Abstract
Summary: Depression is a highly debilitating disorder that has been estimated to affect up to 21% of the world population. Despite the advances in the treatment of depression with selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs), there continue to be many unmet clinical needs with respect to both efficacy and side effects. These needs range from efficacy in treatment resistant patients, to improved onset, to reductions in side effects such as emesis or sexual dysfunction. To address these needs, there are numerous combination therapies and novel targets that have been identified that may demonstrate improvements in one or more areas. There is tremendous diversity in the types of targets and approaches being taken. At one end of a spectrum is combination therapies that maintain the benefits associated with SSRIs but attempt to either improve efficacy or reduce side effects by adding additional mechanisms (5-HT1A, 5-HT1B, 5-HT1D, 5-HT2C, α-2A). At the other end of the spectrum are more novel targets, such as neurotrophins (BDNF, IGF), based on recent findings that antidepressants induce neurogenesis. In between, there are many approaches that range from directly targeting serotonin receptors (5-HT2C, 5-HT6) to targeting the multiplicity of potential mechanisms associated with excitatory (glutamate, NMDA, mGluR2, mGluR5) or inhibitory amino acid systems (GABA) or peptidergic systems (neurokinin 1, corticotropin-releasing factor 1, melanin-concentrating hormone 1, V1b). The present review addresses the most exciting approaches and reviews the localization, neurochemical and behavioral data that provide the supporting rationale for each of these targets or target combinations.
Keywords: Monoamines, glutamate, peptides, neurotrophins, GABA, serotonin
INTRODUCTION
Depression is a prevalent psychiatric disorder with estimates reaching as high as 21% of the world population. Despite the fact it is a psychiatric disorder, the World Health Organization predicts that it will be the second leading cause of death by the year 2020 due to complications arising from stress and the cardiovascular system. Multiple subtypes of depression exist although major depressive disorder or unipolar depression appears to be the predominant diagnosed subtype and has been the primary focus of drug development efforts. Depression is characterized most often by anhedonia or the loss of interest or pleasure in normal daily activities and feelings of sadness. Additional symptoms may include sleep disturbances, a gain or loss of weight accompanied, respectively, by increases or decreases in appetite, recurrent inappropriate feelings of guilt, psychomotor agitation, difficulty concentrating and thinking including indecisiveness and thoughts of death or suicide. The diagnosis as clinically described in the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders -Fourth Edition, 1994) requires that five of the nine major criteria are met during a period persisting for at least 2 weeks.
Although the first drugs discovered to treat depression were serendipitous in nature, the foundation for further development of drugs that modulate monoaminergic neurotransmission was established. The chemical underpinnings of depression for the last 50 years have been referred to as the monoamine hypothesis that postulates that the debilitating and often chronic symptoms of depression result from perturbations in serotonin (5-HT), norepinephrine and/or dopamine transmission. This hypothesis spawns from work done in the late 1950s showing that monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs), which elevate levels of monoamines by preventing their metabolism and blocking their reuptake, respectively, were effective antidepressants.1 Interestingly, further support for the chemical hypothesis of depression is based on clinical data where the side effects of reserpine as an antihypertensive agent in the 1960s suggested that depleting brain monoamines had detrimental effects on mood.2 In the 1980s and 1990s, the MAOIs and TCAs were superseded by the next generation of antidepressants termed the selective serotonin reuptake inhibitors (SSRIs) including fluoxetine (Prozac), paroxetine (Paxil), and sertraline (Zoloft), which are characterized by their ability to preferentially increase serotonin levels. A more recent class of novel antidepressants blocks the reuptake of both serotonin and norepinephrine. Such dual-acting serotonin/norepinephrine reuptake inhibitors (SNRIs), including venlafaxine (Effexor), are reported to exhibit a faster clinical onset of action3–5 and be more effective in treating depression that is refractory to other types of antidepressants.6,7 Bupropion (Wellbutrin), likely by virtue of its ability to block the reuptake of norepinephrine and dopamine, has been touted as an effective antidepressant with less incidence of side effects including sexual dysfunction and weight gain.8 Notably and regardless of their mechanism of action, there continue to be significant unmet clinical needs with respect both to efficacy and side effect profile.
Over the last 10 years, it has also been revealed that depression may be related to neurodegenerative processes that lead to loss of synaptic connectivity and perhaps neuronal networks in limbic brain structures including the hippocampus and cortex. Several reports have now demonstrated that the volume of the hippocampus and prefrontal cortices are decreased and structural imaging studies have shown reduced gray matter volumes in depressed patients.9–11 Notably, the amygdala, which may play a role in the neuronal circuitry involved in regulating mood, appears to be enlarged in patients exhibiting their first major episode of depression but subsequently shrinks in volume with prolonged and recurrent depression.12,13 The significance of this has yet to be determined and raises interesting questions regarding the role of the amygdala in depression. Furthermore, there appear to be functional consequences of this volume loss based upon anatomical correlates related to regional cerebral blood flow and glucose metabolism.14 Additionally, in terms of loss of function, it has been demonstrated and observed that there is a loss of executive memory in depressed patients. The finding that depressed patients frequently exhibit cognitive deficits is well documented and dates back to work done in the 1960s.15 The framework in which one can base the comorbidity of depression and cognitive deficits can certainly be related to overlapping and reciprocal neural networks in the brain.16,17 Nonetheless, it is still not clear if the neurodegenerative aspects play a major role in the etiology of the disease or are secondary as a result of neurotoxicity induced by increased excitatory amino acids such as glutamate, increased cortisol levels, or a decrease in the functioning of neurotrophins.
Despite the advances that have been made in the development of antidepressants, there are clearly still unmet clinical needs that need to be addressed (Table 1). The future generation of successful antidepressants will need to address multiple efficacy parameters or issues that lead to discontinuation such as sexual dysfunction. The current strategies being undertaken are generally directed at symptomatic and/or disease modifying approaches. Indeed there are multiple new approaches in progress to improve current pharmacological means of modulating serotonin or norepinephrine neurotransmission by either combining mechanisms or alternatively selectively stimulating receptor subtypes that may trigger improved efficacy or fewer side effects (FIG. 1). Overall, these approaches take advantage of the current state of understanding as to the role of monoaminergic enhancement strategies. Other innovative approaches are targeting excitatory or inhibitory amino acids and receptors, peptidergic systems, and neurotrophins. This review will bring to light the current progress in these areas with emphasis on the background, rationale and potential advantages, which may arise from these approaches.
TABLE 1.
Unmet Clinical Needs for Antidepressant Drug Development
| Unmet Medical Need |
|---|
|
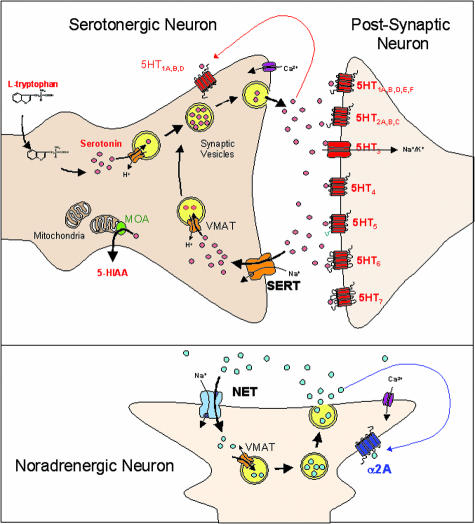
FIG. 1.
Potential targets for novel monoaminergic antidepressants: overview of a serotonergic and noradrenergic synapse defining the multiple targets for drug development.
MONOAMINERGIC STRATEGIES
The monoamine hypothesis of depression postulates that the etiology and pathogenesis of depression arises from central deficiencies in serotonin, norepinephrine, and/or dopamine. Correspondingly, current pharmacotherapies have been developed in an effort to amend these alterations in monoaminergic systems (e.g., SSRIs, SNRIs). Regardless of their mechanism of action, however, a drawback of all marketed antidepressants is the 3- to 5-week delay necessary to achieve therapeutic efficacy. This lag time is thought to reflect the time required for desensitization of the receptors regulating monoamine release (e.g., 5-HT1A, 5-HT2C, and α-2 adrenergic receptors). To potentially accelerate the onset of antidepressant action—as well as limit unwanted side effects—current drug development strategies are focusing on designing new antidepressants with dual and/or triple modes of action. These approaches, along with examples of preclinical and clinical studies, will be highlighted in the following sections.
SSRI/5-HT1A antagonists
The delayed clinical efficacy of SSRIs is believed to result, to a large extent, from the indirect activation of somatodendritic 5-HT1A autoreceptors. A profound body of preclinical literature indicates that acute SSRI treatment increases serotonin levels in various brain regions including the dorsal raphe nuclei. This elevation in serotonin engages inhibitory 5-HT1A autoreceptors residing in the dorsal raphe to inhibit 5-HT cell firing and dampen subsequent 5-HT release in terminal serotonergic brain regions.18 However, following long-term SSRI treatment (14–21 d) 5-HT1A autoreceptors desensitize resulting in more pronounced elevations in serotonin levels compared to acute treatment.19,20 These data suggest that a strategy combining SSRIs with 5-HT1A receptor antagonists would produce robust and more rapid increases in central serotonin levels and likely yield an antidepressant with an accelerated onset of activity. This neurochemical hypothesis is supported by a plethora of microdialysis studies demonstrating that pretreatment with selective 5-HT1A antagonists such as WAY-100635 augments SSRI- and SNRI-induced changes in cortical serotonin levels.21 Preclinical models sensitive to the behavioral effects of serotonergics corroborate these findings as 5-HT1A antagonism is reported to potentiate the antidepressant-like effects of SSRIs in the rodent resident-intruder, social interaction, and schedule-induced polydipsia assays.22–24 Clinical data using this combination strategy demonstrate that the antidepressant activity of SSRIs is accelerated and/or enhanced when combined with the mixed 5-HT1A/β-adrenoceptor antagonist, pindolol25. While the results of these clinical studies remain somewhat equivocal, there is enough collective evidence suggesting that a strategy combining SSRIs with 5-HT1A antagonists may be useful in the clinical management of depression. Correspondingly, several companies have recently published on the synthesis of such dual-acting compounds possessing potent activity as both SSRIs and full/partial 5-HT1A receptor antagonists, some of which are reported to be active in preclinical models of depression.26–28 As some of these dual-acting SSRI/5-HT1A compounds begin their clinical evaluation, it may only be a matter of time to determine whether this approach will represent the newest generation of antidepressants.
SSRI/5-HT2C antagonists
Desensitization of 5-HT2C receptors is routinely reported following chronic SSRI treatment. However, the overall contribution of this molecular change to the antidepressant effects of SSRIs is not well understood. Recent data suggest that 5-HT2C receptor inactivation may play a role in augmenting the neurochemical and behavioral effects of antidepressants. Using in vivo microdialysis techniques in rats, Cremers et al. and others showed that the selective 5-HT2C antagonists, SB 242084 and RS102221, and the nonselective 5-HT2C receptor antagonists, ketanserin and irindalone, potentiate the neurochemical effects of SSRIs on hippocampal and cortical serotonin levels.29,30 Despite the robust neurochemical effects when these agents are combined, 5-HT2C receptor antagonism alone has no significant effects on extracellular serotonin.30,31 Similar to the reported neurochemical effects, this serotonergic combination produces marked augmentation of the antidepressant-like effects of SSRIs in behavioral models of depression and anxiety including the mouse tail suspension test (TST) and schedule-induced polydipsia assay.29,31 Complementary studies done in 5-HT2C receptor null mutant mice show enhanced neurochemical and behavioral (TST) responses to fluoxetine compared to their wild-type littermates.31 Although the precise neural mechanisms mediating these enhanced behavioral and neurochemical responses are unclear, it likely occurs at the postsynaptic level via modifying negative feedback mechanisms. This is evidenced by studies showing that local infusion of RS102221 into serotonergic nerve terminals produces effects on SSRI neurochemistry similar to that of systemically administered 5-HT2C antagonists.29 Overall, these preclinical data show that 5-HT2C antagonism augments the neurochemical and behavioral effects of SSRIs. Moreover, these data highlight a novel strategy of combining both targets, either in a single molecular entity or as adjunctive therapy to already marketed SSRIs, for the potential treatment of depressive disorders.
SSRI/α-2 adrenergic antagonists
The success of SNRIs in the clinic underscores the importance of elevating both norepinephrine and serotonin in the treatment of depression. Ways to enhance the effects of SSRIs via concomitant blockade of serotonin receptors have already been discussed. However, a strategy that targets noradrenergic autoreceptors may have merit in augmenting the neurochemical effects of conventional antidepressants. Several classes of antidepressants, particularly norepinephrine reuptake inhibitors such as reboxetine (Edronax) and the SNRIs, acutely elevate extracellular levels of norepinephrine. The release of norepinephrine can activate presynaptic α-2 adrenergic autoreceptors located on both norepinephrine and dopamine cells causing blunted noradrenergic and dopaminergic responses, respectively. Furthermore, because serotonin efferents from the dorsal raphe are inhibited by local α-2 adrenergic receptors, blocking α-2 receptors may also influence serotonergic.32 Thus, antidepressants, when given in combination with agents that “turn off ” α-2 autoreceptors, can potentially elevate levels of all three monoamines. Neurochemical validation of this hypothesis comes from microdialysis studies showing that α-2 adrenergic antagonists markedly potentiate the ability of antidepressants to increase extracellular levels of norepinephrine, serotonin, and dopamine, depending on the brain region examined.32 Although there are essentially no published data showing that this particular combination strategy is efficacious in preclinical behavioral models of depression, the data from microdialysis studies suggest that α-2 adrenergic antagonism may strengthen the neurochemical effects of antidepressants, and may improve the efficacy of antidepressants in humans. Several arguments support this notion because coadministration of antidepressants with α-2 adrenergic antagonists results in an accelerated down regulation of cortical β-adrenergic receptors.33 In addition, nonselective α-2 adrenergic receptors antagonists such as mirtazapine (Remeron) are reported to possess modest antidepressant activity in their own right.34 Finally, clinical studies emphasize that combining SSRIs with nonselective α-2 receptor antagonists actually shortens the time required to achieve antidepressant activity.35,36 Collectively, these data have ignited considerable chemistry efforts to design and synthesize novel antidepressant molecules that combine monoamine reuptake inhibition with α-2 adrenergic receptor antagonism.37,38
Triple monoamine uptake blockers
Whereas the monoamine hypothesis of depression primarily focuses on the role of norepinephrine and serotonin, a critical role of dopamine in mediating the action of antidepressants was postulated nearly three decades ago.39 Adjunctive treatment with dopamine receptor agonists augments the effects of antidepressants in the rodent forced swim test, whereas, clinically, dopamine agonists have been shown to improve depressive symptomatology in patients refractory to conventional antidepressants.40–43 This evidence, taken together with the clinical success of SSRIs and SNRIs, provides considerable rationale for targeting all three monoamine reuptake sites in the treatment of depression. Moreover, this “broad-spectrum” approach has gained momentum recently as growing clinical and preclinical evidence links core symptoms of depression (i.e., anhedonia) to deficits in dopaminergic transmission. Recently, a single molecule possessing nanomolar inhibition of uptake of all three monoamines has been described and shown to be active in vivo.44 Thus, DOV 21,947 produces antidepressant-like activity in the rodent forced swim and tail suspension tests.44 The success, however, of this compound—as well as the strategy and benefit of combing inhibition of all three monoamines into a single molecule—is still awaiting evaluation in human patients.
Additional multitarget, monoamine strategies
Both transporter and inhibitory autoreceptor mechanisms strictly control the release of biogenic amines into the extracellular environment. For instance, 5-HT1A and 5-HT1B receptors are somatodendritic and terminal autoreceptors, respectively, regulating levels of central serotonin levels. Blockade of 5-HT1B receptors alone has been shown to acutely increase levels of serotonin in the guinea pig frontal cortex and hippocampus as well as augment the effects of SSRIs on serotonin levels.45 Combining the selective 5-HT1A antagonist, WAY-100635, with the 5-HT1B receptor antagonist, SB-224289, produced marked elevations in serotonin levels in the guinea pig.46 These latter results curiously suggest that combining 5-HT1A and 5-HT1B receptor antagonism can elevate serotonin and, consequently, potentially be an effective strategy to treat depression. Additional examples of targeting multiple postsynaptic receptors as putative antidepressant agents include the 5-HT1A agonist/α-2 antagonist, sunepitron, the 5-HT1A agonist/dopamine D2 agonist, roxindole, and α-2 adrenergic antagonist/5-HT2 antagonist, mirtazapine.47 It is also noteworthy that, in addition to their strict regulation of monoamine release, these monoaminergic receptors (e.g., 5-HT1A and α-2 adrenergic receptors) also influence the release of other neurotransmitters such as acetylcholine, which is thought to be procognitive.48 Therefore, targeting the aforementioned receptor systems could yield an effective antidepressant with an added benefit of improving cognitive dysfunction, for example, which is commonly reported in patients suffering from major depressive disorders.
In summary, these strategies seem to efficiently “tweak” the monoaminergic systems in the hopes of developing a more rapid acting antidepressant. However, much needed clinical data regarding the efficacy, safety, and tolerability of such “dual-acting” compounds is eagerly awaited. Perhaps newer approaches targeting convergent, down stream components of the monoamine system (e.g., neurotrophins) and/or nonmonoaminergic systems including GABA and glutamate may ultimately prove beneficial in the clinical management of depression.
Subtype selective serotonergic approaches: 5-HT2C and 5-HT6 agonists
While there are many strategies targeted toward improving the effects of SSRIs by the addition of another mechanism (norepinephrine reuptake inhibition, 5-HT1A antagonist, 5-HT2C antagonist; α-2A antagonist; see above), an alternative strategy involves directly targeting the postsynaptic serotonergic receptors that may more directly mediate the antidepressant and/or anxiolytic effects of SSRIs. SSRIs increase levels of synaptic serotonin that act at 14 different serotonin receptors. The antidepressant and/or anxiolytic effects of SSRIs are likely mediated by one or more of these receptors, but it is unlikely that all 14 play a critical role. The undesired side-effects of SSRIs are also likely mediated via the activation of one or more of these receptors that may be distinct from those that mediate antidepressant or anxiolytic actions. Due to feedback regulation by 5-HT1A, 5-HT1B and 5-HT1D receptors, chronic administration of SSRIs is necessary to sustain increases in serotonin levels in brain regions associated with depression such as frontal cortex, consistent with the 2- to 3-week delay required for antidepressants to become effective.21,49 Therefore, by specifically targeting a postsynaptic serotonin receptor, it may be possible to improve onset of action.
Two potential candidate serotonin receptors for mediating either the antidepressant or anxiolytic effects of SSRIs are the 5-HT2C receptor and the 5-HT6 receptor. Over the last few years, a number of novel 5-HT2C receptor agonists including Org 37684,50 Ro 60-0175,51 WAY-161503,52 YM348,53 WAY-629,54 and WAY-16390955 have been identified and characterized for multiple indications including depression and obsessive-compulsive disorder.55 Ro 60-0175, WAY-161503 and WAY-163909, which are highly structurally diverse, have been more extensively characterized in depression models than the other recently identified 5-HT2C receptor agonists. The first 5-HT6 receptor agonists (LY586713 and WAY-466) have also been identified and are being evaluated as potential treatments for depression and/or anxiety.56,57
5-HT2C receptor agonists show antidepressant-like effects in multiple animal models of depression. For example, 5-HT2C receptor agonists decrease immobility time and increase swimming time in the forced swim test in rats in a manner comparable with SSRIs.58 The effects of the 5-HT2C receptor agonists and SSRIs in the rat forced swim test are antagonized by 5-HT2C receptor antagonists consistent with the role for 5-HT2C receptors in mediating antidepressant-like effects of 5-HT2C receptor agonists and SSRIs.58 5-HT2C receptor agonists are also effective in additional models of antidepressant action including the DRL-72 s (DRL = differential reinforcement of low rate) model, the resident-intruder model, the olfactory bulbectomy model and the chronic mild stress model.51,59,60 In both the chronic mild stress model and in the olfactory bulbectomy model, antidepressants typically require 2–3 weeks of administration to show effectiveness. 5-HT2C receptor agonists are effective by day 3 in the chronic mild stress model and are effective following short-term treatment (<1 week) in the olfactory bulbectomy model, consistent with rapid onset antidepressant-like effects (Rosenzweig-Lipson, S., unpublished observation).51,59,61 Taken together, these results suggest that 5-HT2C receptor agonists have therapeutic potential as rapid onset antidepressants.
5-HT2C agonists may also be beneficial in the treatment of some anxiety disorders such as obsessive-compulsive disorder and panic anxiety. Several lines of evidence suggest that 5-HT2C receptor agonists may be effective treatments for obsessive-compulsive disorder. 5-HT2C knockout mice exhibit compulsive-like behaviors.62 5-HT2C receptor agonists are effective in animal models of compulsive behavior, such as schedule-induced polydipsia (SIP), 8-hydroxy-2-dipropyl-aminotetralin-induced scratching in squirrel monkeys, marble burying, and excessive eating of palatable foods.51,63 The acute effects of 5-HT2C agonists in SIP contrast with the effects of serotonin reuptake inhibitors that typically require chronic administration in this model.24,51,64 It has been suggested that SIP may also be an appropriate model for detecting the onset of activity of antidepressant compounds insomuch as combining the activity of 5-HT1A or 5-HT1B antagonists with SSRIs results in an acute effect in this model.24 5-HT2C agonists are also effective in a model of panic anxiety.65 Taken together, these results suggest that 5-HT2C agonists may be effective rapid onset treatments for obsessive-compulsive disorder and panic anxiety.
The introduction of novel 5-HT6 receptor agonists has allowed for the investigation of their potential in anxiety and depression. Current therapeutic agents for the treatment of anxiety disorders include benzodiazepines and SSRIs that act either directly or indirectly to modulate GABAergic neurotransmission. Benzodiazepines, which act as positive allosteric modulators of the GABAA receptor/Cl− ion channel complex, enhance GABA signaling following receptor stimulation. SSRIs may enhance levels of GABA as predicted from recent imaging studies in humans.66 Interestingly, immunohistochemical studies suggest that 5-HT6 receptors are colocalized on GABAergic neurons.67 In neurochemical studies, both WAY-181187 and WAY-466 consistently elevate levels of GABA in multiple brain regions associated with anxiety including frontal cortex and amygdala (Schechter, L., unpublished observations). The neurochemical effects of 5-HT6 agonists can be blocked by 5-HT6 receptor antagonists, indicative of 5-HT6 receptor activation in the mediation of these effects. Additional supporting neurochemical evidence is based on the effects of 5-HT6 receptor agonists on stimulated glutamate release. Both WAY-181187 and WAY-466 attenuate stimulated glutamate release in brain slices (Schechter, L., unpublished observations). Under stressful situations, glutamatergic neurotransmission may increase in cortical and limbic systems and may be associated with anxiety symptoms as well as hippocampal atrophy. These hypoglutamatergic effects may also be beneficial in obsessive-compulsive disorder, which may involve increased levels of glutamate and dopamine.68,69 To that end, in electrophysiological studies, chronic administration of a 5-HT6 receptor agonist decreases the basal firing rate of the A9 dopaminergic cell body (substantia nigra compacta) that would be expected to decrease stereotypic behavior, which is one of the cardinal symptoms of the disease. Moreover, 5-HT6 agonists are effective acutely in a schedule-induced polydipsia model, indicative of a rapid onset anti-OCD-like effect (Schechter, L., unpublished observations). Taken together, these results are suggestive of potential anxiolytic-like activity for 5-HT6 agonists.
5-HT6 receptor agonists may also play a role in depression. Antidepressants, including SSRIs, upregulate BDNF gene expression.70 One candidate 5-HT receptor for mediating these changes in BDNF is the 5-HT6 receptor. The 5-HT6 agonist LY586713 upregulates BDNF mRNA in the hippocampus following either acute or short-term (4 day) treatment; effects which can be blocked by a 5-HT6 antagonist.56 Additional studies investigating the role of 5-HT6 agonists in depression will be required to further elucidate the role of this receptor.
STRATEGIES TO TARGET EXCITATORY AMINO ACIDS
The NMDA receptor is an ionotropic glutamate receptor with highest densities located in cortico-limbic regions of the brain. Extracellular glutamate concentrations are enhanced by various stressors, like tail pinch and restraint,71,72 and an involvement of the NMDA receptor became apparent in the modulation of stress-induced glutamate responses. Furthermore, chronic antidepressant administration can influence NMDA receptor function and receptor binding profiles, as well as generate regional alterations in mRNA expression that encodes multiple NMDA receptor subunits.73,74 The NMDA receptor is composed of an oligomer of neuromodulatory subunits including at least one NR1 entity necessary for channel function and several other sites termed NR2A-NR2D that associate to form the channel.75 The regulatory subunits include a glycine-sensitive binding site, two voltage-sensitive magnesium and zinc sites (involved in blocking NMDA responses) and two polyamine sites (either stimulatory or inhibitory). With this in mind, the direction of major research efforts for the treatment of depression and affective disorders now encompasses the development of compounds that regulate the target-rich environment within the NMDA receptor complex.
An extensive library of noncompetitive NMDA antagonists (e.g., MK-801, memantine, ketamine) that reduce glutamatergic transmission at the NMDA receptor have demonstrated antidepressant-like effects in animal models, including forced swim and tail suspension tests, inescapable stressors, and in learned helplessness.76,77 In clinical trials, Berman et al. reported the first placebo-controlled, double-blind study assessing the therapeutic effects of a single infusion dose of ketamine in unipolar depressives.78 Their observations indicated that the antidepressant effects of ketamine appeared only after plasma clearance of the drug and supported a rebound hypothesis in which NMDA receptor blockade possibly triggers therapeutic neurogenesis. There is evidence from preclinical studies in rats that subanesthetic doses of ketamine applied subchronically can enhance neurogenesis in the hippocampal subgranular zone.79 The potential antidepressant efficacy of another NMDA antagonist, felbamate is under phase II evaluation for treatment-resistant bipolar depression.80 Felbamate, a 2-phenyl-1, 3-propanediol dicarbamate, is a potent nonsedative anticonvulsant whose clinical effect may be related to the inhibition of NMDA currents through NR1-2B subunits, with additional inhibitory activity at AMPA/kainate and dihydropyridine-sensitive calcium channels; however, its precise mode of action remains unclear.
Glycine and D-serine are endogenous ligands that potentiate NMDA receptor-mediated neurotransmission by association with the NMDA/glycine-sensitive binding site. The site is activated by the presence of endogenous glycine and is required for receptor activation by L-glutamate. Chronic exposure to imipramine and ECS treatment was reported to induce adaptive changes at the glycine-sensitive site.81 d-Cycloserine is a partial agonist at the glycine-sensitive site and exhibits anxiolytic-like activity in the fear-potentiated startle response,82 and the Vogel conflict drinking test at relatively high doses (200–300 mg/kg) in rats.83 However, attempts to synthesize glycine and/or d-serine amino acid analogs with selective agonist activity at the NMDA/glycine binding site have so far remained unsuccessful.
The importance of the polyamine site in NMDA receptor function and especially its relevance to animal behavior is subject to debate. Polyamines can alter the function of NMDA receptors via the polyamine site(s) and the NR2B subunit renders NMDA receptors particularly sensitive to potentiation by polyamines. At the stimulatory polyamine site, both spermine and spermidine can increase the binding of open channel blockers, such as MK-801, and of l-glutamate and glycine to their respective sites. Ifenprodil, a competitive antagonist at the stimulatory polyamine site has an overall negative modulatory effect on the receptor, decreasing the binding of MK-801 and its analogs.84 Ifenprodil, at a relatively low dose, has a favorable anxiolytic-like profile without associated deficits in working memory in mice using the plus-maze paradigm.85 This suggests that selective modulators of the polyamine site to promote anxiolytic effects might provide distinct therapeutic advantages over other noncompetitive NMDA antagonists by avoiding loss of NMDA-dependent working memory and operating through a different NMDA-dependent circuitry.
AMPA and kainate receptors mediate the majority of fast excitatory glutamatergic transmission in the brain and their distribution is similar to that observed for NMDA receptors with highest densities present in the cerebral cortical and hippocampal areas, septum and striatum. The AMPA receptors work in concert with NMDA receptors to mediate the primary depolarization required to unblock NMDA receptors and to trigger synaptic strengthening. The receptor is composed of combinations of subunits, termed, GluR1-4, forming allosteric modulatory sites that represent targets for fine-tuning glutamatergic activity by pharmacologic means. Because these receptors are prone to rapid desensitization following stimulation, direct AMPA agonists are unlikely to be therapeutically useful. One class of compounds, termed AMPA receptor-positive modulators or AMPAkines, have been developed that potentiate AMPA receptor transmission in the presence of agonist (e.g., glutamate, AMPA) and reduce the rate of receptor desensitization and/or deactivation.86,87 Several chemical classes of AMPAkines have been reported, including pyrrolidones (piracetam, aniracetam), benzothiadiazides (cyclothiazide), benzoylpiperidines (CX516, CX546), and more recently, the biarylopropylsulfonamides (LY392098, LY404187, LY451646). AMPAkines, such as LY392098 and LY451646, exhibit dose-dependent antidepressant-like effects in the forced-swim and tail suspension tests.88–90
The AMPA receptor has been functionally associated to a variety of signal transduction events involving G proteins, Src-family kinases, and the neurogenic MAP kinase (MAPK) pathway.91 The dentate gyrus region appears to be particularly sensitive to AMPAkines and raises the possibility that subtle modulation of AMPA receptors may provide a useful strategy to activate neurotrophic MAPK cascades. In vivo studies have demonstrated the ability of AMPAkines to promote BDNF levels. For example, short-term chronic (5 days) treatment with LY451646 and LY404187 increased BDNF protein levels in neurons of the dentate gyrus.92 Furthermore, effective antidepressant doses of LY451646 were well within the range of those required to raise BDNF protein and mRNA expression in hippocampus.88 At present, it is unknown if the antidepressant-like actions of AMPAkines are causally related to changes in BDNF expression. However, if BDNF is proven to be a major contributor to antidepressant effects, AMPAkines may represent a faster onset of action versus biogenic-amine based antidepressants which increase BDNF mRNA following long-term chronic (2–3 weeks) treatment.93 An investigation of CNS-penetrant AMPAkines in the treatment of depression is clearly warranted and could provide additional advantages over current therapies such as an alleviation of cognitive dysfunction and improved working memory.
The metabotropic glutamate receptors (mGluRs) exert longer-term, modulatory effects on glutamatergic neurotransmission compared to the ionotropic receptors. They consist of a family of eight G protein-coupled receptors separated into three groups (group 1: mGluR1 and mGluR5; group II: mGluR2 and mGluR3; group III: mGluR4, mGluR6-mGluR8) on the basis of effector coupling, ligand sensitivity and molecular homologies.94 The discovery of compounds that selectively modulate the heterogeneous family of G protein-coupled mGluRs is still in its infancy. Targeting of the glutamate binding site has provided limited success in the development of small molecules with high-affinity, selectivity, potency, and bioavailability. An alternative approach has relied upon the identification of mGluR allosteric modulators; these bind in a noncompetitive manner to site(s) distal to the glutamate recognition site and can profoundly influence glutamate-induced responses.
Group I negative allosteric modulators (NAMs) block glutamate-induced signaling, and in general, have proven attractive as potential pharmacological agents for the treatment of depression and anxiety disorders. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP) represent the very first mGluR5 NAMs to be described with high selectivity and potency, and good systemic activity.95 mGluR5 NAMs demonstrate antidepressant-like activity in the tail suspension test in mice, and in passive-avoidance learning in the olfactory bulbectomy model of depression in rats, as well as anxiolytic-like effects in several animal models of anxiety including elevated plus maze, fear potentiated startle, Vogel conflict drinking, ultrasonic vocalization, four-plate and social interaction in mice.96–98 Additional support for the involvement of mGluR5 receptors in anxiety came from mGluR5 knockout mice that displayed an anxiolytic-like phenotype in several stress-induced hyperthermic paradigms.99 The mechanism of mGluR5 NAMs to alleviate anxiety and depression is under investigation. In a recent in vivo microdialysis study, MPEP attenuated stress-induced increases in prefrontal cortical noradrenaline levels in rats,100 suggesting that the anxiolytic-like effects of mGluR5 NAMs may be influenced by a noradrenergic component.
mGluR1 receptors also belong to the group I family, yet have been studied much less intensively than the mGluR5 subtype. The selective mGluR1 antagonist, JNJ16259685, exerted anxiolytic-like properties after acute administration in the Vogel conflict drinking test but not in the zero maze, and after a 2-week dosing period in lick suppression studies.101 Furthermore, direct injection of the mGluR1 antagonist, CPCOOEt, into the hippocampus induced anticonflict effects in the Vogel conflict drinking study, which suggested that blockade of mGluR1 in the hippocampus may be an important glutamatergic site for anxiolytic activity.102 Notably, the anxiolytic-like effects seen following mGluR1 antagonism appear task-dependent whereby prominent effects seen in a conflict procedure are not observed in a task based on spontaneous exploration.101
Group II receptor subtypes are negatively linked to adenylyl cyclase through Gi-protein coupling and serve to inhibit endogenous glutamate release, particularly during conditions of glutamate excess from the synaptic cleft. Group II mGluRs are highly localized to the forebrain regions and limbic structures. Preclinical antidepressant effects were reported for group II mGluR antagonists. MGS0039 and LY341495 are new, potent, and selective group II mGluR antagonists that exhibit antidepressant-like effects in rodent models of depression such as the forced swim and tail suspension tests.103 Moreover, microdialysis studies demonstrated that MGS0039 can elevate serotonin levels in the mPFC,104 and that both MGS0039 and LY341495 can increase the firing rate of serotonin neurons within the dorsal raphe nucleus.105 These observations suggest that mGluR2/3 antagonists elicit antidepressant-like effects in part by facilitating serotonergic neurotransmission. It has also been reported that stimulation of postsynaptic AMPA receptors plays a role in mediating the pharmacological effects of LY341495 and MGS0039. Whereas group II mGluR antagonists are potential candidates as antidepressants, preclinical anxiolytic effects have been reported for group II mGluR agonists. Clinical data suggest that the group II agonist LY354740 was effective in reducing fear-of-shock-induced startle potentiation and subjective anxiety in normal volunteers without being sedative.106 To the extent that group II agonists ultimately prove effective as anxiolytics, it would suggest that preclinical glutamatergic hyperactivity might be more relevant to anxiety disorders.
Group III mGluRs have been the least investigated and documented of the three groups, because pharmacological tools with the requisite receptor subtype selectivity are lacking. Group III receptors are typically presynaptic, localized to limbic-system nuclei, and are associated with inhibition of glutamate and/or GABA within the hippocampus and hypothalamus.107 Like the group II receptor subtypes, they are coupled to the Gi-protein adenylyl cyclase pathway. To date, all group III agonists synthesized have proven to be systemically inactive relying on direct central injection of compounds and/or the characterization of knockout animals for the elucidation of their functional properties. Intrahippocampal injection of the selective group III agonists ACPT-1 (also known as L-serine-O-phosphate, L-SOP) and HomoAMPA produced a dose-dependent anxiolytic-like effect in the Vogel conflict drinking test in rats and their actions were reversed by CPPG, a group III antagonist.108 An antidepressant-like effect of ACPT-I after intraventricular injections was also described in the forced swim test.109 Recently, N-phenyl-7-(hydroxyimino) cyclopropa[b] chromen-1a-carboxamide was identified as an mGluR4 positive allosteric modulator, and demonstrated dose-dependent anticonflict effects in Vogel conflict drinking in rats after administration into the basolateral amygdala, a key structure in the regulation of anxiety.110
The generation of specific mGluR7 and mGluR8 receptor knockout animals has enabled further elucidation of the putative physiological and pathological roles for these group III receptor subtypes. mGluR7 is the most widely distributed presynaptic auto- and heteroreceptor in the brain being abundant in regions such as the hippocampus, amygdala, and the locus coeruleus. The mGluR7 receptor is thought to provide mainly negative feedback, limiting l-glutamate release at synapses in a frequency-dependent manner.111 In mice, the targeted gene deletion of mGluR7 generated a phenotypic antidepressant-like behavior in the forced swim and tail suspension tests as well as anxiolytic-like activity in the light-dark box, elevated plus maze, and stress-induced hyperthermia.112 mGluR7 blockade might prevent glutamate-mediated inhibition of GABAergic release resulting in elevated GABA levels,113 thus facilitating the anxiolytic phenotype in mGluR7 knockout mice. In contrast, mGluR8-deficient mice demonstrated an increase in anxiety-related behavior in the elevated plus maze and subtle performance deficits in some learning tasks.114,115 The development of selective mGluR8 receptor compounds should help dissect out the relevant contribution of this receptor subtype to key limbic-system pathways associated with anxiogenesis and the potential clinical benefits of mGluR8 agonists.
At the present time, only a minority of glutamate-based approaches is currently being exploited as novel pharmaceutical therapies. Drug development for glutamatergic neurotransmitters is substantially behind that for other systems (e.g., monoamine-based approaches) and was initially focused on the small molecular synthesis of direct agonists and antagonists of the various ionotropic glutamate receptors. Unfortunately, the therapeutic utility of competitive and uncompetitive NMDA receptor antagonists is greatly hampered by an adverse side effect profile such as psychosis and memory loss leaving unresolved the ideal therapeutic role of NMDA receptor antagonist treatment in the management of persistent depression and anxiety.
THE ROLE OF GABA IN DEPRESSION—TARGETS FOR NOVEL ANTIDEPRESSANTS?
GABA is the primary inhibitory neurotransmitter in the CNS. GABA has been implicated in a number of psychiatric disorders including schizophrenia and affective disorders. A number of studies have been carried out to assess the concentration of GABA in CSF or plasma in patients suffering from psychiatric disorders. The most consistent results are from studies in depressed patients. A number of research groups have reported CSF levels of GABA to be significantly decreased in depressed patients.116–118 Furthermore, studies of plasma levels of GABA in depressed patients concur with these findings.119 Using proton magnetic resonance spectroscopy, Sanacora and colleagues120 have measured cortical GABA concentrations in vivo. Occipital cortex GABA concentrations in depressed patients were found to be significantly lower than in healthy controls. Subsequent studies demonstrated that these low levels of GABA were normalized after SSRI treatment. Interestingly, low levels of GABA in plasma of depressed patients were not reversed by desipramine treatment.121 The decreases in GABA observed in depressed patients do not appear to be associated with changes in GABA uptake binding sites, in the frontal cortex and cingulate gyrus at least.122 Neither GABAB receptors nor glutamic acid decarboxylase (GAD; biosynthetic enzyme for GABA) activity have been found to be altered in depressed suicide victims, whereas GABAA receptor binding in frontal cortex was increased in depressed suicide victims.123,124
Collectively, these findings have raised interest in the manipulation of GABA as a potential target for treating depression. Levels of GABA in the CNS are controlled by the activity of the biosynthetic enzyme for GABA (GAD), the catabolic enzyme, GABA transaminase (GABA-T), and GABA transporters. GABA transporters exist on neuronal and non-neuronal cells; these transporters are the primary means of terminating the activity of GABA in the synapse.
There are two subtypes of GABA receptor, ionotropic GABAA receptors and metabotropic GABAB receptors. Activation of GABAA receptors causes rapid membrane hyperpolarization. GABAA receptors are the predominant inhibitory neurotransmitter receptor in the CNS. As such these receptors represent an important target for a variety of clinically used drugs (including anxiolytics, sedatives, and anticonvulsants). Benzodiazepines act as indirect agonists of GABAA receptors through their activity at the benzodiazepine binding site on this receptor. GABAB receptors are G protein-coupled receptors which, upon activation, cause hyprepolarization of postsynaptic neurones and inhibition of neurotransmitter release from presynaptic nerve terminals. Selective agonists and antagonists exist for both types of GABA receptor. GABAB receptor agonists, such as baclofen, act presynaptically to inhibit the release of excitatory amino acids such as glutamate.
Neuroactive steroids, such as the 3α-reduced metabolites of progesterone, 3α,5α-tetrahydroprogesterone (3α,5α-THP or allopregnanolone), can positively modulate GABAA receptors.125 Interestingly, CSF allopregnanolone levels are significantly lower in depressed patients, and normalized by treatment with fluoxetine or fluvoxamine.126 Similarly, plasma levels of allopregnanolone are reported to be decreased in depression and levels restored by effective antidepressant treatment.127 Fluoxetine and paroxetine have been shown to increase the concentration of allopregnanolone in rat brain.128 Allopregnanolone itself has been shown to have an antidepressant-like effect in rat and mouse forced swim tests.129,130 These effects are thought to be mediated by GABAA receptors as they are blocked by GABAA receptor antagonists.129 Levels of allopregnanolone are also reduced in the frontal cortex of mice that have undergone protracted isolation—-a behavioral manipulation that induces depression-like behaviors. In the rat olfactory bulbectomy model of antidepressant-like activity, removal of the olfactory bulbs caused a significant decrease in frontal cortex levels of allopregnanolone which was reversed by chronic treatment with antidepressants of various classes.131 Interestingly, various SSRIs have been shown to increase allopregnanolone production through increasing the rate of synthesis of allopregnanolone.132
There is a wealth of data implicating GABAB receptors in depression. There are a number of reports in the literature of chronic administration of antidepressants or electroconvulsive therapy increasing the binding and function of GABAB receptors in the frontal cortex of mice and rats.133–137 However, these findings have not been repeated by some groups.138,139 Furthermore, in the olfactory bulbectomy model of depression, removal of the olfactory bulbs was associated with a significant reduction in GABAB, and a significant increase in GABAA receptor densities.140,141 The increase in GABAA receptors was normalized by chronic treatment with antidepressants.142 In the learned helplessness model of antidepressant activity, GABA release is diminished in the hippocampus in helpless rats and this is reversed by desipramine treatment in line with the effects on helpless behavior.141 Interestingly, the selective GABAB receptor antagonist, CGP36742, has been shown to be efficacious in the rat learned helplessness model of antidepressant activity.143 Another GABAB antagonist, CGP56433, also shows antidepressant-like effects in the rat forced swim test.144 Interestingly, GABAB1 knockout mice have an antidepressant-like phenotype in the forced swim test.145 Furthermore, GABAB receptor antagonists, CGP 36742, CGP 56433A, and CGP 56999A, produce rapid increases in nerve growth factors, NGF and BDNF in neocortex and hippocampus, an effect also seen after chronic administration of antidepressants (see Neurotrophins).146
The putative role of GABA, GABAA, and GABAB receptors in depression could be mediated directly by GABA or via other neurotransmitter systems. There are pieces of evidence linking GABAB receptors to noradrenergic and serotonergic systems. For example, administration of GABAB receptor antagonists has also been demonstrated to cause downregulation of β-adrenoceptors—an effect common to chronic administration of a number of types of antidepressants.135,147 The GABAB antagonist, phaclofen, as well as the GABAA receptor antagonist, bicuculline, increased norepinephrine release in the median preoptic nucleus in vivo. Conversely, locally applied agonists of GABAA and GABAB receptors (muscimol and baclofen, respectively) decreased dialysate levels of norepinephrine in the same area. These data indicate that GABAA and GABAB receptors are involved in the control of norepinephrine release in this part of the rat brain.148 Given the established role of norepinephrine in depression and the treatment thereof, such effects on the noradrenergic system could contribute to the antidepressant effects of GABA receptor ligands.
There are also considerable data supporting a relationship between GABAergic and serotonergic systems in the CNS. Allopregnanolone has been demonstrated to directly affect the serotonin system. In rats treated with allopregnanolone for 7 days, the firing rats of serotonergic neurones in the dorsal raphe were increased.149 Given the hypothesis that serotonergic neurotransmission is reduced in depression, such an effect could contribute to the antidepressant-like effects of allopregnanolone. Administration of the GABAB receptor agonist, baclofen, increases serotonin release from the dorsal raphe as well as, to a lesser extent, striatum.150 Local infusion of the GABAA receptor antagonist, bicuculline, increases serotonin release in the dorsal raphe, indicating that GABA afferents exert a tonic inhibitory influence on serotonin neurones in the dorsal raphe.151 Given the serotonin hypothesis of depression, the efficacy of GABAA receptor agonists, and GABAB receptor antagonists could, at least in part, be attributed to these effects on serotonergic transmission. In terms of behavioral effects of GABAergic drugs, the profile of the GABAB antagonist, CGP56433 in the forced swim test indicates a serotonin-mediated effect; CGP56433 decreases immobility and increases swimming, a profile comparable with fluoxetine.144 Depletion of serotonin prevents the effects of GABAB receptor antagonists in this model, further suggesting that the effect involves an interaction with serotonin.144 Reports in the literature also support an interaction between GABAergic and dopaminergic systems. Local application of a GABAA receptor antagonist (bicuculline) increases striatal dopamine release, whereas the GABAA receptor agonist muscimol has the opposite effect.152 Administration of a GABAB receptor agonist (baclofen) has no effect on striatal dopamine levels alone; however, it has been shown to attenuate nicotine-, morphine-, and cocaine-evoked dopamine release in the shell of the nucleus accumbens.153
In summary, GABA is strongly implicated in depression such that GABA receptors are potential targets for the development of novel antidepressants. As the GABAergic system is closely linked to monoaminergic neurotransmitter systems, manipulations of the GABAergic system are very likely to affect other neurotransmitter systems and vice versa. To what extent the effects of antidepressants on the GABAergic system contribute to the efficacy of these drugs as antidepressants remains to be determined.
CENTRAL PEPTIDERGIC SYSTEMS AS TARGETS FOR NOVEL ANTIDEPRESSANT DEVELOPMENT
In the area of depression research, interest in central peptide systems has focused on the high-profile efforts targeting receptors of the central substance P [neurokinin 1 (NK1)] and corticotropin-releasing factor (CRF1) systems. This has led to the development of numerous compounds now in clinical trials for depression. In addition to NK1 and CRF1, however, interest has also fallen on receptors involved in mediating the effects of other central peptidergic systems. These include examples such as melanin-concentrating hormone (MCH) and arginine vasopressin, which are discussed below. Whereas interest in peptidergic systems as platforms for antidepressant development has grown, it is important to note the frustration recognized by the lack of success in the clinic to date. In this section, we will provide a brief overview of several central peptidergic systems, which have been implicated in the pathophysiology of depression, and are currently considered as emerging targets for novel antidepressant development.
Substance P
Substance P (SP) is an undecapeptide member of the tachykinin family of mammalian neuropeptides, which also include neurokinin A and neurokinin B. SP is the most abundantly expressed of the tachykinins in the CNS, and had originally been shown to modulate pain transmission in the spinal cord.154 SP also acts as a neuromodulator in the CNS acting to regulate an array of stress-related behaviors, autonomic control of cardiovascular and respiratory function, as well as emetic reflexes.155–157
The rationale behind the SP system as a target for depression has been reiterated frequently and is assembled from several lines of evidence. Firstly, the expression profile of SP and its receptors is observed within regions of the CNS that are traditionally associated with the regulation of stress responses (e.g., amygdala, hypothalamus, hippocampus, and frontal cortex). Secondly, both acute and chronic stressors (e.g., immobilization, foot shock, maternal separation) have been shown to increase SP content (synthesis/release) in these areas.158–160 Central administration of SP or NK1 agonists induces stress-related behaviors in animal models.160–162 Elevated levels of SP in plasma and CSF are observed in patients with depression.163
The effects that SP exerts are primarily mediated through the NK1 receptor, and subsequently this receptor has emerged as a target for antidepressant development. NK1 is a member of the class-A GPCR family of receptors that also include NK2 and NK3.164 In the CNS, autoradiographic and immunohistochemical techniques have revealed extensive expression of NK1 receptors in regions involved in modulating affective behaviors, and the neurochemical response to stress (e.g., hypothalamus, hippocampus, nucleus accumbens, raphe nucleus).165 SP exhibits inhibitory effects on monoaminergic neurotransmission under physiological conditions that are mediated through NK1, and NK1 antagonists have been shown to enhance firing rates of dopaminergic, noradrenergic, and serotonergic neurons.166 Collectively, these data have helped support the rationale behind the antidepressant hypothesis of NK1 receptor antagonism, and has resulted in the development of numerous NK1-selective antagonists as potentially novel antidepressants. To date, numerous NK1-selective antagonists have been developed and reported by drug companies to demonstrate antidepressant-like profiles in a range of preclinical animal models.167
Despite the preclinical evidence, the track record of NK1 antagonism in humans has been one of disappointment. This has been largely influenced by the rather high-profile failure of aprepitant in the clinic. In initial phase II studies, aprepitant had shown promising antidepressant results in a small population of depressed patients, but fell short of reaching efficacy in five larger phase III studies, thus failing to provide Merck a proof-of-concept for NK1 antagonism.168 Skepticism in NK1 antagonism as a novel mechanism of action following these results, although widespread in the Pharmaceutical Discovery community, has not slowed interest in the continued clinical development of other NK1 antagonists. This is evidenced by appearance of numerous other NK1 antagonists in clinical development (Table 2).
TABLE 2.
Compounds Targeting Peptidergic Receptors with Potential Utility as Antidepressants
| Peptide System | Receptor Target | Compound | Receptor Pharmacology | Probable Clinical Phase |
|---|---|---|---|---|
| SP | NK1 | GW823296 | Antagonist | I |
| GW679769 | Antagonist | II | ||
| GW597599 (Vestipitant) | Antagonist | II | ||
| R673 | Antagonist | II | ||
| CP-122,721 | Antagonist | II | ||
| L-759274 | Antagonist | II | ||
| NK2 | SR48968 | Antagonist | III | |
| CRF | CRF1 | DMP696 | Antagonist | I |
| DMP904 | Antagonist | I | ||
| GW876008 | Antagonist | I | ||
| AAG561 | Antagonist | I | ||
| TS-041 | Antagonist | I | ||
| AVP | V1b | SSR149415 | Antagonist | I |
In contrast to NK1, comparatively less interest has fallen on the NK2 receptor as target for depression. Clinical development of NK2 antagonists has historically focused on inflammatory conditions such as obstructive airways disease.169 However, support from preclinical findings in rodent models has suggested potential for novel antidepressant-like activity for different, receptor-specific, approach of the same system.170 Adding to the antidepressant rationale, NK2 antagonism has also been shown to attenuate stress induced increases in locus coeruleus firing and norepinephrine release in the prefrontal cortex.171 Compounds such as SR48968 (Saredutant) have now entered clinical trials for depression where this approach will to be tested.
Novel approaches that target the central SP systems have also been contemplated, which feature molecules that combine NK1 antagonism with selective serotonin reuptake inhibition (SSRI). This has been based in large part by observations from neurochemical evidence. For instance, increases in extracellular serotonin levels elicited with SSRI (paroxetine) treatment are significantly more robust in NK1−/− mice when compared with wild-type controls.172 Preclinical evidence appears to support this approach as coadministration of an NK1 antagonist (GR205171 or L733060) and an SSRI (paroxetine) produced similar increases in cortical extracellular serotonin, as well as antidepressant-like activity in preclinical models of depression.173 Together, the evidence implies that the antidepressant-like effects of NK1 antagonism may be mediated through modulation of serotonergic activity, which may be potentiated by also including a SSRI component. Small molecules that combine NK1 antagonism with serotonin reuptake inhibition (NK1/SSRI) have now been reported, and these compounds exhibit antidepressant-like activity in animal models sensitive to either SSRI and NK1 antagonists.174
CRF
CRF is a 41-amino acid peptide.175 The CRF system extends throughout the CNS and plays an important role integrating the body's endocrine, autonomic, immune, and behavioral responses to stress.176,177 Through neurosecretory terminals in the median eminence, CRF-synthesizing neurons of the hypothalamic paraventricular nucleus (PVN) release CRF into portal circulation of the anterior pituitary, where CRF stimulates release of ACTH from corticotrophs into peripheral circulation. This positions CRF as an important mediator of hypothalamic-pituitary-adrenal (HPA) axis activity. CRF projections are also observed in numerous extrahypothalamic sites, including key limbic areas (e.g., amygdala, bed nucleus of the stria terminalis), consistent with its involvement in affective behavioral responses to stress.177 CRF neurons are also found in several brain stem nuclei (e.g., locus coeruleus, nucleus of solitary tract) involved in controlling autonomic components of the stress response.177
Several lines of evidence have linked hyperactivity of the central CRF system with depression in humans. The best understood link is drawn from the well-described role of CRF as the principle mediator of ACTH release from the pituitary, which is central to regulation of the HPA axis. This is relevant to depression as hyperactivity of the HPA axis is one of the most consistent clinical findings in depressed patients, and can be normalized after successful antidepressant treatment.178,179 Increased levels of CRF are directly associated with HPA disturbances in subpopulations of depressed patients.179,180 Moreover, elevated levels of CRF in CSF, decreased CRF receptor binding in the frontal cortex, and increased numbers of CRF neurons in the PVN are all observed in depressed patients.181,182
The biological effects of CRF are mediated by two class-B GPCRs, CRF1 and CRF2. CRF1 has emerged as the target of interest for antidepressant development based on the following lines of evidence. CRF1 is the receptor subtype expressed on corticotrophs of the anterior pituitary, and thus responsible for mediating CRF effects on ACTH release and the HPA axis. CRF1 antagonists exhibit the ability, in preclinical animal models, to block many of the behavioral and endocrine responses to stress. For example, CP-154,526 (antalarmin), one of the first CRF1 antagonists to reach clinical trials in humans in depression, produces antidepressant-like activity in learned helplessness (rats) and chronic mild stress (mice) paradigms, as well as attenuation of stress-induced hyperthermia, distress vocalizations, and cortical norepinephrine release.183–185 DMP696, SSR125543, and R278995/CRA0450 are examples of other CRF1 antagonists that have been developed which also have antidepressant-like activity reported in preclinical rodent models, helping to further support the proof of concept for this mechanism of action.186,187
Despite the preclinical picture for CRF1 antagonism as a novel mechanism of action for antidepressant development, only one compound to date, R121919 (Janssen), has demonstrated antidepressant efficacy in clinical trials.188 Unfortunately, clinical development of this compound was ultimately discontinued (believed to be because of hepatotoxicity), and the initial report was never confirmed in larger studies. This has left the field to wait for the outcome of clinical evaluation of the numerous other CRF1 antagonists that have undergone development as antidepressants.
MCH receptors
MCH is a 19-amino acid cyclic neuropeptide synthesized by neurosecretory cells of the mammalian lateral hypothalamus and zona incerta.189 MCH-synthesizing neurons of these nuclei project throughout the CNS comprising a broad circuitry of innervation, modulating areas involved in regulating energy homeostasis, feeding and mood-related behaviors, arousal, sensorimotor integration, and autonomic control.190,191
Two GPCRs mediate the effects MCH in primates, MCH1-R and MCH2-R. To date, MCH1-R (also known as somatostatin-like receptor 1 or SLC-1) is the only subtype identified in rats. Drug discovery interest in the central MCH system has historically focused on targeting the effects of this peptide on feeding behavior (orexigenic) and energy homeostasis (metabolic), with MCH1-R antagonism emerging as a novel approach for development of anorectic and anti-obesity compounds. Evidence, however, has also implicated the MCH system in regulating mood and the stress response. Local administration of MCH into the nucleus accumbens shell has been reported to produce depressant-like behavioral effects in the rat forced swim test.191 MCH also produces stimulatory effects on HPA axis reactivity, as evidenced by the increases in circulating ACTH and cortisol levels reported following central administration of MCH or direct infusion of MCH into the hypothalamic paraventricular nucleus.192 MCH also increases CRF release from hypothalamic explants, an effect that could be blocked by a selective SLC-1 (rat ortholog of MCH1-R) antagonist.192
MCH1-R antagonists have been the preferred approach for targeting the central MCH system for antidepressant development. Although T-226296 was the first MCH1-R selective antagonist to be reported, SNAP-7941 was the first compound to have behavioral effects in preclinical models of depression reported. SNAP-7941 produced antidepressant-like effects in the rat forced swim test similar to those observed with an SSRI (fluoxetine).193 Several other companies have since reported the synthesis of other MCH1-R antagonist compounds (see Table 2) exhibiting similar antidepressant-like profiles in preclinical models.194,195 Collectively, the results from preclinical profiling of MCH1-R antagonists support a rationale for this novel mechanism of action for depression. As is evident with other peptidergic receptor targets, despite a complete preclinical rationale, the clinical utility of MCH1-R antagonists as antidepressants awaits evaluation.
Arginine vasopressin
Arginine vasopressin (AVP) is a cyclic nonapeptide synthesized exclusively by neurosecretory cells of the CNS with a diverse array of biological functions based on differences in sites of release. When released into peripheral circulation from the posterior pituitary, AVP is responsible for the classic endocrine functions described for this neurohormone (e.g., vasoconstriction, glycogen metabolism, antidiuresis). In the CNS, AVP acts as a neuromodulator/neurotransmitter regulating a range of CNS-mediated functions that include learning and memory, social behaviors, circadian rhythmicity, thermoregulation, and autonomic function. AVP released into the portal circulation from the median eminence is also known to directly modulate CRF effects on ACTH release and the HPA axis.
The central vasopressinergic system has been examined as a platform for psychiatric drug development, including depression.196 The central vasopressinergic system acts on several key neural substrates underlying aspects of the depression endophenotype, including monaminergic systems and those regulating memory, pain sensitivity, synchronization of biological rhythms, the timing/quality of R.E.M. sleep, and regulation of fluid and electrolyte homeostasis.197 Disturbances (hyperactivity) in vasopressinergic activity have also been reported clinically in patients with depression.198,199 Together, this has led many to hypothesize the utility of central vasopressinergic receptor antagonism as a potentially novel antidepressant strategy.
The biological activity of AVP is mediated through a phylogenetically related family of class-A GPCRs; V1a, V2, and V1b, and the oxytocin receptor (OTR) to a lesser extent. Of these receptors, the V3R has emerged as the most tractable candidate for antidepressant development. V1b mediates AVP regulation of corticotroph function in the anterior pituitary, and plays an important role in regulating stress responsiveness of CRF-mediated ACTH release and the HPA axis. Receptors are also present throughout key areas (e.g., hippocampus, lateral septum, frontal cortex) involved in regulation of the behavioral responses to stress.200 The lack of truly selective V1b ligands has historically complicated efforts to elucidate the roles of this receptor in the behavioral responses to stress implied by its central distribution. The relevance of this was revealed following the report of the first V1B-selective antagonist, SSR149415.201 Subsequent behavioral profiling of SSR149415 in a wide range of preclinical models of depression and stress behavior provided the evidence needed to support the hypothesis of V3R antagonism as a novel mechanism of action for antidepressant activity. V1b blockade with SSR149415 has also been shown to produce antidepressant-like activity in both acute (forced swim) and chronic (chronic mild stress, subordination stress) rodent behavioral paradigms.202 SSR149415 also demonstrates the ability to attenuate stress-induced changes in endocrine (ACTH release from anterior pituitary), neurochemical (tail pinch norepinephrine release), and autonomic (hyperthermia) activity.202,203 Although these observations have helped to support the hypothesis of V1b antagonism as a novel antidepressant strategy, they are based on the preclinical profile of a single compound, SSR149415, which is currently in clinical trials. More confidence in this mechanism of action awaits the development and preclinical profiling of additional V1b antagonists.
NEUROTROPHINS AS NOVEL ANTIDEPRESSANT DRUGS
The majority of currently marketed antidepressant drugs act directly on serotonergic or noradrengeric neurotransmission. An alternate (or possibly complementary), hypothesis is that neurotrophic factors are involved in or mediate the mechanism of antidepressant drugs. This theory comes from converging lines of data. First, antidepressant drugs require at least 2 weeks administration to see clinical efficacy.204 This time lag may represent a necessity for long-term adaptations and alterations in downstream signaling pathways, such as neurotrophic pathways, before a therapeutic effect is seen.70 Secondly, many researchers have hypothesized that depression can arise from the failure of the CNS to exhibit the appropriate synaptic plasticity in response to stress, which may be offset or reversed by neurotrophic support induced by antidepressant treatments. In support of this are multiple reports, detailed below, that chronic antidepressant treatments activate long-term changes in neurotrophic factor expression and neurotrophic signaling pathways, and that the activation of these factors is a common mechanism of effect of antidepressants.205 Finally, the recent interest in the idea that neurogenesis, or the birth and survival of new neurons, is involved in antidepressant action again leads to the investigation of neurotrophic factors as mediators of antidepressant action.206 Targets of neurotrophic factors or their pathways may represent a novel treatment for depression.
The NGF family of neurotrophins consists of NGF, BDNF, and neurotrophins-3 and -4 (NT-3 and NT-4). These have been grouped as a family due to the high homology of their receptors. There are two classes of neurotrophin receptors; a low-affinity p75 receptor, which is common to all neurotrophins, and the high-affinity trk receptors, which are associated with specific neurotrophins and encode transmembrane receptor tyrosine kinases (RTKs) that mediate multiple signaling pathways. It has been shown that trkA is the receptor for NGF, trkB is the receptor for BDNF and NT-4, and trkC is the receptor for NT-3. However, there is cross talk between some of these receptors; NT-3 can also bind to trkA and trkB, but with lower affinity than to trkC. To date, NGF, NT-3, and NT-4 and their receptors have not been implicated in antidepressant action, and antidepressant research has centered on BDNF and trkB.
An important regulator of BDNF gene expression is the transcription factor cAMP response element-binding protein (CREB). CRE elements in the promoter region of BDNF indicate that it is a downstream target of CREB, although BDNF may induce CREB phosphorylation as well.207 BDNF and CREB are involved in multiple CNS functions that all fall under the umbrella of “synaptic plasticity.”70 Given that one hypothesis of depression is a dysfunction in synaptic plasticity, either by genetic, biochemical, or environmental factors, both CREB and BDNF have been implicated in antidepressant action.
This hypothesis is supported by the findings that chronic, but not acute, antidepressant treatment increases mRNA and protein levels of BDNF and CREB levels in the rat hippocampus and cortex, indicating that upregulation of these factors is one mechanism by which antidepressants may exert their effects.205 Importantly, the chronic time course corresponds to the time course necessary for clinical efficacy. Stress has been shown to downregulate BDNF, and this is reversed by chronic antidepressant treatment. In addition, rolipram, a phosphodiesterase-IV inhibitor that activates the cAMP cascade and increases BDNF, has been shown to have antidepressant-like effects in animal models.208 Clinically, increased hippocampal BDNF levels have been observed in patients taking antidepressants, along with decreased serum BDNF levels in untreated depressed subjects.209 Postmortem studies have shown decreased hippocampal trkB and BDNF mRNA in suicides compared with controls.210
BDNF acutely administered directly into the lateral ventricles or hippocampus has been shown to produce antidepressant-like effects in the forced swim and learned helplessness paradigms.211,212 Interestingly, these antidepressant-like effects may be localized to the hippocampus, given that infusion of BDNF into the VTA produces prodepressive effects.213 A similar effect of anatomical specificity has been seen with CREB; acute overexpression of CREB in the dentate gyrus produces antidepressant-like responses, whereas CREB expression in the nucleus accumbens produces the opposite effect.214,215 These data indicate that the antidepressant-like effects of neurotrophic factors and their pathways may be anatomically restricted or localized.
The finding that antidepressants increase hippocampal BNDF has received much attention. Although not all laboratories are able to demonstrate fluoxetine-induced increases in BDNF, possibly due to variability in experimental protocols, other antidepressant classes, and ECS continue to provide links between antidepressant action and increased BDNF.216,217 The net effect of increased BDNF expression after chronic antidepressant treatment is not merely the effect of simple activation of the BDNF gene. The BDNF gene has four differentially regulated promoters, all of which have the net effect of increased BDNF expression. Recent research has shown that chronic and acute ECS, desipramine, tranylcypromine, and fluoxetine regulate BDNF mRNA via regulation of different exon-specific promoters.217 Although the physiological significance of this is currently unknown, future drug development may center on more specific activation of the four BDNF transcripts.
Animal models of neurotrophic and antidepressant function have yielded varied results. A number of lines of BDNF heterozygous (+/−) knockouts display a reduction in BDNF, but without any accompanying changes in baseline behavior or reactivity to stress.218 Other lines of +/− BDNF mice display altered synaptic responses but no specific phenotype has been identified.219 These animal lines reveal that a partial loss of BDNF is not enough to affect baseline behavior, but this interpretation is compromised by the fact that these transgenic animals had a loss of BNDF from birth and may have evolved compensatory responses as adults.
The animal studies have also investigated the necessity for an intact neurotrophic system to produce an antidepressant response. Saarelainen et al.220 have reported that a line of trkB heterozygous knockout mice, as well as a line of BDNF heterozygous knockout mice, had no change in baseline behavior, but were resistant to the effects of antidepressants. However, Conti et al.216 reported that a line of CREB-deficient mutant mice respond normally to antidepressants but without the expected increase in BDNF. This indicates that there may be BDNF and CREB-dependent as well as BDNF and CREB-independent pathways for some of the pharmacologic actions of antidepressants.
The ability to generate conditional knockout animals is a powerful new technology that has been used in recent studies. The use of an inducible knockout system was employed to delete forebrain BDNF. These knockout mice did not demonstrate a depressive-like phenotype that might have been predicted with the loss of BDNF but had an attenuated response to antidepressant administration in the forced swim test.221 Taken together, the animal studies point to a role of BDNF in antidepressant action and provide evidence that antidepressants may work via a neurotrophic mechanism.
Recently, there has been a focus on GABAergic drugs as putative antidepressants, given that ECS and fluoxetine have been shown to activate the GABAB receptor. In addition, GABAB receptor antagonists have been shown to be antidepressant-like in animal models of depression, and also increase BNDF mRNA and protein.222 This increase in BDNF occurs after acute administration of these compounds, in contrast to the chronic treatment needed with clinically used antidepressants. Although this is still an emerging field, the data indicate that GABAB may represent an alterative downstream pathway by which to activate BDNF.
Given that a common mechanism of effect of antidepressants is to increase CREB and neurotrophic factors, and that neurotrophic factors are antidepressant in the hippocampus, the hypothesis is that a drug that produces increased levels of BDNF would be an effective antidepressant. However, there are no current therapies that directly use neurotrophic factors for depression or any other neurological diseases. One of the difficulties in designing a neurotrophic factor therapy for CNS disorders is the inability of these factors to cross the blood-brain barrier, which prevents entrance into the brain.223 In the 1990s, multiple phase III clinical trials were conducted using subcutaneous injection of BNDF, IGF-I, or CNTF to treat symptoms of amyotrophic lateral sclerosis. None of these neurotrophic factors crossed the blood brain barrier and all three clinical trials failed due to lack of efficacy.224
Despite the failure of these studies, given the great therapeutic potential of the neurotrophic factors, alternate drug delivery systems to the brain are currently being investigated. The use of small molecule peptidomimetics that would bind to specific neurotrophin domains has been hypothesized to be a potential approach for addressing this issue,225 although there is no current clinical evidence that these will yield any effective therapy.
In contrast to directly targeting the neurotrophic factors, the multiple factors in the signal transduction cascades activated by either neurotrophic factors or antidepressant drugs represent another avenue of research toward drug discovery. These include, but are not limited to, the phosphotidylinositol-3-kinase-Akt pathway and the extracellularly regulated kinase (ERK)/MAPK cascade.226 These pathways are involved in cell signaling, synaptic plasticity and cell survival; the MAPK cascade itself regulates the transcription factor CREB, and also activates the antiapoptotic factor Bcl-2 and inhibits the proapoptotic factor Bcl-xL/Bcl-2-associated death promoter. The ERK/MAPK cascade is activated by neurotrophins and activated by mood stabilizers and antidepressants.227,228 Delineation of the role of these factors as well as their specificity for antidepressant-like effects may represent another avenue for drug discovery.
It has recently been demonstrated that chronic antidepressant treatments increase cell proliferation and neurogenesis, and that animal models of depression such as the learned helplessness paradigm and restraint stress decrease cell proliferation.229,230 The direct physiological significance of antidepressant-induced neurogenesis has yet to be demonstrated, although blockade of hippocampal cell proliferation via irradiation prevents the behavioral actions of antidepressant drugs.231 These findings have led to the neurogenenic hypothesis of antidepressant activity, which posits that one mechanism of effect of antidepressant drugs is to increase hippocampal cell proliferation and neurogenesis.
These hypotheses have led to the identification of other neurotrophic factors as putative antidepressant compounds. IGF-I is one such recent example. IGF-I administered centrally or peripherally increases adult hippocampal cell proliferation and neurogenesis, and also increases the differentiation of immature cells into neurons.232 To determine whether the neurotrophic factor hypothesis would indeed yield a novel antidepressant-like compound, rats were administered IGF-I via intracerebroventricular administration. In agreement with the hypothesis, IGF-I produced an antidepressant-effect in the forced swim test, which lasted 6 days after infusion.233 Although the mechanism by which the IGF-I induced antidepressant activity occurs is unknown, this finding indicates that the search for drug targets by looking for the neurogenic potential of compounds may provide novel antidepressants.
IGF-I signals though the IGF-I receptor, via a multiprotein signaling complex that activates the MAPK pathway and PI3 kinase pathways.234 These pathways may be related to the in vivo neuorogenic effect of IGF-I, because in vitro, IGF-I has a direct proliferative effect on adult hippocampal progenitor cells via MAPK activation.235
In addition to IGF-I's direct neurogenic effects on hippocampal cells, there may be interactions between the IGF-I, BDNF, and serotonin pathways to induce neurogenesis and antidepressant-like effects. Although the G protein-coupled serotonin receptors are independent from the trkB and IGF-I receptors, their intracellular signaling pathways have been shown to act cooperatively to regulate genes involved in synaptic plasticity. Serotonin activates receptors coupled to cAMP production, providing a link between drugs that increase serotonergic neurotransmission and enhanced synaptic plasticity. The effect of agonism or antagonism of serotonin receptor subtypes on cell proliferation and neurogenesis is being investigated and the contribution of neurotrophins to this will enhance our understanding of the mechanisms underlying antidepressant-induced neurogenesis and antidepressant action.236
SUMMARY
It is an exciting time for drug development in depression as there are many new and novel approaches on the horizon. Over the next decade, proof-of-concept studies will be performed in the clinic for a wide array of mechanisms and the true validity of these novel strategies will be enlightening. This will not only include combination molecules taking further advantage of monoaminergic approaches but novel mechanisms yet to be tested in humans. Clearly, the current array of animal models for determining antidepressant activity has been useful in predicting therapeutic efficacy of multiple monoaminergic mechanisms. Notably, mechanisms that do not directly or indirectly modulate monoaminergic mechanisms remain to be fully validated and the development of further animal models may be necessary. To this end, many of the systems described in this review such as glutamatergic, GABAergic, neurotrophic, and peptidergic systems have links to the serotonergic system and modulate serotonin levels or alter serotonergic firing. The question of whether a compound that does not affect monoaminergic neurotransmission can be an effective antidepressant remains to be answered.
In conclusion, the most successful novel approaches will be those that not only demonstrate preclinical antidepressant-like effects, but those that target the unmet clinical needs and lead to long-term disease modification. For many of the approaches described in this review, clinical testing will determine the extent to which these approaches show distinct advantages over existing therapies and finally demonstrate the true innovation associated with these novel mechanisms.
REFERENCES
- 1.Eriksson E. Antidepressant drugs: does it matter if they inhibit the reuptake of noradrenaline or serotonin. Acta Psychiatr Scand 101 [Suppl 402]: 12–17, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin FK, Bunney WE Jr. Depressions following reserpine: a reevaluation. Semin Psychiatry 3: 435–448, 1971. [PubMed] [Google Scholar]
- 3.Clerc GE, Ruimy P, Verdeay-Pailles J. A double blind comparison of venlafaxine and fluoxetine in patients hospitalized for major depression and melancholia. Intl Clin Psychopharmacol 9: 138–143, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Guelfi JD, White C, Hackett D, Guichoux JY, Magni G. Effectiveness of venlafaxine in patients hospitalized for major depression and melancholia. J Clin Psychiatry 56: 450–458, 1995. [PubMed] [Google Scholar]
- 5.Benkert O, Grunder G, Wetzel H. Is there an advantage to venlafaxine in comparison with other antidepressants? Hum Psychopharmacol 12: 53–64, 1997. [Google Scholar]
- 6.de Montigny C, Debonnel G, Bergeron R, St Andre E, Blier P. Venlafaxine in treatment resistant depression: open label multicentre study. Am Coll Neuropharmacol 34: 158, 1995. [Google Scholar]
- 7.Nierenberg AA, Feigner JP, Rudolph R, Cole JO, Sullivan J. Venlafaxine for treatment resistant unipolar depression. J Clin Psychopharmacol 4: 419–423, 1994. [PubMed] [Google Scholar]
- 8.Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry 6: 159–166, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 93: 3908–3913, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duman RS, Charney DS. Cell atrophy and loss in major depression. Biol Psychiatry 45: 1083–1084, 1999. [DOI] [PubMed] [Google Scholar]
- 11.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 100: 1387–1392, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 159: 1112–1118, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry 53: 338–344, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol 12: 527–544, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Cronholm B, Ottosson JO. Memory functions in endogenous depression before and after electroconvulsive therapy. Arch Gen Psychiatry 5: 193–199, 1961. [DOI] [PubMed] [Google Scholar]
- 16.Soares JC, Mann JJ. The anatomy of mood disorders—review of structural neuroimaging studies. [Comment]. Biological Psychiatry 41: 86–106, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry 54: 515–528, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci 19: 378–383, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Dawson LA, Nguyen HQ, Smith DL, Schechter LE. Effect of chronic fluoxetine and WAY-100635 treatment on serotonergic neurotransmission in the frontal cortex. J Psychopharmacol 16: 145–152, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Kreiss DS, Lucki I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J Pharmacol Exp Ther 274: 866–876, 1995. [PubMed] [Google Scholar]
- 21.Beyer CE, Boikess S, Luo B, Dawson LA. Comparison of the effects of antidepressants on norepinephrine and serotonin concentrations in the rat frontal cortex: an in-vivo microdialysis study. J Psychopharmacol 16: 297–304, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell PJ, Redfern PH. Potentiation of the time-dependent, antidepressant-induced changes in the agonistic behaviour of resident rats by the 5-HT1A receptor antagonist, WAY-100635. Behav Pharmacol 8: 585–606, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Duxon MS, Starr KR, Upton N. Latency to paroxetine-induced anxiolysis in the rat is reduced by co-administration of the 5-HT(1A) receptor antagonist WAY100635. Br J Pharmacol 130: 1713–1719, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogg S, Dalvi A. Acceleration of onset of action in schedule-induced polydipsia: combinations of SSRI and 5-HT1A and 5-HT1B receptor antagonists. Pharmacol Biochem Behav 77: 69–75, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Blier P, Bergeron R. The use of pindolol to potentiate antidepressant medication. J Clin Psychiatry 59 [Suppl 5]: 16–23; discussion 24–15, 1998. [PubMed] [Google Scholar]
- 26.Oficialdegui AM, Martinez J, Perez S, Heras B, Irurzun M, Palop JA, et al. Design, synthesis and biological evaluation of new 3-[(4-aryl)piperazin-1-yl]-1-arylpropane derivatives as potential antidepressants with a dual mode of action: serotonin reuptake inhibition and 5-HT1A receptor antagonism. Farmaco 55: 345–353, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Mewshaw RE, Zhou D, Zhou P, Shi X, Hornby G, Spangler T, et al. Studies toward the discovery of the next generation of antidepressants. 3. Dual 5-HT1A and serotonin transporter affinity within a class of N-aryloxyethylindolylalkylamines. J Med Chem 47: 3823–3842, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Hughes ZA, Starr KR, Langmead CJ, Hill M, Bartoszyk GD, Hagan JJ, et al. Neurochemical evaluation of the novel 5-HT1A receptor partial agonist/serotonin reuptake inhibitor, vilazodone. Eur J Pharmacol 510: 49–57, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Cremers TIFH, Bosker FJ, den Boer JA, Westerink BHC, Wikstrøm HV, Hogg S, et al. 5-HT2C antagonists augment the antidepressant effects of SSRIs. Proceedings of the 10th International Conference on in Vivo Methods, Stockholm, Sweden, 2003.
- 30.Mørk A, Hogg S. Augmentation of paroxetine by the 5-HT2 antagonist, irindalone: evidence for increased efficacy. Proceedings of the 10th International Conference on in Vivo Methods, Stockholm, Sweden, 2003.
- 31.Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mork A, et al. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology 29: 1782–1789, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Gobert A, Rivet JM, Cistarelli L, Melon C, Millan MJ. α2-Adrenergic receptor blockade markedly potentiates duloxetine- and fluoxetine-induced increases in noradrenaline, dopamine, and serotonin levels in the frontal cortex of freely moving rats. J Neurochem 69: 2616–2619, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Scott JA, Crews FT. Rapid decrease in rat brain β adrenergic receptor binding during combined antidepressant α-2 antagonist treatment. J Pharmacol Exp Ther 224: 640–646, 1983. [PubMed] [Google Scholar]
- 34.Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIS, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry 14: 175–182, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Cappiello A, McDougle CJ, Malison RT, Heninger GR, Price LH. Yohimbine augmentation of fluvoxamine in refractory depression: a single-blind study. Biol Psychiatry 38: 765–767, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Sanacora G, Berman RM, Cappiello A, Oren DA, Kugaya A, Liu N, et al. Addition of the α2-antagonist yohimbine to fluoxetine: effects on rate of antidepressant response. Neuropsychopharmacology 29: 1166–1171, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Cordi AA, Berque-Bestel I, Persigand T, Lacoste JM, Newman-Tancredi A, Audinot V, et al. Potential antidepressants displayed combined α(2)-adrenoceptor antagonist and monoamine uptake inhibitor properties. J Med Chem 44: 787–805, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Andres JI, Alcazar J, Alonso JM, Alvarez RM, Bakker MH, Biesmans I, et al. Discovery of a new series of centrally active tricyclic isoxazoles combining serotonin (5-HT) reuptake inhibition with α2-adrenoceptor blocking activity. J Med Chem 48: 2054–2071, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Randrup A, Braestrup C. Uptake inhibition of biogenic amines by newer antidepressant drugs: relevance to the dopamine hypothesis of depression. Psychopharmacology (Berl) 53: 309–314, 1977. [DOI] [PubMed] [Google Scholar]
- 40.Maj J, Rogoz Z. Synergistic effect of pramipexole and sertraline in the forced swimming test. Pol J Pharmacol 51: 471–475, 1999. [PubMed] [Google Scholar]
- 41.Bouckoms A, Mangini L. Pergolide: an antidepressant adjuvant for mood disorders? Psychopharmacol Bull 29: 207–211, 1993. [PubMed] [Google Scholar]
- 42.Izumi T, Inoue T, Kitagawa N, Nishi N, Shimanaka S, Takahashi Y, et al. Open pergolide treatment of tricyclic and heterocyclic antidepressant-resistant depression. J Affect Disord 61: 127–132, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Sporn J, Ghaemi SN, Sambur MR, Rankin MA, Recht J, Sachs GS, et al. Pramipexole augmentation in the treatment of unipolar and bipolar depression: a retrospective chart review. Ann Clin Psychiatry 12: 137–140, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. Antidepressant-like actions of DOV 21,947: a “triple” reuptake inhibitor. Eur J Pharmacol 461: 99–104, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Roberts C, Price GW, Jones BJ. The role of 5-HT(1B/1D) receptors in the modulation of 5-hydroxytryptamine levels in the frontal cortex of the conscious guinea pig. Eur J Pharmacol 326: 23–30, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Hughes ZA, Dawson LA. Differential autoreceptor control of extracellular 5-HT in guinea pig and rat: species and regional differences. Psychopharmacology (Berl) 172: 87–93, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Millan MJ. The role of monoamines in the actions of established and “novel” antidepressant agents: a critical review. Eur J Pharmacol 500: 371–384, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Kushida K, Ishida K, Kikuta J, Kato M, Uchiyama T, Taguchi K. α2-Adrenoceptor modulates the release of acetylcholine from the rostral ventrolateral medulla in response to morphine. Biol Pharm Bull 26: 1548–1551, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Dawson LA, Nguyen HQ. The role of 5-HT(1A) and 5-HT(1B/1D) receptors on the modulation of acute fluoxetine-induced changes in extracellular 5-HT: the mechanism of action of (+/−)pindolol. Neuropharmacology 39: 1044–1052, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Leyson D, Kelder J. Ligands for the 5-HT2C receptor as potential antidepressants and anxiolytics. In: Trends in drug research (van der Groot J, ed). Amsterdam: Elsevier; 49–61, 1998.
- 51.Martin JR, Bos M, Jenck F, Moreau J, Mutel V, Sleight AJ, et al. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther 286: 913–924, 1998. [PubMed] [Google Scholar]
- 52.Welmaker GS, Nelson JA, Sabalski JE, Sabb AL, Potoski JR, Graziano D, et al. Synthesis and 5-hydroxytryptamine (5-HT) activity of 2,3,4,4a-tetrahydro-1H-pyrazino[1,2-a]quinoxalin-5-(6H)ones and 2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,2-a]quinoxalines. Bioorg Med Chem Lett 10: 1991–1994, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Kimura Y, Hatanaka K, Naitou Y, Maeno K, Shimada I, Koakutsu A, et al. Pharmacological profile of YM348, a novel, potent and orally active 5-HT2C receptor agonist. Eur J Pharmacol 483: 37–43, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Sabb AL, Vogel RL, Welmaker GS, Sabalski JE, Coupet J, Dunlop J, et al. Cycloalkyl[b][1,4]benzodiazepinoindoles are agonists at the human 5-HT2C receptor. Bioorg Med Chem Lett 14: 2603–2607, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Dunlop J, Sabb AL, Mazandarani H, Zhang J, Kalgaonker S, Shukhina E, et al. WAY-163909 [(7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1h i]indole], a novel 5-hydroxytryptamine 2C receptor-selective agonist with anorectic activity. J Pharmacol Exp Ther 313: 862–869, 2005. [DOI] [PubMed] [Google Scholar]
- 56.De Foubert G, Murray TK, O'Neill MJ, Zetterström TSC. 5-HT6 receptor-selective upregulation of brain-derived neurotrophic factor mRNA in the rat brain. Federation of European Neuroscience Societies, 2004.
- 57.Schechter LE, Smith DL, Li P, Lin Q, Rosenzweig-Lipson S, Robichaud A, et al. Pharmacological profile of a novel and selective 5-HT6 receptor agonist: WAY-466. The EPHAR Satellite Meeting on Serotonin, Porto, Portugal, 2004.
- 58.Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther 295: 1120–1126, 2000. [PubMed] [Google Scholar]
- 59.Moreau JL, Bos M, Jenck F, Martin JR, Mortas P, Wichmann J. 5HT2C receptor agonists exhibit antidepressant-like properties in the anhedonia model of depression in rats. Eur Neuropsychopharmacol 6: 169–175, 1996. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell PJ, Redfern PH. Animal models of depressive illness: the importance of chronic drug treatment. Curr Pharm Des 11: 171–203, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Moreau JL, Jenck F, Martin JR, Perrin S, Haefely WE. Effects of repeated mild stress and two antidepressant treatments on the behavioral response to 5HT1C receptor activation in rats. Psychopharmacology (Berl) 110: 140–144, 1993. [DOI] [PubMed] [Google Scholar]
- 62.Chou-Green JM, Holscher TD, Dallman MF, Akana SF. Compulsive behavior in the 5-HT2C receptor knockout mouse. Physiol Behav 78: 641–649, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Bos M, Jenck F, Martin JR, Moreau JL, Sleight AJ, Wichmann J, et al. Novel agonists of 5HT2C receptors. Synthesis and biological evaluation of substituted 2-(indol-1-yl)-1-methylethylamines and 2-(indeno[1,2-b]pyrrol-1-yl)-1-methylethylamines. Improved therapeutics for obsessive compulsive disorder. J Med Chem 40: 2762–2769, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Woods A, Smith C, Szewczak M, Dunn RW, Cornfeldt M, Corbett R. Selective serotonin re-uptake inhibitors decrease schedule-induced polydipsia in rats: a potential model for obsessive compulsive disorder. Psychopharmacology (Berl) 112: 195–198, 1993. [DOI] [PubMed] [Google Scholar]
- 65.Jenck F, Moreau JL, Berendsen HH, Boes M, Broekkamp CL, Martin JR, et al. Antiaversive effects of 5HT2C receptor agonists and fluoxetine in a model of panic-like anxiety in rats. Eur Neuropsychopharmacol 8: 161–168, 1998. [DOI] [PubMed] [Google Scholar]
- 66.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry 159: 663–665, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Woolley M, Marsden C, Sleight A, Fone K. Co-localization of 5-HT6 receptors on GABAergic but not cholinergic neurones in the adult brain. Society for Neuroscience Satellite Symposium: Serotonin: from the molecule to the clinic, San Diego, CA, 2001.
- 68.Carlsson ML. On the role of cortical glutamate in obsessive-compulsive disorder and attention-deficit hyperactivity disorder, two phenomenologically antithetical conditions. Acta Psychiatr Scand 102: 401–413, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Stein DJ. Neurobiology of the obsessive-compulsive spectrum disorders. Biol Psychiatry 47: 296–304, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Russo-Neustadt AA, Chen MJ. Brain-derived neurotrophic factor and antidepressant activity. Curr Pharm Des 11: 1495–1510, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience 77: 65–73, 1997. [DOI] [PubMed] [Google Scholar]
- 72.Lowy MT, Wittenberg L, Yamamoto BK. Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. J Neurochem 65: 268–274, 1995. [DOI] [PubMed] [Google Scholar]
- 73.Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry 29: 23–26, 1996. [DOI] [PubMed] [Google Scholar]
- 74.Petrie RX, Reid IC, Stewart CA. The N-methyl-D-aspartate receptor, synaptic plasticity, and depressive disorder. A critical review. Pharmacol Ther 87: 11–25, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist 11: 37–49, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Papp M, Moryl E. Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur J Pharmacol 263: 1–7, 1994. [DOI] [PubMed] [Google Scholar]
- 77.Kos T, Popik P. A comparison of the predictive therapeutic and undesired side-effects of the NMDA receptor antagonist, memantine, in mice. Behav Pharmacol 16: 155–161, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354, 2000. [DOI] [PubMed] [Google Scholar]
- 79.Keilhoff G, Bernstein HG, Becker A, Grecksch G, Wolf G. Increased neurogenesis in a rat ketamine model of schizophrenia. Biol Psychiatry 56: 317–322, 2004. [DOI] [PubMed] [Google Scholar]
- 80.National Institute of Mental Health (NIMH). A double-blind randomized placebo-controlled trial of felbamate in treatment resistant bipolar depression. Year initiated: 2002. Clinical Trials Identifier: NCT00034229.
- 81.Paul IA, Layer RT, Skolnick P, Nowak G. Adaptation of the NMDA receptor in rat cortex following chronic electroconvulsive shock or imipramine. Eur J Pharmacol 247: 305–311, 1993. [DOI] [PubMed] [Google Scholar]
- 82.Anthony EW, Nevins ME. Anxiolytic-like effects of N-methyl-D-aspartate-associated glycine receptor ligands in the rat potentiated startle test. Eur J Pharmacol 250: 317–324, 1993. [DOI] [PubMed] [Google Scholar]
- 83.Klodzinska A, Chojnacka-Wojcik E. Anticonflict effect of the glycineB receptor partial agonist, D-cycloserine, in rats. Pharmacological analysis. Psychopharmacology (Berl) 152: 224–228, 2000. [DOI] [PubMed] [Google Scholar]
- 84.Murray F, Kennedy J, Hutson PH, Elliot J, Huscroft I, Mohnen K, et al. Modulation of [3H]MK-801 binding to NMDA receptors in vivo and in vitro. Eur J Pharmacol 397: 263–270, 2000. [DOI] [PubMed] [Google Scholar]
- 85.Fraser CM, Cooke MJ, Fisher A, Thompson ID, Stone TW. Interactions between ifenprodil and dizocilpine on mouse behaviour in models of anxiety and working memory. Eur Neuropsychopharmacol 6: 311–316, 1996. [DOI] [PubMed] [Google Scholar]
- 86.Lynch G. Memory and the brain: unexpected chemistries and a new pharmacology. Neurobiol Learn Mem 70: 82–100, 1998. [DOI] [PubMed] [Google Scholar]
- 87.Skolnick P, Legutko B, Li X, Bymaster FP. Current perspectives on the development of non-biogenic amine-based antidepressants. Pharmacol Res 43: 411–423, 2001. [DOI] [PubMed] [Google Scholar]
- 88.Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair.” Pharmacol Biochem Behav 70: 187–192, 2001. [DOI] [PubMed] [Google Scholar]
- 89.Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098). Neuropharmacology 40: 1028–1033, 2001. [DOI] [PubMed] [Google Scholar]
- 90.Knapp RJ, Goldenberg R, Shuck C, Cecil A, Watkins J, Miller C, et al. Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. Eur J Pharmacol 440: 27–35, 2002. [DOI] [PubMed] [Google Scholar]
- 91.Wang JQ, Tang Q, Parelkar NK, Liu Z, Samdani S, Choe ES, et al. Glutamate signaling to Ras-MAPK in striatal neurons: mechanisms for inducible gene expression and plasticity. Mol Neurobiol 29: 1–14, 2004. [DOI] [PubMed] [Google Scholar]
- 92.Mackowiak M, O'Neill MJ, Hicks CA, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology 43: 1–10, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Duman RS. Synaptic plasticity and mood disorders. Mol Psychiatry 7 [Suppl 1]: S29–S34, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Pin JP, Acher F. The metabotropic glutamate receptors: structure, activation mechanism and pharmacology. Curr Drug Targets CNS Neurol Disord 1: 297–317, 2002. [DOI] [PubMed] [Google Scholar]
- 95.Spooren WP, Gasparini F, Salt TE, Kuhn R. Novel allosteric antagonists shed light on mglu(5) receptors and CNS disorders. Trends Pharmacol Sci 22: 331–337, 2001. [DOI] [PubMed] [Google Scholar]
- 96.Tatarczynska E, Klodzinska A, Chojnacka-Wojcik E, Palucha A, Gasparini F, Kuhn R, et al. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol 132: 1423–1430, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pilc A, Klodzinska A, Branski P, Nowak G, Palucha A, Szewczyk B, et al. Multiple MPEP administrations evoke anxiolytic- and antidepressant-like effects in rats. Neuropharmacology 43: 181–187, 2002. [DOI] [PubMed] [Google Scholar]
- 98.Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, et al. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology 29: 1971–1979, 2004. [DOI] [PubMed] [Google Scholar]
- 99.Brodkin J, Bradbury M, Busse C, Warren N, Bristow LJ, Varney MA. Reduced stress-induced hyperthermia in mGluR5 knockout mice. Eur J Neurosci 16: 2241–2244, 2002. [DOI] [PubMed] [Google Scholar]
- 100.Page ME, Szeliga P, Gasparini F, Cryan JF. Blockade of the mGlu5 receptor decreases basal and stress-induced cortical norepinephrine in rodents. Psychopharmacology (Berl) 179: 240–246, 2005. [DOI] [PubMed] [Google Scholar]
- 101.Steckler T, Lavreysen H, Oliveira AM, Aerts N, Van Craenendonck H, Prickaerts J, et al. Effects of mGlu1 receptor blockade on anxiety-related behaviour in the rat lick suppression test. Psychopharmacology (Berl) 179: 198–206, 2005. [DOI] [PubMed] [Google Scholar]
- 102.Tatarczynska E, Klodzinska A, Kroczka B, Chojnacka-Wojcik E, Pilc A. The antianxiety-like effects of antagonists of group I and agonists of group II and III metabotropic glutamate receptors after intrahippocampal administration. Psychopharmacology (Berl) 158: 94–99, 2001. [DOI] [PubMed] [Google Scholar]
- 103.Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, et al. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology 46: 457–467, 2004. [DOI] [PubMed] [Google Scholar]
- 104.Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res 1042: 92–98, 2005. [DOI] [PubMed] [Google Scholar]
- 105.Kawashima N, Karasawa J, Shimazaki T, Chaki S, Okuyama S, Yasuhara A, et al. Neuropharmacological profiles of antagonists of group II metabotropic glutamate receptors. Neurosci Lett 378: 131–134, 2005. [DOI] [PubMed] [Google Scholar]
- 106.Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress 6: 189–197, 2003. [DOI] [PubMed] [Google Scholar]
- 107.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299: 12–20, 2001. [PubMed] [Google Scholar]
- 108.Palucha A, Tatarczynska E, Branski P, Szewczyk B, Wieronska JM, Klak K, et al. Group III mGlu receptor agonists produce anxiolytic- and antidepressant-like effects after central administration in rats. Neuropharmacology 46: 151–159, 2004. [DOI] [PubMed] [Google Scholar]
- 109.Tatarczynska E, Palucha A, Szewczyk B, Chojnacka-Wojcik E, Wieronska J, Pilc A. Anxiolytic- and antidepressant-like effects of group III metabotropic glutamate agonist (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (ACPT-I) in rats. Pol J Pharmacol 54: 707–710, 2002. [PubMed] [Google Scholar]
- 110.Stachowicz K, Klak K, Klodzinska A, Chojnacka-Wojcik E, Pilc A. Anxiolytic-like effects of PHCCC, an allosteric modulator of mGlu4 receptors, in rats. Eur J Pharmacol 498: 153–156, 2004. [DOI] [PubMed] [Google Scholar]
- 111.Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron 46: 89–102, 2005. [DOI] [PubMed] [Google Scholar]
- 112.Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der Putten H. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci 17: 2409–2417, 2003. [DOI] [PubMed] [Google Scholar]
- 113.Somogyi P, Dalezios Y, Lujan R, Roberts JD, Watanabe M, Shigemoto R. High level of mGluR7 in the presynaptic active zones of select populations of GABAergic terminals innervating interneurons in the rat hippocampus. Eur J Neurosci 17: 2503–2520, 2003. [DOI] [PubMed] [Google Scholar]
- 114.Linden AM, Johnson BG, Peters SC, Shannon HE, Tian M, Wang Y, et al. Increased anxiety-related behavior in mice deficient for metabotropic glutamate 8 (mGlu8) receptor. Neuropharmacology 43: 251–259, 2002. [DOI] [PubMed] [Google Scholar]
- 115.Gerlai R, Adams B, Fitch T, Chaney S, Baez M. Performance deficits of mGluR8 knockout mice in learning tasks: the effects of null mutation and the background genotype. Neuropharmacology 43: 235–249, 2002. [DOI] [PubMed] [Google Scholar]
- 116.Gerner RH, Hare TA. CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. Am J Psychiatry 138: 1098–1101, 1981. [DOI] [PubMed] [Google Scholar]
- 117.Gold BI, Bowers MB Jr, Roth RH, Sweeney DW. GABA levels in CSF of patients with psychiatric disorders. Am J Psychiatry 137: 362–364, 1980. [DOI] [PubMed] [Google Scholar]
- 118.Kasa K, Otsuki S, Yamamoto M, Sato M, Kuroda H, Ogawa N. Cerebrospinal fluid γ-aminobutyric acid and homovanillic acid in depressive disorders. Biol Psychiatry 17: 877–883, 1982. [PubMed] [Google Scholar]
- 119.Petty F, Sherman AD. Plasma GABA levels in psychiatric illness. J Affect Disord 6: 131–138, 1984. [DOI] [PubMed] [Google Scholar]
- 120.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OAC, et al. Reduced cortical γ-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 56: 1043–1047, 1999. [DOI] [PubMed] [Google Scholar]
- 121.Petty F, Steinberg J, Kramer GL, Fulton M, Moeller FG. Desipramine does not alter plasma GABA in patients with major depression. J Affect Disord 29: 53–56, 1993. [DOI] [PubMed] [Google Scholar]
- 122.Sundman-Eriksson I, Allard P. [3H]Tiagabine binding to GABA transporter-1 (GAT-1) in suicidal depression. J Affect Disord 71: 29–33, 2002. [DOI] [PubMed] [Google Scholar]
- 123.Cheetham SC, Crompton MR, Katona CL, Parker SJ, Horton RW. Brain GABAA/benzodiazepine binding sites and glutamic acid decarboxylase activity in depressed suicide victims. Brain Res 460: 114–123, 1988. [DOI] [PubMed] [Google Scholar]
- 124.Cross JA, Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain GABAB binding sites in depressed suicide victims. Psychiatry Res 26: 119–129, 1988. [DOI] [PubMed] [Google Scholar]
- 125.Paul SM, Purdy RH. Neuroactive steroids. FASEB J 6: 2311–2322, 1992. [PubMed] [Google Scholar]
- 126.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA 95: 3239–3244, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ströhle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, et al. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry 45: 274–277, 1999. [DOI] [PubMed] [Google Scholar]
- 128.Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci USA 93: 12599–12604, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3 α-hydroxy-5 α-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav 67: 137–143, 2000. [DOI] [PubMed] [Google Scholar]
- 130.Molina-Hernandez M, Tellez-Alcantara NP, Perez Garcia J, Olivera Lopez JI, Teresa Jaramillo M. Antidepressant-like actions of intra-accumbens infusions of allopregnanolone in ovariectomized Wistar rats. Pharmacol Biochem Behav 80: 401–409, 2005. [DOI] [PubMed] [Google Scholar]
- 131.Uzunova V, Wrynn AS, Kinnunen A, Ceci M, Kohler C, Uzunov DP. Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur J Pharmacol 486: 31–34, 2004. [DOI] [PubMed] [Google Scholar]
- 132.Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA 96: 13512–13517, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gray JA, Green AR. Increased GABAB receptor function in mouse frontal cortex after repeated administration of antidepressant drugs or electroconvulsive shocks. Br J Pharmacol 92: 357–362, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lloyd KG, Thuret F, Pilc A. Upregulation of γ-aminobutyric acid (GABA) B binding sites in rat frontal cortex: a common action of repeated administration of different classes of antidepressants and electroshock. J Pharmacol Exp Ther 235: 191–199, 1985. [PubMed] [Google Scholar]
- 135.Pratt GD, Bowery NG. Repeated administration of desipramine and a GABAB receptor antagonist, CGP 36742, discretely up-regulates GABAB receptor binding sites in rat frontal cortex. Br J Pharmacol 110: 724–735, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sands SA, Reisman SA, Enna SJ. Effect of antidepressants on GABAB receptor function and subunit expression in rat hippocampus. Biochem Pharmacol 68: 1489–1495, 2004. [DOI] [PubMed] [Google Scholar]
- 137.Szekely AM, Barbaccia ML, Costa E. Effect of a protracted antidepressant treatment on signal transduction and [3H](-)-baclofen binding at GABAB receptors. J Pharmacol Exp Ther 243: 155–159, 1987. [PubMed] [Google Scholar]
- 138.Cross JA, Horton RW. Are increases in GABAB receptors consistent findings following chronic antidepressant administration? Eur J Pharmacol 141: 159–162, 1987. [DOI] [PubMed] [Google Scholar]
- 139.McManus DJ, Greenshaw AJ. Differential effects of antidepressants on GABAB and β-adrenergic receptors in rat cerebral cortex. Biochem Pharmacol 42: 1525–1528, 1991. [DOI] [PubMed] [Google Scholar]
- 140.Dennis T, Beauchemin V, Lavoie N. Differential effects of olfactory bulbectomy on GABAA and GABAB receptors in the rat brain. Pharmacol Biochem Behav 46: 77–82, 1993. [DOI] [PubMed] [Google Scholar]
- 141.Martin P, Pichat P, Massol J, Soubrie P, Lloyd KG, Puech AJ. Decreased GABA B receptors in helpless rats: reversal by tricyclic antidepressants. Neuropsychobiology 22: 220–224, 1989. [DOI] [PubMed] [Google Scholar]
- 142.Dennis T, Beauchemin V, Lavoie N. Antidepressant-induced modulation of GABAA receptors and β-adrenoceptors but not GABAB receptors in the frontal cortex of olfactory bulbectomised rats. Eur J Pharmacol 262: 143–148, 1994. [DOI] [PubMed] [Google Scholar]
- 143.Nakagawa Y, Sasaki A, Takashima T. The GABAB receptor antagonist CGP36742 improves learned helplessness in rats. Eur J Pharmacol 381: 1–7, 1999. [DOI] [PubMed] [Google Scholar]
- 144.Slattery DA, Desrayaud S, Cryan JF. GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J Pharmacol Exp Ther 312: 290–296, 2005. [DOI] [PubMed] [Google Scholar]
- 145.Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABAB receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology 29: 1050–1062, 2004. [DOI] [PubMed] [Google Scholar]
- 146.Heese K, Otten U, Mathivet P, Raiteri M, Marescaux C, Bernasconi R. GABAB receptor antagonists elevate both mRNA and protein levels of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) but not neurotrophin-3 (NT-3) in brain and spinal cord of rats. Neuropharmacology 39: 449–462, 2000. [DOI] [PubMed] [Google Scholar]
- 147.Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol 33: 109–147, 1993. [DOI] [PubMed] [Google Scholar]
- 148.Sakamaki K, Nomura M, Hatakenaka S, Miyakubo H, Tanaka J. GABAergic modulation of noradrenaline release in the median preoptic nucleus area in the rat. Neurosci Lett 342: 77–80, 2003. [DOI] [PubMed] [Google Scholar]
- 149.Robichaud M, Debonnel G. Modulation of the firing activity of female dorsal raphe nucleus serotonergic neurons by neuroactive steroids. J Endocrinol 182: 11–21, 2004. [DOI] [PubMed] [Google Scholar]
- 150.Abellan MT, Jolas T, Aghajanian GK, Artigas F. Dual control of dorsal raphe serotonergic neurons by GABAB receptors. Electrophysiological and microdialysis studies. Synapse 36: 21–34, 2000. [DOI] [PubMed] [Google Scholar]
- 151.Tao R, Auerbach SB. Influence of inhibitory and excitatory inputs on serotonin efflux differs in the dorsal and median raphe nuclei. Brain Res 961: 109–120, 2003. [DOI] [PubMed] [Google Scholar]
- 152.Matsumoto M, Kanno M, Togashi H, Ueno K, Otani H, Mano Y, et al. Involvement of GABAA receptors in the regulation of the prefrontal cortex on dopamine release in the rat dorsolateral striatum. Eur J Pharmacol 482: 177–184, 2003. [DOI] [PubMed] [Google Scholar]
- 153.Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse 50: 1–6, 2003. [DOI] [PubMed] [Google Scholar]
- 154.Minamino N, Masuda H, Kangawa K, Matsuo H. Regional distribution of neuromedin K and neuromedin L in rat brain and spinal cord. Biochem Biophys Res Commun 124: 731–738, 1984. [DOI] [PubMed] [Google Scholar]
- 155.Rupniak NM, Kramer MS. Discovery of the antidepressant and anti-emetic efficacy of substance P receptor (NK1) antagonists. Trends Pharmacol Sci 20: 485–490, 1999. [DOI] [PubMed] [Google Scholar]
- 156.Saito R, Takano Y, Kamiya HO. Roles of substance P and NK(1) receptor in the brainstem in the development of emesis. J Pharmacol Sci 91: 87–94, 2003. [DOI] [PubMed] [Google Scholar]
- 157.Culman J, Unger T. Central tachykinins: mediators of defence reaction and stress reactions. Can J Physiol (Lond) Pharmacol 73: 885–891, 1995. [DOI] [PubMed] [Google Scholar]
- 158.Takayama H, Ota Z, Ogawa N. Effect of immobilization stress on neuropeptides and their receptors in rat central nervous system. Regul Pept 15: 239–248, 1986. [DOI] [PubMed] [Google Scholar]
- 159.Vaupel R, Jarry H, Schlomer HT, Wuttke W. Differential response of substance P-containing subtypes of adrenomedullary cells to different stressors. Endocrinology 123: 2140–2145, 1988. [DOI] [PubMed] [Google Scholar]
- 160.Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 281: 1640–1645, 1998. [DOI] [PubMed] [Google Scholar]
- 161.Unger T, Carolus S, Demmert G, Ganten D, Lang RE, Maser-Gluth C, et al. Substance P induces a cardiovascular defense reaction in the rat: pharmacological characterization. Circ Res 63: 812–820, 1988. [DOI] [PubMed] [Google Scholar]
- 162.Van Wimersma Greidanus TB, Maigret C. Grooming behavior induced by substance P. Eur J Pharmacol 154: 217–220, 1988. [DOI] [PubMed] [Google Scholar]
- 163.Rimon R, Le Greves P, Nyberg F, Heikkila L, Salmela L, Terenius L. Elevation of substance P-like peptides in the CSF of psychiatric patients. Biol Psychiatry 19: 509–516, 1984. [PubMed] [Google Scholar]
- 164.Regoli D, Boudon A, Fauchere JL. Receptors and antagonists for substance P and related peptides. Pharmacol Rev 46: 551–599, 1994. [PubMed] [Google Scholar]
- 165.Quartara L, Maggi CA. The tachykinin NK1 receptor. Part II: distribution and pathophysiological roles. Neuropeptides 32: 1–49, 1998. [DOI] [PubMed] [Google Scholar]
- 166.Adell A. Antidepressant properties of substance P antagonists: relationship to monoaminergic mechanisms? Curr Drug Targets CNS Neurol Disord 3: 113–121, 2004. [DOI] [PubMed] [Google Scholar]
- 167.Herpfer I, Lieb K. Substance P receptor antagonists in psychiatry: rationale for development and therapeutic potential. CNS Drugs 19: 275–293, 2005. [DOI] [PubMed] [Google Scholar]
- 168.Montgomery SA, Keller M, Ball W, Morrison M, Snavely D, Liu G, et al. S. 13.05 peptide approaches in the treatment of major depression—lack of efficacy of the substance P (neurokinin1 receptor) antagonist aprepitant. Eur Neuropsychopharmacol 14: S136–S137, 2004. [Google Scholar]
- 169.Joos GF, Pauwels RA. Tachykinin receptor antagonists: potential in airways diseases. Curr Opin Pharmacol 1: 235–241, 2001. [DOI] [PubMed] [Google Scholar]
- 170.Griebel G, Perrault G, Soubrie P. Effects of SR48968, a selective non-peptide NK2 receptor antagonist on emotional processes in rodents. Psychopharmacology (Berl) 158: 241–251, 2001. [DOI] [PubMed] [Google Scholar]
- 171.Steinberg R, Alonso R, Griebel G, Bert L, Jung M, Oury-Donat F, et al. Selective blockade of neurokinin-2 receptors produces antidepressant-like effects associated with reduced corticotropin-releasing factor function. J Pharmacol Exp Ther 299: 449–458, 2001. [PubMed] [Google Scholar]
- 172.Froger N, Gardier AM, Moratalla R, Alberti I, Lena I, Boni C, et al. 5-Hydroxytryptamine (5-HT)1A autoreceptor adaptive changes in substance P (neurokinin 1) receptor knock-out mice mimic antidepressant-induced desensitization. J Neurosci 21: 8188–8197, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guiard BP, Przybylski C, Guilloux JP, Seif I, Froger N, De Felipe C, et al. Blockade of substance P (neurokinin 1) receptors enhances extracellular serotonin when combined with a selective serotonin reuptake inhibitor: an in vivo microdialysis study in mice. J Neurochem 89: 54–63, 2004. [DOI] [PubMed] [Google Scholar]
- 174.Ryckmans T, Berton O, Grimee R, Kogej T, Lamberty Y, Pasau P, et al. Dual NK(1) antagonists—serotonin reuptake inhibitors as potential antidepressants. Part 2: SAR and activity of benzyloxyphenethyl piperazine derivatives. Bioorg Med Chem Lett 12: 3195–3198, 2002. [DOI] [PubMed] [Google Scholar]
- 175.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213: 1394–1397, 1981. [DOI] [PubMed] [Google Scholar]
- 176.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 15: 71–100, 1990. [DOI] [PubMed] [Google Scholar]
- 177.Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp 172:5–21; discussion 21–29, 1993. [DOI] [PubMed]
- 178.Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav 43: 60–66, 2003. [DOI] [PubMed] [Google Scholar]
- 179.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160: 1–12, 1999. [DOI] [PubMed] [Google Scholar]
- 180.Kasckow JW, Baker D, Geracioti TD Jr. Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides 22: 845–851, 2001. [DOI] [PubMed] [Google Scholar]
- 181.Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found Symp 172: 296–308; discussion 308–216, 1993. [DOI] [PubMed] [Google Scholar]
- 182.Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology 28: 1328–1335, 2003. [DOI] [PubMed] [Google Scholar]
- 183.Mansbach RS, Brooks EN, Chen YL. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur J Pharmacol 323: 21–26, 1997. [DOI] [PubMed] [Google Scholar]
- 184.Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry 27: 625–631, 2003. [DOI] [PubMed] [Google Scholar]
- 185.Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, et al. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther 301: 333–345, 2002. [DOI] [PubMed] [Google Scholar]
- 186.Overstreet DH, Griebel G. Antidepressant-like effects of CRF1 receptor antagonist SSR125543 in an animal model of depression. Eur J Pharmacol 497: 49–53, 2004. [DOI] [PubMed] [Google Scholar]
- 187.Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, et al. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur J Pharmacol 485: 145–158, 2004. [DOI] [PubMed] [Google Scholar]
- 188.Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 34: 171–181, 2000. [DOI] [PubMed] [Google Scholar]
- 189.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, et al. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol 319: 218–245, 1992. [DOI] [PubMed] [Google Scholar]
- 190.Lembo PM, Grazzini E, Cao J, Hubatsch DA, Pelletier M, Hoffert C, et al. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat Cell Biol 1: 267–271, 1999. [DOI] [PubMed] [Google Scholar]
- 191.Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci 25: 2933–2940, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kennedy AR, Todd JF, Dhillo WS, Seal LJ, Ghatei MA, O'Toole CP, et al. Effect of direct injection of melanin-concentrating hormone into the paraventricular nucleus: further evidence for a stimulatory role in the adrenal axis via SLC-1. J Neuroendocrinol 15: 268–272, 2003. [DOI] [PubMed] [Google Scholar]
- 193.Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, et al. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med 8: 825–830, 2002. [DOI] [PubMed] [Google Scholar]
- 194.Chaki S, Funakoshi T, Hirota-Okuno S, Nishiguchi M, Shimazaki T, Iijima M, et al. Anxiolytic- and antidepressant-like profile of ATC0065 and ATC0175: nonpeptidic and orally active melanin-concentrating hormone receptor 1 antagonists. J Pharmacol Exp Ther 313: 831–839, 2005. [DOI] [PubMed] [Google Scholar]
- 195.Takekawa S, Asami A, Ishihara Y, Terauchi J, Kato K, Shimomura Y, et al. T-226296: a novel, orally active and selective melanin-concentrating hormone receptor antagonist. Eur J Pharmacol 438: 129–135, 2002. [DOI] [PubMed] [Google Scholar]
- 196.Ring RH. The central vasopressinergic system: examining the opportunities for psychiatric drug development. Curr Pharm Des 11: 205–225, 2005. [DOI] [PubMed] [Google Scholar]
- 197.Gold PW, Goodwin FK, Reus VI. Vasopressin in affective illness. Lancet 1: 1233–1236, 1978. [DOI] [PubMed] [Google Scholar]
- 198.van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, et al. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology 17: 284–292, 1997. [DOI] [PubMed] [Google Scholar]
- 199.de Winter RF, van Hemert AM, DeRijk RH, Zwinderman KH, Frankhuijzen-Sierevogel AC, Wiegant VM, et al. Anxious-retarded depression: relation with plasma vasopressin and cortisol. Neuropsychopharmacology 28: 140–147, 2003. [DOI] [PubMed] [Google Scholar]
- 200.Hernando F, Schoots O, Lolait SJ, Burbach JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology 142: 1659–1668, 2001. [DOI] [PubMed] [Google Scholar]
- 201.Serradeil-Le Gal C, Wagnon J, Simiand J, Griebel G, Lacour C, Guillon G, et al. Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo-2,3-di-hydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J Pharmacol Exp Ther 300: 1122–1130, 2002. [DOI] [PubMed] [Google Scholar]
- 202.Griebel G, Stemmelin J, Gal CS, Soubrie P. Non-peptide vasopressin V1b receptor antagonists as potential drugs for the treatment of stress-related disorders. Curr Pharm Des 11: 1549–1559, 2005. [DOI] [PubMed] [Google Scholar]
- 203.Griebel G, Simiand J, Stemmelin J, Gal CS, Steinberg R. The vasopressin V1b receptor as a therapeutic target in stress-related disorders. Curr Drug Targets CNS Neurol Disord 2: 191–200, 2003. [DOI] [PubMed] [Google Scholar]
- 204.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 34: 13–25, 2002. [DOI] [PubMed] [Google Scholar]
- 205.Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 25: 836–844, 2001. [DOI] [PubMed] [Google Scholar]
- 206.Malberg JE, Schechter LE. Increasing hippocampal neurogenesis: a novel mechanism for antidepressant drugs. Curr Pharm Des 11: 145–155, 2005. [DOI] [PubMed] [Google Scholar]
- 207.Conti AC, Blendy JA. Regulation of antidepressant activity by cAMP response element binding proteins. Mol Neurobiol 30: 143–155, 2004. [DOI] [PubMed] [Google Scholar]
- 208.Takahashi T, Nowakowski RS, Caviness VS Jr. BUdR as an S-phase marker for quantitative studies of cytokinetic behaviour in the murine cerebral ventricular zone. J Neurocytol 21: 185–197, 1992. [DOI] [PubMed] [Google Scholar]
- 209.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 50: 260–265, 2001. [DOI] [PubMed] [Google Scholar]
- 210.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 60: 804–815, 2003. [DOI] [PubMed] [Google Scholar]
- 211.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav 56: 131–137, 1997. [DOI] [PubMed] [Google Scholar]
- 212.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22: 3251–3261, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry 54: 994–1005, 2003. [DOI] [PubMed] [Google Scholar]
- 214.Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry 49: 753–762, 2001. [DOI] [PubMed] [Google Scholar]
- 215.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA Jr. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21: 7397–7403, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci 22: 3262–3268, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology 45: 553–563, 2003. [DOI] [PubMed] [Google Scholar]
- 218.Chourbaji S, Hellweg R, Brandis D, Zorner B, Zacher C, Lang UE, et al. Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res 121: 28–36, 2004. [DOI] [PubMed] [Google Scholar]
- 219.Olofsdotter K, Lindvall O, Asztely F. Increased synaptic inhibition in dentate gyrus of mice with reduced levels of endogenous brain-derived neurotrophic factor. Neuroscience 101: 531–539, 2000. [DOI] [PubMed] [Google Scholar]
- 220.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 23: 349–357, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA 101: 10827–10832, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Froestl W, Gallagher M, Jenkins H, Madrid A, Melcher T, Teichman S, et al. SGS742: the first GABA(B) receptor antagonist in clinical trials. Biochem Pharmacol 68: 1479–1487, 2004. [DOI] [PubMed] [Google Scholar]
- 223.Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J Neurochem 70: 1781–1792, 1998. [DOI] [PubMed] [Google Scholar]
- 224.Pollack SJ, Harper SJ. Small molecule Trk receptor agonists and other neurotrophic factor mimetics. Curr Drug Targets CNS Neurol Disord 1: 59–80, 2002. [DOI] [PubMed] [Google Scholar]
- 225.Bruno MA, Clarke PB, Seltzer A, Quirion R, Burgess K, Cuello AC, et al. Long-lasting rescue of age-associated deficits in cognition and the CNS cholinergic phenotype by a partial agonist peptidomimetic ligand of TrkA. J Neurosci 24: 8009–8018, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron 38: 157–160, 2003. [DOI] [PubMed] [Google Scholar]
- 227.Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci 23: 7311–7316, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dawson TM, Ginty DD. CREB family transcription factors inhibit neuronal suicide. Nat Med 8: 450–451, 2002. [DOI] [PubMed] [Google Scholar]
- 229.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28: 1562–1571, 2003. [DOI] [PubMed] [Google Scholar]
- 230.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809, 2003. [DOI] [PubMed] [Google Scholar]
- 232.Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci 20: 2896–2903, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res 1037: 204–208, 2005. [DOI] [PubMed] [Google Scholar]
- 234.Kurihara S, Hakuno F, Takahashi S. Insulin-like growth factor-I-dependent signal transduction pathways leading to the induction of cell growth and differentiation of human neuroblastoma cell line SH-SY5Y: the roles of MAP kinase pathway and PI 3-kinase pathway. Endocr J 47: 739–751, 2000. [DOI] [PubMed] [Google Scholar]
- 235.Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, et al. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci 24: 23–40, 2003. [DOI] [PubMed] [Google Scholar]
- 236.Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 29: 450–460, 2004. [DOI] [PubMed] [Google Scholar]