Abstract
Defensins are key participants in mucosal innate defense. The varied antimicrobial activity and differential distribution of defensins at mucosal sites indicate that peptide repertoires are tailored to site-specific innate defense requirements. Nonetheless, few studies have investigated changes in peptide profiles and function after in vivo pathogen challenge. Here, we determined defensin profiles in urethral secretions of healthy men and men with Chlamydia trachomatis- and Neisseria gonorrhoeae-mediated urethritis by immunoblotting for the epithelial defensins HBD1, HBD2, and HD5 and the neutrophil defensins HNP1 to -3 (HNP1-3). HBD1 was not detectable in secretions, and HBD2 was only induced in a small proportion of the urethritis patients; however, HD5 and HNP1-3 were increased in C. trachomatis infection and significantly elevated in N. gonorrhoeae infection. When HNP1-3 levels were low, HD5 appeared mostly as the propeptide; however, when HNP1-3 levels were >10 μg/ml, HD5 was proteolytically processed, suggesting neutrophil proteases might contribute to HD5 processing. HD5 and HNP1-3 were bactericidal against C. trachomatis and N. gonorrhoeae, but HD5 activity was dependent upon N-terminal processing of the peptide. In vitro proteolysis of proHD5 by neutrophil proteases and analysis of urethral secretions by surface-enhanced laser desorption ionization substantiated that neutrophils contribute the key convertases for proHD5 in the urethra during these infections. This contrasts with the small intestine, where Paneth cells secrete both proHD5 and its processing enzyme, trypsin. In conclusion, we describe a unique defensin expression repertoire in response to inflammatory sexually transmitted infections and a novel host defense mechanism wherein epithelial cells collaborate with neutrophils to establish an antimicrobial barrier during infection.
The integrity of the reproductive organs is a requirement for optimal fertility, and this can be compromised by sexually transmitted infections (7, 11, 20). In men, the penile urethra is the portal of entry for pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis, and urethritis is the most common clinical syndrome (8). N. gonorrhoeae is a gram-negative bacterium that typically associates via the asialoglycoprotein receptor with urethral epithelial cells in men to cause an acute pyogenic infection with a substantial neutrophil influx (23). C. trachomatis is an obligate intracellular gram-negative bacterium with a unique replicative life cycle in columnar epithelial cells, generally causing a more prolonged infection and which evokes an adaptive immune response (41). Occasionally, these infections can also ascend into deeper organs such as the epididymis, where the final stages of sperm maturation occur, or into the prostate (19, 72, 77). Intermittent protection of the urethra may be provided by periodic flushing with semen and urine, which both contain antimicrobial activity (1, 15, 46, 76); however, the molecules contributing to the continuous innate local immune defense of the urethra and involved in the early events following specific pathogen challenge have not been investigated. A basic understanding of the regulation and expression of innate mediators in this unique environment may provide information that could contribute to the development of new intervention strategies for sexually transmitted infections (STIs).
Antimicrobial peptides are a well-recognized component of the innate immune repertoire at mucosal surfaces (25). In humans, the defensins are a major family of antimicrobial peptides with two subfamilies, the alpha and beta defensins, which differ by the pairing arrangement of their six invariant cysteines into three disulfide bridges (43). In addition to controlling microbial growth, recent reports indicate that defensins exert multiple additional activities, including chemoattraction for cells of the adaptive immune system and the initiation of cytokine release (4, 13, 59). The alpha defensins are made by neutrophils during hematopoiesis (human neutrophil peptides HNP1 to -4) (50) and by select epithelial cells (human defensins 5 and 6). HD5 is constitutively produced by Paneth cells in the small intestine and the squamous epithelium of the vagina and ectocervix (39, 63), but expression is regulated in the endocervix and endometrium both by female hormones and by infection (63). Recently, HD5 has been also has been demonstrated in testis (15). In humans, the beta defensins (HBD1, -2, and -3) are predominantly produced by epithelial cells. Expression of HBD1 is largely constitutive, but HBD2 and -3 are induced during infection and upon stimulation with proinflammatory cytokines or microbial products (14, 27, 33, 45, 76).
Defensins, like other antimicrobial peptides, are synthesized as preproproteins, which must be proteolytically cleaved to liberate potent antimicrobial peptides (2, 26, 31, 35, 47, 79, 82). The mature peptides have a broad spectrum of antimicrobial activity via disruption of microbial target membranes, and their N-terminal processing is an important factor in their functional activity (28, 75). Although processing of the neutrophil defensin prepropeptides only occurs intracellularly (31, 74) by an as-yet-unidentified enzyme, some epithelial defensins are modified after their secretion. For example, Paneth cell-derived HD5 is stored as a propeptide and is only cleaved upon secretion by Paneth cell-derived trypsin (16, 61, 28), and HBD1 is secreted into the urinary collection system as mature peptide that subsequently undergoes further N-terminal modification (76).
In this study, our objective was to define the specific expression profile of lumenal defensin forms in the male urethra and to determine whether defined STIs physically and functionally changed these forms. By collecting urethral lavages from healthy men and men with Neisseria gonorrhoeae, Chlamydia trachomatis, and nongonococcal nonchlamydial infections, we identified a unique defensin expression repertoire at this mucosal site and a novel processing mechanism for HD5, in which neutrophil proteases are responsible for the liberation of mature epithelial cell-derived peptides during infection.
MATERIALS AND METHODS
Urethral lavages.
All studies were approved by the institutional review boards of participating institutions. Urethral lavages were obtained from male subjects who attended the Boston Medical Center Sexually Transmitted Diseases Clinic. Men were excluded from participating in the study if they were under 18 years of age, were taking steroids, had an autoimmune disease, were human immunodeficiency virus seropositive, or were infected with herpes simplex virus and actively shedding. Three urethral swabs were first obtained and used for screening by Gram's stain to ascertain the presence (or absence) of urethritis (defined as >4 polymorphonuclear cells, neutrophil granulocytes [PMN]/high-power field) and to identify gram-negative intracellular diplococci (a presumptive diagnosis of gonorrhea) and for N. gonorrhoeae and C. trachomatis testing, either by culture and/or nucleic acid amplification test (NAAT). To collect urethral lavages, a 16-gauge pediatric catheter was inserted approximately 1 to 2 cm into the urethra, which exceeded the area previously sampled, followed by the gentle instillation of 3 ml of sterile saline via a syringe that had been attached distally to the catheter. The saline was allowed to dwell in the urethra for 30 seconds while keeping the urethral orifice manually sealed around the catheter, and then the saline was slowly drawn back into the syringe. This collection manner hence sampled a portion of the penile urethra that had not been previously in contact with the swabs. Lavages were immediately centrifuged to remove cells; the resulting supernatants were aliquoted and stored at −70°C until further analysis could be undertaken. Four sample sets meeting the following group criteria were included in the study: (i) healthy or “normal” (N), no urethritis or gonorrhea by Gram's stain and negative for N. gonorrhoeae and C. trachomatis by culture and/or NAAT (n = 5); (ii) chlamydial urethritis (CT), urethritis by Gram's stain, C. trachomatis positive by culture and/or NAAT, and N. gonorrhoeae negative by Gram's stain, culture and/or NAAT (n = 5); (iii) gonococcal urethritis (GC), urethritis by Gram's stain also including gram-negative intracellular diplococci, and N. gonorrhoeae positive, but C. trachomatis negative, by culture and/or NAAT (n = 5); and (iv) nongonococcal nonchlamydial urethritis (NGU), urethritis by Gram's stain, N. gonorrhoeae negative by Gram's stain, and both N. gonorrhoeae and C. trachomatis negative by culture and/or NAAT (n = 5).
Recombinant defensins.
Recombinant HD5 propeptide (amino acids [aa] 20 to 94) was biosynthesized using the baculovirus/insect cell culture system as previously described (60). To obtain further-processed HD5, purified proHD5 was cleaved with bovine trypsin (for HD5 aa 56 to 94 and aa 63 to 94) [28] or neutrophil proteases (for HD5 aa 53 to 94, 56 to 94, 57 to 94, 62 to 94, and 64 to 94; see below) and then subjected to reverse-phase high-performance liquid chromatography (HPLC) purification on a C18 column (61). HNP1, HBD1 (the predominant native forms HBD1-40 and HBD1-44 with lengths of 40 and 44 amino acids, respectively), and HBD2 originated from previously purified peptide stocks (45, 76). Peptide concentrations were determined either based on absorption at 280 nm and the individual absorption coefficients calculated with GPMAW 5.1 software (Lighthouse Data, Denmark) or, for HD5 peptide mixtures, by quantitative immunoblotting.
Immunoblotting.
Urethral lavages were thawed and immediately centrifuged at 800 × g for 10 min to remove residual particulates, and the resulting supernatants were treated for 30 min at 60°C to ensure inactivation of residual infectious material. Aliquots were then lyophilized and subjected to acid urea-polyacrylamide gel electrophoresis (AU-PAGE) for HD5, HNP1 to -3 (HNP1-3), and HBD1 (61, 26, 76) and sodium dodecyl sulfate (SDS)-Tricine-PAGE for HBD2 (33). Unless otherwise specified, all incubations were at room temperature. After electrophoretic separation, proteins were blotted onto Millipore PSQ membrane and fixed to the membrane for 30 min with 0.05% glutaraldehyde in Tris-buffered saline (TBS) (for HD5 and HNP1-3) or for 10 min in a formalin vapor chamber (for HBD1 and HBD2). After blocking in 0.75% nonfat milk powder in phosphate-buffered saline (PBS) for 30 min at 37°C, membranes were transferred into a 1:1,000 dilution of defensin-specific polyclonal rabbit antibodies in 0.25% nonfat milk powder in PBS supplemented with 0.01% thimerosal. After overnight incubation, membranes were washed three times in TBS with 0.1% bovine serum albumin (BSA) for 10 min each and then immersed in a 1:2,000 dilution of the secondary antibody (Immunopure; alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G; Pierce, Rockford, IL) for 1 h, washed as above, incubated for 5 min in TBS, and developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (Research Products Inc., Mt. Prospect, IL). Defensins were quantified by comparing resulting band intensities with a serial dilution of recombinant standard protein. Data were graphed with SigmaPlot software 7.0, and statistics were calculated using SigmaStat software, version 2.0.
To detect trypsin, aliquots of human neutrophil granule extract and urethral washes were subjected to immunoblotting as described above, employing AU-PAGE fixation with glutaraldehyde, the monoclonal antibodies mixture from QED Bioscience Inc., diluted 1:1,000 (pooled clones 13401 to 13404), and goat-anti-mouse alkaline phosphatase-conjugated secondary antibodies (Pierce).
In vitro processing of precursor proHD5 by neutrophil proteases.
Human neutrophil granules were prepared as previously described and resuspended in a buffer consisting of 200 mM sodium acetate, pH 4.0, 10 mM CaCl2 (granules from 2 × 108 PMN/ml buffer), briefly sonicated on ice, and extracted overnight at 4°C (30). Insoluble material was removed by centrifugation, and the resulting supernatant was diluted 1:20 in 100 mM Tris-150 mM NaCl (pH 7.5). Leukozyme, a partially purified extract from purulent human sputum containing among other proteins elastase, proteinase 3, and traces of cathepsin G, was also tested, as were the following purified human proteases: neutrophil elastase, neutrophil proteinase 3, and trypsin (all enzymes from Elastin Products Company Inc., Owensville, MI). In vitro cleavage experiments were conducted according to the methods of Panyutich et al. (58) in 100 mM Tris-150 mM NaCl adjusted to pH 6.0, 7.5, and 8.0. Briefly, for each time point, 4 μg of recombinant proHD5 was dissolved in 1 μl 0.01% BSA and mixed with 5 μl of neutrophil granule extract, or 4 μl of assay buffer and 1 μl of proteases adjusted to 250 μg/ml in 0.01% BSA, or solvent only, and incubated at 37°C. At selected time points, samples were placed on ice and diluted 1:2 in 5% acetic acid to prevent further proteolysis. Two microliters was lyophilized and subjected to AU-PAGE followed by anti-HD5 immunoblotting, and the remainder was subjected to mass spectrum analysis by matrix-assisted laser desorption ionization-time-of-flight analysis or to AU-PAGE followed by Western blotting to PSQ membrane and N-terminal sequencing at the Microchemical and Proteomics Facility, Emory University, Atlanta, Ga.
To obtain sufficient purified material to test the antimicrobial activity of these neutrophil protease-processed peptides, the protocol was scaled up and cleavage products were subjected to reverse-phase HPLC purification as described previously (61), but with the following acetonitrile gradient with 0.1% trifluoroacetic acid (TFA) as pairing ion: 5% acetonitrile for 5 min, to 22% in 17 min, to 32% in 20 min, to 40% in 8 min, and to 60% in 10 min. HD5-positive fractions were identified by anti-HD5 immunoblotting and tested for purity by silver-stained SDS-PAGE. The N termini of HD5 cleavage products (over 95% pure) were further identified by matrix-assisted laser desorption ionization-time-of-flight analysis and N-terminal amino acid sequencing.
SELDI-TOF analysis of urethral lavages.
Urethral lavage samples were first captured by H50 (reversed phase/hydrophobic) Ciphergen ProteinChip arrays (Ciphergen Biosystems, Freemont, California) according to the manufacturer's protocol. Each ProteinChip array was bulk washed twice with 50% methanol for 5 min and allowed to air dry for 1 h. Next, each individual spot was pretreated twice with 5% methanol, 0.1% TFA for 2 min. The prewetting solution was removed and replaced with either 100 ng of synthetic peptide or 2 μl of urethral lavages. The ProteinChip was then placed in a humid chamber and placed on a rotating platform for 30 min. The spots were then washed three times with 5 μl of 10% acetonitrile-0.1% TFA for 2 min with rocking. The last wash was removed, and the spots were allowed to air dry for 10 min. One microliter of freshly prepared 25-mg/ml α-cyano-4-hydroxy-cinnamic acid (Sigma) in 50% acetonitrile-0.5% TFA was added as the energy-absorbing matrix and allowed to air dry. All-in-one peptide (Ciphergen Biosystems) was prepared as recommended and used to calibrate the chip reader. The masses of each protein spot were then subjected to surface-enhanced laser desorption ionization-time of flight (SELDI-TOF) and analyzed using the ProteinChip Biology System (Ciphergen Biosystems).
For all spots the following parameters were used: high mass was set to 10,000 Da and optimized between 3,000 and 9,000 Da, the starting laser intensity was set to 150 and the detector sensitivity was set to 10, and spots were focused by optimization to the center. The SELDI acquisition parameters were set to 20, Δ to 5, transients per to 5, and ending position to 80. The warming positions were set to two shots with an intensity of 155; the warming shots were not included in the spectra. The SELDI technique has been previously used successfully for defensin identification (18). The recorded masses were analyzed with GPMAW 5.11 software from Lighthouse Data, and masses consistent with a complete C terminus and ≤0.8% mass deviation from the expected average masses were considered here as matching defensin peptides.
Antimicrobial assays.
The activities of defensins against N. gonorrhoeae (ATCC 31426) were analyzed using a microtiter-based CFU spot assay. All incubations were at 37°C with 5% CO2. For each assay, a fresh isolate was prepared from a frozen stock culture (in TSB with 20% glycerol) on chocolate agar plates. Four to five isolated colonies were inoculated into 10 ml of GC broth (Difco) supplemented with 22 mM glucose, 0.5 mM sodium bicarbonate, and vitamins (BBL IsoVitaleX, diluted 1:100) and incubated for 24 h slanted at a 45° angle (48). Thereafter, 0.8 ml of the top liquid layer was transferred into a glass tube and adjusted to McFarland 0.5 standard in fresh culture medium (∼5 × 107 CFU/ml) and further diluted 1:20 in assay medium (70% RPMI 1640 medium supplemented with glucose, sodium bicarbonate, and IsoVitaleX as above). Antimicrobial peptides were prepared as 10-fold-concentrated stocks in 0.01% acetic acid. In a nonbinding surface microtiter plate (Costar), 90 μl of bacterial stock was admixed with 10 μl of peptide solution or solvent only as control. Immediately thereafter (time zero; for control only) and after 3 h of incubation in a CO2 GasPak system (Becton Dickinson) at 200 rpm, aliquots were transferred into a new microtiter plate (general assay plate; Costar) and serially diluted in duplicates in 1:5 steps in supplemented GC broth using a multichannel pipetter. From each dilution row, 6 μl was spotted twice onto predried chocolate agar plates (Chocolate II agar; BD Diagnostic Systems, Sparks, MD). Colonies per spot were enumerated after 24 h of incubation. Only spots containing 3 to 30 colonies and only dilutions that produced at least two countable spots were included in the calculations. Undiluted assay samples were also viewed under the light microscope to ensure that a decrease in CFU/ml was not the effect of bacterial clumping.
HD5 activity was also tested against Escherichia coli ML35p (42). Isolated colonies were inoculated into 50 ml Trypticase soy broth, and after 18 h of incubation at 37°C, the bacteria were adjusted to McFarland 0.5 and the assay was further performed as described above for N. gonorrhoeae.
C. trachomatis serovar F, a common urogenital isolate, was utilized for in vitro antimicrobial studies. The ability of defensins to prevent chlamydial infection was determined by preincubating the peptides with purified elementary bodies (EB) and titrating out infectious activity on monolayers of the Ishikawa endometrial epithelial cell line (36) in triplicates in 96-well tissue culture plates, using a modification of the technique described by Yasin et al. (81). In brief, for each triplicate, 2.5 μl of EB containing 1.2 × 103 viable EB diluted in 100 μl SPG medium (200 mM sucrose, 4 mM KH2PO4, 9 mM Na2HPO4, 4 mM glutamic acid), with and without various defensin peptide concentrations, was incubated for 0 and 45 min at 37°C, unless stated otherwise, in polypropylene microtubes. Immediately prior to chlamydia inoculation, the Ishikawa cells were washed with PBS, and then 33 μl EB mixture was added. Plates were centrifuged for 40 min at 250 × g at room temperature, and then the supernatants were removed and replaced with 200 μl of cell culture medium supplemented with 2.0 μg/ml cycloheximide. Thereafter, Ishikawa cells were incubated for an additional 40 h at 37°C with 5% CO2, fixed in 10% ice-cold methanol for 10 min, and stained using an antibody specific for chlamydial lipopolysaccharide (9), which was visualized using the APAAP detection system (Biogenix) and counterstaining with aqueous hematoxylin. Inclusion bodies, a measure of chlamydial infectious activity, were microscopically enumerated using a 40× objective (Olympus culture microscope model CK 40; Olympus America, Melville, NY).
All antimicrobial assay data were graphed with SigmaPlot software 7.0, and statistics were calculated using SigmaStat software, version 2.0.
RESULTS
During N. gonorrhoeae and C. trachomatis infections, HD5 appears in the urethral lumen as a propeptide.
We collected urethral lavages of both healthy men and men with STIs and determined lumenal defensin profiles and concentrations by immunoblotting and probing for HD5, HBD1, HBD2, and HNP1-3. Four clinical groups were examined (n = 5 per group): a healthy uninfected group, a chlamydial urethritis group, a gonococcal urethritis group, and a group of men diagnosed with nongonococcal nonchlamydial urethritis.
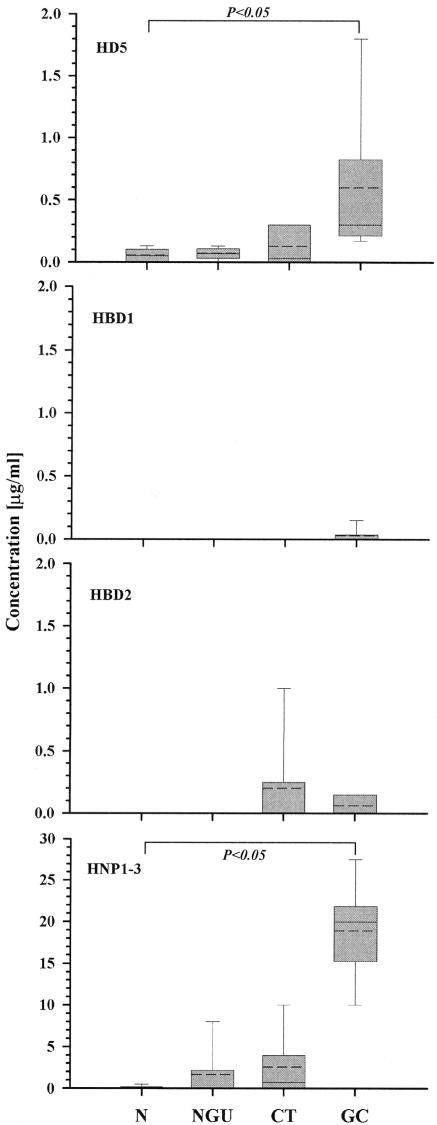
Only traces or low levels of HD5 were detectable in the lavages from subjects without STIs or men with NGU (0.05 ± 0.06 μg/ml and 0.07 ± 0.05 μg/ml, respectively); however, increased concentrations of HD5 were found in the urethral lavages from men with CT (0.13 ± 0.16 μg/ml), and significantly higher concentrations were found in lavages from men with GC (0.6 ± 0.68 μg/ml, reaching up to 1.8 μg/ml; P < 0.05) (Fig. 1). Since a substantial dilution of secretions is caused by injecting saline into the urethra, the actual lumenal HD5 concentrations are estimated to be at least 10-fold higher than this. In contrast to HD5, HBD1 was only detected in a single GC lavage, suggesting that this defensin is retained intracellularly or in intercellular spaces. HBD2 was also up-regulated during inflammation, but only in a small number of the patients with CT or GC. Among the 15 patients with urethritis (NGU, GC, or CT), 12 were positive for HD5, but only 3 were positive for HBD2 at comparable sensitivity in the HD5 and HBD2 immunoblot assays.
FIG. 1.
Defensin profiles in urethral lavages from healthy men and men with STIs. Aliquots of urethral lavages were dialyzed against 5% acetic acid using a 3-kDa molecular weight cutoff membrane, subjected to AU-PAGE (for HD5 and HNP1-3) or SDS-Tricine-PAGE (for HBD1 and HBD2), electroblotted onto PSQ membrane, fixed, blocked, and probed with the appropriate rabbit polyclonal antibody, and defensin bands were visualized with alkaline phosphatase-conjugated polyclonal goat anti-rabbit antibodies and nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate substrate. Resulting band inten-sities were compared to the band intensities of standard peptides, and the approximate defensin concentrations in the various samples were deduced. Graphs were plotted as vertical boxes showing the 10th to 90th percentile, the median (solid line), the mean (dotted line), and the standard deviation of five different subjects per group. Statistical significance was calculated by the Kruskal-Wallis one-way analysis of variance. Note the larger y-axis scale for HNP. N, normal (healthy); NGU, nongonococcal nonchlamydial urethritis; CT, chlamydial urethritis; GC, gonococcal urethritis.
Concentrations of the neutrophil defensins HNP1-3 were lowest in the lavages from the uninfected men, elevated in the lavages from men with NGU and CT and, similar to HD5, significantly increased in men with GC, here reaching concentrations up to 27 μg/ml.
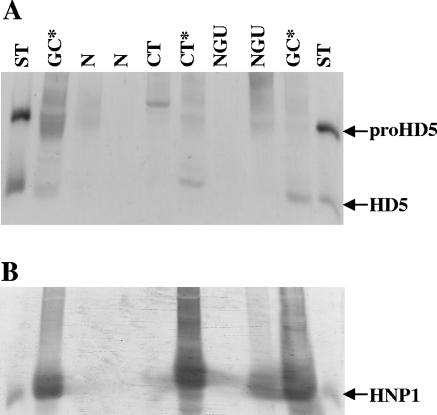
Immunoblot analysis also revealed two distinct profiles of HD5 forms in lavages. When HNP levels were low (<10 μg/ml), HD5 was mostly present as the proform, but in samples containing high levels of HNP (≥10 μg/ml), HD5 was further processed (Fig. 2). Categorical data analysis by Fisher's exact test indicated a significant relationship between HD5 processing and the presence of high concentrations of neutrophil defensin (P < 0.05) (Table 1). Since human neutrophils do not express HD5 (39) and, as determined by immunohistochemistry of inflamed urethral tissue and immunoblot analysis of neutrophil granule extract (unpublished data), our findings suggested that urethral epithelial cells produce and secrete HD5 as a propeptide and that neutrophil proteases could contribute to proHD5 processing.
FIG. 2.
Representative immunoblots of urethral lavages probing for HD5 and HNP1 to -3. Samples were processed as described in the legend for Fig. 1. Equivalents of 100 μl of urethral lavage or 20 ng of standard peptide (proHD5 [aa 20 to 94], HD5 [aa 64 to 94], or HNP1) were subjected to AU-PAGE followed by immunoblotting for HD5 (A) or HNP1-3 (B). ST, peptide standard; N, normal (healthy); NGU, nongonococcal nonchlamydial urethritis; CT, chlamydial urethritis; GC, gonococcal urethritis. *, increased neutrophil counts in the urethral lavage sediment, which were paralleled by strongly elevated HNP concentrations of ≥10 μg/ml. Note the appearance of processed HD5 forms in samples with high HNP concentrations (by Fisher exact test, P < 0.05; see Table 1).
TABLE 1.
Occurrence of processed HD5 among lavages with low and high HNP1-3 concentrationsa
| Processed HD5 | No. of samples with HNP concn of:
|
Total | |
|---|---|---|---|
| <10 μg/ml | ≥10 μg/ml | ||
| No | 11 | 1 | 12 |
| Yes | 3 | 5 | 8 |
| Total | 14 | 6 | 20 |
By Fisher exact test, P = 0.018.
Neutrophil proteases process proHD5 in vitro, and neutrophil protease-cleaved forms are detected in urethral secretions from infected men.
To determine whether neutrophil proteases could participate in proHD5 processing in vivo, we first subjected recombinant proHD5 to in vitro cleavage by neutrophil proteases, identified these products, and then compared the HD5 cleavage products present in the urethral lavages using SELDI mass spectrum analysis.
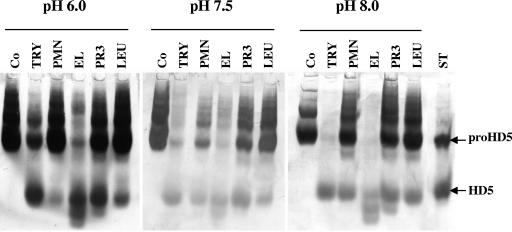
Human neutrophil granule extract, human neutrophil elastase and proteinase 3, and leukocyte extract from purulent sputum all effected proHD5 cleavage in vitro, as did human trypsin (Fig. 3). Trypsin was included for several reasons: it is the key processing enzyme for proHD5 in the small intestine (28), trypsinogen has been previously detected by immunohistochemistry in the male genital tract (57), and we detected trace amounts of trypsin in neutrophils by immunohistochemical staining of inflamed tissue (data not shown) and by immunoblot analysis of neutrophil granule extract (approximately 4 μg trypsin/108 PMN, compared to a reported 50 μg elastase/108 PMN [44], and in some subsequent urethral lavages not included in this study [data not shown]). Whereas proteinase 3, granule extract, and leukocyte extract cleaved proHD5 partially, elastase processed proHD5 considerably at all pHs and nearly completely at pH 8.0. Trypsin also effected almost complete cleavage of proHD5 at pH 8.0. Trypsin produced only one major fragment, identified previously as HD5 (aa 63 to 94) (28), but elastase and proteinase 3 generated at least two major cleavage products. N-terminal sequencing of these products in conjunction with mass spectrum analysis identified the major neutrophil elastase cleavage products as HD5 (aa 53 to 94), HD5 (aa 57 to 94), and HD5 (aa 62 to 94), along with additional minor products as HD5 (aa 56 to 94) and HD5 (aa 64 to 94). The dominant proteinase 3 cleavage products were HD5 (aa 56 to 94) and HD5 (aa 53 to 94), in addition to the minor product HD5 (aa 57 to 94) (Table 2).
FIG. 3.
In vitro cleavage of proHD5 by neutrophil proteases. Experiments were conducted in 100 mM Tris-150 mM NaCl adjusted to various pHs. Purified enzymes were employed at a ratio of 1 molecule per 100 molecules of proHD5. After 2 h of incubation at 37°C, samples were placed on ice and diluted 1:2 in 5% acetic acid to prevent further proteolysis. Aliquots representing 100 ng proHD5 originally added to the reaction tubes were lyophilized and subjected to AU-PAGE followed by anti-HD5 immunoblotting. Co, control, no proteases; TRY, trypsin; PMN, neutrophil granule extract; EL, human neutrophil elastase; PR3, human neutrophil proteinase 3; LEU, leukozyme, human sputum leukocyte extract; ST, 50 ng recombinant proHD5 (aa 20 to 94) and HD5 (aa 56 to 94). Note: proHD5 tends to form multimeric aggregates that appear as additional slower migrating bands with lower intensity.
TABLE 2.
Identification of neutrophil protease cleavage products of proHD5 in urethral lavagesd
| Protease | HD5 peptidea | Cleavage siteb | Expected masse | Patient | Detected masse | Deviation (%) |
|---|---|---|---|---|---|---|
| EL, PR | 53-94 | S52*A53 | 4,610.27 | ND | ND | |
| EL, PR | 56-94 | R55*T56 | 4,269.8c | NGU2533 | 4,255 | 0.33 |
| CT2192 | 4,250 | 0.37 | ||||
| CT2309 | 4,254 | 0.35 | ||||
| GC2403 | 4,288 | 0.42 | ||||
| EL, PR | 57-94 | T56*S57 | 4,168.74 | N2361 | 4,136 | 0.77 |
| GC2414 | 4,150 | 0.43 | ||||
| EL | 62-94 | A61*R62 | 3,738.32c | GC2182 | 3,755 | 0.45 |
| GC2403 | 3,741 | 0.08 | ||||
| EL | 64-94 | A63*T64 | 3,511.05c | CT2192 | 3,517 | 0.17 |
| CT2309 | 3,517 | 0.17 | ||||
| GC2182 | 3,506 | 0.14 | ||||
| GC2403 | 3,518 | 0.19 | ||||
| GC2414 | 3,518 | 0.19 |
Peptides (aa) identified by N-terminal sequencing and/or MALDI analysis.
Amino acids and their positions flanking the cleavage site (*).
Masses also detected after in vitro cleavage of proHD5 with human neutrophil granule extract.
EL, elastase; PR, proteinase 3; ND, not detected; NGU, nongonococcal nonchlamydial urethritis; CT, chlamydial urethritis; GC, gonococcal urethritis; N, normal (healthy).
Masses (expected and detected) are reported in daltons.
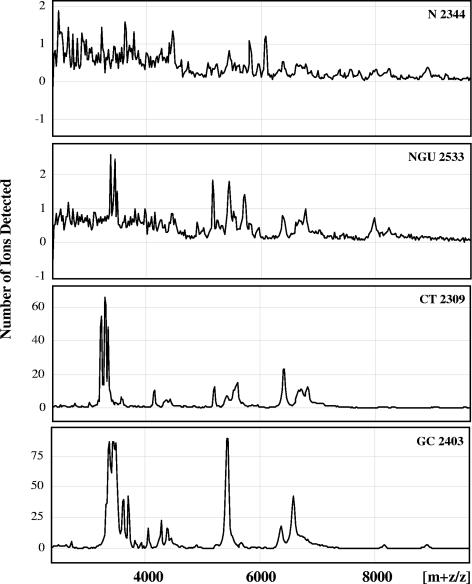
To identify the major biological defensin forms in the urethral lavages, we used SELDI, a highly sensitive technique well suited for analysis of very small volumes of native crude samples without further processing. Although SELDI is not quantitative, this generated important qualitative information on these clinical samples. Each clinical group of patients produced similar mass profiles, and representative mass spectra for each patient group are shown in Fig. 4. Whereas in lavages from healthy (N) men no, or very few, mass peaks in the peptide region (2 to 10 kDa) were detected (median, 0), several such peaks appeared in the NGU group (median, 3), and a larger number of peaks were detected in the CT and GC group (median, 11 and 19, respectively). HNP1-3 were easily identifiable as a distinctive three-peak configuration around the mass ([m+z]/z) of 3,400, reflecting HNP1, HNP2, and HNP3 peptides (expected masses of 3,443, 3,371, and 3,486, respectively). HNP1-3 were detectable in traces in two of five washes from patients without clinical infection (group N) and in all patients with urethritis and generated outstanding peaks in all of the GC patients. Masses consistent with HD5 peptides generated by neutrophil proteases in our in vitro experiments were present in several washes from patients with CT and GC (Table 2). Additional presumptive HD5 masses, including unprocessed proHD5, were also detected in the infected patient groups (Table 3). We cannot completely rule out that some of the masses detected are of microbial origin. However, since all of the predominant HD5 masses were observed in all patient groups with infections, this is unlikely. Masses consistent with HBD2 were detected only in three lavages, and masses consistent with HBD1 could not be detected with certainty (data not shown). In every case, lavages with presumptive defensin masses tested positive in the appropriate immunoblot assay. Of note, we also found masses that could reflect the presence of human defensin 6, which has been located previously in Paneth cells in the small intestine together with HD5 (40, 61). However, because of the unavailability of an antibody and the small sample volumes, we were not able to confirm this by a second technique.
FIG. 4.
SELDI profiles of representative urethral lavages for each patient group. Note the different y-axis scales. HNP1 to -3 peptides produce a characteristic three-peak pattern around a mass ([m+z]/z) of 3,400, reflecting HNP1, HNP2, and HNP3 peptides (expected masses of 3,443, 3,371, and 3,486, respectively). For clarity, individual masses are not shown. See also Tables 2 and 3.
TABLE 3.
Additional masses consistent with HD5 peptides detected in urethral lavages by SELDI
| Group (n = 5) | Detected massa | HD5 peptide match (aa) | Expected mass | Deviation (%) |
|---|---|---|---|---|
| N | None | |||
| NGU | 5,441 | 44-94 | 5,457 | 0.29 |
| 6,373 | 35-94 | 6,387 | 0.22 | |
| 6,665 | 33-94 | 6,643 | 0.33 | |
| CT | 3,410 | 65-94 | 3,409 | 0.03 |
| 5,231 | 46-94 | 5,257 | 0.49 | |
| 5,435 | 44-94 | 5,457 | 0.40 | |
| 6,373 | 35-94 | 6,387 | 0.22 | |
| 6,657 | 33-94 | 6,643 | 0.21 | |
| GC | 3,409 | 65-94 | 3,409 | 0.00 |
| 3,587 | 63-94b | 3,582 | 0.14 | |
| 3,937 | 60-94 | 3,937 | 0.00 | |
| 5,229 | 46-94 | 5,257 | 0.53 | |
| 5,438 | 44-94 | 5,457 | 0.35 | |
| 6,373 | 35-94 | 6,387 | 0.22 | |
| 6,658 | 33-94 | 6,643 | 0.22 | |
| 8,166 | 20-94 | 8,103 | 0.78 |
HD5-mediated antimicrobial activity against N. gonorrhoeae and C. trachomatis is N-terminal processing dependent and distinct from the activity of HBD1 and HBD2.
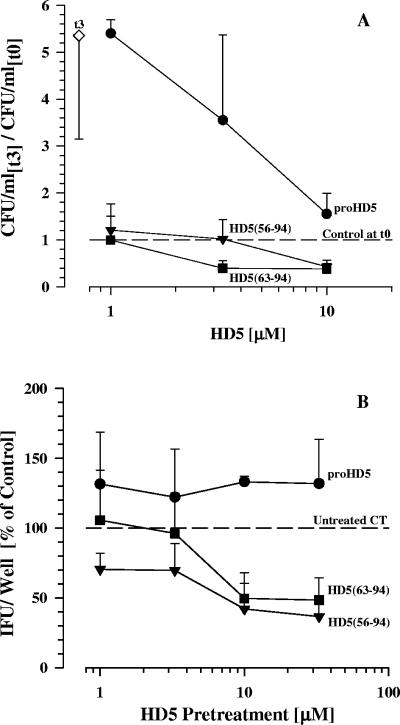
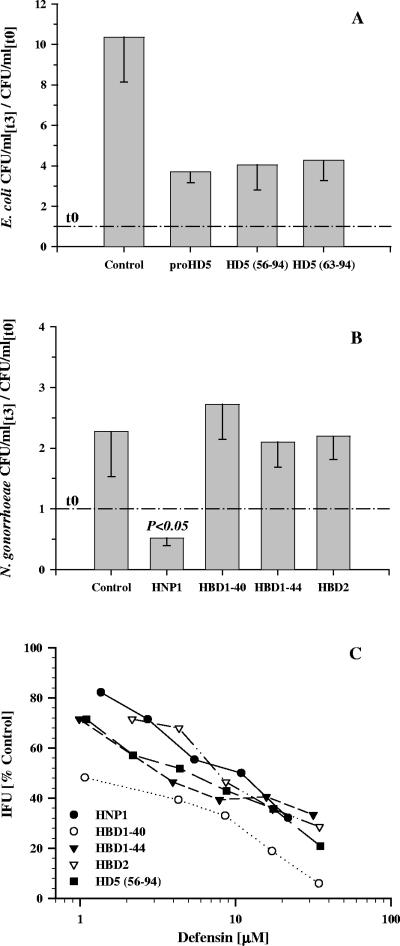
We next investigated whether urethral-processed HD5 was active against N. gonorrhoeae and C. trachomatis and whether its antibacterial activity depended on N-terminal processing. We began with proHD5 and two processed forms that have been described previously in vivo (28, 61). We found that recombinant HD5 exerts bactericidal activity against both microorganisms in a dose- and processing-dependent manner (Fig. 5). At 3.3 μM and 10 μM concentrations or higher, HD5 (aa 63 to 94) and HD5 (aa 56 to 94), respectively, were bactericidal in contrast to unprocessed proHD5, which either slightly enhanced the infectiousness of the pathogen (C. trachomatis) or at most decreased the growth rate (N. gonorrhoeae). Under the same conditions, which included the presence of salts, divalent cations, sucrose, amino acids, and vitamins as likely present in vivo, E. coli was not killed by any of the HD5 forms (Fig. 6A). HBD1 and HBD2 were inactive against N. gonorrhoeae but demonstrated activity against C. trachomatis comparable to HD5. HNP1 demonstrated a bactericidal effect against both microorganisms (Fig. 6B and C).
FIG. 5.
In vitro antibacterial activities of different HD5 forms with various N termini. Dose response relationships with recombinant proHD5 (aa 20 to 94), HD5 (aa 56 to 94), and HD5 (aa 63 to 94), tested at the same molarities, are shown. (A) For antigonococcal activity, defensins were mixed with 24-hour cultures of N. gonorrhoeae adjusted to ∼2 × 106 CFU/ml in 70% RPMI supplemented with glucose and IsoVitaleX, and the number of CFU were determined after a 3-hour incubation. Values are expressed as change over the control at time zero. (B) For anti-C. trachomatis activity, defensins were added to infectious EB in SPG medium for 45 min prior to infection of Ishikawa cells. The inclusion-forming units (IFU)/well were enumerated 40 h after infection by immunohistochemistry using an anti-chlamydia lipopolysaccharide antibody. Data are expressed relative to the IFU/well resulting from infection with untreated chlamydia. Depicted are the means + standard deviations of three experiments.
FIG. 6.
Comparative defensin activity. (A) Various HD5 peptides were tested at a 3.3 μM concentration against E. coli cells under the same conditions as shown above for N. gonorrhoeae. (B) Activities of HNP1, HBD1, and HBD2 at a 3.3 μM concentration against N. gonorrhoeae. In panels A and B, the data depicted are the means minus standard deviations for three experiments; see Fig. 5 for details. (C) Dose-response curve for HNP1, HBD1 and HBD2, and HD5 (aa 56 to 94) for comparison against C. trachomatis. HBD1-40 and HBD1-44 are predominant native forms of HBD1 40 and 44 aminoacids in length, respectively. The assay was conducted as described in the legend for Fig. 5 with the exception that chlamydia were preincubated for 2 h with and without defensins prior to infection of Ishikawa cells. Depicted are the means of duplicates.
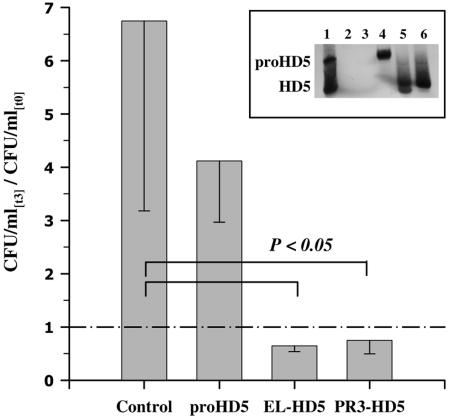
Mixtures of HD5 peptides generated from the precursor proHD5 by the neutrophil proteases elastase or proteinase 3 exhibited significant antibacterial activities against N. gonorrhoeae comparable to individual processed HD5 peptides (Fig. 7). In contrast, when tested against Chlamydia trachomatis, only elastase-processed HD5 peptides exhibited activity, effecting a 28% reduction of chlamydial inclusion bodies at a 20 μM concentration (data not shown). As the HD5 peptide mixtures were tested at a combined concentration, individual peptide concentrations reached about ∼5 μM. This indicates that only certain elastase-processed HD5 forms exert activity against C. trachomatis and further supports the importance of the N terminus of HD5 on its antibacterial activity.
FIG. 7.
Antigonococcal activity of HD5 peptides generated from proHD5 through proteolytic cleavage by neutrophil proteases. Human neutrophil elastase (EL-HD5) and human neutrophil proteinase 3 (PR3-HD5) cleavage products were purified by reverse-phase HPLC and tested at a 5 μM final concentration. For assay details and data presentation, see the legend for Fig. 5A. Depicted are the means minus standard deviations of three experiments. (Insert) Two-microliter aliquots were taken from each sample of one experiment after completion of the incubation and subjected to AU-PAGE, followed by anti-HD5 immunoblotting. Lanes: 1, 50 ng recombinant proHD5 (aa 20 to 94) and HD5 (aa 56 to 94); 2, control at time zero (t0); 3, control after 3 h of incubation (t3); 4, untreated proHD5; 5, EL-generated HD5 peptides; 6, PR3-generated HD5 peptides.
DISCUSSION
In this study we lavaged the urethras of healthy and acutely ill men to examine lumenal defensin forms and to determine the potential roles and interactions of defensin-mediated host defense mechanisms during STIs in vivo. We found that HD5 is the predominant epithelial defensin appearing in the urethral lumen during infection, that it is secreted as propeptide, that its antimicrobial function is activated by N-terminal processing, and that neutrophil proteases likely mediate the required processing at this site. We describe a novel interaction between epithelial cells and neutrophils in the context of an acute infection, wherein epithelial cells provide the antimicrobial propeptide and neutrophils provide the activating convertases. Previously, neutrophil proteases have only been implicated in processing of homologous neutrophil antimicrobial propeptides (46, 58, 68, 70), or distinct epithelial cell types were shown to interact in the context of protection of the germ cells (71).
Similar to our earlier observations in the upper female genital tract (63), we also observed an up-regulation of HD5 in the male urethra during inflammation. This contrasts with the lower female genital tract and the small intestinal mucosa, in which HD5 is constitutively expressed by stratified squamous epithelial cells and Paneth cells, respectively (39). However, these sites are constantly exposed to microbial products, which may lead to continuous cell stimulation (3), unlike the penile urethra and upper female genital tract, which are normally sterile or scarcely populated. We also observed an increase of HBD2 in some patients during infection, in agreement with studies in other epithelia (29, 45, 53), but HD5 seemed to be more readily produced than HBD2. This could be the result of differential defensin gene induction: HBD2 gene induction is primarily NF-κB mediated and additional consensus sequences for activator protein 1 and nuclear factor 6 have been found (52), whereas HD5 does not have consensus sequences for NF-κB but consensus sequences for interferon response factor I and activator proteins 1, 2, and 4 upstream of the HD5 gene (MatInspector match). HBD1 was detected in the urethral washes in only one patient, suggesting that it, and perhaps HBD2, may function here mostly as an intraepithelial barrier against invading microbes. We also observed elevated HNP levels in all the lavages from patients with clinical signs of inflammation, with the highest concentrations in N. gonorrhoeae-infected patients, reflecting the large influx of neutrophils characteristically seen in this infection (37), and in agreement with previous reports on HNP detection in STI-mediated endometritis (78).
Our data indicate that HD5 is primarily secreted into the male urethral lumen as a precursor molecule, similar to earlier findings in the normal female genital tract (63) and in the small intestine (16, 28, 61). However, in the small intestine Paneth cells also codeliver the key processing enzyme trypsin together with the propeptide, which is immediately cleaved, and the fully processed peptide is the predominant form in the intestinal lumen (28). In contrast, in the male urethra, the key processing and activating enzymes for HD5 appear to be delivered by neutrophils predominantly in the context of inflammation, as was indicated by the correlation between processed HD5 forms and neutrophil influx, in vitro cleavage experiments with individual neutrophil proteases and neutrophil granule extract, and matches between the masses of these in vitro-processed HD5 forms and masses of native forms in the urethral washes. Though the destructive action of neutrophil proteases against host tissue has resulted in the development of antiprotease treatments in chronic inflammation (34, 51, 65), our results further caution such usage, as it may result in decreased antimicrobial peptide levels.
Our studies revealed that elastase and proteinase 3, in addition to cleaving C-terminal of alanine residues (which are the reported major cleavage site for these enzymes), also cleaved C-terminal of threonine or serine residues. These cleavage sites have been also reported by others (10, 38, 64, 67). The less-complete cleavage by crude neutrophil granule extract compared to purified neutrophil proteases could be due to the presence of neutrophil-derived protease inhibitors (80). Interestingly, we also detected low levels of trypsin in neutrophils, and in some of the urethral washes we found HD5 peptide forms consistent with complete trypsin processing. Earlier, Paju et al. (57) reported on the presence of trypsinogen in various epithelial cells of the male genital tract, including the prostatic part of urethra and luminal cells of the periurethral glands (62). Hence, trypsin of either epithelial origin or delivered by neutrophils may also contribute to proHD5 processing in the male urethral lumen.
The antimicrobial activities of HD5 against N. gonorrhoeae and C. trachomatis were dependent upon proteolytic processing and were greatly influenced by the N terminus of the peptide. This has been also documented for HD5 activity against intestinal pathogens (28) and for cryptdins, the mouse HD5 homologue (56). Allowing an inactive propeptide to become antimicrobially active only in the context of ongoing inflammation may be a safeguard that prevents antimicrobial action against the normal flora in the male at the urethral orifice and in the female in the vagina. In contrast, there is a requirement for continuous antimicrobial activity in the small intestine because of the dual task of allowing food resorption while preventing microbial invasion from both microbes taken up with food that have survived the gastric passage and microbes ascending from the colon. In addition, processing of HD5 precursor molecules by site-specific proteases could result in a differentialized antimicrobial action of one antimicrobial peptide, thus increasing its functional versatility, optimizing it to balance protection of the normal microbiota and site-specific pathogen-directed action. In our study, N. gonorrhoeae was more susceptible to the neutrophil-processed HD5 forms than C. trachomatis. Increased resistance to neutrophil-mediated immune mechanisms may in part account for the stronger adaptive immune response evoked by C. trachomatis compared to N. gonorrhoeae (41).
Our data reveal distinct defensin profiles in N. gonorrhoeae and C. trachomatis urethritis. HD5 may be more efficient in the clearance of infection with extracellular N. gonorrhoeae than with C. trachomatis infection, as we found much higher levels of HD5 in gonococcal infection. Conversely, HBD1 and HBD2 perhaps may largely contribute to intraepithelial defense. The two peptides did not appear to be secreted in substantial amounts into the urethral lumen and were not active against N. gonorrhoeae but exhibited activity against the obligate intracellular C. trachomatis.
HNP1 also demonstrated activity against N. gonorrhoeae and C. trachomatis, indicating that neutrophils directly provide antimicrobial activity against these STI pathogens. HNP may act in synergy with HD5, and this will be addressed in future studies. Although neutrophil activity against C. trachomatis has been reported previously (81), the activity against N. gonorrhoeae contrasts with earlier findings (69). However, in addition to using a different bacterial strain at a different growth phase, the earlier studies were conducted with an assay buffer based on Neisseria growth medium, whereas we used an RPMI 1640-based assay buffer, mimicking human extracellular fluid. These parameters are likely to affect both the defensin interaction with the bacterial surface and the bacterial response against the antimicrobial peptide.
In the last few years, it has become clear that microbial products can modulate antimicrobial peptide induction through binding to distinct toll-like receptors (5, 12, 17, 21, 22, 32, 33, 49, 55, 66) and that neutrophil-derived proteases participate in cytokine processing and degradation (6, 24), leukocyte chemotaxis (73), and the stimulation of protease-activated receptors (54). Our study of defensin profiles in the male urethra during STI further supports the idea that antimicrobial peptide production is influenced by the pathogen. Moreover, we illustrate an additional modulation of the immune response that may be achieved by site-specific, distinct postsecretory processing of antimicrobial peptides and an orchestrated action of different cell types involved in host defense.
Acknowledgments
This work was supported by grants from the National Institutes of Health (PO1 AI46518 to A.J.Q. and E.P.; RO1 AI40690 to A.J.Q.; U19 AI38515 to S.G., P.A.R., and A.J.Q.; RO1 DE14897 and RO1 HL67871 to G.D.; and the Minority Biomedical Research Support-Research Initiative for Scientific Enhancement to G.C.P. and H.L.) and by a STAR fellowship from the U.S. EPA (to M.K.-P.).
We thank Joseph Politch for assistance in statistical analysis, Charles L. Bevins for critical review of the manuscript, Jan Pohl for expert advice on proteomics, and Shandee Dixon for excellent technical support.
Editor: A. D. O'Brien
REFERENCES
- 1.Avellar, M. C., L. Honda, K. G. Hamil, S. Yenugu, G. Grossman, P. Petrusz, F. S. French, and S. H. Hall. 2004. Differential expression and antibacterial activity of epididymis protein 2 isoforms in the male reproductive tract of human and rhesus monkey (Macaca mulatta). Biol. Reprod. 71:1453-1460. [DOI] [PubMed] [Google Scholar]
- 2.Ayabe, T., D. P. Satchell, P. Pesendorfer, H. Tanabe, C. L. Wilson, S. J. Hagen, and A. J. Ouellette. 2002. Activation of Paneth cell alpha-defensins in mouse small intestine. J. Biol. Chem. 277:5219-5228. [DOI] [PubMed] [Google Scholar]
- 3.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. [DOI] [PubMed] [Google Scholar]
- 4.Bals, R. 2000. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 1:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bals, R., and P. S. Hiemstra. 2004. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 23:327-333. [DOI] [PubMed] [Google Scholar]
- 6.Bank, U., and S. Ansorge. 2001. More than destructive: neutrophil-derived serine proteases in cytokine bioactivity control. J. Leukoc. Biol. 69:197-206. [PubMed] [Google Scholar]
- 7.Berger, R. E. 1999. Acute epididymitis, p. 847-858. In K. K. Holmes, P. F. Sparling, P.-A. Mardh, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Vasserheit (ed.), Sexually transmitted diseases. McGraw-Hill, New York, N.Y.
- 8.Bowie, W. R. 1990. Approach to men with urethritis and urologic complications of sexually transmitted diseases. Med. Clin. North Am. 74:1543-1557. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell, H. D., and P. J. Hitchcock. 1984. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect. Immun. 44:306-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camire, R. M., M. Kalafatis, and P. B. Tracy. 1998. Proteolysis of factor V by cathepsin G and elastase indicates that cleavage at Arg1545 optimizes cofactor function by facilitating factor Xa binding. Biochemistry 37:11896-11906. [DOI] [PubMed] [Google Scholar]
- 11.Cates, W., and R. C. Brunham. 1999. Sexually transmitted diseases and infertility, p. 1079-1087. In K. K. Holmes, P. F. Sparling, P.-A. Mardh, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Vasserheit (ed.), Sexually transmitted diseases. McGraw-Hill, New York, N.Y.
- 12.Chalifour, A., P. Jeannin, J. F. Gauchat, A. Blaecke, M. Malissard, T. N′Guyen, N. Thieblemont, and Y. Delneste. 2004. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers α-defensin production. Blood 104:1778-1783. [DOI] [PubMed] [Google Scholar]
- 13.Chertov, O., D. F. Michiel, L. Xu, J. M. Wang, K. Tani, W. J. Murphy, D. L. Longo, D. D. Taub, and J. J. Oppenheim. 1996. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 271:2935-2940. [DOI] [PubMed] [Google Scholar]
- 14.Chung, W. O., and B. A. Dale. 2004. Innate immune response of oral and foreskin keratinocytes: utilization of different signaling pathways by various bacterial species. Infect. Immun. 72:352-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Com, E., F. Bourgeon, B. Evrard, T. Ganz, D. Colleu, B. Jegou, and C. Pineau. 2003. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol. Reprod. 68:95-104. [DOI] [PubMed] [Google Scholar]
- 16.Cunliffe, R. N., F. R. Rose, J. Keyte, L. Abberley, W. C. Chan, and Y. R. Mahida. 2001. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 48:176-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devine, D. A. 2003. Antimicrobial peptides in defence of the oral and respiratory tracts. Mol. Immunol. 40:431-443. [DOI] [PubMed] [Google Scholar]
- 18.Diamond, D. L., J. R. Kimball, S. Krisanaprakornkit, T. Ganz, and B. A. Dale. 2001. Detection of beta-defensins secreted by human oral epithelial cells. J. Immunol. Methods 256:65-76. [DOI] [PubMed] [Google Scholar]
- 19.Diemer, T., P. Huwe, M. Ludwig, E. W. Hauck, and W. Weidner. 2003. Urogenital infection and sperm motility. Andrologia 35:283-287. [PubMed] [Google Scholar]
- 20.Dohle, G. R. 2003. Inflammatory-associated obstructions of the male reproductive tract. Andrologia 35:321-324. [PubMed] [Google Scholar]
- 21.Duits, L. A., P. H. Nibbering, E. van Strijen, J. B. Vos, S. P. Mannesse-Lazeroms, M. A. van Sterkenburg, and P. S. Hiemstra. 2003. Rhinovirus increases human beta-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol. Med. Microbiol. 38:59-64. [DOI] [PubMed] [Google Scholar]
- 22.Dziarski, R. 2003. Recognition of bacterial peptidoglycan by the innate immune system. Cell Mol. Life Sci. 60:1793-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards, J. L., and M. A. Apicella. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17:965-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Ouriaghli, F., H. Fujiwara, J. J. Melenhorst, G. Sconocchia, N. Hensel, and A. J. Barrett. 2003. Neutrophil elastase enzymatically antagonizes the in vitro action of G-CSF: implications for the regulation of granulopoiesis. Blood 101:1752-1758. [DOI] [PubMed] [Google Scholar]
- 25.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 26.Ganz, T., L. Liu, E. V. Valore, and A. Oren. 1993. Posttranslational processing and targeting of transgenic human defensin in murine granulocyte, macrophage, fibroblast, and pituitary adenoma cell lines. Blood 82:641-650. [PubMed] [Google Scholar]
- 27.Garcia, J. R., F. Jaumann, S. Schulz, A. Krause, J. Rodriguez-Jimenez, U. Forssmann, K. Adermann, E. Kluver, C. Vogelmeier, D. Becker, R. Hedrich, W. G. Forssmann, and R. Bals. 2001. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 306:257-264. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh, D., E. Porter, B. Shen, S. K. Lee, D. Wilk, J. Drazba, S. P. Yadav, J. W. Crabb, T. Ganz, and C. L. Bevins. 2002. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat. Immunol. 3:583-590. [DOI] [PubMed] [Google Scholar]
- 29.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861. [DOI] [PubMed] [Google Scholar]
- 30.Harwig, S. S., T. Ganz, and R. I. Lehrer. 1994. Neutrophil defensins: purification, characterization, and antimicrobial testing. Methods Enzymol. 236:160-172. [DOI] [PubMed] [Google Scholar]
- 31.Harwig, S. S., A. S. Park, and R. I. Lehrer. 1992. Characterization of defensin precursors in mature human neutrophils. Blood 79:1532-1537. [PubMed] [Google Scholar]
- 32.Hertz, C. J., S. M. Kiertscher, P. J. Godowski, D. A. Bouis, M. V. Norgard, M. D. Roth, and R. L. Modlin. 2001. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol. 166:2444-2450. [DOI] [PubMed] [Google Scholar]
- 33.Hertz, C. J., Q. Wu, E. M. Porter, Y. J. Zhang, K. H. Weismuller, P. J. Godowski, T. Ganz, S. H. Randell, and R. L. Modlin. 2003. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J. Immunol. 171:6820-6826. [DOI] [PubMed] [Google Scholar]
- 34.Hiemstra, P. S. 2002. Novel roles of protease inhibitors in infection and inflammation. Biochem. Soc. Trans. 30:116-120. [DOI] [PubMed] [Google Scholar]
- 35.Hiratsuka, T., M. Nakazato, T. Ihi, T. Minematsu, N. Chino, T. Nakanishi, A. Shimizu, K. Kangawa, and S. Matsukura. 2000. Structural analysis of human beta-defensin-1 and its significance in urinary tract infection. Nephron 85:34-40. [DOI] [PubMed] [Google Scholar]
- 36.Holinka, C. F., H. Hata, H. Kuramoto, and E. Gurpide. 1986. Responses to estradiol in a human endometrial adenocarcinoma cell line (Ishikawa). J. Steroid Biochem. 24:85-89. [DOI] [PubMed] [Google Scholar]
- 37.Hook, E. W., and H. H. Handsfield. 1999. Gonococcal infections in the adult, p. 451. In K. K. Holmes, P. F. Sparling, P.-A. Mardh, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserheit (ed.), Sexually transmitted diseases. McGraw-Hill, New York, N.Y.
- 38.Iwata, H., M. Kaibara, N. Dohmae, K. Takio, R. Himeno, and S. Kawakami. 2004. Purification, identification, and characterization of elastase on erythrocyte membrane as factor IX-activating enzyme. Biochem. Biophys. Res. Commun. 316:65-70. [DOI] [PubMed] [Google Scholar]
- 39.Jones, D. E., and C. L. Bevins. 1992. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 267:23216-23225. [PubMed] [Google Scholar]
- 40.Jones, D. E., and C. L. Bevins. 1993. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 315:187-192. [DOI] [PubMed] [Google Scholar]
- 41.Kelly, K. A. 2003. Cellular immunity and Chlamydia genital infection: induction, recruitment, and effector mechanisms. Int. Rev. Immunol. 22:3-41. [DOI] [PubMed] [Google Scholar]
- 42.Lehrer, R. I., A. Barton, K. A. Daher, S. S. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Investig. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehrer, R. I., and T. Ganz. 2002. Defensins of vertebrate animals. Curr. Opin. Immunol. 14:96-102. [DOI] [PubMed] [Google Scholar]
- 44.Liau, D. F., N. X. Yin, and S. F. Ryan. 1993. Isolation of human polymorphonuclear leukocyte elastase by chromatography on immobilized benzamidine. Prep. Biochem. 23:439-447. [DOI] [PubMed] [Google Scholar]
- 45.Liu, A. Y., D. Destoumieux, A. V. Wong, C. H. Park, E. V. Valore, L. Liu, and T. Ganz. 2002. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J. Investig. Dermatol. 118:275-281. [DOI] [PubMed] [Google Scholar]
- 46.Malm, J., O. Sorensen, T. Persson, M. Frohm-Nilsson, B. Johansson, A. Bjartell, H. Lilja, M. Stahle-Backdahl, N. Borregaard, and A. Egesten. 2000. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect. Immun. 68:4297-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michaelson, D., J. Rayner, M. Couto, and T. Ganz. 1992. Cationic defensins arise from charge-neutralized propeptides: a mechanism for avoiding leukocyte autocytotoxicity? J. Leukoc. Biol. 51:634-639. [DOI] [PubMed] [Google Scholar]
- 48.Morse, S. A., S. Stein, and J. Hines. 1974. Glucose metabolism in Neisseria gonorrhoeae. J. Bacteriol. 120:702-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naitza, S., and P. Ligoxygakis. 2004. Antimicrobial defences in Drosophila: the story so far. Mol. Immunol. 40:887-896. [DOI] [PubMed] [Google Scholar]
- 50.Nakazato, M., K. Shiomi, Y. Date, S. Matsukura, K. Kangawa, N. Minamino, and H. Matsuo. 1995. Isolation and sequence determination of 6- and 8-kDa precursors of human neutrophil peptides from bone marrow, plasma and peripheral blood neutrophils. Biochem. Biophys. Res. Commun. 211:1053-1062. [DOI] [PubMed] [Google Scholar]
- 51.Ohbayashi, H. 2002. Neutrophil elastase inhibitors as treatment for COPD. Expert Opin. Investig. Drugs 11:965-980. [DOI] [PubMed] [Google Scholar]
- 52.O'Neil, D. A. 2003. Regulation of expression of beta-defensins: endogenous enteric peptide antibiotics. Mol. Immunol. 40:445-450. [DOI] [PubMed] [Google Scholar]
- 53.O'Neil, D. A., E. M. Porter, D. Elewaut, G. M. Anderson, L. Eckmann, T. Ganz, and M. F. Kagnoff. 1999. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163:6718-6724. [PubMed] [Google Scholar]
- 54.Ossovskaya, V. S., and N. W. Bunnett. 2004. Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 84:579-621. [DOI] [PubMed] [Google Scholar]
- 55.Otte, J. M., K. Kiehne, and K. H. Herzig. 2003. Antimicrobial peptides in innate immunity of the human intestine. J. Gastroenterol. 38:717-726. [DOI] [PubMed] [Google Scholar]
- 56.Ouellette, A. J., D. P. Satchell, M. M. Hsieh, S. J. Hagen, and M. E. Selsted. 2000. Characterization of luminal paneth cell alpha-defensins in mouse small intestine. Attenuated antimicrobial activities of peptides with truncated amino termini. J. Biol. Chem. 275:33969-33973. [DOI] [PubMed] [Google Scholar]
- 57.Paju, A., A. Bjartell, W. M. Zhang, S. Nordling, A. Borgstrom, J. Hansson, and U. H. Stenman. 2000. Expression and characterization of trypsinogen produced in the human male genital tract. Am. J. Pathol. 157:2011-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panyutich, A., J. Shi, P. L. Boutz, C. Zhao, and T. Ganz. 1997. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect. Immun. 65:978-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perregaux, D. G., K. Bhavsar, L. Contillo, J. Shi, and C. A. Gabel. 2002. Antimicrobial peptides initiate IL-1 beta posttranslational processing: a novel role beyond innate immunity. J. Immunol. 168:3024-3032. [DOI] [PubMed] [Google Scholar]
- 60.Porter, E. M., L. Liu, A. Oren, P. A. Anton, and T. Ganz. 1997. Localization of human intestinal defensin 5 in Paneth cell granules. Infect. Immun. 65:2389-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Porter, E. M., M. A. Poles, J. S. Lee, J. Naitoh, C. L. Bevins, and T. Ganz. 1998. Isolation of human intestinal defensins from ileal neobladder urine. FEBS Lett. 434:272-276. [DOI] [PubMed] [Google Scholar]
- 62.Price, B., C. Dennison, H. Tschesche, and E. Elliott. 2000. Neutrophil tissue inhibitor of matrix metalloproteinases-1 occurs in novel vesicles that do not fuse with the phagosome. J. Biol. Chem. 275:28308-28315. [DOI] [PubMed] [Google Scholar]
- 63.Quayle, A. J., E. M. Porter, A. A. Nussbaum, Y. M. Wang, C. Brabec, K. P. Yip, and S. C. Mok. 1998. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am. J. Pathol. 152:1247-1258. [PMC free article] [PubMed] [Google Scholar]
- 64.Rao, N. V., N. G. Wehner, B. C. Marshall, W. R. Gray, B. H. Gray, and J. R. Hoidal. 1991. Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J. Biol. Chem. 266:9540-9548. [PubMed] [Google Scholar]
- 65.Reid, P. T., and J. M. Sallenave. 2001. Neutrophil-derived elastases and their inhibitors: potential role in the pathogenesis of lung disease. Curr. Opin. Investig. Drugs 2:59-67. [PubMed] [Google Scholar]
- 66.Rumio, C., D. Besusso, M. Palazzo, S. Selleri, L. Sfondrini, F. Dubini, S. Menard, and A. Balsari. 2004. Degranulation of paneth cells via toll-like receptor 9. Am. J. Pathol. 165:373-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samis, J. A., E. Kam, M. E. Nesheim, and A. R. Giles. 1998. Neutrophil elastase cleavage of human factor IX generates an activated factor IX-like product devoid of coagulant function. Blood 92:1287-1296. [PubMed] [Google Scholar]
- 68.Scocchi, M., B. Skerlavaj, D. Romeo, and R. Gennaro. 1992. Proteolytic cleavage by neutrophil elastase converts inactive storage proforms to antibacterial bactenecins. Eur. J. Biochem. 209:589-595. [DOI] [PubMed] [Google Scholar]
- 69.Shafer, W. M., X. Qu, A. J. Waring, and R. I. Lehrer. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. USA 95:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi, J., and T. Ganz. 1998. The role of protegrins and other elastase-activated polypeptides in the bactericidal properties of porcine inflammatory fluids. Infect. Immun. 66:3611-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sorensen, O. E., L. Gram, A. H. Johnsen, E. Andersson, S. Bangsboll, G. S. Tjabringa, P. S. Hiemstra, J. Malm, A. Egesten, and N. Borregaard. 2003. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: a novel mechanism of generating antimicrobial peptides in vagina. J. Biol. Chem. 278:28540-28546. [DOI] [PubMed] [Google Scholar]
- 72.Stamm, W. E. 1999. Chlamydia trachomatis infections: progress and problems. J. Infect. Dis. 179(Suppl. 2):S380-S383. [DOI] [PubMed] [Google Scholar]
- 73.Tani, K., F. Ogushi, T. Shimizu, and S. Sone. 2001. Protease-induced leukocyte chemotaxis and activation: roles in host defense and inflammation. J. Med. Investig. 48:133-141. [PubMed] [Google Scholar]
- 74.Valore, E. V., and T. Ganz. 1992. Posttranslational processing of defensins in immature human myeloid cells. Blood 79:1538-1544. [PubMed] [Google Scholar]
- 75.Valore, E. V., E. Martin, S. S. Harwig, and T. Ganz. 1996. Intramolecular inhibition of human defensin HNP-1 by its propiece. J. Clin. Investig. 97:1624-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valore, E. V., C. H. Park, A. J. Quayle, K. R. Wiles, P. B. McCray, Jr., and T. Ganz. 1998. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Investig. 101:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Westrom, L. V. 1996. Chlamydia and its effect on reproduction. J. Br. Fertil. Soc. 1:23-30. [PubMed] [Google Scholar]
- 78.Wiesenfeld, H. C., R. P. Heine, M. A. Krohn, S. L. Hillier, A. A. Amortegui, M. Nicolazzo, and R. L. Sweet. 2002. Association between elevated neutrophil defensin levels and endometritis. J. Infect. Dis. 186:792-797. [DOI] [PubMed] [Google Scholar]
- 79.Wu, Z., A. Prahl, R. Powell, B. Ericksen, J. Lubkowski, and W. Lu. 2003. From pro defensins to defensins: synthesis and characterization of human neutrophil pro alpha-defensin-1 and its mature domain. J. Peptide Res. 62:53-62. [DOI] [PubMed] [Google Scholar]
- 80.Xu, S. Y., M. Carlson, A. Engstrom, R. Garcia, C. G. Peterson, and P. Venge. 1994. Purification and characterization of a human neutrophil lipocalin (HNL) from the secondary granules of human neutrophils. Scand. J. Clin. Lab Investig. 54:365-376. [DOI] [PubMed] [Google Scholar]
- 81.Yasin, B., S. S. Harwig, R. I. Lehrer, and E. A. Wagar. 1996. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect. Immun. 64:709-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yount, N. Y., M. S. Wang, J. Yuan, N. Banaiee, A. J. Ouellette, and M. E. Selsted. 1995. Rat neutrophil defensins. Precursor structures and expression during neutrophilic myelopoiesis. J. Immunol. 155:4476-4484. [PubMed] [Google Scholar]