 18 PCR cycles template plasmid pUC18hcpA was removed by DpnI digestion, and the PCR-amplified linear pUC18hcpA fragment was purified by gel extraction according to the manufacturer's instructions (QIAGEN, Hilden, Germany). A linear cat cassette consisting of the chloramphenicol acetyltransferase gene (cat) was amplified from plasmid pHel2 (24) by PCR with the primers cat-for (5′-TAACTGGAGCATCGATAAGCTTCTAGAGAT-3′) and cat-rev (5′-TATGGTACCTTAAGGGCACCAATAAC-3′) and then blunt end ligated with the linear pUC18hcpA fragment to generate plasmid pUChcpA::cat. Then, a hcpA::cat fragment flanked by 370-bp terminal arms homologous to the desired target site within hcpA was PCR amplified by using oligonucleotides hp211A and hp211B and introduced into H. pylori 2802 by natural transformation. For this purpose, 107 logarithmically growing H. pylori (OD600 = 0.2) were suspended in 1 ml of brucella broth (Difco, Detroit, MI) containing 10% fetal calf serum, and each 1 μg of linear hcpA::cat fragment was added at h 0, 2, and 4 and then incubated for two additional hours. Transformants were selected on Wilkins-Chalgren (Oxoid) agar plates supplemented with 10% defibrinized horse blood, DENT, and 6 μg of chloramphenicol per ml. The hcpA disruption by cat insertion within H. pylori mutant strain 2802 hcpA::cat was verified by PCR analysis and sequencing, as well as by Southern blotting and immunoblotting.
Cloning, expression and purification of HcpA.
The bacterial pRBI-PDI system (54) has been applied for the production of native H. pylori HcpA in E. coli. The hcpA gene lacking the coding region of the endogenous signal peptide (Fig. 2D) was amplified from H. pylori strain 60190 and cloned into the pRBI-PDI plasmid 3′ to the sequence of an E. coli outer membrane protein signal peptide. Therefore, the hcpA-coding region was amplified by PCR from purified genomic DNA of H. pylori 60190 by using oligonucleotides HcpAfor (5′-CCGAGCCAGACGCTAAAGAGC-3′) and HcpArev (5′-TTTAAGCTTTCAGAGAAGTTCTATTTTCAATTCC-3′). Plasmid pRBI-PDI was linearized with StuI and HindIII, and 3′ extensions were blunted by treatment with T4-DNA polymerase and dephosphorylated prior to ligation with the PCR-amplified hcpA gene. The vector pRBI-PDIhcpA obtained was verified by sequencing.
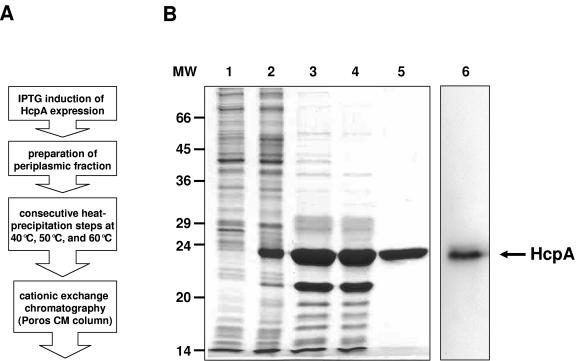
For the expression of HcpA plasmid pRBI-PDIhcpA was transformed into Escherichia coli JM83, and bacteria were induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and 5 mM N-acetylcysteine (Sigma, Deisenhofen, Germany) for 10 h at 25°C. Bacterial cells were harvested by centrifugation (5,000 × g for 15 min at 4°C). The obtained pellet was washed in ice-cold polymyxin B buffer (10 mM MOPS [pH 6.8], 150 mM NaCl, 5 mM EDTA), resuspended on ice in polymyxin B solution (1 mg of polymyxin B [Fluca, Neu-Ulm, Germany]/ml in polymyxin B buffer), and incubated for 1 h on ice. The remaining bacterial cells were then sedimented at 20,000 × g for 30 min at 4°C. The supernatant was subsequently heat precipitated at 40, 50, and 60°C with intervening centrifugation steps for 30 min with 14,000 rpm at 4°C. The resulting supernatant was dialyzed against 50 mM morpholineethanesulfonic acid (MES; pH 6.4). HcpA was then purified by cation-exchange chromatography by using a Poros CM column on a BioCAD 700E fast-performance liquid chromatography gadget (PerSeptive Biosystems, Weiterstadt, Germany) and elution with a 0 to 1 M NaCl gradient. Purity and HcpA content at subsequent purification steps were investigated by using Coomassie blue-stained sodium dodecyl sulfate (SDS)-polyacrylamide gels (Fig. 1B, lanes 2 to 5) and Western blotting (immunoblotting) with a polyclonal rabbit anti-HcpA serum (Fig. 1B, lane 6). The described purification procedure (Fig. 1A) resulted in a protein with an apparent molecular mass of ca. 24 kDa, as expected for N terminally processed HcpA protein with a purity of >95% (Fig. 1B, lane 5). For negative control, E. coli cells producing the α-amylase/trypsin inhibitor (RBI) protein were treated by the same stimulation and purification protocol as described for HcpA-producing E. coli. The resulting sample was entitled the RBI fraction. Identical volumes of purified HcpA and RBI fractions were comparatively used to analyze the influence of E. coli-derived impurities in stimulation assays.
FIG. 1.
Expression and purification of HcpA protein. (A) Flow diagram of HcpA purification process. (B) Coomassie blue-stained SDS-PAGE of HcpA at various purification stages. Lanes: 1, lysate of noninduced cells; 2, lysates of cells 10 h after induction with 1 mM IPTG; 3, periplasmic fraction; 4, heat-precipitated periplasmic protein fraction; 5, HcpA-positive peak fractions obtained from cation-exchange chromatography; 6, immunoblot analysis of purified HcpA by using a HcpA-specific polyclonal rabbit serum. The arrow at the right indicates the position of HcpA. The position of the markers (MW) is given on the left (in kilodaltons).
The generation of a stably transfected Drosophila-Schneider-2 (DS-2) cell line secreting high yields of HcpA (DS-2-HcpA) will be described elsewhere in detail (L. Deml, unpublished data). Briefly, stable transfectants were generated by lipofection by using a transfection deposit containing the expression vector pMt-HcpA and selection plasmid pA5c-DHFR (12) in a molar ratio of 20:1 and subsequent methotrexate selection. The resulting stable transfected DS-2-cell-line (DS2-HcpA) produced and secreted high levels of HcpA (>60 mg/liter) to the serum-free cell culture medium. For HcpA production, DS-2-HcpA cells were grown in cell culture flasks (75-cm2 in surface area; Becton Dickinson, Franklin Lakes, NJ) with 12 ml of serum-free insect XPRESS medium (Cambrex, Walkersville, MD). Cupric sulfate (200 mM) was added at day 2 to the culture medium to induce HcpA expression, and cultures were continued for another 6 to 8 days at 25 to 27°C. The culture supernatant was then precleared by low-speed centrifugation and dialyzed against 50 mM MES (pH 6.4) by using a Spektrapore dialysis membrane (3.5-kDa cutoff). HcpA was further purified from the dialyzed supernatant by cation-exchange chromatography on a Poros CM column (PerSeptive Biosystems) using a 0 to 20% linear gradient of 1 M NaCl. The HcpA protein, eluting at ca. 500 mM NaCl, was collected and dialyzed against 20 mM Tris-HCl (pH 7.5)-50 mM NaCl. This purification procedure resulted in HcpA with a purity of >95%. Lipopolysaccharide (LPS) content in all E. coli and DS-2-HcpA cell-derived HcpA preparations was <0.10 endotoxin units/mg of HcpA (<10 pg/mg of HcpA) as determined by a Limulus amoebocyte lysate QLC-1000 kit (Whittaker Bioproducts, Walkersville, MD).
SDS-PAGE and Western blot analysis.
Bacterial cells or proteins at various stages of purification were denatured in SDS sample puffer for 5 min at 100°C and separated on 15% SDS polyacrylamide gels, and individual proteins were visualized by Coomassie blue staining and immunoblotting. Therefore, proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), which were probed sequentially with a polyclonal rabbit anti-HcpA serum (1:50,000 in 2% nonfat dry milk in PBS; Bio-Rad, Munich, Germany) and a horseradish-peroxidase conjugated goat anti-rabbit IgG antibody (1:3,000 in 2% nonfat dry milk in PBS). Proteins were visualized by using a chemiluminescence Western kit (NOWA; Energene, Regensburg, Germany).
Detection of cytokines.
Female 5- to 6-week old, specific-pathogen-free, Helicobacter species-negative C57BL/6 and BALB/c mice were purchased from Charles River (Sulzfeld, Germany) and Harlan Winkelmann (Borchen, Germany) and housed under specific-pathogen-free conditions. All procedures involving mice were carried out in compliance with the regulations of the German animal protection law. Spleens of naive mice at an age of 50 to 100 days were recovered under sterile conditions, and the obtained splenic single cell suspensions were seeded at 2 × 106 cells per ml in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum and 1% penicillin-streptomycin (all from Gibco-BRL, Eggenstein, Germany) in the presence or absence of recombinant HcpA or H. pylori. As a positive control, splenic cells were stimulated with 5 μg of E. coli LPS (Sigma, Taufkirchen, Germany). If not otherwise indicated, supernatants were removed after 32 to 36 h to determine cytokine release from splenocytes.
Role of IL-12 and IL-2 in IFN-γ production by murine splenocytes.
Spleen cells from naive mice were stimulated with HcpA (2 μg/ml) or H. pylori (multiplicity of infection [MOI] = 1) in the presence or absence of the IgG fraction of a polyclonal goat anti-IL-12 (directed against p35 and p40) or anti-IL-2 antiserum (Sigma-Aldrich, Deisenhofen, Germany). At the indicated time points, concentrations of IL-4, IL-5, IL-6, IL-10, IL-12, tumor necrosis factor alpha (TNF-α), and IFN-γ were determined from the precleared supernatants using either OptEIA mouse cytokine sets (Becton Dickinson, Heidelberg, Germany) or the Bio-Plex protein array system (Bio-Rad) according to manufacturer's instructions.
Statistical analysis.
The results shown are from one single representative experiment (of at least three experiments) and expressed as mean ± the standard deviation (SD) of the mean. The data were analyzed by using the Wilcoxon test (MedCalc Software, Mariakerke, Belgium). P values of <0.05 were considered significant.
RESULTS
Production and secretion of HcpA by different clinical H. pylori isolates.
At present, hardly anything is known about expression and secretion of HcpA by H. pylori isolates. In order to compare the levels of HcpA expression in H. pylori strains isolated from patients suffering from different clinical entities, cultures of five CagA-positive H. pylori strains from ulcer patients (Fig. 2, lanes 1, 2, 3, 4, and 7), two CagA-positive strains from MALT lymphoma patients (lanes 6 and 8), and one CagA-positive (lane 5), as well as four CagA-negative isolates from carcinoma patients (lanes 11 to 14) and three CagA-negative stains from gastritis patients (lanes 9, 10, and 15), were lysed in a buffer containing 8 M urea and analyzed by immunoblotting with an HcpA-specific polyclonal rabbit serum (Fig. 2B and C). Cytosolic RecA protein served as a loading control (Fig. 2A). Here, a protein with an apparent molecular mass of 24 kDa, representing the processed form of HcpA lacking the N-terminal signal sequence was detectable in the lysates from 14 of 15 tested H. pylori strains. The identity of this band was confirmed by the fact that it is present in wt H. pylori 2802 (Fig. 2, lane 1, and Fig. 6C, lane 2) but absent in an isogenic 2802 hcpA::cat strain (Fig. 6C, lane 3). Analyzed H. pylori isolates differed remarkably in their HcpA expression profiles ranging from relatively weak (Fig. 2B, lanes 8 to 10) to substantial HcpA production (Fig. 2B, lanes 1 to 7 and 11 to 15). Interestingly, one H. pylori strain produced an anti-HcpA reactive protein with an increased molecular mass, most probably representing the unprocessed HcpA protein, including the N-terminal signal peptide (Fig. 2B, lane 6). In addition, a protein with an apparent molecular mass of 30 kDa was visualized in all lysates by the HcpA polyclonal antiserum. This band identified as HcpC, an additional member of the Hcp family, which shows the highest sequence homology to HcpA (see Materials and Methods for details). In order to analyze the secretion levels of HcpA, proteins were precipitated from precleared liquid cultures of all H. pylori isolates by sodium deoxycholate-trichloroacetic acid precipitation, and the HcpA content was determined by immunoblotting (Fig. 2C). HcpA secretion was seen in almost all tested H. pylori isolates, but the amounts of secreted HcpA varied significantly between individual isolates. Three H. pylori strains exhibited a strong HcpA secretion (Fig. 2C, lanes 1, 2, and 7) compared to a medium or weak HcpA release observed in eight (lanes 3 to 5, 10, and 12 to 15) and three (lanes 8, 9, and 11) H. pylori isolates, respectively. In two of three H. pylori strains poor HcpA secretion correlated with low HcpA expression (Fig. 2B, lanes 8 and 9), but one H. pylori isolate (Fig. 2B, lane 11) showed a weak release despite strong HcpA expression. No detectable HcpA secretion was observed from the H. pylori isolate producing the elongated anti-HcpA reactive protein (Fig. 2B, lane 6). The mechanisms underlying these obvious differences in HcpA secretion are yet not clear and remain to be elucidated in more detail. However, HcpA release was not due to cell lysis since RecA, a strictly cytoplasmic protein (8), was not detectable from the culture supernatants (data not shown).
Recombinant HcpA induces the secretion of Th1-type and proinflammatory cytokines from splenic cells of naive mice.
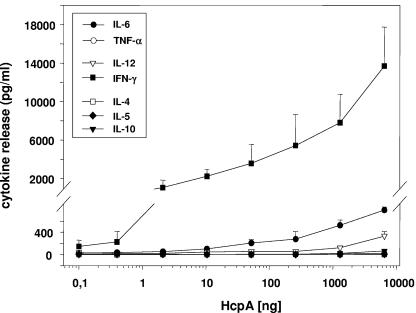
The capacity of H. pylori HcpA to activate innate immune responses was analyzed by using the murine splenocyte model system. Here, single-cell cultures of splenocytes from naive H. pylori-negative BALB/c mice were stimulated with increasing concentrations (0.1 to 10,000 ng/ml) of purified recombinant HcpA, and the production of cytokines was used as a measure for activation of innate immune responses. Incubation of splenic cells from BALB/c (Fig. 3), as well as C57BL/6 mice (data not shown), with HcpA triggered the release of the Th1-polarizing cytokines IL-12 and IFN-γ and elevated levels of the proinflammatory cytokine IL-6. Surprisingly, at high concentrations HcpA also provoked an enhanced IL-10 production (Fig. 3). In contrast, murine splenocytes failed to produce detectable levels of typical Th2-promoting cytokines such as IL-4 and IL-5 in the presence of HcpA. In these experiments HcpA concentrations of 1 ng/ml were sufficient to stimulate a measurable IFN-γ release, whereas significant levels of IL-6, IL-12, and IL-10 were only seen in response to stimulation with HcpA concentrations greater than 50 ng, 1 μg, and 7 μg/ml, respectively. These results strongly suggest HcpA to be a very potent inducer of Th1-polarizing and proinflammatory cytokines from murine splenocytes.
FIG. 3.
Dose dependency of cytokine production induced by purified HcpA in cultures of splenic cells from naive BALB/c mice. Cells were incubated in triplicate with increasing HcpA concentrations, and after 36 h the cytokine levels were determined from the precleared culture supernatant by ELISA as essentially described in Materials and Methods. One representative experiment out of three is shown. The data represent means ± standard deviations (SD).
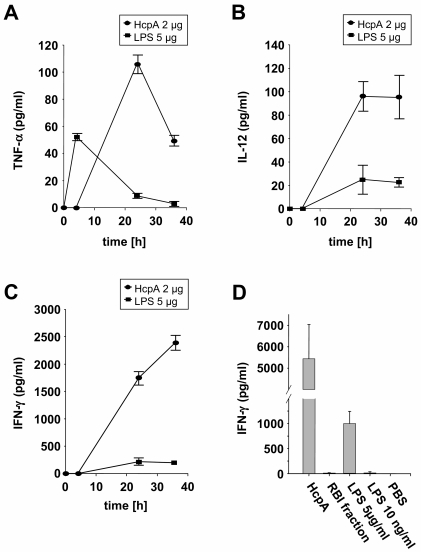
HcpA and LPS differ in the levels and kinetics of induced cytokines.
In order to compare the immunostimulatory properties of HcpA to that of a well-characterized pathogen-associated molecular pattern such as that of LPS, BALB/c mouse-derived splenocytes were stimulated either with 2 μg of E. coli-derived HcpA or 5 μg of E. coli LPS and supernatants were assayed by ELISA for the production of TNF-α, IL-12, and IFN-γ. We found that HcpA and LPS showed similar cytokine profiles but differed remarkably in the kinetics and maximum levels of the individual cytokines. At the tested dose HcpA was approximately two-, five- and tenfold more efficient than E. coli LPS to induce TNF-α (Fig. 4A), IL-12 (Fig. 4B), and IFN-γ (Fig. 4C) secretion, respectively. In addition, LPS and HcpA differed in the kinetics of the production of individual cytokines, in that maximum TNF-α levels were reached after 4 and 24 h, respectively (Fig. 4A).
FIG. 4.
Comparison of HcpA and LPS for the stimulation of cytokine release from splenic cells from naive BALB/c mice. Cells were incubated in triplicate with purified HcpA (2 μg/ml) or LPS (5 μg/ml), and the levels of TNF-α (A), IL-12 (B), and IFN-γ (C) were measured from the precleared culture supernatant at the indicated time points by ELISA essentially as described in Materials and Methods. (D) Murine splenocytes were stimulated for 36 h with purified HcpA (2 μg/ml), RBI fraction (2 μl), LPS (5 μg/ml or 10 ng/ml), and the concentrations of IFN-γ were determined by ELISA. PBS-stimulated splenocytes served as negative controls. One representative experiment out of three is shown. The data represent the mean values ± SD.
Next, we sought to investigate the role of traces of contaminating producer cell-derived substances for the observed immunostimulatory properties of the HcpA protein. An examination of E. coli-derived purified HcpA by the Limulus amebocyte lysate QLC-1000 kit (Whittaker) revealed that the LPS content was <0.10 endotoxin units/mg of HcpA (<10 pg/mg of HcpA). In order to analyze the influence of that highest possible concentration of LPS within the HcpA preparation, splenic cells were stimulated for 36 h with 2 μg of HcpA/ml and 10 pg of LPS/ml and then analyzed for the secretion of IFN-γ. For controls, cells were stimulated with LPS at a concentration of 5 μg/ml (positive control) and PBS (negative control). Furthermore, to analyze the contribution of other E. coli derived impurities, cells were also stimulated with an adequate volume of an α-amylase/trypsin inhibitor (RBI) fraction, which was produced and purified by the same protocol as the HcpA protein. These investigations showed that only HcpA and LPS at the high concentration of 5 μg/ml induced substantial release of IFN-γ. In contrast, only very weak to undetectable IFN-γ production was observed in cultures of splenocytes, which were incubated with LPS concentrations, representing the maximal impurity within HcpA preparations, as well as the RBI fraction (Fig. 4D). In addition, the capacity of HcpA preparations to stimulate cytokine secretion from splenic cells of naive mice was almost completely abrogated by chymotrypsin digestion (data not shown), indicating that the immunostimulatory properties of the HcpA preparation are mediated by a polypeptide. These data were further affirmed by the results of a direct comparison of the stimulatory effects of HcpA preparations derived from E. coli and DS-2 insect cells. Both immunogens from different sources stimulated an almost identical cytokine profile, with the exception of minor differences in the maximum levels of IFN-γ and IL-10 (Table 1). Altogether, these findings strongly suggest that the immunostimulatory effect of HcpA is not due to traces of contaminating producer cell-derived components within HcpA preparation from E. coli.
TABLE 1.
Cytokine secretion from splenic cells of nonimmunized mice upon stimulation with E. coli or DS-2 insect cell-derived HcpAa
| Stimulus | Mean cytokine concn (pg/ml) ± SD
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ | IL-12 | TNF-α | IL-6 | IL-2 | IL-4 | IL-5 | IL-10 | |
| HcpA (E. coli) | 7,810 ± 2,930 | 121 ± 24 | 17 ± 8 | 524 ± 98 | <3 | <8 | <32 | 20 ± 5 |
| HcpA (DS-2) | 1,864 ± 124 | 84 ± 8 | 16 ± 3 | 933 ± 55 | <3 | <8 | <32 | 72 ± 2 |
Splenic cells were isolated from nonimmunized female BALB/c mice and stimulated with 2 μg of E. coli or Drosophila Schneider (DS)-2 cell-derived HcpA, respectively, and supernatants were collected for cytokine assays after 36 h. The results of one representative experiment out of three are shown. Each value represents the mean cytokine level ± the standard deviation of three independent experiments.
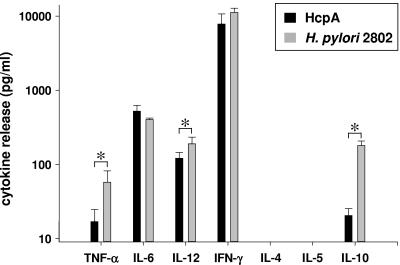
HcpA and H. pylori mediate comparable stimulation of the innate immune system.
Next we sought to analyze the role of HcpA in H. pylori-mediated activation of innate immune responses. To attain more detailed information about the immunomodulatory properties of a viable wild-type H. pylori isolate, murine splenocytes were incubated with increasing MOIs of H. pylori 2802, and IFN-γ release was assayed as a measure for immune activation. These experiments indicated a bell-shaped curve of IFN-γ secretion, revealing significant IFN-γ production at an MOI of >0.1 and reaching maximum release at an MOI of 1, strongly indicating the dose dependency of IFN-γ secretion by live H. pylori (data not shown). A direct comparison of the immunostimulatory properties of recombinant HcpA at a concentration of 2 μg per ml and H. pylori (MOI = 1) resulted in an almost identical profile of secreted proinflammatory and Th1-promoting cytokines (Fig. 5). However, H. pylori induced significantly increased concentrations of IL-10, IL-12, and TNF-α (P < 0.0001) compared to HcpA. Thus, in the model system of cultured murine splenocytes recombinant HcpA exhibits immunostimulatory properties similar to H. pylori strain 2802.
FIG. 5.
Cytokine production from cultured murine splenocytes upon stimulation with H. pylori 2802 or recombinant HcpA. Cells were stimulated with live H. pylori 2802 (MOI = 0.5) or 2 μg of E. coli-derived HcpA/ml, and the levels of the indicated cytokines were determined by ELISA. One representative experiment out of three performed in triplicate cultures is shown. The data represent means ± the SD. ✽, P < 0,0001.
Role of HcpA for the immunomodulatory properties of H. pylori.
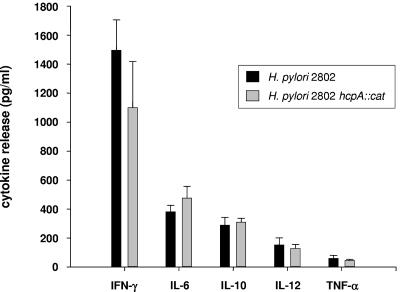
In order to investigate the contribution of HcpA to the immunostimulatory properties of H. pylori, an isogenic HcpA-deficient H. pylori 2802 strain (H. pylori 2802 hcpA::cat) was generated by inserting the chloramphenicol acetyltransferase resistance gene (cat) within hp0211. Transformants were screened by PCR with primers covering the whole ORF to determine the presence of the inserted cat sequence within hp0211. As depicted in Fig. 6A, PCR analysis of two randomly selected chloramphenicol-resistant H. pylori transformants confirmed the integration of the cat resistance gene into the genomic locus of hp0211. The obtained PCR product exhibited exactly the size to be expected for a successful recombination event. Considering the nucleic acid homology of hcpA with hcpC, obtained transformants were further analyzed for a possible misintegration of the cat gene within the genomic hcpC locus. Amplification of the hcpC gene from both the parental strain and the hcpA-deficient transformants revealed no differences in the amplicon size, excluding a misintegration of the cat cassette within the hcpC gene (Fig. 6B). In addition, cytoplasmic (CP) and periplasmic (P) fractions of H. pylori 2802 hcpA::cat (Fig. 6C, lane 3), as well as wild-type H. pylori isolates 2802 and 60190 (Fig. 6C, lanes 1 and 2), were analyzed for the presence of HcpA by immunoblotting. A protein with an apparent molecular mass of 24 kDa, representing the processed form of HcpA lacking the N-terminal signal sequence, was visualized in both CP and P fractions of H. pylori strains 2802 and 60190 but was absent in the isogenic knockout mutant H. pylori 2802 hcpA::cat. As depicted in Fig. 6C, an additional protein, HcpC, was visualized by the HcpA polyclonal antiserum within the cytosolic but not in the periplasmic fraction of all three tested H. pylori strains.
To investigate the role of HcpA in its in vivo background, we comparatively tested the potency of H. pylori 2802, and the isogenic HcpA knockout mutant H. pylori 2802 hcpA::cat to induce IFN-γ production from splenic cells of naive BALB/c mice. Here, the mutant H. pylori hcpA::cat strain stimulated an almost identical release of all tested cytokines (IL-6, IL-10, IL-12, TNF-α, and IFN-γ; P > 0.2) from cultures of murine splenocytes compared to the parental H. pylori 2802 strain (Fig. 7). In addition, both wild-type H. pylori 2802 and the isogenic HcpA knockout strain revealed no cytotoxic effects, as determined by microscopic examination over the incubation period of up to 36 h. These results indicate that other bacterial components such as LPS, flagellin, or probably other members of the Hcp family can compensate for the deletion of secreted HcpA to induce cytokine release from splenic cells.
FIG. 7.
Cytokine production from cultured murine splenocytes induced by wild-type H. pylori 2802 and the isogenic HcpA knockout strain. Splenocytes from naive C57BL/6 mice were incubated with H. pylori at an MOI of 0.5, and the induced cytokine pattern was assessed after 36 h. PBS-stimulated splenocytes served as negative controls. The levels of the indicated cytokines were determined by ELISA. One representative experiment out of four performed in triplicate cultures is shown. The data represent means ± the SD (P > 0.2).
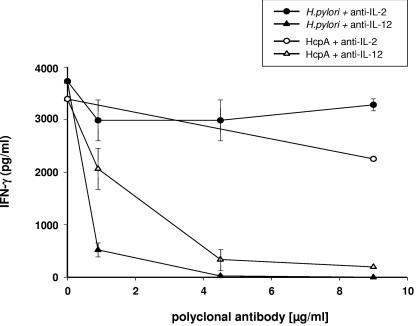
HcpA and H. pylori induced IL-12-dependent IFN-γ production.
IL-12 production by antigen-presenting cells in response to bacterial components has been shown to play a key role in the activation of NK cells and the initiation of Th1-biased cognate immune responses. Therefore, we examined the possible role of IL-12 in promoting IFN-γ secretion upon stimulation with HcpA and H. pylori by using a neutralizing polyclonal anti-IL-12 antibody. In these experiments, induction of IFN-γ release by HcpA and H. pylori was strongly impaired by neutralizing anti-IL-12 but not by neutralizing anti-IL-2 antibodies (Fig. 8). However, taking into account that the IL-12-specific antibody was raised against both IL-12p35 and IL-12p40, we cannot exclude a specific contribution of IL-23 in addition to IL-12 to the observed IFN-γ induction. In these experiments, HcpA-induced IFN-γ secretion by splenic cells was more sensitive to neutralization by IL-12 specific antibodies than H. pylori-mediated IFN-γ production. These data suggest that HcpA mediates an IL-12-dependent release of IFN-γ supporting the onset of Th1-type and proinflammatory immune responses. From these results, we conclude that HcpA is an immunostimulatory bacterial virulence factor which may support the onset of H. pylori-mediated Th1-biased proinflammatory disorder.
FIG. 8.
Dependency of IFN-γ production on IL-12 and IL-2. Splenic cell of naive BALB/c mice were stimulated with H. pylori 2802 or E. coli-derived HcpA in presence of increasing concentrations of anti-IL-12 and anti-IL-2 antibodies. The levels of IFN-γ were determined from the cell culture supernatant after 36 h by ELISA. One representative experiment out of three performed in triplicates is shown. The data represent means ± the SD.
DISCUSSION
Several studies have indicated that infection with H. pylori usually induced Th1-biased immune responses and the production of significant levels of IFN-γ, which seems to be of particular significance for the pathogenesis of H. pylori-associated inflammatory diseases, such as gastritis and peptic ulcer (36, 37). Although it has been assumed that a complex interplay between bacterial cells and APC may be a pivotal factor in the initiation of H. pylori-induced immune activation, the related molecular mechanisms described here have remained obscure. In addition, it remains unclear how these noninvasive bacteria, residing in the gastric mucosa, produce destructive inflammation and cause damage to the underlying epithelial tissue. The present study strongly indicates secreted Helicobacter HcpA to represent a novel mobile virulence factor, which is able to activate the innate immune system for the production of detrimental proinflammatory and Th1-promoting cytokines.
H. pylori HcpA protein belongs to a group of unique and highly disulfide-bridged proteins (8). Mittl and coworkers have recently reported HcpA to possess weak β-lactamase activity and speculated on a role of HcpA in maintenance of cell wall proteoglycan through the bacterial cell cycle (31, 34). However, the described weak enzymatic activity does not exclude in vivo immunomodulatory properties of secreted HcpA. Analysis of whole cellular lysates of 15 different H. pylori isolates obtained from patients suffering from different clinical entities revealed that the majority of strains (12 of 15) showed strong or at least moderate (2 of 15) HcpA expression and secretion of detectable levels of HcpA in 14 of 15 strains. These results clearly indicate that HcpA is an abundantly expressed protein in clinical H. pylori isolates. Furthermore, these results affirm and extend previous observations by others, which provided indirect evidence for the secretion of HcpA by determining specific antibodies from the sera of infected patients (35) or in response to immunization of animals with precipitated proteins from the supernatant of H. pylori cultures (8). It is tempting to speculate whether this finding reflects a role of secreted HcpA in the pathogenesis of H. pylori-induced mucosal inflammation because in some H. pylori-infected patients the titers of HcpA-specific antibodies are substantially increased (35; data not shown). Whether elevated levels of anti-HcpA antibodies are a result of an increased HcpA secretion by more virulent H. pylori strains and thus can be used as a measure for the identification of patients at risk for the development of inflammatory complications remains to be elucidated in further studies. Interestingly, in a comprehensive immunoproteomics study elevated levels of antibodies to HcpC, a closely related member of the Hcp family, were detected in sera from patients with gastric carcinoma (20), indicating a role of Hcp proteins in H. pylori pathogenesis.
The immunostimulatory properties of H. pylori in cultures of murine splenocytes are in accordance with findings in very recent publications by us and others reporting the activation and maturation of immature human dendritic cells upon coculture with H. pylori isolates (22, 28). The observation that HcpA induces a substantial secretion of proinflammatory (IL-6 and TNF-α) and Th1-promoting cytokines (IL-12 and IFN-γ) but no significant release of IL-2 and the Th2-promoting cytokines IL-4 and IL-5 from splenic cells of naive mice emphasizes the strong capacity of HcpA to stimulate innate immune activation. These results indicate that secreted HcpA might also be able to contribute to the activation of the innate immune responses in vivo in mucosal tissues of H. pylori-infected individuals. Thus, HcpA seems to represent an additional bacterial component, which triggers the release of a consorted set of cytokines to instruct the adaptive immune system to initiate Th1-type immune responses. The molecular mechanisms by which HcpA stimulates a Th1-type immune polarization remain to be elucidated in more detail.
Stimulation of cultured splenocytes with recombinant HcpA and H. pylori revealed a dose-dependent induction of Th1 biasing (IL-12 and IFN-γ) and proinflammatory cytokines (TNF-α and IL-6). Both HcpA and H. pylori mediate IFN-γ production from naive splenocytes, which was shown to be suppressible by anti-IL-12 but not by anti-IL-2 antibodies. A possible role of NK cells in the observed IFN-γ production (4, 14, 21) was substantiated by a very recent study by Yun et al. (55), who demonstrated that a yet-unknown secreted factor from H. pylori, together with IL-12, was able to induce IFN-γ production from human NK cells. Whether HcpA is involved in the activation of human or murine NK cells remains to be elucidated in future experiments.
The observation that an isogenic HcpA-deficient H. pylori strain revealed only a slightly decreased capacity to induce cytokine production compared to the corresponding wild-type strain also indicates that secreted HcpA is not the only IFN-γ-inducing component of H. pylori. This is not surprising since several potential nonsecreted virulence factors have been recently considered to stimulate cytokine production that could compensate for the lack of a secreted immunomodulating protein such as HcpA (26, 42, 50, 52). Recognition of H. pylori components by TLR4 (LPS) and TLR5 (flagellin) has been described recently (27, 46), although they activated the immune system to a much lesser extent than LPS and flagellin of other gram-negative bacteria (18, 29, 30, 41). In addition, considering the sequence identity on protein level of up to 66% (36, 39), it seems likely that other members of the family of Helicobacter cysteine-rich proteins (Hcp) may also reveal immunostimulatory properties and thus may contribute to the immune response of the HcpA-deficient H. pylori. However, this suggestion remains to be confirmed in future studies.
In conclusion, our results suggest that HcpA represents a novel H. pylori-secreted protein which may act as a virulence factor to trigger the release of a consorted set of cytokines, thereby instructing the adaptive immune system to initiate Th1-type immune responses. Thus, HcpA may be an interesting target for therapeutic vaccination strategies to modulate H. pylori-mediated immune response. In fact, vaccination with the gene product of ORF 1117, a member of the Hcp family, was shown to confer protection in the BALB/c H. pylori SS1 model (25). Further investigations are necessary to identify the physiological function of HcpA, the target cell(s), and signal transduction events of HcpA-mediated immune activation.
Acknowledgments
We thank Martin Blaser for helpful discussion, R. Glockshuber (Zurich, Switzerland) for providing the expression plasmid pRBI-PDI, and W. Fischer (Munich, Germany) for the gift of an anti-RecA antibody.
This research was supported by the DFG Sonderforschungsbereich 585 TB B3/B4 to W.S.-B., L.D., and N.L. and in part by BMBF Klinische Forschergruppe 01 KI 9952 to W.S.-B. and N.L. P.R.E.M. has been supported by the Swiss National Science Foundation grant 3100-063794.001.
Editor: J. B. Bliska
REFERENCES
- 1.Aihara, M., K. Imagawa, Y. Funakoshi, Y. Ohmoto, and M. Kikuchi. 1998. Effects of rebamipide on production of several cytokines by human peripheral blood mononuclear cells. Dig. Dis. Sci. 43:160S-166S. [PubMed] [Google Scholar]
- 2.Atherton, J. C. 1998. Helicobacter pylori virulence factors. Br. Med. Bull. 54:105-120. [DOI] [PubMed] [Google Scholar]
- 3.Atherton, J. C., R. M. Peek, K. T. Tham, T. L. Cover, and M. J. Blaser. 1997. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112:92-99. [DOI] [PubMed] [Google Scholar]
- 4.Bauditz, J., M. Ortner, M. Bierbaum, G. Niedobitek, H. Lochs, and S. Schreiber. 1999. Production of IL-12 in gastritis relates to infection with Helicobacter pylori. Clin. Exp. Immunol. 117:316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser, M. J. 1987. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology 93:371-383. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, M. J., and J. C. Atherton. 2004. Helicobacter pylori persistence: biology and disease. J. Clin. Investig. 113:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bumann, D., S. Aksu, M. Wendland, K. Janek, U. Zimny-Arndt, N. Sabarth, T. F. Meyer, and P. R. Jungblut. 2002. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 70:3396-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, P., M. S. McClain, M. H. Forsyth, and T. L. Cover. 1998. Extracellular release of antigenic proteins by Helicobacter pylori. Infect. Immun. 66:2984-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crabtree, J. E. 1998. Role of cytokines in pathogenesis of Helicobacter pylori-induced mucosal damage. Dig. Dis. Sci. 43:46S-55S. [PubMed] [Google Scholar]
- 10.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 11.Deml, L., A. Bojak, S. Steck, M. Graf, J. Wild, R. Schirmbeck, H. Wolf, and R. Wagner. 2001. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein. J. Virol. 75:10991-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deml, L., H. Wolf, and R. Wagner. 1999. High level expression of hepatitis B virus surface antigen in stably transfected Drosophila Schneider-2 cells. J. Virol. Methods 79:191-203. [DOI] [PubMed] [Google Scholar]
- 13.Dorrell, N., J. E. Crabtree, and B. W. Wren. 1998. Host-bacterial interactions and the pathogenesis of Helicobacter pylori infection. Trends Microbiol. 6:379-382. [DOI] [PubMed] [Google Scholar]
- 14.Duchmann, R., H. Scherer, M. Neurath, P. Knolle, and z. B. K. Meyer. 1997. Normal interleukin-12 production in individuals with antibodies to Helicobacter pylori. APMIS 105:824-830. [DOI] [PubMed] [Google Scholar]
- 15.Dundon, W. G., M. de Bernard, and C. Montecucco. 2001. Virulence factors of Helicobacter pylori. Int. J. Med. Microbiol. 290:647-658. [DOI] [PubMed] [Google Scholar]
- 16.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 17.Gebert, B., W. Fischer, E. Weiss, R. Hoffmann, and R. Haas. 2003. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301:1099-1102. [DOI] [PubMed] [Google Scholar]
- 18.Gewirtz, A. T., Y. Yu, U. S. Krishna, D. A. Israel, S. L. Lyons, and R. M. Peek, Jr. 2004. Helicobacter pylori flagellin evades Toll-like receptor 5-mediated innate immunity. J. Infect. Dis. 189:1914-1920. [DOI] [PubMed] [Google Scholar]
- 19.Guiney, D. G., P. Hasegawa, and S. P. Cole. 2003. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect. Immun. 71:4163-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas, G., G. Karaali, K. Ebermayer, W. G. Metzger, S. Lamer, U. Zimny-Arndt, S. Diescher, U. B. Goebel, K. Vogt, A. B. Roznowski, B. J. Wiedenmann, T. F. Meyer, T. Aebischer, and P. R. Jungblut. 2002. Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics 2:313-324. [DOI] [PubMed] [Google Scholar]
- 21.Haeberle, H. A., M. Kubin, K. B. Bamford, R. Garofalo, D. Y. Graham, F. El-Zaatari, R. Karttunen, S. E. Crowe, V. E. Reyes, and P. B. Ernst. 1997. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect. Immun. 65:4229-4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hafsi, N., P. Voland, S. Schwendy, R. Rad, W. Reindl, M. Gerhard, and C. Prinz. 2004. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J. Immunol. 173:1249-1257. [DOI] [PubMed] [Google Scholar]
- 23.Harris, P. R., H. L. T. Mobley, G. I. Perez-Perez, M. J. Blaser, and P. D. Smith. 1996. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology 111:419-425. [DOI] [PubMed] [Google Scholar]
- 24.Heuermann, D., and R. Haas. 1998. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257:519-528. [DOI] [PubMed] [Google Scholar]
- 25.Hocking, D., E. Webb, F. Radcliff, L. Rothel, S. Taylor, G. Pinczower, C. Kapouleas, H. Braley, A. Lee, and C. Doidge. 1999. Isolation of recombinant protective Helicobacter pylori antigens. Infect. Immun. 67:4713-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang, J., P. W. O'Toole, P. Doig, and T. J. Trust. 1995. Stimulation of Interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect. Immun. 63:1732-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawahara, T., Y. Kuwano, S. Teshima-Kondo, T. Kawai, T. Nikawa, K. Kishi, and K. Rokutan. 2001. Toll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infection. J. Med. Investig. 48:190-197. [PubMed] [Google Scholar]
- 28.Kranzer, K., A. Eckhardt, M. Aigner, G. Knoll, L. Deml, C. Speth, N. Lehn, M. Rehli, and W. Schneider-Brachert. 2004. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect. Immun. 72:4416-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, S. K., A. Stack, E. Katzowitsch, S. I. Aizawa, S. Suerbaum, and C. Josenhans. 2003. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 5:1345-1356. [DOI] [PubMed] [Google Scholar]
- 30.Luo, Y. H., J. Yan, and Y. F. Mao. 2004. Helicobacter pylori lipopolysaccharide: biological activities in vitro and in vivo, pathological correlation to human chronic gastritis and peptic ulcer. W. J. Gastroenterol. 10:2055-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lüthy, L., M. G. Grutter, and P. R. Mittl. 2002. The crystal structure of Helicobacter pylori cysteine-rich protein B reveals a novel fold for a penicillin-binding protein. J. Biol. Chem. 277:10187-10193. [DOI] [PubMed] [Google Scholar]
- 32.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 33.Merrell, D. S., and S. Falkow. 2004. Frontal and stealth attack strategies in microbial pathogenesis. Nature 430:250-256. [DOI] [PubMed] [Google Scholar]
- 34.Mittl, P. R., L. Lüthy, P. Hunziker, and M. G. Grutter. 2000. The cysteine-rich protein A from Helicobacter pylori is a β-lactamase. J. Biol. Chem. 275:17693-17699. [DOI] [PubMed] [Google Scholar]
- 35.Mittl, P. R., L. Lüthy, C. Reinhardt, and H. Joller. 2003. Detection of high titers of antibody against Helicobacter cysteine-rich proteins A, B, C, and E in Helicobacter pylori-infected individuals. Clin. Diagn. Lab. Immunol. 10:542-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moss, S. F., S. Legon, J. Davies, and J. Calam. 1994. Cytokine gene expression in Helicobacter pylori-associated antral gastritis. Gut 35:1567-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noach, L. A., N. B. Bosma, J. Jansen, F. J. Hoek, S. J. van Deventer, and G. N. Tytgat. 1994. Mucosal tumor necrosis factor-alpha, interleukin-1β, and interleukin-8 production in patients with Helicobacter pylori infection. Scand. J. Gastroenterol. 29:425-429. [DOI] [PubMed] [Google Scholar]
- 38.Nomura, A., and G. N. Stemmermann. 1993. Helicobacter pylori and gastric cancer. J. Gastroenterol. Hepatol. 8:294-303. [DOI] [PubMed] [Google Scholar]
- 39.Parsonnet, J., S. Hansen, L. Rodriguez, A. B. Gelb, R. A. Warnke, E. Jellum, N. Orentreich, J. H. Vogelman, and G. D. Friedman. 1994. Helicobacter pylori infection and gastric lymphoma. N. Engl. J. Med. 330:1267-1271. [DOI] [PubMed] [Google Scholar]
- 40.Parsonnet, J., D. Vandersteeen, J. Goates, R. K. Sibley, J. Pritkin, and Y. Chong. 1991. Helicobacter pylori in intestinal-and diffuse-type adenocarcinomas. J. Natl. Cancer Inst. 83:640-643. [DOI] [PubMed] [Google Scholar]
- 41.Perez-Perez, G. I., V. L. Shepherd, J. D. Morrow, and M. J. Blaser. 1995. Activation of human THP-1 cells and rat bone marrow-derived macrophages by Helicobacter pylori lipopolysaccharide. Infect. Immun. 63:1183-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieder, G., R. Hatz, A. P. Moran, A. Walz, M. Stolte, and G. Enders. 1997. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect. Immun. 65:3622-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawai, N., M. Kita, T. Kodama, T. Tanahashi, Y. Yamaoka, Y. Tagawa, Y. Iwakura, and J. Imanishi. 1999. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect. Immun. 67:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimoyama, T., and J. E. Crabtree. 1998. Bacterial factors and immune pathogenesis in Helicobacter pylori infection. Gut 43(Suppl. 1):S2-S5. [PMC free article] [PubMed] [Google Scholar]
- 45.Sommer, F., G. Faller, P. Konturek, T. Kirchner, E. G. Hahn, J. Zeus, M. Rollinghoff, and M. Lohoff. 1998. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 66:5543-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su, B., P. J. Ceponis, S. Lebel, H. Huynh, and P. M. Sherman. 2003. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect. Immun. 71:3496-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suerbaum, S., C. Josenhans, T. Sterzenbach, B. Drescher, P. Brandt, M. Bell, M. Droge, B. Fartmann, H. P. Fischer, Z. Ge, A. Horster, R. Holland, K. Klein, J. Konig, L. Macko, G. L. Mendz, G. Nyakatura, D. B. Schauer, Z. Shen, J. Weber, M. Frosch, and J. G. Fox. 2003. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc. Natl. Acad. Sci. USA 100:7901-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sundrud, M. S., V. J. Torres, D. Unutmaz, and T. L. Cover. 2004. Inhibition of primary human T-cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc. Natl. Acad. Sci. USA 101:7727-7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talley, N. J., A. R. Zinsmeister, E. P. Di Magno, A. Weaver, H. A. Carpenter, G. I. Perez-Perez, and M. J. Blaser. 1991. Gastric adenocarcinoma and Helicobacter pylori infection. J. Natl. Cancer Inst. 83:1734-1739. [DOI] [PubMed] [Google Scholar]
- 50.Tanahashi, T., M. Kita, T. Kodama, Y. Yamaoka, N. Sawai, T. Ohno, S. Mitsufuji, Y. P. Wei, K. Kashima, and J. Imanishi. 2000. Cytokine expression and production by purified Helicobacter pylori urease in human gastric epithelial cells. Infect. Immun. 68:664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarkkanen, J., T. U. Kosunen, and E. Saksela. 1993. Contact of lymphocytes with Helicobacter pylori augments natural killer cell activity and induces production of gamma interferon. Infect. Immun. 61:3012-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tummuru, M. K. R., S. A. Sharma, and M. J. Blaser. 1995. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol. Microbiol. 18:867-876. [DOI] [PubMed] [Google Scholar]
- 53.Wotherspoon, A. C., C. Ortiz-Hidalgo, M. R. Falzon, and P. G. Isaacson. 1991. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 338:1175-1176. [DOI] [PubMed] [Google Scholar]
- 54.Wunderlich, M., and R. Glockshuber. 1993. In vivo control of redox potential during protein folding catalyzed by bacterial protein disulfide-isomerase (DsbA). J. Biol. Chem. 268:24547-24550. [PubMed] [Google Scholar]
- 55.Yun, C. H., A. Lundgren, J. Azem, A. Sjoling, J. Holmgren, A. M. Svennerholm, and B. S. Lundin. 2005. Natural killer cells and Helicobacter pylori infection: bacterial antigens and interleukin-12 act synergistically to induce gamma interferon production. Infect. Immun. 73:1482-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]