Abstract
Chlamydia trachomatis is the leading cause of sexually transmitted disease in the United States. Effective screening for this agent can facilitate prompt treatment and prevent its sequelae. The recent introduction of liquid-based cytology has made possible the simultaneous screening of cervical intraepithelial lesions and detection of C. trachomatis in a single collection vial. In this study we determined whether cytological fluid could support DNA-based amplification for the detection of C. trachomatis. Three methods were compared, including ramification amplification (RAM), real-time PCR with molecular beacon, and Abbott’s ligase chain reaction (LCx). RAM is a novel, recently introduced, isothermal DNA amplification technique that utilizes a circular probe for target detection and achieves exponential amplification through the mechanism of primer extension, strand displacement, and ramification. Our results show that RAM can detect as few as 10 C. trachomatis elementary bodies in less than 2 h, comparable to results with real-time PCR. Thirty clinical specimens collected in PreservCyt solution were tested by LCx, real-time PCR, and RAM. Among 30 specimens, 15 were positive by PCR and LCx and 14 were positive by RAM. One specimen missed by RAM had an inadequate amount of residual cellular material. Our results show that nucleic acid amplification methods can serve to detect C. trachomatis and presumably other sexually transmitted agents in cytological fluid and that the RAM assay can be an alternative to PCR and LCx because of its simplicity and isothermal amplification.
Chlamydia trachomatis is one of the leading causes of sexually transmitted diseases (STDs) in the Western world. An estimated four million cases occur annually in the United States (2, 10). Since infection with this agent is often asymptomatic (18, 19), many infected individuals are not readily identified and go untreated. Therefore, it is important to identify patients infected with this organism not only to reduce transmission but also to minimize the risk of more serious infections and their sequelae (17). Increasing awareness of the need to contain the spread of chlamydia in the United States has led to recommendations by clinicians and government agencies to adopt widespread screening for this pathogen (15, 20).
Until recently, the gold standard for identifying C. trachomatis has been the culture method. Although specific, this procedure is time consuming and laborious and is unfit for the routine screening of C. trachomatis in clinical specimens (6). With the advent of molecular testing, most laboratories have abandoned the culture method and instead have relied on the use of such sensitive and convenient techniques as PCR (4, 6), ligase chain reaction (4), strand displacement assay (4), and nucleic acid-based amplification (14). Our laboratory has recently invented a novel molecular technique, which we termed the ramification amplification method (RAM) (21–23). The technique utilizes a circular probe (C-probe) for target detection and a capture probe for target isolation. One of the significant advantages of the technique is that there is no need for thermocycling to facilitate the amplification. Furthermore, the combination of magnetic isolation and isothermal DNA amplification makes this assay amenable for clinical diagnosis.
The implementation of fluid-based collection of endocervical scrapes into PreservCyt (Cytyc Corporation, Boxborough, Mass.) for Pap testing facilitates screening of female populations by cytological examination. It follows that a sensitive DNA amplification method could also be used to detect STDs, including human papillomavirus (F. Yarkin, S. Chauvin, N. Konomi, W. Wang, R. Mo, G. Bauchman, A. Diaz, D. Burstein, A. Szporn, E. Hauptman, and D. Y. Zhang, submitted for publication) and C. trachomatis (9) from the same PreservCyt collection vial. The aim of the study was to determine the feasibility of detecting C. trachomatis DNA in a gynecological sample collected in PreservCyt solution by RAM technology and to compare results of RAM assay with those of Abbott’s ligase chain reaction method (LCx) and with a real-time PCR method.
MATERIALS AND METHODS
Sample preparation.
A group of 30 residual endocervical specimens in PreservCyt were selected for this study. Of this group, 15 specimens tested positive and 15 tested negative for C. trachomatis DNA by LCx and real-time PCR. For the RAM assay, 1 ml of each specimen was centrifuged at 14,000 rpm for 15 min in an Eppendorf centrifuge 5417C. The sediment was lysed by incubation at 60°C for 60 min in 50 to 100 μl of a lysis buffer containing 400 mM Tris-HCl buffer (pH 7.5), 5 M guanidium thiocyanate (GTC) (Sigma, St. Louis, Mo.), 0.5% bovine serum albumin (Sigma), 80 mM EDTA, and 0.5% sodium-N-lauroylsarcosine (Sigma). The lysed specimens were stored at −20°C until use.
For real-time PCR testing, DNA was extracted with 1% ethylene glycol monomethyl ether in 2 mM borate buffer (pH 9.5). Briefly, 1 ml of PreservCyt sample was centrifuged at 13,000 rpm for 10 min, the supernatant was decanted, and the cell pellet was then resuspended in a 200-μl aliquot of the extraction fluid. The cell pellet was vortexed, and the suspension was then heated at 98°C for 10 min. The solution containing the extracted DNA was then brought to room temperature and tested by PCR.
RAM assay.
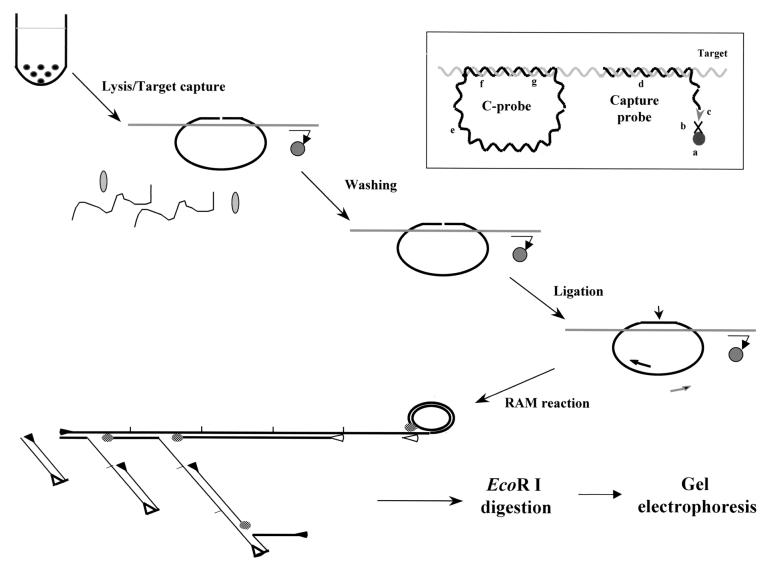
The RAM assay consists of steps including hybridization of the C-probe and capture probe to a target, capture of the hybrid onto magnetic beads, washing of the beads to remove unbound probes and cellular components, ligation of the 3′ and 5′ ends to form a closed C-probe, and amplification by primer extension, strand displacement, and ramification (Fig. 1) (23). Hybridization of the C-probe, capture probe, and target was carried out in an 80-μl reaction mixture containing 2 M GTC, 0.5% bovine serum albumin (Sigma), 80 mM EDTA, 400 mM Tris-HCl (pH 7.5), 0.5% sodium-N-lauroylsarcosine (Sigma), 50 nM phosphorylated C-probe, 2 μM capture probe (Table 1), and 5 to 15 μl of lysed specimens or DNA target. The reaction was incubated at 55°C for 2 h to allow hybrid formation. Three microliters of streptavidin-coated magnetic beads (10 mg/ml; Dynal, Lake Success, N.Y.), 80 μl of 10 mM Tris-HCl (pH 7.5) with 1 mM EDTA, and 2 M NaCl were added to the hybridization mixture and incubated at room temperature for 20 min to allow the hybrids to be captured on the beads through the binding of biotin of the capture probe with streptavidin coated on the bead surface. The beads with bound complex were then washed twice with 400 μl of TE buffer (10 mM Tris-HCl [pH 8] and 1 mM EDTA) at room temperature to remove unhybridized C-probes and other cellular components. Twenty microliters of ligase mixture containing 20 mM Tris-HCl (pH 7.6), 25 mM potassium acetate, 10 mM magnesium acetate, 10 mM dithiothreitol, 1 mM nicotiamide adenosine dinucleotides 0.1% Triton X-100, and 12 U of Taq DNA ligase (New England Biolabs, Beverly, Mass.) were added to the bead pellet and incubated at 60°C for 20 min. Ligation of the 3′ and 5′ ends hybridized to the target allowed the formation of a closed circle that locked on the target. After ligation, the RAM reaction solution was aspirated and the beads were suspended in 50 μl of RAM reaction smixture containing 300 μM deoxynucleoside triphosphate (USB Biochemicals), 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, 0.1% Triton X-100, 1.2 μM concentration of forward and reverse primers, 6.4 U of Bst DNA polymerase large fragment (New England Biolabs), 1 μg of T4 gene 32 protein (USB Biochemicals), and 6% dimethyl sulfoxide. This mixture was incubated at 63°C for 1 h, followed by heating at 95°C for 5 min to inactivate Bst DNA polymerase. Fifteen microliters of RAM reaction products were transferred to 5 μl of 50 mM Tris-HCl buffer (pH 7) containing 15 U of EcoRI (Boehringer Mannheim), 100 mM MgCl2, 1 mM dithiothreitol and incubated at 37°C for 3 h. The digested products were analyzed on a 2% agarose gel.
FIG. 1.
Schematic representation of the RAM assay. Target DNA, capture probe, C-probe, and paramagnetic bead are added to hybridization buffer to allow formation of a hybrid complex. The hybrid is captured on a paramagnetic bead, allowing extensive washing to remove unbound C-probe and cellular components. The C-probe aligned on the target is linked together by a DNA ligase. RAM amplification is then carried out by the addition of forward (▸) and reverse (◃) primers and DNA polymerase (shaded ovals). The forward primer bound to the C-probe is extended by the polymerase and continues after one round of synthesis by displacing the bound forward primer and its extended product, generating a long ssDNA with repeated sequence. With the reactions going on, multiple reverse primers can bind to the nascent ssDNA as their binding sites are available. Each bound reverse primer will be extended and displace the downstream primers and their extended products. The forward primer binding sites of the displaced ssDNA are then available for the forward primer to bind and extend similarly, thus forming a large ramifying DNA complex. Finally, the RAM products are examined by gel electrophoresis after EcoRI digestion. Insert: the C-probe hybridizes to the target through their complementary regions (f and g), and sequence at the noncomplementary region (e) is generic for the binding of primers. The C-probe-target hybrid is captured on a paramagnetic bead (a) through the binding of the biotin moiety (c) of the capture probe (d) to streptavidin (b) coated on the beads.
TABLE 1.
Sequences of circular probe, capture probe, RAM and PCR primers, and synthetic target sequencea
| Probes (no. of nucleotides) | Sequence (5′ to 3′) |
|---|---|
| C-probe (124) | GGTTTTGTCTTCGTAACTCGCTCCGGATGTCTGTGTATCTGCTAACCAAGAGCAACTACACGAATTCTCGATTAGGTTACTGCGATTAGCACAAGCTCTACAAGAGTACATCGGTCAACGAAGA |
| Capture probe (43) | Biotin-AAGAGCTTAAGAACCGTCAGACAGAAAAGAGGATTATTATAAC |
| RAM primer-1 (25) | CTTGTGCTAATCGCAGTAACCTAAT (forward) |
| RAM primer-2 (23) | ACCAAGAGCAACTACACGAATTC (reverse) |
| C. trachomatis target (97) | TCCGGAGCGAGTTACGAAGACAAAACC|TCTTCGTTGACCGATGTACTCTTGTAGAAA |
| GTTATAATAATCCTCTTTTCTGTCTGACGGTTCTTAAGC | |
| Molecular beacon (42) | CCGTCACTGGGAGAAAGAAATGGTAGGTTGTTGGAATGACGG |
| PCR primer 1 (20) | TCTTTTCTCTCTGACGGTTC |
| PCR primer 2 (20) | AGGTTGGAGATTAGTCAGAT |
Single underlines indicate sequences complementary to C. trachomatis EM; italic letters indicate restriction enzyme EcoRI recognition site (GAATTC); underline and bold letters indicate the binding region for RAM primer 1; dotted underline and bold letters indicate the binding region for RAM primer 2. For the C. trachomatis target, the single underlines indicate C-probe binding region; the vertical bar indicate contact sites of the 3′ and 5′ ends of the C-probe; double underline indicates the capture probe binding region. The underlined section in the molecular beacon highlights the stem portion of the molecular beacon.
Real-time PCR detection of C. trachomatis DNA.
The PCR amplification of C. trachomatis DNA was performed with a primer set (Table 1) that is specific to the KL1 and KL2 region of the C. trachomatis plasmid (12, 13). This set of primers amplified a 79-bp sequence. Amplification product was detected with a molecular beacon of 42 nucleotides in length (Table 1). Real-time PCR amplification and detection were achieved using the LightCycler PCR System (Roche Molecular Biochemicals, Indianapolis, Ind.) as described previously (12). The reaction was carried out in a 20-μl mixture containing 50 mM KCl, 10 mM Tris-HCl buffer (pH 8.3), 8.33 mM MgCl2, 200 μM of each deoxynucleoside triphosphate, 0.75 M betaine, 1 μM concentrations of each primer, 1 U of Platinum Taq Polymerase (Perkin Elmer Cetus, P.E. Roche Molecular System, Inc., Branchburg, N.J.), 50 nM molecular beacon, and 4 μl of target DNA. Typical thermocycling conditions included an initial denaturation step at 95°C for 1 min followed by 45 cycles each consisting of a denaturation step at 95°C for 5 s, primer and molecular beacon annealing steps at 48°C (20 s) and 53°C (22 s), and an extension step at 72°C (10 s).
LCx assay.
Abbott’s LCx assay was performed according to the manufacturer’s instructions but on DNA that was extracted from cells that were pelleted from PreservCyt solution. Unless otherwise indicated, the DNA from the cells was extracted by Abbott’s LCx urine protocol (manufacturer’s insert sheet, no. 3B21-24).
RESULTS
Analytical sensitivity of RAM assay.
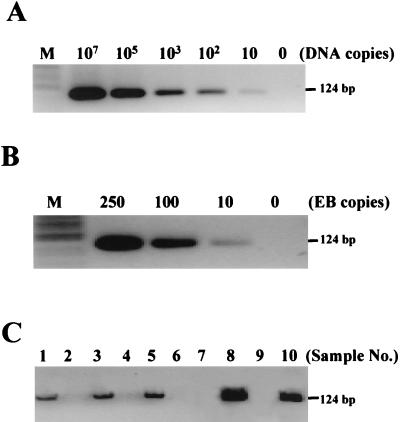
The analytical sensitivity of the RAM assay was determined with a synthetic C. trachomatis DNA target (Table 1). The DNA was diluted in 10-fold serials from 107 to 101 molecules and was used for RAM assay. Our results show that the lowest number of targets detected by this assay was 10 molecules (Fig. 2A), and the reactions were confirmed by finding the correct products (124 bp) in each lane after digestion with EcoRI, establishing the C-probe as their source. In the absence of target molecule, no DNA was produced, proving the target-dependent amplification of the C-probe. The assay sensitivity was further determined using C. trachomatis elementary bodies (EB) collected in Cytyc PreservCyt solution. The C. trachomatis EB were obtained from infected McCoy cell culture and quantified by direct counts after fluorescent antibody staining (1). The C. trachomatis EB were lysed in 5 M GTC, diluted to 250, 100, and 10 copies, and used for the RAM assay. The results in Fig. 2B show that the assay detected as few as 10 EB copies, comparable to the sensitivity of real-time PCR (data not shown).
FIG. 2.
Detection of C. trachomatis by RAM assay. (A) The RAM reactions were initiated with the synthetic C. trachomatis targets from 107 to 10 molecules as described in Materials and Methods. The RAM products were examined on a 2% agarose gel after digestion with EcoRI. The results show that 10 molecules of C. trachomatis targets were detected. (B) The sensitivity was further confirmed with C. trachomatis EB that were diluted to 250, 100, and 10 copies. As few as 10 copies of C. trachomatis EB can be detected. Lane 0, no target present. (C) RAM products amplified from 10 patient-derived specimens (no. 1 through no. 10) were examined through agarose gel electrophoresis after EcoRI digestion. The presence of a 124-bp band indicates positivity for C. trachomatis.
Detection of C. trachomatis in patient samples.
The samples were initially tested by real-time PCR and LCx for C. trachomatis and then by the RAM assay. Of the 30 samples, 15 samples tested positive for C. trachomatis DNA and 15 yielded a negative C. trachomatis DNA result by PCR and LCx (Table 2). All samples positive by PCR were also positive by LCx, and all that were negative by PCR were also negative by LCx. In the case of PCR, the criterion used to determine the presence of C. trachomatis DNA in the sample was a cycle that yielded a linear increase in detectable fluorescence signal (threshold cycle). In the case of LCx, the diagnostic status of the samples was determined on the basis of the manufacturer’s recommended sample/cutoff (S/CO) ratios.
TABLE 2.
Summary of the results of RAM, PCR, and LCx
| Result by RAM | No. of samples
|
|||
|---|---|---|---|---|
| PCR
|
LCx
|
|||
| Positive | Negative | Positive | Negative | |
| Positive | 14 | 0 | 14 | 0 |
| Negative | 1 | 15 | 1 | 15 |
Evaluation of the specimens by RAM revealed that of the 15 C. trachomatis-positive samples, only 14 specimens tested positive for C. trachomatis DNA (Table 2). The one specimen (no. 11) testing negative by RAM that was positive by PCR and LCx contained negligible amounts of cellular material as determined by inspection. Of the remaining 15 specimens that tested negative by PCR and LCx, all yielded a negative RAM result, confirming the high specificity of the RAM assay for C. trachomatis DNA. Furthermore, RAM accurately detected a wide range of C. trachomatis DNA in clinical samples, as indicated by PCR cycles (24 to 44 cycles) and S/CO ratio (4.39 to 1.85). For example, one of the samples (no. 20) had a low copy number of C. trachomatis DNA, since it required 44 cycles to become positive by PCR and had a low S/CO ratio (1.85) by LCx. Fig. 2C shows the agarose gel of the RAM products that were generated from PreservCyt samples 1 through 10.
DISCUSSION
We describe a novel strategy that combines magnetic isolation of target DNA/C-probe hybrids, target-specific ligation of C-probe, and isothermal amplification of the ligated C-probe. One-step isolation of C. trachomatis DNA with paramagnetic beads offers several advantages. First, dissolution of residual cellular material in 5 M GTC lyses cells, denatures proteins, inactivates nucleases, and relaxes DNA helices. Subsequent reduction of the GTC concentration to 2 M promotes the rapid formation of hybrids between target DNA and C-probes. Second, the capture of DNA-C-probe hybrids on paramagnetic beads allows extensive washing to remove all other components in the specimen that may inhibit ligation and PCR (7). In addition, removal of nontarget nucleic acids present in the specimen and unbound C-probes significantly reduces the probability of false-positive signals and background. Third, the use of magnetic isolation eliminates complicated, laborious manual DNA extraction procedures and enables the high-throughput processing of a large number of specimens in a clinical laboratory setting (7).
The circular probe has several unique features (22, 23). First, the formation of a closed C-probe requires target-specific ligation of the C-probe, therefore significantly increasing assay specificity. The 5′ end of the C-probe must align perfectly with the 3′ end on a target DNA in order for ligation to occur (7, 11). Furthermore, the stringent requirement of ligation makes detection of a single nucleotide polymorphism possible (11). Second, the C-probe can be amplified with a set of generic primers that bind to the loop region of the C-probe (Fig. 1) (7), making multiplex detection of different targets possible. It has been shown that because of the efficiency of different PCR primer pairs, multiplexing of PCR is sometimes difficult to perform (3, 16). In our case, different C-probes specific for different targets can be amplified with the same set of primers, eliminating competition among primers. This offers great advantages for the detection of most sexually transmitted agents (i.e., human papillomavirus, C. trachomatis, and Neisseria gonorrhoeae) in a single reaction from a single collection vial. Third, the circular probe allows a long single-stranded DNA (ssDNA) to form with up to thousands of repeats (21). This ssDNA can be further amplified by the ramification process as described previously. Since no temperature cycling is required, the reaction can be carried out in a simple water bath, obviating the use of an expensive thermocycler.
The liquid-based ThinPrep method of sample collection and slide preparation has significantly improved cytology diagnosis (8) and has allowed the use of PreservCyt specimens in addition to the Pap test, for the detection of such STD agents as C. trachomatis (12), N. gonorrhoeae (12), human papillomavirus (Yarkin et al., submitted), and herpes simplex virus (5). Our results with specimens that were stored in the methanol-based PreservCyt solution showed that under the conditions of testing, PreservCyt had no apparent inhibitory effect on PCR, ligase chain reaction, and RAM and that no components of PreservCyt interfered with the functionality of the molecular beacon that was used for the detection of amplified PCR products. Like PCR, the RAM assay also proved sensitive and detected as few as 10 copies of C. trachomatis DNA.
In summary, the RAM assay offers a viable alternative to PCR and LCx for the detection of C. trachomatis DNA from PreservCyt. With minor modifications, this assay can be used with molecular beacon technology for the real-time detection of C. trachomatis DNA and other STD agents in PreservCyt (data not shown). The high sensitivity and specificity of the RAM assay coupled with its ease of use thus encourages a further investigation of this technique.
REFERENCES
- 1.An, Q., J. Liu, W. O’Brien, G. Radcliffe, D. Buxton, S. Popoff, W. King, M. Vera-Garcia, L. Lu, J. Shah, J. Klingler, and D. M. Oliver. 1995. Comparison of characteristics of Q beta replicase-amplified assay with competitive PCR assay for Chlamydia trachomatis. J. Clin. Microbiol. 33:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control. 1985. Chlamydia trachomatis infections: Policy guidelines for prevention and control. Morb. Mortal. Wkly. Rep. 34(Suppl.):53–73. [Google Scholar]
- 3.Cha, T. A., J. Kolberg, B. Irvine, M. Stempien, E. Beall, M. Yano, A. L. Choo, M. Houghton, G. Kuo, J. H. Han, and M. S. Urdea. 1991. Use of a signature nucleotide sequence of hepatitis C virus for detection of viral RNA in human serum and plasma. J. Clin. Microbiol. 29:2528–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyck, E. V., M. Ieven, S. Pattyn, L. V. Damme, and M. Laga. 2001. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by enzyme immunoassay, culture, and three nucleic acid amplification tests. J. Clin. Microbiol. 39:1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiel-Gan, M. D., C. F. Villamil, S. R. Mandavilli, M. E. Ludwig, and G. J. Tsongalis. 1999. Rapid detection of HSV from cytologic specimens collected into ThinPrep fixative. Acta Cytol. 43:1034–1038. [DOI] [PubMed] [Google Scholar]
- 6.Hsuih, T., A. Guichon, A. Diaz, E. J. Bottone, R. Sperling, and D. Y. Zhang. 1995. Chlamydial infection in a high-risk population: comparison of Amplicor PCR and Gen-Probe PACE II for diagnosis. Adolesc. Pediatr. Gynecol. 8:71–76. [Google Scholar]
- 7.Hsuih, T. C. H., Y. N. Park, C. Zaretsky, F. Wu, S. Tyagi, F. R. Kramer, R. Sperling, and D. Y. Zhang. 1996. Novel, ligation-dependent PCR assay for detection of hepatitis C virus in serum. J. Clin. Microbiol. 34:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchinson, M. L., D. J. Zahniser, M. E. Sherman, R. Herrero, M. Alfaro, M. C. Bratti, A. Hildesheim, A. T. Lorincz, M. D. Greenberg, J. Morales, and M. Schiffman. 1999. Utility of liquid-based cytology for cervical carcinoma screening: results of a population-based study conducted in a region of Costa Rica with a high incidence of cervical carcinoma. Cancer 87:48–55. [DOI] [PubMed] [Google Scholar]
- 9.Inhorn, S. L., P. J. Wand, T. C. Wright, K. D. Hatch, J. Hallum, and B. B. Lentrichia. 2001. Chlamydia trachomatis and Pap testing from a single, fluid-based sample. J. Reprod. Med. 46:155–162. [PubMed] [Google Scholar]
- 10.Judson, F. N. 1985. Assessing the number of genital chlamydial infections in the United States. J. Reprod. Med. 30:269–272. [PubMed] [Google Scholar]
- 11.Landegren, U., R. Kaiser, J. Sanders, and L. Hood. 1988. A ligase-mediated gene detection technique. Science 241:1077–1080. [DOI] [PubMed] [Google Scholar]
- 12.Lentrichia, B. B., M. A. Cohenford, S. Hecht, E. Coffman, B. D’Zuira, and P. Thomas. 2001. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae from liquid-based PAP collection using a novel extraction method and rapid thermal cycling with real-time detection by molecular beacons, p.67–73. In International Proceedings Sexually Transmitted Infections. Monduzzi Editore, Berlin, Germany.
- 13.Mahony, J. B., K. E. Luinstra, M. Tyndall, J. W. Sellors, J. Krepel, and M. Chernesky. 1995. Multiplex PCR for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in genitourinary specimens. J. Clin. Microbiol. 33:3049–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahony, J. B., X. Song, S. Chong, M. Faught, T. Salonga, and J. Kapala. 2001. Evaluation of the NucliSens Basic Kit for detection of Chlamydia trachmatis and Neisseria gonorrhoeae in genital tract specimens using nucleic acid sequence-based amplification of 16S rRNA. J. Clin. Microbiol. 39:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangione-Smith, R., J. O’Leary, and E. A. McGlymm. 1999. Health and cost-benefits of chlamydia screening in young women. Sex. Transm. Dis. 26:309–316. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto, M., M. Baba, E. Kodama, K. Sekine, T. Takagi, R. Kasukawa, and S. Shigeta. 1993. Detection of hepatitis C virus genome in human serum by multi-targeted polymerase chain reaction. J. Med. Virol. 41:6–10. [DOI] [PubMed] [Google Scholar]
- 17.Scholes, D., A. Stergachis, F. Heidrich, H. Andrilla, K. Holmes, and W. Stamm. 1996. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N. Engl. J. Med. 334:1362–1366. [DOI] [PubMed] [Google Scholar]
- 18.Shafer, M. A., V. Prager, J. Schalwitz, E. Vaughan, B. Moscicki, R. Brown, C. Wibbelsman, and J. Schachter. 1987. Prevalence of urethral Chlamydia trachomatis and Neisseria gonorrhoeae among asymptomatic, sexually active adolescent boys. J. Infect. Dis. 156:223–224. [DOI] [PubMed] [Google Scholar]
- 19.Smith, J. W., R. E. Rogers, B. P. Katz, J. F. Brickler, P. L. Lineback, B. V. D. Pol, and R. B. Jones. 1987. Diagnosis of chlamydial infection in women attending antenatal and gynecologic clinics. J. Clin. Microbiol. 25:868–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamm, W. E., M. E. Guinan, C. Johnson, T. Starcher, K. K. Holmes, and McCormack. 1984. Effect of treatment regimens for Neisseria gonorrhoeae on simultaneous infection with Chlamydia trachomatis. N. Engl. J. Med. 310:545–549. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, D. Y., M. Brandwein, T. Hsuih, and H. B. Li. 2001. Ramification amplification: a novel isothermal DNA amplification method. Mol. Diagn. 6:141–150. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, D. Y., M. Brandwein, T. C. H. Hsuih, and H. Li. 1998. Amplification of target-specific, ligation-dependent circular probe. Gene 211:277–285. [DOI] [PubMed] [Google Scholar]
- 23.Zhang, D. Y., W. Zhang, X. Li, and Y. Konomi. 2001. Detection of rare DNA targets by isothermal ramification amplification. Gene 247:209–216. [DOI] [PubMed] [Google Scholar]