Abstract
Helicobacter pylori persistently infects the human stomach and can cause gastritis, gastric ulceration, and gastric cancer. The type IV secretion system (TFSS) of virulent H. pylori strains translocates the CagA protein, inducing the dephosphorylation of host cell proteins and leading to changes in the morphology or shape of AGS gastric epithelial cells. Furthermore, the TFSS is involved in the induction of proinflammatory cytokines. While the H. pylori genes required for TFSS function have been investigated systematically, little is known about possible host cell factors involved. We infected 19 different mammalian cell lines individually with H. pylori and analyzed CagA translocation, dephosphorylation of host cell proteins, chemokine secretion (interleukin-8 and macrophage inflammatory protein 2), and changes in cellular phenotypes. Our results demonstrate that not only bacterial but also host cell factors determine the cellular response to infection. The identification of such unknown host cell factors will add to our understanding of host-pathogen interactions and might help in the development of new therapeutic strategies.
Helicobacter pylori is a gram-negative bacterium which infects the human gastric mucosa. It is estimated that about 50% of all humans carry H. pylori in their stomach (7, 12). The persistent infection induces a state of chronic gastric inflammation that frequently remains asymptomatic. In some patients, however, the infection causes disease, such as peptic or gastric ulceration, the development of a mucosa-associated lymphoid tissue lymphoma, or even gastric cancer (25). It is not yet clear why only some people develop more severe forms of disease despite the high prevalence of H. pylori in the human population. Certainly, host genetic factors play a role in determining the clinical outcome of the infection (14). On the other hand, H. pylori virulence factors also play a role in pathogenesis, since virulent strains are associated with more aggressive tissue damage and an increased risk of a severe clinical outcome (8). Finally, environmental factors such as nutrition are also thought to be important (38). Thus, the disease outcome is determined by a combination of host, bacterial, and environmental factors.
The major genetic difference between more virulent and less virulent H. pylori strains is the so-called cytotoxin-associated gene pathogenicity island (cag PAI). The cag PAI carries up to 31 genes which encode a type IV secretion system (TFSS) that appears to play a key role in H. pylori pathogenesis (2, 7). TFSSs enable bacteria to inject effector molecules into the cytoplasm of host cells (10, 26). In recent years, the function of the TFSS in the interaction of H. pylori with host cells has been studied intensively. The only protein known to be translocated by the H. pylori TFSS is the CagA protein, which is also encoded by a gene in the cag PAI. After translocation, CagA becomes phosphorylated on tyrosine residues by Src family kinases (34, 37) and induces rearrangements of the host cell actin cytoskeleton (“hummingbird phenotype”) (32). CagA has been reported to interact with several host cell proteins and to stimulate various signaling processes in the host (25). Moreover, tyrosine-phosphorylated CagA inhibits c-Src, which leads to tyrosine dephosphorylation of the cellular Src substrates cortactin and ezrin (33, 35).
Besides the translocation of CagA, the H. pylori TFSS also mediates the induction of a proinflammatory response in host cells. Recently, it was shown that this involves the delivery of peptidoglycan to host cells via the TFSS (41). Translocated peptidoglycan is detected by the intracellular pathogen recognition molecule Nod1, which activates a signaling cascade leading to the activation of eukaryotic transcription factors such as nuclear factor kappa B (NF-κB). These transcription factors stimulate the synthesis and secretion of a range of inflammatory mediators, such as interleukin-8 (IL-8). Collectively, the combined effects of the cag PAI on the actin cytoskeleton and the proinflammatory responses of host cells are thought to play a central role in pathogenesis.
The H. pylori factors required for CagA translocation, the stimulation of IL-8 secretion, and induction of the hummingbird phenotype have been investigated by systematic mutagenesis of cag PAI genes (15, 36) and by comparisons of different clinical H. pylori isolates (5). A functional analysis of H. pylori strains lacking individual genes of the cag PAI has revealed that most genes are required for the induction of the proinflammatory response, changes in cellular morphology or shape, and CagA translocation. Much less is known about possible host cell components involved in these processes.
For this study, we investigated whether host cell determinants are required for the function of the H. pylori TFSS. For this purpose, we infected 19 different mammalian cell lines with wild-type H. pylori and isogenic knockout mutants of selected cag PAI genes. In order to determine the function of the TFSS, we monitored CagA translocation, host cell protein dephosphorylation, chemokine secretion, and changes in the cellular phenotype. Our findings provide the first clear experimental evidence of a role for different host factors in the induction of cag PAI-mediated responses.
MATERIALS AND METHODS
H. pylori strains.
The H. pylori strains P1 and P12 and the isogenic knockout mutants P1ΔcagA, P1ΔvirB11, and P12ΔPAI have been described previously (6, 29). The cagA and virB11 genes correspond to the loci HP0547 and HP0525, respectively, in the database of The Institute for Genome Research (http://www.tigr.org). The P12ΔPAI strain lacks the entire cag pathogenicity island. H. pylori wild-type strains were cultivated on horse serum agar plates supplemented with vancomycin (10 μg/ml). The mutants were cultivated on the same type of plates with additional chloramphenicol or kanamycin. Bacteria were incubated for 2 days at 37°C in an anaerobic jar containing a Campygen gas mix of 5% O2, 10% CO2, and 85% N2 (Oxoid).
Eukaryotic cell lines.
Nineteen different eukaryotic cell lines were analyzed for TFSS-dependent host cell responses (Table 1). Cells were grown in the indicated media and subcultured every 2 to 3 days.
TABLE 1.
Cell lines used for this study
| Cell line | Reference or sourcea (no.) | Cell type | Growth mediumb |
|---|---|---|---|
| 293T | ATCC (CRL 11268) | Human embryonal kidney | DMEM (Gibco BRL), 10% fetal bovine serum (FBS; Gibco BRL), 2 mM l-glutamine, 1 mM sodium pyruvate |
| AGS | ATCC (CRL 1739) | Human gastric adenocarcinoma | RPMI 1640 (Gibco BRL), 10% FBS, 2 mM l-glutamine |
| CHO K1 | ATCC (CCL 61) | Chinese hamster ovary | MEM alpha (Gibco BRL), 10% FBS |
| Cos1 | ATCC (CRL 1650) | Green African monkey kidney | RPMI 1640 (Gibco BRL), 10% FBS, 2 mM l-glutamine |
| GLC4 | 42 | Human lung carcinoma | RPMI 1640 (Gibco BRL), 10% heat-inactivated FBS, 2 mM l-glutamine |
| Hec1.b | ATCC (HTP 113) | Human endometrial adenocarcinoma | RPMI 1640 (Gibco BRL), 10% FBS, 2 mM l-glutamine |
| Hela | ATCC (CCL 2) | Human cervix epithelial carcinoma | RPMI 1640 (Gibco BRL), 10% FBS, 2 mM l-glutamine |
| HepG2 | ATCC (HB 8065) | Human hepatocellular carcinoma | RPMI 1640 (Gibco BRL), 10% FBS, 2 mM l-glutamine |
| HL | ECACC (96121720) | Human lung, fibroblast-like | RPMI 1640 (Gibco BRL), 10% FBS, 2 mM l-glutamine |
| HT29 | ATCC (HTB 38) | Human colon adenocarcinoma | Mc Coy's 5A (Gibco BRL), 10% FBS |
| J774.A | DSMZ (ACC 170) | Mouse monocytic macrophages | RPMI 1640 (Gibco BRL), 10% heat-inactivated FBS, 2 mM l-glutamine |
| Kato3 | ATCC (HTB 103) | Human gastic carcinoma | RPMI 1640 (Gibco BRL), 10% heat-inactivated FBS, 2 mM l-glutamine |
| L929 | ATCC (CCL 1) | Mouse fibroblasts | RPMI 1640 (Gibco BRL), 10% FBS, 2 mM l-glutamine |
| MDCK | ATCC (CCL 34) | Canine kidney | RPMI 1640 (Gibco BRL), 10% FBS, 2 mM l-glutamine |
| MKN-28 | 27 | Human gastric adenocarcinoma | RPMI 1640 (Gibco BRL), 10% heat-inactivated FBS, 2 mM l-glutamine |
| MKN-45 | DSMZ (ACC 409) | Human gastric adenocarcinoma | RPMI 1640 (Gibco BRL), 10% heat-inactivated FBS, 2 mM l-glutamine |
| SR4987 | DSMZ (ACC 323) | Mouse stromal fibroblast-like | Mc Coy's 5A (Gibco BRL), 10% FBS |
| SYF+src | ATCC (CRL 2498) | Mouse fibroblasts overexpressing Src | DMEM (Gibco BRL), 10% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate |
| THP1 | ATCC (TIB 202) | Human monocytes from acute monocytic leukemia | RPMI 1640 (Gibco BRL), 10% heat-inactivated FBS, 2 mM l-glutamine |
ATCC, American Type Culture Collection (www.atcc.org); DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (www.dsmz.de); ECACC, European Collection of Cell Cultures (www.ecacc.org.uk).
DMEM, Dulbecco's modified Eagle's medium; MEM, minimum essential medium.
Infection assays.
Eukaryotic cell lines were cultivated in six-well tissue culture dishes in 1 ml/well medium for 2 days to reach monolayers of 70 to 80% confluence. H. pylori was suspended in phosphate-buffered saline and added to the cells at a multiplicity of infection of 100. After incubation in a 5% CO2-95% air incubator for 20 h, the phenotype was analyzed by phase-contrast microscopy. The cell culture supernatants were collected for IL-8 and Mip-2 enzyme-linked immunosorbent assays (ELISAs) and stored at −20°C. Cells were washed once with ice-cold phosphate-buffered saline containing 1 mM Na3VO4 (Sigma-Aldrich), harvested with a rubber policeman in the same buffer, and pelleted together with attached bacteria at 600 × g and 4°C.
SDS-PAGE and immunoblotting.
Cell pellets with attached bacteria were mixed with equal amounts of 4× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer (125 mM Tris-HCl, pH 6.8, 200 mM SDS, 40% [vol/vol] glycerol, 10% [vol/vol] β-mercaptoethanol, 0.4% bromphenol blue) and boiled for 5 min. Proteins were separated by SDS-PAGE in 6% or 10% polyacrylamide gels and blotted onto polyvinylidene difluoride membranes (Immobilon-P; Millipore) using standard protocols. Membranes were blocked in TBS-T (140 mM NaCl, 25 mM Tris-HCl, pH 7.4, 0.1% Tween 20) with 3% bovine serum albumin for 1 h at room temperature. Tyrosine-phosphorylated proteins were detected with the monoclonal phosphotyrosine-specific antibody PY99 (Santa Cruz Biotechnology) at a 1:3,000 dilution in TBS-T for 1 h at room temperature. CagA was detected with a rabbit anti-CagA antibody (Austral Biologicals). Blots were washed three times with TBS-T and incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (Amersham Pharmacia Biotech) for 1 h. After three more washing steps, the bound antibodies were detected with the Renaissance Western blot system for enhanced chemiluminescence immunostaining (ICN Biochemicals). The same blot was used for both anti-phosphotyrosine and anti-CagA immunoblotting. Between blotting procedures, the membranes were stripped for 30 min at 50°C in stripping buffer (62.5 mM Tris-HCl, pH 6.7, 100 mM 2-mercaptoethanol, 2% SDS).
Chemokine ELISA.
The amounts of IL-8 and Mip-2 secreted into the cell culture medium after 20 h of infection were determined by a sandwich enzyme immunoassay using the ELISA systems of BioSource International for IL-8 detection and of R&D Systems for Mip-2 detection. The experiments were performed according to the manufacturer's instructions. Samples from at least three independent experiments were measured in triplicate. Due to differences in sequence or the absence of the IL-8 and Mip-2 genes, we were unable to detect chemokine secretion by the animal cell lines MDCK, Cos-1, and CHOK-1.
In vitro phosphorylation assay.
In order to investigate the ability of host cell lysates to phosphorylate CagA in vitro, we performed in vitro kinase assays. H. pylori wild-type strain P1 and the corresponding knockout mutant P1ΔcagA were harvested from agar plates (optical density at 550 nm = 0.9) and resuspended in 2 ml cold kinase buffer (25 mM HEPES, pH 7.0, 150 mM NaCl, 10 mM MgCl2, 1% NP-40, 5 mM dithiothreitol, 1 mM Na3VO4, Complete protease inhibitors [Hoffmann-La Roche]). For bacterial lysis, the bacteria were sonicated on ice (Branson sonifier 450). Additionally, the bacteria were homogenized by 20 passages through a syringe. The eukaryotic cells were pelleted, resuspended in 1 ml cold kinase buffer, and lysed using a syringe as described above. Each bacterial lysate (50 μl) was mixed with 50 μl of the eukaryotic cell lysate. ATP was added to reach a final concentration of 40 μM. The reaction samples were incubated for 5 min at 30°C. To stop the phosphorylation reaction, we added 4× SDS-PAGE buffer and boiled the samples for 5 min. To monitor the in vitro phosphorylation of CagA, SDS-PAGE and immunoblotting were performed.
RESULTS
A representative collection of cell lines derived from the human stomach, from other human tissues, and from various animals was chosen to investigate the role of host cell factors in the function of the H. pylori TFSS (Table 1). All cell lines were infected with the H. pylori wild-type strain P1 and with isogenic cagA and virB11 knockout mutants. After 20 h of infection, we analyzed CagA translocation, host cell tyrosine dephosphorylation, the secretion of chemokines, and changes in the morphology or shape of cells. The results from the experiments with all 19 cell lines are summarized in Table 2.
TABLE 2.
Responses of 19 mammalian cell lines to infection with H. pylori strain P1
| Cell type | Cell line | Organism | Tissue | CagA phosphory- lationa | CagA pro- cessing | Host cell protein dephosphory- lation | Cellular phenotype | IL-8 secretionb |
|---|---|---|---|---|---|---|---|---|
| Human gastric cells | AGS | Human | Stomach | +++ | − | + | Hummingbird (CagA dependent) | + |
| MKN-45 | Human | Stomach | +++ | + | + | + | ||
| MKN-28 | Human | Stomach | ++ | − | − | + | ||
| Kato 3 | Human | Stomach | +++ | − | + | + | ||
| Human nongastric cells | HT29 | Human | Colon | ++ | − | + | + | |
| HeLa | Human | Cervix | ++ | − | − | + | ||
| GLC4 | Human | Lung | − | − | − | − | ||
| Hec1.b | Human | Endometrium | + | − | + | + | ||
| 293T | Human | Kidney | ++ | − | + | + | ||
| HL | Human | Lung | ++ | − | − | + | ||
| THP1 | Human | Blood | + | + | − | Homotypic aggregation (TFSS | + | |
| HepG2 | Human | Liver | ++ | − | + | dependent) | + | |
| Nonhuman nongastric | CHO K1 | Hamster | Ovary | − | − | − | ND | |
| cells | Cos-1 | African green monkey | Kidney | + | − | − | ND | |
| J774.A | Mouse | Blood | ++ | + | − | +(Mip-2) | ||
| MDCK | Dog | Kidney | − | − | − | ND | ||
| SR 4987 | Mouse | Bone marrow | + | − | − | − (Mip-2) | ||
| SYF+src | Mouse | Connective tissue | + | − | − | − (Mip-2) | ||
| L929 | Mouse | Connective tissue | + | − | − | − (Mip-2) |
Intensity of CagA-tyrosine phosphorylation relative to that in AGS cells (see Fig. 1A for further details).
At least a twofold increase in IL-8 secretion compared to that by P1ΔvirB11 is indicated by “+”(see Fig. 2). With the mouse cell lines, we analyzed the secretion of the murine IL-8 analog Mip-2, as indicated. Proinflammatory responses in CHO K1, Cos-1, and MDCK cells could not be determined (ND). For details, see Materials and Methods.
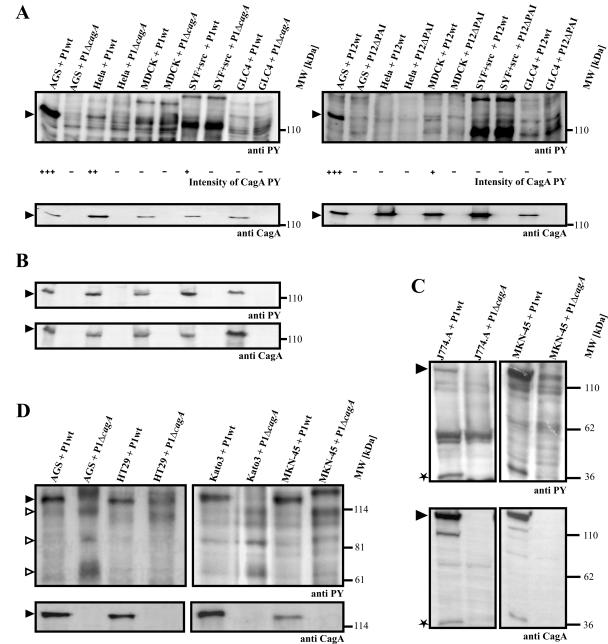
In order to study CagA translocation, we took advantage of the fact that CagA becomes tyrosine phosphorylated in the host cell. Therefore, tyrosine phosphorylation can be used as a readout system for CagA translocation. Infected cells were harvested together with the attached bacteria, and CagA tyrosine phosphorylation was analyzed by SDS-PAGE and immunoblotting with antiphosphotyrosine antibodies. A representative selection of the cell lines we tested is shown in Fig. 1A. We observed strong CagA tyrosine phosphorylation upon infection of human AGS gastric epithelial cells with H. pylori strain P1, as expected (Fig. 1A, left panels). Interestingly, the infection of nongastric human cervical epithelioid carcinoma cells (HeLa) resulted in a significantly lower level of CagA tyrosine phosphorylation. In mouse fibroblasts (SYF+src), CagA tyrosine phosphorylation was close to the detection limit. Finally, we were unable to detect CagA tyrosine phosphorylation in human lung cell carcinoma and canine kidney cells (GLC4 and MDCK, respectively). In order to test whether the lack of CagA phosphorylation in some cell lines is also observed during infection with other H. pylori isolates, we also performed experiments with the strain P12. As a control, we used an isogenic mutant lacking the entire cag pathogenicity island (P12ΔPAI). We did not detect CagA phosphorylation during the infection of HeLa, SYF+src, and GLC4 cells with H. pylori strain P12 (Fig. 1A, right panels). These results demonstrate that the observed host cell dependence of CagA phosphorylation also holds true for other H. pylori isolates besides P1. The differences between P1 and P12 are in agreement with the observation that bacterial factors play a critical role in functions mediated by the TFSS (5, 15, 36). Since we wanted to concentrate our study on differences between host cells rather than on differences between bacterial strains, we performed our subsequent experiments with a single well-characterized H. pylori isolate (strain P1). Notably, among the 19 cell lines tested, the highest level of CagA phosphorylation was observed with human gastric cell lines (Table 2). Most nongastric human cells showed decreased CagA phosphorylation. Finally, very weak or no CagA tyrosine phosphorylation was detected upon infection of most nonhuman cells.
FIG. 1.
CagA translocation, CagA processing, and dephosphorylation of host cell proteins during H. pylori infection of different cell lines. Cells were infected with the wild-type strain P1 (A, left panels) or strain P12 (A, right panels) or the isogenic P1ΔcagA and P12ΔPAI knockout mutants for 20 h at a multiplicity of infection of 100. CagA tyrosine phosphorylation and processing were analyzed by immunoblotting with antiphosphotyrosine (anti-PY) and anti-CagA antibodies. (A) The degree of CagA translocation varies between cell lines and bacterial strains (upper panels, arrowheads). The detection of tyrosine-phosphorylated CagA indicates its translocation into host cells. Upon P1 infection, strong phosphorylation was detected in gastric epithelial cells (e.g., AGS cells) and weak phosphorylation was detected in HeLa cells. No CagA phosphorylation was observed in MDCK and GLC4 cells. During P12 infection, CagA phosphorylation was only detected in AGS cells. Similar amounts of CagA from the attached bacteria were present in all samples (lower panels). (B) Lysates from all cell lines had similar abilities to phosphorylate CagA in vitro. H. pylori lysates and host cell lysates were combined and incubated with ATP at 30°C for 5 min. (C) In J774.A and MKN-45 cells, processing of CagA to a 35-kDa fragment was observed (stars). (D) CagA-induced tyrosine dephosphorylation of host cell proteins (open arrowheads) in cell lines such as AGS, HT29, Kato3, and MKN-45.
A possible explanation for this observation is that the H. pylori strain P1 does not adhere to cell lines such as GLC4 and MDCK. However, a microscopic inspection of the infected cells did not reveal significant differences in H. pylori adhesion (data not shown). Moreover, blotting with an anti-CagA antibody showed that similar amounts of CagA from the attached bacteria were present in the samples (Fig. 1A, bottom panels). It is therefore unlikely that differences in H. pylori adhesion account for the variation in CagA translocation.
An alternative explanation is that the differences in CagA phosphorylation are not directly correlated to differences in CagA translocation, but rather reflect variations in cellular kinase activity. In order to investigate this issue, we performed in vitro phosphorylation experiments with all 19 cell lines (Fig. 1B). We found that lysates from all cell lines were able to phosphorylate CagA in vitro. Thus, the variation in CagA tyrosine phosphorylation during infection evidently results from different levels of CagA translocation. Collectively, these results suggest that H. pylori does not arbitrarily inject CagA into any mammalian cell line. On the contrary, there is apparently some degree of cell type specificity involved in the process of type IV secretion.
Translocated CagA has been found to be processed into smaller fragments during infections of phagocytic cells (24, 29). Consistent with this observation, we also observed a p35CagA fragment in J774.A (Fig. 1C, left panels) and THP1 (data not shown) cells. Additionally, we were able to detect the processing of CagA in the human gastric cell line MKN-45 (Fig. 1C, right panels). The molecular mass of the smaller fragment in MKN-45 cells correlates with that of the p35CagA fragment detected in J774.A cells (Fig. 1C, stars).
Tyrosine-phosphorylated CagA has been shown to inhibit the tyrosine kinase c-Src in AGS gastric epithelial cells, which results in dephosphorylation of the Src substrates cortactin and ezrin (33, 35). We found that the dephosphorylation of host cell proteins did not occur in all cell lines where CagA phosphorylation could be detected. Only seven human cell lines showed this effect, namely, the gastric epithelial cell lines AGS, MKN-45, and Kato3, the human colon cell line HT29 (Fig. 1D), the endometrial cell line Hec1.b, the kidney epithelial cell line 293T, and the liver epithelial cell line HepG2 (data not shown). This dephosphorylation clearly depends on CagA, because it did not occur when cells were infected with an isogenic cagA knockout mutant.
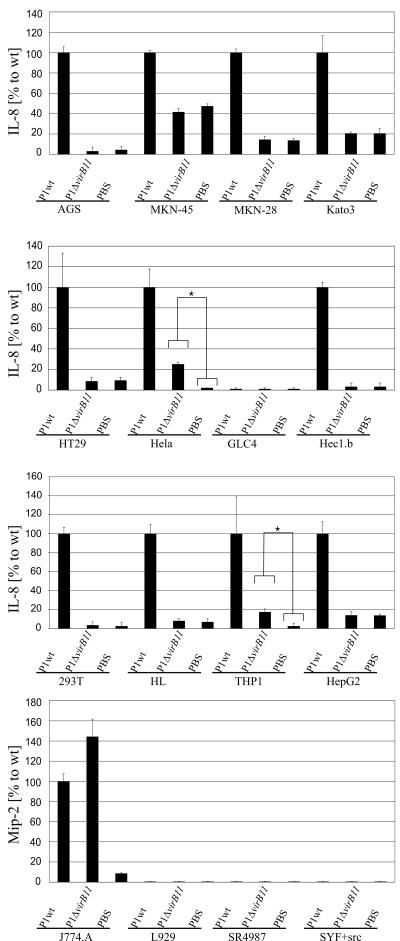
Besides the translocation of CagA, the H. pylori TFSS has also been shown to mediate proinflammatory responses in a CagA-independent manner. We investigated this proinflammatory response by measuring IL-8 secretion into the cell culture supernatant during infection by ELISA (Fig. 2). We found that infection with wild-type H. pylori stimulated IL-8 secretion by all human cell lines except GLC4. Intriguingly, GLC4 was also the only human cell line in which CagA translocation could not be observed. Again, the inability of wild-type H. pylori to stimulate IL-8 secretion by GLC4 cells indicates that some degree of cell type specificity is involved in this process. It might also be possible that GLC4 cells have a general defect in IL-8 synthesis.
FIG. 2.
Chemokine secretion from H. pylori-infected human and murine cell lines. Cells were infected for 20 h with the H. pylori wild-type strain (P1wt) or the type IV secretion-defective knockout mutant P1ΔvirB11. IL-8 or Mip-2 secreted into the medium was determined by ELISA. The amounts of chemokine secretion are presented as percentages relative to the amount during wild-type infection, except for GLC4, L929, SR4987, and SYF+src cells, from which no secretion was detected at all. For most cell lines, chemokine secretion was dependent on the function of the TFSS. In addition, HeLa and THP1 cells showed some IL-8 secretion upon infection withP1ΔvirB11. The Mann-Whitney test indicated that this low level of IL-8 secretion was significantly higher than that in noninfected cells (P < 0.05, asterisks). Infection of the mouse cell line J774.A with P1ΔvirB11 induced a slightly higher Mip-2 secretion level than infection with the wild-type strain. Error bars indicate standard deviations.
IL-8 production was strongly reduced when cells were infected with the isogenic virB11 knockout mutant in which the TFSS is nonfunctional. For most of the human cell lines, infection with P1ΔvirB11 did not increase IL-8 secretion compared to that in noninfected controls. This result indicates that the proinflammatory response indeed largely depends on the H. pylori TFSS. Only HeLa and THP1 cells secreted a small amount of IL-8 in a virB11-independent manner. Therefore, these cell lines are apparently able to respond to H. pylori infection by an additional mechanism which does not depend on type IV secretion. However, TFSS-dependent IL-8 secretion is considerably stronger than TFSS-independent IL-8 secretion in both HeLa and THP1 cells.
Since mice do not harbor an IL-8 gene, instead we determined the concentration of the functional analog Mip-2 in the supernatants of the four murine cell lines. We only detected Mip-2 secretion upon infection of the macrophage-like J774.A cells. Interestingly, Mip-2 secretion by these cells was independent of the TFSS, as the Mip-2 level induced by the virB11 knockout mutant was even slightly higher than that induced by wild-type bacteria.
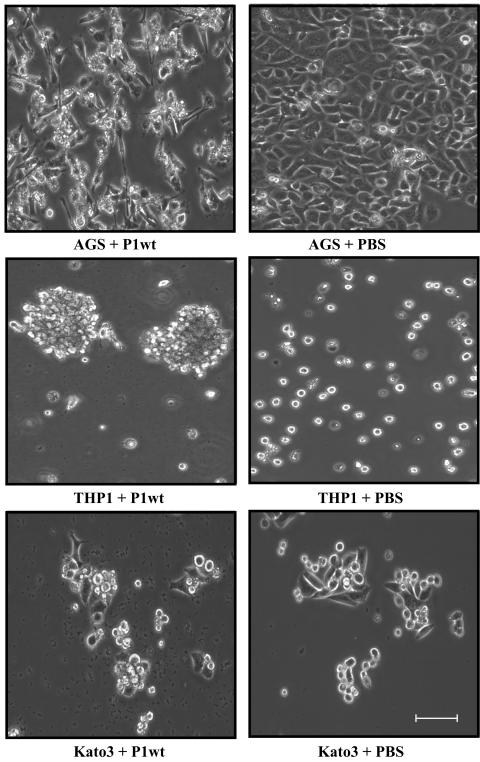
CagA translocation has been shown to induce elongation and scattering of AGS gastric epithelial cells, which result in the formation of a striking cellular phenotype (hummingbird phenotype). We investigated changes in cell morphology after 20 h of infection with wild-type P1 or with the cagA and virB11 knockout mutants (Fig. 3; Table 2). Remarkably, only 2 of the 19 cell lines showed a clear change in morphology in response to H. pylori infection. As expected, we found that wild-type H. pylori induce the hummingbird phenotype of AGS cells. Neither infection with the cagA knockout strain nor that with the virB11 knockout strain caused the hummingbird phenotype, confirming that it depends on CagA translocation through the TFSS. When we infected the macrophage-like cell line THP1, we observed the formation of large cell aggregates. In contrast, noninfected cells grew mainly as a single cell suspension. Aggregation was found to be dependent on the TFSS, as it did not occur during infection with the virB11 knockout strain. CagA, however, appears to be dispensable because the cagA knockout mutant was still able to cause aggregation (data not shown). Apart from the frequently observed cellular vacuolization caused by the vacuolating cytotoxin VacA, we did not observe major changes in morphology or shape upon infection of the 17 other cell lines. Thus, changes in phenotype during infection are apparently the exception rather than the rule.
FIG. 3.
Phenotypes of H. pylori-infected host cells. Infected cells were analyzed by phase-contrast microscopy. Only AGS cells showed the clearly defined hummingbird phenotype (upper panels). The hummingbird phenotype requires CagA, as it was not observed after infection with P1ΔcagA (not shown). THP1 cells formed aggregates after infection with the wild-type strain (middle panels). Aggregation is independent of CagA but requires the TFSS, as it was induced upon infection with the cagA but not the virB11 knockout mutant (not shown). Kato3 cells and all other cell lines tested did not reveal any obvious change in phenotype upon infection (lower panels). Bar, 100 μm.
DISCUSSION
The TFSS encoded by the cag PAI of virulent H. pylori strains is known to induce several host cell responses which are believed to play a role in disease development. While the H. pylori genes involved in this process have been analyzed systematically, little is known about host cell factors which might also be required. For this study, we systematically analyzed cag PAI-dependent host cell responses in 19 different mammalian cell lines. Our results demonstrate that host cell factors play a critical role in determining the outcome of an H. pylori infection.
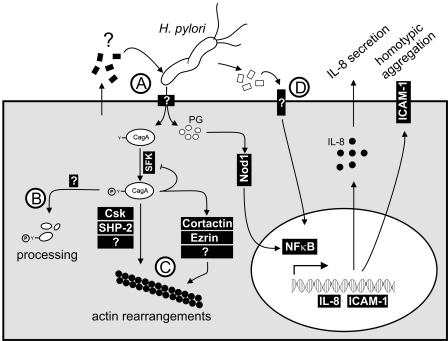
Based on our data, we have compiled a model of the roles of host cell factors required for the cellular responses induced by the H. pylori TFSS. The first step where host cell factors appear to be involved is the translocation of CagA by the TFSS (Fig. 4A). Since we found that CagA is not translocated into all cell lines, the function of the TFSS obviously depends on a host cell factor(s). A possible explanation could be that a specific host cell receptor directly binds to components of the H. pylori TFSS. Alternatively, the type IV secretion machinery might be activated by specific host cell factors that are sensed by H. pylori.
FIG. 4.
Model of the roles of host cell factors required for the cellular responses mediated by the H. pylori TFSS. H. pylori translocates CagA, which is phosphorylated by Src family kinases (SFK). Tyrosine-phosphorylated CagA has been shown to interact with Csk and SHP-2, to inactivate Src family kinases, and to induce tyrosine dephosphorylation of cortactin and ezrin and rearrangements of the actin cytoskeleton. The TFSS also translocates peptidoglycan (PG), which activates the intracellular Nod1 receptor, activates the transcription factor NF-κB, and stimulates the expression and secretion of IL-8 and the upregulation of ICAM-1 on the cell surface. (A) Host cell factors appear to be required for CagA and peptidoglycan translocation. These factors could be inducers or cellular receptors of the TFSS. (B) Processing of CagA only occurs in some cell lines, indicating that specific cellular proteases are involved. (C) The hummingbird phenotype is only observed with AGS cells. Thus, some unknown host cell factors are apparently required for its induction. (D) HeLa and THP1 cells secrete some IL-8 in a TFSS-independent manner, indicating that another receptor can detect H. pylori in these cells.
After translocation, CagA becomes phosphorylated on tyrosine residues by Src family kinases. We did not detect differences in the abilities of host cell lysates to phosphorylate CagA in vitro, indicating that the difference lies in the translocation event. This result was expected, as Src family kinases are ubiquitously expressed in different mammalian cell types (39). In contrast, processing of CagA into smaller fragments appears to be largely restricted to cells of the hematopoietic lineage, as reported previously (24, 29). The simplest explanation is that these cells express a protease which is absent from most other cell types. However, processing of CagA by MKN-45 cells indicates that this protease might not be absolutely specific for hematopoietic cells (Fig. 4B). Additionally, processing of CagA is occasionally also observed in the gastric epithelial cell line AGS (4). Further experiments are needed to resolve this issue.
Another interesting observation is that we were unable to detect major changes in cell morphology during the infection of most cell lines (Fig. 3). Specifically, we found that the hummingbird phenotype occurs only during infection of AGS gastric epithelial cells. Some molecular details showing how this striking phenotype is induced have recently emerged (Fig. 4C) (7). For example, phosphorylated CagA binds to the tyrosine phosphatase SHP-2 and the tyrosine kinase Csk (17, 40). Moreover, tyrosine-phosphorylated CagA inhibits the catalytic activity of c-Src, which results in the dephosphorylation of Src substrates, the actin binding proteins cortactin and ezrin (33, 35), and some other host cell proteins that have not yet been identified (6, 30). Finally, CagA was also shown to bind to Grb2 and c-Met and to disrupt the apical junction complexes of epithelial cells (1, 11, 22). Since these last events are independent of CagA tyrosine phosphorylation, however, it is unlikely that they play a direct role in the induction of the hummingbird phenotype, which strictly depends on CagA tyrosine phosphorylation (3).
The observation that the hummingbird phenotype is restricted to AGS cells indicates that some of the required factors are absent from other cell lines. It might also be possible that some cell lines possess inhibitory factors that are absent from AGS cells. Interestingly, we found that tyrosine dephosphorylation of host cell proteins is frequently observed in most cell lines where substantial CagA translocation occurs. Although we have not unambiguously identified these proteins, their apparent molecular weight suggests that they are identical to proteins that become dephosphorylated in AGS cells. Thus, it is improbable that differences in host protein dephosphorylation can account for the different phenotypic outcomes. Similarly, Csk and SHP-2 are ubiquitously expressed, indicating that they also are not the factors responsible for the differences (28, 39). Apparently, some pieces of this puzzle are still missing. It will be a challenge to identify the relevant factors in order to understand how the H. pylori CagA protein subverts the actin cytoskeleton in AGS cells.
The proinflammatory response is thought to be induced by the translocation of peptidoglycan by the H. pylori TFSS (41). In agreement with this model, we found that for all human cell lines tested, strong IL-8 secretion was only observed during infection with the wild-type strain P1, not with the virB11 knockout mutant. However, we observed a small but significant amount of IL-8 secretion upon infection of HeLa and THP1 cells with the virB11 mutant. This indicates that in these cells an additional mechanism exists which detects H. pylori and stimulates a proinflammatory response independent of type IV secretion, although to a much lower extent (Fig. 4D). Toll-like receptors (TLRs) are attractive candidates for alternative cellular sensors of H. pylori infection. While H. pylori flagellins fail to activate TLR5 (16, 20), intact bacteria were recently shown to activate TLR2 (21). Interestingly, infection of the murine macrophage-like cell line J774.A resulted in a strong cag PAI-independent secretion of Mip-2. This indicates that in mouse macrophages the alternative mechanisms of H. pylori-induced inflammation are much more pronounced.
Proinflammatory signaling induced by H. pylori results in the activation of the eukaryotic transcription factors NF-κB and AP-1. This in turn up-regulates the expression of several proinflammatory cytokines and chemokines, including IL-8. In contrast to the case for the hummingbird phenotype, we found that all human cell lines positive for CagA translocation also secrete IL-8 (Table 2), suggesting that the Nod1 cascade is functional in many different cell types. Interestingly, neither IL-8 secretion nor CagA translocation was observed in GLC4 cells, suggesting that the same host cell factors might be required for both CagA and peptidoglycan translocation.
Besides IL-8 secretion, NF-κB activation has also been shown to enhance the expression and surface exposure of intercellular cell adhesion molecule 1 (ICAM-1). ICAM-1 binds to its receptor, lymphocyte function-associated protein 1, on other cells, which results in the homotypic aggregation of H. pylori-infected cells growing in suspension (23). In agreement with this report, we observed a homotypic aggregation of THP1 cells.
Collectively, our results demonstrate that host cell factors play a critical role at different levels in cellular responses mediated by the H. pylori TFSS. Most interestingly, specific host cell factors are apparently required for the ability of H. pylori to translocate effector molecules into host cells. This is in marked contrast to the TFSSs of other pathogens. For example, the Dot/Icm system of Legionella pneumophila is able to inject effectors into both protozoa and mammalian cells (26). Similarly, the type IV machinery encoded by the tumor-inducing plasmid of Agrobacterium tumefaciens can deliver DNA into both plant cells and human cell lines (19).
There are several possibilities for how the observed host cell specificity of the responses induced by the H. pylori TFSS could be explained. One potential explanation is that specific host cell factors might be required to activate the TFSS. This activation could operate at the level of protein expression. For example, this is the case for the type III secretion system of Yersinia spp. (31). However, the abundant expression of CagA in the absence of host cells indicates that the translocation itself, rather than CagA expression, is repressed. Indeed, despite its abundant expression, CagA is not secreted without host cell contact (9). This is reminiscent of the situation in Shigella flexneri, where effector proteins are stored in the bacterial cytoplasm before contact with host cells. In this case, translocation is triggered by a variety of factors, such as extracellular matrix proteins, bile salts, and Congo red (18). In a similar manner, the H. pylori TFSS might be activated by a specific factor secreted by (or present on the surfaces of) only some host cells.
An alternative explanation is that a receptor on the host cell surface is required for the contact between components of the secretion system and the plasma membrane. In the case of A. tumefaciens, the VirE2 protein encoded by the bacterium forms a channel in the host cell membrane through which substrate translocation is thought to proceed (13). No such factor has yet been identified for H. pylori. It is tempting to speculate that H. pylori uses host cell proteins as membrane channels for the TFSS. Until now, however, there has been little experimental evidence in favor of this hypothesis. Interestingly, Amieva and coworkers were able to adapt H. pylori strain G27 to MDCK cells by 4 months of cocultivation (1). In contrast to the wild-type strain, the adapted bacteria were able to translocate a small but significant amount of CagA. Apparently, the high genetic variability of H. pylori allows the bacterium to change the specificity of its type IV secretion system within a rather short timescale.
In summary, our comparative analysis of different mammalian cell lines has revealed that unknown host cell factors are required for the function of the H. pylori TFSS. When extrapolated to the in vivo situation, these results support the notion that the disease outcome is not only determined by bacterial factors but also by host determinants. The identification of these factors will shed new light on the molecular details of the host-pathogen interaction and might help in the development of new therapeutic strategies to fight infection.
Acknowledgments
We thank Anna Walduck for valuable comments on the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Amieva, M. R., R. Vogelmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backert, S., Y. Churin, and T. F. Meyer. 2002. Helicobacter pylori type IV secretion, host cell signalling and vaccine development. Keio J. Med. 51(Suppl. 2):6-14. [DOI] [PubMed] [Google Scholar]
- 3.Backert, S., S. Moese, M. Selbach, V. Brinkmann, and T. F. Meyer. 2001. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 42:631-644. [DOI] [PubMed] [Google Scholar]
- 4.Backert, S., E. C. Muller, P. R. Jungblut, and T. F. Meyer. 2001. Tyrosine phosphorylation patterns and size modification of the Helicobacter pylori CagA protein after translocation into gastric epithelial cells. Proteomics 1:608-617. [DOI] [PubMed] [Google Scholar]
- 5.Backert, S., T. Schwarz, S. Miehlke, C. Kirsch, C. Sommer, T. Kwok, M. Gerhard, U. B. Goebel, N. Lehn, W. Koenig, and T. F. Meyer. 2004. Functional analysis of the cag pathogenicity island in Helicobacter pylori isolates from patients with gastritis, peptic ulcer, and gastric cancer. Infect. Immun. 72:1043-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backert, S., E. Ziska, V. Brinkmann, U. Zimny-Arndt, A. Fauconnier, P. R. Jungblut, M. Naumann, and T. F. Meyer. 2000. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol. 2:155-164. [DOI] [PubMed] [Google Scholar]
- 7.Blaser, M. J., and J. C. Atherton. 2004. Helicobacter pylori persistence: biology and disease. J. Clin. Investig. 113:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaser, M. J., and D. E. Berg. 2001. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Investig. 107:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bumann, D., S. Aksu, M. Wendland, K. Janek, U. Zimny-Arndt, N. Sabarth, T. F. Meyer, and P. R. Jungblut. 2002. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 70:3396-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cascales, E., and P. J. Christie. 2003. The versatile bacterial type IV secretion systems. Nat. Rev. Microbiol. 1:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Churin, Y., L. Al-Ghoul, O. Kepp, T. F. Meyer, W. Birchmeier, and M. Naumann. 2003. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell Biol. 161:249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covacci, A., J. L. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 13.Dumas, F., M. Duckely, P. Pelczar, P. Van Gelder, and B. Hohn. 2001. An Agrobacterium VirE2 channel for transferred-DNA transport into plant cells. Proc. Natl. Acad. Sci. USA 98:485-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Omar, E. M., M. Carrington, W. H. Chow, K. E. McColl, J. H. Bream, H. A. Young, J. Herrera, J. Lissowska, C. C. Yuan, N. Rothman, G. Lanyon, M. Martin, J. F. Fraumeni, Jr., and C. S. Rabkin. 2001. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature 412:499. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, W., J. Puls, R. Buhrdorf, B. Gebert, S. Odenbreit, and R. Haas. 2001. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42:1337-1348. [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz, A. T., Y. Yu, U. S. Krishna, D. A. Israel, S. L. Lyons, and R. M. Peek, Jr. 2004. Helicobacter pylori flagellin evades Toll-like receptor 5-mediated innate immunity. J. Infect. Dis. 189:1914-1920. [DOI] [PubMed] [Google Scholar]
- 17.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683-686. [DOI] [PubMed] [Google Scholar]
- 18.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunik, T., T. Tzfira, Y. Kapulnik, Y. Gafni, C. Dingwall, and V. Citovsky. 2001. Genetic transformation of HeLa cells by Agrobacterium. Proc. Natl. Acad. Sci. USA 98:1871-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, S. K., A. Stack, E. Katzowitsch, S. I. Aizawa, S. Suerbaum, and C. Josenhans. 2003. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 5:1345-1356. [DOI] [PubMed] [Google Scholar]
- 21.Mandell, L., A. P. Moran, A. Cocchiarella, J. Houghton, N. Taylor, J. G. Fox, T. C. Wang, and E. A. Kurt-Jones. 2004. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via Toll-like receptor 2 but not Toll-like receptor 4. Infect. Immun. 72:6446-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mimuro, H., T. Suzuki, J. Tanaka, M. Asahi, R. Haas, and C. Sasakawa. 2002. Grb2 is a key mediator of Helicobacter pylori CagA protein activities. Mol. Cell 10:745-755. [DOI] [PubMed] [Google Scholar]
- 23.Moese, S., M. Selbach, T. F. Meyer, and S. Backert. 2002. cag+ Helicobacter pylori induces homotypic aggregation of macrophage-like cells by up-regulation and recruitment of intracellular adhesion molecule 1 to the cell surface. Infect. Immun. 70:4687-4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moese, S., M. Selbach, U. Zimny-Arndt, P. R. Jungblut, T. F. Meyer, and S. Backert. 2001. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: processing or breakage? Proteomics 1:618-629. [DOI] [PubMed] [Google Scholar]
- 25.Moss, S. F., and S. Sood. 2003. Helicobacter pylori. Curr. Opin. Infect. Dis. 16:445-451. [DOI] [PubMed] [Google Scholar]
- 26.Nagai, H., and C. R. Roy. 2003. Show me the substrates: modulation of host cell function by type IV secretion systems. Cell. Microbiol. 5:373-383. [DOI] [PubMed] [Google Scholar]
- 27.Naito, Y., I. Kino, K. Horiuchi, and D. Fujimoto. 1984. Promotion of collagen production by human fibroblasts with gastric cancer cells in vitro. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 46:145-154. [DOI] [PubMed] [Google Scholar]
- 28.Neel, B., H. Gu, and L. Pao. 2003. The ′Shp'ing news: SH2 domain containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28:284-293. [DOI] [PubMed] [Google Scholar]
- 29.Odenbreit, S., B. Gebert, J. Puls, W. Fischer, and R. Haas. 2001. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell Microbiol. 3:21-31. [DOI] [PubMed] [Google Scholar]
- 30.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 31.Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 32.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selbach, M., S. Moese, S. Backert, P. R. Jungblut, and T. F. Meyer. 2004. The Helicobacter pylori CagA protein induces tyrosine dephosphorylation of ezrin. Proteomics 4:2961-2968. [DOI] [PubMed] [Google Scholar]
- 34.Selbach, M., S. Moese, C. R. Hauck, T. F. Meyer, and S. Backert. 2002. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 277:6775-6778. [DOI] [PubMed] [Google Scholar]
- 35.Selbach, M., S. Moese, R. Hurwitz, C. R. Hauck, T. F. Meyer, and S. Backert. 2003. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 22:515-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selbach, M., S. Moese, T. F. Meyer, and S. Backert. 2002. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect. Immun. 70:665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein, M., F. Bagnoli, R. Halenbeck, R. Rappuoli, W. J. Fantl, and A. Covacci. 2002. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 43:971-980. [DOI] [PubMed] [Google Scholar]
- 38.Stoicov, C., R. Saffari, X. Cai, C. Hasyagar, and J. Houghton. 2004. Molecular biology of gastric cancer: Helicobacter infection and gastric adenocarcinoma: bacterial and host factors responsible for altered growth signaling. Gene 341:1-17. [DOI] [PubMed] [Google Scholar]
- 39.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 40.Tsutsumi, R., H. Higashi, M. Higuchi, M. Okada, and M. Hatakeyama. 2003. Attenuation of Helicobacter pylori CagA × SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J. Biol. Chem. 278:3664-3670. [DOI] [PubMed] [Google Scholar]
- 41.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 42.Zijlstra, J. G., E. G. de Vries, and N. H. Mulder. 1987. Multifactorial drug resistance in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 47:1780-1784. [PubMed] [Google Scholar]