Abstract
Neisseria meningitidis, an important cause of bacterial meningitis and septicemia worldwide, is associated with high mortality and serious sequelae. Natural immunity against meningococcal disease develops with age, but the specificity and functional activity of natural antibodies associated with protection are poorly understood. We addressed this question by using a selected subset of prevaccination sera (n = 26) with convergent or discrepant serum bactericidal activity (SBA) and infant rat protective activity (IRPA) against the serogroup B meningococcal strain 44/76-SL (B:15:P1.7,16) from Icelandic teenagers (B. A. Perkins et al., J. Infect. Dis. 177:683-691, 1998). The sera were analyzed by opsonophagocytic activity (OPA) assay, immunoblotting, immunoglobulin G (IgG) quantitation against live meningococcal cells by flow cytometry, and enzyme immunosorbent assay (EIA). High levels of SBA and OPA were reflected in distinct IgG binding to major outer membrane proteins and/or lipopolysaccharide in immunoblots. However, we could not detect any specific antibody patterns on blots that could explain IRPA. Only IgM antibody to group B capsular polysaccharide (B-PS), measured by EIA, correlated positively (r = 0.76, P < 0.001) with IRPA. Normal human sera (NHS; n = 20) from healthy Finnish children of different ages (7, 14, and 24 months and 10 years) supported this finding and showed an age-related increase in IRPA that coincided with the acquisition of B-PS specific IgM antibody. The protection was independent of complement-mediated bacterial lysis, as detected by the inability of NHS to augment SBA in the presence of human or infant rat complement and the equal protective activity of NHS in rat strains with fully functional or C6-deficient complement.
Neisseria meningitidis remains an important cause of meningitis and septicemia worldwide. Natural immunity against meningococcal disease develops with age, associated with an increase in serum bactericidal activity (SBA) (15). For serogroup A and C meningococci, SBA is primarily dependent on antibody to capsular polysaccharide (PS) (15, 60), and vaccines based on either plain PS or PS conjugated to a protein carrier are effective against disease caused by these serogroups (5, 41, 53). This does not hold true for serogroup B meningococci, against which SBA has been attributed to be primarily against noncapsular antigens (60, 62), since the purified capsular PS is poorly immunogenic (61). Nevertheless, antibodies to group B capsular polysaccharide (B-PS), predominantly of the immunoglobulin M (IgM) isotype, are naturally present in the majority of the adult population (16, 25). Specific immune responses to B-PS have been observed in the majority of adults and in 30% of children recovering from serogroup B meningococcal disease (3, 16, 17). Due to the relatively low avidity (28) and the poor bactericidal activity of anti-B-PS antibodies, especially in the presence of human complement (62), their contribution to protective immunity against serogroup B meningococci has been considered questionable.
The efficacy of the Norwegian and Cuban serogroup B vaccines, based on outer membrane vesicles (OMV) derived from their respective epidemic strains, has been proven in separate clinical trials (7, 48), and their immunogenicity has been compared in clinical trials among teenagers in Iceland (39) and among infants, toddlers, and adults in Chile (50). Although the antibody responses in these trials have been analyzed extensively (19, 20, 39, 43, 48, 50, 56), there is still much uncertainty about the specificity and functional mechanisms of antibodies providing protection against serogroup B disease. In essence, although there is evidence that SBA is associated with the protection afforded by OMV vaccines (19), lack of SBA in nonimmune sera does not necessarily predict disease susceptibility (39). Indeed, it is likely that also phagocytic killing plays an important role for protection against meningococcal disease (1, 9, 44, 47). Thus, other techniques, both functional, such as opsonophagocytic (2, 33) and whole-blood assay (32), and nonfunctional assays (34, 38, 55, 56), as well as assessment of active (49) or passive protection in animal models (21, 51), have been evaluated for providing additional information about the mechanisms of protective immunity or even for providing correlates of protection against group B meningococcal disease.
We have previously evaluated a 25% stratified subset of sera from the Icelandic study (39) collected before and after vaccination for passive protection in infant rats (52a). There we showed that while infant rat protective activity (IRPA) correlated to some extent with both SBA and anti-OMV IgG concentrations measured by enzyme immunosorbent assay (EIA), many prevaccination sera were highly protective against challenge with the Norwegian vaccine strain 44/76-SL (B:15:P1.7,16) without having SBA and vice versa. The aim of the present study was to assess the specificity and functional activity of natural antibodies responsible for IRPA against strain 44/76-SL. To this end, four subsets of prevaccination sera of Icelandic teenagers with convergent or discrepant SBA and IRPA results ( 52a) were analyzed by EIA, immunoblotting, IgG quantitation against live meningococcal cells by flow cytometry, and opsonophagocytic activity (OPA) assay. Normal human sera (NHS), collected from healthy Finnish children of different ages, were used to evaluate the development of IRPA, and rat strains with fully functional or C6 deficient complement served to assess the importance of complement-mediated bacterial lysis for protection.
(These data were presented in part at the 13th International Pathogenic Neisseria Conference in Oslo, Norway [M. Toropainen, L. Saarinen, K. Bolstad, T. E. Michaelsen, E. Rosenqvist, E. Wedege, A. Aase, and H. Käyhty, Abstracts 13th International Pathogenic Neisseria Conference, p. 303, 2002].)
MATERIALS AND METHODS
Serum samples.
Characteristics of the Icelandic study participants, the immunizations, SBA, and anti-OMV IgG concentrations have been described elsewhere (39). Previously, a 25% stratified sample (n = 92), sorted on the basis of SBA responses, was drawn by Brian Plikaytis at the Centers for Diseases Control and Prevention (Atlanta, Ga.) from the original study subjects (n = 391) from whom prevaccination samples (bleed 1) and postvaccination samples drawn 6 weeks after the second dose (bleed 3) were available. These sera were assayed for IRPA against the Norwegian vaccine strain 44/76-SL (B:15:P1.7,16) and the Cuban vaccine strain Cu385 (B:4:P1.19,15) (52a). From the IRPA and SBA data with strain 44/76-SL, the prevaccination sera fell into four different categories: IRPA positive, SBA positive sera (n = 24) (category I); IRPA negative, SBA positive sera (n = 22) (category II); IRPA positive, SBA negative sera (n = 18) (category III); and IRPA negative, SBA negative sera (n = 28) (category IV). For the present study, six to seven representative sera from each category were selected on the basis of the availability of >0.2-ml volumes and analyzed for functional and specific antibodies as described below.
In addition, NHS (n = 20) collected from healthy Finnish children of four different age groups (7, 14, and 24 months and 10 years) were used in SBA and IRPA studies. These sera had been collected in connection with previous immunogenicity studies (26) and stored frozen at −20°C until used. Informed consent and approvals from the ethics committees covered the use of these sera in the present study.
Absorption of sera with aluminum hydroxide gel-bound B-PS.
Equal volumes (0.1 ml) of aluminum hydroxide gel suspension (2% Alhydrogel; Brenntag Biosector, Denmark) were mixed with B-PS (received from Monique Moreau, Aventis Pasteur, Marcy l'Etoile, France) or C-PS (NIBSC, Potters Bar, United Kingdom) (1 mg/ml for both PS), and the suspensions were incubated for 45 min at room temperature. The aluminum hydroxide gel-PS complex [0.1 mg of PS per 3 mg of Al(OH)3] was pelleted by centrifugation at 10,000 rpm for 5 min, and the supernatant was discarded. To each tube, 0.15 ml of undiluted serum was added and incubated for 2 h at 4°C with occasional mixing. The tubes were then centrifuged at 10,000 rpm for 5 min, and the supernatant was collected and assayed for IRPA and for PS-specific antibody by EIA as described below.
Bacterial strains and growth conditions.
N. meningitidis strains 44/76-SL and Cu385 have been described previously (14, 48). For IRPA studies, the strains were passaged in rats (46) three (44/76-SL) or four (Cu385) times and stored in skimmed milk at −70°C. After rat passaging, both strains expressed the L3,7,9 immunotype, as determined by colony blotting with L3,7,9 (4A8-B2) (58) and L8 (MN43F8.10) specific monoclonal antibodies (received from B. Kuipers, NVI, Bilthoven, The Netherlands). Both strains expressed similar amounts of capsular PS as estimated by whole-cell EIAs (52). The inoculum for the protection assays was prepared as previously described (51).
SBA assay.
SBA data for the Icelandic sera were received through B. D. Plikaytis. SBA was measured according to Centers for Diseases Control and Prevention SBA assay protocol with plate-grown bacteria as described elsewhere with 25% human plasma as the exogenous complement source (39).
SBA of the sera from Finnish children was determined with human serum from an individual without bactericidal antibodies to 44/76-SL or serum from 5- to 6-day-old HsdCpb:WU rat pups (Harlan, The Netherlands) as exogenous complement sources at a final concentration of 20%. The reaction mixture had a final volume of 50 μl, and Hanks balanced salt solution with Mg2+ and Ca2+ was the dilution buffer. A mixture containing twofold serially diluted, heat-inactivated (56°C, 30 min) serum (25 μl), bacteria (15 μl, corresponding to ca. 100 CFU), and complement serum (10 μl) was incubated in duplicate wells at 37°C on a rotatory shaker at 220 rpm for 1 h. Reaction was stopped by placing the plates on ice, and 25-μl samples from each well were spotted on supplemented proteose peptone (Difco) agar plates, followed by incubation overnight at 37°C in 5% CO2. After colony counting, the results were expressed as the reciprocal of the highest serum dilution giving 50% killing of the inoculum. Sera with titers of <4 were considered SBA negative, and those with titers of ≥4 were considered SBA positive.
Opsonophagocytic assay.
OPA was measured at the Norwegian Institute of Public Health (NIPH; Oslo, Norway) as respiratory burst (RB) by flow cytometry with polymorphonuclear leukocytes (PMNL) from a donor heterozygous for the FcgRIIa allotype as effector cells and live 44/76-SL cells grown on plates to log phase as the target, as previously described (2). Human serum without any antibody activity against the target strain was used as complement source at a final concentration of 10%. The percentage of effector cells that had undergone RB was determined, and the results were expressed as the reciprocal of the highest serum dilution giving RB above the cutoff line of 15% cells. Sera with titers of <2 were considered OPA negative, and those with titers of ≥2 were considered OPA positive.
Quantification of IgG by flow cytometry.
Antimeningococcal IgG antibodies were quantified at the NIPH with live, log-grown 44/76-SL cells by flow cytometry as described previously (2). Twofold dilutions of a reference plasma (quantified by EIA against OMVs) was used as an internal standard to create a standard curve with concentration (range, 0.07 to 9.0 μg/ml) on the abscissa and median fluorescence intensity on the ordinate, to which the median fluorescence intensities of test samples were interpolated by using GraphPad Prism software (GraphPad, San Diego, CA).
Immunoblotting.
Immunoblotting was carried out at the NIPH as described previously (55) with deoxycholate-extracted OMVs (14) from strain 44/76-SL as antigen. Strips loaded with OMV antigens were incubated with 1:200 dilutions of human sera in the absence and presence of 0.15% Empigen BB to detect antibody binding to conformation-dependent epitopes (54). The intensities of IgG binding to different OMV antigens (Omp85, FetA [FrpB of 70 kDa], P1.7,16 PorA, P3.15 PorB, Rmp [class 4 protein], OpcA, OpaJ [class 5.5 protein], lipopolysaccharide [LPS] of immunotypes L3 and L8, and unidentified antigens with higher molecular masses of ∼50 kDa or in the lower range of 20 to 25 kDa) were determined visually. By visual determination, the immunoreactive bands were scored on a scale from 0 to 4, where scores between 0 and 1.5, between 2 and 2.5, and between 3 and 4 represented no or weak binding, medium binding, and strong binding, respectively.
OMV EIA.
The OMV EIA data for the Icelandic study sera were obtained from B. D. Plikaytis. As reported previously (39), these data were obtained at the NIPH and the Finlay Institute, respectively, using OMVs from their respective vaccine preparations as solid-phase antigen in their OMV EIA (43).
B-PS EIA.
IgM and IgG antibodies to meningococcal group B capsular polysaccharide (B-PS) were measured by EIA as described previously (4), using B-PS noncovalently complexed to methylated human serum albumin as coating antigen. The results are expressed as titers, i.e., reciprocals of serum dilution giving an optical density of 0.3 measured at 450 nm. The sera were assayed starting at 1:50 dilution. Negative samples were assigned a titer of 1:10.
Infant rat protection assay.
The passive protection experiments were done as described previously (51). In brief, outbred HsdCpb:WU (Harlan Nederland, Horst, The Netherlands) or inbred, complement component C6-deficient PVG (received from Mohamed R. Daha, LUMC, Leiden, The Netherlands) (24) 5- to 7-day-old infant rats (five rats/serum with sera from Finnish children and six rats/serum with sera from Icelandic teenagers) were injected intraperitoneally with 0.1 ml of 1:10-diluted serum 1 to 2 h before the intraperitoneal bacterial challenge of ca. 105 (Finnish NHS) or 106 (Icelandic prevaccination sera) CFU/pup in a final volume of 0.1 ml. Saline was used as a negative control for protection (five to six rats/group). Development of bacteremia was assessed by culturing blood samples taken 6 h after challenge. The limit of detection for blood cultures was 103 CFU/ml. Animals with sterile cultures were assigned a value of 0.3 times the detection limit, i.e., 3 × 102 CFU/ml of blood.
A protection index (PI) was generated based on the reduction in geometric mean (GM) concentration of bacteria in blood (CFU/ml). The PI was equivalent to the fold decrease in GM CFU/ml in each experimental group of animals relative to the control group of the same day. For each serum, the PI was calculated as the GM CFU/ml for control animals/GM CFU/ml for serum-treated animals. Sera with PIs of <1 were assigned a value of 1. A PI of 10 (i.e., a ≥10-fold reduction in GM CFU/ml of blood in the experimental group compared to the control group) was used as the cutoff value of protective activity. Thus, sera with PIs of <10 were considered IRPA negative and those with PIs of ≥10 were considered IRPA positive. All experimental protocols were reviewed by the Institutional Laboratory Animal Committee and approved by the Provincial Board.
Statistical methods.
All statistical analyses were done with log-transformed data. For calculation of GMs, sera with SBA or OPA titers of <2 were assigned a value of 1. Differences in GMs between different groups were calculated with two-tailed t test assuming equal variances (SPSS, Inc., Chicago, Ill.). Pearson correlation coefficients were calculated with SPSS software. For all comparisons, a P value of <0.05 was considered significant.
RESULTS
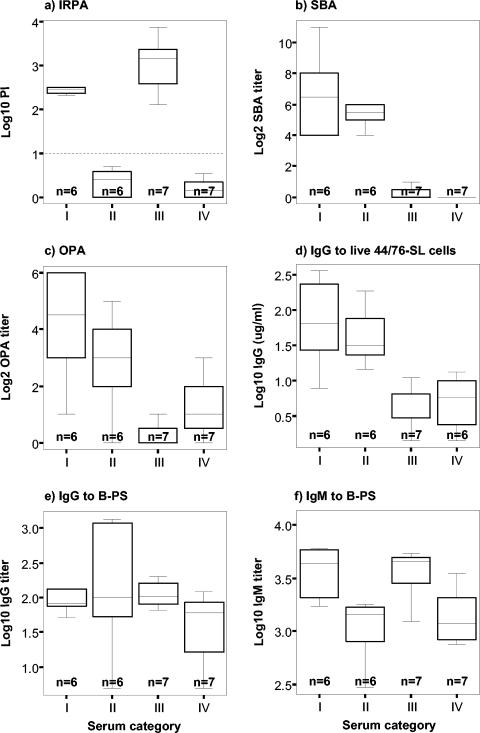
Figure 1a shows the IRPA PI values, and Fig. 1b shows the SBA titers against strain 44/76-SL for the four categories of Icelandic prevaccination sera. The OMV EIA data of these sera resembled closely the data obtained by the SBA assay: no significant differences in anti-OMV IgG concentrations between IRPA-positive, SBA-positive category I and IRPA-negative, SBA-positive category II sera, or IRPA-positive, SBA-negative category III and IRPA-negative, SBA-negative category IV sera were found (data not shown).
FIG. 1.
Summary “box-and-whiskers” plots based on the median, quartile, and extreme antibody values for the prevaccination sera from Icelandic teenagers evaluated for functionality (IRPA [a], SBA [b], OPA [c]) and quantity of meningococcal specific antibodies ([d to f]). On basis of the convergence or discrepancy of the IRPA and SBA data with the 44/76-SL (B:15:P1.7,16) strain, sera were divided into four different categories: category I, IRPA-positive, SBA-positive sera; category II, IRPA-negative, SBA-positive sera; category III, IRPA-positive, SBA-negative sera; and category IV, IRPA-negative, SBA-negative sera. The designated cutoff value (PI = 10) for IRPA is indicated by the horizontal dotted line.
Opsonophagocytic activity.
To find out whether mechanisms other than SBA might be responsible for IRPA, the OPA of the sera was evaluated with PMNL as effector cells and live 44/76-SL cells as the target. The results were similar to those obtained with SBA assay (Fig. 1c) with no significant differences between categories I and II or III and IV, respectively.
The highest OPA titers (GM = 18, 95% confidence interval [CI] = 6 to 53) were found for category I in which all (6 of 6) sera were OPA positive. Category II sera showed similar, albeit ∼2.5-fold-lower titers than category I sera (GM = 7, 95% CI = 3 to 19, P = 0.24). Five of the six (83%) sera were OPA positive. Category III sera showed negligible titers (GM = 1, 95% CI = 1 to 2); only two of seven (29%) sera were OPA positive. Category IV sera showed similar low titers as category III sera (GM = 2, 95% CI = 1 to 4, P = 0.07); five of the seven (71%) sera were OPA positive.
Antibody quantitation against viable meningococci.
IgG antibody binding to live 44/76-SL cells was determined by flow cytometry. Again, the results were similar to those obtained with the SBA assay (Fig. 1d). No significant differences in antibody levels between IRPA-positive and IRPA-negative category I and II sera or the category III and IV sera, were found.
The highest IgG levels (GM = 64 μg/ml, 95% CI = 21 to 202 μg/ml) were found for category I sera. Category II sera had similar, albeit ∼1.5-fold-lower IgG levels than category I (GM = 41 μg/ml, 95% CI = 20 to 86 μg/ml, P = 0.53). Category III had ∼10-fold-lower IgG levels (GM = 4 μg/ml, 95% CI = 2 to 6 μg/ml) than category I or II. Category IV sera had low IgG levels similar to those of category III sera (GM = 5 μg/ml, 95% CI = 3 to 9 μg/ml, P = 0.62).
Antibody specificity detected on immunoblots.
The possible association between antibody specificity and IRPA was analyzed by blotting the sera against OMV from strain 44/76-SL, followed by evaluation of the IgG binding intensity to several outer membrane components (Omp85, FetA, P1.7,16 PorA, P3.15 PorB, Rmp, OpcA, OpaJ, and L3 and L8 immunotypes) and some unknown antigens. The majority of the sera (19 of 26, 73%) showed medium or strong signals to one or more of the antigens. Although large individual variations in the antibody specificities were observed, all sera in categories I and II (n = 12) demonstrated such signals, thus roughly resembling the findings of SBA and IgG levels (data not shown). Among the 14 SBA-negative sera in category III and IV, only one serum showed strong signals, six gave medium signals, and the remaining seven sera gave no or negligible signals. No differences in the antigen binding patterns between IRPA-positive and IRPA-negative category I and II sera or category III and IV sera were detected that could account for a specific protective activity.
Antibody to B-PS.
Since antibodies to capsular PS cannot be detected by immunoblots, we used EIA to measure B-PS-specific IgG and IgM antibodies. With the exception of three individual sera with titers of >1,000 (one individual in category I and two individuals in category II), the IgG titers to B-PS were very low in all categories (GM IgG titer range of 36 to 127), with 15 of 26 (58%) of the sera having a titer of ≤100 and 22 of 26 (85%) having a titer of ≤200. No significant differences in titers between IRPA-positive and IRPA-negative category I and II sera or category III and IV sera that could explain the protection were found (Fig. 1e). In contrast, IgM titers to B-PS were significantly higher among the IRPA-positive, SBA-positive sera than the IRPA-negative, SBA-positive sera (GM IgM titers of 3,662 versus 1,064, P = 0.006), and among the IRPA-positive, SBA-negative sera than the IRPA-negative, SBA-negative sera (GM IgM titers of 3,868 versus 1,378, P = 0.013) (Fig. 1f). No significant differences in IgM titers between SBA-positive and SBA-negative sera were found.
Correlation between IRPA and in vitro antibody measurements against strain 44/76-SL.
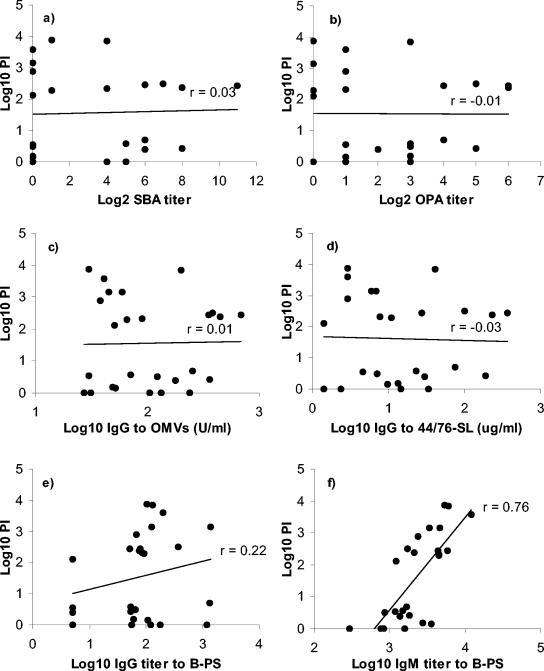
Figure 2 shows the correlation between PI and functional (SBA and OPA) or quantitative (EIAs and flow cytometric assay) antibody measurements. Only IgM antibody to B-PS measured by EIA correlated positively (r = 0.76, P < 0.001) with IRPA against strain 44/76-SL (Fig. 2f).
FIG. 2.
Correlations between IRPA and functional (SBA [a] and OPA [b]) or quantitative antibody (c to f) assays for the prevaccination sera from Icelandic teenagers (n = 26). Strain 44/76-SL (B:15:P1.7,16) was used in IRPA, SBA, OPA, and preparation of the OMVs, and in measurement of IgG binding to live cells. (a) SBA titers versus protection indices (PIs); (b) OPA titers versus PIs; (c) anti-OMV IgG concentrations versus PIs; (d) IgG binding to live 44/76-SL cells versus PIs; (e) anti-B-PS IgG concentrations versus PIs; (f) anti-B-PS IgM concentrations versus PIs.
Development of protection against strain 44/76-SL as a function of age.
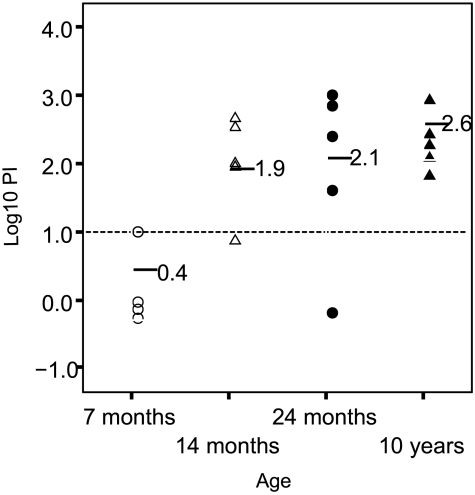
Since the Icelandic study sera were from teenagers of a narrow age group (16 to 19 years), the tracing of development of natural immunity was not possible. To study this, a set of NHS, collected from Finnish children of different ages (7, 14, and 20 months and 10 years), was tested for IRPA against strain 44/76-SL, and the results were compared to SBA and B-PS specific IgG and IgM concentrations. Since the source of complement may affect SBA assay results (62), both human and infant rat sera were used as the exogenous complement source.
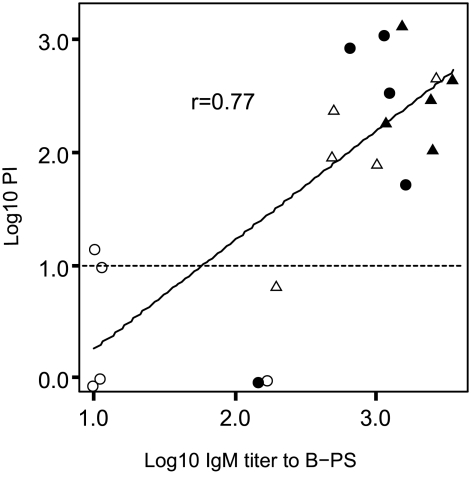
There was a clear, age-related increase in PI against 44/76-SL that coincided with acquisition of B-PS specific IgM antibody. At the age of 7 months, only one of the five (20%) individual sera was IRPA positive (PI of ≥10) (Fig. 3). At the age of 10 years, all individuals (five of five) had reached this level (Fig. 3). In SBA assays, similar low titers were obtained regardless of the complement source used; only one (5%) serum was SBA positive (titer of 4 with infant rat serum as the complement source). The positive control serum gave essentially the same titer regardless of the complement source used. Similarly to the Icelandic teenager sera, only low IgG titers to B-PS were found (GM = 27, 95% CI = 14 to 51). Again, only IgM antibody to B-PS correlated positively (r = 0.77, P < 0.001) with IRPA against strain 44/76-SL (Fig. 4).
FIG. 3.
Age-related increase in IRPA against strain 44/76-SL (B:15:P1.7,16). Sera collected from Finnish children of the four indicated ages were used (n = 5/group). Horizontal lines, GMs of PIs at different ages; horizontal dotted line, designated cutoff value (PI = 10) for IRPA. Datum points are separated by 10% (i.e., jittered) to show individual datum points.
FIG. 4.
Correlation between anti-B-PS IgM antibody concentrations and PIs for sera from Finnish children (n = 20) of four different age groups (○, 7 months; ▵, 14 months; •, 24 months; ▴, 10 years). The horizontal dotted line indicates the cutoff value (PI = 10) for IRPA.
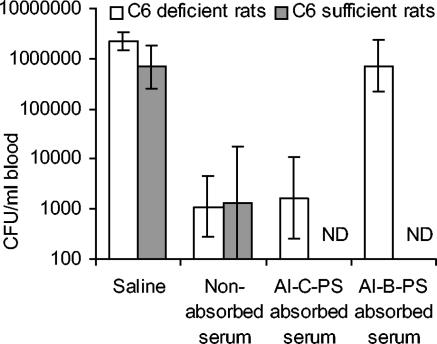
Protection by nonabsorbed and aluminum hydroxide gel-bound B-PS absorbed sera in complement component C6-deficient rats.
To confirm the importance of B-PS specific IgM antibody to protection against strain 44/76-SL, two IRPA-positive NHS (samples 105 and 109) of sufficient volumes from 10-year-old Finnish children were absorbed with aluminum hydroxide gel-bound B-PS (Al-B-PS) to deplete B-PS specific antibody, and the nonabsorbed and absorbed sera were assayed for IRPA. To further assess whether protection afforded by these sera was independent of direct bacterial lysis, as suggested by the lack of SBA with either human or infant rat serum as the complement source, a rat strain with a deficiency in the terminal part of the complement pathway (C6 deficiency), i.e., in the generation of the membrane attack complex, was used.
The absorption of B-PS specific IgM antibody was efficient and specific. In both sera, the IgM titers to B-PS decreased by >99% after Al-B-PS absorption (serum 105 from 6,400 to 80 and serum 109 from 3,600 to <50). No significant reduction in B-PS specific IgM titers was seen after absorption of the sera with aluminum hydroxide gel-bound meningococcal serogroup C capsular polysaccharide (Al-C-PS). The anti-B-PS IgG titers, which were low already before the absorptions (<200), remained essentially the same.
Figure 5 shows the IRPA results obtained with nonabsorbed, Al-B-PS-absorbed and Al-C-PS-absorbed NHS 105. For comparison, data obtained previously with the complement-sufficient rat strain are included. Both saline-treated rat strains developed similar bacteremia (GM bacteremia level 2.2 × 106 compared to 6.9 × 105 CFU/ml of blood, P = 0.06). The nonabsorbed serum was equally protective in both rat strains. As expected, absorption with Al-C-PS did not reduce protective activity. This was in contrast to absorption with Al-B-PS that completely abolished protective activity. Similar results were obtained with serum 109 (data not shown).
FIG. 5.
Influence of B-PS antibody depletion on passive protection in complement component C6-deficient rats. A serum from a 10-year-old Finnish child was absorbed with Al-B-PS or Al-C-PS. The results are shown as the GM CFU values per ml of blood ± the 95% CI from n = 5 rats. ND, not determined.
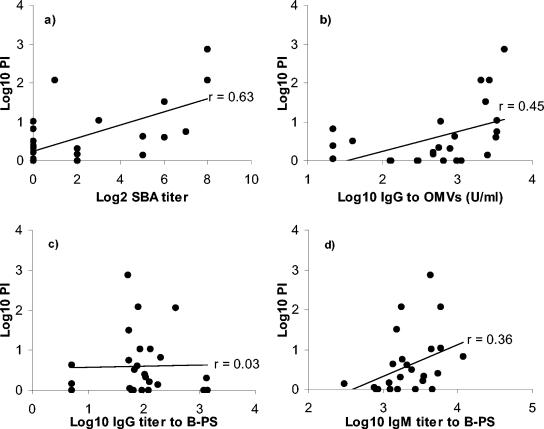
Correlation between IRPA and in vitro antibody measurements against strain Cu385.
To summarize, only IgM antibodies to B-PS correlated positively with IRPA against the 44/76-SL strain. In the light of the significantly higher protection rate conferred by the Icelandic prevaccination sera against strain 44/76-SL than against strain Cu385 (B:4:P1.19,15) (46% versus 12% [52a] ), which expressed an identical capsular PS, it was of interest to study whether a similar relationship between PI and anti-B-PS IgM concentration also existed for strain Cu385. For this purpose, IRPA data obtained with the Cu385 strain for the Icelandic serum subset were examined for correlation to SBA, OMV EIA, and anti-B-PS IgG and IgM concentrations. Although there was a trend toward higher anti-B-PS IgM titers in IRPA positive (n = 6) than IRPA negative (n = 20) sera (GM IgM titers of 3,467 versus 1,860, P = 0.11), the correlation between PI and anti-B-PS IgM concentration (r = 0.36, P = 0.07) did not reach statistical significance (Fig. 6d). Only SBA (r = 0.63, P < 0.01) and anti-OMV IgG antibody concentration (r = 0.45, P < 0.05) showed significant positive correlation with IRPA against strain Cu385 (Fig. 6a and b).
FIG. 6.
Correlations between IRPA and functional (SBA) (a) or quantitative antibody (b to d) assays for prevaccination sera from Icelandic teenagers (n = 26). Strain Cu385 (B:4:P1.19,15) was used in IRPA and SBA analyses and for preparing the OMVs. (a) SBA titers versus PIs; (b) anti-OMV IgG concentrations versus PIs; (c) anti-B-PS IgG concentrations versus PIs; (d) anti-B-PS IgM concentrations versus PIs.
DISCUSSION
We have evaluated functional and specific antibodies in prevaccination sera from Icelandic teenagers with convergent or discrepant SBA and IRPA results and found that only IgM antibody to B-PS correlated with IRPA against the serogroup B meningococcal strain 44/76-SL (B:15:P1.7,16). Experiments with NHS collected from healthy Finnish children of different ages (7, 14, and 24 months and 10 years) supported this finding. The importance of anti-B-PS IgM antibody for protection was confirmed by the complete loss of IRPA by NHS after Al-B-PS absorption. The protection against 44/76-SL seemed to be independent of direct lysis of the bacteria, as demonstrated by the inability of half of the IRPA positive Icelandic sera and the majority (13 of 14) of the Finnish NHS to promote in vitro bacterial killing in the SBA assay (titer of <4). In support of these findings, the NHS showed equal protective activity in a complement-sufficient rat strain and in a rat strain with a deficiency (C6 deficiency) in the generation of the membrane attack complex. OPA with human PMNL as the effector cells did not, however, explain the protective activity of the IRPA-positive but SBA-negative Icelandic sera since five of seven (71%) of these sera were also OPA negative (titers of <2). These findings contrast with the general conception of the minor importance of B-PS specific antibodies for protection against serogroup B meningococcal disease; due to the low avidity, especially of IgG (28), and the divergent reports of the functional activity of B-PS specific antibodies in SBA assays with human complement (27, 62), which probably arose from differences in experimental procedures (27), the role of these antibodies in protection against meningococcal infection has been challenged. On the other hand, monoclonal antibodies of human (6, 40) and mouse (11, 31, 51) origin with specificity to B-PS have previously been shown to be protective in animal models of serogroup B meningococcal or Escherichia coli K1 bacteremia, the latter bearing an immunochemically identical polysialic acid capsule (23).
It is well recognized that the use of complement from heterologous species (especially rabbits) may greatly enhance the SBA of human anti-PS antibodies compared to the activity with human complement (18, 27, 62). Thus, to exclude the possibility that the discrepancy between IRPA and SBA data was due to species-specific differences in the efficiency of complement to lyse B-PS antibody-targeted bacterial cells, we used sera from both humans and infant rats as the exogenous complement source in SBA assays with NHS. Similar low or undetectable activities were found irrespective of the complement source. Furthermore, two NHS were equally protective in vivo in rat strains with functional or C6-deficient complement. These data suggested that a mechanism(s) other than direct bacterial lysis, such as opsonophagocytosis (40), was responsible for the anti-B-PS mediated bacterial clearance, similar to previous findings in the chicken embryo model that has an active mononuclear phagocyte system but lacks functional complement (13). These findings are also in accordance with in vitro results showing that when generation of the membrane attack complex has been prevented (depletion of C8 from pooled normal human serum), serogroup B meningococci, although relatively resistant to NHS-mediated SBA, are efficiently ingested and killed by neutrophils with the indispensable augmentation of complement; heat inactivation of the NHS completely abolished phagocytic activity (44).
Thus, another aim of the present study was to find out whether the lack of correlation between IRPA and SBA could be explained by PMNL-mediated OPA, especially in IRPA-positive but SBA-negative Icelandic prevaccination sera. Somewhat unexpectedly, only low or even undetectable OPA activities were found. At present, we have no experimental data to explain the mechanism(s) behind the protection in IRPA-positive but SBA- and OPA-negative sera. One explanation could be the sensitivity of the OPA assay. PMNL lack Fcμ receptors, and therefore opsonization by IgM antibody is strictly dependent on complement activation via the classical and/or the alternative complement pathway. At low complement concentrations (<10%) activation of complement occurs mainly through the classical pathway (59). However, previous studies comparing monoclonal IgG and IgM antibodies against meningococcal surface PorA protein revealed IgM to be more active than IgG in OPA under conditions similar to those used in the present study (29, 30). Also, when different concentrations of complement serum were tested with a human monoclonal IgM anti-P1.16 PorA antibody, no change in sensitivity was observed at serum concentrations between 10 and 50% (unpublished observations). Thus, alternatively, in infant rats, the macrophages of the peritoneum or mononuclear phagocyte system (13), rather than PMNL, might have been responsible for bacterial clearance. It is well recognized that Fc and especially C3 receptors on macrophages play a crucial role in the clearance of other well-opsonized encapsulated bacteria from the bloodstream (8, 10, 35, 37, 42) and even in the absence of opsonins, pattern recognition molecules, especially the class A scavenger receptor, mediate meningococcal uptake by human bone marrow culture-derived macrophages (36).
We used immunoblotting, EIA, and flow cytometry to characterize the specificity and quantity of antibodies to meningococcal noncapsular and capsular antigens that might be responsible for IRPA against strain 44/76-SL. On blots, most of the prevaccination sera (73%) showed either medium or strong IgG binding to one or more of the outer membrane antigens of strain 44/76-SL. Such responses were not unexpected since 31% of all participants were previously identified as carriers of meningococci during the trial (39); of the carried isolates, 25% expressed PorA and/or PorB epitopes present on strain 44/76-SL, whereas 36% expressed corresponding epitopes present on strain Cu385 (56).
In general, high SBA and OPA titers were reflected in distinct IgG binding to whole meningococcal cells (measured by flow cytometry) and to the major outer membrane proteins and/or LPS on immunoblots. However, we could not detect any specific antibody patterns on the blots that could explain IRPA; three of seven of the IRPA-positive but SBA-negative sera were negative by immunoblotting. Since the blotting was performed with deoxycholate-extracted OMV as antigen, more loosely attached outer membrane antigens or cellular antigens will have escaped detection. In contrast, the anti-B-PS IgM antibody levels were approximately three times higher among IRPA-positive, SBA-positive sera than among IRPA-negative, SBA-positive sera and among IRPA-positive, SBA-negative sera than among IRPA-negative, SBA-negative sera. Although we cannot completely exclude the possibility that IgM or IgG antibody to noncapsular antigens might have also contributed to IRPA, the total loss of protection by NHS after IgM depletion by Al-B-PS absorption and the very low anti-capsular IgG concentrations suggested that it was mainly due to anti-B-PS IgM antibodies.
Similar to previous studies (16, 25), the B-PS specific antibodies in both the Icelandic sera and NHS of Finnish children were predominantly of the IgM isotype, whereas low or undetectable anti-B-PS IgG concentrations were found, especially in NHS collected from the young Finnish children. In a previous study, to increase the specificity of the assay, serum samples were assayed in the presence or absence of soluble MenB PS, and only the inhibitable fraction of the binding signal was used to calculate the anti-B-PS antibody concentrations (16). In our study, detection of B-PS specific IgM antibodies by a slightly different EIA, one adapted from that originally described by Arakere and Frasch (4), seemed very specific, as seen from the nearly complete inhibition (>99% reduction in titer) of IgM antibody binding after Al-B-PS absorption. In contrast and similar to previous studies (16, 25), IgG titers to B-PS remained essentially the same after adsorption, suggesting that a significant part of the signal represented nonspecific antibody binding or that the conditions used in the absorptions did not favor efficient IgG binding, probably due to the low avidity of IgG antibodies (28).
Since the Icelandic study sera were from teenagers (16 to 19 years old), the relatively high percentage (46% with strain 44/76-SL) of IRPA-positive prevaccination sera found in our previous study (52a) and the high prevalence (100%) of anti-B-PS IgM-positive sera in the present study were not unexpected. In order to study the kinetics of the development of IRPA and its possible association with anti-B-PS IgM antibodies, an additional set of sera collected from Finnish children of different ages (7 months to 10 years) was assayed for protective activity against strain 44/76-SL. A clear age-related increase in IRPA was found that closely coincided with the acquisition of B-PS specific IgM antibodies.
In another study (52a), we used two meningococcal strains sharing an identical capsular polysaccharide and LPS immunotype in IRPA studies with the Icelandic sera. Assuming that IgM activity to B-PS was responsible for the protection against strain 44/76-SL with the prevaccination sera (and NHS), the less apparent association of anti-B-PS IgM to protection against strain Cu385 was somewhat surprising. However, similar differences in the susceptibility of serogroup B strains to B-PS specific antibody have also been described previously. In a study by Mandrell et al. (27), the concentration of human monoclonal anti-B-PS IgM antibody needed to elicit killing in vitro in SBA assays varied from 0.03 to as much as >100 μg/ml, depending on both the monoclonal antibody clone and serogroup B meningococcal target strain used. Thus, a higher concentration of antibodies (i.e., lower serum dilution) may have been needed to confer protection against the Cu385 strain. Unfortunately, the insufficient volumes of Icelandic sera prevented the reassessment this question.
The reason for the differences in target strain susceptibility to anti-B-PS antibody is not clear but could be related to quantitative (59) and/or qualitative (22) differences in antibody-mediated deposition of opsonic C3 fragments to the bacterial surface. The activated metastable C3 has a strong preference for terminal sugar residues (45), such as the terminal galactose of lacto-N-neotetraose (LNnT) on meningococcal LPS, whereas the sialylation of LPS has been shown to inhibit bacterial killing of NHS by masking the LNnT structure (12). Thus, differences in the expression of free LNnT (not determined in the present study) or other (unidentified) C3 receptor molecules might have accounted for strain-specific differences in the anti-B-PS IgM-mediated bacterial clearance.
To conclude, we have shown that natural human IgM antibodies to serogroup B-PS can protect against experimental meningococcal bacteremia in infant rats even in the absence of complement-mediated lytic activity, thereby extending the importance of subbactericidal antibody concentrations in protection from serogroup C (57) meningococci also to group B organisms and emphasizing the importance of using multiple assays when protective immunity against meningococcal disease is measured. Before extending our findings to protection in humans, however, several factors need to be addressed. First, although our study showed no significant differences between the in vitro functional activity of meningococcal antibodies in the presence of human or infant rat complement, relatively little is known about the function of human antibodies in experimental animals in the presence of complement and phagocytic cells from heterologous species compared to their presumed function in humans. Second, the mechanism(s) behind the protective activity of IRPA-positive but SBA- and OPA-negative sera and the different sensitivities of meningococcal strains expressing an identical capsular PS to B-PS specific antibodies would be important to investigate. All of this highlights the complexity of the in vitro and in vivo assessment of protective immunity against group B meningococcus.
Acknowledgments
We are grateful to P. Helena Mäkelä for critical reading of the manuscript, to Mohamed R. Daha for providing the C6-deficient rat strain, to Brian Plikaytis for providing the SBA and OMV EIA data, and to Monique Moreau for providing B-PS. We thank Liisa Pyhälä for providing facilities for experimental work with animals and the staffs at the animal facilities of KTL in Helsinki and Kuopio for their comprehensive care of the animals.
This study was funded partially by the World Health Organization Global Programme for Vaccines and Immunization, Vaccine Research, and Development (contracts V23/181/74 and V23/181/118) and the National Meningitis Trust (United Kingdom).
Editor: J. N. Weiser
REFERENCES
- 1.Aase, A., G. Bjune, E. A. Høiby, E. Rosenqvist, A. K. Pedersen, and T. E. Michaelsen. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect. Immun. 63:3531-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aase, A., E. A. Høiby, and T. E. Michaelsen. 1998. Opsonophagocytic and bactericidal activity mediated by purified IgG subclass antibodies after vaccination with the Norwegian group B meningococcal vaccine. Scand. J. Immunol. 47:388-396. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, J., L. Berthelsen, and I. Lind. 1997. Measurement of antibodies against meningococcal capsular polysaccharides B and C in enzyme-linked immunosorbent assays: toward an improved surveillance of meningococcal disease. Clin. Diagn. Lab. Immunol. 4:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arakere, G., and C. E. Frasch. 1991. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect. Immun. 59:4349-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artenstein, M. S., R. Gold, J. G. Zimmerly, F. A. Wyle, H. Schneider, and C. Harkins. 1970. Prevention of meningococcal disease by group C polysaccharide vaccine. N. Engl. J. Med. 282:417-420. [DOI] [PubMed] [Google Scholar]
- 6.Azmi, F. H., A. H. Lucas, H. L. Spiegelberg, and D. M. Granoff. 1995. Human immunoglobulin M paraproteins cross-reactive with Neisseria meningitidis group B polysaccharide and fetal brain. Infect. Immun. 63:1906-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjune, G., E. A. Høiby, J. K. Grønnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nøkleby, and E. Rosenqvist. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 8.Bohnsack, J. F., and E. J. Brown. 1986. The role of the spleen in resistance to infection. Annu. Rev. Med. 37:49-59. [DOI] [PubMed] [Google Scholar]
- 9.Bredius, R. G., B. H. Derkx, C. A. Fijen, T. P. de Wit, M. de Haas, R. S. Weening, J. G. van de Winkel, and T. A. Out. 1994. Fc gamma receptor IIa (CD32) polymorphism in fulminant meningococcal septic shock in children. J. Infect. Dis. 170:848-853. [DOI] [PubMed] [Google Scholar]
- 10.Brown, E. J., S. W. Hosea, and M. M. Frank. 1983. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev. Infect. Dis. 5(Suppl. 4):S797-S805. [DOI] [PubMed] [Google Scholar]
- 11.Cross, A. S., W. Zollinger, R. Mandrell, P. Gemski, and J. Sadoff. 1983. Evaluation of immunotherapeutic approaches for the potential treatment of infections caused by K1-positive Escherichia coli. J. Infect. Dis. 147:68-76. [DOI] [PubMed] [Google Scholar]
- 12.Estabrook, M. M., J. M. Griffiss, and G. A. Jarvis. 1997. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect. Immun. 65:4436-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frasch, C. E., L. Parkes, R. M. McNelis, and E. C. Gotschlich. 1976. Protection against group B meningococcal disease. I. Comparison of group-specific and type-specific protection in the chick embryo model. J. Exp. Med. 144:319-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Frøholm, A. K. Lindbak, B. Mogster, E. Namork, U. Rye, G. Stabbetorp, R. Winsnes, B. Aase, and O. Closs. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67-79. [PubMed] [Google Scholar]
- 15.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granoff, D. M., S. K. Kelsey, H. A. Bijlmer, L. Van Alphen, J. Dankert, R. E. Mandrell, F. H. Azmi, and R. J. Scholten. 1995. Antibody responses to the capsular polysaccharide of Neisseria meningitidis serogroup B in patients with meningococcal disease. Clin. Diagn. Lab. Immunol. 2:574-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiss, J. M., B. L. Brandt, D. D. Broud, D. K. Goroff, and C. J. Baker. 1984. Immune response of infants and children to disseminated infections with Neisseria meningitidis. J. Infect. Dis. 150:71-79. [DOI] [PubMed] [Google Scholar]
- 18.Griffiss, J. M., and D. K. Goroff. 1983. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J. Immunol. 130:2882-2885. [PubMed] [Google Scholar]
- 19.Holst, J., B. Feiring, J. E. Fuglesang, E. A. Høiby, H. Nøkleby, I. S. Aaberge, and E. Rosenqvist. 2003. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine 21:734-737. [DOI] [PubMed] [Google Scholar]
- 20.Høiby, E. A., E. Rosenqvist, L. O. Frøholm, G. Bjune, B. Feiring, H. Nøkleby, and E. Rønnild. 1991. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH Ann. 14:147-155. [PubMed] [Google Scholar]
- 21.Infante, J. F., O. Marrero, S. Sifontes, G. Sierra, C. Campa, E. Caro, M. Gutierrez, A. Malberti, V. Capo, M. Farinas, and E. Munoz. 1994. Evaluation of the efficacy of human antimeningococcal immunoglobulin G in infant rats experimentally infected with Neisseria meningitidis group B. Arch. Med. Res. 25:455-461. [PubMed] [Google Scholar]
- 22.Jarvis, G. A., and N. A. Vedros. 1987. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect. Immun. 55:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasper, D. L., J. L. Winkelhake, W. D. Zollinger, B. L. Brandt, and M. S. Artenstein. 1973. Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J. Immunol. 110:262-268. [PubMed] [Google Scholar]
- 24.Leenaerts, P. L., R. K. Stad, B. M. Hall, B. J. Van Damme, Y. Vanrenterghem, and M. R. Daha. 1994. Hereditary C6 deficiency in a strain of PVG/c rats. Clin. Exp. Immunol. 97:478-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leinonen, M., and C. E. Frasch. 1982. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect. Immun. 38:1203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mäkelä, P. H., H. Käyhty, T. Leino, K. Auranen, H. Peltola, N. Ekström, and J. Eskola. 2003. Long-term persistence of immunity after immunization with Haemophilus influenzae type b conjugate vaccine. Vaccine 22:287-292. [DOI] [PubMed] [Google Scholar]
- 27.Mandrell, R. E., F. H. Azmi, and D. M. Granoff. 1995. Complement-mediated bactericidal activity of human antibodies to poly alpha2→8 N-acetylneuraminic acid, the capsular polysaccharide of Neisseria meningitidis serogroup B. J. Infect. Dis. 172:1279-1289. [DOI] [PubMed] [Google Scholar]
- 28.Mandrell, R. E., and W. D. Zollinger. 1982. Measurement of antibodies to meningococcal group B polysaccharide: low avidity binding and equilibrium binding constants. J. Immunol. 129:2172-2178. [PubMed] [Google Scholar]
- 29.Michaelsen, T. E., O. Ihle, K. J. Beckstrom, T. K. Herstad, J. Kolberg, E. A. Hoiby, and A. Aase. 2003. Construction and functional activities of chimeric mouse-human immunoglobulin G and immunoglobulin M antibodies against the Neisseria meningitidis PorA P1.7 and P1.16 epitopes. Infect. Immun. 71:5714-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaelsen, T. E., O. Ihle, K. J. Beckstrom, T. K. Herstad, R. H. Sandin, J. Kolberg, and A. Aase. 2003. Binding properties and antibacterial activities of V-region identical, human IgG and IgM antibodies, against group B Neisseria meningitidis. Biochem. Soc. Trans. 31:1032-1035. [DOI] [PubMed] [Google Scholar]
- 31.Moreno, C., J. Hewitt, K. Hastings, and D. Brown. 1983. Immunological properties of monoclonal antibodies specific for meningococcal polysaccharides: the protective capacity of IgM antibodies specific for polysaccharide group B. J. Gen. Microbiol. 129:2451-2456. [DOI] [PubMed] [Google Scholar]
- 32.Morley, S. L., M. J. Cole, C. A. Ison, M. A. Camaraza, F. Sotolongo, N. Anwar, I. Cuevas, M. Carbonero, H. C. Campa, G. Sierra, and M. Levin. 2001. Immunogenicity of a serogroup B meningococcal vaccine against multiple Neisseria meningitidis strains in infants. Pediatr. Infect. Dis. J. 20:1054-1061. [DOI] [PubMed] [Google Scholar]
- 33.Naess, L. M., T. Aarvak, A. Aase, F. Oftung, E. A. Høiby, R. Sandin, and T. E. Michaelsen. 1999. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine 17:754-764. [DOI] [PubMed] [Google Scholar]
- 34.Naess, L. M., F. Oftung, A. Aase, L. M. Wetzler, R. Sandin, and T. E. Michaelsen. 1998. Human T-cell responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine. Infect. Immun. 66:959-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noel, G. J., D. M. Mosser, and P. J. Edelson. 1990. Role of complement in mouse macrophage binding of Haemophilus influenzae type b. J. Clin. Investig. 85:208-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peiser, L., M. P. De Winther, K. Makepeace, M. Hollinshead, P. Coull, J. Plested, T. Kodama, E. R. Moxon, and S. Gordon. 2002. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect. Immun. 70:5346-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelkonen, S., and G. Pluschke. 1989. Roles of spleen and liver in the clearance of Escherichia coli K1 bacteremia in infant rats. Microb. Pathog. 6:93-102. [DOI] [PubMed] [Google Scholar]
- 38.Perez, O., M. Lastre, J. Lapinet, G. Bracho, M. Diaz, C. Zayas, C. Taboada, and G. Sierra. 2001. Immune response induction and new effector mechanisms possibly involved in protection conferred by the Cuban anti-meningococcal BC vaccine. Infect. Immun. 69:4502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Høiby, E. Rosenqvist, J. Holst, H. Nøkleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 40.Raff, H. V., D. Devereux, W. Shuford, D. Abbott-Brown, and G. Maloney. 1988. Human monoclonal antibody with protective activity for Escherichia coli K1 and Neisseria meningitidis group B infections. J. Infect. Dis. 157:118-126. [DOI] [PubMed] [Google Scholar]
- 41.Ramsay, M. E., N. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 42.Rogers, D. E. 1960. Host mechanisms which act to remove bacteria from the blood stream. Bacteriol. Rev. 24:50-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenqvist, E., E. A. Høiby, G. Bjune, K. Bryn, O. Closs, B. Feiring, A. Klem, H. Nøkleby, and L. O. Frøholm. 1991. Human antibody responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine: results from ELISA studies. NIPH Ann. 14:169-179. [PubMed] [Google Scholar]
- 44.Ross, S. C., P. J. Rosenthal, H. M. Berberich, and P. Densen. 1987. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J. Infect. Dis. 155:1266-1275. [DOI] [PubMed] [Google Scholar]
- 45.Sahu, A., T. R. Kozel, and M. K. Pangburn. 1994. Specificity of the thioester-containing reactive site of human C3 and its significance to complement activation. Biochem. J. 302:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saukkonen, K. 1988. Experimental meningococcal meningitis in the infant rat. Microb. Pathog. 4:203-211. [DOI] [PubMed] [Google Scholar]
- 47.Schlesinger, M., R. Greenberg, J. Levy, H. Kayhty, and R. Levy. 1994. Killing of meningococci by neutrophils: effect of vaccination on patients with complement deficiency. J. Infect. Dis. 170:449-453. [DOI] [PubMed] [Google Scholar]
- 48.Sierra, G. V., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195-207. [PubMed] [Google Scholar]
- 49.Sifontes, S., J. F. Infante, P. Perez, E. Caro, G. Sierra, and C. Campa. 1997. The hyperferremic mouse model for the evaluation of the effectiveness of VA-MENGOC-BC against Neisseria meningitidis B clinical isolates. Arch. Med. Res. 28:41-45. [PubMed] [Google Scholar]
- 50.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Høiby, J. Holst, H. Nøkleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 51.Toropainen, M., H. Käyhty, L. Saarinen, E. Rosenqvist, E. A. Høiby, E. Wedege, T. Michaelsen, and P. H. Mäkelä. 1999. The infant rat model adapted to evaluate human sera for protective immunity to group B meningococci. Vaccine 17:2677-2689. [DOI] [PubMed] [Google Scholar]
- 52.Toropainen, M., L. Saarinen, P. van der Ley, B. Kuipers, and H. Käyhty. 2001. Murine monoclonal antibodies to PorA of Neisseria meningitidis show reduced protective activity in vivo against B:15:P1.7,16 subtype variants in an infant rat infection model. Microb. Pathog. 30:139-148. [DOI] [PubMed] [Google Scholar]
- 52a.Toropainen, M., L. Saarinen, E. Wedege, K. Bolstad, P. H. Mäkelä, and H. Käyhty. Passive protection in the infant rat protection assay by sera taken before and after vaccination of teenagers with serogroup B meningococcal outer membrane vesicle (OMV) vaccines. Vaccine, in press. [DOI] [PubMed]
- 53.Wahdan, M. H., F. Rizk, A. M. el-Akkad, A. A. el-Ghoroury, R. Hablas, N. I. Girgis, A. Amer, W. Boctar, J. E. Sippel, E. C. Gotschlich, R. Triau, W. R. Sanborn, and B. Cvjetanovic. 1973. A controlled field trial of a serogroup A meningococcal polysaccharide vaccine. Bull. W. H. O. 48:667-673. [PMC free article] [PubMed] [Google Scholar]
- 54.Wedege, E., K. Bryn, and L. O. Frøholm. 1988. Restoration of antibody binding to blotted meningococcal outer membrane proteins using various detergents. J. Immunol. Methods 113:51-59. [DOI] [PubMed] [Google Scholar]
- 55.Wedege, E., E. A. Høiby, E. Rosenqvist, and G. Bjune. 1998. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect. Immun. 66:3223-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wedege, E., B. Kuipers, K. Bolstad, H. van Dijken, L. O. Frøholm, C. Vermont, D. A. Caugant, and G. van den Dobbelsteen. 2003. Antibody specificities and effect of meningococcal carriage in Icelandic teenagers receiving the Norwegian serogroup B outer membrane vesicle vaccine. Infect. Immun. 71:3775-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Welsch, J. A., and D. Granoff. 2004. Naturally acquired passive protective activity against Neisseria meningitidis group C in the absence of serum bactericidal activity. Infect. Immun. 72:5903-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verheul, A. F., A. J. Kuipers, A. K. Braat, H. A. Dekker, C. C. Peeters, H. Snippe, and J. T. Poolman. 1994. Development, characterization, and biological properties of meningococcal immunotype L3,7,(8),9-specific monoclonal antibodies. Clin. Diagn. Lab. Immunol. 1:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogel, U., A. Weinberger, R. Frank, A. Muller, J. Kohl, J. P. Atkinson, and M. Frosch. 1997. Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis. Infect. Immun. 65:4022-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams, J. N., G. R. Jones, M. Christodoulides, and J. E. Heckels. 2003. Serological correlates of protection against meningococci in a cohort of university students, before and during an outbreak of serogroup C infection. J. Infect. Dis. 187:1433-1441. [DOI] [PubMed] [Google Scholar]
- 61.Wyle, F. A., M. S. Artenstein, B. L. Brandt, E. C. Tramont, D. L. Kasper, P. L. Altieri, S. L. Berman, and J. P. Lowenthal. 1972. Immunologic response of man to group B meningococcal polysaccharide vaccines. J. Infect. Dis. 126:514-521. [DOI] [PubMed] [Google Scholar]
- 62.Zollinger, W. D., and R. E. Mandrell. 1983. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect. Immun. 40:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]