Abstract
Trichomonas vaginalis infection is highly prevalent worldwide and is associated with poor birth outcomes and enhanced human immunodeficiency virus transmission. Traditional detection methods rely on microscopic examination of vaginal specimens (wet mount) and culture, which can be insensitive and time-consuming. More than 3,000 women attending two sexually transmitted disease clinics were enrolled in this cross-sectional study to evaluate urine-based PCR for detection of T. vaginalis using a combined reference standard of wet mount and culture from vaginal swab. The prevalence of trichomoniasis in the population was 16.7% (502 of 3,009 women) using the reference standard. PCR with urine combined with agarose gel-based detection was 66.9% sensitive and 98.3% specific compared to the reference standard. Detection of PCR products using an unlabeled enzyme-linked immunosorbent assay (ELISA) improved the sensitivity to 86.4%, but specificity fell to 86.1%. Using a digoxigenin-labeled ELISA for detection of amplified T. vaginalis DNA from urine, the sensitivity and specificity of the PCR improved to 90.8 and 93.4%, respectively, compared to wet mount or culture from vaginal swabs. For clinical research settings in which vaginal specimens are not available and culture conditions are not feasible, urine-based PCR-ELISA may be useful for the detection of trichomoniasis in women.
The urogenital protozoan parasite Trichomonas vaginalis is the most common nonviral sexually transmitted pathogen in developing and industrialized countries (20, 21, 23). Infections in women cause vaginitis, urethritis, and cervicitis (4, 22); are associated with poor birth outcomes (3, 6, 17); and may enhance transmission of the human immunodeficiency virus (8, 10). Traditionally, diagnosis of trichomoniasis in women has relied on microscopic examination of wet mount preparations made directly from vaginal secretions. Although rapid and inexpensive, this technique is relatively insensitive (26) and requires collection of a vaginal sample. Microscopic examination of cultures is more sensitive but is time-consuming and more expensive. Diagnosis by either wet mount or culture depends heavily on skilled and experienced microscopists and requires the presence and maintenance of viable, motile trichomonads.
In recent years, urine-based molecular amplification detection assays for the bacterial sexually transmitted pathogens Chlamydia trachomatis and Neisseria gonorrhoeae have proven invaluable for diagnosis in routine patient care and for epidemiological research (1, 19, 24). The increased sensitivity over culture and antigen-based detection methods combined with the use of a noninvasive specimen and simple collection and storage parameters has led to the widespread use of these assays. This has been particularly useful in field research settings where urine is the only biological specimen available and culture conditions cannot be achieved. Accordingly, we sought to develop and validate a PCR-based assay with urine for the detection of T. vaginalis.
Several PCR-based diagnostic assays for trichomoniasis using vaginal specimens (7, 12, 25) and urine (11, 13, 28) from women have recently been described. Lawing et al. found that PCR from vaginal swabs was equivalent to culture (11). In the present report, we evaluated urine-based PCR using previously described primers (9) combined with agarose gel-based and enzyme-linked immunosorbant assay (ELISA) detection methods in a large-scale validation study. Estimates of sensitivity and specificity of the urine-based amplification tests were determined by comparison to a combined reference standard of wet mount and culture-positive vaginal swab specimens.
MATERIALS AND METHODS
Study population.
The cross-sectional study was conducted in the sexually transmitted disease (STD) clinics of two county health departments in central North Carolina. Women were enrolled between October 1998 and March 2000. Eligible subjects were between the ages of 18 and 65, spoke English, and denied use of oral or topical metronidazole during the 4 weeks prior to specimen collection. Women not meeting these criteria and those who refused to participate in the study were excluded.
Three thousand nine women agreed to participate in the study. Urine specimens were received for PCR testing from 2,930 women. The final study population comprises 2,147 women for whom the time and temperature limits for urine storage and transportation were maintained, as described below.
The study was approved by the Committee on the Protection of Human Subjects and the Institutional Review Board of the University of North Carolina at Chapel Hill.
Clinical data and specimen collection.
A medical history was recorded for all subjects according to the standard of care in the STD clinics. The standardized history form included questions on symptoms, past history of an STD, and sexual risk behaviors. In addition, subjects were asked the timing of the last urinary void prior to their clinic visit and frequency of douching. For this analysis, symptomatic women were defined as those with an abnormal vaginal discharge; subjects without a reported or observed abnormal discharge were considered asymptomatic.
Routine specimens were obtained from subjects during standardized pelvic examinations. Vaginal swabs were collected for wet mount microscopy and T. vaginalis culture; endocervical swabs were obtained for Gram staining, N. gonorrhoeae cultures, and C. trachomatis LCR (Abbott Diagnostics, Abbott Park, Ill.) or EIA (Wampole Laboratories, Cranbury, N.J.). After the physical examination, subjects were asked to provide up to 20 ml of first-void urine in marked, sterile specimen containers. Patients were instructed not to pour excess urine from the container if the 20-ml mark was exceeded. For PCR detection of T. vaginalis, specimen collection, storage, transport, and time limits for processing were based on parameters established for LCR detection of N. gonorrhoeae and C. trachomatis from urine (Abbott Diagnostics). Thus, urine specimens were stored at 4°C and transported on ice within 3 days of collection to the Microbiology Core Laboratory at the University of North Carolina at Chapel Hill.
Wet mount microscopy and T. vaginalis culture.
Immediately after collection, vaginal swabs were placed in sterile tubes containing 0.5 ml of normal saline and agitated. One drop of the saline mixture was placed on a glass slide with a coverslip and examined at a magnification of ×200. A positive result was defined as the presence of one or more trichomonads with characteristic morphology and motility. The InPouch TV culture system (Biomed, San Jose, Calif.) was immediately inoculated with a second vaginal swab according to the manufacturer’s instructions. Pouch cultures were examined microscopically on day 2 or 3 and again on day 5 after inoculation. A positive result was defined as the presence of motile trichomonads at any time; a negative result was defined as the absence of motile trichomonads at all readings.
T. vaginalis PCR from urine.
To prevent bias in interpretation, persons performing PCR were unaware of wet mount and culture results until after amplification results were recorded. During development of the urine-based PCR assay for T. vaginalis, several combinations of specimen preparation and amplified product detection methods were tested. Initially, urine was processed using a proteinase K treatment method. One milliliter of first void urine was centrifuged at 15,000 × g for 15 min. The pellet was resuspended in 0.5 ml of phosphate-buffered saline (PBS), centrifuged again and the final pellet was resuspended in 0.05 ml of sterile water. Proteinase K was added to a final concentration of 0.04 mg/ml, the solution was incubated for ≥1 h at 56°C, and the enzyme was inactivated for 10 min at 95°C. Prepared specimens were frozen at −20°C until PCR was performed, using 0.01 ml as the template. Reaction products from specimens prepared in this way were detected by agarose gel electrophoresis as previously described (8).
We tested a second specimen preparation technique using the Amplicor CT Urine Specimen Prep kit (Roche Diagnostic Systems, Indianapolis, Ind.). One milliliter of first void urine was processed according to the manufacturer’s instructions and frozen at −20°C until PCR was performed, using 0.05 ml as template. Specimens prepared by this procedure performed as well as or better than those prepared with the proteinase K treatment. Since the completion of this study, Amplicor has modified the CT urine prep buffers and procedures. The currently available kits perform equally well for trichomonas PCR; however, this study was conducted entirely with the earlier version of the product. PCR products from specimens prepared in this way were detected using one of the ELISA methods described below.
Oligonucleotide primers TVK3 and TVK7 (9) were used for all PCRs. These primers specifically amplify a 312-bp sequence from repetitive DNA in the T. vaginalis genome. PCR with TVK3 and TVK7 is negative with human DNA, other organisms found in the human genitourinary tract, and other Trichomonas species (9). Amplification reactions contained 20 pmol of each primer; 0.2 mM (each) dATP, dCTP, dGTP, and dTTP or dUTP; 2.5 U of Taq DNA polymerase (Gibco BRL, Grand Island, N.Y.); and 4 mM MgCl2 in 1× PCR buffer (Gibco BRL) in a final volume of 0.1 ml. AmpErase (uracil N-glycosylase; Applied Biosystems, Foster City, Calif.) was included in PCRs for detection by ELISA, and dUTP was substituted for dTTP in the reaction mixture. PCR consisted of an initial 5-min incubation at 90°C followed by 35 cycles of denaturation at 90°C for 1 min, annealing at 60°C for 30 s, and extension at 72°C for 2 min. Purified T. vaginalis DNA and sterile water were used as positive and negative controls, respectively.
ELISA detection of PCR products.
For the ELISA without label, Immulon 2 HB microtiter plates (Dynex Technologies, Chantilly, Va.) were coated (40 ng/well) with TVK probe (5′ TTCATGTCCTCTCCAAGCGTA 3′) in 1 M ammonium acetate. Plates were incubated at 37°C for 10 to 20 h, washed five times with phosphate-buffered saline containing 1 mM EDTA, and stored at 4°C in sealed plastic bags containing a dessicant pouch for up to 4 weeks. PCR was performed with 5′-biotinylated TVK3 and TVK7 for use with this ELISA. Amplified products were detected using the Amplicor CT detection kit according to the manufacturer’s instructions except that TVK probe-coated plates were substituted for the kit plates. In this procedure, a streptavidin-horseradish peroxidase conjugate binds amplified targets captured by the probe, a colorimetric substrate is added, and absorbance is measured at 450 nm. Samples and PCR positive and negative controls were tested in duplicate on each plate. In addition, a biotinylated oligonucleotide corresponding to the reverse complement of the TVK probe (40 ng/well) was used as an ELISA positive control for each plate. Tests were considered valid if the absorbances of ELISA and PCR positive controls were ≥1.0 and those of PCR negative controls were <0.1. The limit of detection for PCR combined with the unlabeled ELISA was between 1 and 10 organisms per PCR mixture prepared from urine. The average standard error on a typical plate was 14.5% of the mean for duplicate determinations.
We tested a second ELISA method using PCR with TVK3 and digoxigenin (DIG)-labeled TVK7 and biotinylated TVK probe (the TVK probe hybridizes to the amplified DNA strand generated with the TVK7 primer). PCR products were detected using the PCR ELISA DIG detection kit (Roche Diagnostic Systems) according to the manufacturer’s instructions. In this procedure, microtiter plates are precoated with streptavidin, which binds amplified target-probe complexes via the biotinylated TVK probe. The DIG-labeled target is recognized by an anti-DIG antibody-enzyme conjugate, a colorimetric substrate is added, and absorbance is measured at 405 nm. Samples and PCR positive and negative controls were tested in duplicate along with a DIG-labeled oligonucleotide corresponding to the reverse complement of the TVK probe as an ELISA positive control for each plate. Tests were considered valid if the absorbances of ELISA positive controls were ≥1.0, those of PCR positive controls were ≥2.0, and those of PCR negative controls were <0.2. The limits of detection for PCR combined with the DIG ELISA were two organisms per PCR mixture prepared from phosphate-buffered saline suspensions and eight organisms per PCR mixture prepared from urine. The average standard error on a typical plate was 3.0% of the mean for duplicate determinations.
Data analysis.
Data were entered into databases created and maintained in Microsoft Excel 2000 and EpiInfo (version 6.04; Centers for Disease Control and Prevention, Atlanta, Ga.). Clinical and laboratory data were double entered by different study personnel. Discrepant records were resolved by review of chart information. Data analysis was performed using SAS version 7 (SAS Institute, Cary, N.C.) and STATA version 6.0 (Stata Corp., College Station, Tex.).
For evaluation of the performance of the PCR assays, the reference standard was based on the combined results of wet mount microscopy and culture from vaginal swabs. Presence of trichomoniasis was defined as the presence of motile trichomonads by either test. Absence of trichomoniasis was defined as the absence of motile trichomonads by both tests. Estimates of the sensitivity and specificity and 95% confidence intervals (CIs) were computed for each PCR assay using standard methods. Nonparametric receiver-operating characteristic (ROC) analysis was performed for the two PCR-ELISAs. Sensitivity and specificity of the PCR were calculated for every possible ELISA absorbance cutoff value. Sensitivity was plotted against 1 - specificity at each value to obtain the ROC curve. The absorbance value corresponding to the upper leftmost point of the ROC curve is the ELISA cutoff point that jointly optimizes sensitivity and specificity.
T. vaginalis culture and wet mount are known to be imperfect tests. In particular, the sensitivity of these tests is recognized to be less than 1. As a result, specificity estimates using wet mount or culture as the reference standard are subject to reference test bias. We assessed the potential impact of this bias by adjusting the estimates of specificity using the formula of Staquet et al. (27). We assumed the sensitivity and specificity of the combined reference standard to be 0.70 and 1.0, respectively. This method also assumes the reference standard tests and PCR are independent. Under the assumption that the specificity of the reference standard is 1.0, the sensitivity estimates for the new tests are unbiased (27).
RESULTS
Patient population.
Of 4,015 women attending the two county health department STD clinics invited to participate in the study, 3,009 met the inclusion criteria. Only 2% of invited women refused to participate in the study. The majority of included and excluded subjects comprised African-Americans. Caucasians were the next most common in both groups. Hispanic women were more likely to be excluded, primarily due to the English-language-only inclusion criterion.
Women presenting to the clinics were most likely to seek medical attention because of symptoms, for STD screening, or because they had partners who had been diagnosed with an STD (Table 1). Twenty-three women (0.8% of the study population) reported a partner who had been diagnosed with T. vaginalis infection. Sixty-five percent of the women in the study were symptomatic.
TABLE 1.
Demographics of study invitees and clinical characteristics of study participants
| Characteristic | No. (%) in study population (n = 3,009) | No. (%) excluded (n = 1,006) |
|---|---|---|
| Race or ethnicity | ||
| African-American | 2,449 (81.4) | 721 (71.7) |
| Caucasian | 471 (15.7) | 106 (10.5) |
| Latina | 52 (1.7) | 144 (14.3) |
| Other | 35 (1.2) | 7 (0.7) |
| Not known | 2 (0.1) | 28 (2.8) |
| Age (yr)a | ||
| <18 | 0 | 204 (20.3) |
| 18–25 | 1,587 (52.7) | 389 (38.7) |
| 26–35 | 939 (31.3) | 261 (25.9) |
| 36–45 | 405 (13.5) | 108 (10.7) |
| >46 | 78 (2.6) | 19 (1.9) |
| Mean (SD) | 27.1 (7.8) | 25.2 (8.3) |
| Reason for exclusion | ||
| Age, <18 yr | 204 (20.3) | |
| Language | 143 (14.2) | |
| Metronidazole | 80 (8.0) | |
| Refused | 88 (8.7) | |
| Unable to void | 119 (11.8) | |
| Otherb | 372 (37.0) | |
| Reason for visitc | ||
| Symptoms | 1,954 (64.9) | |
| STD screening | 683 (22.7) | |
| STD contact | 377 (12.5) | |
| T. vaginalis contact | 23 (0.8) | |
| Referral | 14 (0.5) | |
| Complaintc | ||
| Discharge | 1,884 (62.8) | |
| Genital itch | 835 (27.8) | |
| Vaginal irritation | 716 (23.9) | |
| Abdominal pain | 706 (23.5) | |
| Dysuria | 462 (15.4) | |
| Genital ulcer | 97 (3.2) | |
| Skin rash | 128 (4.3) | |
| Other | 566 (18.9) | |
| STDs diagnosedd | ||
| C. trachomatis | 226 (10.4) | |
| N. gonorrhoeae | 174 (6.0) | |
| T. vaginalis | 502 (16.7) | |
| Bacterial vaginosise | 1,147 (38.1) | |
| Syphilis | 9 (0.3) |
Twenty-five women for whom age was unknown were excluded.
Other reasons for exclusion were improper specimen collection and scheduling conflicts.
Numbers exceed 100% because some women presented to the clinic for multiple reasons.
C. trachomatis diagnosed by EIA or LCR; N. gonorrhoeae diagnosed by culture; T. vaginalis diagnosed by wet mount microscopy and/or culture; bacterial vaginosis diagnosed by observation of three of the following: white or gray discharge, fishy odor of discharge, two or more clue cells per microscopic field, and vaginal pH of >4.5; syphilis diagnosed clinically, and includes all stages of syphilis as well as cases with stage unknown.
Bacterial vaginosis is included with classical STDs due to its association with certain risk factors and because it is most often diagnosed at STD clinics.
Bacterial vaginosis was the most commonly diagnosed reproductive tract infection, occurring in 38.1% of the study population. Chlamydial infection and gonorrhea prevalences were within the expected ranges for this study population.
Five hundred two women had trichomoniasis detected by wet mount microscopy or culture. Sixty-five percent (n = 286) of all trichomoniasis cases were positive by both wet mount microscopy and culture, 62 cases (12.3%) were detected by wet mount microscopy only, and 122 cases (24.3%) were detected by culture only.
Among the 1,194 study subjects diagnosed with an STD, 22% (n = 256) were diagnosed with at least two of gonorrhea, chlamydial infection, trichomoniasis, or bacterial vaginosis. Thirty-nine women (3.3%) were diagnosed with three of the four diseases, and two women (0.2%) were diagnosed with all four.
Performance of PCR for T. vaginalis using different detection methods.
The development and refinement of the PCR-based T. vaginalis detection assay included the use of several methods for detecting amplified products. Initially, PCR products were visualized as ethidium bromide-stained bands of approximately 300 bp resolved by agarose gel electrophoresis as previously described by our laboratory and others (8, 11, 28). A total of 1,513 urine specimens were tested using this method, 1,038 from symptomatic women and 475 from asymptomatic women. Using the combined reference standard of a positive wet mount or culture from a vaginal swab, urine-based PCR with gel detection performed with an overall sensitivity of 66.9% and specificity of 98.3% (Table 2).
TABLE 2.
T. vaginalis urine-based PCR performance with specimens from symptomatic and asymptomatic subjects
| Subject group | PCR products detected by:
|
|||||
|---|---|---|---|---|---|---|
| Agarose gel
|
Unlabeled ELISA
|
|||||
| n | % Sensitivity (95% CI) | % Specificity (95% CI) | n | % Sensitivity (95% CI) | % Specificity (95% CI) | |
| Asymptomatic | 475 | 64.3 (44.1, 80.7) | 100.0 (97.4, 100.0) | 186 | 80.0 (51.4, 94.7) | 82.2 (71.1, 89.8) |
| Symptomatic | 1,038 | 68.8 (61.8, 75.1) | 98.2 (97.0, 99.0) | 451 | 85.9 (76.7, 92.0) | 87.5 (83.5, 90.6) |
| Overall | 1,513 | 66.9 (60.8, 72.5) | 98.3 (97.4, 98.9) | 637 | 86.4 (78.6, 91.8) | 86.1 (83.2, 88.7) |
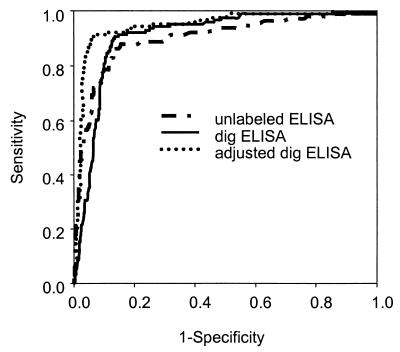
In an effort to increase the sensitivity of the PCR assay, we adopted an ELISA detection method using microtiter plates coated with an unlabeled oligonucleotide probe specific for an internal sequence of the amplified product which was generated with biotin-labeled primers as described in Materials and Methods. A total of 637 urine specimens were tested using this method: 451 from symptomatic women and 186 from asymptomatic women. A ROC curve was constructed comparing urine-based PCR with the unlabeled ELISA to the combined reference standard from a vaginal swab (Fig. 1). Sensitivity and specificity for this assay were jointly maximized at an absorbance cutoff of ≥0.500, yielding a sensitivity of 86.4% and specificity of 86.1% (Table 2).
FIG. 1.
ROC analysis of PCR-ELISAs for detection of T. vaginalis in women’s urine. ROC curves were plotted for the unlabeled ELISA, the DIG ELISA, and adjusted sensitivity values for the DIG ELISA.
The imperfect performance of urine PCR with the unlabeled ELISA prompted further modification of the detection method. In the third and final version of the urine-based PCR assay for T. vaginalis, amplified products were labeled with DIG, the internal probe was biotinylated, and the microtiter plates for the ELISA were coated with streptavidin as described in Materials and Methods. A total of 780 urine specimens were tested using this method (hereafter referred to as T. vaginalis PCR-ELISA): 549 from symptomatic women and 231 from asymptomatic women. ROC curves for the two ELISA detection methods are shown in Fig. 1. The absolute absorbance value corresponding to the cutoff point with the optimal joint sensitivity and specificity was much higher for T. vaginalis PCR-ELISA (3.0 versus 0.5 for the unlabeled ELISA).
Wet mount microscopy and culture are recognized to have virtually perfect specificity but imperfect sensitivity for detection of T. vaginalis. Thus, the observed specificity of T. vaginalis PCR-ELISA using wet mount or culture as the sole reference standard is likely underestimated. To examine the degree of this underestimation, we calculated an adjusted specificity using the formula of Staquet et al. (27). Assuming that wet mount or culture has a sensitivity of 70% and that the tests are independent, the sensitivity and estimated specificity of T. vaginalis PCR-ELISA were calculated at different absorbance cutoffs. The effect of the specificity adjustment on the ROC curve is shown in Fig. 1. Using an absorbance of 3.0 as the cutoff for the T. vaginalis PCR-ELISA resulted in an overall sensitivity of 90.8% and an adjusted specificity of 93.4% (Table 3). If the sensitivity of the reference standard is actually higher than our assumed value, the specificity of the new test will be slightly lower; if the sensitivity of wet mount or culture is actually lower, the specificity of T. vaginalis PCR-ELISA will be higher.
TABLE 3.
Urine-based T. vaginalis PCR-ELISA performance with specimens from symptomatic and asymptomatic subjects
| Subject group | n | % Sensitivity (95% CI) | % Specificity (95% CI) | Adjusted specificitya (%) |
|---|---|---|---|---|
| Asymptomatic | 231 | 89.5 (65.5, 98.2) | 83.5 (73.9, 90.2) | 90.7 |
| Symptomatic | 549 | 91.8 (84.1, 96.2) | 87.2 (83.6, 90.0) | 95.3 |
| Overall | 780 | 90.8 (84.2, 95.0) | 86.1 (83.2, 88.7) | 93.4 |
Adjusted specificity using the formula of Staquet et al. (27); assumes wet mount or culture has sensitivity of 70% and specificity of 100% and that tests are independent.
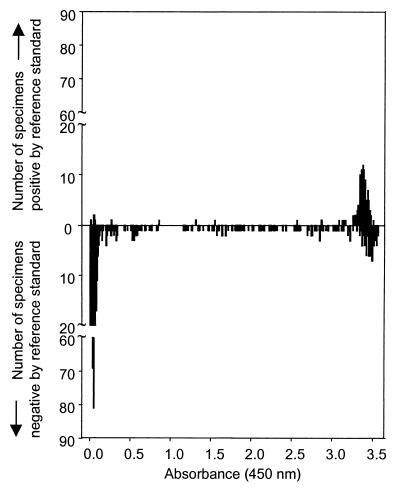
To provide additional insight into the performance of the T. vaginalis PCR-ELISA, we examined the distribution of absorbance values for the assay of urine specimens from subjects that tested positive or negative using the combined reference standard from a vaginal swab (Fig. 2). The histograms display two distinct peaks, with the first at absorbances below 0.3 containing specimens predominantly from women who were reference standard negative (n = 131). The second peak occurred at absorbances greater than 3.0 and contained primarily specimens from women who were reference standard positive but also a substantial number of women who were reference standard negative (n = 649). Fewer specimens (3.8% of reference standard positives and 17.4% of reference standard negatives) yielded absorbances between the two peaks.
FIG. 2.
Distribution of DIG ELISA absorbance values for T. vaginalis PCR using women’s urine. Histograms show data from women who were positive (upper panel) or negative (lower panel) by the reference standard of wet mount and culture from vaginal swabs.
Clinical factors affecting T. vaginalis PCR-ELISA performance.
We compared the performance of T. vaginalis PCR-ELISA using urine specimens from patients with different clinical characteristics. The test performed with equivalent sensitivity and specificity in patients with or without an abnormal vaginal discharge; a history of douching; or the presence of chlamydial infection, gonorrhea, or bacterial vaginosis (data not shown).
The volume of urine collected and time elapsed since the previous void were predicted to affect the performance of T. vaginalis PCR-ELISA. At higher specimen volumes, the expected concentration of organisms in urine is lower. Likewise, the more recent the previous void, the lower the expected concentration of organisms in the test specimen. Although subjects were instructed to collect only the first 20 ml of first-void urine, many women were unable to comply with these instructions. Specimen volumes ranged from 2 to 100 ml, with a mean volume of 30.8 ml (standard error of the mean, 11.9 ml). The assay was slightly more sensitive with urine specimens with smaller volumes (≤20 ml) and when more than 1.5 h had elapsed since the previous void (Table 4).
TABLE 4.
Effect of specimen collection parameters on T. vaginalis PCR-ELISA performance
| Specimen parameter | n | % Sensitivity (95% CI) | % Specificity (95% CI) | % Adjusted specificitya (%) |
|---|---|---|---|---|
| Urine volume (ml) | ||||
| 20 | 186 | 100.0 (82.2, 100.0) | 84.7 (78.0, 89.6) | 90.1 |
| >20 | 594 | 88.9 (81.0, 93.9) | 86.6 (83.2, 89.5) | 94.6 |
| Time since last void (h) | ||||
| >1.5 | 268 | 91.3 (78.3, 97.2) | 86.9 (81.6, 90.9) | 94.6 |
| 1.5 | 139 | 85.7 (66.4, 95.3) | 87.4 (79.4, 92.7) | 96.2 |
Adjusted specificity using the formula of Staquet et al. (27); assumes wet mount or culture has sensitivity of 70% and specificity of 100% and that tests are independent.
DISCUSSION
The urine-based T. vaginalis PCR-ELISA performed with an overall sensitivity of 90.8% and an adjusted specificity of 93.4% compared to wet mount and culture from vaginal swabs. Detection of amplified target DNA using an ELISA provided an objective result and improved the sensitivity of the urine based PCR assay compared to that achieved with gel electrophoresis. We used two different strategies in the development of T. vaginalis PCR-ELISA. The unlabeled ELISA relied on hybridization of PCR products to an unlabeled oligonucleotide probe coated onto the wells of a microtiter plate. Although the sensitivity of PCR combined with the unlabeled ELISA was improved compared with gel-based detection, the assay specificity was decreased, and there was unacceptable variation in the absorbance values of duplicate samples. We attributed the poor performance of the unlabeled ELISA to the uncontrolled orientation of the capture probe bound to the microtiter plate and inconsistent accessibility of the probe for hybridization with amplified target DNA. Using streptavidin-coated microtiter plates to bind the biotin-labeled capture probe in the DIG ELISA dramatically improved the performance of the assay. Absorbance values for positive specimens were considerably higher than those with the unlabeled ELISA, and variation between duplicate values was minimal.
Other studies using PCR for detection of T. vaginalis in women’s urine report sensitivities between 64 and 100% and specificities from 97 to 100% (11, 13, 28). While these studies are similar in their use of nucleic acid amplification technology, important differences in urine collection and processing, PCR primers, and detection methods make direct comparisons between assays difficult. In a recent comparative study, the primers used in the current analysis (TVK3 and TVK7) performed better than other previously reported PCR primer pairs for T. vaginalis detection in cervicovaginal swab specimens (T. Crucitti, E. Van Dyck, S. Abdellati, and M. Laga, Abstr. Int. Congr. Sex. Transm. Dis., p. 37, 2001). In the T. vaginalis PCR-ELISA described here, 1 ml of first-void urine was concentrated and processed without DNA extraction or purification, whereas DNA was extracted from the sediment of up to 50 ml of first-void urine before amplification in other studies (11, 13, 28).
As with evaluation of any new diagnostic test based on nucleic acid amplification, the selection of a reference standard was central to the design and outcome of this study. The choice of vaginal wet mount microscopy and culture as the reference standard is patient based, reflecting an acceptable reference standard for trichomoniasis in women. Though T. vaginalis can be detected on the vaginal epithelium and in urine, results from both sites are not always concordant. In general, vaginal specimens are more often positive than urine, whether trichomonads are detected by wet mount and culture or by PCR (11, 18). Consequently, diagnosis from urine assay alone is likely to underestimate the true extent of trichomoniasis in a given population of women. In a pilot study, we compared urine culture to wet mount and culture from vaginal swabs for detection of T. vaginalis in our patient population. Detection from vaginal specimens was more sensitive than that from urine; no additional cases of trichomoniasis were identified in urine cultures beyond those identified in vaginal specimens (data not shown). Thus, we did not compare the PCR assays to culture of urine specimens only. Such a comparison would have overestimated test performance, especially given the predominance of infections detected by vaginal preparations only.
Although there are clearly limitations to urine compared to vaginal specimens for T. vaginalis detection in women, the choice of urine for validation of PCR in this study reflected several considerations for large-scale population-based studies. While self-collected vaginal swabs may have performed better than urine, there are field studies under way and in the planning stages for which even this minimally invasive sampling is difficult. Since urine-based amplification tests for gonorrhea and chlamydia are routinely used, development of a test for trichomoniasis that would not require an additional specimen was desirable. In addition, urine can be obtained from women and men with uniform specimen collection procedures, making urine a valuable, if imperfect, specimen.
Comparative studies such as this one, in which an amplification test with the potential for high sensitivity is judged against a less-sensitive culture method, produce a now-familiar dilemma: how to estimate specificity when it is likely that some positive results classified as false positives are, in fact, true positives. Discrepant analysis, which subjects discordant results to additional testing and reclassification, has been used in many previous evaluations of new amplification tests. However, discrepant analysis does not provide valid estimates of sensitivity or specificity, and its use has been discouraged (5, 14–16). As an alternative to discrepant analysis, we algebraically adjusted specificity estimates for T. vaginalis PCR-ELISA (27). Using reasonable assumptions for the performance of wet mount microscopy and culture, this procedure is unlikely to overestimate the specificity of the new test. We did not adjust the sensitivity estimate, because we assumed the specificity of our combined reference standard to be 1.0. However, our sensitivity may be slightly overestimated due to the potential for concordant false negatives for PCR and the reference standard. The estimated sensitivity of the PCR assay is based upon specimens positive by the combined reference standard. One would predict that the sensitivity would be lower among women with trichomoniasis but a negative combined reference test (16). If these results could be incorporated into the sensitivity estimate, the figure would be lowered slightly.
The observed and adjusted specificities of the T. vaginalis PCR-ELISA are lower than expected for an amplification test. This relatively low specificity could potentially result from the reference test bias noted above (possibly with insufficient adjustment algebraically), specimen contamination, or inadequate primer specificity. There are two pieces of evidence suggesting that the observed specificity for the PCR-ELISA is an underestimate due to reference test bias. Careful examination of the ROC curve (Fig. 1 [dig ELISA]) reveals that there is an early deviation of the curve from the vertical axis. The specificity drops off rapidly, even at very high cutoff values. This shape of the ROC curve is consistent with the misclassification of truly positive specimens as false positives. Similarly, the substantial number of subjects with positive PCR assays at high absorbances in women with negative wet mounts and cultures (Fig. 2) suggests that a significant number of women with trichomoniasis, correctly identified by the PCR, are being misclassified because of the low sensitivity of the reference standard. Typically, the probability of a false-positive result is greater near the chosen cutoff, rather than at more extreme values. These observations indicate that the specificity may actually be even higher than the adjusted values.
The relatively uncomplicated urine collection and processing procedures adopted for this study make T. vaginalis PCR-ELISA an attractive assay for the detection of T. vaginalis in certain research settings. However, the very features that make this assay useful from a practical perspective may also explain its relative insensitivity compared to urine-based nucleic acid amplification assays for other STD agents. Unlike the gram-negative bacterial pathogens N. gonorrhoeae and C. trachomatis, the protozoan T. vaginalis lacks a protective outer membrane. Thus, trichomonads are more fragile than most bacteria and may not survive or remain physically intact during urine storage and centrifugation before the target DNA can be concentrated and further processed. The addition of a preservative or protectant to urine immediately after collection may stabilize parasites and further improve the performance of this assay. In a recent report, published after the completion of the present study, Williams et al. used Fuji culture medium in this context with promising results (J. A. Williams, N. J. Smith, and V. Van Der Pol, Abstr. Int. Congr. Sex. Transm. Dis., p. 37, 2001).
PCR inhibitors represent another problem in the development of urine-based T. vaginalis detection assays. In experiments using laboratory-grown trichomonads added to urine from study subjects, approximately 9% of specimens showed evidence of inhibition (data not shown). Inhibition was abolished when smaller template volumes were used for PCR amplification or when preparations were extracted with phenol-chloroform and precipitated with sodium acetate; however, we did not systematically assess inhibitory activity in urines throughout the study. The addition of an internal amplification control and interpretation guidelines for inhibitory specimens should greatly improve the performance of urine-based amplification assays for detection of T. vaginalis.
Despite the high prevalence of T. vaginalis infection worldwide (20, 21, 23) and the association of trichomoniasis with adverse pregnancy outcomes (3, 6, 17) and more recently with increased HIV transmission (2, 10), efforts to improve diagnosis of this STD have not kept pace with diagnostic developments for gonorrhea and chlamydia. Particularly for large-scale, population-based studies conducted without clinic or immediate laboratory access, reliable and sensitive urine-based testing for T. vaginalis is necessary. The current study was specifically designed to fulfill this need. Accordingly, urine specimen collection, storage, and transport conditions implemented in this study were kept simple so that these conditions could be reproduced in field studies. Urine specimen processing was likewise simplified using sample volumes and commercially available kit reagents optimized for batch handling in typical laboratory microcentrifuges. PCR combined with ELISA for detection of T. vaginalis in women’s urine performed well compared to wet mount microscopy and culture from vaginal swabs. Further improvements in the assay and validation of the test using specimens from men are currently under way. The T. vaginalis PCR-ELISA should be useful for the detection of this under-studied organism in settings where microscopy and culture are not practical.
. .
Acknowledgments
This work was supported by NIH Sexually Transmitted Diseases Clinical Trials Unit contract N01 AI75329, NIH Sexually Transmitted Diseases Cooperative Research Centers grant UO1 AI31496, and the NIH National Study of Adolescent Health: Survey 2000 grant P01 HD31921. W.C.M. received support through the Clinical Associate Physician Program of the General Clinical Research Center (RR00046), Division of Research Resources, National Institutes of Health.
We thank Zenaida Klepovic, Karen Lau, Linda Brown, Gail Lieblang, Karen Best, and the clinicians and patients at the county health departments for enrollment and specimen collection. Karen Lau and Lashonda Bryant were instrumental in data entry, and Robert Krysiak, Natasha Harvey, and Jay Gratz provided excellent laboratory assistance. We are grateful to James Williams and Barbara Van Der Pol at Indiana University School of Medicine for providing the TVK probe sequence prior to its publication. We thank David Savitz and Lola Stamm for critically reading the manuscript.
REFERENCES
- 1.Carroll, K. C., W. E. Aldeen, M. Morrison, R. Anderson, D. Lee, and S. Mottice. 1998. Evaluation of the Abbott LCx ligase chain reaction assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine and genital swab specimens from a sexually transmitted disease clinic population. J. Clin. Microbiol. 36:1630–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen, M. S., I. F. Hoffman, R. A. Royce, P. Kazembe, J. R. Dyer, C. Costello Daly, D. Zimba, P. L. Vernazza, M. Maida, S. A. Fiscus, and J. J. Eron. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 349:1868–1875. [DOI] [PubMed] [Google Scholar]
- 3.Cotch, M. F., J. G. Pastorek II, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Regan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, and G. G. Rhoads. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex. Transm. Dis. 24:353–360. [DOI] [PubMed] [Google Scholar]
- 4.Fouts, A. C., and S. J. Kraus. 1980. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J. Infect. Dis. 141:137–143. [DOI] [PubMed] [Google Scholar]
- 5.Hadgu, A. 1996. The discrepancy in discrepant analysis. Lancet 348:592–593. [DOI] [PubMed] [Google Scholar]
- 6.Heine, P., and J. A. McGregor. 1993. Trichomonas vaginalis: a reemerging pathogen. Clin. Obstet. Gynecol. 36:137–144. [DOI] [PubMed] [Google Scholar]
- 7.Heine, R. P., H. C. Wiesenfeld, R. L. Sweet, and S. S. Witkin. 1997. Polymerase chain reaction analysis of distal vaginal specimens: a less invasive strategy for detection of Trichomonas vaginalis. Clin. Infect. Dis. 24:985–987. [DOI] [PubMed] [Google Scholar]
- 8.Hobbs, M. M., P. Kazembe, A. W. Reed, W. C. Miller, E. Nkata, D. Zimba, C. C. Daly, H. Chakraborty, M. S. Cohen, and I. Hoffman. 1999. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sex. Transm. Dis. 26:381–387. [DOI] [PubMed] [Google Scholar]
- 9.Kengne, P., F. Veas, N. Vidal, J. L. Rey, and G. Cuny. 1994. Trichomonas vaginalis: repeated DNA target for highly sensitive and specific polymerase chain reaction diagnosis. Cell. Mol. Biol. 40:819–831. [PubMed] [Google Scholar]
- 10.Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, N. Nzila, J. Goeman, F. Behets, V. Batter, and M. Alary. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95–102. [DOI] [PubMed] [Google Scholar]
- 11.Lawing, L. F., S. R. Hedges, and J. R. Schwebke. 2000. Detection of trichomonosis in vaginal and urine specimens from women by culture and PCR. J. Clin. Microbiol. 38:3585–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madico, G., T. C. Quinn, A. Rompalo, K. T. McKee, Jr., and C. A. Gaydos. 1998. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J. Clin. Microbiol. 36:3205–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayta, H., R. H. Gilman, M. M. Calderon, A. Gottlieb, G. Soto, I. Tuero, S. Sanchez, and A. Vivar. 2000. 18S ribosomal DNA-based PCR for diagnosis of Trichomonas vaginalis. J. Clin. Microbiol. 38:2683–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAdam, A. J. 2000. Discrepant analysis: how can we test a test? J. Clin. Microbiol. 38:2027–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller, W. C. 1998. Bias in discrepant analysis: when two wrongs don’t make a right. J. Clin. Epidemiol. 51:219–231. [DOI] [PubMed] [Google Scholar]
- 16.Miller, W. C. 1998. Can we do better than discrepant analysis for new diagnostic test evaluation? Clin. Infect. Dis. 27:1186–1193. [DOI] [PubMed] [Google Scholar]
- 17.Minkoff, H., A. N. Grunebaum, R. H. Schwarz, J. Feldman, M. Cummings, W. Crombleholme, L. Clark, G. Pringle, and W. M. McCormack. 1984. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am. J. Obstet. Gynecol. 150:965–972. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed, O. A., C. R. Cohen, D. Kungu, M. A. Kuyoh, J. A. Onyango, J. J. Bwayo, M. Welsh, and P. J. Feldblum. 2001. Urine proves a poor specimen for culture of Trichomonas vaginalis in women. Sex. Transm. Infect. 77:78–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palladino, S., J. W. Pearman, I. D. Kay, D. W. Smith, G. B. Harnett, M. Woods, L. Marshall, and J. McCloskey. 1999. Diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae genitourinary infections in males by the Amplicor PCR assay of urine. Diagn. Microbiol. Infect. Dis. 33:141–146. [DOI] [PubMed] [Google Scholar]
- 20.Petrin, D., K. Delgaty, R. Bhatt, and G. Garber. 1998. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 11:300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn, T. C., and J. N. Kreiger. 1990. Trichomoniasis, p. 358. In K. S. Warren and A. A. F. Mahmoud (ed.), Tropical and geographic medicine, 2nd ed. McGraw-Hill, New York, N.Y.
- 22.Rein, M. F. 1990. Clinical manifestation of urogenital trichomoniasis in women, p. 225–234. In B. M. Honigberg (ed.), Trichomonads parasitic in humans. Springer-Verlag, New York, N.Y.
- 23.Rein, M. F., and M. Muller. 1990. Trichomonas vaginalis and trichomoniasis, p. 480. In K. K. Holmes, P.-A. Mardh, P. F. Sparling, and P. Wiesner (ed.), Sexually transmitted diseases, 2nd ed. McGraw-Hill, New York, N.Y.
- 24.Schachter, J. 1983. Urine as a specimen for diagnosis of sexually transmitted diseases. Am. J. Med. 75:93–97. [DOI] [PubMed] [Google Scholar]
- 25.Shaio, M. F., P. R. Lin, and J. Y. Liu. 1997. Colorimetric one-tube nested PCR for detection of Trichomonas vaginalis in vaginal discharge. J. Clin. Microbiol. 35:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spence, M. R., D. H. Hollander, J. Smith, L. McCaig, D. Sewell, and M. Brockman. 1980. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex. Transm. Dis. 7:168–171. [DOI] [PubMed] [Google Scholar]
- 27.Staquet, M., M. Rozencweig, Y. J. Lee, and F. M. Muggia. 1981. Methodology for the assessment of new dichotomous diagnostic tests. J. Chron. Dis. 34:599–610. [DOI] [PubMed] [Google Scholar]
- 28.van Der Schee, C., A. van Belkum, L. Zwijgers, E. van Der Brugge, E. L. O’Neill, A. Luijendijk, T. van Rijsoort-Vos, W. I. van Der Meijden, H. Verbrugh, and H. J. Sluiters. 1999. Improved diagnosis of Trichomonas vaginalis infection by PCR using vaginal swabs and urine specimens compared to diagnosis by wet mount microscopy, culture, and fluorescent staining. J. Clin. Microbiol. 37:4127–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]