Abstract
Staphylococci can cause a wide spectrum of infections, including endocarditis, osteomyelitis, and sepsis, which is reflected by the numerous virulence factors they produce, among them a recently identified new class of adhesins, namely, the multifunctional autolysins/adhesins. Here we report the identification and molecular characterization of Aaa, a novel autolysin/adhesin from Staphylococcus aureus. The gene encoding Aaa was cloned from the clinical isolate Staphylococcus aureus 4074. DNA sequence analysis revealed that aaa encodes a deduced protein of 334 amino acids with a predicted molecular mass of 35.8 kDa. Aaa contains three N-terminal repetitive sequences that comprise features of a peptidoglycan-binding domain, the LysM domain. The expression of aaa by Escherichia coli and its subsequent characterization revealed that Aaa possesses bacteriolytic activity as well as adhesive properties, such as binding to extracellular matrix proteins. Real-time biomolecular interaction analysis demonstrated that the interaction of Aaa with fibrinogen, fibronectin, and vitronectin is dose dependent and saturable and occurs with a high affinity. Furthermore, we demonstrate that Aaa binds to the Aα and Bβ chains of fragment D of fibrinogen. Immunofluorescence microscopy revealed that Aaa is located at the cell surface. Finally, an aaa knockout mutant showed reduced adherence to surface-adsorbed fibrinogen and fibronectin, strongly suggesting a role for Aaa in the colonization of host factor-coated polymer surfaces and/or host tissue.
Staphylococcus aureus is a major human pathogen responsible for a variety of community-acquired and nosocomial infections, ranging from mild skin diseases, abscesses, and soft tissue diseases to life-threatening infections such as osteomyelitis, endocarditis, pneumonia, and sepsis (37). The adherence of bacteria to host tissue or to implanted medical devices plays a crucial role in the development of disease. The adherence of bacteria to biological or artificial surfaces can occur either directly or via bridging molecules, such as the extracellular matrix and plasma proteins fibrinogen (Fg), fibronectin (Fn), and vitronectin (Vn), and may be followed by proliferation of the bacteria into multilayered cell clusters, leading to the formation of a three-dimensional structure of microorganisms known as a biofilm. Various genes for host factor-binding proteins from S. aureus that typically belong to the MSCRAMM (microbial surface components recognizing adhesive matrix molecules) family (49) have been cloned and sequenced (8, 11, 13, 24, 25, 28, 29, 41, 48, 50). However, the mechanisms leading to colonization are not yet completely understood.
Recently, a new class of staphylococcal adhesins, the autolysins/adhesins, was proposed. Members include AtlE from Staphylococcus epidermidis (20), the homologous Aas protein from Staphylococcus saprophyticus (22), and AtlC from Staphylococcus caprae (1). These surface-associated proteins have both enzymatic (amidase and glucosaminidase) and adhesive functions (1, 17, 20, 22). AtlE is involved in initial attachment of the cells to a polymer surface and in biofilm formation. It also binds to Vn, suggesting a role not only in colonizing polymer surfaces but also in colonizing host factor-coated material and host tissue. Recent results demonstrated the importance of AtlE in S. epidermidis pathogenicity: an atlE mutant strain was significantly less virulent than the wild type in an intravascular catheter-associated infection model in rats (55). Aas and AtlC bind to Fn, and Aas also agglutinates sheep erythrocytes (1, 22). Recently, we identified a novel autolysin with adhesive functions, Aae, from S. epidermidis (21). Here we describe the molecular characterization of Aaa, a homologous autolysin/adhesin from S. aureus. Furthermore, our results demonstrate that Aaa is located at the cell surface, has bacteriolytic activity, and is involved in the adherence of S. aureus to surface-adsorbed Fg and Fn.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The clinical strain S. aureus 4074 (19) was used to clone the aaa gene and to generate the aaa-deficient mutant. The protein A-deficient strain S. aureus Wood 46 (44) was used for immunofluorescence microscopy; the presence of the aaa gene in that strain was confirmed by PCR (see below). Recombinant plasmids prepared in Escherichia coli were passaged in the restriction-negative mutant S. aureus SA113 (27). Cloning hosts were E. coli INVαF′ (Invitrogen, Groningen, The Netherlands) and E. coli TG1.
For cloning of aaa, the vector pCR2.1 (Invitrogen) was used, and for the expression of a six-His-tagged fusion protein, the Qiaexpress vector pQE30 (QIAGEN, Hilden, Germany) was used. The aaa-deficient mutant was constructed by using the plasmids pEC4 and pBT9 (9).
Staphylococci were routinely cultivated in brain heart infusion (BHI) broth or Trypticase soy broth (TSB). E. coli strains were grown in Luria-Bertani (LB) medium. Trypticase soy agar (TSA), BHI agar, and LB agar contained 1.4% agar. Selection for resistance to antibiotics in E. coli was performed with 100 μg/ml ampicillin and 200 μg/ml erythromycin, and selection for that in S. aureus was performed with 10 μg/ml chloramphenicol and 5 μg/ml erythromycin.
DNA manipulations, transformation, PCR, DNA sequencing, and websites.
DNA manipulations and the transformation of E. coli were performed according to standard procedures (56). S. aureus was transformed with plasmid DNA by electroporation (36). Plasmid DNA was isolated using a QIAGEN plasmid kit according to the instructions of the manufacturer (QIAGEN). Chromosomal DNA from S. aureus was isolated according to the procedure of Marmur (40). PCR was carried out with Taq DNA polymerase (Boehringer Mannheim) or with PCR High Fidelity Supermix (Invitrogen) in accordance with the protocol of the supplier. The primers were synthesized by MWG-Biotech (Ebersberg, Germany).
The following primers were used (restriction sites are underlined). For cloning and sequencing of the aaa gene, the primers were primer CH55 (5′-CAG GGA TCC GGA AAG TTA AGC AAG AGG AGG-3′ [BamHI site]) and primer CH57 (5′-GCA CTG CAG CAT TAT ATA TTT ATA TAC GTA AGA C-3′ [PstI site]). For the construction of the six-His-Aaa fusion protein, the primers were primer CH94 (5′-CAG GGA TCC GCT ACA ACT CAC ACA GTA AAA CCG GG-3′ [BamHI site]) and CH57 (see above). For the construction of the aaa-deficient mutant, the primers were AaaKn1 (5′-CAA ATT CAG ATG CAG TAA AGC C-3′ [including nucleotides 517 to 538 upstream of the aaa start codon]), AaaKn2 (5′-GTT TAA GCA CTG TAG GAA CCG-3′ [including nucleotides 1315 to 1335 downstream of the aaa start codon]), AaaKn3 (5′-CAG GTC GAC CCG CAT TTG CTT GAG TTG CCG C-3′ [including nucleotides 55 to 76 of the aaa gene and a SalI site]), and AaaKn4 (5′-GCT AAT AAC TGG GAT AAC GCA GCG G-3′ [including nucleotides 769 to 793 downstream of the aaa start codon]).
To determine the prevalence of aaa, the aaa gene was amplified from genomic DNAs of various S. aureus clinical isolates and S. aureus strains Wood 46 and Mu50 by using the primers CH56 (5′-CAG GGA TCC GTG CAA AAA AAA GTA ATT GCA GC-3′ [BamHI site]) and CH57 (see above).
The PCR-amplified aaa gene was cloned into the vector pCR2.1 by use of a TA cloning kit (Invitrogen) according to the manufacturer's instructions. The DNA sequences of both strands of aaa were determined by the dideoxy chain termination method using a cycle sequencing protocol with a GeneAmp PCR system 2400 (Perkin-Elmer) on an ABI Prism 310 genetic analyzer (Perkin-Elmer) or by MWG-Biotech using a LI-COR DNA sequencer.
The DNA and deduced protein sequences were analyzed using the program PC/GENE (IntelliGenetics). The protein sequences were compared with those of known proteins using the programs BLASTP (2) and FASTA (51). Alignments were done using the program ClustalW from the European Bioinformatics Institute (Cambridge, United Kingdom). Homologous aaa or its promoter sequences were analyzed from S. aureus strains COL (The Institute for Genomic Research [http://www.tigr.org/]), N315 (34), Mu50 (34), MW2 (3), NCTC8325 (26), MSSA476, and MRSA252 (both from The Wellcome Trust Sanger Institute [http://www.sanger.ac.uk/Projects/S_aureus/]). The Pfam accession number of the LysM motif is Pf01476 and is available at http://pfam.wustl.edu and at http://www.sanger.ac.uk/Software/Pfam (4, 31, 54). The signal peptide of Aaa was predicted by using the program SignalP at http://www.cbs.dtu.dk/services/SignalP/.
Construction and purification of six-His-Aaa fusion protein and production of anti-Aaa antiserum.
For the construction of the His-tagged fusion protein, the primers CH94 and CH57 were used to amplify the aaa gene without the nucleotide sequences encoding the signal peptide from the genomic DNA of S. aureus 4074, introducing a BamHI site at the 5′ end and a PstI site at the 3′ end. The PCR-amplified fragment was cloned into the vector pQE30 so that the aaa gene was in frame with the His codons. A representative clone that expressed the aaa gene was designated E. coli(pQAaa). The respective His-tagged fusion protein was purified under denaturing conditions via its His tag using Ni-nitrilotriacetic acid (Ni-NTA) affinity chromatography (Ni-NTA spin kit; QIAGEN) according to the protocol of the supplier. The yield of the fusion protein was in the range of 150 μg per 10 ml culture volume, as determined by the Pierce BCA protein assay (Pierce Europe B.V., BA oud-Beijerland, The Netherlands). For use in Biacore experiments, ligand affinity blot analysis, Western blot analysis, and immunization of rabbits, the Aaa protein solution was dialyzed against 0.1 M phosphate buffer (pH 7.0) to remove urea. The purified Aaa protein was used to immunize rabbits, which was performed by Eurogentec (Belgium) according to their standard immunization program.
Protein isolation, SDS-PAGE, detection of bacteriolytic activity, and Western blot and ligand affinity blot analysis.
Surface-associated proteins of S. aureus 4074 and the aaa mutant were prepared from cultures that were grown overnight in BHI broth at 37°C. The cells were harvested by centrifugation, and the cell pellet was resuspended in 1 volume of Laemmli (SDS) sample buffer and then heated for 5 min at 95°C. Crude cell lysates of E. coli(pQAaa) were prepared from noninduced and induced (by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside [IPTG] and continued growth for 5 h) cultures by harvesting the cells, resuspending the cell pellet in sample buffer, and heating the suspension for 5 min at 95°C. Additionally, as a negative control, a crude cell lysate was prepared from an induced culture of E. coli(pQE30). After centrifugation, 10 μl of supernatant containing SDS-soluble, surface-associated staphylococcal proteins, 10 μl of cell lysate from E. coli, or 4 μl of the purified protein (containing 1.5 μg) was subjected to SDS-PAGE (10% or 12.5% separation gel and 4.5% stacking gel). Proteins were stained with Coomassie brilliant blue R250 (0.1%).
Zymographic analysis of the bacteriolytic activities of protein preparations was performed as described earlier (20). Briefly, surface-associated proteins or crude cell lysates were isolated as described above and separated by SDS-PAGE on a polyacrylamide gel containing heat-inactivated staphylococcal cells (0.05%) as a substrate in the separation gel (12.5%). After electrophoresis, the gels were washed and then incubated in phosphate buffer at 37°C. Bands with bacteriolytic activity were observed as clear zones in the opaque gel. After photography against a dark background, the clear zones appeared as dark bands.
For Western blot analysis, surface-associated proteins were prepared and separated by SDS-PAGE as described above and then transferred to a nitrocellulose membrane (Schleicher & Schuell). The membranes were then blocked in Tris-buffered saline (TBS)-5% bovine serum albumin (BSA) (overnight) and washed three times with TBS-0.05% Tween 20 (TBST). Afterwards, the nitrocellulose membranes were incubated for 2.5 h with the anti-Aaa antiserum diluted 1:2,000 in TBST-1% normal goat serum (NGS) (Dako Cytomation GmbH, Hamburg, Germany). As a negative control, incubation was performed in TBST-1% NGS with (1:2,000) or without preimmune serum. The reaction of proteins with specific antibodies was detected by incubation (1.5 h) with an anti-rabbit immunoglobulin G (IgG)-alkaline phosphatase (AP) conjugate (Dako) diluted 1:2,000 in TBST-1% NGS and by the subsequent color reaction.
To determine the binding domains of Fg for Aaa, we performed ligand affinity blot analysis. For this, the disulfide bonds between the Fg chains (Aα, Bβ, and γ chains) were reduced by heating Fg (Calbiochem, Darmstadt, Germany) in sample buffer (Bio-Rad). Additionally, fragments D (85-kDa protein band under nonreducing conditions; Calbiochem) and E (50-kDa protein band under nonreducing conditions; Calbiochem) of human Fg were tested. The Fg chains (5 μg per lane) or Fg fragments D and E under nonreducing conditions (5 μg per lane) were separated by SDS-PAGE and electrotransferred to a nitrocellulose membrane. Afterwards, the membranes were blocked in TBST-5% BSA and then incubated with 2 μg/ml purified Aaa in TBST-1% BSA. As a negative control, incubation was performed in TBST-1% BSA without a ligand or with 2 μg/ml six-His-dihydrofolate reductase (purified from a culture of E. coli harboring the control plasmid pQE40 [QIAGEN]) in TBST-1% BSA. Bound proteins were detected by incubation with a Ni-NTA-AP conjugate (QIAGEN) diluted 1:3,500 in TBST-1% BSA and by the subsequent color reaction (Bio-Rad).
Real-time BIA for quantification of molecular interactions.
Experiments were performed on a BIAcore 2000 instrument according to the general procedures recommended by the manufacturer. Aaa was immobilized on the sensor chip C1 to analyze its interaction with the extracellular matrix proteins Fg, Fn, and Vn. For immobilization, Aaa was covalently coupled to the sensor chip surface C1 via primary amine groups. To prepare the surface of the sensor chip, a freshly prepared 0.1 M glycine-NaOH (pH 12), 0.3% Triton X-100 solution was injected at a flow rate of 20 μl/min (twice, with 30 μl each time). Afterwards, to activate the sensor chip, a mixture of 50 μl NHS-EDC [0.1 M N-hydroxysuccinimide (NHS) and N-ethyl-N′-(3-dimethylaminopropyl)-carbodiimide (EDC)] (amine coupling kit; Biacore AB) was injected at a flow rate of 5 μl/min. The autolysin Aaa (approximately 8 μg/ml) in 10 mM sodium acetate buffer (pH 5.0) was immobilized on the surface. Remaining NHS-ester groups on the sensor chip surface were then blocked by the injection of 55 μl of 1 M ethanolamine (pH 8.5) (amine coupling kit; Biacore AB). Nonspecific binding of the extracellular matrix proteins to the sensor surface was avoided by coating the surface with 0.2 mg/ml BSA in HBS buffer (10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, 0.005% surfactant, pH 7.4) at a flow rate of 5 μl/min. HBS buffer was also used as the running buffer. After Aaa was immobilized on the C1 sensor chip surface (211 pg Aaa/0.26 mm2), extracellular matrix proteins in increasing concentrations were injected over the sensor surface for the kinetic studies at a flow rate of 30 μl/min. The binding of extracellular matrix proteins was monitored and presented in an overlay plot of the sensorgrams (a plot of resonance units versus time). Kinetic data were determined at 25°C. For some experiments, Fg was preincubated with different concentrations of peptides (HHLGGAKQAGDV, GRGDSP [both from Bachem AG, Weil am Rhein, Germany] and FYQVLNMPNLNA [synthesized by Eurogentec]) before injection over the sensor surface. For regeneration of the sensor chip surface after each injection, 100 mM glycine (pH 10.5) was used, resulting in a return to baseline. Kinetic data were analyzed using biomolecular interaction analysis (BIA) evaluation software (version 3.1) from Biacore AB as described previously (32).
Immunofluorescence microscopy.
Immunofluorescence microscopy was performed essentially as described before (20). Briefly, an aliquot (500 μl) of a culture of S. aureus strain Wood 46, which is deficient in the IgG-binding protein A, was washed in phosphate-buffered saline (PBS; 500 μl) and then incubated with 500 μl of rabbit anti-Aaa antibodies or preimmune serum diluted 1:500 in PBS for 2 h at 37°C. Afterwards, the cells were washed three times with PBS, and bound rabbit antibodies were detected by incubation with 500 μl of fluorescein-conjugated anti-rabbit IgG (Sigma) diluted 1:500 in PBS for 1 h at 37°C. Thereafter, cells were washed three times with PBS and resuspended in 200 μl of distilled water. Aliquots (15 μl) were applied to slides, air dried, and viewed with a fluorescence microscope (Zeiss, Oberkochen, Germany).
Construction of an aaa-deficient mutant in S. aureus by gene replacement (aaa::Eryr). (i) Cloning of the aaa gene.
The aaa gene was amplified from the chromosomal DNA of S. aureus 4074 by PCR with primers AaaKn1 and AaaKn2, yielding a 1,873-bp DNA fragment. The amplified DNA fragment was cloned into the vector pCR2.1, creating plasmid pA2.
(ii) Insertion of the ermB gene.
Plasmid pA2 was amplified by inverse PCR using primers AaaKn3 and AaaKn4, which deleted an internal 693-bp fragment of the aaa gene, yielding a 5.1-kb DNA fragment with a SalI restriction site at the 5′ end and a blunt end at the 3′ end. The deleted fragment was then replaced by the 1.4-kb erythromycin cassette ermB taken from plasmid pEC4, creating the 6.5-kb plasmid pA210. The aaa-ermB-aaa fragment was isolated from plasmid pA210 as a 2.6-kb SstI and XbaI fragment that was then ligated to the temperature-sensitive shuttle vector pBT9. E. coli was transformed with the ligation mixture, and the resulting plasmid was designated pBA3.
(iii) Insertional inactivation of aaa in S. aureus 4074.
Plasmid pBA3 was propagated in strain SA113 and then transferred to S. aureus 4074 by electroporation. For gene disruption experiments, one transformant was cultivated in TSB with chloramphenicol (10 μg/ml) at 30°C overnight. The culture was diluted 1:1,000 in fresh medium containing erythromycin (5 μg/ml) and grown at 44°C. After an identical second culture, a 1:1,000 dilution was plated onto TSA containing erythromycin (5 μg/ml) and incubated at 44°C. Resulting colonies were checked for resistance to chloramphenicol. Successful recombination in erythromycin-resistant and chloramphenicol-sensitive colonies was verified by PCR.
Adherence of S. aureus 4074 and its aaa mutant to Fg- and Fn-coated coverslips.
To quantify the capacity of strains to adhere to surface-adsorbed Fg or Fn, we performed a radiometric adherence assay essentially as described before (15, 62). Briefly, 50-μl quantities of a solution of Fn or Fg (200 μg/ml) were adsorbed onto polymethylmethacrylate coverslips (60 min at 37°C). As a negative control, binding to BSA (5 mg/ml) was estimated. Coverslips were then washed three times with PBS and transferred into tubes. For radiolabeling, bacteria were grown for 4 h in Mueller-Hinton broth supplemented with [3H]thymidine as described previously (30) and then washed three times. Thereafter, coverslips were incubated with 1 ml of Ca2+-Mg2+-PBS containing labeled staphylococci (4 × 107 CFU) and 0.5% BSA in a stirred water bath (60 min at 37°C). Afterwards, unbound bacteria were removed from coverslips by washing three times with PBS, and adherent radioactivity was determined.
Statistical analysis.
Data are given as means ± standard errors of the means. Statistical analysis was performed using the Wilcoxon matched-pair signed rank test for nonparametric data. P values of <0.05 were considered to indicate statistically significant differences.
Nucleotide sequence accession number.
The EMBL/GenBank/DDBJ accession number of the aaa DNA sequence is AJ250906.
RESULTS
Identification of the autolysin/adhesin Aaa in S. aureus and cloning of the aaa gene.
Recently, we identified a novel autolysin with adhesive properties in S. epidermidis (21). We found that S. aureus produces a homologous protein, which we termed Aaa (autolysin/adhesin from S. aureus), and we sought to characterize its function in more detail. We amplified a DNA fragment containing the aaa gene by PCR from S. aureus 4074 genomic DNA, yielding a 1,060-bp DNA fragment. The DNA fragment was cloned into the vector pCR2.1 in E. coli, creating plasmid pCAaa.
Nucleotide sequence of aaa and amino acid sequence analysis of the deduced protein.
The nucleotide sequence of the cloned aaa gene was determined for both strands. aaa consists of 1,002 nucleotides and encodes a deduced protein of 334 amino acids (aa) with a predicted molecular mass of 35.8 kDa. It starts with the start codon GTG and is preceded by a putative ribosome-binding site at a distance of 8 bp. Putative −10 (CATAAT; nucleotides −79 to −84) and −35 (ATGTCA; nucleotides −110 to −104) promoter sequences were deduced from homologous DNA sequences from strain S. aureus Col. Aaa contains a putative signal peptide in the first 25 aa. The predicted aaa gene product is composed of 32.4% hydrophobic, 7.5% basic, and 3.3% acidic aa. The theoretical pI value of Aaa is 9.67. The aa sequence of Aaa is 100% identical to those of the putative homologous proteins from S. aureus strains COL, N315 (34), MW2 (3), NCTC8325 (26), and MSSA476 and shares 97.9% identical aa with Aaa from strain MRSA252. The aaa gene of strain Mu50 (34) encodes a putative protein with only 267 aa. Its predicted aa sequence is 100% identical to the aa sequence of Aaa from strain 4074 except for a 67-aa deletion from aa 90 to aa 157.
A sequence comparison of Aaa with known proteins in databases revealed two domains. The N-terminal domain of Aaa contains three direct repeated sequences. The repeats Aaa-2 (66 aa, starting at Y-93) and Aaa-3 (65 aa, starting at Y-160) are 65% identical, and the repeat Aaa-1 (56 aa, starting at H-29) shares only 32% identical aa with Aaa-3. The Aaa repeats are highly homologous to a consensus sequence that has been termed the LysM domain (for lysin motif) (Fig. 1A). The LysM domain is a part of various cell wall lytic enzymes (4, 31, 54). The N-terminal Aaa repeats are homologous to those of other autolysins that harbor the LysM domain, including the 35-kDa autolysin/adhesin Aae from S. epidermidis (three repeats, 71% identity) (21), the 51-kDa cell wall-associated endopeptidase LytF from Bacillus subtilis (five repeats, 42% identity) (38), the putative 35-kDa endopeptidase LytE from B. subtilis (three repeats, 42% identity) (39), the 70-kDa N-acetylmuramoyl-l-alanine amidase from Enterococcus faecalis (five repeats, 37% identity) (6), and the 50-kDa invasion-associated protein p60 of Listeria species (three repeats, 35% identity) (10) (Fig. 1A). The C-terminal domain of Aaa (starting at S-225) shows the highest similarity to the 35-kDa autolysin/adhesin Aae from S. epidermidis (87% identical aa) (21), the secretory, highly antigenic, in vivo-expressed protein SsaA from S. epidermidis (56% identical aa) (35), ORF1 from S. aureus (55% identical aa) (K. Wieland and F. Götz, unpublished), and the extracellular protein SceB from Staphylococcus carnosus (53.6% identical aa) (B. Krismer, J. Montenbruck, and F. Götz, unpublished) (Fig. 1B). The functions of the last three proteins remain unknown.
FIG. 1.
(A) Alignment of the deduced amino acid sequences of the repetitive sequences of the autolysin/adhesin Aaa (334 aa) from S. aureus 4074 (Aaa-1 to Aaa-3), Aae (324 aa) from S. epidermidis (Aae-1 to Aae-3), LytE (334 aa) from B. subtilis (LytE-1 to LytE-3), LytF (488 aa) from B. subtilis (LytF-1 to LytF-5), p60 (524 aa) from Listeria ivanovii (P60-1 to P60-3), and N-acetylmuramoyl-l-alanine amidase (641 aa) from E. faecalis (Am-1 to Am-5) (only the first 44 amino acids of the repeats are shown). The consensus sequence indicates the LysM domain that is proposed to have a general peptidoglycan-binding function. Bold letters indicate amino acids that match the consensus sequence. The letters in the second and third line in the consensus sequence show alternative amino acids. (B) Alignment of the deduced C-terminal amino acid sequences of the autolysin/adhesin Aaa from S. aureus 4074, Aae from S. epidermidis (324 aa), the secretory antigen SsaA (257 aa) from S. epidermidis, ORF1 (255 aa) from S. aureus (EMBL database accession number X97985), and the extracellular protein SceB (263 aa) from S. carnosus (EMBL database accession number U96107). Asterisks indicate identical amino acids, colons indicate very similar amino acids, and dots indicate somewhat similar amino acids. Gaps (dashes) were filled in to maximize homologies.
Expression and purification of six-His-Aaa in E. coli.
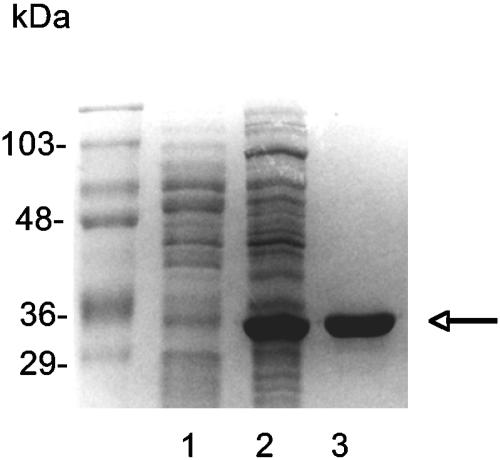
For expression of the aaa gene in E. coli, the PCR-amplified fragment was cloned into the expression vector pQE30. A representative clone expressing the aaa gene contained the plasmid pQAaa. Subsequently, Aaa was purified from E. coli(pQAaa) via its His tag using Ni-NTA affinity chromatography. SDS-PAGE of the affinity-purified fusion protein revealed an approximately 36-kDa protein. A protein with the same size was present in a cell lysate of an induced culture of E. coli(pQAaa) and was absent from cell lysates of an induced culture of E. coli(pQE30) (not shown) and a noninduced culture of E. coli(pQAaa) (Fig. 2).
FIG. 2.
Expression and purification of six-His-Aaa. The image shows an SDS-PAGE gel (12.5% separation gel) with crude cell lysates (10 μl) of noninduced E. coli(pQAaa) (lane 1), IPTG-induced E. coli(pQAaa) (lane 2), and six-His-Aaa (1.5 μg) purified from E. coli(pQAaa) (lane 3). The arrow indicates the 35.8-kDa His-tagged Aaa protein purified from E. coli(pQAaa). The same protein band was absent from crude extracts of E. coli(pQAaa) before induction. Marker proteins are shown on the left.
Bacteriolytic activity of Aaa.
To analyze the bacteriolytic activity of Aaa, the His-tagged Aaa protein as well as cell surface-associated S. aureus proteins and cell lysates of E. coli(pQAaa) and E. coli(pQE30) was investigated using zymographic analysis. SDS-PAGE was performed with a separation gel containing heat-inactivated S. carnosus, S. aureus, or Micrococcus luteus cells as a substrate for lytic enzymes. Aaa extracted from the S. aureus surface as well as His-tagged Aaa produced by E. coli exhibited bacteriolytic activity when S. carnosus cells (Fig. 3) and S. aureus cells (not shown) were used as the substrate. Smaller proteins than the 35.8-kDa Aaa protein that showed bacteriolytic activity presumably represented degradation products of Aaa (Fig. 3). No bacteriolytic activity was detected with cell lysates from noninduced cultures of E. coli(pQAaa) (Fig. 3) or induced cultures of E. coli(pQE30) (not shown). No Aaa-associated bacteriolytic activity was detected when M. luteus cells were used as the substrate in the separation gel (not shown).
FIG. 3.
Zymographic analysis of bacteriolytic activity of Aaa. The image shows an SDS-PAGE gel of crude cell lysates (10 μl) of noninduced E. coli(pQAaa) (lane 1), IPTG-induced E. coli (pQAaa) (lane 2), six-His-Aaa (1.5 μg) purified from E. coli(pQAaa) (lane 3), and cell surface-associated proteins (10 μl) of S. aureus 4074 (lane 4). The separation gel (12.5%) contained heat-inactivated S. carnosus cells (0.05%) as a substrate for bacteriolytic enzymes. Bacteriolytic activity is visible as a clear zone after incubation in phosphate buffer. The arrows indicate Aaa-associated bacteriolytic activity.
Quantification of Fg-, Fn-, and Vn-binding activity of Aaa by real-time BIA.
Biomolecular interaction analysis (BIA), based on the optical detection of surface plasmon resonance, was performed to study the adhesive properties of the autolysin Aaa. In BIA, interactions are analyzed between a ligand immobilized on a sensor chip and an analyte, which is in solution and flows over the surface of the chip. The use of this system allowed the study of the interaction of the autolysin Aaa (ligand) with the extracellular matrix proteins Fg, Fn, and Vn (analytes) without the need to label any of the proteins. After Aaa was immobilized on the C1 chip, various concentrations of Fg (concentrations ranging from 100 nM to 1,200 nM), Fn (concentrations ranging from 200 nM to 1,600 nM), or Vn (concentrations ranging from 500 nM to 1,300 nM) were sequentially injected over the immobilized protein for kinetic studies. The sensorgrams of the binding of the matrix proteins to Aaa were monitored as shown in Fig. 4. The interactions of Aaa with Fg, Fn, and Vn were dose dependent and saturable (Fig. 4A to C). In control experiments, Aaa did not bind to BSA (Fig. 4D). From the quantitative data on the kinetics of these interactions, the dissociation constants (KD) were determined to be 12.3 nM for the interaction of Aaa with Fg, 22.1 nM for the interaction of Aaa with Vn, and 29.9 nM for the interaction of Aaa with Fn. To further characterize the binding regions, competition experiments were performed. For these experiments, the peptides HHLGGAKQAGDV and GRGDSP, which represent different binding sites in Fg for the platelet receptor GPIIb/IIIa (16, 33), and FYQVLNMPNLNA, which represents the ligand-binding site in protein A (unpublished results), were used. In a previous study, we found that protein A binds to von Willebrand factor (15) and also to Fg (unpublished results). We found that the peptide FYQVLNMPNLNA dose-dependently inhibited the interaction of Fg with Aaa (Fig. 4G). In contrast, the peptides HHLGGAKQAGDV (Fig. 4E) and GRGDSP (Fig. 4F) had no effect.
FIG. 4.
Determination of binding of Fg (A, E, F, and G), Fn (B), Vn (C), and BSA (D) to the immobilized autolysin/adhesin Aaa using the BIAcore system. After Aaa was immobilized on the C1 chip surface, Fg, Fn, Vn, and BSA were injected over the chip surface at a flow rate of 30 μl/min. The binding of Fg, Fn, Vn, and BSA was monitored and presented in overlay plots of the sensorgrams (plots of resonance units [RU] versus time). (A) Concentrations of Fg (from bottom to top): 100 nM, 200 nM, 300 nM, 400 nM, 500 nM, 700 nM, 800 nM, and 1,200 nM; (B) concentrations of Fn (from bottom to top): 200 nM, 300 nM, 400 nM, 700 nM, 1,200 nM, and 1,600 nM; (C) concentrations of Vn (from bottom to top): 500 nM, 600 nM, 700 nM, 800 nM, 900 nM, 1,000 nM, 1,100 nM, 1,200 nM, and 1,300 nM; (D) concentrations of BSA (from bottom to top): 0 nM, 100 nM, 500 nM, 1,000 nM, 2,000 nM, 5 μM, and 10 μM; (E) concentrations of Fg and peptide HHLGGAKQAGDV (from bottom to top): 0 nM Fg and 1,000 nM peptide, 100 nM Fg and 1,000 nM peptide, and 100 nM Fg and 0 nM peptide; (F) concentrations of Fg and peptide GRGDSP (from bottom to top): 0 nM Fg and 900 nM peptide, 100 nM Fg and 900 nM peptide, 100 nM Fg and 750 nM peptide, 100 nM Fg and 500 nM peptide, 100 nM Fg and 250 nM peptide, 100 nM Fg and 100 nM peptide, and 100 nM Fg and 0 nM peptide; (G) concentrations of Fg and peptide FYQVLNMPNLNA (from bottom to top): 0 nM Fg and 900 nM peptide, 100 nM Fg and 900 nM peptide, 100 nM Fg and 500 nM peptide, 100 nM Fg and 250 nM peptide, 100 nM Fg and 100 nM peptide, 100 nM Fg and 50 nM peptide, and 100 nM Fg and 0 nM peptide.
Surface location of Aaa.
To detect the surface location of Aaa, an antiserum against Aaa that was raised in rabbits was used for immunofluorescence microscopy. The anti-Aaa antiserum strongly reacted with cells of the protein A-deficient strain S. aureus Wood 46, indicating the cell surface location of Aaa (Fig. 5A). Much less immunofluorescence was detected with the preimmune serum (Fig. 5B). However, there was some immunofluorescence detectable with the preimmune serum, which may be due to the IgG-binding and surface-associated protein Eap (14), which also seemed to react with the anti-Aaa antiserum and the preimmune serum in Western blot analysis (Fig. 6).
FIG. 5.
Detection of Aaa at the cell surface by immunofluorescence microscopy. Cells of the protein A-deficient strain S. aureus Wood 46 grown overnight in BHI broth were incubated with the anti-Aaa antiserum raised in rabbits (A) or with preimmune serum (B). Bound antibodies were detected with fluorescein-conjugated anti-rabbit IgG antibodies. Cells were viewed with a fluorescence microscope. Wood 46 cells reacted with the anti-Aaa antiserum, indicating the cell surface location of Aaa. In comparison, the reaction of the cells with the preimmune serum was much weaker. Magnification, ×1,000.
FIG. 6.
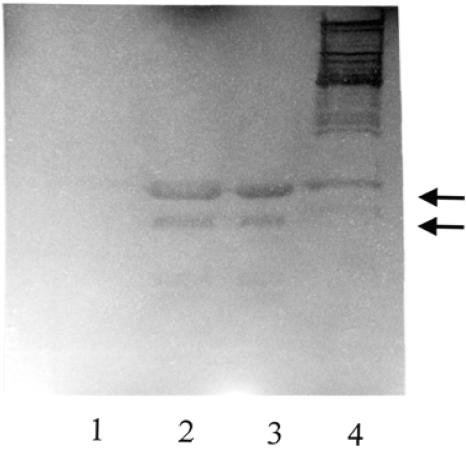
Surface-associated proteins (10 μl) from S. aureus 4074 (lanes 1) and the aaa mutant (lanes 2) were separated by SDS-PAGE (10% separation gel), stained with Coomassie brilliant blue (A), and subjected to zymographic analysis (B) or Western blot analysis (C). A 35.8-kDa protein with bacteriolytic activity (B) that reacted with the anti-Aaa antiserum (C) was missing from the aaa mutant (indicated by an arrow). The sizes of the marker proteins are indicated on the left (A; Bio-Rad prestained marker [M]) and the right (C; MBI Fermentas prestained marker [M]).
Characterization of aaa-deficient mutant.
To analyze the function of Aaa in the context of the bacterial cell, we constructed an aaa-deficient mutant from S. aureus 4074 by gene replacement and compared it to its wild type.
(i) Confirmation of the aaa mutant.
To verify that the 35.8-kDa autolysin Aaa was missing from protein preparations of the aaa mutant, surface-associated proteins were analyzed by zymographic analysis and Western blot analysis (Fig. 6). Zymographic analysis confirmed that a 35.8-kDa protein with bacteriolytic activity was present in the S. aureus wild type but missing from the aaa mutant (Fig. 6B). The larger protein bands with bacteriolytic activity presumably represent the products of proteolytic cleavage from the Atl precursor molecule. In Western blot analysis, a 35.8-kDa protein that reacted with the anti-Aaa antiserum was detected in protein preparations of the wild-type strain but was missing from protein preparations of the aaa mutant (Fig. 6C). In control experiments, no specific binding of Aaa to the preimmune serum or to the anti-digoxigenin-AP conjugate was observed (not shown). A larger and a smaller protein that reacted with the anti-Aaa antiserum and also with the preimmune serum (not shown) may represent Eap (also referred to as Map or p70) and its degradation products, respectively, which were reported to have an IgG-binding function (14). Like Aaa, Eap does not seem to be covalently linked to the cell surface and thus can be extracted from the cell surface by SDS (25).
(ii) Colony morphology.
Previously, we found that the colony morphology of the autolysin mutant S. epidermidis atlE differed from that of its wild type (18): the atlE mutant formed rough and dull colonies instead of shiny colonies like the wild type. In contrast, there was no difference in colony morphology phenotypes on TSA between the aaa mutant and its wild type, strain 4074 (not shown).
(iii) Growth in liquid culture and cell aggregation.
Autolysins are involved in cell division and cell separation. Therefore, we compared the growth of the aaa mutant in liquid culture with that of its wild type. The aaa mutant revealed the same growth curve in TSB as the wild type, and both strains reached the same end OD578 (data not shown). Cell aggregation is indicative of a loss of an enzyme involved in cell separation (20, 61, 64). To clarify whether Aaa is involved in cell separation, we observed liquid cultures of the aaa mutant and its wild type in TSB by using phase-contrast microscopy and could not detect any difference in cell cluster formation. With both strains, there were mainly single cells, pairs, and tetrads visible (not shown).
(iv) Adherence to surface-adsorbed Fg and Fn.
To examine whether Aaa is involved in the adherence of S. aureus cells to Fg- or Fn-coated surfaces, we performed a radiometric adherence assay. Compared to that of the S. aureus wild type, the adherence of the aaa mutant to Fg and Fn was reduced approximately 50%, which was statistically significant (Fig. 7). With both strains, binding to Fg was more pronounced than binding to Fn. The adherence of the strains to BSA was comparable and very low.
FIG. 7.
Adherence of S. aureus 4074 and its aaa mutant to immobilized Fg and Fn adsorbed to polymethylmethacrylate coverslips. [3H]thymidine-labeled S. aureus 4074 or S. aureus aaa (4 × 107 CFU/ml) was incubated with coverslips that were preadsorbed with Fg, Fn, or BSA. Afterwards, the bacterial suspension was removed, the coverslips were washed, the adherent radioactivity was determined, and the CFU were calculated. Experiments were performed three times in quintuplicate. Results are means ± standard errors of the means. Statistical analysis was performed using the Wilcoxon matched-pair signed rank test for nonparametric data. P values of <0.05 were considered to indicate statistically significant differences. *, P < 0.05 for comparison with wild-type strain S. aureus 4074. Black bars, S. aureus 4074; gray bars, aaa mutant.
Identification of Aaa-binding domains of Fg and Fn by ligand affinity blot analysis.
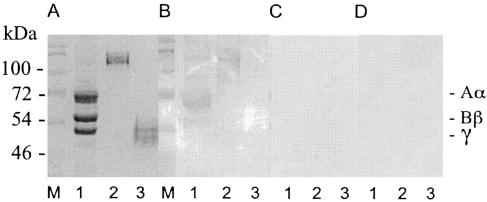
Each Fg molecule consists of three pairs of nonidentical polypeptide chains (AαBβγ) that are arranged into three globular domains, one central domain (E), and two distal domains (D), which are created after digestion with plasmin. To identify the binding domains for Aaa on Fg, the Aα, Bβ, and γ chains were analyzed in a ligand affinity blot using six-His-Aaa as a probe followed by a Ni-NTA-AP conjugate. Aaa bound to the Aα chains and, to a lesser extent, to the Bβ chains (Fig. 8B). Furthermore, fragments D and E of human Fg (nonreduced) were analyzed in a ligand affinity blot. Aaa bound to the D fragment but not to the E fragment (Fig. 8B). In the negative controls, no significant binding of the Ni-NTA-AP conjugate was detected (Fig. 8C and D). To identify the binding domain of Fn for Aaa, human Fn was digested with the protease thermolysin, which produces a 29-kDa N-terminal Fn fragment and three larger polypeptide fragments (46). In ligand affinity blot analysis, Aaa was detected binding to the 29-kDa Fn fragment (not shown).
FIG. 8.
Determination of binding domains of Fg for Aaa by ligand affinity blot analysis. Fragments D (lanes 2) and E (lanes 3) of Fg (nonreduced) and the Fg chains (Aα, Bβ, and γ chains [reduced]) (lanes 1) were separated by SDS-PAGE (10% separation gel) (A) and transferred to nitrocellulose filters that were probed with six-His-Aaa (B) or six-His-dihydrofolate reductase (C) or without a ligand (D), followed by a Ni-NTA-alkaline phosphatase conjugate. The sizes of the marker proteins (MBI Fermentas prestained marker [M]) and the positions of the Aα, Bβ, and γ chains of Fg are indicated.
Prevalence of aaa.
To determine the prevalence of the aaa gene among clinical S. aureus isolates, we performed a PCR analysis using primers CH56 and CH57. An approximately 1-kb fragment representing the aaa gene was found to be present in all 30 clinical S. aureus strains tested as well as in strain Wood 46 (not shown). This indicates a very high prevalence of the aaa gene among clinical S. aureus isolates. PCR with the chromosomal DNA of strain Mu50 revealed a 0.8-kb fragment, which reflects the deletion that we identified within the aaa gene in the genome of Mu50 (see above; not shown).
DISCUSSION
Recently, we reported the identification and cloning of an autolysin with adhesive properties, Aae, from S. epidermidis (21). Here we describe the molecular characterization of Aaa, a multifunctional autolysin/adhesin from S. aureus that is 76% identical to Aae. Furthermore, this study deals with the construction and characterization of an aaa-negative mutant. Generally, bacterial cell wall lytic enzymes or autolysins are peptidoglycan hydrolases with multiple functions. They are not only involved in cellular processes, such as cell division, cell separation, and cell wall turnover (for a review on Bacillus subtilis autolysins, see reference 59), but are also thought to be involved in pathogenesis. In vivo studies have shown that some autolysis-defective mutants are less virulent than their wild-type strains, including an atlE mutant of S. epidermidis (55), an iap-negative mutant of Listeria monocytogenes (53), an ami mutant of L. monocytogenes (42), and a lytA mutant of Streptococcus pneumoniae (7). The 60-kDa invasion-associated protein p60 (Iap) from L. monocytogenes seems to be involved in adherence to and invasion of eukaryotic cells, and an iap mutant is strongly impaired in the ability to spread between host cells (47, 53). Another autolysin from L. monocytogenes, Ami, also seems to function in adherence to eucaryotic cells (43). The eucaryotic cell-binding activity of Ami has been located to the cell wall-anchoring domain (42). This domain consists of GW-containing repeats. Such GW repeats are also present within the repeat regions of the homologous autolysins Atl from S. aureus, AtlE from S. epidermidis, and Aas from S. saprophyticus (23, 42). Like these major staphylococcal autolysins, Aaa contains three repetitive sequences, but they are not homologous to the GW repeats. Instead, they are highly homologous to a consensus sequence that is part of several peptidoglycan hydrolases, namely, the LysM (lysin motif) domain (Fig. 1). Recently, the LysM domain of the major autolysin AcmA of Lactococcus lactis has been demonstrated to bind to peptidoglycan (60). The peptidoglycan-binding function of the LysM motif in Aaa might also be responsible for its surface-associated location, because Aaa does not have a C-terminal anchor region with the LPXTG motif that is typical for surface proteins of gram-positive bacteria (57). The LysM domain has also been found in proteins that are not involved in cell wall metabolism but that have been associated with adherence and pathogenesis, such as the elastin-binding protein EbpS of S. aureus (one copy of the LysM domain located in the C-terminal portion) (12), the immunoglobulin-binding protein A of S. aureus (one copy of the LysM domain located near the C terminus) (58), and intimin, which is an outer membrane protein required for the attachment of enteropathogenic E. coli to mammalian cells (one copy of the LysM domain located near the N terminus) (5). The function of the LysM domain in protein A and EbpS is not known. However, a function in anchoring these proteins to the cell surface seems unlikely, because protein A has a C-terminal anchor region with the LPXTG motif (57) and EbpS is an integral membrane protein (12).
Aaa was found to bind to the D fragment of Fg and, more precisely, to the Aα and Bβ chains of the protein. These chains are also recognized by other staphylococcal Fg-binding proteins, such as Aae from S. epidermidis (21), Efb from S. aureus, which binds to the Aα chains (45), and Fbe from S. epidermidis, which binds to the Bβ chains (52). Using BIA, the interactions between Aaa and extracellular matrix proteins were found to be dose dependent and saturable. The resulting KD values were determined to be between 12.3 nM for the interaction of Aaa with Fg and 29.9 nM for the interaction of Aaa with Fn. Thus, our results indicate a very high affinity of the autolysin/adhesin Aaa for extracellular matrix proteins. Competition experiments revealed that the interaction of Fg with Aaa was inhibited by the peptide FYQVLNMPNLNA in a dose-dependent manner, confirming the specificity of the interaction between Fg and Aaa. This peptide represents a portion of protein A that we identified as a ligand-binding site (unpublished results). Therefore, we concluded that protein A and Aaa bind to the same site within Fg, which seems to be independent of the binding sites (HHLGGAKQAGDV and RGD) in Fg for the platelet receptor GPIIb/IIIa.
Aaa exhibits lytic activity against S. carnosus and S. aureus (not shown) cells but not against M. luteus cells (not shown). Thus, it is likely that Aaa does not possess amidase and glucosaminidase activities, both of which cleave the peptidoglycans of staphylococcal as well as M. luteus cells. Aaa might have an endopeptidase activity that cleaves the amide bonds in the peptide cross-links. Further analyses are necessary to identify the enzymatic activity of Aaa.
To gain a better understanding of the function of Aaa, we constructed an aaa-deficient mutant. The aaa mutant did not differ from the wild type in its colony morphology, growth rate, and cell cluster formation, suggesting that Aaa does not play a role in cell separation or, more probably, that the function of Aaa in cell separation may have been taken over by other autolysins, among them the major autolysin Atl, which seemed to be more strongly expressed in the aaa mutant than in the wild type (Fig. 6B). Analogous to the recent finding that an agr mutation in S. epidermidis led to increased biofilm formation due to an increased expression of atlE (63), the increased expression of atl might also be responsible for the observed, slightly more pronounced capacity (approximately 1.7-fold) of the aaa mutant for biofilm formation than that of the S. aureus wild type (data not shown).
Immunofluorescence microscopy indicated that Aaa is located at the cell surface, which is the prerequisite for acting as an adhesin. Compared with the S. aureus wild type, the aaa mutant revealed an approximately 50% reduced adherence to surface-adsorbed Fg and Fn. This suggests that among several other S. aureus factors, which typically belong to the MSCRAMM (microbial surface components recognizing adhesive matrix molecules) adhesin family (49), Aaa might be involved in the colonization of host factor-coated material or host tissue by S. aureus. The surface location of Aaa and the high prevalence of the aaa gene among clinical S. aureus isolates indicate its possible suitability as a vaccine candidate. Analyses of the aaa-negative mutant in animal models are warranted to determine the role that Aaa plays in staphylococcal pathogenicity.
Acknowledgments
We thank S. Weber and K. Schörmann for excellent technical assistance and B. Specht (Inventus BioTec, Münster, Germany) for performing the BIA. R. Brückner is acknowledged for providing plasmids pEC4 and pBT9. Sequence data for identification and cloning of the aaa gene were obtained from the Institute for Genomic Research website (http://www.tigr.org).
This work was supported by the German National Research Foundation for Specialized Research Center no. 293, project A6, Münster, Germany.
Editor: J. T. Barbieri
REFERENCES
- 1.Allignet, J., P. England, I. Old, and N. El Solh. 2002. Several regions of the repeat domain of the Staphylococcus caprae autolysin, AtlC, are involved in fibronectin binding. FEMS Microbiol. Lett. 213:193-197. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819-1827. [DOI] [PubMed] [Google Scholar]
- 4.Bateman, A., E. Birney, R. Durbin, S. R. Eddy, R. D. Finn, and E. L. Sonnhammer. 1999. Pfam 3.1: 1313 multiple alignments and profile HMMs match the majority of proteins. Nucleic Acids Res. 27:260-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman, A., and M. Bycroft. 2000. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299:1113-1119. [DOI] [PubMed] [Google Scholar]
- 6.Beliveau, C., C. Potvin, J. Trudel, A. Asselin, and G. Bellemare. 1991. Cloning, sequencing, and expression in Escherichia coli of a Streptococcus faecalis autolysin. J. Bacteriol. 173:5619-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry, A. M., and J. C. Paton. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden, M. K., and J. I. Flock. 1994. Cloning and characterization of a gene for a 19 kDa fibrinogen-binding protein from Staphylococcus aureus. Mol. Microbiol. 12:599-606. [DOI] [PubMed] [Google Scholar]
- 9.Brückner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. [DOI] [PubMed] [Google Scholar]
- 10.Bubert, A., M. Kuhn, W. Goebel, and S. Kohler. 1992. Structural and functional properties of the p60 proteins from different Listeria species. J. Bacteriol. 174:8166-8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung, A. I., S. J. Projan, R. E. Edelstein, and V. A. Fischetti. 1995. Cloning, expression, and nucleotide sequence of a Staphylococcus aureus gene (fbpA) encoding a fibrinogen-binding protein. Infect. Immun. 63:1914-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downer, R., F. Roche, P. W. Park, R. P. Mecham, and T. J. Foster. 2002. The elastin-binding protein of Staphylococcus aureus (EbpS) is expressed at the cell surface as an integral membrane protein and not as a cell wall-associated protein. J. Biol. Chem. 277:243-250. [DOI] [PubMed] [Google Scholar]
- 13.Flock, J. I., G. Froman, K. Jonsson, B. Guss, C. Signas, B. Nilsson, G. Raucci, M. Höök, T. Wadstrom, and M. Lindberg. 1987. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 6:2351-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujigaki, Y., Y. Yousif, T. Morioka, S. Batsford, A. Vogt, A. Hishida, and M. Miyasaka. 1998. Glomerular injury induced by cationic 70-kD staphylococcal protein; specific immune response is not involved in early phase in rats. J. Pathol. 184:436-445. [DOI] [PubMed] [Google Scholar]
- 15.Hartleib, J., N. Kohler, R. B. Dickinson, G. S. Chhatwal, J. J. Sixma, O. M. Hartford, T. J. Foster, G. Peters, B. E. Kehrel, and M. Herrmann. 2000. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood 96:2149-2156. [PubMed] [Google Scholar]
- 16.Hawiger, J., M. Kloczewiak, M. A. Bednarek, and S. Timmons. 1989. Platelet receptor recognition domains on the alpha chain of human fibrinogen: structure-function analysis. Biochemistry 28:2909-2914. [DOI] [PubMed] [Google Scholar]
- 17.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Götz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heilmann, C., and F. Götz. 1998. Further characterization of Staphylococcus epidermidis transposon mutants deficient in primary attachment or intercellular adhesion. Zentbl. Bakteriol. 287:69-83. [DOI] [PubMed] [Google Scholar]
- 19.Heilmann, C., M. Herrmann, B. E. Kehrel, and G. Peters. 2002. Platelet-binding domains in 2 fibrinogen-binding proteins of Staphylococcus aureus identified by phage display. J. Infect. Dis. 186:32-39. [DOI] [PubMed] [Google Scholar]
- 20.Heilmann, C., M. Hussain, G. Peters, and F. Götz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 21.Heilmann, C., G. Thumm, G. S. Chhatwal, J. Hartleib, A. Uekötter, and G. Peters. 2003. Identification and characterization of a novel autolysin (Aae) with adhesive properties from Staphylococcus epidermidis. Microbiology 149:2769-2778. [DOI] [PubMed] [Google Scholar]
- 22.Hell, W., H. G. Meyer, and S. G. Gatermann. 1998. Cloning of aas, a gene encoding a Staphylococcus saprophyticus surface protein with adhesive and autolytic properties. Mol. Microbiol. 29:871-881. [DOI] [PubMed] [Google Scholar]
- 23.Hell, W., S. Reichl, A. Anders, and S. Gatermann. 2003. The autolytic activity of the recombinant amidase of Staphylococcus saprophyticus is inhibited by its own recombinant GW repeats. FEMS Microbiol. Lett. 227:47-51. [DOI] [PubMed] [Google Scholar]
- 24.Hussain, M., K. Becker, C. von Eiff, G. Peters, and M. Herrmann. 2001. Analogs of Eap protein are conserved and prevalent in clinical Staphylococcus aureus isolates. Clin. Diagn. Lab. Immunol. 8:1271-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain, M., A. Haggar, C. Heilmann, G. Peters, J.-I. Flock, and M. Herrmann. 2002. Insertional inactivation of eap in Staphylococcus aureus strain Newman confers reduced staphylococcal binding to fibroblasts. Infect. Immun. 70:2933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iandolo, J. J., V. Worrell, K. H. Groicher, Y. Qian, R. Y. Tian, S. Kenton, A. Dorman, H.-G. Jia, S. Lin, P. Loh, S. Qi, H. Zhu, and B. A. Roe. 2002. Comparative analysis of the genomes of the temperate bacteriophages phi 11, phi 12 and phi 13 of Staphylococcus aureus 8325. Gene 289:109-118. [DOI] [PubMed] [Google Scholar]
- 27.Iordanescu, S., and M. Surdeanu. 1976. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J. Gen. Microbiol. 96:277-281. [DOI] [PubMed] [Google Scholar]
- 28.Jönsson, K., D. McDevitt, M. H. McGavin, J. M. Patti, and M. Höök. 1995. Staphylococcus aureus expresses a major histocompatibility complex class II analog. J. Biol. Chem. 270:21457-21460. [DOI] [PubMed] [Google Scholar]
- 29.Jönsson, K., C. Signas, H. P. Müller, and M. Lindberg. 1991. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur. J. Biochem. 202:1041-1048. [DOI] [PubMed] [Google Scholar]
- 30.Jonsson, P., M. Lindberg, I. Haraldsson, and T. Wadström. 1985. Virulence of Staphylococcus aureus in a mouse mastitis model: studies of alpha hemolysin, coagulase, and protein A as possible virulence determinants with protoplast fusion and gene cloning. Infect. Immun. 49:765-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joris, B., S. Englebert, C. P. Chu, R. Kariyama, L. Daneo-Moore, G. D. Shockman, and J. M. Ghuysen. 1992. Modular design of the Enterococcus hirae muramidase-2 and Streptococcus faecalis autolysin. FEMS Microbiol. Lett. 70:257-264. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson, R., A. Michaelsson, and L. Mattsson. 1991. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J. Immunol. Methods 145:229-240. [DOI] [PubMed] [Google Scholar]
- 33.Kloczewiak, M., S. Timmons, T. J. Lukas, and J. Hawiger. 1984. Platelet receptor recognition site on human fibrinogen. Synthesis and structure-function relationship of peptides corresponding to the carboxy-terminal segment of the gamma chain. Biochemistry 23:1767-1774. [DOI] [PubMed] [Google Scholar]
- 34.Kuroda, M., T. Ohta, I. Ushiyama, T. Baba, H. Yuzawa, I. Kobayashi, C. Longzhu, A. Oguchi, K.-I. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R.-I. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshina, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 35.Lang, S., M. A. Livesley, P. A. Lambert, W. A. Littler, and T. S. Elliott. 2000. Identification of a novel antigen from Staphylococcus epidermidis. FEMS Immunol. Med. Microbiol. 29:213-220. [DOI] [PubMed] [Google Scholar]
- 36.Lee, J. 1993. Electrotransformation of staphylococci. Methods Mol. Biol. 47:209-216. [DOI] [PubMed] [Google Scholar]
- 37.Lowy, F. D. 1998. Medical progress: Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 38.Margot, P., M. Pagni, and D. Karamata. 1999. Bacillus subtilis 168 gene lytF encodes a gamma-d-glutamate-meso-diaminopimelate muropeptidase expressed by the alternative vegetative sigma factor, sigmaD. Microbiology 145:57-65. [DOI] [PubMed] [Google Scholar]
- 39.Margot, P., M. Wahlen, A. Gholamhoseinian, P. Piggot, and D. Karamata. 1998. The lytE gene of Bacillus subtilis 168 encodes a cell wall hydrolase. J. Bacteriol. 180:749-752. (Erratum, 180: 2272.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marmur, J. 1961. A procedure for isolation of deoxyribonucleic acid from microorganisms. J. Mol. Biol. 3:208-218. [Google Scholar]
- 41.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 42.Milohanic, E., R. Jonquieres, P. Cossart, P. Berche, and J.-L. Gaillard. 2001. The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol. Microbiol. 39:1212-1224. [DOI] [PubMed] [Google Scholar]
- 43.Milohanic, E., B. Pron, P. Berche, and J. L. Gaillard. 2000. Identification of new loci involved in adhesion of Listeria monocytogenes to eukaryotic cells. European Listeria Genome Consortium. Microbiology 146:731-739. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen, T., B. Ghebrehiwet, and E. I. B. Peerschke. 2000. Staphylococcus aureus protein A recognizes platelet gC1qR/p33: a novel mechanism for staphylococcal interactions with platelets. Infect. Immun. 68:2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palma, M., O. Shannon, H. C. Quezada, A. Berg, and J. I. Flock. 2001. Extracellular fibrinogen-binding protein, Efb, from Staphylococcus aureus blocks platelet aggregation due to its binding to the alpha-chain. J. Biol. Chem. 276:31691-31697. [DOI] [PubMed] [Google Scholar]
- 46.Pankov, R., and K. M. Yamada. 2002. Fibronectin at a glance. J. Cell Sci. 115:3861-3863. [DOI] [PubMed] [Google Scholar]
- 47.Park, J. H., Y. S. Lee, Y. K. Lim, S. H. Kwon, C. U. Lee, and B. S. Yoon. 2000. Specific binding of recombinant Listeria monocytogenes p60 protein to Caco-2 cells. FEMS Microbiol. Lett. 186:35-40. [DOI] [PubMed] [Google Scholar]
- 48.Park, P. W., J. Rosenbloom, W. R. Abrams, and R. P. Mecham. 1996. Molecular cloning and expression of the gene for elastin-binding protein (ebpS) in Staphylococcus aureus. J. Biol. Chem. 271:15803-15809. [DOI] [PubMed] [Google Scholar]
- 49.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Höök. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 50.Patti, J. M., H. Jonsson, B. Guss, L. M. Switalski, K. Wiberg, M. Lindberg, and M. Höök. 1992. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J. Biol. Chem. 267:4766-4772. [PubMed] [Google Scholar]
- 51.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pei, L., M. Palma, M. Nilsson, B. Guss, and J. I. Flock. 1999. Functional studies of a fibrinogen binding protein from Staphylococcus epidermidis. Infect. Immun. 67:4525-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pilgrim, S., A. Kolb-Maurer, I. Gentschev, W. Goebel, and M. Kuhn. 2003. Deletion of the gene encoding p60 in Listeria monocytogenes leads to abnormal cell division and loss of actin-based motility. Infect. Immun. 71:3473-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ponting, C. P., L. Aravind, J. Schultz, P. Bork, and E. V. Koonin. 1999. Eukaryotic signalling domain homologues in archaea and bacteria. Ancient ancestry and horizontal gene transfer. J. Mol. Biol. 289:729-745. [DOI] [PubMed] [Google Scholar]
- 55.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Götz. 2001. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 57.Schneewind, O., D. Mihaylova-Petkov, and P. Model. 1993. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 12:4803-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shuttleworth, H. L., C. J. Duggleby, S. A. Jones, T. Atkinson, and N. P. Minton. 1987. Nucleotide sequence analysis of the gene for protein A from Staphylococcus aureus Cowan 1 (NCTC8530) and its enhanced expression in Escherichia coli. Gene 58:283-295. [DOI] [PubMed] [Google Scholar]
- 59.Smith, T. J., S. A. Blackman, and S. J. Foster. 2000. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology 146:249-262. [DOI] [PubMed] [Google Scholar]
- 60.Steen, A., G. Buist, K. J. Leenhouts, M. E. Khattabi, F. Grijpstra, A. L. Zomer, G. Venema, O. P. Kuipers, and J. Kok. 2003. Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J. Biol. Chem. 278:23874-23881. [DOI] [PubMed] [Google Scholar]
- 61.Sugai, M., H. Komatsuzawa, T. Akiyama, Y.-M. Hong, T. Oshida, Y. Miyake, T. Yamaguchi, and H. Suginaka. 1995. Identification of endo-β-N-acetylglucosaminidase and N-acetylmuramyl-l-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. J. Bacteriol. 177:1491-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaudaux, P. E., F. A. Waldvogel, J. J. Morgenthaler, and U. E. Nydegger. 1984. Adsorption of fibronectin onto polymethylmethacrylate and promotion of Staphylococcus aureus adherence. Infect. Immun. 45:768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vuong, C., C. Gerke, G. A. Somerville, E. R. Fischer, and M. Otto. 2003. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J. Infect. Dis. 188:706-718. [DOI] [PubMed] [Google Scholar]
- 64.Wuenscher, M. D., S. Kohler, A. Bubert, U. Gerike, and W. Goebel. 1993. The iap gene of Listeria monocytogenes is essential for cell viability, and its gene product, p60, has bacteriolytic activity. J. Bacteriol. 175:3491-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]