Abstract
Enterohemorrhagic Escherichia coli (EHEC) is a bacterial pathogen that is associated with several life-threatening diseases for humans. The combination of protein E-mediated cell lysis to produce EHEC ghosts and staphylococcal nuclease A to degrade DNA was used for the development of an oral EHEC vaccine. The lack of genetic material in the oral EHEC bacterial-ghost vaccine abolished any hazard of horizontal gene transfer of resistance genes or pathogenic islands to resident gut flora. Intragastric immunization of mice with EHEC ghosts without the addition of any adjuvant induced cellular and humoral immunity. Immunized mice challenged at day 55 showed 86% protection against lethal challenge with a heterologous EHEC strain after single-dose oral immunization and 93.3% protection after one booster at day 28, whereas the controls showed 26.7% and 30% survival, respectively. These results indicate that it is possible to develop an efficacious single-dose oral EHEC bacterial-ghost vaccine.
Enterohemorrhagic Escherichia coli (EHEC) strains, including those of the serotype O157:H7, are enteric bacteria which were recognized as human pathogens in 1982 (41). E. coli O157:H7 characteristically causes “attaching and effacing” lesions along the intestinal mucosa, where it triggers an inflammatory response sometimes developing into a severe hemorrhagic colitis, especially in the elderly (45, 52). Furthermore, this bacterium produces Shiga toxin types 1 and 2, as well as type 2 variants and an endotoxin (15, 26, 50), resulting in systemic damage, including hemolytic uremic syndrome (45). Current treatment is limited largely to supportive care, as no specific regimen against an EHEC infection exists and the use of antibiotics is not recommended. One major reason for not using antibiotics is the liberation of toxins from the bacterium following antibiotic treatment, as this can worsen the clinical course (48).
The major reservoir for E. coli O157:H7 is cattle, which harbor this organism in their intestinal tracts (18, 49), especially in the lymphoid follicle-dense mucosa at the terminal rectum (36). Usually the bacteria are isolated from healthy animals, and just in young animals, an initial episode of diarrhea occurs. Fecal contamination of meat during slaughter, the use of feces as fertilizer, and the contamination of drinking water are major ways by which this organism can enter the human food chain (6, 37, 49).
EHEC O157:H7 also belongs to category B bioterrorism diseases/agents. The vaccination of cattle to prevent or clear up colonization with EHEC O157:H7 is aimed at interrupting EHEC infections in ruminant animals and thereby preventing its transmission to humans (5, 52). The inclusion of an EHEC vaccine into a combination with other diarrheal vaccines for humans would be of great benefit to counter bioterrorism and to help prevent the spread of the disease in children and the elderly. Presently, several candidate vaccines against EHEC are under development (7, 52) and have been tested in mouse models (16, 23, 28, 44). It is believed that a vaccine that inhibits the organism from colonizing the intestinal tracts of both cattle and humans reflects the most promising way to prevent the infection (16, 29, 31). To inhibit the adherence of the pathogen to the mucosa, a vaccine that includes all important antigenic cell surface factors is needed (6, 29). The oral-immunization route mimics the natural path of infection and should be capable of eliciting local immunity in the gut (5).
Bacterial ghosts are produced by the controlled expression of φX174 lysis gene E. E-mediated lysis of bacteria results in the formation of empty bacterial cell envelopes, which have the same cell surface composition as their living counterparts. They display all surface components in a natural nondenatured form and are able to induce a strong mucosal immune response (for reviews, see references 24 and 25). Even highly sensitive and fragile structures like pili are well protected by this technology, as was demonstrated recently for Vibrio cholerae ghosts expressing the toxin-coregulated pilus (12). Furthermore, Pasteurella haemolytica ghosts, which induce immunity in cattle (35), exhibit a broader spectrum of protection in rabbits than chemically inactivated Pasteurella haemolytica (34).
In conventional, nonviable whole-cell vaccines, antigenic epitopes are heavily impaired by physical or chemical processing treatments which are not used in bacterial-ghost technology (14, 46). In addition, conventional subunit vaccines produced from many microorganisms are often less immunogenic, and adjuvants have to be added to the vaccine formulation. Bacterial ghosts themselves show adjuvant properties (39) and represent an excellent carrier system for foreign antigens (11, 21, 40).
To avoid the presence of pathogenic islands and antibiotic resistance genes in the bacterial-ghost vaccine preparation, the DNA is completely degraded by a nuclease in combination with the protein E-mediated lysis system (17). The thermostable nuclease (EC 3.1.4.7) of Staphylococcus aureus (SNUC) cleaves either single- or double-stranded DNA and RNA into nucleotides, acting as a phosphodiesterase (1-3, 19). SNUC is fully dependent on Ca2+, and supplementation with Mg2+ has a stimulatory effect on DNase activity (8). Apart from its natural host, the staphylococcal nuclease has been expressed in its active form in various gram-positive and gram-negative bacteria (4, 10, 30, 47). Recently, the E-mediated lysis and SNUC inactivation of E. coli K-12 strain NM522 has been described in detail (17), and in this study, the method was adapted for the first time to an EHEC strain at the fermentor scale. The EHEC O157:H7 ghosts produced by the combination of E-mediated lysis and SNUC activity were tested in a mouse model without the addition of any adjuvant as an oral vaccine and exhibited protection against lethal challenge even at a single-dose application.
MATERIALS AND METHODS
Lysis plasmid pML1 and construction of plasmid pSUNCIQ3.
Plasmid pML1 (46) carries lysis gene E under the transcriptional control of the phage λ PR cI857 system. Induction of gene E expression is achieved by a temperature shift from 28°C to 42°C of bacterial cultures harboring pML1 (46).
The staphylococcal nuclease A gene is under the control of a strong synthetic promoter, A1-O4/O3, and nuclease expression was achieved by the addition of 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG).
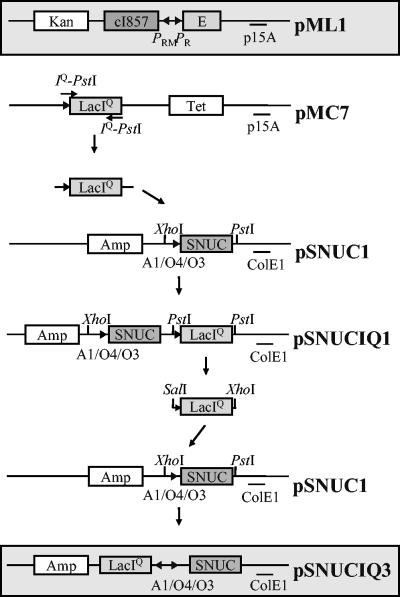
For this study, to obtain a SNUC expression vector that can be used independently of a chromosomally or F′ factor-encoded repressor gene, the lacIq gene was introduced into pSNUC1 (17) to give pSNUCIQ1 and pSNUCIQ3. A 1,300-bp PCR fragment containing the lacIq gene was generated by PCR amplification with plasmid pMC7 as the template and primers to introduce PstI restriction sites at the termini. This fragment was cloned into the corresponding site of pSNUC1, resulting in plasmid pSNUCIQ1 (Fig. 1). Plasmid pSNUCIQ1 was digested with SalI and XhoI, and the 1,300-bp DNA fragment containing the lacIq gene was inserted into the corresponding sites of plasmid pSUNC1, resulting in plasmid pSNUCIQ3 (Fig. 1).
FIG. 1.
Schematic drawing of plasmid pML1 and construction of plasmid pSNUCIQ3. Plasmid pML1, encoding the lysis protein E, and plasmid pSNUCIQ3, encoding SNUC, were used for the coexpression of gene E and SNUC in E. coli O157:H7. The expression of the killing genes was triggered either by the addition of a chemical inducer (pSNUCIQ3) or by a thermal shift from 28°C to 42°C (pML1). E, lysis gene E; Amp, ampicillin resistance gene; Kan, kanamycin resistance gene; Tet, tetracycline resistance gene; ColE1 and p15A, origins of replication; A1-O4/O3, synthetic, chemically inducible promoter; PRMand PR, rightward “maintenance” and rightward promoters of bacteriophage lambda, respectively; cI857, gene encoding the thermosensitive repressor for the lambda PR promoter; lacIq, gene encoding the repressor for the synthetic A1-O4/O3 promoter; XhoI, PstI, and SalI, restriction sites used for the construction of pSNUCIQ3.
Bacterial strains and growth conditions.
EHEC serotype O157:H7 (strains CIP 103571 and CIP 105282, both expressing Stx1 and Stx2) was obtained from the Collection de l'Institut Pasteur, Paris, France.
The lysis plasmid pML1 (46) was cotransformed with the plasmid pSNUCIQ3 (Fig. 1) into EHEC strain CIP 105282 as described by Sambrook et al. (42) and grown in Luria-Bertani (LB) broth supplemented with kanamycin (50 μg/ml) and ampicillin (100 μg/ml). Cultures were supplemented with 1% glucose during overnight incubations and subsequently washed twice with LB prior to further lysis experiments (17). For full activity of the expressed nuclease, 1 mM MgCl2 and 10 mM CaCl2 were added. Growth and lysis of the bacteria were monitored by measuring the optical density at 600 nm (OD600). Samples were taken at various time points during growth, and viable cell counts were determined using a spiral plater (WASP system; Don Whitley Scientific, Ltd., West Yorkshire, United Kingdom).
Production of EHEC O157:H7 bacterial ghosts.
EHEC CIP 105282 ghosts were produced in a 10-liter fermentor (Meredos, Bovenden, Germany) with a stirring rate of 350 rpm and 3.5 liters of air per min. No antifoam was added, and pH values were stable in the range (6.5 to 7.5) necessary for successful E-mediated lysis (32). To induce nuclease expression, IPTG was added to the EHEC cultures at an OD600 of 0.3. Protein E-mediated lysis was induced 45 min later by a temperature shift from 28°C to 42°C. MgCl2 and CaCl2 were added 90 min after induction of lysis. The number of CFU within the bacterial samples taken during lysis and nuclease treatment was determined using the spiral plater. Samples were serially diluted in 0.85% NaCl, inoculated onto LB agar plates, and incubated at 28°C overnight. Preparation of the total DNA of the ghost samples and electrophoretic analysis were done as described recently in detail (17).
The ghosts were collected by centrifugation 6 h after lysis induction and washed three times with 0.85% NaCl solution (with 1/3, 1/6, and finally 1/12 of the starting culture volume). The final cell pellet was resuspended in 20 ml distilled water and freeze-dried for 24 h. Ten milligrams of the lyophilized ghost preparations was inoculated in LB, incubated for 1 week at 28°C, and analyzed for living-cell counts by plating on LB agar plates.
Experimental animals.
Inbred mice (BALB/c, male, 4 weeks old) were obtained from the vivarium of the State Research Centre of Virology and Biotechnology “Vector” (Koltsovo, Novosibirsk region, Russia). Animals were placed into individual cages with autoclaved food and water available ad libitum.
Oral vaccination of mice with EHEC ghosts and challenge scheme.
Mice were divided into four groups, A to D, with different schemes of immunization. All mice were deprived of food 24 h before oral immunization. Ten minutes before immunization and challenge, 30 μl of 10% sodium hydrocarbonate was given orally.
Mice from group A, divided into two subgroups (group A1, 35 mice, and group A2, 30 mice), were immunized once on day 0. Group B mice, divided into two subgroups (group B1, 35 mice, and group B2, 30 mice), were immunized twice, once on day 0 and once on day 28. For all immunizations, the oral dose was 1 mg of freeze-dried EHEC ghost strain CIP 105282 (corresponding to 4.8 × 109 dead bacterial ghosts) in 30 μl of phosphate-buffered saline (PBS), applied through a soft polyethylene catheter. Group C mice (the control for group A), divided into two subgroups (group C1, 35 mice, and group C2, 30 mice), were immunized with a placebo (30 μl of PBS) once on day 0. Mice from group D (the control for group B), divided into two subgroups (group D1, 35 mice, and group D2, 30 mice), were immunized with a placebo (30 μl of PBS) twice, once on day 0 and once on day 28. On day 55, all mice were orally challenged with 108 CFU of heterologous EHEC strain CIP 103571.
For specific antibody determinations, blood was taken from the orbital sinuses of the mice at various time points from subgroups A1, B1, C1, and D1 under methoxyflurane anesthesia. Colon lavage samples (9) as well as spleen samples (22) were taken after the animals were killed by decapitation. Three mice were used for each time point on day 0, in weekly intervals after the immunizations, and after the challenge.
Mice from subgroups A2, B2, C2, and D2 were used as controls of mortality after challenge. All mice remaining after the experiment were killed on day 76 (corresponding to day 21 postchallenge) by CO2.
Infection confirmation.
Before challenge and on days 1 to 8, 14, and 21 postchallenge, all mice were examined for the presence of EHEC O157:H7 in feces, by use of a diagnostic reagent E. coli O157 latex test kit (Oxoid Ltd., Basingstoke, Hampshire, England) according to the manufacturer's instructions. A positive result with the O157 latex reagent was interpreted as large clumps of agglutinated latex and bacteria with partial or complete clearing of the background latex within 1 to 2 min, similar to the positive-control suspension provided by the kit.
Antibody determinations.
The presence of specific immunoglobulin A (IgA) and IgG antibodies against EHEC ghosts in sera and in the colon lavage samples was determined by an enzyme-linked immunosorbent assay (9). Briefly, 100 μl carbonate-bicarbonate buffer (pH 9.6) containing 0.5 μg EHEC CIP 105282 ghosts was added to each well of a microtiter plate (MaxiSorp surface assay plates; Nunc, Denmark) and incubated for 12 h at 4°C. The plate was washed three times with PBS containing 0.05% Tween 20 (PBS-T) and blocked for 1 h at 37°C with blocking buffer containing 0.1% milk protein in PBS. After the plate was washed three times with PBS-T, sera from immunized and control mice, at a starting dilution of 1:4 in PBS-T, were titrated through a twofold-dilution series. Plates were incubated at 37°C for 2 h and washed four times with PBS-T. One hundred microliters of goat anti-mouse IgG or IgA conjugated with horseradish peroxidase (ICN), at dilutions of 1:10,000 for IgG and 1:5,000 for IgA, was added per well and incubated for another hour at 37°C. Plates were washed four times with PBS-T. Then, o-phenylenediamine (FAST o-phenylenediamine dihydrochloride tablet sets; Sigma) was used according to the manufacturer's instructions (100 μl/well), and plates were incubated at room temperature for about 30 min. The reaction was stopped with 50 μl of 0.5 M H2SO4, and the absorbance was read at 490 nm.
ELISpot analysis.
Spleen cells (1 × 105 cells/well) were activated with EHEC CIP 105282 ghosts at 0.5 μg/100 μl or inactivated measles antigen (Edmonston strain) at 0.5 μg/100 μl as a nonspecific antigen. As a control, spleen cells left without any activation were used. An enzyme-linked immunospot (ELISpot) assay was carried out with specific gamma interferon (IFN-γ) antibodies according to the manufacturer's instructions (ELISpot mouse IFN-γ; R&D Systems, Inc., Minneapolis, Minn.).
Cell proliferation assay.
Spleen cells (1 × 105 cells/well) were cultured in 100 μl of RPMI medium as described previously (22). Cells were stimulated with EHEC CIP 105282 ghosts at 5 μg/ml as a specific antigen, with inactivated measles antigen (Edmonston strain) at 2 μg/ml as a nonspecific antigen, or with concanavalin A (ConA) at 5 μg/ml as a mitogen. Incubation was carried out at 37°C in a 5% CO2-humidified atmosphere for 96 h. XTT {2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide} and PMS (phenazine methosulfate) (Sigma) were added as previously described (22, 43) before the final 8 h of a 4-day culture, and the absorbance was read at 450 nm. Results were calculated as the ratio between the control (spleen cells left without any activation) OD and a sample OD and are presented as an index.
Statistical analysis.
Statistical significance was determined by Student's t or the chi-square test. P values of <0.05 were considered significant.
RESULTS
Production of nucleic acid-free Escherichia coli O157:H7 ghosts.
Lysis plasmid pML1 was cotransformed with the plasmid pSNUCIQ3 into EHEC strain CIP 105282. This vector combination (Fig. 1) allowed the induction of gene E by a temperature shift and SNUC by the addition of IPTG independently of each other.
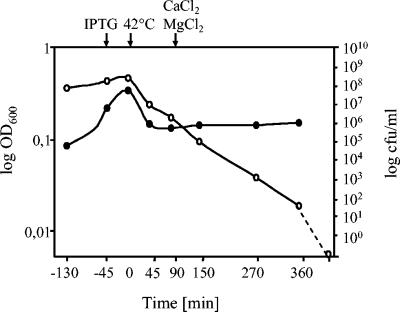
EHEC strain CIP 105282(pML1, pSNUCIQ3) was grown with aeration in a total volume of 7 liters, and the OD and live-cell counts were followed during growth and lysis (Fig. 2). The OD of the culture decreased during the first hour after induction of gene E expression due to cell lysis and remained constant for the next 5 h until the bacterial ghosts were harvested. The number of CFU decreased after expression of gene E and SNUC until the time of ghost harvest. Six hours after the temperature shift for lysis induction, the viability of the culture decreased by 8 orders of magnitude. Taking the number of viable cells at the time point of 90 min (constant OD), it was calculated that the lysis efficiency was at least 99%. However, light microscopy indicated that a higher value than 99% is likely (99.99%). The contribution of SNUC to cell killing was an additional 3 to 4 logs, as determined by viable cell counts of E. coli O157:H7 ghost preparations with and without induction of SNUC expression. Gel electrophoretic analysis of extracted DNA from the EHEC O157:H7 ghosts produced by expression of protein E and SNUC showed fragments less than 100 bp in size.
FIG. 2.
Growth and lysis kinetics of E. coli O157:H7 strain CIP 105282(pML1, pSNUCIQ3). Growth and lysis were monitored by the measurement of the OD600 (•) and the determination of the number of CFU (○). The dashed line indicates the reduction of cell viability to zero after the freeze-drying process. The time points of supplementation with IPTG (−45 min), the temperature shift to 42°C (0 min), and the addition of CaCl2 and MgCl2 (90 min) are indicated by arrows at the top of the figure.
After freeze-drying of the washed ghosts, no survivors were found after 7 days of enrichment growth conditions in an amount of 10 mg EHEC ghosts, corresponding to 10 times the immunization dose.
EHEC O157:H7 ghost immunization of mice and heterologous challenge.
After the first (group A) and the second (group B) oral immunizations with EHEC O157:H7 ghosts, the mice did not change their behavior and did not show any signs of illness. Before the oral challenge with 108 viable EHEC O157:H7 (strain CIP 103571) cells, the feces samples of all mice were negative for the presence of E. coli O157. Postchallenge, immunized mice were positive for E. coli O157 from days 1 to 3. In contrast, sham-immunized mice showed positive results for E. coli O157 from days 1 to 7 postchallenge. After 7 days, feces determinations for E. coli O157 challenge bacteria were negative for all surviving mice and remained negative until day 21 postchallenge, at which time point all mice were killed.
A clear difference in survival rate (Table 1) was seen between the immunized group and the group that received the placebo. In group A2 (one oral immunization at day 0), 26 mice survived (86.6%), whereas in group B2 (two oral immunizations, at days 0 and 28), 28 mice survived (93.3%) after the heterologous challenge at day 55. There was no statistical difference in survival rate between the two immunized groups (A2 and B2). In the control groups, eight mice from group C2 survived (26.7%) and nine mice from group D2 survived (30%).
TABLE 1.
Survival rates among immunized and control mice after infection with E. coli O157:H7
| Group | No. of surviving mice/no. of dead mice on postchallenge day:
|
Survival rate (%) | |||
|---|---|---|---|---|---|
| 3 | 5 | 10 | 21 | ||
| A2a | 30/0 | 26/4 | 26/4 | 26e/4 | 86.6 |
| B2b | 30/0 | 28/2 | 28/2 | 28e/2 | 93.3 |
| C2c | 30/0 | 14/16 | 8/22 | 8/22 | 26.7 |
| D2d | 30/0 | 20/10 | 9/21 | 9/21 | 30.0 |
Mice immunized with EHEC ghosts once (day 0).
Mice immunized with EHEC ghosts twice (day 0 and day 28).
Mice immunized with PBS once (control for group A2).
Mice immunized with PBS twice (control for group B2).
P > 0.001.
The conditions of the mice were monitored daily for 3 weeks postchallenge. Disease manifestations appeared on days 4 to 7 after the challenge and were as follows: slowing of activity, no stimulus reaction, anorexia, and convulsions before death.
Antibody determination.
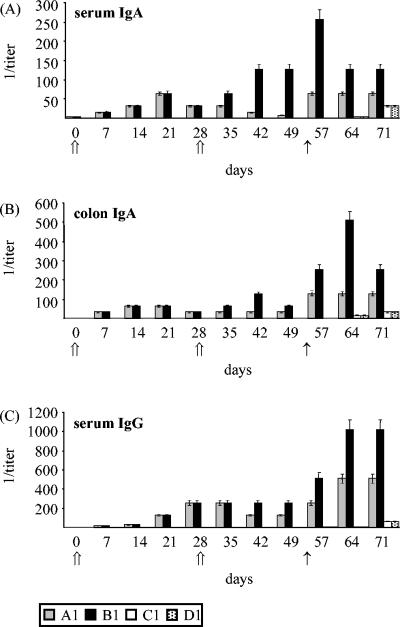
Titers of specific IgA and IgG antibodies against EHEC O157:H7 in serum and colon samples were analyzed by enzyme-linked immunosorbent assay, using EHEC ghosts as the antigen (Fig. 3). In immunized mice, IgG antibodies were found in sera (Fig. 3C) but not in colon samples, whereas IgA antibodies were found in both serum (Fig. 3A) and colon (Fig. 3B) samples. No specific antibodies were found in the placebo groups during the immunization period. Mice in group A1 (immunized orally at day 0) showed the highest levels of IgA in sera at day 21 postimmunization but already on day 14 in the colon samples. The titers decreased in both serum and colon samples after day 21. Animals immunized twice, at days 0 and 28 (group B1), showed the highest IgA titers both in colon (P < 0.01) and in serum (P < 0.001) samples on day 42. The titer stayed constant until challenge in the serum samples but decreased in the colon samples at day 49. IgG antibodies in sera (Fig. 3C) reached the highest peaks on day 28 for mice immunized only on day 0 (group A1) and for mice immunized on days 0 and 28 (group B1). In the latter group, antibody titers did not change after booster immunization until challenge, whereas the titers in animals immunized only at day 0 dropped to half. All antibody determinations showed an increase in titer after exposure to the pathogen (challenge), with an evident and statistical (P < 0.01 to 0.001) prevalence of antibodies in group B1. EHEC-specific IgG and IgA were absent in serum and intestine samples from the control groups (C and D) until day 64. After day 64, the survivors of the control groups showed increases in specific-antibody titers for serum IgG, serum IgA, and colon IgA.
FIG. 3.
Antibody levels in mice following oral immunization with EHEC O157:H7 ghosts and challenge with E. coli O157:H7. Each value represents the mean for three mice. (A) Serum IgA antibody titers against EHEC O157:H7 ghosts. (B) Colon IgA antibody titers against EHEC O157:H7 ghosts. (C) Serum IgG antibody titers against EHEC O157:H7 ghosts. Mice were immunized intragastrically with 1 mg EHEC O157:H7 ghosts once (group A) or twice (group B) (⇑). All mice were challenged with 1 × 108 CFU EHEC O157:H7 (CIP 103571) on day 55 (↑).
ELISpot analysis for the detection of antigen-dependent IFN-γ-secreting cells.
An ELISpot analysis was performed to determine the cellular side of the immune response induced by EHEC O157:H7 ghosts, as measured by the level of IFN-γ production of spleen cells (Table 2). Mice from control groups C1 and D1 did not show any changes in IFN-γ levels during the entire observation period. A statistically significant increase (P < 0.01) of counted spots (each spot representing an individual IFN-γ-secreting cell) was observed in both groups A1 and B1 after the first immunization with EHEC CIP 105282 ghosts. The production of IFN-γ was statistically higher (P < 0.01, 47 spots on day 49) in animals that were immunized twice (group B1) than in those that were immunized once (group A1) (32 spots on day 35). The number of counted spots was highest for spleen cells taken after the challenge in both groups. In group A1, counted spots rose up to 42, whereas in group B1, they rose up to 56. The in vitro stimulation of spleen cells with measles antigen (unrelated antigen) showed spot numbers up to 5.5, compared to 2 for the control cells, which were not stimulated with any antigen.
TABLE 2.
Results of ELISpot analysis of spleen cells in mice
| Group | Antigena | No. of counted spots (mean ± SD) at dayb:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 57 | 64 | 71 | ||
| A1c | Ghosts | 5.6 ± 0.34 | 8.3 ± 0.34 | 11.0 ± 0.68 | 16.0 ± 0.68 | 28.0 ± 0.68 | 32.0 ± 1.36 | 32.0 ± 1.02 | 30.0 ± 1.02 | 36.0 ± 1.02 | 38.0 ± 1.36 | 42.0 ± 1.36 |
| Measles | 2.3 ± 0.32 | 3.0 ± 0.02 | 4.0 ± 0.68 | 5.5 ± 0.68 | 4.0 ± 0.68 | 4.0 ± 0.68 | 5.0 ± 1.02 | 4.0 ± 0.68 | 4.0 ± 0.68 | 4.0 ± 0.68 | 4.0 ± 0.68 | |
| Control | 0 | 1.0 ± 0.34 | 0.7 ± 0.59 | 2.0 ± 0.68 | 0.7 ± 0.34 | 1.0 ± 0.68 | 1.0 ± 0.68 | 1.0 ± 0.68 | 1.0 ± 0.68 | 0.7 ± 0.34 | 0.7 ± 0.34 | |
| B1d | Ghosts | 5.4 ± 0.32 | 8.3 ± 0.34 | 11.0 ± 1.02 | 16.0 ± 0.68 | 28 ± 1.02 | 34 ± 1.02 | 42.0 ± 1.02 | 47.0 ± 1.36 | 52.0 ± 1.02 | 54.0 ± 1.36 | 56.0 ± 1.36 |
| Measles | 2.0 ± 0.28 | 2.6 ± 0.34 | 4.0 ± 1.02 | 4.0 ± 0.68 | 4.0 ± 0.68 | 5.0 ± 0.68 | 5.0 ± 1.02 | 5.0 ± 1.02 | 4.0 ± 0.68 | 5.0 ± 1.02 | 5.0 ± 1.02 | |
| Control | 0 | 1.0 ± 0.68 | 1.0 ± 0.68 | 2.0 ± 0.34 | 0.7 ± 0.34 | 1.0 ± 0.68 | 0.7 ± 0.34 | 0.7 ± 0.34 | 1.0 ± 0.68 | 1.0 ± 0.34 | 07 ± 0.34 | |
| C1e | Ghosts | 5.2 ± 0.42 | 6.2 ± 0.34 | 6.0 ± 0.42 | 6.1 ± 0.62 | 5.6 ± 0.42 | 6.0 ± 0.64 | 6.0 ± 0.82 | 5.8 ± 0.42 | 6.0 ± 0.63 | 8.0 ± 1.16 | 8.6 ± 1.26 |
| Measles | 2.3 ± 0.20 | 2.4 ± 0.12 | 2.2 ± 0.43 | 2.4 ± 0.53 | 2.7 ± 0.52 | 2.8 ± 0.38 | 2.4 ± 0.42 | 2.6 ± 0.58 | 2.4 ± 0.58 | 4.0 ± 0.58 | 4.0 ± 0.42 | |
| Control | 0 | 1.0 ± 0.20 | 0.7 ± 0.59 | 1.2 ± 0.26 | 0.9 ± 0.24 | 1.0 ± 0.48 | 1.0 ± 0.28 | 1.0 ± 0.28 | 1.0 ± 0.42 | 1.2 ± 0.32 | 1.4 ± 0.24 | |
| D1f | Ghosts | 5.1 ± 0.44 | 5.8 ± 0.26 | 6.2 ± 0.42 | 6.0 ± 0.58 | 6.2 ± 0.56 | 6.0 ± 0.42 | 5.8 ± 0.62 | 6.0 ± 0.64 | 6.8 ± 0.64 | 7.2 ± 0.82 | 8.6 ± 0.84 |
| Measles | 2.2 ± 0.26 | 2.2 ± 0.24 | 2.6 ± 0.42 | 2.4 ± 0.48 | 2.1 ± 0.42 | 2.8 ± 0.40 | 2.4 ± 0.26 | 2.4 ± 0.26 | 2.8 ± 0.42 | 3.6 ± 0.54 | 3.8 ± 1.02 | |
| Control | 0 | 1.0 ± 0.24 | 1.4 ± 0.48 | 1.1 ± 0.34 | 1.2 ± 0.24 | 1.1 ± 0.28 | 1.2 ± 0.24 | 1.2 ± 0.22 | 1.2 ± 0.40 | 1.4 ± 0.24 | 1.4 ± 0.26 | |
Cells were activated with EHEC O157:H7 ghosts as a specific antigen or with inactivated measles as a nonspecific antigen. Cells left without activation were used as a negative control.
Values are the average results from three mice per time point and represent the number of counted spots in 1 × 105 cells. Day 55 was the day of challenge. Day 0 was the day of first immunization for all groups. Groups A1 and B1 were immunized with EHEC ghosts; and groups C1 and D1 were given a placebo immunization with PBS. Day 28 was the day of second immunization for groups B1 (EHEC ghosts) and D1 (PBS). Day 55 was the day of challenge.
Mice immunized with EHEC ghosts once (day 0).
Mice immunized with EHEC ghosts twice (day 0 and day 28).
Mice immunized with PBS once (control for group A1).
Mice immunized with PBS twice (control for group B1).
Cell proliferation assay.
Spleen cells were used to do a cell proliferation assay (Table 3). Mice from control groups C1 and D1 showed no differences in the cell proliferation indexes during the whole observation period. After stimulation with measles antigen (unrelated antigen), proliferation values between 1.02 and 1.05 were found. After stimulation with ConA (mitogen), values between 2.0 and 2.4 were found. Stimulation with EHEC CIP 105282 ghosts led to proliferation of cells in both groups A1 and B1, but cell proliferation was higher for group B1. There was a statistical (P < 0.05) difference in spleen cell proliferation indexes between day 0 and day 28 in both groups. The highest values were obtained after the challenge for both groups (1.6 for group A1 and 1.8 for group B1). However, the differences in values on day 49 (before challenge) and on day 57 (after challenge) were not statistically significant.
TABLE 3.
Cell proliferation index of spleen cells in mice
| Group | Antigen or mitogena | Amt of cell proliferation (mean ± SD) at dayb:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 57 | 64 | 71 | ||
| A1c | Ghosts | 1.04 ± 0.08 | 1.10 ± 0.10 | 1.40 ± 0.10 | 1.46 ± 0.10 | 1.46 ± 0.10 | 1.4 ± 0.10 | 1.42 ± 0.08 | 1.40 ± 0.08 | 1.46 ± 0.10 | 1.52 ± 0.10 | 1.60 ± 0.1 |
| Measles | 1.04 ± 0.08 | 1.02 ± 0.08 | 1.02 ± 0.08 | 1.03 ± 0.08 | 1.04 ± 0.12 | 1.02 ± 0.08 | 1.04 ± 0.12 | 1.03 ± 0.08 | 1.02 ± 0.10 | 1.03 ± 0.08 | 1.04 ± 0.10 | |
| ConA | 2.0 ± 0.10 | 2.20 ± 0.10 | 2.20 ± 0.10 | 2.20 ± 0.10 | 2.20 ± 0.08 | 2.30 ± 0.12 | 2.20 ± 0.10 | 2.40 ± 0.20 | 2.30 ± 0.10 | 2.30 ± 0.08 | 2.20 ± 0.08 | |
| B1d | Ghosts | 1.04 ± 0.08 | 1.10 ± 0.08 | 1.40 ± 0.08 | 1.40 ± 0.08 | 1.46 ± 0.08 | 1.50 ± 0.10 | 1.60 ± 0.18 | 1.62 ± 0.08 | 1.70 ± 0.08 | 1.76 ± 0.10 | 1.80 ± 0.08 |
| Measles | 1.04 ± 0.10 | 1.03 ± 0.08 | 1.02 ± 0.08 | 1.02 ± 0.08 | 1.04 ± 0.08 | 1.04 ± 0.10 | 1.04 ± 0.10 | 1.03 ± 0.10 | 1.04 ± 0.10 | 1.03 ± 0.10 | 1.05 ± 0.08 | |
| ConA | 2.0 ± 0.10 | 2.20 ± 0.10 | 2.20 ± 0.10 | 2.20 ± 0.10 | 2.20 ± 0.08 | 2.30 ± 0.10 | 2.30 ± 0.08 | 2.20 ± 0.10 | 2.20 ± 0.10 | 2.10 ± 0.10 | 2.30 ± 0.08 | |
| C1e | Ghosts | 1.02 ± 0.08 | 1.08 ± 0.10 | 1.10 ± 0.10 | 1.10 ± 0.10 | 1.08 ± 0.10 | 1.10 ± 0.10 | 1.08 ± 0.08 | 1.06 ± 0.08 | 1.16 ± 0.10 | 1.22 ± 0.10 | 1.21 ± 0.10 |
| Measles | 1.04 ± 0.08 | 1.04 ± 0.08 | 1.06 ± 0.08 | 1.04 ± 0.08 | 1.06 ± 0.12 | 1.04 ± 0.08 | 1.04 ± 0.12 | 1.03 ± 0.08 | 1.08 ± 0.10 | 1.06 ± 0.08 | 1.04 ± 0.10 | |
| ConA | 2.10 ± 0.10 | 2.20 ± 0.10 | 2.22 ± 0.10 | 2.21 ± 0.10 | 2.22 ± 0.10 | 2.26 ± 0.12 | 2.21 ± 0.10 | 2.36 ± 0.16 | 2.28 ± 0.10 | 2.30 ± 0.08 | 2.28 ± 0.08 | |
| D1f | Ghosts | 1.02 ± 0.08 | 1.06 ± 0.08 | 1.08 ± 0.08 | 1.04 ± 0.08 | 1.06 ± 0.08 | 1.05 ± 0.10 | 1.06 ± 0.18 | 1.08 ± 0.08 | 1.12 ± 0.08 | 1.16 ± 0.10 | 1.20 ± 0.08 |
| Measles | 1.02 ± 0.10 | 1.02 ± 0.08 | 1.03 ± 0.06 | 1.02 ± 0.08 | 1.04 ± 0.08 | 1.04 ± 0.10 | 1.04 ± 0.10 | 1.04 ± 0.10 | 1.04 ± 0.08 | 1.04 ± 0.10 | 1.04 ± 0.08 | |
| ConA | 2.10 ± 0.10 | 2.20 ± 0.10 | 2.20 ± 0.10 | 2.21 ± 0.10 | 2.20 ± 0.06 | 2.27 ± 0.10 | 2.28 ± 0.08 | 2.25 ± 0.12 | 2.22 ± 0.10 | 2.18 ± 0.10 | 2.30 ± 0.08 | |
Cells were activated with EHEC O157:H7 ghosts as a specific antigen, inactivated measles as a nonspecific antigen, or ConA as a mitogen.
Values are the average results from three mice per time point and represent the ratios between control cells (left without any activation) and samples. Day 0 was the day of first immunization for all groups. Groups A1 and B1 were immunized with EHEC ghosts, and groups C1 and D1 were given a placebo immunization with PBS. Day 28 was the day of second immunization for groups B1 (EHEC ghosts) and D1 (PBS). Day 55 was the day of challenge.
Mice immunized with EHEC ghosts once (day 0).
Mice immunized with EHEC ghosts twice (day 0 and day 28).
Mice immunized with PBS once (control for group A1).
Mice immunized with PBS twice (control for group B1).
DISCUSSION
Modern vaccines should represent a stable and cold chain-independent dry powder for noninvasive administration and should be safe and efficacious for infants as well as the elderly (51). In the model study presented here, it is evident that EHEC O157:H7 ghosts given orally as a resuspension of freeze-dried material fulfill the requirements for the first half of the previous statement. The protective immunity against a lethal challenge induced in mice gives hope that, in cattle and humans, similar protective levels might be achieved.
Bacterial ghosts provide all surface antigens in their natural conformations (12, 20), and because of the induced immune response after immunization with EHEC O157:H7 ghosts, it can be assumed that the adherence of the challenge bacteria to the target cells (16, 29, 31) has been hampered by the induction of antibodies against surface antigens. Antibodies against surface antigens, like intimin, EspA, EspB, Tir, and lipopolysaccharide (LPS), were previously detected in humans after EHEC infections (6, 29). It is possible that anti-O157 LPS antibodies may protect against EHEC O157:H7-induced illnesses (6, 52). The properties of LPS present in the outer membranes of the bacterial ghosts have been reported previously (12). For bacterial-ghost vaccines, the LPS content has no effect when delivered orally. The minimal toxicity, even after intravenous administration of cell-associated LPS in comparison to free LPS, does not limit the use of bacterial ghosts as a candidate vaccine (33).
The bacterial-ghost candidate vaccine against E. coli O157:H7 was produced by the coexpression of gene E and SNUC. Since SNUC is a nuclease, ghost production was accompanied by the degradation of the genetic material of the host cells. The expression of gene E led to empty bacterial cell envelopes (ghosts), and the staphylococcal nuclease caused intracellular degradation of the host and plasmid DNA in the E. coli O157:H7 ghost preparation, as has been demonstrated previously for E. coli K-12 strain NM522 (17). The results presented herein show that the coexpression of lysis gene E and SNUC, followed by freeze-drying, resulted in the total inactivation of EHEC O157:H7 strain CIP 105282, representing a vaccine formulation free of bacterial and plasmid DNA. No live cells were detected in enrichment cultures of EHEC ghost samples with 10 times the amount of the immunization dose. Thus, this freeze-dried, nonliving vaccine candidate is safe for mice and has the potential to be used as an oral vaccine without a hazard for horizontal gene transfer of resistance genes or pathogenic islands to the resident gut flora of the vaccinated animal or person. For convenience of application and safety, an oral route is preferred, especially for children. Conventionally prepared, nonliving-full-cell or subunit bacterial vaccine preparations have been shown to be less immunogenic when delivered orally. Mucosal adjuvants are added to these vaccine formulations to elicit protective immunity against enteric pathogens, even though the negative side effects of adjuvants are well known (24, 39). Since bacterial ghosts carry immunostimulatory compounds that have adjuvant properties, such as LPS, lipids, and peptidoglycan, no additional adjuvants are necessary. These intrinsic adjuvant properties of bacterial ghosts were shown to activate the innate immune system as well as the acquired immune response. Bacterial ghosts have been tested in different animal models for their ability to induce mucosal immune responses (for reviews, see references 24, 39, and 51). In cell culture experiments, bacterial ghosts were taken up very efficiently by dendritic cells and macrophages expressing plasmid-borne marker genes which were delivered together with the bacterial ghosts (27, 38).
Oral immunization of mice with EHEC ghosts revealed the formation of both cellular and humoral immune responses. This was confirmed by the results of the cell proliferation test, the levels of IFN-γ production by spleen cells, and the levels of specific IgG and IgA in serum and colon samples. A strong booster response for all determined antibodies was observed following oral challenge with the pathogen. IgG was not found in the colon samples, supporting the suggestion that systemic IgG antibodies do not egress to the intestinal mucosa (5) and emphasizing the advantage of the oral route of immunization. Locally acting IgA directed against the LPS O side chain of EHEC O157:H7 might also be involved in the resistance of the bacteria in colonizing the gut (7). The production of IFN-γ by spleen cells isolated from immunized animals indicates the stimulation of both the cellular and the humoral immune responses. Recently, recombinant V. cholerae ghosts carrying Chlamydia trachomatis antigens were shown to induce a chlamydia-specific Th1 response by measuring the IFN-γ production by spleen T cells (11). Protection against lethal challenge has also been detected in rabbits orally immunized with V. cholerae ghosts (13).
Even more important is the ability of EHEC ghosts to confer immunity against a lethal challenge given 55 days after a single oral dose to mice. The immunized mice showed high rates of protection (86.6% to 93.3%) to the challenge with the heterologous EHEC strain (EHEC CIP 103571) in comparison to the negative control (26.7% to 30%). Shu and Gill (44) reported a morbidity of BALB/c mice of about 45% after feeding the mice Bifidobacterium lactis strain HN019 intragastrically prior to challenge with EHEC O157:H7, in contrast to a morbidity of about 75% for the negative control.
Further, it is promising that the single immunization was equally as protective as double immunizations against the lethal challenge in mice, since the difference in survival rates was not statistically significant. This shows that the achievable systemic and mucosal immunity was adequately matured and provided a high level of protection after the first immunization. However, antibody levels and IFN-γ production in the animals that were immunized twice led us to speculate that mild natural infections with the pathogen or related bacteria after the immunization will confer long-lasting protective immunity.
Bacterial ghosts as candidate vaccines and carriers of foreign viral and/or bacterial antigens are under development as multivalent vaccines against diarrheal diseases of humans and might represent new, improved nonliving bacterial vaccines with excellent safety properties and high immunological potential.
Acknowledgments
We thank Cesar V. Mujer, VitalProbes, Inc., for critical reading of the manuscript.
This work has been supported by BIRD-C GmbH & CoKEG, Vienna, Austria.
Editor: A. D. O'Brien
REFERENCES
- 1.Alexander, M., L. A. Heppel, and J. Hurwitz. 1961. The purification and properties of micrococcal nuclease. J. Biol. Chem. 236:3014-3019. [PubMed] [Google Scholar]
- 2.Anfinsen, C. B., P. Cuatrecasas, and H. Taniuchi. 1971. Staphylococcal nuclease: chemical properties and catalysis, p. 177-201. In P. Boyer (ed.), The enzymes, vol. 4. Academic Press, New York, N.Y. [Google Scholar]
- 3.Anfinsen, C. B., A. N. Schechter, and H. Taniuchi. 1972. Some aspects of the structure of staphylococcal nuclease. II. Studies in solution. Cold Spring Harbor Symp. Quant. Biol. 36:249-255. [PubMed] [Google Scholar]
- 4.Boynton, Z. L., J. J. Koon, E. M. Brennan, J. D. Clouart, D. M. Horowitz, T. U. Gerngross, and G. W. Huisman. 1999. Reduction of cell lysate viscosity during processing of poly(3-hydroxyalkanoates) by chromosomal integration of the staphylococcal nuclease gene in Pseudomonas putida. Appl. Environ. Microbiol. 65:1524-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlan, J. W., A. D. Cox, R. KuoLee, A. Webb, and M. B. Perry. 1999. Parenteral immunization with a glycoconjugate vaccine containing the O157 antigen of Escherichia coli O157:H7 elicits a systemic humoral immune response in mice, but fails to prevent colonization by the pathogen. Can. J. Microbiol. 45:279-286. [PubMed] [Google Scholar]
- 6.Conlan, J. W., R. KuoLee, A. Webb, and M. B. Perry. 1999. Salmonella landau as a live vaccine against Escherichia coli O157:H7 investigated in a mouse model of intestinal colonization. Can. J. Microbiol. 45:723-731. [PubMed] [Google Scholar]
- 7.Conlan, J. W., and M. B. Perry. 1998. Susceptibility of three strains of conventional adult mice to intestinal colonization by an isolate of Escherichia coli O157:H7. Can. J. Microbiol. 44:800-805. [DOI] [PubMed] [Google Scholar]
- 8.Cuatrecasas, P., S. Fuchs, and C. B. Anfinsen. 1967. Catalytic properties and specificity of the extracellular nuclease of Staphylococcus aureus. J. Biol. Chem. 242:1541-1547. [PubMed] [Google Scholar]
- 9.Douce, G., M. M. Giuliani, V. Giannelli, M. G. Pizza, R. Rappuoli, and G. Dougan. 1998. Mucosal immunogenicity of genetically detoxified derivatives of heat labile toxin from Escherichia coli. Vaccine 16:1065-1073. [DOI] [PubMed] [Google Scholar]
- 10.Dutton, E. K., S. A. Ottum, T. C. Bolken, C. A. Franke, and D. E. Hruby. 2000. Expression of active monomeric and dimeric nuclease A from the gram-positive Streptococcus gordonii surface protein expression system. Protein Expr. Purif. 19:158-172. [DOI] [PubMed] [Google Scholar]
- 11.Eko, F. O., W. Lubitz, L. McMillan, K. Ramey, T. T. Moore, G. A. Ananaba, D. Lyn, C. M. Black, and J. U. Igietseme. 2003. Recombinant Vibrio cholerae ghosts as a delivery vehicle for vaccinating against Chlamydia trachomatis. Vaccine 21:1694-1703. [DOI] [PubMed] [Google Scholar]
- 12.Eko, F. O., U. B. Mayr, S. R. Attridge, and W. Lubitz. 2000. Characterization and immunogenicity of Vibrio cholerae ghosts expressing toxin-coregulated pili. J. Biotechnol. 83:115-123. [DOI] [PubMed] [Google Scholar]
- 13.Eko, F. O., T. Schukovskaya, E. Y. Lotzmanova, V. V. Firstova, N. V. Emalyanova, S. N. Klueva, A. L. Kravtzov, L. F. Livanova, V. V. Kutyrev, J. U. Igietseme, and W. Lubitz. 2003. Evaluation of the protective efficacy of Vibrio cholerae ghost (VCG) candidate vaccines in rabbits. Vaccine 21:3663-3674. [DOI] [PubMed] [Google Scholar]
- 14.Eko, F. O., A. Witte, V. Huter, B. Kuen, S. Furst-Ladani, A. Haslberger, A. Katinger, A. Hensel, M. P. Szostak, S. Resch, H. Mader, P. Raza, E. Brand, J. Marchart, W. Jechlinger, W. Haidinger, and W. Lubitz. 1999. New strategies for combination vaccines based on the extended recombinant bacterial ghost system. Vaccine 17:1643-1649. [DOI] [PubMed] [Google Scholar]
- 15.Fujii, J., T. Kita, S.-I. Yoshida, T. Takeda, H. Kobayashi, N. Tanaka, K. Ohsato, and Y. Mizuguchi. 1994. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H− in mitomycin-treated mice. Infect. Immun. 62:3447-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funatogawa, K., T. Ide, F. Kirikae, K. Saruta, M. Nakano, and T. Kirikae. 2002. Use of immunoglobulin enriched bovine colostrum against oral challenge with enterohaemorrhagic Escherichia coli O157:H7 in mice. Microbiol. Immunol. 46:761-766. [DOI] [PubMed] [Google Scholar]
- 17.Haidinger, W., U. B. Mayr, M. P. Szostak, S. Resch, and W. Lubitz. 2003. Escherichia coli ghost production by expression of lysis gene E and staphylococcal nuclease. Appl. Environ. Microbiol. 69:6106-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hancock, D. D., T. E. Besser, D. H. Rice, E. D. Ebel, D. E. Herriott, and L. V. Carpenter. 1998. Multiple sources of Escherichia coli O157 in feedlots and dairy farms in the northwestern USA. Prev. Vet. Med. 35:11-19. [DOI] [PubMed] [Google Scholar]
- 19.Heins, J. N., J. R. Suriano, H. Taniuchi, and C. B. Anfinsen. 1967. Characterization of a nuclease produced by Staphylococcus aureus. J. Biol. Chem. 242:1016-1020. [PubMed] [Google Scholar]
- 20.Huter, V., A. Hensel, E. Brand, and W. Lubitz. 2000. Improved protection against lung colonization by Actinobacillus pleuropneumoniae ghosts: characterization of a genetically inactivated vaccine. J. Biotechnol. 83:161-172. [DOI] [PubMed] [Google Scholar]
- 21.Huter, V., M. P. Szostak, J. Gampfer, S. Prethaler, G. Wanner, F. Gabor, and W. Lubitz. 1999. Bacterial ghosts as drug carrier and targeting vehicles. J. Control. Release 61:51-63. [DOI] [PubMed] [Google Scholar]
- 22.Ignatyev, G. M., S. A. Kaliberov, A. T. Godneva, A. V. Tverdochlebov, L. A. Pereboeva, I. V. Patrusheva, and E. A. Kashentseva. 1995. Immunity parameters in mice of different lines infected with Machupo or Lassa viruses, p. 250-253. In Proceedings of the 3rd Congress, European Society for Veterinary Virology. Fondation Marcel Mérieux, Lyon, France.
- 23.Ishikawa, S., K. Kawahara, Y. Kagami, Y. Isshiki, A. Kaneko, H. Matsui, N. Okada, and H. Danbara. 2003. Protection against Shiga toxin 1 challenge by immunization of mice with purified mutant Shiga toxin 1. Infect. Immun. 71:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalava, K., F. O. Eko, E. Riedmann, and W. Lubitz. 2003. Bacterial ghosts as carrier and targeting systems for mucosal antigen delivery. Expert Rev. Vaccines 2:45-51. [DOI] [PubMed] [Google Scholar]
- 25.Jalava, K., A. Hensel, M. Szostak, S. Resch, and W. Lubitz. 2002. Bacterial ghosts as vaccine candidates for veterinary applications. J. Control. Release 85:17-25. [DOI] [PubMed] [Google Scholar]
- 26.Karpman, D., H. Connell, M. Svensson, F. Scheutz, P. Alm, and C. Svanborg. 1997. The role of lipopolysaccharide and Shiga-like toxin in a mouse model of Escherichia coli O157:H7 infection. J. Infect. Dis. 175:611-620. [DOI] [PubMed] [Google Scholar]
- 27.Kudela, P., S. Paukner, U. B. Mayr, D. Cholujova, Z. Schwarczova, J. Sedlak, J. Bizik, and W. Lubitz. 2005. Bacterial ghosts as novel efficient targeting vehicles for DNA delivery to the human monocyte-derived dendritic cells. J. Immunother. 28:136-143. [DOI] [PubMed] [Google Scholar]
- 28.LeBlanc, J., I. Fliss, and C. Matar. 2004. Induction of a humoral immune response following an Escherichia coli O157:H7 infection with an immunomodulatory peptidic fraction derived from Lactobacillus helveticus-fermented milk. Clin. Diagn. Lab. Immunol. 11:1171-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Y., E. Frey, A. M. R. Mackenzie, and B. B. Finlay. 2000. Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect. Immun. 68:5090-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liebl, W., A. J. Sinskey, and K.-H. Schleifer. 1992. Expression, secretion, and processing of staphylococcal nuclease by Corynebacterium glutamicum. J. Bacteriol. 174:1854-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovett, R. A. 1998. Training a molecular gun on killer E. coli. Science 282:1404. [DOI] [PubMed] [Google Scholar]
- 32.Lubitz, W., G. Halfmann, and R. Plapp. 1984. Lysis of Escherichia coli after infection with phiX174 depends on the regulation of the cellular autolytic system. J. Gen. Microbiol. 130:1079-1087. [DOI] [PubMed] [Google Scholar]
- 33.Mader, H. J., M. P. Szostak, A. Hensel, W. Lubitz, and A. G. Haslberger. 1997. Endotoxicity does not limit the use of bacterial ghosts as candidate vaccines. Vaccine 15:195-202. [DOI] [PubMed] [Google Scholar]
- 34.Marchart, J., G. Dropmann, S. Lechleitner, T. Schlapp, G. Wanner, M. P. Szostak, and W. Lubitz. 2003. Pasteurella multocida- and Pasteurella haemolytica-ghosts: new vaccine candidates. Vaccine 21:3988-3997. [DOI] [PubMed] [Google Scholar]
- 35.Marchart, J., M. Rehagen, G. Dropmann, M. P. Szostak, S. Alldinger, S. Lechleitner, T. Schlapp, S. Resch, and W. Lubitz. 2003. Protective immunity against pasteurellosis in cattle, induced by Pasteurella haemolytica ghosts. Vaccine 21:1415-1422. [DOI] [PubMed] [Google Scholar]
- 36.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. E. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen, S. J., G. Miller, T. Breuer, M. Kennedy, C. Higgins, J. Walford, G. McKee, K. Fox, W. Bibb, and P. Mead. 2002. A waterborne outbreak of Escherichia coli O157:H7 infections and hemolytic uremic syndrome: implications for rural water systems. Emerg. Infect. Dis. 8:370-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paukner, S., P. Kudela, G. Kohl, T. Schlapp, S. Friederichs, and W. Lubitz. 2005. DNA-loaded bacterial ghosts efficiently mediate reporter gene transfer and expression in macrophages. Mol. Ther. 11:215-223. [DOI] [PubMed] [Google Scholar]
- 39.Riedmann, E. M., J. M. Kyd, A. W. Cripps, and W. Lubitz. Adjuvant properties of bacterial ghosts. Vaccine, in press.
- 40.Riedmann, E. M., J. M. Kyd, A. M. Smith, S. Gomez-Gallego, K. Jalava, A. W. Cripps, and W. Lubitz. 2003. Construction of recombinant S-layer proteins (rSbsA) and their expression in bacterial ghosts—a delivery system for the nontypeable Haemophilus influenzae antigen Omp26. FEMS Immunol. Med. Microbiol. 37:185-192. [DOI] [PubMed] [Google Scholar]
- 41.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York, N.Y.
- 43.Scudiero, D. A., R. H. Shoemaker, K. D. Paull, A. Monks, S. Tierney, T. H. Nofziger, M. J. Currens, D. Seniff, and M. R. Boyd. 1988. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 48:4827-4833. [PubMed] [Google Scholar]
- 44.Shu, Q., and H. S. Gill. 2001. A dietary probiotic (Bifidobacterium lactis HN019) reduces the severity of Escherichia coli O157:H7 infection in mice. Med. Microbiol. Immunol. 189:147-152. [DOI] [PubMed] [Google Scholar]
- 45.Su, C., and L. J. Brandt. 1995. Escherichia coli O157:H7 infection in humans. Ann. Intern. Med. 123:698-714. [DOI] [PubMed] [Google Scholar]
- 46.Szostak, M. P., A. Hensel, F. O. Eko, R. Klein, T. Auer, H. Mader, A. Haslberger, S. Bunka, G. Wanner, and W. Lubitz. 1996. Bacterial ghosts: non-living candidate vaccines. J. Biotechnol. 44:161-170. [DOI] [PubMed] [Google Scholar]
- 47.Takahara, M., D. W. Hibler, P. J. Barr, J. A. Gerlt, and M. Inouye. 1985. The ompA signal peptide directed secretion of staphylococcal nuclease A by Escherichia coli. J. Biol. Chem. 260:2670-2674. [PubMed] [Google Scholar]
- 48.Tarr, P. I., M. A. Neill, D. L. Christie, and D. E. Anderson. 1988. Escherichia coli O157:H7 hemorrhagic colitis. N. Engl. J. Med. 318:1697. [PubMed] [Google Scholar]
- 49.Verweyen, H. M., H. Karch, M. Brandis, and L. B. Zimmerhackl. 2000. Enterohemorrhagic Escherichia coli infections: following transmission routes. Pediatr. Nephrol. 14:73-83. [DOI] [PubMed] [Google Scholar]
- 50.Wadolkowski, E. A., L. M. Sung, J. A. Burris, J. E. Samuel, and A. D. O'Brien. 1990. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect. Immun. 58:3959-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walcher, P., U. B. Mayr, C. Azimpour-Tabrizi, F. O. Eko, W. Jechlinger, P. Mayrhofer, T. Alefantis, C. V. Mujer, V. G. DelVecchio, and W. Lubitz. 2004. Antigen discovery and delivery of subunit vaccines by nonliving bacterial ghost vectors. Expert Rev. Vaccines 3:681-691. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. 1999. New frontiers in the development of vaccines against enterotoxinogenic (ETEC) and enterohaemorrhagic (EHEC) E. coli infections. Part II. Enterohaemorrhagic (EHEC) E. coli vaccines. Wkly. Epidemiol. Rec. 74:105-111. [PubMed] [Google Scholar]