Abstract
Campylobacter jejuni 81-176 is capable of extensive replication within human monocytic cell vacuoles and induces apoptotic death via cytolethal distending toxin.
Our understanding of the interaction of Campylobacter jejuni with immune effector cells is limited. C. jejuni can survive phagocytosis by human mononuclear cells and remain viable for extended periods (9), induce secretion of chemokines (8), and mediate apoptosis (20). C. jejuni elaborates a well-characterized toxin, cytolethal distending toxin (CjCDT), that induces cell cycle arrest and interleukin 8 (IL-8) secretion from intestinal epithelial cells in culture (2, 7, 13, 22). CDTs from other bacteria are also known to target immune cells and ultimately induce apoptosis (3, 19, 21). There have been two reports of apoptotic responses to C. jejuni (20, 24), and both suggested that mechanisms independent of CDT were responsible. Here, we confirm earlier observations of long-term campylobacter survival and replication within monocytes and the induction of apoptosis. Since CDTs of other pathogens induce apoptosis of lymphocytes (1, 3, 17, 19, 21), we reexamined the effect of CjCDT on human monocytes in culture.
Growth of C. jejuni in human 28SC monocyte cultures.
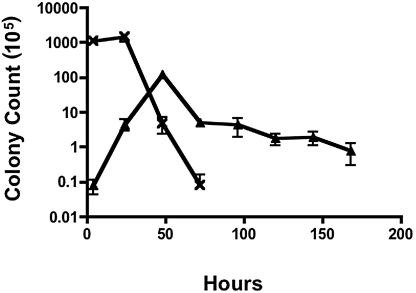
The human monocyte line 28SC was infected with 81-176 at a multiplicity of infection (MOI) of 100:1 for 2 h. Extracellular bacteria were killed with 100 μg/ml gentamicin for an additional 2-h incubation. The cells were washed and reincubated, and bacterial counts were enumerated at various times. Peak levels of 81-176 were recovered from 48-hour cultures (Fig. 1), representing an approximate 3-log-unit increase in bacterial counts (from approximately 104 CFU/ml following gentamicin treatment to 107 CFU/ml after 48 h). Viable campylobacters were recovered up to day 7, consistent with the results of Kiehlbauch et al. (9). In contrast, in the absence of monocytes, 81-176 viability dropped rapidly, and no bacteria could be cultured after 72 h. Thus, replication of C. jejuni within monocytes was more extensive than the limited replication following internalization into epithelial cells (12).
FIG. 1.
Survival of C. jejuni 81-176 in monocytic cell cultures. C. jejuni 81-176 was added to 28SC human monocytic cells grown in Iscove's modified Dulbecco's medium with 4 mM l-glutamine at an MOI of 100 bacteria to each monocyte. After a 2-h infection, extracellular campylobacters were killed with gentamicin (100 μg/ml) for an additional 2 h, and the cells were washed and resuspended in medium free of antibiotics. At the indicated time points, culture cells were harvested and lysed with 0.2% Triton X-100. Viable campylobacters were enumerated by plate counts. Assay controls were established by culturing the inoculating dose of 81-176 in tissue culture medium without monocytic cells. ▴, 81-176-infected 28SC cells; ×, 81-176 control cultured without 28SC cells. The data represent the mean and standard deviation of three independent experiments.
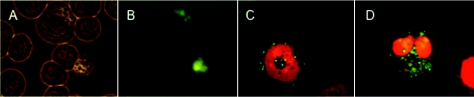
Green fluorescent protein (GFP)-tagged 81-176 was imaged within 28SC cells using fluorescence microscopy. Generalized fluorescence (Fig. 2B) could be seen intracellularly within enlarged and irregularly shaped areas of cells (Fig. 2A). Using live/dead staining, the C. jejuni organisms appeared to be localized within vacuoles, either as single or multiple bacteria (Fig. 2C and D). The bacteria appeared motile within the vacuoles, consistent with expression of GFP under the control of the σ28 promoter of the major flagellin (5).
FIG. 2.
Images of 28SC cells infected with C. jejuni 81-176. (A and B) Cells infected with 81-176 carrying pCPE111/28/GFP under phase (A) or fluorescence (B). The GFP gene from plasmid pWM1007 (16) was PCR amplified and cloned into plasmid pCPE111/28, a kanamycin resistance shuttle plasmid (23). This expression vector is similar to the chloramphenicol-resistant vector described by Larsen et al. (14). (C and D) Cells infected with wild-type 81-176 and stained with BacLight live/dead stain. The monocytes in panels C and D are dead but contain live C. jejuni organisms in vacuoles. 28SC cells were inoculated as described in the legend to Fig. 1.
Induction of chemokines from 28SC cells is independent of CDT.
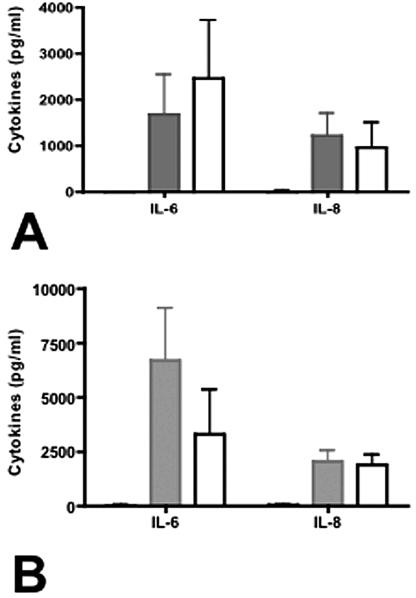
Viable campylobacters mediated chemokine responses from 28SC cells in 24-h cultures (Fig. 3A). 81-176 induced 1,689 ± 848 pg/ml IL-6 and 1,231 ± 479 pg/ml IL-8 in nine independent experiments. Since CDT is localized to bacterial membranes and is known to induce IL-8 secretion from intestinal epithelial cells (7), we compared the abilities of purified membrane proteins from DS105, a cdtA mutant deficient in CDT activity, and 81-176 to induce chemokine secretion from monocytes. When inoculated with 2 μg/ml bacterial proteins as described previously (6, 7), chemokine responses to 81-176 and DS105 proteins were similar (Fig. 3B). 81-176 and DS105 proteins induced 6,700 ± 2,409 and 3,324 ± 2,042 pg/ml IL-6 and 2,073 ± 467 and 1,909 ± 436 pg/ml IL-8, respectively. These data are consistent with previous reports implicating bacterial components, such as lipooligosaccharide, and not CjCDT in cytokine induction from monocytes (8).
FIG. 3.
Chemokine induction by the human monocytic cell line 28SC. (A) Induction of IL-6 and IL-8 by whole cells of C. jejuni. Live C. jejuni 81-176 or the cdt mutant DS105 was added to 28SC cells at an MOI of 100:1 as described previously, and IL-6 and IL-8 secretion levels were determined by enzyme-linked immunosorbent assay (ELISA) after 24 h of incubation. The P values for IL-6 and IL-8 secretion comparing the wild type to DS105 were 0.18 and 0.09, respectively. (B) Induction of IL-6 and IL-8 by membrane fractions from 81-176 or DS105. Bacterial membrane protein (2 μg) was added to each ml of 28SC culture maintained at 2.5 × 105 to 1.0 × 106 cells/ml. At 24 h, the culture supernatants were collected and assayed for IL-6 and IL-8 by ELISA. The P values for IL-6 and IL-8 secretion comparing the wild type to DS105 were 0.74 and 0.65, respectively. P values were calculated assuming unequal variance. Black solid, uninoculated control; grey solid, 81-176 inoculated; white, DS105 inoculated. The black bars are barely visible because of the low background level. The data represent the means and standard deviations of three to nine independent experiments.
Programmed cell death (PCD).
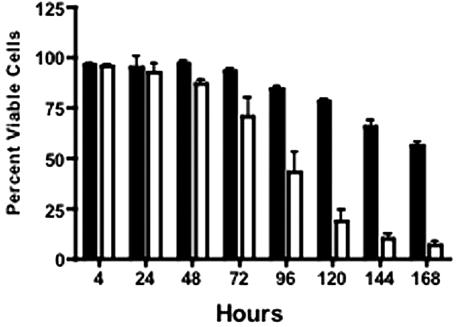
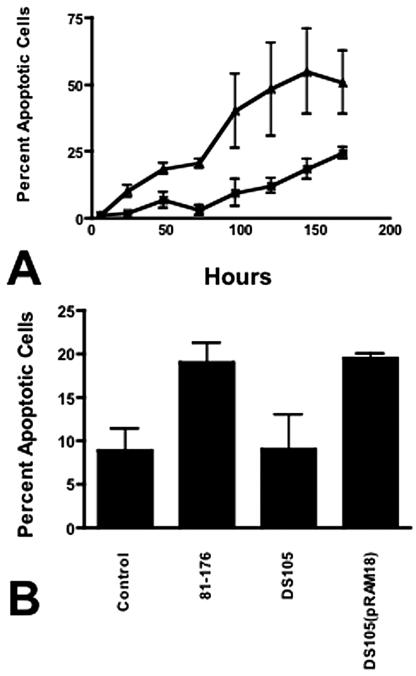
Uninfected 28SC cell viability was >84% at 96 h (Fig. 4) and fell to 56.5% ± 0.7% by 168 h. In contrast, the viability of cells infected with 81-176 was <75% by 72 h and continued to decline to 7.2% ± 2.6% at 168 h. Annexin V binding to surface phosphatidylserine (PS) (15) was measured using the Guava Technologies Nexin assay (Guava Technologies, Hayward, CA). C. jejuni induced apoptosis of 28SC cells at levels significantly greater than that seen in uninoculated cultures (Fig. 5A). Only 9.5% ± 5.0% of cells in control cultures bound annexin V after 96 h, and this progressed to 24.4% ± 2.2% at 168 h. In contrast, by 48 h, 18.6% ± 2% of 81-176-infected monocytes were apoptotic. Maximal levels were evident at 144 h, when 55% ± 15% of the cells expressed surface PS.
FIG. 4.
Monocyte viability was impacted by C. jejuni 81-176. 28SC cells were inoculated with C. jejuni 81-176 as described in the legend to Fig. 1. At the indicated time points, 28SC cells cultured with or without 81-176 were mixed 1:1 with Guava ViaCount reagent and assessed for viability through flow cytometry. The mean percentage of culture cells impervious to the dye plus 1 standard deviation was reported. Black solid, 28SC uninoculated; white, 81-176 inoculated. The data are representative of three independent experiments.
FIG. 5.
Programmed cell death of monocytes. (A) Kinetics of induction of apoptosis. 28SC cells were inoculated with 81-176 as described in the legend to Fig. 1. At the indicated time points, tissue culture cells were harvested and assayed for annexin V binding to surface PS via the Guava Nexin assay. Control cultures consisted of 28SC cells cultured in parallel without bacteria. The values reported are the mean percentages of cultured cells exhibiting early to intermediate signs of apoptosis (annexin V+ and 7-aminoactinomycin D−) ± 1 standard deviation. ▴, 81-176-inoculated 28SC cells; ×, control uninoculated 28SC cells. The data are representative of four independent experiments. (B) Induction of apoptosis by Campylobacter CDT. 28SC monocyte cultures were inoculated with 2 μg membrane protein fractions from 81-176, DS105, or DS105(pRAM18) per ml tissue culture. Control wells were incubated in parallel without inoculum or stimulation. At 48 h, the eukaryotic cells were harvested and assessed for PCD through surface PS expression and annexin V binding. The data are presented as mean percentages of cells expressing surface PS plus 1 standard deviation. P values were calculated assuming unequal variance. Statistical conclusions were verified through nonparametric Wilcoxon ranked sum tests.
CjCDT mediates apoptosis of 28SC cells.
CjCDT has been shown to localize to membranes (6, 7). Membrane proteins (2 μg/ml) from 81-176, the cdtA mutant DS105, and its complement, DS105(pRAM18) (7), were added to 28SC cultures. Wild-type 81-176 membranes induced surface PS expression on 19.0% ± 2.3% of culture cells by 48 h (Fig. 5B) (P ≤ 0.001). Membranes of DS105 cdtA mediated PS expression on 9.0% ± 4% of the cells (P = 0.92). Membranes isolated from DS105(pRAM18), the CDT mutant complemented in trans, induced PS expression at levels comparable to those of the wild type.
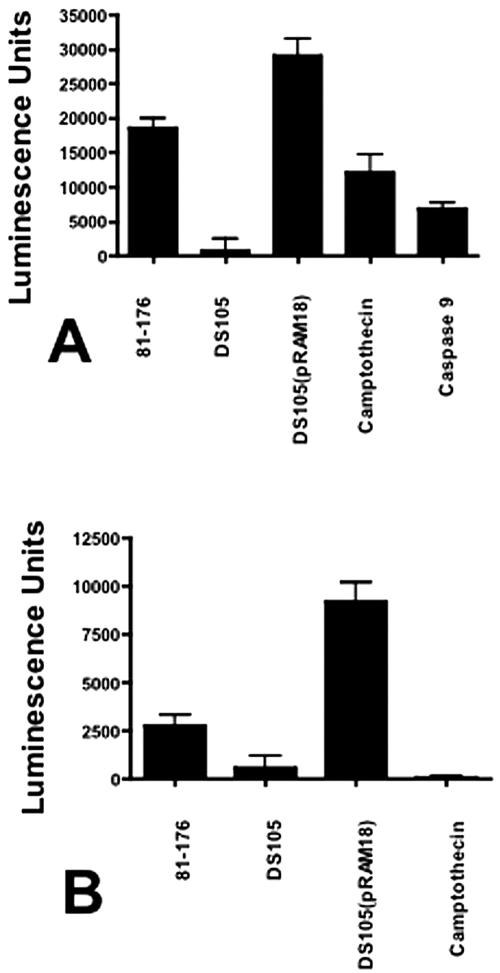
To verify CjCDT-induced apoptosis by an alternate method, activation levels of pre- and postmitochondrial caspases following exposure to C. jejuni membranes were measured. In 8-h cultures, caspase 9, and to a lesser extent caspase 8, activities correlated with the presence of CjCDT (Fig. 6A and B). 81-176 membranes mediated 18,546 ± 1,486 luminescence units for caspase 9 (P ≤ 0.0001). Cultures inoculated with DS105 membranes induced 793 ± 1,759 units (P = 0.37). The highest activity was seen in cultures inoculated with DS105(pRAM18) membrane fractions (29,129 ± 2,477 units; P ≤ 0.0001). Similar results were observed for caspase 8 activity (Fig. 6B). These data indicate that caspases associated with intrinsic and external apoptosis pathways were activated in response to CjCDT (4), consistent with other CDTs (1, 3, 17, 19, 21).
FIG. 6.
Caspase activation by C. jejuni membrane fractions. Human monocytic cell cultures (28SC) were inoculated with 2 μg campylobacter membrane proteins per ml of culture as described in the legends to Fig. 3 and 5. After 8 h in culture, the cells were harvested and assessed for caspase 8 and caspase 9 activities via the Promega Caspase-Glo 8 and 9 assays. One unit of recombinant caspase 8 or caspase 9 (BIOMOL, Plymouth Meeting, PA) was used as a positive control. In these assays, 1 unit of recombinant caspase 9 produced 6,838 ± 864 luminescence units. One unit caspase 8 produced 178,254 ± 12,611 luminescence units (data not shown). 28SC cells were pulsed with 6.2 μM camptothecin for 2 h as a positive control for apoptosis. (A) Caspase 9 activity. (B) Caspase 8 activity. The values are reported as mean luminescence units plus 1 standard deviation. P values were calculated assuming unequal variance. The data are representative of four independent experiments.
Both previous reports of induction of apoptosis by C. jejuni in either chicken lymphocytes (24) or human monocytes (20) indicated that PCD was independent of CjCDT, although limited data were presented. Siegesmund et al. (20) reported that apoptosis of THP-1 monocytes required, instead, secretion of the Cia (campylobacter invasion antigen) proteins that are secreted through the flagellar secretion apparatus (10, 11, 18). We constructed site-specific insertional mutants of 81-176 defective in ciaB or flgB, a component of the flagellar basal body. Both mutants would be expected to be defective in secretion of the Cia proteins (10). However, both mutants induced levels of apoptosis comparable to those of 81-176 (data not shown).
C. jejuni can survive intracellularly in both intestinal epithelial cells and monocytes. The DNA damage induced by CjCDT appears to exert different effects on each cell type, which is likely a function of the p53 status of the line (1). In epithelial cells, CjCDT causes a G2/M block and induces high levels of chemokines. In contrast, release of proinflammatory chemokines from infected monocytes appears to be independent of CjCDT, and the cytotoxic effect is manifested by apoptosis. Survival within macrophages likely enhances the localized inflammatory response to C. jejuni and may also provide the bacteria with an immunologically privileged site and a mechanism for replication and dissemination within the host (12).
Acknowledgments
We thank Cheryl Ewing and Anahita Kiavand for plasmid and mutant constructions, Shahida Baqar and Bob Lin for their expert advice on flow cytometry, and Chad Porter for help with statistical analysis.
This work was funded by the Military Infectious Diseases Program work unit no. 6000.RAD1.DA3.A0308.
Editor: J. T. Barbieri
REFERENCES
- 1.Cortes-Bratti, X., C. Karlsson, T. Lagergard, M. Thelestam, and T. Frisan. 2001. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via DNA damage checkpoint pathways. Infect. Immun. 276:5296-5302. [DOI] [PubMed] [Google Scholar]
- 2.Elwell, C. A., and L. A. Dreyfus. 2000. DNase I homologous residues in CdtB are critical for cytolethal distending toxin mediated cell cycle arrest. Mol. Microbiol. 37:952-963. [DOI] [PubMed] [Google Scholar]
- 3.Gelfanova, V., E. J. Hansen, and S. M. Spinola. 1999. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect. Immun. 67:6394-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green, D. R. 2000. Apoptotic pathways: paper wraps stone blunts scissors. Cell 102:1-4. [DOI] [PubMed] [Google Scholar]
- 5.Guerry, P., R. A. Alm, M. E. Power, S. M. Logan, and T. J. Trust. 1991. Role of two genes in Campylobacter motility. J. Bacteriol. 173:4757-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickey, T. E., S. Baqar, A. L. Bourgeois, C. P. Ewing, and P. Guerry. 1999. Campylobacter jejuni stimulated secretion of interleukin-8 by INT407 cells. Infect. Immun. 67:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickey, T. E., A. L. McVeigh, D. A. Scott, R. E. Michietutti, A. Bixby, S. A. Carroll, A. L. Bourgeois, and P. Guerry. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 68:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, M. A., S. Totemeyer, D. J. Maskell, C. E. Bryant, and P. A. Barrow. 2003. Induction of proinflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni. Infect. Immun. 71:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiehlbauch, J. A., R. A. Albach, L. L. Baum, and K. P. Chang. 1985. Phagocytosis of Campylobacter jejuni and its intracellular survival in mononuclear phagocytes. Infect. Immun. 48:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konkel, M. E., B. J. Kim, V. Rivera-Amill, and S. G. Garvis. 1999. Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 32:691-701. [DOI] [PubMed] [Google Scholar]
- 11.Konkel, M. E., J. D. Klena, V. Rivera-Amill, M. R. Monteville, D. Biswas, B. Raphael, and J. Mickelson. 2004. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J. Bacteriol. 186:3296-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopecko, D. J., L. Hu, and K. J. M. Zaal. 2001. Campylobacter jejuni-microtubule-dependent invasion. Trends Microbiol. 9:389-396. [DOI] [PubMed] [Google Scholar]
- 13.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease-1 like protein. Science 290:354-357. [DOI] [PubMed] [Google Scholar]
- 14.Larsen, J. C., C. Szymanski, and P. Guerry. 2004. N-linked protein glycosylation is required for full competence in Campylobacter jejuni 81-176. J. Bacteriol. 186:6508-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin, S. J., C. P. M. Reutelingsperger, A. J. McGahon, J. A. Rader, R. C. A. A. Van Schie, D. M. LaFace, and D. R. Green. 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182:1545-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, W. G., A. H. Bates, S. T. Horn, M. T. Brandl, M. R. Wachtel, and R. E. Mandrell. 2000. Detection on surfaces and in Caco-2 cells of Campylobacter jejuni transformed with new gfp, yfp, and cfp marker plasmids. Appl. Environ. Microbiol. 66:5426-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohara, M., T. Hayashi, Y. Kusunoki, M. Miyauchi, T. Takata, and M. Sugai. 2004. Caspase-2 and caspase-7 are involved in cytolethal distending toxin-induced apoptosis in Jurkat and MOLT-4 T-cell lines. Infect. Immun. 72:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera-Amill, V., B. J. Kim, J. Keshu, and M. E. Konkel. 2001. Secretion of the virulence associated Campylobacter invasion antigens from Campylobacter jejuni requires a stimulatory signal. J. Infect. Dis. 183:1607-1616. [DOI] [PubMed] [Google Scholar]
- 19.Shenker, B. J., R. H. Hoffmaster, A. Zekavat, N. Yamaguchi, E. T. Lally, and D. Demuth. 2001. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J. Immunol. 167:435-441. [DOI] [PubMed] [Google Scholar]
- 20.Siegesmund, A. M., M. E. Konkel, J. D. Klena, and P. F. Mixter. 2004. Campylobacter jejuni infection of differentiated THP-1 macrophages results in interleukin 1β release and caspase-1-independent apoptosis. Microbiology 150:561-569. [DOI] [PubMed] [Google Scholar]
- 21.Svensson, L. A., A. Tarkowski, M. Thelestam, and T. Lagergard. 2001. The impact of Hemophilus ducreyi cytolethal disetending toxin on cells involved in the immune response. Microb. Pathog. 30:157-166. [DOI] [PubMed] [Google Scholar]
- 22.Whitehouse, C. A., P. B. Balbo, E. C. Pesci, D. L. Cottle, P. M. Mirabito, and C. L. Pickett. 1998. Campylobacter jejuni cytolethal distending toxin causes a G2 phase cell cycle block. Infect. Immun. 66:1934-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao, R., R. A. Alm, T. J. Trust, and P. Guerry. 1993. Construction of new Campylobacter cloning vectors and a new chloramphenicol mutational cassette. Gene 130:127-130. [DOI] [PubMed] [Google Scholar]
- 24.Zhu, J., R. J. Meinersmann, K. L. Hiett, and D. L. Evans. 1999. Apoptotic effect of outer-membrane proteins from Campylobacter jejuni on chicken lymphocytes. Curr. Microbiol. 38:244-249. [DOI] [PubMed] [Google Scholar]