Abstract
One-month-old dexamethasone-immunosuppressed Mongolian gerbils were challenged with 1 oocyst to 2 × 105 oocysts from two isolates genotyped as Cryptosporidium hominis and C. parvum (genotype 2), respectively. A similar dose-dependent gut infection was obtained, and the initial genotype maintained for 21 to 22 days. The data suggest that immunosuppressed gerbils provide a reliable rodent model of persistent C. hominis infection.
Human cryptosporidiosis is caused by the coccidian parasites Cryptosporidium sp., which are considered emerging pathogens (8, 14, 24). Their entire life cycle takes place in the intestinal epithelium, and transmission occurs in the form of environmentally resistant oocysts by the fecal-oral route and/or through contaminated food and/or water (9, 14, 31). In humans, the most commonly detected genotypes are Cryptosporidium hominis (i.e., C. parvum genotype 1 recognized as a distinct species) and the bovine C. parvum genotype 2 (23, 38). C. hominis naturally infects humans almost exclusively, and experimentally, C. hominis infections were obtained in calves, lambs, and pigs with at least some isolates but not in rodents commonly used for propagating C. parvum genotype 2, with some exceptions (2, 3, 13, 14, 32, 38, 40). In spite of recent advances, the pathogenesis of cryptosporidiosis is presently poorly understood, and a limited number of effective anticryptosporidial therapeutic agents is available. The aim of the present study was to investigate the infectivity of C. hominis in an alternative laboratory rodent, the Mongolian gerbil (jird, Meriones unguiculatus, Muridae, Rodentia).
Four- to five-week-old 15- to 36-g gerbils, free of Cryptosporidium sp. oocysts before the study, hosted in individual heat-sterilized cages and given heat-sterilized rodent food and water ad libitum, were handled according to the regulations of the French Ministry of Agriculture. Isolate Cp/H4 was obtained from a human immunodeficiency virus-infected patient, and isolate MN was experimentally maintained in calves (kindly provided by R. Mancassola and M. Naciri, INRA, Nouzilly, France). Viable (>80%, excluding propidium iodide) oocysts were purified from fecal samples as previously described and stored in phosphate-buffered saline (pH 7.2) at 4°C for less than 3 months before use (20). Genomic DNA was extracted from purified oocysts by using a standard phenol-chloroform-ethanol procedure. PCR amplification was performed with the primers Cp.E (5′-GGA TGG GTA TCA GGT AAT AAG AA-3′) and Cp.Z (5′-CCA CTA GCC CAG TTC TGA CTC TCT GG-3′) corresponding to the COWP gene (33). PCR products were digested with RsaI, and the genotype was established according to characteristic PCR-restriction fragment length polymorphism (RFLP) patterns. Gerbils were injected with 0.80 mg of dexamethasone (Qualimed, Puteaux, France)/animal every second day from 10 days before oocyst ingestion to day 21 postinfection and divided into groups that ingested either 1, 10, 102, 2 × 102, 2 × 103, or 2 × 105 oocysts. Fecal smears were microscopically examined at days 7, 14, and 21 postinfection by using Heine staining, and 24-h shedding was expressed as the mean blind oocyst count per phase-contrast microscopic field (×400 magnification) from 30 microscopic fields by two independent investigators. In six animals/group that were killed on day 22 postinfection, distal ileal segments fixed in 10% formalin were cut, embedded in paraffin for histological examination of 4-μm sections stained with hematoxylin-eosin-saffron, and considered infected if at least one cryptosporidial developmental form was observed within an epithelial cell. Isolation and genotyping of shed oocysts were performed as described above. DNA from ileal samples was extracted by using the QIAGEN DNeasy tissue kit (QIAGEN Gmbh, Courtaboeuf, France), and genotypes were determined as described above. The significance of differences in distributions between groups was assessed by using Student unpaired t tests, thus assuming “normal-like” distributions of data.
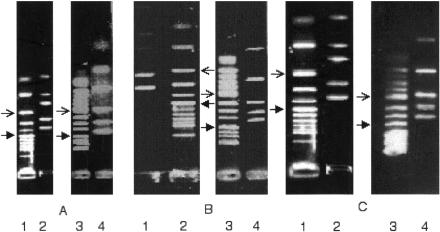
As depicted in Fig. 1A and B, oocysts from isolates Cp/H4 and MN exhibited C. hominis and C. parvum type 2 genotypes, respectively. Ingestion of 100 oocysts to 2 × 105 oocysts resulted in sustained intestinal infection, as evidenced by oocyst shedding monitored for 3 weeks with no significant difference between isolates (P > 0.05; Table 1). For both isolates, oocyst shedding was maximum by day 14 (P < 0.05). In all animals challenged with 2 × 103 to 2 × 105 oocysts, parasites were histologically detected in ilea, predominantly in the distal portion. Ileal infection was found in five of six and two of six animals challenged with 200 and 100 oocysts, respectively, and was absent in animals challenged with 10 or 1 oocyst. No mucosa alteration was present, with the same histological aspect in C. hominis and C. parvum type 2-infected gerbils. In shed oocysts and ileal samples obtained on day 21 or 22, the initial genotype of ingested oocysts was maintained in all animals infected with either 2 × 103 or 2 × 105, as documented by the typical PCR-RFLP patterns depicted in Fig. 1C and D.
FIG. 1.
PCR-RFLP Cryptosporidium genotype characterization of ingested and shed oocysts and ileum samples from infected Mongolian gerbils. Typical patterns from individual experiments. (A) Ingested oocysts, uncut PCR. Lane 1, control DNA size markers, fragments ranging in size from 24 to 726 bp (φX174 DNA/Hinf; Promega, Madison, WI); lanes 2 and 3, typical 550-bp Cryptosporidium product (C. hominis isolate Cp/H4 and C. parvum genotype 2 isolate MN, respectively). (B) Ingested oocysts and RFLP characterization of PCR products. Lanes 1 and 3, 11 control double-stranded DNA fragments ranging in size from 100 to 1,500 bp (100-bp ladder; Promega); lane 2, C. hominis pattern of isolate Cp/H4 (360-, 263-, 130-, 110-, and 80-bp bands); lane 4, C. parvum genotype 2 pattern of isolate MN (456-, 300-, 180-, 100-, and 69-bp bands). (C) Shed oocysts from Cryptosporidium sp.-infected Mongolian gerbils (day 21 postinfection). RFLP characterization of PCR products. Lane 1, C. hominis pattern in animals infected with isolate Cp/H4; lane 2, 11 control double-stranded DNA fragments (100-bp ladder; Promega); lane 3, control size DNA markers (φX174 DNA/Hinf; Promega); lane 4, C. parvum genotype 2 pattern in animals infected with isolate MN. (D) Ileum samples (day 22 postinfection) from Cryptosporidium sp.-infected Mongolian gerbils and RFLP characterization of PCR products. Lanes 1 and 3, 11 control double-stranded DNA fragments (100-bp ladder; Promega); lane 2, C. hominis pattern in animals infected with isolate Cp/H4; lane 4, C. parvum genotype 2 pattern in animals infected with isolate MN. Arrows, 500 and 200 bp.
TABLE 1.
Oocyst shedding in immunosuppressed Mongolian gerbils infected with C. hominis Cp/H4 and C. parvum MN oocysts
| Isolate | No. of ingested oocysts/animal | No. of gerbils | Mean no. of shed oocysts in feces ± SDa
|
||
|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | |||
| Cp/H4 | 2 × 105 | 7 | 14.4 ± 0.9 | 19.4 ± 0.9 | 11.0 ± 1.4 |
| 2 × 103 | 7 | 14.6 ± 0.4 | 19.7 ± 0.7 | 11.1 ± 1.1 | |
| 2 × 102 | 7 | 7.6 ± 1.3 | 23.1 ± 1.5 | 11.7 ± 1.1 | |
| 100 | 7 | 5.3 ± 1.9 | 12.8 ± 4.8 | 6.3 ± 2.4 | |
| 10 | 7 | NF | NF | NF | |
| 1 | 7 | NF | NF | NF | |
| MN | 2 × 105 | 7 | 11.1 ± 0.8 | 17.0 ± 1.1 | 14.1 ± 0.8 |
| 2 × 103 | 6 | 11.5 ± 1.1 | 20.6 ± 1.2 | 17.1 ± 1.7 | |
| 2 × 102 | 6 | 10.8 ± 2.2 | 18.6 ± 0.9 | 11.5 ± 1.1 | |
| 100 | 9 | 6.5 ± 3.0 | 9.6 ± 4.3 | 7.3 ± 3.5 | |
| 10 | 6 | NF | NF | NF | |
| 1 | 6 | NF | NF | NF | |
| Controlb | 0 | 11 | NF | NF | NF |
That is, the mean number of oocysts/10 microscopic fields at various days after oocyst ingestion. NF, none found.
Control, noninfected control animals.
In the present study, a corticosteroid immunosuppression procedure was adapted from rat cryptosporidiosis models since no infection was obtained in immunocompetent gerbils (data not shown) (6, 30). This differs from other intestinal protozoal pathogen infections, to which immunocompetent adult gerbils were found experimentally to be at least partially permissive, including Cryptosporidium muris with protracted chronic infections in methylprednisolone immunosuppressed animals (18). The present regimen did not result in C. parvum genotype 2 oocyst shedding in animals older than 6 weeks (data not shown), and permissive 4- to 6-week-old animals were used further in the present study.
The lowest infective oocyst dose was 100/gerbil, which compared favorably with previous experimental models of C. parvum infection in rodents (21). In immunocompromised adult and suckling rodents, doses ranged usually from 104 to 107oocysts/animal, and some isolates were found to be infective at doses as low as 25 oocysts/animal or close to the “one-oocyst level” (4, 5, 10, 15, 17, 19, 21, 26, 35, 39). The sensitivity of 100 oocysts/animal reached in the present model was included within the 1- to 1,000-oocyst range that was considered infective in immunocompetent humans from direct and indirect evidence (7, 11, 25).
The subdivision of C. parvum into two species, C. hominis and C. parvum, has recently been proposed to replace the type 1 and 2 subspecies designations, respectively (23). C. hominis is considered as not naturally infective for animals with few exceptions and, experimentally, some C. hominis isolates were infective at high doses for calves, lambs, and piglets but not for cats, dogs, and rodents, with some exceptions (3, 8, 12, 13, 14, 22, 23, 27, 32, 36). To our knowledge, the present findings represent the first report of reproducible and stable experimental C. hominis infection in the Mongolian rodent gerbil. In contrast, the evidence thus far has clearly demonstrated that C. parvum genotype 2 infects a wide range of mammals, including bovines and humans (24, 28, 31).
Genotyping of isolates was based on a consensual PCR-RFLP analysis (16, 33, 37). Isolates Cp/H4 and MN exhibited consistently a typical COWP PCR-RFLP profile of the C. hominis and C. parvum type 2 genotype, respectively, which does not exclude the presence of a mixed infection since the sensitivity of commonly used C. parvum PCR-RFLP assays has not been thoroughly examined, and identification of a single genotype is not definitive evidence for the presence of a single genotype (29, 34). In gerbils, C. hominis infection, as well as C. parvum genotype 2 infection, was stably maintained for 3 weeks in shed oocysts and ileal samples, a finding consistent with the ingestion of single-genotype oocysts or, alternatively, indicating that displacement of C. parvum type 1 by type 2, which was noted in piglets, did not occur in gerbils (1). Infected animals did not present with clinical symptoms such as diarrhea, wasting, or weight loss, and no mortality was noted (data not shown). No differences in the rates of oocyst shedding, in the presence of parasite forms seen in ileal villi, or in ileal histology were found between animals infected with either C. hominis or C. parvum genotype 2. In gnotobiotic piglets, infection was associated with more severe disease, moderate to severe villous/mucosal attenuation, and marked lymphoid hyperplasia throughout the intestine for C. parvum and mild to moderate lesions restricted to the ileum and colon for C. hominis (23, 27). In humans, differences in oocyst shedding have been reported between C. hominis and C. parvum genotype 2 cryptosporidiosis (38).
The present data suggest that Mongolian immunosuppressed gerbils provide an easy-to-work experimental C. hominis infection model. Although it does not meet all of the criteria applicable to the human disease, this infection model should be helpful in studies of the pathogenesis of infection and in evaluating the effectiveness of anticryptosporidial agents.
Acknowledgments
This study was supported by grants from Romark Research Foundation, Programme Seine-Aval (SAIII 2004 1-20) and the Direction Régionale de l'Action Sanitaire et Sociale de Haute Normandie (Rouen, France).
The authors thank Dr. Naciri for the kind gift of C. parvum oocysts.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Akiyoshi, D. E., S. Mor, and S. Tzipori. 2003. Rapid displacement of Cryptosporidium parvum type 1 by type 2 in mixed infections in piglets. Infect. Immun. 71:5765-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyoshi, D. E., X. Feng, M. A. Buckholt, G. Widmer, and S. Tzipori. 2002. Genetic analysis of a Cryptosporidium parvum human genotype 1 isolate passaged through different host species. Infect. Immun. 70:5670-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awad-El-Kariem, F. M., H. A. Robinson, F. Petry, V. McDonald, D. Evans, and D. D. Casemore. 1998. Differentiation between human and animal isolates of Cryptosporidium parvum using molecular and biological markers. Parasitol. Res. 84:297-301. [DOI] [PubMed] [Google Scholar]
- 4.Blagburn, B. L., K. L. Drain, T. M. Land, R. G. Kinard, P. H. Moore, D. S. Lindsay, D. A. Patrick, D. W. Boykin, and R. R. Tidwell. 1998. Comparative efficacy evaluation of dicationic carbazole compounds, nitazoxanide, and paromomycin against Cryptosporidium parvum infections in a neonatal mouse model. Antimicrob. Agents Chemother. 42:2877-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boher, Y., I. Perez-Schael, G. Caceres-Dittmar, G. Urbina, R. Gonzalez, G; Kraal, and F. J. Tapia. 1994. Enumeration of selected leukocytes in the small intestine of BALB/c mice infected with Cryptosporidium parvum. Am. J. Trop. Med. Hyg. 50:145-151. [PubMed] [Google Scholar]
- 6.Brasseur, P., D. Lemeteil, and J. J. Ballet. 1988. Rat model for human cryptosporidiosis. J. Clin. Microbiol. 26:1037-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chappell, C. L., P. C. Okhuysen, C. R. Sterling, and H. L. DuPont. 1996. Cryptosporidium parvum: intensity of infection and oocyst excretion patterns in healthy volunteers. J. Infect. Dis. 173:232-236. [DOI] [PubMed] [Google Scholar]
- 8.Chen, X. M., J. S. Keithly, C. V. Paya, and N. F. LaRusso. 2002. Cryptosporidiosis. N. Engl. J. Med. 346:1723-1731. [DOI] [PubMed] [Google Scholar]
- 9.Current, W. L., and L. S. Garcia. 1991. Cryptosporidiosis. Clin. Microbiol. Rev. 4:325-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaunay, A., G. Gargala, X. Li, L. Favennec, and J. J. Ballet. 2000. Quantitative flow cytometric evaluation of maximal Cryptosporidium parvum oocyst infectivity in a neonate mouse model. Appl. Environ. Microbiol. 66:4315-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuPont, H. L., C. L. Chappell, C. R. Sterling, P. C. Okhuysen, J. B. Rose, and W. Jakubowski. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332:855-859. [DOI] [PubMed] [Google Scholar]
- 12.Ebeid, M., A. Mathis, A. Pospischil, and P. Deplazes. 2003. Infectivity of Cryptosporidium parvum genotype I in conventionally reared piglets and lambs. Parasitol. Res. 90:232-235. [DOI] [PubMed] [Google Scholar]
- 13.Giles, M., K. A. Webster, J. A. Marshall, J. Catchpole, and T. M. Goddard. 2001. Experimental infection of a lamb with Cryptosporidium parvum genotype 1. Vet. Rec. 149:523-525. [DOI] [PubMed] [Google Scholar]
- 14.Guerrant, R. L. 1997. Cryptosporidiosis: an emerging, highly infectious threat. Emerg. Infect. Dis. 3:51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harp, J. A. 1999. Oral dosing of neonatal mice with sucrose reduces infection with Cryptosporidium parvum. J. Parasitol. 85:952-955. [PubMed] [Google Scholar]
- 16.Jiang, J., and L. Xiao. 2003. An evaluation of molecular diagnostic tools for the detection and differentiation of human-pathogenic Cryptosporidium spp. J. Eukaryot. Microbiol. 50:542-547. [DOI] [PubMed] [Google Scholar]
- 17.Korich, D. G., Marshall, M. M., Smith, H. V., J. O'Grady, Z. Bukhari, C. R. Fricker, J. P. Rosen, and J. L. Clancy. 2000. Inter-laboratory comparison of the CD-1 neonatal mouse logistic dose-response model for Cryptosporidium parvum oocysts. J. Eukaryot. Microbiol. 47:294-298. [DOI] [PubMed] [Google Scholar]
- 18.Koudela, B., D. Modry, and J. Vitovec. 1998. Infectivity of Cryptosporidium muris isolated from cattle. Vet. Parasitol. 76:181-188. [DOI] [PubMed] [Google Scholar]
- 19.Lacroix, S., R. Mancassola, M. Naciri, and F. Laurent. 2001. Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: role of tumor necrosis factor alpha in protection. Infect. Immun. 69:1635-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, X., P. Brasseur, P. Agnamey, D. Lemeteil, L. Favennec, J. J. Ballet, and J. F. Rossignol. 2003. Long-lasting anticryptosporidial activity of nitazoxanide in an immunosuppressed rat model. Folia Parasitol. 50:19-22. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay, D. S. 1997. Laboratory models of cryptosporidiosis, p. 209-223. In R. Faye (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Inc., Boca Raton, Fla.
- 22.Morgan, U. M., L. Xiao, B. D. Hill, P. O'Donoghue, J. Limor, A. Lal, and R. C. Thompson. 2000. Detection of the Cryptosporidium parvum “human” genotype in a dugong (Dugong dugon). J. Parasitol. 86:1352-1354. [DOI] [PubMed] [Google Scholar]
- 23.Morgan-Ryan, U. M., A. Fall, L. A. Ward, N. Hijjawi, I. Sulaiman, R. Fayer, R. C. Thompson, M. Olson, A. Lal, and L. Xiao. 2002. Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J. Eukaryot. Microbiol. 49:433-440. [DOI] [PubMed] [Google Scholar]
- 24.O'Donoghue, P. J. 1995. Cryptosporidium and cryptosporidiosis in man and animals. Int. J. Parasitol. 25:139-195. [DOI] [PubMed] [Google Scholar]
- 25.Okhuysen, P. C., C. L. Chappell, J. H. Crabb, C. R. Sterling, and H. L. DuPont. 1999. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 180:1275-1281. [DOI] [PubMed] [Google Scholar]
- 26.Okhuysen, P. C., S. M. Rich, C. L. Chappell, K. A. Grimes, G. Widmer, X. Feng, and S. Tzipori. 2002. Infectivity of a Cryptosporidium parvum isolate of cervine origin for healthy adults and interferon-γ knockout mice. J. Infect. Dis. 185:1320-1325. [DOI] [PubMed] [Google Scholar]
- 27.Pereira, S. J., N. E. Ramirez, L. Xiao, and L. A. Ward. 2002. Pathogenesis of human and bovine Cryptosporidium parvum in gnotobiotic pigs. J. Infect. Dis. 186:715-718. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez, N. E., L. A. Ward, and S. Sreevatsan. 2004. A review of the biology and epidemiology of cryptosporidiosis in humans and animals. Microbes Infect. 6:773-785. [DOI] [PubMed] [Google Scholar]
- 29.Reed, C., G. D. Sturbaum, P. J. Hoover, and C. R. Sterling. 2002. Cryptosporidium parvum mixed genotypes detected by PCR-restriction fragment length polymorphism analysis. Appl. Environ. Microbiol. 68:427-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehg, J. E., M. L. Hancock, and D. B. Woodmansee. 1988. Characterization of a dexamethasone-treated rat model of cryptosporidial infection. J. Infect. Dis. 158:1406-1407. [DOI] [PubMed] [Google Scholar]
- 31.Rose, J. 1997. Environmental ecology of Cryptosporidium parvum and public health implications. Annu. Rev. Public Health 18:135-161. [DOI] [PubMed] [Google Scholar]
- 32.Sang-Mee Guk, Tai-Soon Yong, Soon-Jung Park, Jae-Hwan Park and Jong-Yil Chai. 2004. Genotype and animal infectivity of a human isolate of Cryptosporidium parvum in the Republic of Korea. Korean J. Parasitol. 42:85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spano, F., L. Putignani, J. McLauchlin, D. P. Casemore, and A. Cristanti. 1997. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol. Lett. 150:209-217. [DOI] [PubMed] [Google Scholar]
- 34.Tanriverdi, S., M. O. Arslan, D. E. Akiyoshi, S. Tzipori, and G. Widmer. 2003. Identification of genotypically mixed Cryptosporidium parvum populations in humans and calves. Mol. Biochem. Parasitol. 130:13-22. [DOI] [PubMed] [Google Scholar]
- 35.Upton, S. J., and H. H. Gillock. 1996. Infection dynamics of Cryptosporidium parvum in ICR outbred suckling mice. Folia Parasitol. 43:101-106. [PubMed] [Google Scholar]
- 36.Widmer, G., D. Akiyoshi, M. A. Buckholt, X. Feng, S. M. Rich, K. M. Deary, C. A. Bowman, P. Xu, Y. Wang, X. Wang, G. A. Buck, and S. Tzipori. 2000. Animal propagation and genomic survey of a genotype 1 isolate of Cryptosporidium parvum. Mol. Biochem. Parasitol. 108:187-197. [DOI] [PubMed] [Google Scholar]
- 37.Widmer, G., L. Lin, V. Kapur, X. Feng, and M. S. Abrahamsen. 2002. Genomics and genetics of Cryptosporidium parvum: the key to understanding cryptosporidiosis. Microbes Infect. 4:1081-1090. [DOI] [PubMed] [Google Scholar]
- 38.Xiao, L., U. M. Ryan. 2004. Cryptosporidiosis: an update in molecular epidemiology. Curr. Opin. Infect. Dis. 17:483-490. [DOI] [PubMed] [Google Scholar]
- 39.Yang, S., S. K. Benson, C. Du, and M. C. Healey. 2000. Infection of immunosuppressed C57BL/6N adult mice with a single oocyst of Cryptosporidium parvum. J. Parasitol. 86:884-887. [DOI] [PubMed] [Google Scholar]
- 40.Zhou, L., H. Kassa, M. L. Tischler, and L. Xiao. 2004. Host-adapted Cryptosporidium spp. in Canada geese (Branta canadensis). Appl. Environ. Microbiol. 70:4211-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]