Abstract
Multiple-antibiotic-resistant Salmonella enterica serotype Typhimurium is a food-borne pathogen that has been purported to be more virulent than antibiotic-sensitive counterparts. The paradigm for this multiresistant/hyperpathogenic phenotype is Salmonella enterica serotype Typhimurium phage type DT104 (DT104). The basis for the multiresistance in DT104 is related to an integron structure designated SGI1, but factors underlying hyperpathogenicity have not been completely identified. Since protozoa have been implicated in the alteration of virulence in Legionella and Mycobacterium spp., we attempted to assess the possibility that protozoa may contribute to the putative hypervirulence of DT104. Our study reveals that DT104 can be more invasive, as determined by a tissue culture invasion assay, after surviving within protozoa originating from the bovine rumen. The enhancement of invasion was correlated with hypervirulence in a bovine infection model in which we observed a more rapid progression of disease and a greater recovery rate for the pathogen. Fewer DT104 cells were recovered from tissues of infected animals when protozoa were lysed by preinfection chemical defaunation of the bovine or ovine rumen. The protozoan-mediated hypervirulence phenotype was observed only in DT104 and other Salmonella strains, including serovars Agona and Infantis, possessing SGI1.
Salmonella enterica is a major cause of food-borne illnesses throughout the world (32). Salmonellosis can present as a self-limiting diarrheal disease that does not require antimicrobial therapy. However, severe diarrhea and/or systemic infection can occur, and thus antibiotic treatment is needed. Unfortunately, numerous Salmonella strains have become resistant to multiple antibiotics. This is especially true for Salmonella enterica serotype Typhimurium, a pathogen with a broad host range. Within this serotype there exist numerous subgroups based on phage type with one particular strain, namely, phage type DT104, emerging as the predominant multiresistant Salmonella strain (30). S. enterica serotype Typhimurium phage type DT104 (DT104) is often resistant to five or more antibiotics as the result of the acquisition of an integron structure, designated Salmonella genomic island 1 (SGI1) (2), that contains genes encoding resistance to five different antibiotics (3).
Besides demonstrating the multiresistant phenotype, DT104 also appears to be more virulent, especially in cattle. This is underscored by the finding that calves infected with DT104 are 13 times more likely to die than are calves infected with antibiotic-sensitive S. enterica serotype Typhimurium (14). Additionally, humans are two to three times more likely to be hospitalized upon DT104 infection (36). The basis for the putative “hypervirulence” is unclear since enhanced virulence might be difficult to separate from treatment failures and/or selective pressures.
Explanations for hypervirulence in Salmonella include acquisition and expression of exogenous virulence genes or overexpression of innate virulence genes. Numerous studies have failed to identify virulence genes unique to DT104; thus, the latter possibility seemed more likely. Since intestinal cell invasion by Salmonella is a major factor for initiating systemic illness (15) and since certain genetic manipulations in Salmonella enterica serotype Typhimurium can result in a hyperinvasive/hypervirulent phenotype (24), enhanced invasiveness was previously examined as a possible explanation for DT104 hypervirulence. Two previous in vitro studies demonstrated that DT104 is not inherently hyperinvasive (1, 5).
It is possible, however, that DT104 may be hyperinvasive in the presence of certain environmental signals, such as antibiotics, or in the presence of other microbes such as bacteria, viruses, yeast, or protozoa. Carlson et al. demonstrated that hyperinvasion was not observed in the presence of antibiotics (7). Commensal microbes can moderately augment Salmonella invasion (25), although this appears to be more applicable to host-adapted strains and not to serotypes with broad host ranges such as serotype Typhimurium. Perhaps the most relevant scenario is the finding that protozoa can increase the invasiveness of Legionella pneumophila (10). Since the bovine rumen contains a wide variety and a large quantity of protozoa and since Legionella and Salmonella share some invasive characteristics (42), it seemed possible that rumen protozoa (RPz) could play a part in the putative hypervirulent phenotype of DT104. Thus, the goal of this study was to examine the relationship between RPz, Salmonella invasion, and Salmonella pathogenicity.
MATERIALS AND METHODS
Bacterial strains and preparation.
Bacterial strains are summarized in Table 1 with strain 98-420 (4) serving as the model strain for Salmonella enterica serotype Typhimurium phage type DT104. Bacteria were stored in cryopreservation tubes containing 50% glycerol-50% culture medium at −70°C and grown on Lennox L broth or agar (GIBCO BRL) with antibiotics such as ampicillin (Sigma; 32 μg/ml), chloramphenicol (Sigma Chemicals; 32 μg/ml), kanamycin (Sigma Chemicals; 64 μg/ml), or zeocin (Invitrogen; 25 μg/ml). Bacteria used in all invasion assays and in vivo experiments were transformed with pECFP (16), a pCRII-Blunt (Invitrogen) plasmid containing genes encoding enhanced cyan fluorescent protein (ECFP), kanamycin resistance, and zeocin resistance. However, one strain (MW55) of DT104 was transformed with pCRII-Blunt containing the gene encoding ECFP but lacking the zeocin resistance gene (pECFPΔzeo). Transformation with ECFP-encoding plasmids was performed in order to visually distinguish bacteria of interest from bacteria normally present in rumen fluid.
TABLE 1.
Summary of strains and plasmids used in this studya
| Strain or plasmid designation | SGI1 status | Antibiogramb | Note | Reference |
|---|---|---|---|---|
| 98-420 (DT104) | + | PentaR | DT104 phage type | 4 |
| EE419 | − | Tet | Hyperinvasive | 24 |
| SL1344 | − | PanSen | 41 | |
| BJ68 | − | Tet (via Tn5) | Noninvasive; Tn5 insertion in sipC | 28 |
| TH16 (DT104) | − | PanSen | Wild-type isolate lacking SGI1 | 4 |
| MW54 (DT104) | + | StrSuTetZeo (latter via TnZeo) | TnZeo insertion in pse-1; naturally occurring truncation of floR | This study |
| MW55 (DT104) | + | StrSuTetZeo | Noninvasive (sipD point mutation) subclone of MW54 | This study |
| MW55/pInvasin | + | AmpStrSuTetZeo | Invasive (Yersinia-based) MW55 transformant | This study |
| LNWI(−)slyA (DT104) | + | PentaR | Unable to survive within macrophages | 8 |
| 202/37 and 76 | − | PentaR | 13, 35 | |
| 798 | − | PentaR | Phage type DT208 | 17 |
| Serotype Agona | + or − | PentaR or PanSen, respectively | 2 | |
| Serotype Infantis | + or − | PentaR or PanSen, respectively | 4 | |
| Serotype Newport | − | PentaR or PanSen | 4 | |
| Serotype Dublin | − | PentaR or PanSen | Bovine adapted | 20 |
| Serotypes Gallinarum, Senftenberg, Montevideo, Heidelberg, Panama, Choleraesuis, and Enteritidis | − | PanSen | S. enterica serotype Gallinarum is noninvasive for mammalian cells | 4 |
| pECFP | NAc | KanZeo | Encodes ECFP | 16 |
| pECFPΔzeo | NA | Kan | ECFP for MW55 | This study |
| pKLP103 | NA | Amp | Encodes SipC | 28 |
| pKLP104 | NA | Kan | Encodes SipD | 28 |
| pRW27 | NA | Cm | Encodes SipB | This study |
| pInvasin | NA | Amp | Encodes invasin | 22 |
All strains are Salmonella enterica serotype Typhimurium unless indicated otherwise.
Amp, ampicillin; Cm, chloramphenicol; PentaR, pentaresistant with the AmpCmStrSuTet antibiogram; Kan, kanamycin; PanSen, pan-sensitive; Str, streptomycin; Su, sulfamethoxazole; Tet, tetracycline; Zeo, zeocin.
NA, not applicable.
Isolation of RPz for in vitro studies.
Approximately 100 ml of postprandial rumen fluid was removed from a 12-year-old nonlactating Jersey cow fed a standard hay and grain diet. Fluid was removed through a rumen fistula that was surgically introduced approximately 9 years previously. Rumen fluid was then filtered to remove large particulate matter and mixed with an equal volume of Coleman's buffer D (12). Protozoa were then allowed to settle for 2 h under CO2. Settled protozoa were aspirated and washed twice with approximately 45 ml Coleman's buffer D and then centrifuged for 20 s at 230 × g. Pelleted protozoa were resuspended in 30 ml Coleman's buffer D under CO2. One milliliter was used for enumeration, and 3 ml (approximately 105 RPz) was used in each invasion assay. Genera of protozoa observed included Eudiplodinium, Metadinium, Polyplastron, Isotricha, Entodinium, Ophryoscolex, and Diplodinium, with Isotricha predominating.
Salmonella invasion assays following survival within RPz, bovine macrophages, or HEp-2 cells.
HEp-2 cells and bovine macrophages (33) were separately maintained in RPMI 1640 (GIBCO) containing 10% fetal bovine serum at 37°C in a 5% CO2 humidified incubator. Approximately 109 bacteria were added to approximately 105 RPz, bovine macrophages, or HEp-2 cells. The Salmonella-RPz mixture was then gently rolled for 16 h at 37°C in a sealed 5-ml glass tube whereas the Salmonella-tissue culture (bovine macrophages or HEp-2 cells) incubations were performed using adherent cells in tissue culture dishes for 16 h at 37°C in a 5% CO2 humidified incubator. For the antiengulfment experiments, cytochalasin D (Sigma Chemicals) was added (1 μg/ml) (27) to the Salmonella-eukaryotic cell mixture for 16 h at 37°C. At the end of the 16-h incubation period, extracellular Salmonella organisms were killed using 300 μg/ml florfenicol (Schering-Plough). Eukaryotic cells (i.e., RPz, HEp-2, or bovine macrophages) were then lysed for 60 s at 4,800 rpm using 2.5 mM glass beads and a mini-Bead Beater (Biospec Products). We found that this procedure lysed 70 to 75% of the RPz. The lysate was centrifuged at 15,000 rpm for 2 min and then resuspended in 350 μl Lennox L broth. Of the 350 μl, 25 μl was used for selective enumeration and 300 μl was used for an invasion assay performed in triplicate (i.e., 100 μl/well).
Invasion assays were performed as described previously using HEp-2 cells (5) with the exception that enumerations were performed using agar plates containing 25 μg/ml zeocin. Briefly, bacteria were incubated with HEp-2 cells for various times (0 to 1 h) at 37°C in a 5% CO2 humidified incubator with a multiplicity of infection equal to approximately 40. Extracellular bacteria were killed using 300 μg/ml florfenicol during a 2-h incubation period at 37°C in a 5% CO2 humidified incubator. HEp-2 cells were then lysed using 1% Triton X at 37°C, and lysates were plated on selective media and grown overnight at 37°C, followed by enumeration of green colonies on the next day. Percent invasion was calculated by dividing CFU recovered by CFU added.
Creation of DT104 strains MW55 and MW55/pInvasin.
To determine if the RPz-mediated effects were specific for Salmonella invasion processes, we engineered a DT104 strain that invades tissue culture cells as a result of expressing the Yersinia enterocolitica protein designated invasin (22). The first step was to create an ampicillin-sensitive DT104, by deleting the SGI1 beta-lactamase gene designated pse-1 (3), since the plasmid encoding invasin (pInvasin) contains an ampicillin resistance marker. A chloramphenicol-sensitive, due to a naturally occurring truncation in floR (4), and kanamycin-sensitive strain of DT104 was subjected to insertional mutagenesis using a transposon (TnZeo) containing the zeocin resistance gene (8) followed by selection of ampicillin-sensitive colonies.
The second step was to create a noninvasive DT104 from a pse-1::TnZeo insertional mutant. An ampicillin-sensitive DT104 subclone (designated MW54) was subjected to a high-throughput invasion assay (6) in which noninvading bacteria, i.e., those remaining extracellular after 3 to 4 h of incubation with eukaryotic cells, were collected and propagated for subsequent invasion assays. Percent invasion began to wane after the 70th consecutive assay and became negligible after the 236th consecutive invasion assay. The resulting pool of noninvasive MW54 subclones was then cotransformed with pKLP103 (ampicillin resistance) (28), pKLP104 (kanamycin resistance) (28), and pRW27 (a pBAD vector [Invitrogen] containing floR [37] instead of the beta-lactamase gene), which respectively encode the invasion-conferring proteins SipD, SipC, and SipB (23). One clone, designated MW55/pKLP103pKLP104pRW27, was found to be invasive, suggesting a mutation in the sipB-sipC-sipD operon (28). Subsequent transformations and invasion assays revealed that MW55/pKLP103 was invasive. DNA sequencing identified a point mutation in sipD that resulted in a premature stop codon for MW55.
The third step was to create an MW55 subclone that invades using invasin as its primary invasion protein. To do so, MW55 was cotransformed with pInvasin plus pCRII-Blunt (Invitrogen) containing ECFP (16) but lacking the zeocin resistance gene (i.e., pECFPΔzeo). The zeocin resistance gene was deleted from pCRII-Blunt/ECFP by restriction digestion, using AatII and XmaI (New England BioLabs), followed by blunting with mung bean nuclease (New England BioLabs) and then self-ligation using T4 DNA ligase (New England BioLabs). Fluorescent MW55/pInvasin/pECFPΔzeo transformants were then selected using zeocin (resistance conferred by the transposon), ampicillin (resistance conferred by pInvasin), and kanamycin (resistance conferred by pECFPΔzeo).
In vivo infection experiments.
Salmonella cells and rumen fistula-derived RPz were incubated as described above. Calves were infected with Salmonella recovered from RPz (n = 3 calves) or with RPz still containing Salmonella (n = 6 calves). RPz or RPz lysates were resuspended in 350 μl of Lennox L broth, of which 25 μl was used for bacterial enumeration while 325 μl (approximately 4 × 108 CFU of Salmonella) was used for infection. The 325 μl was placed in a gelatin capsule, and the capsule was orally introduced into 1- to 2-week-old Holstein calves (approximately 50 to 100 lb each) immediately followed by 500 ml of commercial milk replacer. Control calves (n = 7) were challenged with DT104 exposed to RPz buffer (n = 3) or DT104 present in HEp-2 cells (n = 4). Calves were monitored for changes in appetite, stool consistency, and rectal temperature every 8 to 12 h. At 36 h postinfection, calves were euthanized using xylazine (1 mg/lb of body weight, intramuscularly; Phoenix Laboratories) and pentobarbital (2.6 mg/lb, intravenously; Fort Dodge Laboratories). The 36-hour time point was arbitrarily derived based on empirical evidence from the first calf infected with Salmonella exposed to RPz. This calf was severely dehydrated with labored breathing, and thus, euthanasia was chosen at this point. Thus, all calves were euthanized at 36 h followed by a necropsy in which tissues were aseptically removed. Tissues collected included the spleen and mesenteric lymphatic tissue (e.g., ileocecal lymph nodes, celiac lymph nodes, and gut-associated lymphoid tissues).
Ligated loop experiments were performed by inoculating approximately 4 × 108 CFU of DT104/pECFP, either recovered from RPz or exposed to RPz culture medium, into 5-cm sections of ligated ileal sections. After 25 min, ileal sections were excised and everted and then washed three times with phosphate-buffered saline (PBS). Sections were then soaked for 1 h in PBS containing 300 μg/ml florfenicol in order to kill extracellular bacteria. The ileal sections were then removed from the medium and rinsed in PBS followed by scraping of the lumen with a scalpel blade. Scrapings were placed in 250 μl of Lennox L broth containing 0.1% Triton X for 30 min. Lysates were then plated on selective medium, i.e., Lennox L agar containing 25 μg/ml zeocin, for enumeration of bacteria. Animal experiments were approved by the Animal Care and Use Committee at the National Animal Disease Center (protocol 3462).
Salmonella most-probable-number experiments.
Tissue samples (1.5 to 3 g) taken from calves were homogenized with a rubber mallet and a stomacher. Homogenates were then subjected to the most-probable-number enumeration procedure using a series of selective medium preparations (40) containing 25 μg/ml zeocin. The identity of Salmonella strains was confirmed using fluorescence, antiserum/agglutination-based serogrouping, and PCR as described previously using the cmlA-tetR amplicon (4).
Defaunation procedures.
Eight-week-old Jersey calves (approximately 50 lb) or adult sheep (approximately 150 lb; various breeds) were fed a standard alfalfa and grain diet. Feed was withheld for 24 h, and then animals were orally drenched with 0.7 mg/kg of body weight of dioctyl sodium sulfosuccinate (DSS; Sigma). Following the first dose of DSS, animals were fed 50% the normal volume of alfalfa. The next day animals were given a second oral dose of DSS (0.7 mg/kg) and then fed the normal volume of alfalfa and grain. Control animals were orally drenched with water instead of DSS. Animals were infected, as described above, with approximately 4 × 108 CFU of DT104/pECFP at 12 h following the second dose of DSS or water. Animals were monitored as described above. At 36 h postinfection, animals were euthanized as described above and spleen samples were aseptically removed. Additionally, 50 ml of abomasal fluid was removed for RPz isolation and enumeration.
Quantitative PCR.
Tissues (100 mg) from infected animals were aseptically chopped into smaller pieces and processed for DNA isolation using the DNeasy tissue kit according to the directions recommended by the manufacturer (QIAGEN, Valencia, Calif.). Since 100 mg of tissue was used for each sample, the volume of reagents used was four times that indicated in the kit directions. Total DNA was eluted in 200 μl of elution buffer, and the concentration of isolated DNA was determined using a Biospec Mini spectrophotometer (Shimadzu, Torrance, CA).
Total DNA isolated from infected tissues was amplified using primers (31) specific for detection of a 250-bp sipB-sipC gene fragment of Salmonella. Briefly, 5-μl aliquots of DNA (containing 48, 140, or 310 ng of total DNA from spleen, lymph node, or ileal tissue, respectively) were added to 45 μl of a PCR mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 10 mM Na2 EDTA, 3 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 600 nM forward and reverse primers, and 2.5 units of AmpliTaq Gold DNA polymerase (PE Applied Biosystems, Foster City, CA). Samples were heated to 95°C for 10 min and subjected to 40 cycles of denaturation at 94°C for 30 s, annealing for 30 s at 55°C, and polymerization for 30 s at 72°C. The final extension was carried out at 72°C for 10 min followed by incubation of samples at 4°C. The amplification products were electrophoresed through a 4% agarose gel (Cambrex Bioscience Rockland, Inc., Rockland, ME), and DNA bands were visualized by staining the gel in ethidium bromide solution. The molecular size of visible bands was estimated from a DNA-sizing ladder (50-bp ladder; Gibco-BRL).
Statistical analyses.
Statistical analysis was performed using an analysis of variance with Scheffe's F test for multiple comparisons. Comparisons were made between tissues or between time points (e.g., lymph nodes versus spleen or 8 h versus 36 h, respectively) or between RPz-dependent and RPz-independent data. Analyses were performed using StatView (SAS Institute).
RESULTS
Hyperinvasiveness in DT104 recovered from rumen protozoa.
Various Salmonella bacteria were incubated with RPz, recovered from RPz, and then evaluated for alterations in virulence using a HEp-2 cell invasion assay. In order to visually distinguish Salmonella from other bacteria present in and around RPz, all Salmonella bacteria were transformed with a plasmid encoding a fluorescent protein (16). This transformation also enabled the selection of Salmonella using zeocin, an antibiotic that most bacteria are sensitive to. As shown previously, this plasmid has no impact upon invasion (16).
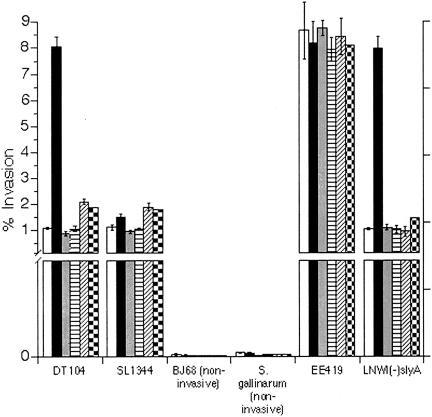
As depicted in Fig. 1, DT104 was markedly more invasive than standard invasion controls after recovery from RPz. Smaller increases in invasiveness were noted following recovery from bovine macrophages or HEp-2 cells. The hyperinvasive state of DT104 was comparable to that of EE419, a recombinant S. enterica serotype Typhimurium strain that is significantly hyperinvasive due to overexpression of hilA (24). RPz had no effect on the invasiveness of antibiotic-sensitive Salmonella enterica serotype Typhimurium strain SL1344 (41), a strain that is a model for studying Salmonella invasion (26). Hyperinvasion was also not observed for DT104 exposed to RPz culture medium (control), RPz lysates, or RPz that were presumably incapable of engulfing bacteria due to the presence of the actin cytoskeletal rearrangement inhibitor cytochalasin D. For the cytochalasin D experiments, however, less than 1% of Salmonella bacteria were recovered, except for EE419, where up to 4% were recovered (Table 2).
FIG. 1.
Assessment of 1-h HEp-2 cell invasion for Salmonella recovered from RPz. Strain designations are summarized in Table 1. Black bars correspond to Salmonella recovered from RPz. Controls included Salmonella exposed to RPz culture medium (open bars), Salmonella exposed to RPz lysates (gray bars), Salmonella recovered from RPz that were exposed to cytochalasin D (horizontally hatched bars), Salmonella recovered from bovine macrophages (diagonally hatched bars), and Salmonella recovered from HEp-2 cells (checkered bars). Percent invasion equals 100(CFU recovered/CFU added).
TABLE 2.
Relationship between survival within RPz, RPz-mediated hyperinvasion, and SGI1 status for Salmonella and other multiresistant pathogens
| Strain identification | No. of strains assessed | RPz-mediated hyperinvasion | SGI1 status | % Recovered from RPzg |
|---|---|---|---|---|
| DT104a | 12 | Yes | + | 38 ± 3 (<1) |
| S. enterica serotype Typhimurium (pan-sensitive and multiresistant); non-DT104 | 10 | No | − | 40 ± 1 (<1) |
| LNWIΔslyA (DT104) | 1 | Yes | + | <1 (<1) |
| S. enterica serotype Agona | 1 | Yes | + | 37 ± 5 |
| S. enterica serotype Infantis | 1 | Yes | + | 41 ± 2 |
| S. enterica, various serotypesb | 10 | No | − | 36 ± 4 [<1] |
| MW55/pInvasin (DT104) | 1 | Noc | +f | 33 ± 8 |
| BJ68 | 1 | Nod | − | 32 ± 4 (<1) |
| EE419 | 1 | Noe | − | 44 ± 3 (4 ± 0.5) |
| S. enterica serotype Gallinarum | 1 | Nod | − | 31 ± 6 (<1) |
Includes DT104-related phage types DT193, DT120, and U302.
Includes one strain of pan-sensitive S. enterica serotype Agona, one strain of pan-sensitive S. enterica serotype Infantis, and one pan-sensitive strain of S. enterica serotype Panama.
Invasion is independent of Salmonella invasion proteins.
Noninvasive.
Constitutively hyperinvasive.
SGI1 has a transposon in the beta-lactamase gene and a naturally occurring truncation in the chloramphenicol resistance gene.
Numbers in parentheses indicate percent recovered in the presence of cytochalasin D. Number in brackets indicates percent recovered for S. enterica serotype Panama.
An invasive phenotype was not conferred on noninvasive S. enterica serotype Typhimurium strain BJ68 (sipC deletion [28]) or on S. enterica serotype Gallinarum, a poultry strain that is noninvasive for mammalian cells (39). However, both of these strains were recovered from RPz at a percentage similar to that of DT104 as shown in Table 2. Interestingly, EE419 was capable of invading RPz in the presence of cytochalasin, although the percent recovered from RPz was approximately 1/10 of that observed in the absence of cytochalasin.
Since the hyperinvasive phenotype could be a result of enhanced intracellular survival, the RPz-mediated phenotype was also assessed in a strain of DT104 that is unable to survive in macrophages because of a deletion in slyA (8). This strain, LNWI(−)slyA, was capable of exhibiting the RPz-mediated hyperinvasive phenotype (Fig. 1), although only a small percentage of bacteria were able to survive in RPz (Table 2). One strain of S. enterica serotype Panama was also not able to survive within RPz, although the few colonies recovered were not hyperinvasive (Table 2).
As an additional control, Salmonella bacteria were incubated with RPz medium in the absence of RPz and then placed in a well containing both RPz and HEp-2 cells. HEp-2 invasion was measured after RPz were washed away and extracellular Salmonella bacteria were killed. The percent invasion for DT104 was 1.06 ± 0.14 while that for SL1344 was 1.15 ± 0.17 in this control experiment.
Temporally related onset of hyperinvasiveness in DT104 recovered from rumen protozoa.
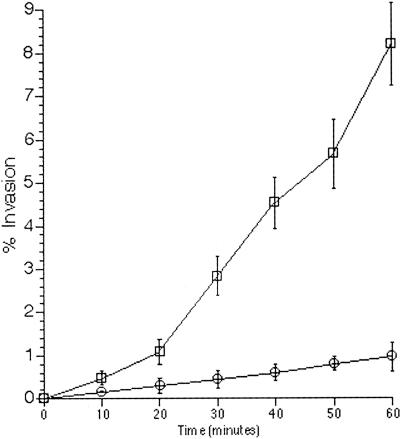
As shown in Fig. 2, the enhancement of invasiveness in DT104 can be observed as early as 20 min following recovery from RPz. These studies provided the basis for evaluating invasion in an in vivo ileal ligated loop model shown in Fig. 5.
FIG. 2.
Evaluation of the temporal nature of RPz-mediated DT104 hyperinvasion of HEp-2 cells. Time points correspond to length of DT104-HEp-2 cell coincubation. Squares represent the invasion of DT104 recovered from RPz, while the circles correspond to DT104 exposed to RPz culture medium. Percent invasion equals 100(CFU recovered/CFU added).
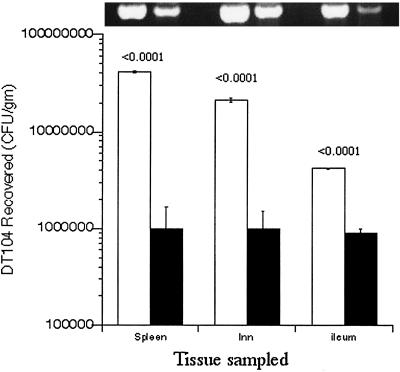
FIG. 5.
Quantitation of DT104 recovered from calves experimentally challenged with the pathogen. (Bottom, graph) Enumeration of Salmonella bacteria recovered from tissues (spleen, lymph nodes [lnn], or ileum) removed from calves challenged with DT104. For spleen and lymph node studies, DT104 was either recovered from RPz (open bars, n = 3), present in RPz (open bars, n = 6), exposed to RPz culture medium (filled bars, n = 3), or present in HEp-2 cells (filled bars, n = 4). The data represent most-probable numbers of DT104 recovered from calves at 36 h following an oral infection. For ileal studies, ligated loops were employed and the data represent CFU recovered at 25 min following ileal inoculation. Ligated loops were inoculated with DT104 recovered from either RPz (open bars, n = 3) or HEp-2 cells (filled bars, n = 3). P values, comparing RPz-dependent and RPz-independent data, are given above the RPz-dependent data. (Top, gel photo) Agarose gel electrophoresis of 250-bp sipB-C amplicons (4) obtained from PCR amplification of infected tissues corresponding to that present in the graph. For each tissue, a standard amount of DNA was used for amplification as described in Materials and Methods.
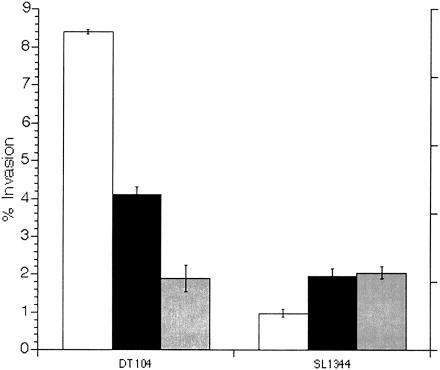
Transient RPz-mediated hyperinvasiveness in DT104.
To determine the duration of the effect on invasion, colonies recovered from RPz-dependent invasion assays were immediately used in consecutive RPz-independent invasion assays. As shown in Fig. 3, the RPz-mediated effect on invasion was transient. Hyperinvasion was evident yet not as robust in the second assay and was not observed in the third assay. Interestingly, but not surprisingly, invasion for SL1344 initially increased after the first recovery from HEp-2 cells but stabilized thereafter. A similar small increase in invasion was noted for both SL1344 and DT104 recovered from HEp-2 cells or bovine macrophages (Fig. 1).
FIG. 3.
Transient hyperinvasion of HEp-2 cells (1 h) for DT104 exposed to RPz. Open bars represent bacteria isolated from RPz, i.e., the first invasion assay. Black bars represent the second invasion assay, i.e., bacteria recovered from the first assay. Gray bars represent the third invasion assay, i.e., bacteria recovered from the second assay. Percent invasion equals 100(CFU recovered/CFU added).
Salmonella hyperinvasiveness related to the presence of the DT104 integron structure SGI1.
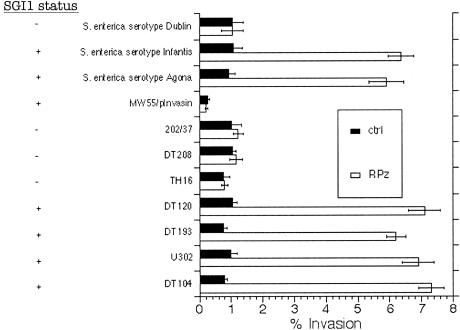
To assess the possible relationship between the RPz-mediated hyperinvasiveness and SGI, we assessed invasion in other SGI-bearing strains of Salmonella. As shown in Fig. 4, other SGI-bearing Salmonella strains were also hyperinvasive after recovery from RPz. These Salmonella strains include S. enterica serotype Agona (2); S. enterica serotype Infantis (4); and the DT104-related phage types U302, DT193, and DT120 (17). No effect was observed for strain TH16, a wild-type strain of phage type DT104 that lacks SGI1 (4). Hyperinvasion was not observed in several multiresistant Salmonella strains lacking SGI1. These strains included S. enterica serotype Typhimurium phage type DT208 (17), S. enterica serotype Typhimurium strains 202/37 (35) and 76 (13), and S. enterica serotype Dublin (20). The RPz-mediated effect on invasion was also not observed in Salmonella strain MW55/pInvasin, a multiresistant DT104 strain engineered to invade using a nonspecific mechanism conferred by the invasin gene of Yersinia (22).
FIG. 4.
Evaluation of the relationship between RPz-mediated hyperinvasion of HEp-2 cells (1 h) and SGI1. Strain designations are summarized in Table 1, while the SGI1 status is indicated on the left. Black bars represent the controls (ctrl), which correspond to exposure to RPz culture medium, while open bars represent Salmonella recovered from RPz. Percent invasion equals 100(CFU recovered/CFU added).
Table 2 summarizes the conserved nature of the RPz-mediated hyperinvasive phenotype in Salmonella possessing SGI1. The phenotype was observed only in strains possessing SGI1. However, the phenotype was dependent upon Salmonella-specific invasion genes, as evidenced by MW55/pInvasin, which contains SGI1 yet invades cells using a nonspecific mechanism.
In vivo studies employing DT104 exposed to RPz.
In vivo studies were undertaken to potentially correlate the RPz-mediated effect on invasion and the reports of DT104 hypervirulence in calves (14). To investigate this possibility, DT104 cells were recovered from RPz and immediately inoculated into neonatal calves. Additionally, DT104-loaded RPz were inoculated into neonatal calves. Neonatal calves were chosen since they are a good model for assessing Salmonella virulence and since they are natively free of RPz until about 6 to 8 weeks of age.
By inoculation of the DT104 or DT104-loaded RPz into milk, the inocula will bypass the rumen (albeit nonfunctional in neonatal calves) and be deposited in the abomasum, which is the “true” stomach of ruminants. Abomasal enzymes and acid lyse the RPz, thus liberating DT104, leading to translocation into the small intestine, the preferred invasion site for Salmonella (11). As shown in Fig. 5, significantly more DT104 cells were isolated from mesenteric lymph nodes and spleen for calves inoculated with DT104 recovered from or present in RPz. Additionally, significantly more RPz-exposed DT104 cells were recovered from the ileum in 25-min ligated loop experiments. Statistical analyses revealed P values of <0.0001 despite the relatively small numbers of animals, i.e., nine principals and seven controls, that were used in the oral infection studies.
Quantitative PCR (top portion of Fig. 5) was performed in order to assess the possibility that pathogen load may be equivalent, i.e., in RPz-dependent and RPz-independent data, but that nonviable DT104 may predominate in calves challenged with DT104 not exposed to RPz. These assays confirm that significantly more Salmonella DNA was present in tissues from animals infected with DT104 exposed to RPz. That is, differences in DT104 viability do not appear to be an issue.
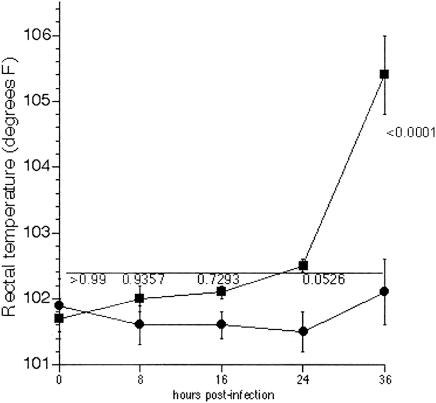
As shown in Fig. 6, pyrexia was evident only at 36 h in calves infected with DT104 exposed to RPz. At 16 and 24 h postinfection there appears to be a difference in rectal temperatures, although these differences turned out to be statistically insignificant. This is especially true at 16 h postinfection, where neither group had rectal temperatures above normal (∼102.4°F, horizontal line).
FIG. 6.
Assessment of the onset of pyrexia in calves infected with DT104 exposed to RPz. Rectal temperatures were determined every 8 to 12 h for calves infected with DT104 exposed to RPz (squares, n = 6) or DT104 not exposed to RPz (circles, n = 4). P values, comparing RPz-dependent and RPz-independent data, are given above the RPz-dependent data. The upper limit for normal bovine rectal temperature (∼102.4°F) is indicated by the horizontal line.
To confirm the role of RPz in the putative hypervirulence of DT104, rumens of calves and sheep were defaunated prior to oral challenge with DT104. Defaunation was performed using DSS, a surfactant that lyses RPz. Sheep were additionally utilized since a defaunation dosing schedule has been established for these ruminants (38). As shown in Table 3, fewer DT104 cells were recovered from defaunated calves and sheep.
TABLE 3.
Quantitation of DT104 recovered from spleens obtained from calves and sheep that were defaunated prior to experimental challenge with the pathogena
| Host | Treatment | CFU/g of spleen |
|---|---|---|
| Calves | Water | 5.4 × 108 ± 6.7 × 107 |
| DSS | 2.4 × 107 ± 5.7 × 106 (P < 0.0001 versus calves treated with water) | |
| Sheep | Water | 8.5 × 108 ± 8.3 × 107 |
| DSS | 3.3 × 107 ± 5.2 × 106 (P < 0.0001 versus sheep treated with water) |
Calves and sheep were defaunated using DSS or treated with water as described in Materials and Methods. Enumeration of RPz from the rumen revealed that defaunated animals had no detectable RPz while nondefaunated animals possessed 104 to 105 RPz/mL. The data represent most-probable numbers of DT104 recovered from calves (n = 6) or sheep (n = 6) at 36 hours following an oral infection. P values were derived using an analysis of variance with Scheffe's F test for multiple comparisons. Comparisons were made for water versus DSS treatments within an animal species.
DISCUSSION
The objective of this project was to identify a possible relationship between RPz and enhanced virulence in multiple-antibiotic-resistant Salmonella. RPz are active predators of microbes in the rumen, and as evidenced by the equivalent recovery of noninvasive strain BJ68 and invasive DT104 from RPz, Salmonella appears to mostly enter RPz passively. Most microbes engulfed by protozoa are digested as food, but some bacterial pathogens, like Salmonella, appear to be resistant to destruction in digestive vacuoles (18) and can even replicate within protozoa (34). This capability may represent an unappreciated reservoir for pathogens and influence the carrier status of animals. Survival in protozoal vacuoles can also select for pathogens with enhanced virulence traits (9, 10).
Our studies indicate that DT104, the most prevalent multiresistant Salmonella type (30), is more pathogenic after survival inside RPz. This increase in pathogenicity appears to be related to enhanced invasion of eukaryotic cells, and not an increase in intracellular survival, since hyperinvasiveness could be observed in DT104 with diminished abilities to survive in macrophages. Additionally, it appears that this hyperinvasive phenotype extends to other Salmonella strains that possess the DT104 integron structure designated SGI1. Our hypothesis is that the enhancement of DT104 invasion is the result of an overactivation of invasion genes while inside the challenging environment of the protozoa. The hyperinvasive DT104 is then released from RPz after normal digestive lysis of RPz in the abomasum followed by translocation into the intestine, where invasion ensues.
The SGI1-dependent nature of the hypervirulence suggests that an SGI1 component may be upregulating invasion while DT104 survives within the protozoal vacuoles. SGI1 is a 43-kb chromosomal segment of genes inserted between thdF and a retron (between thdF and yidY in serotypes Agona [2] and Infantis [unpublished observations]). Approximately 13 kb is devoted to antibiotic resistance genes while the other 30 kb is characterized as plasmid-related and insertion-related genes with 10 unknown open reading frames. Studies in our laboratory have tentatively identified one of these open reading frames as a regulator of the RPz-mediated phenotype, although the molecular basis for upregulating invasion is not clear (unpublished observations).
RPz-mediated hyperinvasiveness was comparable to the invasiveness of Salmonella enterica serotype Typhimurium EE419 (EE419), a recombinant strain that constitutively expresses a global regulator of invasion (hilA), thereby maximizing invasion (24). For both DT104 and EE419, hyperinvasiveness is transient, although hyperinvasive mutants can be continually propagated for EE419. Specifically, DT104 hyperinvasion wanes yet EE419 hyperinvasion can be maintained by selecting for the smallest postinvasion colonies. DT104 colonies isolated postinvasion, however, are uniform, thus representing a homogenous population. The postinvasion heterogeneity observed for EE419 is likely due to repositioning of the transposon, as a means of selecting for healthier clones, in an alternative genomic location. In both situations, there is a tendency to restore the invasiveness to a baseline level that is less deleterious to the bacterium.
Invasion was unaffected for MW55/pInvasin, a recombinant DT104 strain that possesses SGI1 but weakly (compared to Salmonella) invades eukaryotic cells by means of a nonspecific mechanism conferred by a Yersinia protein (22). While Salmonella and Yersinia have some common invasive characteristics (15, 29), it would seem to be more appropriate to assess RPz-mediated alterations in cellular invasion for Shigella flexneri due to numerous virulence-related similarities between Salmonella and Shigella (19, 21). This line of research was not pursued since SGI1 has not been reported in Shigella and since Shigella is not a pathogen of ruminants. Nonetheless, it appears that the RPz-mediated effects are not ubiquitous for other invasive pathogens.
Based on our findings, one preventative strategy for pathogen control may be periodic defaunation of the rumen, which would temporarily eliminate RPz. While protozoa are a normal part of the rumen microbiota, the temporary removal of these microbes does not appear to have deleterious effects upon ruminants (38). The results of this project may suggest that periodic defaunation may indeed be warranted.
In summary, our study suggests that DT104 hypervirulence is related to a transient enhancement of enteroinvasive capabilities. This enhancement is facilitated by existence within RPz, a finding that is consistent with the observation that cattle are the species most susceptible to DT104 infection (14). Future studies will be aimed at determining the molecular mechanisms for the RPz-mediated upregulation of invasion and assessing the feasibility and effectiveness of periodic rumen defaunation. These observations have broad implications relating to microbial pathogenesis, rumen microbial ecology, and pathogen reservoir status.
Acknowledgments
We thank Ruth Willson and Deb Lebo for technical assistance, Sandy Johnson for secretarial assistance, and Brad Jones and Jim Harp for reading the manuscript.
This work was partially supported by the National Cattlemen's Beef Association.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Editor: J. D. Clements
REFERENCES
- 1.Allen, C., P. Fedorka-Cray, A. Vazquez-Torres, M. Suyemoto, C. Altier, L. Ryder, F. Fang, and S. Libby. 2001. In vitro and in vivo assessment of Salmonella enterica serovar Typhimurium DT104 virulence. Infect. Immun. 69:4673-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd, D., G. Peters, A. Cloeckaert, K. Boumedine, E. Chaslus-Dancla, H. Imberechts, and M. Mulvey. 2001. Complete nucleotide sequence of a 43-kilobase genomic island associated with the multidrug resistance region of Salmonella enterica serovar Typhimurium DT104 and its identification in phage type DT120 and serovar Agona. J. Bacteriol. 183:5725-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briggs, C. E., and P. M. Fratamico. 1999. Molecular characterization of an antibiotic resistance gene cluster of Salmonella typhimurium DT104. Antimicrob. Agents Chemother. 43:846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson, S. A., L. F. Bolton, C. E. Briggs, H. S. Hurd, V. K. Sharma, P. Fedorka-Cray, and B. D. Jones. 1999. Detection of Salmonella typhimurium DT104 using multiplex and fluorogenic PCR. Mol. Cell. Probes 13:213-222. [DOI] [PubMed] [Google Scholar]
- 5.Carlson, S. A., M. Browning, K. E. Ferris, and B. D. Jones. 2000. Identification of diminished tissue culture invasiveness among multiple antibiotic resistant Salmonella typhimurium DT104. Microb. Pathog. 28:37-44. [DOI] [PubMed] [Google Scholar]
- 6.Carlson, S. A., T. A. Casey, M. T. Wu, B. D. Hammes, and B. D. Jones. 2002. A high-throughput genetic system for assessing the inhibition of proteins: identification of antibiotic resistance and virulence targets and their cognate inhibitors in Salmonella. Anal. Biochem. 310:72-83. [DOI] [PubMed] [Google Scholar]
- 7.Carlson, S. A., R. M. Willson, A. J. Crane, and K. E. Ferris. 2000. Evaluation of invasion-conferring genotypes and antibiotic-induced hyperinvasive phenotypes in multiple antibiotic resistant Salmonella typhimurium DT104. Microb. Pathog. 28:373-378. [DOI] [PubMed] [Google Scholar]
- 8.Carlson, S. A., Z. P. McCuddin, and M. Wu. 2005. SlyA regulates the collagenase-mediated cytopathic phenotype in multiresistant Salmonella. Microb Pathog. 38:181-187. [DOI] [PubMed] [Google Scholar]
- 9.Cirillo, J., S. Falkow, L. Tompkins, and L. Bermudez. 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cirillo, J. D., S. Falkow, and L. S. Tompkins. 1994. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect. Immun. 62:3254-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark, M. A., M. A. Jepson, N. L. Simmons, and B. H. Hirst. 1994. Preferential interaction of Salmonella typhimurium with mouse Peyer's patch M cells. Res. Microbiol. 145:543-552. [DOI] [PubMed] [Google Scholar]
- 12.Coleman, G., and D. Reynolds. 1982. The uptake of bacteria and amino acids by Ophryoscolex caudatus, Diploplastron affine and some other rumen Entodiniomorphid protozoa. J. Appl. Bacteriol. 52:135-144. [Google Scholar]
- 13.Espinasse, F., R. Gheorghiu, A. Poiata, R. Labia, and M. H. Nicolas-Chanoine. 1997. Reduced susceptibility to co-amoxiclav in Escherichia coli, Salmonella typhimurium and Klebsiella pneumoniae isolated in Romania between 1985 and 1993. J. Antimicrob. Chemother. 39:103-106. [DOI] [PubMed] [Google Scholar]
- 14.Evans, S., and R. Davies. 1996. Case control study of multiple-resistant Salmonella typhimurium DT104 infection of cattle in Great Britain. Vet. Rec. 139:557-558. [PubMed] [Google Scholar]
- 15.Formal, S., T. Hale, and P. Sansonetti. 1983. Invasive enteric pathogens. Rev. Infect. Dis. 4:S702-S707. [DOI] [PubMed] [Google Scholar]
- 16.Frana, T., and S. Carlson. 2001. Development and use of a plasmid encoding green fluorescence protein in multiple antibiotic resistant Salmonella. BioTechniques 30:28-32. [DOI] [PubMed] [Google Scholar]
- 17.Frana, T., S. Carlson, and R. Griffith. 2001. Relative distribution and conservation of genes encoding aminoglycoside-modifying enzymes in Salmonella enterica serotype Typhimurium phage type DT104. Appl. Environ. Microbiol. 67:445-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaze, W., N. Burroughs, M. Gallagher, and E. Wellington. 2003. Interactions between Salmonella typhimurium and Acanthamoeba polyphaga, and observation of a new mode of intracellular growth within contractile vacuoles. Microb. Ecol. 46:358-369. [DOI] [PubMed] [Google Scholar]
- 19.Groisman, E. A., and H. Ochman. 1993. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 12:3779-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helmuth, R., and A. Seiler. 1986. Epidemiology and chromosomal location of genes encoding multiresistance in Salmonella dublin. J. Antimicrob. Chemother. 18:179-181. [DOI] [PubMed] [Google Scholar]
- 21.Hueck, C. J., M. J. Hantman, V. Bajaj, C. Johnston, C. A. Lee, and S. I. Miller. 1995. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol. Microbiol. 18:479-490. [DOI] [PubMed] [Google Scholar]
- 22.Isberg, R. R., D. L. Voorhis, and S. Falkow. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 50:769-778. [DOI] [PubMed] [Google Scholar]
- 23.Kaniga, K., S. Tucker, D. Trollinger, and J. E. Galan. 1995. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J. Bacteriol. 177:3965-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, C. A., B. D. Jones, and S. Falkow. 1992. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc. Natl. Acad. Sci. USA 89:1847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyczak, J. B. 2003. Commensal bacteria increase invasion of intestinal epithelium by Salmonella enterica serovar Typhi. Infect. Immun. 71:6610-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills, S., and B. Finlay. 1994. Comparison of Salmonella typhi and Salmonella typhimurium invasion, intracellular growth and localization in cultured human epithelial cells. Microb. Pathog. 17:409-423. [DOI] [PubMed] [Google Scholar]
- 27.Moffat, J., and L. Tompkins. 1992. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect. Immun. 60:296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penheiter, K. L., N. Mathur, D. Giles, T. Fahlen, and B. D. Jones. 1997. Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer's patches. Mol. Microbiol. 24:697-709. [DOI] [PubMed] [Google Scholar]
- 29.Pulkkinen, W. S., and S. I. Miller. 1991. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J. Bacteriol. 173:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridley, A., and E. J. Threlfall. 1998. Molecular epidemiology of antibiotic resistance genes in multiresistant epidemic Salmonella typhimurium DT 104. Microb. Drug Resist. 4:113-118. [DOI] [PubMed] [Google Scholar]
- 31.Sharma, V., and S. Carlson. 2000. Simultaneous detection of Salmonella strains and Escherichia coli O157:H7 with fluorogenic PCR and single-enrichment-broth culture. Appl. Environ. Microbiol. 66:5472-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slutsker, L., S. F. Altekruse, and D. L. Swerdlow. 1998. Foodborne diseases. Emerging pathogens and trends. Infect. Dis. Clin. N. Am. 12:199-216. [DOI] [PubMed] [Google Scholar]
- 33.Stabel, J., and T. Stabel. 1995. Immortalization and characterization of bovine peritoneal macrophages transfected with SV40 plasmid DNA. Vet. Immunol. Immunopathol. 45:211-220. [DOI] [PubMed] [Google Scholar]
- 34.Tezcan-Merdol, D., M. Ljungstrom, J. Winiecka-Krusnell, E. Linder, L. Engstrand, and M. Rhen. 2004. Uptake and replication of Salmonella enterica in Acanthamoeba rhysodes. Appl. Environ. Microbiol. 70:3706-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tosini, F., P. Visca, I. Luzzi, A. M. Dionisi, C. Pezzella, A. Petrucca, and A. Carattoli. 1998. Class 1 integron-borne multiple-antibiotic resistance carried by IncFI and IncL/M plasmids in Salmonella enterica serotype Typhimurium. Antimicrob. Agents Chemother. 42:3053-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wall, P., D. Morgan, K. Lamden, M. Ryan, M. Griffin, E. Threlfall, L. Ward, and B. Rowe. 1994. A case control study of infection with an epidemic strain of multiresistant Salmonella typhimurium DT104 in England and Wales. Commun. Dis. Rep. Rev. 4:R130-R135. [PubMed] [Google Scholar]
- 37.White, D. G., C. Hudson, J. J. Maurer, S. Ayers, S. Zhao, M. D. Lee, L. Bolton, T. Foley, and J. Sherwood. 2000. Characterization of chloramphenicol and florfenicol resistance in Escherichia coli associated with bovine diarrhea. J. Clin. Microbiol. 38:4593-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams, A. G., and G. S. Coleman. 1997. The rumen protozoa, p. 73-139. In P. N. Hobson and C. S. Stewart (ed.), The rumen microbial ecosystem. Chapman & Hall, London, United Kingdom.
- 39.Wilson, R., J. Elthon, S. Clegg, and B. Jones. 2000. Salmonella enterica serovars Gallinarum and Pullorum expressing Salmonella enterica serovar Typhimurium type 1 fimbriae exhibit increased invasiveness for mammalian cells. Infect. Immun. 63:4782-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood, R., and R. Rose. 1992. Populations of Salmonella typhimurium in internal organs of experimentally infected carrier swine. Am. J. Vet. Res. 53:69-76. [PubMed] [Google Scholar]
- 41.Wray, C., and W. J. Sojka. 1978. Experimental Salmonella typhimurium in calves. Res. Vet. Sci. 25:139-143. [PubMed] [Google Scholar]
- 42.Zusman, T. M. Feldman, E. Halperin, and G. Segal. 2004. Characterization of the icmH and icmF genes required for Legionella pneumophila intracellular growth, genes that are present in many bacteria associated with eukaryotic cells. J. Bacteriol. 72:3398-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]