Abstract
The high accessibility of the skin and the presence of immunocompetent cells in the epidermis makes this surface an attractive route for needle-free administration of vaccines. However, the lining of the skin by the stratum corneum is a major obstacle to vaccine delivery. In this study we examined the effect of skin barrier disruption on the immune responses to the cross-reacting material CRM197, a nontoxic mutant of diphtheria toxin (DTx) that is considered as a vaccine candidate. Application of CRM197, together with cholera toxin (CT), onto the tape-stripped skin of mice elicited antibody responses that had anti-DTx neutralizing activity. Vaccine delivery onto mildly ablated skin or intact skin did not elicit any detectable anti-CRM197 antibodies. Mice immunized with CRM197 alone onto the tape-stripped skin mounted a vigorous antigen-specific proliferative response. In contrast, the induction of cellular immunity after CRM197 deposition onto mildly ablated or intact skin was adjuvant dependent. Furthermore, epidermal cells were activated and underwent apoptosis that was more pronounced when the stratum corneum was removed by tape stripping. Overall, these findings highlight the potential for transcutaneous delivery of CRM197 and establish a correlation between the degree of barrier disruption and levels of antigen-specific immune responses. Moreover, these results provide the first evidence that the development of a transcutaneous immunization strategy for diphtheria, based on simple and practical methods to disrupt the skin barrier, is feasible.
The high accessibility of the skin and the presence of immunocompetent cells in the epidermis make this surface an attractive route for needle-free administration of vaccines (7, 9, 17). However, the lining of the skin by the stratum corneum is a major obstacle to vaccine delivery. Advances in drug delivery have created new opportunities to successfully breach the skin barrier using devices that work with one or both of the following two methods: change of the skin's physical environment and application of a driving force (18). A common characteristic of all of these methods is the disruption to a various degree (depending on the method) of the skin barrier. After this damage, the skin immune system senses dangerous signals, and Langerhans cells (LCs) and keratinocytes are activated to protect the body, repair the barrier and reestablish the epidermal homeostasis (16, 23). Disruption of the skin barrier also increases the percutaneous penetration of antigens that access more easily the LCs that reside at the basal layer of the epidermis. LCs play a sentinel role in the epidermis and initiate immune responses by presenting antigens to T lymphocytes at the regional lymph nodes (4).
Since the skin provides an attractive interface for simple, practical, and injection-free delivery of vaccines in the present study we sought to examine the immunogenicity of the cross-reacting material CRM197, a nontoxic mutant of diphtheria toxin (DTx) after application onto the intact or barrier disrupted skin. Protection against Corynebacterium diphtheriae is mainly focused on the induction of anti-toxin neutralizing antibody responses using nontoxic forms of DTx. The currently available vaccines contain diphtheria toxin treated with formaldehyde (diphtheria toxoid [DT]). Although vaccination with DT was successful, it is considered an antigen of low purity and high heterogeneity and causes reactions in adults (6). This is mainly because detoxification of DTx with formaldehyde cannot be controlled and results in a heterogeneous product, which shows lot-to-lot variation in its physicochemical and immunochemical properties (12, 15). The CRM197 mutant bears a glycine-to-glutamic acid mutation at position 52 in the A subunit of the toxin, which eliminates enzymatic function, but the molecule still binds to receptors on sensitive cells (12). It can be obtained at very high purity and is safe in humans since it is currently used as a carrier protein for Haemophilus influenzae type b, meningococcal C, and pneumococcal conjugate polysaccharide vaccines. Therefore, CRM197 could be a promising candidate vaccine to elicit protective antibodies against C. diphtheriae by providing antigenic and immunogenic consistency between different lots. Moreover, it will not require confirmation of lack of toxicity and the reversal to toxin that is normally necessary for chemically inactivated products.
Our findings demonstrated that the disruption of the skin barrier resulted in the potentiation of CRM197-specific humoral and cellular immune responses. Although the studies were conducted in mice that lack the binding receptor for DTx and are species that are generally considered to be of low sensitivity to immunization with DTx (12), the elicited anti-CRM197 antibody responses had a neutralizing activity higher than the acceptable levels considered to provide clinical immunity against diphtheria. Furthermore, we observed the induction of apoptosis and activation of epidermal cells that was more pronounced when the stratum corneum was removed by tape stripping and correlated with the high immunogenicity of CRM197. These findings highlight the potential of the skin for vaccination against C. diphtheriae and the effect of skin disruption on the immune responses to CRM197.
MATERIALS AND METHODS
Immunization procedure.
Prior to immunization the skin of BALB/c mice (six mice per group) was treated as follows. (i) The skin of a small surface area of the abdomen was tape stripped with six strokes using a facial strip wax (Jolen, France) and then the antigen solution was applied topically (protocol A). (ii) The stratum corneum of a small surface area of the abdomen was mildly ablated using a razor (the hair was removed by shaving, and no cuts were observed), and the antigen solution was applied topically after 5 h (protocol B). (iii) Finally, the hair of a small surface area of the abdomen was trimmed to prevent disruption of the cornified layer of the epidermis and the antigen solution was applied topically after 48 h (protocol C). In all cases, the treated surface area of the skin was hydrated for 5 min prior to antigen application. After blotting the skin with a dry tissue, 50 μl of antigen solution in phosphate-buffered saline (PBS) was applied topically as a mixture of (i) various doses of CRM197 (Chiron, Siena, Italy) (20, 10, or 5 μg) with 20 μg of CT (Sigma-Aldrich, St. Louis, MO) or (ii) various doses of CRM197 (20, 10, or 5 μg) applied alone. Booster immunizations were given on days 21 and 42 after priming by applying onto barrier-disrupted or intact skin the same dose and vaccine formulation as described for priming. During the immunization procedure mice had sufficient anesthetic to immobilize them for ca. 1 h (to limit grooming activity and to allow for antigen absorption) by intramuscular injection of a solution of ketamine-xylazine. One hour after skin exposure to the antigen, the site of topical application was thoroughly washed with water.
Preparation of lung homogenates.
Lungs were weighted and suspended (10% [wt/vol]) in PBS containing 0.1% bovine serum albumin (no protease inhibitors were included). After homogenization, samples were centrifuged to remove cell debris and stored at −20°C until use.
ELISA for measurement of antibody responses.
To measure the anti-CRM197 antibody response, Nunc Maxisorb 96-well plates were coated with 100 μl of CRM197 antigen (Chiron, Siena, Italy)/well at 1.5 μg/ml in carbonate buffer pH 9.6 at 4°C overnight. The plates were then washed and blocked with 5% skimmed milk solution in 0.05% PBS-Tween (PBS-T) for 1 h. Serum samples were diluted across the plate starting at 1:100 and incubated at 37°C for 2 h. Antigen-specific antibodies were detected by using horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) antibody (Sigma A9044) diluted 1:2,000 in 1% skimmed milk solution in PBS-T. Enzyme substrate solution ABTS ([2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)]; Sigma P-9941) in 0.05 M phosphate-citrate buffer was added, the reaction was allowed to develop for 30 min, and the plates were read at 405 nm. The results were expressed as titers using as a cutoff value the optical density of the control serum ± two standard deviations (SD). Mucosal anti-CRM197 IgA and IgG antibody responses were measured on Nunc Maxisorb 96-well plates coated with 50 μl of CRM197 antigen/well at 2.5 μg/ml in carbonate buffer (pH 9.6) at 4°C overnight. The subsequent enzyme-linked immunosorbent assay (ELISA) steps were similar to those described previously (1).
Anti-DTx neutralizing Vero cell assay.
This assay relies on the inhibition of a cytotoxic dose of DTx, and the endpoint was taken as the highest serum dilution protecting the cells (24). Briefly, in 96-flat well plastic culture plates 50-μl serial dilutions of each individual serum were mixed with 50 μl of DTx at 0.25 mLf (limit of flocculation)/ml. One Lf of reference toxin is equivalent to 0.4 μg of toxin (22). Plates were incubated at 37°C for 1 h. At the end of the incubation period 50 μl of a Vero cell suspension (in minimum essential medium supplemented with 10% fetal calf serum and 2% antibiotic-antimycotic solution) containing 4 × 105 cells/ml was added into each well, and the plates were incubated at 37°C for 7 days. At the end of the incubation period, 10 μl of a methylthiazoletetrazolium (MTT; Sigma catalog no. M2128) solution was added to each well, and sealed plates were incubated at 37°C for a further 4 h. Viable cells were extracted with a 10% (wt/vol) lauryl sulfate (Sigma catalog no. L4509) in a 50% (vol/vol) solution of dimethyl formamide (80-2039-80; Novabiochem, Switzerland) in distilled water, and plates were read at 570 nm. The sensitivity of the assay was calculated as 0.0075 IU of diphtheria antitoxin/ml using calibrated in house mouse serum to DTaP. The results were expressed in IU/ml relative to mouse serum to DTaP, which was previously calibrated against International Standards for diphtheria antitoxin (WHO 1st IS, DI, NIBSC lot no. 01/544), and results were presented as the average IU of six individual samples ± the standard error of the mean (SEM).
Measurement of proliferative T-cell responses.
Spleens were aseptically removed 5 weeks after the final boost and a single cell suspension was prepared for each group in RPMI 1640 medium (Life Technologies, Gergy-Pontoise, France) supplemented with 100 IU/ml of gentamicin, 2 mM l-glutamine, 25 mM HEPES, and 1% inactivated autologous mouse serum. Viable splenocytes (4 × 105/well) were cultured in 0.2-ml volumes in the presence of various concentrations of CRM197 in 5% CO2 at 37°C for 5 days. At 18 h before the end of the culture, cells were pulsed with 1 μCi of [3H]thymidine (6.7mCi/mmol; ICN, Orsay, France), and incorporation was measured by using a Matrix 9600 direct beta counter (Packard, Downers Grove, IL). The results were expressed as stimulation indices (SI) of the mean counts per minute (cpm) from quadruplicate cultures in the presence of antigen divided by mean cpm of hexaplicate cultures with medium only. Values of ≥2 were considered positive. Supernatants collected after 3 day culture were tested for their ability to support the proliferation of an interleukin-2 (IL-2)-dependent cell line (CTLL-2) by incubating the cells (2 × 105/ml) for 24 h. Cells were pulsed with 1 μCi of [3H]thymidine for 7 h, and incorporation was measured as described above. A standard curve determined with known concentrations of recombinant mouse IL-2 (Pharmingen, San Diego, CA) was used as an internal control to calculate the concentration of secreted IL-2 in triplicate cultures. In all cases background values from medium alone were subtracted.
Cytokine ELISA.
Culture supernatants collected after 3 days incubation were assayed for the presence of gamma interferon (IFN-γ) and IL-6 by using a double-sandwich ELISA and commercial antibodies from Pharmingen and polyvinyl Falcon plates (Oxnard, CA). All steps were performed according to the manufacturers' recommendations. The results were expressed as mean cytokine concentration (pg/ml) ± the SD after extrapolation from a standard curve prepared with a reference cytokine (Pharmingen). In all cases average background values from medium alone were subtracted.
Preparation and analysis of epidermal cell suspensions.
Skin from the ventral surface of abdomen and dorsal and ventral halves of split ears (used as controls of normal skin) was incubated in 0.5% trypsin for 2 h to separate the epidermis from the underlying dermis. A single-cell suspension was obtained by mechanical disruption upon pipetting and pushing though a wire mesh (100 μm). The resulting cell suspension was washed, resuspended in PBS containing 2% fetal calf serum, and stained with the following monoclonal antibodies: rat anti-mouse CD11b (clone M1/70, biotin conjugated), rat anti-mouse I-Ad (clone AMS-32.1, fluorescein isothiocyanate [FITC] conjugated), or isotype-matched rat control antibodies. The CD11b staining was detected by incubation with streptavidin-phycoerythrin. Cell death was then evaluated by the detection of phosphatidylserine expression by flow cytometry after the addition of FITC-annexin V (BD Pharmingen, San Diego, CA). All antibodies were purchased from BD Pharmingen. Analysis was performed on a FACscan flow cytometer (BD Biosciences) using CellQuest software (BD Biosciences).
Statistical analysis.
Data were analyzed by using the Student t test. Differences were considered significant at P < 0.05.
RESULTS
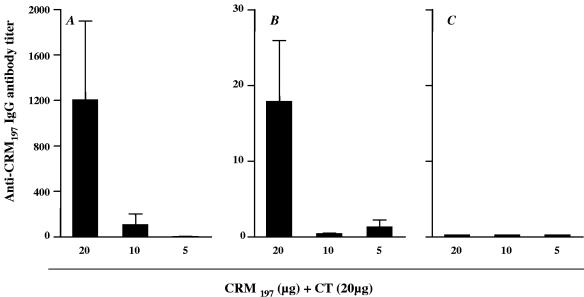
Systemic and mucosal antibody responses to CRM197 after its application onto the intact or barrier disrupted skin. Three applications of 20, 10, or 5 μg of CRM197 alone onto the intact or barrier-disrupted skin did not elicit any detectable antibody responses (data not shown). However, when CT was used as an adjuvant good anti-CRM197 antibody responses were measured in serum samples of mice immunized with 20 μg of CRM197 applied onto the tape-stripped skin (Fig. 1A) (P < 0.05). However, it is important to note that, despite the fact that all mice responded, there was a great variation in the observed immune responses, which reflects the nature of the immunization procedure. These antibodies were IgG1, and no IgG2a response was detected (data not shown). A dose of 10 μg of CRM197 was weakly immunogenic in two of six mice (P = 0.053 compared to 20 μg of CRM197), whereas 5 μg of CRM197 did not elicit any detectable antibodies (Fig. 1A). When CRM197 was applied together with CT onto mildly ablated skin, responses were just detectable at the immunizing dose of 20 μg, whereas it was not immunogenic on the intact skin (Fig. 1B and C, respectively).
FIG. 1.
Immunogenicity of CRM197 after transcutaneous immunization. Groups of six mice were immunized by applying 20, 10, or 5 μg of CRM197, together with 20 μg of CT, onto tape-stripped skin (A), mildly ablated skin (B), or intact skin (C). Serum samples collected 2 weeks after the second boost were assayed by ELISA for the presence of anti-CRM197-specific antibodies. The results represent average antibody titers of six mice ± the SEM. The scale for panel C is the same as for panel B.
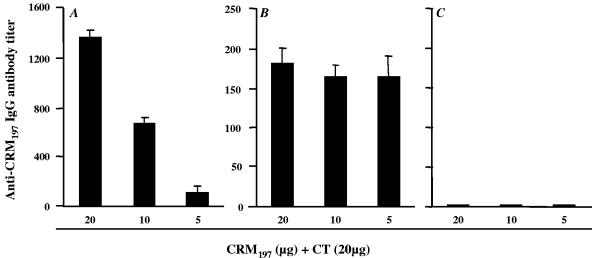
Since transcutaneous immunization is a procedure that elicits mucosal antibody responses (1, 5, 8, 10), samples of lung homogenates were tested for the presence of anti-CRM197-specific IgA and IgG antibodies. There was no detectable antigen-specific IgA antibody response in any of the samples of mice immunized with CRM197 alone or together with CT (data not shown). However, antigen-specific IgG antibodies were measured in lung homogenates of mice after application of CRM197, together with CT, onto the tape-stripped or mildly ablated skin (Fig. 2A and B) but not onto the intact skin (Fig. 2C) 5 weeks after the final boost. In the tape-stripped group antibody titers correlated with the immunizing dose of CRM197 and were higher than those measured in mice which had their skin mildly ablated. When CRM197 was applied without adjuvant there was no detectable IgG antibody response in any of the tested groups of mice (data not shown).
FIG. 2.
Mucosal anti-CRM197 IgG antibody responses in lung homogenates elicited after application of CRM197 together with CT onto tape stripped (A), mildly ablated (B), or intact skin (C). The results represent average antibody titers of pools of lung homogenates (collected 5 weeks after the final boost) of six mice in each group tested in triplicates ± the SD. The scale for panel C is the same as for panel B.
Induction of protective levels of anti-diphtheria toxin neutralizing antibodies.
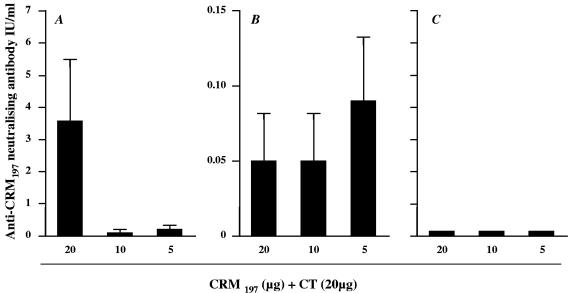
The capacity of serum antibodies to neutralize DTx was evaluated in vitro (24). In the present study the average protective level of toxin neutralizing activity was 3.7 IU/ml (range, 0.2 to 9.6 IU/ml) with all mice responding when 20 μg of CRM197 was applied together with CT onto the tape-stripped skin (Fig. 3A). Immunization with 10 or 5 μg of CRM197, together with CT, elicited a weak neutralizing antibody response in one and two mice of six per group, respectively (Fig. 3A), whereas topical application of antigen onto mildly ablated skin gave low neutralizing titers in two of six mice for all tested doses of CRM197 (Fig. 3B) (P = 0.05 compared to tape-stripped skin). Mice immunized onto their intact skin did not elicit any detectable neutralizing antibodies (Fig. 3C).
FIG. 3.
Anti-DTx neutralizing antibodies measured in vitro. Serum samples elicited after application of 20, 10, or 5 μg of CRM197, together with 20 μg of CT, onto tape-stripped skin (A), mildly ablated skin (B), or intact skin (C) (were collected 2 weeks after the second boost and tested for neutralizing activity. The titers are expressed as average IU/ml ± the SEM of six mice. The scale for panel C is the same as for panel B.
Sera of mice immunized subcutaneously with one dose of 100 μg of DT without adjuvant or absorbed to aluminum hydroxide had neutralizing titers ranging from 0.2 to 0.5 IU/ml and 10 to 20 IU/ml, respectively, 6 weeks after priming. In another study, reference mouse sera collected 2 weeks after injection of 50 μg of CRM197 subcutaneously twice (6 weeks apart) without an adjuvant or absorbed to AL(OH) had a very low neutralizing activity of <0.01 IU/ml or an activity of 0.2 IU/ml at best (0.01 to 0.2 IU/ml), respectively (data not shown).
Antigen-specific T-cell responses and cytokine secretion after application of CRM197 onto intact or barrier-disrupted skin.
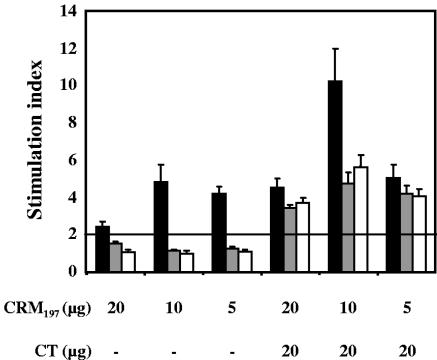
Immunization experiments using different skin pretreatment protocols prior to topical application of CRM197 were performed to demonstrate their effect on antigen-specific T-cell responses. Three doses of CRM197 were tested either alone or in combination with CT as an adjuvant. Splenocytes of mice immunized with CRM197 alone proliferated strongly only when the skin barrier was disrupted by tape stripping (Fig. 4). In contrast, no proliferation was observed in splenocyte cultures of mice which skin was mildly ablated or intact (Fig. 4). This finding also highlights the nonmitogenic nature of CRM197 used in the in vitro assays. When CRM197 was coadministered with CT, strong proliferative responses were measured in all groups of mice, irrespective of the skin pretreatment they received. A dose of 10 μg of CRM197 was shown to be the most antigenic. In all cases proliferative responses were dose dependent (data not shown), and only the maximum proliferation obtained at 1 μg of CRM197/culture is indicated in Fig. 4.
FIG. 4.
Proliferative responses of splenocytes of mice immunized with CRM197 alone or together with CT by application onto tape-stripped (▪), mildly ablated (░⃞), or intact skin (□). Spleens from each group of mice were aseptically removed 5 weeks after the final boost and cultured in the presence of 1 μg of CRM197/culture for 5 days. At 18 h before the end of the culture, cells were pulsed with 1μCi of 3[H]thymidine, and incorporation was measured. The results represent SI ± the SD. Counts twice higher than the medium alone were considered positive.
Besides the strong antigen-specific proliferation observed after transcutaneous immunization, high levels of IL-2, IFN-γ, and IL-6 were measured in the supernatants of cultured splenocytes (Table 1). All three cytokines were predominantly measured in groups of mice receiving antigen, together with CT, irrespective of the state of their skin barrier (Table 1). There was no IL-10 detected in any of the culture supernatants (data not shown).
TABLE 1.
Cytokine profile of proliferating splenocytes of mice immunized with CRM197 alone or together with CT by application onto tape-stripped, mildly ablated, or intact skina
| Skin | Amt (μg) of CRM197 | Alone or plus CT | Mean concn ± SD |
||
|---|---|---|---|---|---|
| IL-2 (U/ml) | IL-6 (pg/ml) | IFNγ (pg/ml) | |||
| Tape-stripped skin | 20 | Alone | 0.1 ± 0.1 | 0 | 0 |
| + CT | 4.4 ± 2.5 | 280 ± 219 | 2,484 ± 260 | ||
| 10 | Alone | 0.8 ± 0.1 | 0 | 0 | |
| + CT | 2.8 ± 1.4 | 445 ± 325 | 0 | ||
| 5 | Alone | 0.7 ± 0.1 | 0 | 244 ± 141 | |
| + CT | 0.3 ± 0.1 | 0 | 0 | ||
| Mildly ablated skin | 20 | Alone | 0.4 ± 0.1 | 0 | 0 |
| + CT | 6.6 ± 3.8 | 1,322 ± 395 | 0 | ||
| 10 | Alone | 0.2 ± 0.1 | 120 ± 50 | 0 | |
| + CT | 6.2 ± 1.1 | 3,130 ± 774 | 0 | ||
| 5 | Alone | 0 | 0 | 0 | |
| + CT | 6.5 ± 2.2 | 845 ± 184 | 1,669 ± 21 | ||
| Intact skin | 20 | Alone | 0 | 0 | 0 |
| + CT | 9 ± 0.6 | 270 ± 49 | 648 ± 466 | ||
| 10 | Alone | 0 | 0 | 0 | |
| + CT | 23 ± 0.8 | 3,698 ± 579 | 9,531 ± 1,947 | ||
| 5 | Alone | 0 | 0 | 0 | |
| + CT | 14.3 ± 0.1 | 1,161 ± 319 | 3,707 ± 977 | ||
Spleens from each group of mice were aseptically removed 5 weeks after the final boost, and the splenocytes were cultured in the presence of an optimum dose (1 μg/culture) of CRM197 for 3 days. Collected supernatants were assayed for IL-2 secretion by using the IL-2-dependent cell line (CTLL-2), whereas IFN-γ and IL-6 secretion was measured with a commercially available ELISA kit. In each case, a standard curve with known concentrations of recombinant mouse cytokine was used as an internal control to calculate the concentration of secreted cytokine. Data represent the mean of quadruplicates or triplicates ± the SD.
Effect of barrier disruption on epidermal cells.
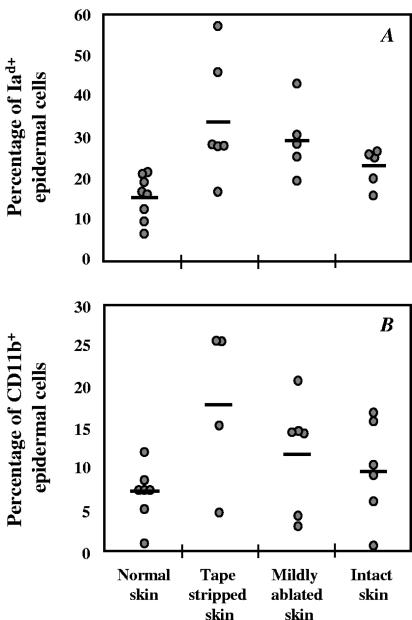
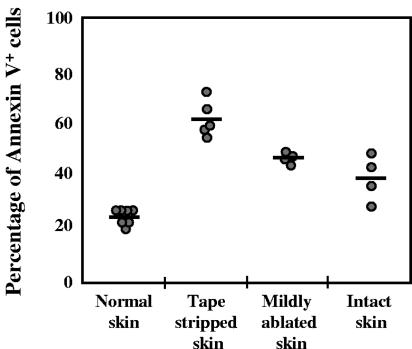
Since disruption of the skin barrier triggers inflammation, we examined its effects on epidermal cells. Figure 5A and B shows the upregulation of expression of class II major histocompatibility complex (MHC) and CD11b molecules, which was more pronounced when the stratum corneum was removed by tape stripping. However, the differences between the three groups of mice treated with the immunizing protocols A, B, and C were not significant (P > 0.05). Tape stripping also increased the expression of the ICAM-1 adhesion molecule (data not shown). In addition, the epidermal cells undergo apoptosis with percentages of apoptotic cells correlating to the degree of barrier disruption (Fig. 6). In particular, the skin of mice that was tape stripped exhibited significantly higher apoptosis than that measured in the mildly ablated or intact skin (P < 0.005).
FIG. 5.
Effect of skin barrier disruption on the phenotype of epidermal cells. Epidermal single-cell suspensions were obtained from tape-stripped, mildly ablated, or intact skin and analyzed for levels of MHC class II (Iad) and CD11b. The percentages of Iad (A)- and CD11b (B)-positive cells are represented by a circle for each mouse of a group of five to eight mice. The average expression is represented by a horizontal line.
FIG. 6.
Effect of skin barrier disruption on the apoptosis cell death of epidermal cells. Epidermal single-cell suspensions were obtained from tape-stripped, mildly ablated, or intact skin and cell death was evaluated by the detection of phosphatidylserine expression by flow cytometry using FITC-annexin V. The percentage of FITC-annexin V-positive cells are represented by a circle for each mouse of a group of four to seven mice. The mean of FITC-annexin V-positive cells per group is represented by a horizontal line.
DISCUSSION
In an effort to design a needle-free vaccination strategy against diphtheria, we applied the nontoxic mutant CRM197 onto the intact or barrier-disrupted skin and examined the induction of protective levels of anti-DTx neutralizing antibodies and antigen-specific cellular immune responses. Our findings demonstrated that CRM197 was immunogenic and elicited anti-CRM197 antibodies when coapplied with CT onto the tape-stripped skin. These antibodies had high neutralizing capacity against DTx. Application of CRM197, together with CT, onto the tape-stripped skin or mildly ablated skin also elicited mucosal IgG but no IgA antibody responses in the lungs, highlighting the potential of transcutaneous immunization to elicit both systemic and mucosal immunity (1, 8-11, 14). An interesting finding of the present study was the demonstration that the degree of skin barrier disruption correlated with levels of anti-CRM197 antibody responses. This suggests that the removal of the stratum corneum had facilitated the percutaneous penetration of CRM197 at sufficient concentrations to trigger an antibody response. Studies by Kahlon et al. (13) have shown that the application of ovalbumin (OVA), together with CT, onto the tape-stripped skin of mice enhanced the magnitude of anti-OVA antibody responses compared to those elicited after application onto the non-tape-stripped skin. Similar observations by Glenn et al. (9) using CT B, tetanus toxoid, or split-virus influenza vaccine as immunogens have highlighted the importance of combining hydration and some disruption of the skin barrier to improve antigen delivery and therefore enhance immune responses.
Transcutaneous immunization is a procedure that elicits potent antigen-specific cellular responses (2, 3, 13, 19). In the present study we observed the induction of strong proliferative responses when CRM197 was applied alone onto the tape-stripped skin, and the magnitude of the response was increased in the presence of CT. In contrast, application of antigen onto mildly ablated skin or onto intact skin required the addition of an adjuvant to induce detectable proliferative responses. These findings are consistent with published data by Seo et al. (21) demonstrating the capacity of a peptide antigen to elicit peptide and tumor-specific CD8+-T-cell responses after its application without adjuvant onto the tape-stripped skin. The observed cellular responses did not correlate in all cases with the antibody responses. This suggests that the minimum antigenic dose required for efficient priming of B and T cells differ. Moreover, cytokines such as IL-2, IFN-γ, and IL-6 but not IL-10 were measured in the supernatants of cultured splenocytes of mice immunized with CRM197 in the presence of CT. Overall, the highest levels of these cytokines were observed in mice, where the antigen was deposited onto the intact skin with lower responses seen in the group that the skin was tape stripped. This suggests that the number of proliferating antigen-specific T cells might be higher in the former group. The higher proliferative responses observed in the tape-stripped group (Fig. 4) probably reflects a bystander effect due to the destruction of the skin barrier. Despite the fact that the cytokine profile was predominantly Th1, subclasses of anti-CRM197 antibodies were IgG1 with no detectable IgG2a, which suggests that transcutaneous immunization elicited a mixed Th1/Th2 response.
Skin barrier disruption in addition to abrogating the barrier function of the skin is also immunostimulatory. In particular, after barrier disruption there is a chain of molecular events that leads to the secretion of proinflammatory cytokines such as tumor necrosis factor alpha, IL-1α, IL-1β, and granulocyte-macrophage colony-stimulating factor by the keratinocytes (16). The secretion of tumor necrosis factor alpha and IL-1β in particular promote the migration of LCs from the epidermis to regional lymph nodes (4). Recent studies have shown that tape stripping and transcutaneous immunization with ADP-ribosylating exotoxins activated LCs and stimulated their migration, respectively (9, 11, 13). In the present study we extended these observations by demonstrating the activation of epidermal cells that had increased expression of class II MHC molecules and numbers of CD11b+ cells (bearing an integrin that is a key molecule for phagocytic leukocyte trafficking) infiltrating the epidermis of tape-stripped skin that could explain in part the observed potent immune responses. Moreover, tape stripping had a significant effect on epidermal cells increasing their apoptotic cell death, a finding that is consistent with the notion that apoptosis is an important mechanism to maintain tissue homeostasis by limiting cutaneous inflammation. The observed apoptosis might be one of the mechanisms contributing to the strong immunogenicity of antigens after application onto the tape-stripped skin. After transcutaneous immunization, apoptotic epidermal cells are likely to be taken up by immature LCs which, together with the signals provided by the adjuvant, condition the LCs to initiate T-cell responses at the regional lymph nodes (19). As a result, apoptotic bodies will be processed and presented more efficiently than the free antigen that reaches the epidermis through diffusion mainly via the intercellular space.
Overall, the findings of the present study demonstrated the potential of the skin for the delivery of CRM197 and established a correlation between the degree of barrier disruption and the induction of antigen-specific immune responses. Despite the fact that mice lack the required receptors for DTx binding, which significantly reduces the immunogenicity of CRM197 (12), the present study highlighted that the development of a noninvasive immunization strategy for diphtheria based on transcutaneous immunization is feasible, and the establishment of principles for its effective delivery using simple and practical methods to disrupt the skin barrier would potentiate the protective immune responses. Moreover, the presence of CT played an important role in enhancing immune responses. Even though this adjuvant is toxic when administered mucosally, it does not exert any toxicity when applied transcutaneously. Therefore, it appears that the skin microenvironment harnesses the immunogenic and adjuvant potential of CT while neutralizing its harmful effects. Nevertheless, alternative adjuvants, which are less toxic or do not pose the risk of autoimmunity (20), such as nontoxic mutants of ADP-ribosylating exotoxins (19) or ligands of Toll receptors (19, 25), might be the adjuvants of choice for future human applications.
Acknowledgments
This study was in part financed by the Centre National de la Recherche Scientifique (CNRS) and the National Institute for Biological Standards and Control (NIBSC). None of the authors has competing financial interests.
We thank B. Jessel and G. Amann (IBMC-CNRS) for excellent animal husbandry.
Editor: J. D. Clements
REFERENCES
- 1.Beignon, A.-S., J.-P. Briand, S. Muller, and C. D. Partidos. 2001. Immunization onto bare skin with heat-labile enterotoxin of Escherichia coli enhances immune responses to co-administered protein and peptide antigens and protects mice against lethal toxin challenge. Immunology 102:344-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beignon, A.-S., J.-P. Briand, S. Muller, and C. D. Partidos. 2002. Immunization onto bare skin with synthetic peptides: immunomodulation with a CpG-containing oligodeoxynucleotide and effective priming of influenza virus-specific CD4+ T cells. Immunology 105:204-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belyakov, I. M., S. A. Hammond, J. D. Ahlers, G. M. Glenn, and J. A. Berzofsky. 2004. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J. Clin. Investig. 113:998-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cumberbatch, M., R. J. Dearman, C. E. Griffiths, and I. Kimber. 2000. Langerhans cell migration. Clin. Exp. Dermatol. 25:413-418. [DOI] [PubMed] [Google Scholar]
- 5.Enioutina, E. Y., D. Visic, and R. A. Daynes. 2000. The induction of systemic and mucosal immune responses to antigen-adjuvant compositions administered into the skin: alterations in the migratory properties of dendritic cells appears to be important for stimulating mucosal immunity. Vaccine 18:2753-2767. [DOI] [PubMed] [Google Scholar]
- 6.Galazka, A. M., and S. E. Robertson. 1996. Immunization against diphtheria with special emphasis on immunization of adults. Vaccine 14:845-857. [DOI] [PubMed] [Google Scholar]
- 7.Glenn, G. M., M. Rao, G. R. Matyas, and C. R. Alving. 1998. Skin immunization made possible by cholera toxin. Nature 391:851. [DOI] [PubMed] [Google Scholar]
- 8.Glenn, G. M., T. Scharton-Kersten, R. Vassell, C. P. Mallett, T. L. Hale, and C. R. Alving. 1998. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J. Immunol. 161:3211-3214. [PubMed] [Google Scholar]
- 9.Glenn, G. M., R. T. Kenney, L. R. Ellingsworth, S. A. Frech, S. A. Hammond, and J. P. Zoeteweij. 2003. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev. Vaccines. 2:253-267. [DOI] [PubMed] [Google Scholar]
- 10.Gockel, C. M., S. Bao, and K. W. Beagley. 2000. Transcutaneous immunization induces mucosal and systemic immunity: a potent method for targeting immunity to the female reproductive tract. Mol. Immunol. 37:537-544. [DOI] [PubMed] [Google Scholar]
- 11.Guebre-Xabier, M., S. A. Hammond, D. E. Epperson, J. Yu, L. Ellingsworth, and G. M. Glenn. 2003. Immunostimulant patch containing heat-labile enterotoxin from Escherichia coli enhances immune responses to injected influenza virus vaccine through activation of skin dendritic cells. J. Virol. 77:5218-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta, R. K., R. J. Collier, R. Rappuoli, and G. R. Siber. 1997. Differences in the immunogenicity of native and formalinized cross reacting material (CRM197) of diphtheria toxin in mice and guinea pigs and their implications on the development and control of diphtheria vaccine based on CRMs. Vaccine 15:1341-1343. [DOI] [PubMed] [Google Scholar]
- 13.Kahlon, R., Y. Hu, C. H. Orteu, A. Kifayet, J. D. Trudeau, R. Tan, and J. P. Dutz. 2003. Optimization of epicutaneous immunization for the induction of CTL. Vaccine 21:2890-2899. [DOI] [PubMed] [Google Scholar]
- 14.Mawas, F., M. Peyre, A. S. Beignon, L. Frost, G. Del Giudice, R. Rappuoli, S. Muller, D. Sesardic, and C. D. Partidos. 2004. Successful induction of protective antibody responses against Haemophilus influenzae type b and diphtheria after transcutaneous immunization with the glycoconjugate polyribosyl ribitol phosphate-cross-reacting material 197 vaccine. J. Infect. Dis. 190:1177-1182. [DOI] [PubMed] [Google Scholar]
- 15.Metz, B., W. Jiskoot, W. E. Hennink, D. J. Crommelin, and G. F. Kersten. 2003. Physicochemical and immunochemical techniques predict the quality of diphtheria toxoid vaccines. Vaccine 22:156-167. [DOI] [PubMed] [Google Scholar]
- 16.Nickoloff, B. J., and Y. Naidu. 1994. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J. Am. Acad. Dermatol. 30:535-546. [DOI] [PubMed] [Google Scholar]
- 17.Partidos, C. D. 2003. Antigens onto bare skin: a “painless” paradigm shift in vaccine delivery. Expert Opin. Biol. Ther. 3:895-902. [DOI] [PubMed] [Google Scholar]
- 18.Partidos, C. D. 2003. Delivering vaccines into the skin without needles and syringes. Expert Rev. Vaccines 2:753-761. [DOI] [PubMed] [Google Scholar]
- 19.Partidos, C. D., A. S. Beignon, J. P. Briand, and S. Muller. 2004. Modulation of immune responses with transcutaneously deliverable adjuvants. Vaccine 22:2385-2390. [DOI] [PubMed] [Google Scholar]
- 20.Riminton, D. S., R. Kandasamy, D. Dravec, A. Basten, and A. G. Baxter. 2004. Dermal enhancement: bacterial products on intact skin induce and augment organ-specific autoimmune disease. J. Immunol. 172:302-309. [DOI] [PubMed] [Google Scholar]
- 21.Seo, N., Y. Tokura, T. Nishijima, H. Hashizume, F. Furukawa, and M. Takigawa. 2000. Percutaneous peptide immunization via corneum barrier-disrupted murine skin for experimental tumor immunoprophylaxis. Proc. Natl. Acad. Sci. USA 97:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sesardic, D., C. Prior, A. Daas, and K. H. Buchheit. 2003. Collaborative study for establishment of the European Pharmacopoeia BRP batch 1 for diphtheria toxin. PharmaEuropa Biol. 1:7-21. [PubMed] [Google Scholar]
- 23.Williams, I. R., and T. S. Kupper. 1996. Immunity at the surface: homeostatic mechanisms of the skin immune system. Life Sci. 58:1485-1507. [DOI] [PubMed] [Google Scholar]
- 24.Winsnes, R., D. Sesardic, A. Daas, and P. Rigsby. 2002. A Vero cell method for potency testing of diphtheria vaccines. Dev. Biol. 111:141-148. [PubMed] [Google Scholar]
- 25.Zuber, A. K., A. Brave, G. Engstrom, B. Zuber, K. Ljungberg, M. Fredriksson, R. Benthin, M. G. Isaguliants, E. Sandstrom, J. Hinkula, and B. Wahren. 2004. Topical delivery of imiquimod to a mouse model as a novel adjuvant for human immunodeficiency virus (HIV) DNA. Vaccine 22:1791-1798. [DOI] [PubMed] [Google Scholar]