Abstract
Yersinia pestis, the causative agent of plague, secretes LcrV (low-calcium-response V or V antigen) during infection. LcrV triggers the release of interleukin 10 (IL-10) by host immune cells and suppresses proinflammatory cytokines such as tumor necrosis factor alpha and gamma interferon as well as innate defense mechanisms required to combat the pathogenesis of plague. Although immunization of animals with LcrV elicits protective immunity, the associated suppression of host defense mechanisms may preclude the use of LcrV as a human vaccine. Here we show that short deletions within LcrV can reduce its immune modulatory properties. An LcrV variant lacking amino acid residues 271 to 300 (rV10) elicited immune responses that protected mice against a lethal challenge with Y. pestis. Compared to full-length LcrV, rV10 displayed a reduced ability to release IL-10 from mouse and human macrophages. Furthermore, the lipopolysaccharide-stimulated release of proinflammatory cytokines by human or mouse macrophages was inhibited by full-length LcrV but not by the rV10 variant. Thus, it appears that LcrV variants with reduced immune modulatory properties could be used as a human vaccine to generate protective immunity against plague.
Plague epidemics have likely killed more people worldwide than any other infectious disease (36, 54). Mammals, in particular rats, prairie dogs, and gerbils, are the primary reservoir of Yersinia pestis, and human disease transmission occurs by flea bites, aerosol, or contact (15, 17). Flea bite-transmitted Y. pestis infection leads to bubonic plague, with characteristic lymph node swellings and disease symptoms that progress frequently to systemic infection and pneumonia (7, 14). Aerosol transmission of virulent Y. pestis, whether during plague epidemics or deliberate dissemination, precipitates pneumonic plague, a rapidly fatal disease with few characteristic symptoms (28). Considering the ubiquitous zoonotic nature of the disease and the possible illegitimate use of Y. pestis as a weapon, there is an urgent need for vaccine development to protect humans against bubonic and pneumonic plague (36).
Some, but not all, bubonic plague victims survive the disease, even without therapy, and appear to develop immunity (12, 48). Burrows discovered Y. pestis LcrV as a protective antigen which stimulates humoral immune responses in experimental animals that afford protection against plague infection (10, 11). Based on these observations, several laboratories developed recombinant LcrV subunit vaccines, either alone or in combination with other Y. pestis proteins, and demonstrated that a humoral immune response to LcrV confers plague protection in experimental animals (2, 21, 25, 31, 32, 47). Brubaker and colleagues first showed that LcrV injection of animals stimulates the release of interleukin 10 (IL-10), a cytokine that suppresses innate immune functions (8, 34). LcrV injection also prevents the release of proinflammatory cytokines, such as gamma interferon (IFN-γ) or tumor necrosis factor alpha (TNF-α), during plague or other bacterial infections (34). Recent results suggested that the immune modulatory properties of LcrV involve the signaling functions of Toll-like receptor 2 (TLR2) and CD14, imposing a systemic suppression of innate immune functions that prohibits the use of LcrV as a plague vaccine in humans (40-42).
LcrV is secreted in massive amounts via the type III pathway of Y. pestis during infection, and mutations that abrogate the bacterial expression of LcrV or the type III machinery render Yersinia variants avirulent (10, 43). PcrV, the Pseudomonas aeruginosa homolog of LcrV, is also secreted during infection and can be exploited as a protective antigen to prevent Pseudomonas lung infections (39). In contrast to LcrV secreted by pathogenic Yersinia spp. (Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis) (6, 8), PcrV neither activates IL-10 release nor prevents proinflammatory cytokine responses during infection, suggesting that the immune modulatory and protective antigen properties of LcrV may represent separable entities (see Fig. 1 for a comparison of Yersinia LcrV and Pseudomonas PcrV). To test this hypothesis, we generated small deletions in LcrV and examined purified recombinant variants for their immune modulatory properties.
FIG. 1.
Amino acid sequence alignment of LcrV proteins from Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica with P. aeruginosa PcrV. Residues that are identical to those in Y. pestis LcrV are not color coded, whereas blue marks similar amino acids and red indicates dissimilar amino acids. Residues 31 to 49, encompassing the LcrV peptide of Y. enterocolitica that is sufficient to activate IL-10 secretion, are underlined (40).
MATERIALS AND METHODS
Construction of recombinant rLcrV (rLcrV) expression vectors.
The Y. pestis strain KIM coding sequence of lcrV was PCR amplified with primers specifying abutted NdeI and BamHI restriction sites (Table 1). Amplified DNA fragments were cloned into pCR2.1 (Invitrogen), recombinant plasmids were isolated, and cloned insertions were verified by DNA sequencing. Inserts containing the correct sequence were excised by NdeI/BamHI cleavage and subcloned into the expression vector pET16b (Novagen) cut with the same enzymes to yield prLcrV. To generate rV1 and rV11 deletions (Fig. 2), lcrV DNA fragments with 5′ or 3′ truncations were amplified with oligonucleotide primers that annealed at the appropriate sites and harbored abutted NdeI (5′ truncation) and BamHI (3′ truncation) restriction sites, respectively. PCR products were cloned into pET16b as described above. To generate internal 30-codon deletions, lcrV was PCR amplified with two primer pairs, resulting in 5′ and 3′ coding fragments that were joined by ligation at a unique KpnI restriction site generated from nucleotide sequences that were abutted to PCR primers (Table 1). Fragments were amplified by PCR, cloned into pCR2.1 (Invitrogen), excised with restriction enzymes, ligated together, and subcloned as joined NdeI/BamHI fragments into pET16b. P. aeruginosa PcrV was amplified using the same restriction sites and was also cloned into pET16b. The primers for all constructs described herein are listed in Table 1.
TABLE 1.
Oligonucleotides used for construction of LcrV variants
| Plasmid | Deletion | Sequence of forward primer | Sequence of reverse primer |
|---|---|---|---|
| pEM1 | Δ1-30 | AACATATGGTTTTAGAAGAATTGGTTCAGTT | AAGGATCCTTTACCAGACGTGTCATC |
| pEM2 | Δ31-60 | AAGGTACCAATAGAGTAATTACTGATGATATC | AAGGATCCTTTACCAGACGTGTCATC |
| pEM3 | Δ61-90 | AACATATGATTAGAGCCTACGAACAA | AAGGTACCGGCAAAAACCTCCGAATCTTT |
| pEM3 | Δ61-90 | AAGGTACCGACAACCAACTGCAAAATGG | AAGGATCCTTTACCAGACGTGTCATC |
| pEM4 | Δ91-120 | AACATATGATTAGAGCCTACGAACAA | AAGGTACCATAATGACCGCCTTTAAGAATG |
| pEM4 | Δ91-120 | AAGGTACCGTAATGCATTTCTCTTTAACCG | AAGGATCCTTTACCAGACGTGTCATC |
| pEM5 | Δ121-150 | AACATATGATTAGAGCCTACGAACAA | AAGGTACCTGCCATGAACGCCCGCAAT |
| pEM5 | Δ121-150 | AAGGTACCAGCAAGTTGCGTGAAGAATTA | AAGGATCCTTTACCAGACGTGTCATC |
| pEM6 | Δ151-180 | AACATATGATTAGAGCCTACGAACAA | AAGGTACCACGGGCATCACCATGATGAT |
| pEM6 | Δ151-180 | AAGGTACCAGTGGCACCATAAATATCCAT | AAGGATCCTTTACCAGACGTGTCATC |
| pEM7 | Δ181-210 | AACATATGATTAGAGCCTACGAACAA | AAGGTACCACTAGACAGATGCTTATTAATTT |
| pEM7 | Δ181-210 | AAGGTACCGCAGAGTACAAAATTCTCGAG | AAGGATCCTTTACCAGACGTGTCATC |
| pEM8 | Δ211-240 | AACATATGATTAGAGCCTACGAACAA | AAGGTACCGCTGGCTTTAAAAATCTCTTCA |
| pEM8 | Δ211-240 | AAGGTACCGGAAGTGAGAATAAAAGAACC | AAGGATCCTTTACCAGACGTGTCATC |
| pEM9 | Δ241-270 | AACATATGATTAGAGCCTACGAACAA | AAGGTACCAAGAAAGTCCTTTATCGAGACT |
| pEM9 | Δ241-270 | AAGGTACCACCACCTGCTCGGATAAGT | AAGGATCCTTTACCAGACGTGTCATC |
| pEM10 | Δ271-300 | AACATATGATTAGAGCCTACGAACAA | AAGGTACCGGCAAAGTGAGATAATTCATTAT |
| pEM11 | Δ301-326 | AACATATGATTAGAGCCTACGAACAA | AAGGATCCTGAATTAAAACGTGATGTAATATC |
FIG. 2.
Expression and purification of recombinant LcrV and its variants. (A) Full-length wild-type Y. pestis strain KIM LcrV was expressed as an N-terminal decahistidyl fusion protein from the T7 polymerase expression vector pET16b. Recombinant lcrV variant genes encoding staggered 30-amino-acid deletions were generated from PCR-amplified DNA fragments and expressed under the same conditions. (B) rLcrV and rV1 to -11 were purified by affinity chromatography on Ni-NTA and eluted with imidazole. After Triton X-114 extraction of endotoxin, proteins were purified on Sephadex G25. Protein aliquots were separated by SDS-PAGE and stained with Coomassie brilliant blue. M indicates molecular size markers, with mass assignments in kDa. rLcrV was loaded in lane 1, while lanes 2 to 12 harbor rV1 to rV11, in numerical order.
Expression and purification of rLcrV and rLcrV variants.
Escherichia coli BL21(DE3) (44) carrying prLcrV or any plasmid expressing an rLcrV variant was grown overnight at 37°C in Luria-Bertani medium with 100 μg/ml ampicillin. Bacteria were diluted in fresh medium and grown to an optical density at 600 nm (OD600) of 0.5. T7 polymerase was induced with 1 mM isopropyl-1-thiol-d-galactopyranoside, bacterial growth was continued for 3 hours at 37°C, and cells were harvested by centrifugation at 10,000 × g for 15 min. E. coli cells in a 500-ml culture were disrupted twice in a French pressure cell at 14,000 lb/in2 in 20 ml of 50 mM Tris-HCl (pH 7.5)-150 mM NaCl (column buffer) containing 100 μM phenylmethylsulfonyl fluoride. The lysate was subjected to ultracentrifugation at 100,000 × g for 30 min, and the soluble fraction was applied to a nickel nitrilotriacetic acid (Ni-NTA) column (1-ml bed volume) pre-equilibrated with 20 ml column buffer. The column was washed with 20 volumes of the same buffer, followed by a second washing with 20 volumes of column buffer containing 20 mM imidazole. Bound protein was eluted in 50 mM Tris-HCl (pH 7.5)-150 mM NaCl with either 250 mM or 500 mM imidazole. Purified proteins were subjected to Triton X-114 (Sigma) phase separation to remove endotoxin (1). Purified proteins were subjected to G25 (Amersham) gel filtration chromatography to remove residual Triton X-114 and then retrieved by phosphate-buffered saline (PBS) elution. Lipopolysaccharide (LPS) contamination of the proteins was assayed with Limulus amebocyte lysate (QCL-1000; Cambrex, NJ) and determined to be <1 ng per 100 μg purified protein (39). Protein concentrations were determined by a bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL) or by measuring the absorption at 280 nm. Proteins were flash frozen with dry ice and ethanol and stored at −80°C for further use. To characterize the purified antigens, 1 μg of purified protein was introduced into the ion source of an Agilent Technologies LC/MSD Trap XCT ion-trap mass spectrometry instrument. Average compound masses were derived from observed ion signals and compared to predicted compound masses. For example, the observed and predicted average masses of rLrcV (39,932 Da and 39,932 Da, respectively), rV7 (36,691 Da and 36,691 Da, respectively), and rV10 (36,766 Da and 36,765 Da, respectively) were within experimentally acceptable error rates (0.01%).
Animal immunization and challenge with Y. pestis strain KIM D27.
Groups of 6- to 8-week-old female BALB/c mice (Charles River Labs, MA) were immunized by intramuscular injection into the hind leg with 50 μg of purified protein that was preadsorbed to adjuvant by mixing with 50 μl 50% (vol/vol) Alhydrogel. On day 21 following primary immunization, mice were boosted with a second injection of the same antigen at an equal dose. Blood samples were withdrawn via periorbital bleeding before immunization and on days 7, 14, 28, and 42 after primary immunization. Following blood clotting, sera were retrieved from the supernatants of samples centrifuged at 1,000 × g and used for measurements of antibody production. Immunized animals were challenged on day 43 by retro-orbital injection with 0.1-ml aliquots of either 10 or 1,000 50% lethal doses (LD50s) of Y. pestis strain KIM D27 (1 × 103 or 1 × 105 CFU) (9). For this experiment, Y. pestis KIM D27 was grown in heart infusion broth (HIB; Difco) at 26°C overnight, diluted 1:20 in fresh HIB, and grown for 3 h at 26°C until the culture reached an OD600 of 1.0. Plague bacilli were washed and diluted in sterile PBS. Animals were anesthetized for this procedure by injecting a cocktail of 17 mg/ml of ketamine (Ketsed; Vedco) and 0.7 mg/ml of xylazine (Sigma) intraperitoneally. Mice were infected by retro-orbital injection with bacterial suspensions and observed for 14 days, and deaths were recorded. Surviving animals were euthanized at the end of the observation period. Serum immunoglobulin G (IgG) levels with specific antigen binding activity were determined by a custom enzyme-linked immunosorbent assay (ELISA) at the GLRCE Immunology Core at the University of Chicago. All animal experiments were performed in accordance with institutional guidelines following experimental protocol review and approval by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee.
Macrophage assays.
Peritoneal cavities of 6- to 8-week-old C57BL/6 mice (Jackson Laboratories, ME) were washed with cold, serum-free Hanks' balanced salt solution. Cells were plated in triplicate at a density of 5 × 105 cells/well, using 48-well dishes and serum-free RPMI. After 2 h of incubation at 37°C in an atmosphere with 5% CO2, plates were carefully washed three times with prewarmed serum-free medium to remove nonadherent cells and with fresh RPMI containing 10% fetal calf serum (Gemini Bio-Products, CA), 2 mM l-glutamine (Gemini Bio-Products, CA), 100 U/ml penicillin (Gemini Bio-Products), 100 U/ml streptomycin (Gemini Bio-Products), and 50 μM β-mercaptoethanol. More than 95% of the adherent cell population was composed of macrophages, as determined by morphology and flow cytometric analysis. Macrophage cultures were propagated for 2 hours with or without 1 μg/ml LPS prestimulation. Macrophage preparations were treated with the following reagents: LPS (1 μg/ml), rLcrV (10 μg/ml), rV1 to rV11 (10 μg/ml), rPcrV (10 μg/ml), and PBS. Macrophage culture supernatants were collected 18 h after the addition of proteins and analyzed by ELISAs for the concentrations of IL-10 (BD Biosciences, CA) and TNF-α (R&D Systems, MN) according to the manufacturers' recommendations.
Human monocytic cells.
Human THP-1 cells (ATCC TIB-202) were cultured in RPMI 1640 supplemented with 0.005 mM 2-mercaptoethanol and 10% fetal calf serum and then incubated at 37°C in 5% CO2. THP-1 cells were cultured at a density of 5 × 105 cells/ml and stimulated in triplicate with LPS (1 μg/ml), rLcrV, rV7, rV10, or rV11 (10 μg/ml). Culture supernatants were collected after 18 h of incubation and analyzed by ELISA for IL-10.
RESULTS
rLcrV variants and their immune modulatory properties.
Recombinant LcrV (rLcrV) variants were expressed via T7 polymerase in E. coli strain BL21(DE3) using the pET16b expression vector (Novagen). Proteins with N-terminal decahistidyl tags were purified from bacterial lysates by affinity chromatography on a Ni-NTA column. Following elution with imidazole, contaminating LPS was removed by Triton X-114 extraction. LcrV protein samples were chromatographed on Sephadex G25 and eluted in PBS. The endotoxin (LPS) contamination of samples was analyzed with Limulus amebocyte lysate and determined to be below 1 ng per 100 μg of purified protein (39). The purity and identity of polypeptides were analyzed by Coomassie-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry (see Materials and Methods). Figure 2 displays a diagram and Coomassie-stained SDS-PAGE gel of purified full-length rLcrV and its variants, rV1 to -11, with staggered 30-amino-acid deletions throughout the polypeptide chain.
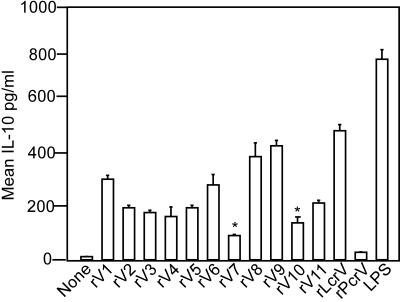
Heesemann and colleagues suggested that the immune modulatory properties of rLcrV result from an interaction of the Y. pestis virulence factor with TLR2 and CD14 on the surfaces of immune cells, particularly murine macrophages (41). To examine the role of primary immune cells during IL-10 release and rLcrV-mediated immune suppression, macrophages were isolated from the peritoneal cavities of C57/BL6 mice. Peritoneal macrophages were treated with 10 μg rLcrV or its variants, and cytokine secretion was analyzed after 18 h of culture (Fig. 3). rLcrV induced a 40-fold increase in the release of IL-10 from peritoneal macrophages compared to that seen with PBS alone, consistent with previous observations (41). As a control, rPcrV, the Pseudomonas protective antigen, failed to induce significant amounts of IL-10 release (39). Although many LcrV variants with short deletions displayed modest decreases in cytokine release, two mutant proteins, rV7 and rV10, triggered only small amounts of IL-10 release, as about five- or fourfold less IL-10, respectively, was found in the culture medium of macrophages than in samples treated with wild-type rLcrV (Fig. 3). Surprisingly, rV9, a variant that did not induce protective immunity (see below), triggered IL-10 secretion by murine macrophages. As a control for the maximum activation of cytokine release, the addition of LPS to macrophage cultures caused a 70-fold increase in IL-10 secretion. Together, these results demonstrate that the addition of purified rLcrV to isolated primary murine immune cells stimulates the release of IL-10, whereas the rV7 and rV10 variants, lacking LcrV amino acids 181 to 210 and 271 to 300, respectively, display significant defects in immune modulation.
FIG. 3.
Induction of IL-10 expression from murine macrophages stimulated with rLcrV and its variants. Murine peritoneal macrophages were incubated with rLcrV or its variants, rPcrV, LPS, or PBS, and IL-10 levels were determined by ELISA after 18 h of incubation. The t test was used to assess the statistical significance of the observed difference between rLcrV and rV7 (P ≤ 0.005) or rV10 (P ≤ 0.001). Data were collected from four independent experiments.
The injection of C57BL/6 mice with LPS or heat-killed Y. pestis activates the release of proinflammatory cytokines such as IFN-γ and TNF-α (34). The simultaneous injection of purified rLcrV with LPS or heat-killed Y. pestis abrogates the murine release of IFN-γ and TNF-α in vivo (34). To test whether rLcrV alone is sufficient to reduce the secretion of proinflammatory cytokines from immune cells activated with LPS, we used murine peritoneal macrophages. The treatment of macrophages with LPS led to an expected 35-fold increase in the secretion of TNF-α (Fig. 4). However, if macrophages were treated with 10 μg rLcrV after 2 h of LPS exposure, the immune cell release of TNF-α was completely blocked (Fig. 4). The addition of rPcrV, rV9, or rV10 caused only a modest reduction in the release of TNF-α from LPS-stimulated macrophages. Together, these data suggest that rLcrV has immune modulatory properties since the addition of rLcrV to murine macrophages not only induced IL-10 but also suppressed LPS-induced TNF-α production. Of importance for vaccine development, TNF-α suppression was reduced in many rLcrV variants, yet only rV10 lost both the ability to induce IL-10 and the ability to suppress TNF-α production. Surprisingly, rV7, a molecule that failed to activate IL-10 release, triggered a significant reduction in the release of TNF-α from LPS-stimulated macrophages, suggesting that the immune modulatory functions of rLcrV may involve multiple signaling pathways in murine macrophages.
FIG. 4.
Immune modulatory properties of rLcrV and its variants. Murine peritoneal macrophages were treated for 2 hours with LPS, washed extensively, and then treated with rLcrV, its variants, or rPcrV. The release of TNF-α after 18 h was determined by ELISA and compared to that of mock (PBS)-stimulated macrophages. The t test was used to assess the statistical significance of the observed difference between LPS and rLcrV (P ≥ 0.001), rV7 (P ≥ 0.02), rV9 (P ≥ 0.15), or rV10 (P ≥ 0.05). Data were collected from four independent experiments.
rLcrV variants and their immune modulatory properties in human THP-1 cells.
To test whether LcrV activates IL-10 secretion in human cells, purified recombinant proteins were added to human THP-1 monocytic cells and incubated for 18 h prior to ELISA analysis of cytokine production. As a control, the addition of LPS to THP-1 cells stimulated IL-10 release 750-fold compared to that seen with PBS-treated cells. The addition of rLcrV activated IL-10 secretion 180-fold, whereas rPcrV did not stimulate any release of the cytokine. Furthermore, rV7 and rV10 displayed defects in IL-10 release by human THP-1 monocytes, similar to our observations with murine macrophages (Fig. 5). These results suggest that the innate immune responses of cultured human macrophages can be suppressed by treatment with rLcrV and that rV10 displays a significant reduction in immunosuppressive properties on murine and human cells.
FIG. 5.
Induction of IL-10 from human monocytic cells stimulated with rLcrV and its variants. Human THP-1 cells were incubated with rLcrV or its variants, rPcrV, LPS, or PBS, and IL-10 levels were determined by ELISA after 18 h of incubation. The t test was used to assess the statistical significance of the observed difference between rLcrV and rV7 (P < 0.006), rV10 (P < 0.007), or rV11 (P < 0.006). Data were collected from three independent experiments.
Immunization with rV10 affords protective immunity against plague.
BALB/c mice were immunized with rLcrV or its variants on days 0 and 21 and challenged with 10 or 1,000 LD50 units of Y. pestis KIM D27 by retro-orbital injection on day 43. As demonstrated previously (32), immunization with full-length rLcrV completely protected experimental animals against plague at both lethal challenge doses (Fig. 6A and Table 2). The immunization of mice with rV1 to -5, rV10, and rV11 also completely protected animals at both lethal challenge doses (Table 2). The protective antigen property of rV9 was only 10% of that observed for rLcrV at the 1,000-LD50 challenge dose. Partial protection at 1,000 LD50 was observed for rV6, rV7, and rV8, with 80%, 80%, and 90% survival, respectively. To assess whether vaccine protection occurred after the immunization of other mouse strains, C57BL/6 mice were immunized with purified protein on days 0 and 21 and challenged with 10 or 1,000 LD50 units of Y. pestis KIM D27 by retro-orbital injection on day 43. Similar results were obtained for both BALB/c and C57BL/6 mice (Table 3). Figure 6A shows a time-to-death analysis of animal infections, documenting the protective immunity afforded by immunization with rLcrV, rV10, and rV11 and showing that rV9 immunization precipitated almost no protection. We sought to assess the ability of recombinant LcrV molecules to elicit a humoral immune response. Mice immunized with rLcrV proteins were bled on days 0, 14, 28, and 42 for an analysis of V-antigen-specific IgG titers (Fig. 6B and Table 2). Each mouse serum sample was analyzed by ELISA for IgG-specific responses. rLcrV variants, in particular rV10 (Fig. 6B), generated similar immune responses to those induced by LcrV, indicating that the protective antigen properties of rLcrV had not been significantly impaired by the deletion of residues 271 to 300.
FIG. 6.
rV10 protects mice against lethal plague challenge. (A) Mice were immunized on days 0 and 21 by intramuscular injection with 100 μg of purified protein (rLcrV, rV9, rV10, or rV11) emulsified with Alhydrogel or with adjuvant alone. On day 43 after the first immunization, animals were challenged by retro-orbital infection with 1,000 LD50 units of Y. pestis KIM D27, and survival was measured. Fisher's exact test was used to analyze the statistical significance of vaccine protection for mice immunized with either rLcrV or rV10 compared to that for Alhydrogel-treated control animals for data points on days 4 to 14 (P < 0.005). (B) Mean serum IgG titers in blood on day 42 after immunization with rLcrV and its variant proteins. Groups of BALB/c mice were immunized with rLcrV proteins on days 0 and 21. Mice were bled on days 0, 14, 28, and 42. Serum samples were analyzed for rLcrV-specific IgG by a custom ELISA.
TABLE 2.
Immune modulatory and protective antigen properties of LcrV variantsf
| Antigena | Deletion | IgG titerb | IL-10 concnc | TNF-α concnc | No. of animals with 14-day survival/total no. challenged
|
|
|---|---|---|---|---|---|---|
| 10 LD50d | 1,000 LD50e | |||||
| None | NA | NA | 13 (±2) | 42 (±18) | 0/10 | 0/10 |
| rLcrV | None | 28,500 (±11,000) | 481 (±9) | 35 (±4) | 10/10 | 10/10 |
| rV1 | 1-30 | 310,000 (±112,000) | 305 (±17) | 635 (±87) | 10/10 | 10/10 |
| rV2 | 31-60 | 537,000 (±130,000) | 195 (±12) | 698 (±17) | 10/10 | 10/10 |
| rV3 | 61-90 | 70,400 (±10,000) | 180 (±7) | 550 (±30) | 10/10 | 10/10 |
| rV4 | 91-120 | 125,000 (±0) | 163 (±35) | 361 (±20) | 10/10 | 10/10 |
| rV5 | 121-150 | 410,000 (±200,000) | 209 (±8) | 515 (±31) | 10/10 | 10/10 |
| rV6 | 151-180 | 62,500 (±0) | 293 (±25) | 663 (±31) | 10/10 | 8/10 |
| rV7 | 181-210 | 625,000 (±0) | 102 (±12) | 194 (±0) | 10/10 | 8/10 |
| rV8 | 211-240 | 46,000 (±19,000) | 389 (±50) | 497 (±28) | 10/10 | 9/10 |
| rV9 | 241-270 | 49,500 (±15,000) | 413 (±20) | 460 (±53) | 8/9 | 1/10 |
| rV10 | 271-300 | 112,500 (±28,000) | 135 (±18) | 412 (±19) | 10/10 | 10/10 |
| rV11 | 301-326 | 293,000 (±44,000) | 212 (±1.4) | 294 (±36) | 10/10 | 10/10 |
| rPcrV | NA | NA | 26 (±3) | 461 (±53) | ND | ND |
| LPS | NA | NA | 738 (±36) | 634 (±67) | ND | ND |
The purity and identity of antigens were analyzed by Coomassie-stained SDS-PAGE, mass spectrometry, and the Limulus amebocyte lysate test.
Mean(± standard deviation [SD]) IgG titer to rLcrV observed 42 days after immunization of BALB/c mice(five mice in each set), immediately before the plague challenge.
Observed mean(± SD) IL-10 or TNF-α concentration(pg/ml) 15 hours after the addition of rLcrV or its variants to murine primary macrophages.
BALB/c mice challenged with 10 LD50(1 × 103 CFU).
BALB/c mice challenged with 1,000 LD50(1 × 105 CFU).
NA, not applicable; ND, not determined.
TABLE 3.
Protective antigen properties of LcrV variants in C57/BL6 mice
| Antigen | Deletion | IgG titera | No. of animals with 14-day survival/total no. challenged
|
|
|---|---|---|---|---|
| 10 LD50b | 1,000 LD50c | |||
| rLcrV | None | 125,000 (±0) | 10/10 | 9/9d |
| rV9 | 241-270 | 95,500 (±46,000) | 3/10 | 0/10 |
| rV10 | 271-300 | 125,000 (±0) | 10/10 | 10/10 |
| rV11 | 301-326 | 100,000 (±34,000) | 10/10 | 10/10 |
Mean (± SD) IgG titer to rLcrV observed 42 days after immunization (five mice in each set), immediately before the plague challenge.
C57BL/6 mice challenged with 10 LD50 (1 × 102 CFU).
C57BL/6 mice challenged with 1,000 LD50 (1 × 104 CFU).
Only nine animals were included in this experiment.
DISCUSSION
Early attempts at plague immunization employed avirulent live plague bacilli such as the nonpigmented EV76 strain (29, 49). This approach was effective for preventing bubonic plague but too frequently resulted in complications such as inflammation, if not modest infection or even death (5, 30). Nevertheless, the process was utilized in the Soviet Union even though numerous booster immunizations were required for full effectiveness. A similar but less successful approach was the use of killed organisms in the United States (51). The general failure of these approaches has promoted considerable effort towards the development of soluble subunit vaccines containing one or more protective antigens (38, 46). Vaccination with purified recombinant LcrV elicits an immune response that protects experimental animals against plague (2, 21, 25, 31, 32, 47). Other preparations of LcrV, whether alone or as a fusion to the Caf1 (F1) pilin subunit (21), extended these observations, revealing that antibodies against LcrV provide protection against bubonic and pneumonic plague.
Several arguments have been raised both for and against the F1 pilin subunit as a suitable plague vaccine (3, 16). In favor of F1 as a vaccine antigen, plague-infected animals mount an immune response to F1 pilus subunits (4), and immunization with purified F1 alone can raise protective immune responses in mice and guinea pigs (3, 45). However, in contrast to the case for lcrV mutations in the 70-kb pCD1 virulence plasmid, Y. pestis carrying mutations in the caf pilus gene cluster of the 100-kb pMT1 plasmid (23, 27) remains fully virulent in mice and nonhuman primates (16, 19, 50). Furthermore, a challenge of F1-immunized mice or rats with Y. pestis strain CO92 resulted in lethal plague disease with fully virulent caf1 mutant variants that were presumably selected by the presence of a specific antibody (13, 52). A caf1 mutant strain was also isolated from a plague-infected individual during autopsy (53), suggesting that F1 pili may not be absolutely required for the pathogenesis of human plague. Thus, a suitable protective antigen for plague must be essential for virulence to serve in a vaccine, or mutants lacking that antigen will be selected and eventually cause plague disease or death. Considering these arguments for F1, it appears that LcrV is currently the only Y. pestis subunit candidate for vaccine development for use in humans (16).
Nakajima and Brubaker demonstrated that Y. pestis infection in mice is associated with the suppression of endogenous TNF-α and IFN-γ in vivo (33, 34). Furthermore, an exogenous supply of TNF-α and IFN-γ, of rabbit polyclonal antisera against LcrV, or of a monoclonal antibody against LcrV could protect animals from a lethal infection with Y. pestis (34). The injection of purified recombinant LcrV preparations unequivocally demonstrated that LcrV functions as a protective antigen against plague and that antibodies against LcrV can be passively transferred to naive animals to achieve the same effect, which involves simultaneously blockading IL-10 secretion and restoring endogenous TNF-α and IFN-γ release during animal infections with plague bacilli in vivo (34).
LcrV is absolutely required for human or animal infectious disease by three pathogenic Yersinia species, i.e., Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis (6, 26, 43). Heesemann and colleagues showed that TLR2 and CD14, but not TLR1 or TLR4, are required for the release of IL-10 by Yersinia and that deletion of the TLR2 gene resulted in resistance of mice to infections with pathogenic Yersinia spp. (40-42). Taken together, these observations not only document the central importance of LcrV in disease establishment and modulating host immune functions and its use as a protective vaccine antigen but also point to the suppression of host immune responses as a serious obstacle for the vaccination of humans with purified recombinant LcrV.
To identify minimal components for plague vaccines, presumed linear epitopes of LcrV were divided into peptide segments of 30 amino acids or into truncated LcrV molecules lacking 100 or more amino acids (22, 37). Together, these studies showed that immunization with small linear peptide epitopes (30 amino acids) provides no protection against plague, whereas large truncations of LcrV can elicit at least some protective immunity. However, previous studies did not take into consideration the LcrV-mediated IL-10 release and left unresolved whether plague vaccines without immunosuppressive properties can be generated (22, 37). Guided by previous results, we aimed at developing LcrV vaccines with short deletions, preserving the maximum amount of protein sequence and protective antigen property while reducing or abolishing the immune modulatory properties of this virulence factor. Our experiments generated short, 30-amino-acid deletions in LcrV and tested purified proteins for their immunosuppressive properties. A deletion near the C terminus of LcrV, 271-300, satisfied our experimental criteria and displayed significant defects in immune suppression without reducing the protective properties of plague vaccines. Another mutant, rV7, was also unable to stimulate large amounts of IL-10 secretion, but the rV7 polypeptide continued to suppress TNF-α release by LPS-stimulated macrophages. Figure 7 displays the three-dimensional structure of LcrV and the positions of amino acids 271 to 300 within the C-terminal α-12 helix, engaged in a helical coiled coil with α-7 that connects the N- and C-terminal globular domains of LcrV (18, 35). Deletions of residues 271 to 300, i.e., of helix α-12, do not appear to collapse the structural fold of LcrV, as purified rV10 (or any other variant reported here) retained solubility in sedimentation assays, similar to rLcrV (data not shown).
FIG. 7.
Amino acid residues 271 to 300 are positioned in the C-terminal helix of LcrV. The image shows a three-dimensional model of LcrV as reported previously (18), with residues 274 to 300 colored in purple. Residues 1 to 27, 48 to 60, 89 and 90, and 263 to 273 were omitted because no interpretable electron density was observed in the crystallographic structure (18). The ribbon diagram was generated with DeepView PDB in conjunction with POV-Ray software (20).
Heesemann and colleagues tested whether synthetic peptides derived from the LcrV sequence were sufficient to stimulate immune responses of CD14/TLR2-cotransfected HEK293 cells and identified peptide V7 (LcrV residues 31 to 49 [VLEELVQLVKDKKIDISIK]), which is almost completely conserved in Yersinia LcrV but absent from Pseudomonas PcrV (Fig. 1) (41). They also reported that C-terminal truncations of LcrV, including the truncation of residues 271 to 300 identified here, retained the ability to induce IL-10 release or suppress TNF-α secretion in C3H/HeJ peritoneal macrophages in a CD14/TLR2-dependent manner (24, 41). These results, together with our observation that large C-terminal truncations of LcrV stimulate IL-10 release (data not shown), suggest then that the introduction of short, discrete LcrV deletions may induce conformational changes in rV10. Even though the molecular determinants of LcrV that alone (residues 31 to 49) are presumed to be sufficient for TLR2/CD14 interaction have not been removed, the predicted conformational changes in rV10 seem to prohibit interactions with pattern recognition receptors of the innate immune system such as TLR2 and CD14 (24). rV2 (deletion of residues 31 to 60) completely failed to suppress TNF-α release from LPS-stimulated macrophages, but it promoted 40% of the IL-10 release of wild-type rLcrV (Fig. 3 and 4). Thus, although LcrV residues 31 to 49 alone are sufficient to promote IL-10 release by immune cells, the deletion of this sequence does not abolish the immune modulatory properties of truncated LcrV molecules, suggesting that additional elements of LcrV may interact with pattern recognition receptors. Although the precise molecular properties of the LcrV interaction with TLR2/CD14 have yet to be elucidated, our data provide the first evidence of plague vaccines that do not suppress innate immune responses of mouse or human macrophages and that may be useful for plague vaccination in animals and, perhaps, humans.
Acknowledgments
We thank Wade Williams (University of Chicago) for help with LcrV structural analysis, John Xu (University of Illinois, Urbana-Champaign) for critical comments throughout this work, and members of the Immunology Core of the GLRCE for expert assistance with ELISAs.
All authors acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (GLRCE; NIH award 1-U54-AI-057153).
Editor: V. J. DiRita
REFERENCES
- 1.Aida, Y., and M. J. Pabst. 1990. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 132:191-195. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, G. W., Jr., S. E. C. Leary, E. D. Williamson, R. C. Titball, S. C. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 64:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, G. P., D. G. Heath, G. W. Anderson, Jr., S. L. Welkos, and A. M. Friedlander. 1996. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 64:2180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker, E. E., H. Sommer, L. W. Foster, E. Meyer, and K. F. Meyer. 1952. Studies on immunization against plague. I. The isolation and characterization of the soluble antigen of Pasteurella pestis. J. Immunol. 68:131-145. [PubMed] [Google Scholar]
- 5.Bartelloni, P. J., J. D. Marshall, and D. C. Cavanaugh, Jr. 1973. Clinical and serological responses to plague vaccine U.S.P. Mil. Med. 138:720-722. [PubMed] [Google Scholar]
- 6.Bergmann, T., S. Hakansson, A. Forsberg, L. Norlander, A. Macellaro, A. Backman, I. Bolin, and H. Wolf-Watz. 1991. Analysis of V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of lcrH and lcrV. J. Bacteriol. 173:1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boisier, P., L. Rahalison, M. Rasolomaharo, M. Ratsitorahina, M. Mahafaly, M. Razafimahefa, J.-M. Duplantier, L. Ratsifasomanana, and S. Chanteau. 2002. Epidemiologic features of four successive annual outbreaks of bubonic plague in Mahajanga, Madagascar. Emerg. Infect. Dis. 8:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker, R. R. 2003. Interleukin-10 and the inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brubaker, R. R. 1969. Mutation rate to non-pigmentation in Pasteurella pestis. J. Bacteriol. 98:1404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrows, T. W. 1956. An antigen determining virulence in Pasteurella pestis. Nature 177:426-427. [DOI] [PubMed] [Google Scholar]
- 11.Burrows, T. W. 1957. Virulence of Pasteurella pestis. Nature 179:1246-1247. [DOI] [PubMed] [Google Scholar]
- 12.Burrows, T. W. 1963. Virulence of Pasteurella pestis and immunity to plague. Ergeb. Mikrobiol. 37:59-113. [DOI] [PubMed] [Google Scholar]
- 13.Burrows, T. W., and G. A. Bacon. 1958. The effect of loss of different virulence determinants on the virulence and immunogenicity of strains of Pasteurella pestis. Br. J. Exp. Pathol. 39:278-291. [PMC free article] [PubMed] [Google Scholar]
- 14.Butler, T. 1983. Plague and other Yersinia infections. Plenum Press, New York, N.Y.
- 15.Craven, R. B., G. O. Maupin, M. L. Beard, T. J. Quan, and A. M. Barnes. 1993. Reported cases of human plague infections in the United States 1970-1991. J. Med. Entomol. 30:758-761. [DOI] [PubMed] [Google Scholar]
- 16.Davis, K. J., D. L. Fritz, M. L. M. Pitt, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopithecus aethiops). Arch. Pathol. Lab. Med. 120:156-163. [PubMed] [Google Scholar]
- 17.Davis, S., M. Begon, L. De Bruyn, V. S. Ageyev, N. L. Klasovskiy, S. B. Pole, H. Viljugrein, N. C. Stenseth, and H. Leirs. 2004. Predictive thresholds for plague in Khazakhstan. Science 304:736-738. [DOI] [PubMed] [Google Scholar]
- 18.Derewenda, U., A. Mateja, Y. Devedjiev, K. M. Routzahn, A. G. Evdokimov, Z. S. Derewenda, and D. S. Waugh. 2004. The structure of Yersinia pestis V-antigen, an essential virulence factor and mediator of immunity against plague. Structure 12:301-306. [DOI] [PubMed] [Google Scholar]
- 19.Friedlander, A. M., S. L. Welkos, P. L. Worsham, G. P. Andrews, D. G. Heath, G. W. Anderson, Jr., L. M. Pitt, J. Estep, and K. Davis. 1995. The relationship between virulence and immunity as revealed in recent studies of the F1 capsule of Yersinia pestis. Clin. Infect. Dis. 21:S178-S181. [DOI] [PubMed] [Google Scholar]
- 20.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 21.Heath, D. G., G. W. Anderson, Jr., J. M. Mauro, S. L. Welkos, G. P. Andrews, J. J. Adamovicz, and A. M. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 16:1131-1137. [DOI] [PubMed] [Google Scholar]
- 22.Hill, J., S. E. C. Leary, K. Griffin, E. D. Williamson, and R. W. Titball. 1997. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun. 65:4476-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu, P., J. Elliott, P. McCready, E. Skowronski, J. Garnes, A. Kobayashi, R. Brubaker, and E. Garcia. 1998. Structural organization of virulence-associated plasmids of Yersinia pestis. J. Bacteriol. 180:5192-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopp, E., and R. Medzhitov. 2002. A plague on host defense. J. Exp. Med. 196:1009-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leary, S. E. C., E. D. Williamson, K. F. Griffin, P. Russell, S. M. Eley, and R. W. Titball. 1995. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 63:2854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, V. T., C. Tam, and O. Schneewind. 2000. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J. Biol. Chem. 275:36869-36875. [DOI] [PubMed] [Google Scholar]
- 27.Lindler, L. E., G. V. Plano, V. Burland, G. F. Mayhew, and F. R. Blattner. 1998. Complete DNA sequence and detailed analysis of the Yersinia pestis KIM5 plasmid encoding murine toxin and capsular antigen. Infect. Immun. 66:5731-5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer, K. 1961. Pneumonic plague. Bacteriol. Rev. 25:249-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer, K. F. 1970. Effectiveness of live or killed plague vaccines in man. Bull. W.H.O. 42:653-666. [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer, K. F., G. Smith, L. E. Foster, J. D. Marshall, and D. C. Cavanaugh. 1974. Plague immunization. IV. Clinical reactions and serological responses to inoculations of Haffkine and freeze-dried plague vaccine. J. Infect. Dis. 129:S30-S36. [DOI] [PubMed] [Google Scholar]
- 31.Motin, V. L., R. Nakajima, G. B. Smirvov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 62:4192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motin, V. M., Y. A. Nedialkov, and R. R. Brubaker. 1996. V antigen-polyhistidine fusion peptide: binding to LcrH and active immunity against plague. Infect. Immun. 64:4313-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilles, M. L. 2004. Dissecting the structure of LcrV from Yersinia pestis, a truly unique virulence protein. Structure 12:357-358. [DOI] [PubMed] [Google Scholar]
- 36.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pullen, J. K., G. W. Anderson, S. L. Welkos, and A. M. Friedlander. 1998. Analysis of the Yersinia pestis V protein for the presence of linear antibody epitopes. Infect. Immun. 66:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russel, P., S. M. Eley, S. E. Hibbs, R. J. Manchee, A. J. Stagg, and R. W. Titball. 1995. A comparison of plague vaccine, USP, and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine 13:1551-1556. [DOI] [PubMed] [Google Scholar]
- 39.Sawa, T., T. L. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 40.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 41.Sing, A., D. Rost, N. Tvardovaskaia, A. Roggenkamp, A. Wiedemann, C. Kirschning, J. M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sing, A., N. Tvardovaskaia, D. Rost, C. Kirschning, H. Wagner, and J. Heesemann. 2003. Contribution of Toll-like receptors 2 and 4 in an oral Yersinia enterocolitica mouse infection model. Int. J. Med. Microbiol. 293:341-348. [DOI] [PubMed] [Google Scholar]
- 43.Skrzypek, E., and S. C. Straley. 1995. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J. Bacteriol. 177:2530-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 45.Titball, R. W., A. M. Howells, P. C. Oyston, and E. D. Williamson. 1997. Expression of the Yersinia pestis capsular antigen (F1 antigen) on the surface of an aroA mutant of Salmonella typhimurium induces high levels of protection against plague. Infect. Immun. 65:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Titball, R. W., and E. D. Williamson. 2004. Yersinia pestis (plague) vaccines. Expert Opin. Biol. Ther. 4:965-973. [DOI] [PubMed] [Google Scholar]
- 47.Une, T., and R. R. Brubaker. 1984. Roles of V antigen in promoting virulence and immunity in yersiniae. J. Immunol. 133:2226-2230. [PubMed] [Google Scholar]
- 48.Wake, A., H. Morita, and M. Wake. 1978. Mechanisms of long and short term immunity to plague. Immunology 34:1045-1052. [PMC free article] [PubMed] [Google Scholar]
- 49.Welkos, S., M. L. Pitt, M. Martinez, A. Friedlander, P. Vogel, and R. Tammariello. 2002. Determination of the virulence of the pigmentation-deficient and pigmentation-/plasminogen activator-deficient strains of Yersinia pestis in nonhuman primate and mouse models of pneumonic plague. Vaccine 20:2206-2214. [DOI] [PubMed] [Google Scholar]
- 50.Welkos, S. L., K. M. Davis, L. M. Pitt, P. L. Worsham, and A. M. Friedlander. 1995. Studies on the contribution of the F1 capsule-associated plasmid pFra to the virulence of Yersinia pestis. Contrib. Microbiol. Immunol. 13:299-305. [PubMed] [Google Scholar]
- 51.Williams, J. E., and D. C. Cavanaugh. 1979. Measuring the efficacy of vaccination in affording protection against plague. Bull. W.H.O. 57:309-313. [PMC free article] [PubMed] [Google Scholar]
- 52.Williams, J. E., D. N. Harrison, and D. C. Cavanaugh. 1974. Cryptic infection of rats with non-encapsulated variants of Yersinia pestis. Trans. R. Soc. Trop. Med. Hyg. 69:171-172. [DOI] [PubMed] [Google Scholar]
- 53.Winter, C. C., W. B. Cherry, and M. D. Moody. 1960. An unusual strain of Pasteurella pestis isolated from a fatal case of human plague. Bull. W.H.O. 23:408-409. [PMC free article] [PubMed] [Google Scholar]
- 54.Ziegler, P. 1991. The black death. Alan Sutton Publishing Inc., Wolfeboro Falls, N.H.