Abstract
Aims
To analyse the correlation between the physician categories defined by the 3C classification (crystal-complication-compliance) and the ocular manifestations of nephropathic cystinosis.
Methods
The last visit data of 64 patients aged between 2 and 64 attending the centre for management of cystinosis were reviewed. Each patient had been placed into one of four categories by the clinician based on disease severity. The correlation between these categories and markers of the disease was assessed using Pearson’s correlation.
Results
Photophobia (0.647, p<0.001), visual acuity (−0.695, p<0.001), Gahl’s score (0.603, p<0.001), optical coherence tomography (OCT)% (0.713, p<0.001) and in vivo confocal microscopy (IVCM)% (0.845, p<0.001), showed a strong, highly significant correlation between key signs and symptoms and the 3C classification. Corneal complications were strongly correlated with the 3C classification with scores of 0.802 (p<0.001), 0.634 (p<0.001), 0.726 (p<0.001) and 0.677 (p<0.001) for band keratopathy, keratitis, neovascularisation and corneal ulceration, respectively. 75% of those classified as most severe had all four complications. The use of artificial tears and ciclosporin strongly correlated with the categorisation, 0.574 (p<0.001) and 0.631 (p<0.001), respectively. With all cystinosis markers, the 3C classification showed a stronger correlation than age and crystal scores by Gahl’s and OCT. Category and age were strongly correlated (0.656, p<0.001). There was a moderate negative correlation with therapeutic compliance with cysteamine eye-drops and categorisation (−0.422, p<0.001). The compliance pattern observed may help to explain why the disease progresses in some patients.
Conclusion
3C classification is a reliable tool to categorise ocular cystinosis and can support clinical management decisions allowing more reliable comparison of datasets.
Keywords: Inflammation, Cornea, Visual perception, Neovascularisation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Pinxten et al provided a framework for ocular assessment in cystinosis. The cornerstone of this approach was the assessment of cystine crystals. Most studies look at cystine crystal density as a surrogate marker for disease severity and progression.
WHAT THIS STUDY ADDS
The physician crystal-complication-compliance (3C)-classification provides a holistic view of all ocular markers of cystinosis. The 3C classification was more robust than classification by age, or observations of crystal density in assessing disease severity.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The new classification could become a standard assessment for clinical management and support in-practice decision-making.
Introduction
Cystinosis is a lysosomal autosomal recessive storage disease occurring in 1/100 000–200 000 live births caused by a mutation in the cystinosis gene 17p13. It results in a dysfunction of the lysosomal membrane transport protein, cystinosin, which transports cystine out of the lysosome.1 2 Over 100 identified mutations give rise to variable clinical presentations of the disease.3 4
The accumulation of cystine within cells leads to cystine crystal formation. The resulting organ damage is detected first in the kidneys. Over time crystals accumulate in other organs throughout the body including the eyes.5
Cystine crystals can develop in all ocular structures. The most described ocular manifestation of cystinosis is crystal deposition in the cornea.6 7 Crystal accumulation increases with age and gradually leads to photophobia, blepharospasm, superficial punctate keratopathy and recurrent corneal erosions.6,8 In older patients, filamentous keratopathy, band keratopathy and peripheral corneal neovascularisation also occur.7,9
Crystals can start to accumulate in the eye as early as 3 months old. However, patient symptoms are seen later as crystal accumulation increases. Typically, crystals are observed across all layers of the cornea between 1 and 2 years old.10
The first and most frequently reported ocular symptom is photophobia7 8 which occurs in some patients under five. By 10 years old, and without intervention, the entire periphery of the stroma and endothelium in the eye contains cystine crystals. Progressive photophobia is observed in 50% of patients and blepharospasm is also observed in some.11 By the second or third decade of life visual acuity, colour vision, peripheral and night vision are significantly reduced, and retinal blindness can occur.12
Improvements in transplantation and management mean more patients reach adulthood and experience the ocular manifestations of cystinosis. Early detection and proactive treatment with cysteamine eye-drops are recommended.13,15 Early treatment with, and strict adherence to, cysteamine therapy has a considerable positive impact on the long-term prognosis of ocular cystinosis.15,18
Over the last 10 years, our clinicians routinely scored patients based on the ocular signs and symptoms of cystinosis, the degree of corneal complications and the reported compliance with therapy. We term this the crystal-complication-compliance (3C)-classification, and it defines our clinical management.
This study looks at data from 64 patients and assesses the correlation between the clinician-reported 3C classification and objective markers of the disease.
Methods
Data collection
The medical records of 64 patients with a diagnosis of cystinosis, assessed between 2013 and June 2023, were analysed. The review included patients who were seen on a one-off or infrequent basis or who were regularly seen by the physician. Only data from the last visit were included in the analysis.
The patient record provided the following for each patient: gender, age, and physician observed and recorded 3C categorisation. The four categories can be broadly described as crystal deposition without corneal complications and with good treatment compliance (Cat1), symptoms developing without corneal complications and poor compliance (Cat2), widespread crystal deposition, classical symptoms and corneal complications developing with good compliance (Cat3) and widespread symptoms with severe corneal complications and poor compliance (Cat4).
Routine practice records best-corrected visual acuity (BCVA), photophobia and assesses the eye by slit lamp examination, OCT and IVCM.1115,17
BVCA scores are expressed from 0 to 1 where 1 represents 20/20 vision. Variables were compared using linear correlation which would be affected by using a LogMAR scale. Photophobia scores were based on physician assessment on a 0–5 scale.19 The Corneal Cystine Crystal Scale by Gahl (Gahl’s Score) was collected, where corneal crystal density is scored on a 0–3 scale based on slit-lamp investigation. Anterior-segment OCT analysis scores, where the depth of the crystal deposition is expressed as a percentage of corneal thickness, were recorded. IVCM scores were also recorded which quantifies the percentage of crystal deposition in each corneal layer to provide an overall score from 0 to 28, the higher the score the greater the overall corneal cystine crystal deposits.
The presence or absence of the following corneal complications was included in the analysis: keratitis, neovascularisation, band keratopathy and ulceration. Keratitis was defined as a lack of superficial epithelium on corneal staining scoring 1–5 on the Oxford scale, where 4 and 5 are considered severe. Neovascularisation was graded from 1 to 3, where 1 and 2 had peripheral corneal vascularisation of <2 mm or >2 mm, respectively; 3 (considered severe) included the central cornea. Band keratopathy, and corneal ulceration involving the underlying stroma, were considered severe, and their presence or absence was recorded.
The use of additional therapies for treating the complications of cystinosis,20 21 namely artificial tears and ciclosporin, was analysed.
All patients were being treated with Cystadrops (cysteamine ophthalmic solution, 0.37%).13 Compliance with therapy was self-assessed and patients (or their caregiver/parent) were asked how many instillations of cysteamine eye-drops were taken each day in the period leading up to the appointment.
All patients received a macular OCT.
Data analysis
The data were analysed by category, age and where relevant by Gahl’s score/OCT. The correlation between the key signs, symptoms, complications and compliance with therapy was assessed using Pearson’s correlation which normalises the covariance and takes values from −1 to +1 (±0.300 to ±0.599=medium correlation; ±0.600 to ±1.000=strong correlation). Only correlations with p<0.001 are reported (designated by *). Correlations between Gahl’s score and OCT are seen in online supplemental data.
Results
64 patients were identified. One was excluded from the analysis as this patient was considered to have adult-onset cystinosis. The patient was 63 years old, had full BCVA and no photophobia or corneal complications. Their Gahl’s score was 1.5.
Demographics
63 patients with nephropathic cystinosis, of which 39 were female and 24 were male, with an age range of 2–64 years (mean 23.14 years) were included. There were 33 regular patients and 30 infrequent patients (figure 1a).
Figure 1. Age and 3C classification of patient cohort: (a) table outlining total patient sample by 3C classification, age and gender; (b) graph demonstrating patient cohort by 3C classification category grouped by age; (c) graph demonstrating patient cohort age group grouped by 3C classification category. 3C, crystal-complication-compliance.
Age and physician classification
Increasing age is associated with a higher category. Mean ages were 15.12 (Cat1), 19.38 (Cat2), 32.90 (Cat3) and 36.14 (Cat4). In Cat1, 80% of patients were <21 years. In Cat4, 75% were ≥31 years and all were ≥21 years.
The Pearson’s correlation between age and category was 0.656* (figure 1b,c).
Symptoms of cystinosis
Photophobia (n=63)
The mean photophobia score increased by category. Cat1 was 0.58, Cat2 was 1.69, Cat3 was 1.70 and Cat4 was 2.83. Cat1 had 52% of patients without photophobia. By Cat3 80% of patients scored ≥1, and in Cat4, 83% scored 3–4.
Photophobia progressed with age and presented earlier than loss of visual acuity. In patients <11 years, 46.2% had no photophobia and 92.3% had photophobia scores <2. In the 11 to <21 years group, 25.0% had no photophobia and 70.0% scored 1–3. In those ≥31 years, 73.6% had a photophobia score ≥2.
Pearson’s correlation versus photophobia was 0.647* for category and 0.442* for age. The correlation between Gahl’s score and OCT was 0.660* and 0.626*, respectively (figure 2a,b).
Figure 2. Graphs depicting physician-assessed signs and symptoms of cystinosis categorised by 3C classification category and age: (a) photophobia by 3C classification category; (b) photophobia by age group; (c) BCVA by 3C classification category; (d) BCVA by age group; (e) Gahl’s score by 3C classification category; (f) Gahl’s score by age group. BCVA, best-corrected visual acuity; 3C, crystal-complication-compliance.
Visual acuity (n=63
BCVA decreased as disease severity increased. In Cat1, there was no loss of visual acuity. In Cats 2–4, BCVA is 0.98, 0.78 and 0.47, respectively. In Cat2, 94% had a score of 0.9–1.0. In Cat3, 40% still scored 1.0. In Cat4, 58% scored ≤0.5, but two patients’ BCVA was 1.
The age group analysis revealed 97% of patients <21 years old had BCVA of 1.0. BCVA worsened from 21 years onwards. The mean BCVA scores by decade were 1.00 (<11 years), 0.95 (11 to <21 years), 0.89 (21 to <31 years), 0.63 (31 to <41 years) and 0.62 (41 to <51 years). The patient ≥51 years had a score of 0.2.
Pearson’s correlation was strong for category, (−0.695*) and age, (−0.662*). The correlation between Gahl’s score and OCT was lower at −0.451* and −0.570*, respectively (figure 2c,d).
Signs of cystinosis: crystal deposition
Gahl’s score (n=63)
The mean Gahl’s score increased with category. Cat1=1.70, Cat2=2.13, Cat3=2.39 and Cat4=2.73. In Cat1, 64% had a Gahl’s score of ≤1.5. In Cat2, 0% had a score ≤1.5. In Cat3, 30% scored ≤1.5, 70% scored ≥2.5. In Cat4, 92% had a score ≥2.5 including 6 of the 7 patients with a score of 3.
Gahl’s score increased with age. The mean Gahl’s score was 1.33 (<11 years), 1.88 (11 to <21 years), 2.19 (21 to <31 years), 2.71(31 to <41 years). There was a strong correlation between Gahl’s score and category of 0.603*; with age it was 0.447* (figure 2e,f).
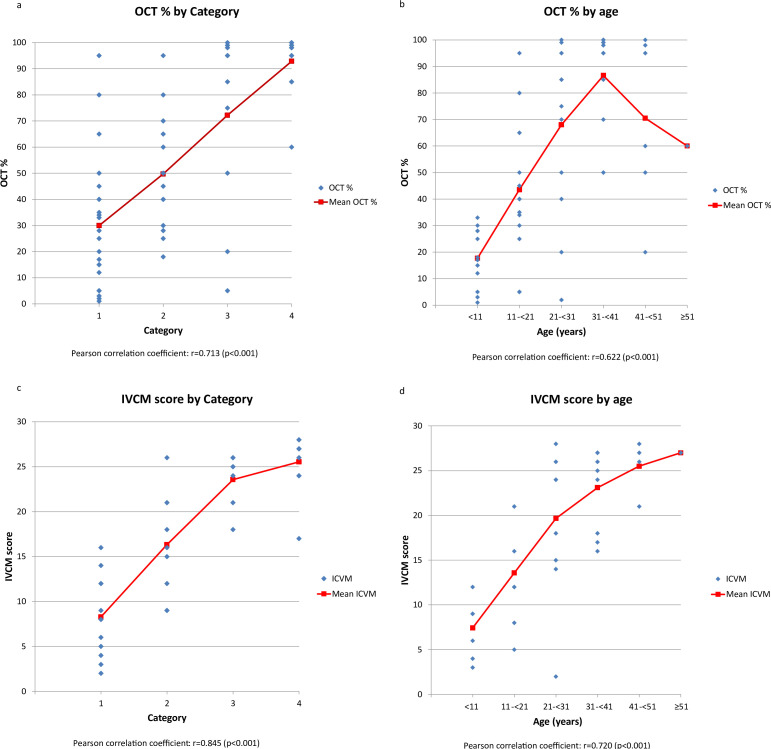
OCT (n=62)
One patient, aged 11 to <21 and in Cat1, did not have an OCT assessment at their last visit. The mean OCT% score increased with each category. Cat1 was 30%, Cat2 was 50%, Cat 3 was 72% and Cat4 was 92%. In Cat4, 92% of patients had an OCT% >80%. In Cat1, by contrast, 88% had an OCT% score of ≤50% with 25% scoring ≤10%.
In general OCT% increased with age. In those <11 years, 100% had a score ≤33%. In those 11 to <21 years, this was 22%. 75% of those 21 to <31 years had an OCT ≥50%. In those ≥31 years, 95% had an OCT ≥50%.
The correlation for OCT% with age or category was stronger than Gahl’s score. The correlation between OCT% and age was 0.622*, and the category was 0.713* (figure 3a,b).
Figure 3. Graphs depicting physician-assessed signs and symptoms of cystinosis categorised by 3C classification category and age: (a) OCT% score by 3C classification category; (b) OCT% score by age group; (c) IVCM score by 3C classification category; (d) IVCM score by age group. 3C, crystal-complication-compliance; IVCM, in vivo confocal microscopy; OCT, optical coherence tomography.
IVCM (n=38)
25 patients did not have an IVCM score recorded. The mean IVCM score increased with category. Cat1 was 8.27, Cat2: 16.33, Cat3: 23.57 and Cat4: 25.55. In Cat1, all had an IVCM score ≤16 with 64% scoring ≤10. In Cat4, 91% scored ≥21, 73% scored ≥25 and no one scored ≤16.
IVCM score increased with age. The mean for those <11 y was 7.43 and all scored <15. In those ≥31 years, all had an IVCM score ≥16; 80% scored ≥21 and 53% scored 26–30. IVCM shows a strong positive correlation with age (0.720*) and category (0.845*).
Gahl’s score and OCT, which also measure crystal accumulation, are strongly correlated with IVCM at 0.785* and 0.875*, respectively (figure 3c,d).
Corneal complications: individually assessed (n=63)
Four corneal complications are routinely assessed in our clinic: ulceration, band keratopathy, neovascularisation and keratitis.
Ulceration affected no one in Cat1 or 2 but occurred in 20% and 75% of patients in Cat3 and 4, respectively.
No patients in Cat1, 19% in Cat 2, 90% in Cat3 and 92% in Cat4 had band keratopathy.
No patients in Cat1, 19% (3) in Cat2 (all at grade 1), 50% (5) in Cat3 and 83% (10) in Cat4 had neovascularisation. Of four patients in grade 3, one was in Cat3 and three in Cat4.
No patients had severe keratitis. Patients with keratitis included one patient (4%) in category 1 (grade 1), three (19%) in category 2 (2 at grade 1, 1 at grade 2), four (40%) in category 3 and ten (83%) in category 4. Of three patients in grade 3, one was in Cat3 and two were in Cat4.
With increasing age, there were increasing corneal complications and in patients ≥31 years, 79% had band keratopathy, 63% keratitis, 79% neovascularisation and 42% ulceration.
There were medium and strong correlations between the individual corneal complications and age. Correlation scores were 0.687*—band keratopathy; 0.560*—keratitis; 0.643*—neovascularisation and 0.490*—ulceration. Similarly, the correlation between Gahl’s score and OCT was medium strong. Gahl’s scores were 0.584*, 0.507*, 0.519* and 0.447*, respectively; OCT scores were 0.684*, 0.600*, 0.621* and 0.551*, respectively.
The correlation was strong with category across the same parameters with scores of 0.802*, 0.634*, 0.726* and 0.677*, respectively.
Corneal complications collectively assessed
The mean number of complications per person by age group was as follows: 0.00 (<11 years), 0.21 (11 to <21 ears), 1.50 (21 to <31 years), 2.58 (31 to <41 years), 2.50 (41 to <50 years) and 4 in the person >51 years. The mean number of complications by Cat was 0.04-Cat1; 0.56-Cat2; 2.00-Cat3 and 3.50-Cat4.
In Cat1, only one patient presented with a corneal complication, keratitis, and this was the mildest form. In Cat2, 69% were without corneal complications. Of those with 4 complications, 18% were in Cat3 and 82% in Cat4.
The correlations between the number of complications per person and age, Gahl’s score, OCT and category were 0.690*, 0.605*, 0.717* and 0.833*, respectively (figure 4a,b).
Figure 4. Graphs depicting number of corneal complications per cystinosis patient by 3C classification category and age: (a) number of corneal complications per patient by 3C classification category; (b) number of corneal complications per patient by age group. 3C, crystal-complication-compliance.
The use of additional therapy (n=63)
Two types of additional therapy were reviewed: artificial tears and ciclosporin. Artificial tears are used to decrease irritation19 and ciclosporin14 21 to reduce inflammation.
Artificial tears were used in 12% of patients in Cat1, 31% in Cat2, 90% in Cat3 and 75% in Cat4. One patient was treated with ciclosporin in Cat1 and Cat2; in Cat3 this was 50% and in Cat4, 75%.
Artificial tears were used in only 8% of patients aged <11 years rising to 74% in those ≥31 years. Ciclosporin was used in 58% of patients ≥31 years. No ciclosporin was used in the first decade of life, and only 17% of patients between 11 and <31 years received it.
The Pearson’s coefficients for artificial tears were 0.504* (age) and 0.574* (category). The correlation for ciclosporin was 0.538*(age) and 0.631 (category)* (figure 5a,b).
Figure 5. Graphs depicting the use of ocular therapies within the patient cohort by 3C classification category and age: (a) the use of ocular therapies additional to Cystadrops, namely ciclosporin and artificial tears, by 3C classification category; (b) the use of ocular therapies additional to Cystadrops, namely ciclosporin and artificial tears, by age group; (c) Cystadrops therapy compliance by 3C classification category; (d) Cystadrops therapy compliance by age group. 3C, crystal-complication-compliance.
The use of cysteamine eye-drops (n=63)
All patients in this cohort were prescribed Cystadrops. Analysis by category showed the highest mean number of daily instillations was Cat1 at 3.1, with 40% reporting use four times/day. Cat 2 was 2.4 applications/day with a single patient <11 years reporting four per day. Cat3 averaged 3.0 applications/day with 30% reporting four applications/day. In category 4, applications fell to a mean of 1.8 per day.
There is a negative correlation between the use of Cystadrops and age (−0.367*) and category (−0.422*) (figure 5c,d).
Discussion
The progression of cystinosis in the cornea is based on the specific genetic mutation when treatment was started, the commitment of the patients and their caregivers to therapy, and the management by the clinical team.
We have used our clinical judgement to place patients into four categories at the time of consultation which helps us with our management approach. We wanted to assess whether this management approach was robust and decided on this retrospective analysis to determine its potential merits.
While we are specifically looking at corneal cystinosis, we need to be aware of the potential impact of the posterior segment of the eye on some of the parameters measured. Visual acuity for instance is dependant not only on the cornea but also the posterior segment of the eye as well as a patient’s general well-being. Without full assessment of the posterior segment of the eye we cannot exclude the possible contribution of retinal disease to poorer vision in the older age group. However, all patients received a macular OCT, and no obvious macular oedema was found (data are not shown). This provides confidence that we are observing the corneal manifestations of the disease. All patients included for analysis were treated with oral cysteamine therapy.
The data analysed are from a large cohort of patients. We can build confidence that it reaffirms what we know about the disease. The method of analysing a single time point, the last visit, is robust. We can see from the data the expected progression of the disease. When looking at crystal accumulation (Gahl’s score, OCT and IVCM) there was a very strong, highly significant correlation between them and 3C classification.
We see early photophobia: 54% of patients <11 years had photophobia while visual acuity remained good. 97% of patients from 0 to <21 years retained full visual acuity. We also observed that crystal accumulation corresponded with visual acuity and photophobia. The method of assessment is important. IVCM correlated more strongly to visual acuity and photophobia than did OCT and Gahl’s score (data are shown in online supplemental file) and OCT was more strongly correlated to visual acuity than Gahl’s score. This perhaps confirms the limitations of Gahl’s score particularly as the disease gets more severe. It also supports the notion that IVCM is the gold standard ocular assessment tool for cystine crystals.
The presence and number of corneal complications increased, both as the patient aged, and the disease grew more severe. These reaffirmations of what we know allow us to be confident in the 3C classification.
When we looked at photophobia and visual acuity, there was a strong correlation between these factors and the 3C classification. We would expect photophobia and crystal deposition scores to be strongly correlated as this is the first presenting symptom of the disease. The correlations for Gahl’s score and OCT were strong and very similar to those of the 3C classifiaction. The correlation between the 3C classification and visual acuity was stronger than for both Gahl’s score and OCT.
The 3C classification was correlated more strongly than age, Gahl’s score and OCT for corneal complications whether this assessment was done by individual complication or whether we reviewed the number of complications a patient has. The correlations were strong and highly significant between the 3C classification and corneal complications. We also looked at the severity of complication in the case of neovascularisation and keratitis and were able to show the pattern applied (data are not shown).
Corneal complications, once they become more severe, have no effective surgical or medical interventions. Using IVCM, we have observed that at the very late stage of cystinosis, there is decreased crystal accumulation due to the loss of corneal collagen supports (data are not shown). The cornea becomes empty with no internal structure. The only potential treatment options are stem-cell-based therapy or corneal transplant.22 23 With corneal transplantation, topical treatment is still necessary because cystinosin-deficient host cells can reinvade into the transplanted cornea.23
Measurements of compliance are difficult, but we observed a pattern emerging from the patients’ reported use of cysteamine eye-drops. Compliance was good in Cat1. Patients were younger and often the instillation was being delivered or encouraged by a parent. Cat2 saw daily use fall, potentially due to the patient getting older and managing their own condition at a time when the disease is not particularly troublesome. In Cat3 compliance rose, maybe as a reaction to their increasing clinical complications and concerns for the future. Daily use fell again in Cat4, probably due to the impact of the corneal complications making it difficult to apply therapy.
It has been reported that inflammatory cell density within the central part of the cornea is less than 50 cells/mm2.24 However, in cystinosis patients with high levels of photophobia and nerve alterations high levels of inflammatory cell up to 800 cells/mm are observed.25 This is the rationale for the use of ciclosporin in these patients and ciclosporin use correlates with the 3C classification. Similarly, there is a good correlation between the 3C classification and the use of artificial tears. Artificial tears can be used for several clinical reasons but are more widely used as the disease progresses as shown in this study.
To examine the potential replicability of our categories, we used linear discriminant analysis (LDA), which attempts to express one categorial dependent variable as a linear combination of other measurements (data are not shown). Although LDA is not a method tailored for our dataset, an LDA model developed from the 38 patients with full ICVM and OCT data achieved 84% overall accuracy in assigning these patients to the correct 3C category. Of 11 patients in category 1, it assigned 10 to category 1 and 1 to category 2. Of 11 patients in category 4, all were assigned correctly, but one category 3 patient was labelled as category 4. No patient was mislabelled by more than one category.
In attempting to help others use our 3C classification, we have drawn the following from the analysis (see online supplemental data.
Cat1: Patients with evidence of crystals but with low to moderate crystal accumulation scores. No loss of BCVA, mild or no photophobia. No corneal complications. Patients will likely be under 20 years with good compliance to ocular therapy.
Cat2: Patients with evidence of crystals with moderate crystal accumulation with an OCT likely to be >30%. Photophobia is more prevalent although still mild to moderate. BCVA remains largely unaffected. No ulceration or band keratopathy but potentially mild keratitis or neovascularisation might be observed. Patients are likely to be 10–30 years and levels of compliance will be lower than Cat1.
Cat3: Extensive evidence of crystals with OCT score >50% and often higher. Photophobia scores of 2–3 with all patients ≥1. BCVA is affected in most although not all. The presence of two or more corneal complications is observed with 90% with band keratopathy. Patients are likely to be 20–45 years with compliance improving over Cat2.
Cat4: Crystal deposition widespread with an OCT score >80%. The likelihood is the photophobia score will be three or 4. BCVA will be moderately or severely affected. All patients will have corneal complications with most having three corneal complications. The likelihood of ulceration is high. Compliance is the lowest in this group.
The 3C classification showed a strong correlation with all markers of disease progression. It supports differential management strategies of patients with ocular cystinosis. The classification could support comparisons of outcomes between cohorts and be the basis of patient categorisation for prospective and retrospective clinical studies. Verification in other centres would be the first step to consider its wider applicability.
Supplementary material
Footnotes
Funding: Editorial assistance and data analysis support was provided by Steve Calder Smith, Joey Calder Smith, Covostra and Michael Smith. Article processing charges were funded by Recordati Rare Diseases.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and the study followed the tenets of the Declaration of Helsinki, approved by the CPP Ile-de-France V Ethics Committee (10793). Participants gave informed consent to participate in the study before taking part.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
References
- 1.Adamson MD, Andersson HC, Gahl WA. Cystinosis. Semin Nephrol. 1989;9:147–61. [PubMed] [Google Scholar]
- 2.Town M, Jean G, Cherqui S, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319–24. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 3.Anikster Y, Shotelersuk V, Gahl WA. CTNS mutations in patients with cystinosis. Hum Mutat. 1999;14:454–8. doi: 10.1002/(SICI)1098-1004(199912)14:6<454::AID-HUMU2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 4.Attard M, Jean G, Forestier L, et al. Severity of phenotype in cystinosis varies with mutations in the CTNS gene: predicted effect on the model of cystinosin. Hum Mol Genet. 1999;8:2507–14. doi: 10.1093/hmg/8.13.2507. [DOI] [PubMed] [Google Scholar]
- 5.Elmonem MA, Veys KR, Soliman NA, et al. Cystinosis: a review. Orphanet J Rare Dis. 2016;11:47. doi: 10.1186/s13023-016-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gahl WA, Kuehl EM, Iwata F, et al. Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab. 2000;71:100–20. doi: 10.1006/mgme.2000.3062. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser-Kupfer MI, Caruso RC, Minkler DS, et al. Long-term ocular manifestations in nephropathic cystinosis. Arch Ophthalmol. 1986;104:706–11. doi: 10.1001/archopht.1986.01050170096030. [DOI] [PubMed] [Google Scholar]
- 8.Nesterova G, Gahl W. Nephropathic cystinosis: late complications of a multisystemic disease. Pediatr Nephrol. 2008;23:863–78. doi: 10.1007/s00467-007-0650-8. [DOI] [PubMed] [Google Scholar]
- 9.Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347:111–21. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- 10.Tsilou E, Zhou M, Gahl W, et al. Ophthalmic manifestations and histopathology of infantile nephropathic cystinosis: report of a case and review of the literature. Surv Ophthalmol. 2007;52:97–105. doi: 10.1016/j.survophthal.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang H, Labbé A, Le Mouhaër J, et al. A New Viscous Cysteamine Eye Drops Treatment for Ophthalmic Cystinosis: An Open-Label Randomized Comparative Phase III Pivotal Study. Invest Ophthalmol Vis Sci. 2017;58:2275–83. doi: 10.1167/iovs.16-21080. [DOI] [PubMed] [Google Scholar]
- 12.Lyseng-Williamson KA. Cystadrops® (cysteamine hydrochloride 0.55% viscous eye-drops solution) in treating corneal cystine crystal deposits in patients with cystinosis: a profile of its use. Drugs Ther Perspect. 2017;33:195–201. doi: 10.1007/s40267-017-0398-6. [DOI] [Google Scholar]
- 13.https://www.ema.europa.eu/en/documents/product-information/cystadrops-epar-product-information_en.pdf Available.
- 14.https://www.ema.europa.eu/en/documents/orphan-review/recommendation-maintenance-orphan-designation-time-marketing-authorisation-cystadrops-mercaptamine_en.pdf Available.
- 15.Biswas S, Gaviria M, Malheiro L, et al. Latest Clinical Approaches in the Ocular Management of Cystinosis: A Review of Current Practice and Opinion from the Ophthalmology Cystinosis Forum. Ophthalmol Ther. 2018;7:307–22. doi: 10.1007/s40123-018-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labbé A, Baudouin C, Deschênes G, et al. A new gel formulation of topical cysteamine for the treatment of corneal cystine crystals in cystinosis: the Cystadrops OCT-1 study. Mol Genet Metab. 2014;111:314–20. doi: 10.1016/j.ymgme.2013.12.298. [DOI] [PubMed] [Google Scholar]
- 17.Liang H, Labbé A, Baudouin C, et al. Long-term follow-up of cystinosis patients treated with 0.55% cysteamine hydrochloride. Br J Ophthalmol. 2021;105:608–13. doi: 10.1136/bjophthalmol-2020-316450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levtchenko E, Servais A, Hulton SA, et al. Expert guidance on the multidisciplinary management of cystinosis in adolescent and adult patients. Clin Kidney J. 2022;15:1675–84. doi: 10.1093/ckj/sfac099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labbé A, Niaudet P, Loirat C, et al. In vivo confocal microscopy and anterior segment optical coherence tomography analysis of the cornea in nephropathic cystinosis. Ophthalmology. 2009;116:870–6. doi: 10.1016/j.ophtha.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Wei Z, Su Y, Su G, et al. Effect of artificial tears on dynamic optical quality in patients with dry eye disease. BMC Ophthal. 2022;22 doi: 10.1186/s12886-022-02280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keklikci U, Soker SI, Sakalar YB, et al. Efficacy of topical cyclosporin A 0.05% in conjunctival impression cytology specimens and clinical findings of severe vernal keratoconjunctivitis in children. Jpn J Ophthalmol. 2008;52:357–62. doi: 10.1007/s10384-008-0577-z. [DOI] [PubMed] [Google Scholar]
- 22.Cherqui S. Hematopoietic Stem Cell Gene Therapy for Cystinosis: From Bench-to-Bedside. Cells. 2021;10 doi: 10.3390/cells10123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emma F, Nesterova G, Langman C, et al. Nephropathic cystinosis: an international consensus document. Nephrol Dial Transplant. 2014;29 Suppl 4:iv87–94. doi: 10.1093/ndt/gfu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhivov A, Stave J, Vollmar B, et al. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch Clin Exp Ophthalmol. 2005;243:1056–61. doi: 10.1007/s00417-004-1075-8. [DOI] [PubMed] [Google Scholar]
- 25.Liang H, Baudouin C, Tahiri Joutei Hassani R, et al. Photophobia and Corneal Crystal Density in Nephropathic Cystinosis: An In Vivo Confocal Microscopy and Anterior-Segment Optical Coherence Tomography Study. Invest Ophthalmol Vis Sci. 2015;56:3218. doi: 10.1167/iovs.15-16499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.