Abstract
Telomere protection and maintenance are accomplished through the coordinated actions of telomere-specific DNA binding proteins and their interacting partners. The fission yeast ortholog of human TRF1/2, Taz1, binds telomeric DNA and regulates numerous aspects of telomere function. Here, we ask which aspects of Taz1 function are mediated through its interacting proteins, Rap1 and Rif1. We demonstrate that rap1+ deletion phenocopies some, but not all, aspects of taz1Δ telomere dysfunction, while Rif1 exhibits a very different functional spectrum. Rap1 acts in a Taz1-dependent pathway to prevent chromosome end fusions and regulate telomeric 3′ overhang formation, while Rif1 is dispensable for these functions. Telomerase inhibition by Taz1 is mediated by two separate pathways, one involving Rap1 and the other involving Rif1. In contrast, Taz1 is uniquely required to prevent chromosomal entanglements and missegregation at cold temperatures. Strikingly, while rap1+ deletion exacerbates the cold sensitivity of taz1Δ cells, rif1+ deletion restores full viability. Thus, Rap1 and Rif1 are each required for a subset of the functions of Taz1, but each acquires Taz1-independent functions in its absence. Furthermore, Taz1 can function independently of its known binding partners.
Keywords: DNA repair, Rap1, Rif1, Taz1, telomere
Introduction
Chromosome ends assemble specialized nucleoprotein structures, known as telomeres, that prevent fusion and attrition of chromosome ends. Telomeric DNA consists of simple sequence repeats, TTAGGG in vertebrates and closely related sequences in most other eukaryotes (e.g. TTAC(A)G1–6 in fission yeast), terminating in a 3′ overhang of the G-rich strand. The telomere repeats recruit sequence-specific binding proteins and proteins that bind to telomeres through protein–protein interactions. Together, these constituents form the telomere ‘cap', the structure that inhibits inappropriate recombinational, nucleolytic and end-joining activities, all of which profoundly disrupt genomic integrity if allowed at telomeres (Ferreira et al, 2004). In addition, telomeres recruit and control telomerase, the ribonucleoprotein enzyme that synthesizes telomeric DNA repeats (Smogorzewska and De Lange, 2004).
In fission yeast, double-stranded telomere sequences are bound by Taz1, an ortholog of human TRF1 and TRF2 (Cooper et al, 1997; Li et al, 2000). Taz1 is a key component of the telomere cap, as its loss results in numerous manifestations of telomere dysfunction. taz1+ deletion leads to elongation of the double-stranded telomeric repeat tracts, deregulation of the 3′ single-stranded overhang (Cooper et al, 1997; Tomita et al, 2003), loss of telomeric chromatin structure and derepression of telomere-adjacent transcription. Under conditions that favor high levels of nonhomologous end joining (NHEJ), taz1Δ cells accumulate NHEJ-mediated end-to-end chromosome fusions and lose viability (Ferreira and Cooper, 2001; Tuzon et al, 2004). At cold temperatures, taz1Δ cells activate the DNA damage and spindle assembly checkpoints and display broken and entangled DNA, chromosome missegregation and reduced viability (Miller and Cooper, 2003).
Two Taz1-interacting proteins, Rap1 and Rif1 (Chikashige and Hiraoka, 2001; Kanoh and Ishikawa, 2001), have homologs in budding yeast and humans but display intriguing interspecies differences in function and telomeric recruitment. Budding yeast lacks a Taz1/TRF ortholog, and its double-stranded telomere region is bound directly by ScRap1, which has dual roles as a major telomere regulator and a regulator of 60–75% of Pol II transcription in growing cells; this latter function confers lethality upon deletion of RAP1 (Shore, 1994, 2001; Warner, 1999). Mutations in ScRap1 result in altered telomere length regulation and loss of telomeric silencing (Sussel and Shore, 1991; Kyrion et al, 1992, 1993). Elevated rates of chromosome loss are seen upon mutation or overexpression of ScRap1, suggesting a role for Rap1 in telomere ‘capping' (Conrad et al, 1990; Kyrion et al, 1992), and indeed, a new study shows that ScRap1 prevents NHEJ-mediated fusions (Pardo and Marcand, 2005).
Like fission yeast Rap1 and unlike budding yeast Rap1, human RAP1 lacks DNA binding activity and localizes to telomeres via an interaction with TRF2 (Li et al, 2000). Overexpression of full-length or truncated forms of hRAP1 leads to telomerase-dependent telomere elongation, presumably by titrating telomerase inhibitory factors away from telomeres (Li and de Lange, 2003). These results suggest that, like yeast Rap1, hRAP1 is a negative regulator of telomerase. The involvement of hRAP1 in other aspects of human telomere biology, including telomere end protection, has so far not been described.
Budding yeast Rif1 interacts with telomeres by binding to ScRap1, and inhibits telomere elongation by regulating the accessibility of the telomere to telomerase activity (Hardy et al, 1992; Teixeira et al, 2004). In the absence of telomerase, Rif1 inhibits the formation of so-called ‘type II survivors' that use a Rad50-dependent recombination pathway to maintain telomeres (Teng et al, 2000). Any involvement of Rif1 in telomere end protection remains to be described. Surprisingly, human RIF1 appears neither to localize to functional telomeres nor to play a role in their maintenance or ‘capping'. Rather, hRIF1 is involved in the intra-S-phase DNA damage response pathway, and may function during anaphase as it localizes along a subset of midzone microtubules at this time (Silverman et al, 2004; Xu and Blackburn, 2004). Thus, Rap1 and Rif1 appear to function differently in budding yeast and humans.
Deletion of fission yeast rap1+ leads to telomere elongation, derepression of telomeric silencing and meiotic defects, all reminiscent of the phenotypes seen in taz1Δ cells (Chikashige and Hiraoka, 2001; Kanoh and Ishikawa, 2001). These observations suggested that at least some Taz1 functions are mediated by recruitment of Rap1 to telomeres. To address this issue directly, a chimeric protein was engineered in which Rap1 was fused to the C-terminal 167 aa of Taz1 that contains the 63 aa DNA binding domain (Chikashige and Hiraoka, 2001). This fusion protein localized to telomeres and largely restored meiotic telomere function in taz1Δ rap1Δ strains, suggesting that a key function of Taz1 during meiosis is to recruit Rap1. However, the ability of this fusion protein to fulfill other functions of Taz1 was not addressed. Fission yeast rif1Δ cells show slightly (approximately two-fold) elongated telomeres and a partial loss of meiotic spore viability (Kanoh and Ishikawa, 2001).
To gain insights into the components of a functional telomere ‘cap' and to assess the interplay between Rap1, Rif1 and Taz1, we evaluated single, double and triple mutants for a panel of telomere-related phenotypes. We found that Rap1 acts collectively with Taz1 to regulate telomere length and 3′ overhang formation and to protect telomeres from NHEJ. However, unlike taz1Δ cells, rap1Δ mutants are not cold sensitive, revealing that Taz1 acts independently of Rap1 to protect cells from cold-induced chromosomal defects. Nonetheless, in cells lacking Taz1, deletion of rap1+ exacerbates cold-induced defects. Remarkably, rif1+ deletion suppresses the cold sensitivity of taz1Δ cells. Our results suggest that Taz1 recruits distinct functional telomere complexes, at least one involving Rap1 and at least one independent of Rap1, and that Rap1 and Rif1 have Taz1-independent telomere activities.
Results
Telomere length regulation
Deletion of taz1 or rap1 results in very long telomeres while deletion of rif1 leads to modest telomere elongation (Cooper et al, 1997; Chikashige and Hiraoka, 2001; Kanoh and Ishikawa, 2001). To study the interdependence of these three genes, we deleted rap1+ or rif1+ in wild-type (wt) and various mutant strain backgrounds. As previously reported, deletion of taz1+ or rap1+ resulted in an ∼10-fold increase in telomere length and length heterogeneity (from ca. 300 bp in wt to 3–5 kbp in taz1Δ or rap1Δ), while loss of rif1+ led to an ∼2-fold increase in telomere length compared to wt cells (Figure 1A). We also confirmed that while deletion of rap1+ or rif1+ has no effect on the length of taz1Δ telomeres, the double mutant rap1Δrif1Δ has longer telomeres than those found in either single gene deletion or in taz1Δ cells. Intriguingly, the triple mutant rap1Δrif1Δtaz1Δ exhibited shorter telomeres than those in rap1Δrif1Δ strains. Thus, Taz1 appears to have both negative and positive roles in regulating telomere length, with its negative role being mediated by two separate pathways, one involving Rap1 and the other involving Rif1.
Figure 1.

Analysis of telomere length and 3′ telomeric overhang formation in various telomere mutants. (A) Telomere length. Genomic DNA was digested with Apa1, resolved on a 1% agarose gel, blotted and hybridized with a telomeric probe. (B) 3′ telomeric overhangs. EcoR1-digested genomic DNA was resolved on a 1% agarose gel and then hybridized under nondenaturing or denaturing conditions in duplicate gels to a G-strand-specific telomere probe. Due to the fragment size requirements for in-gel hybridization, wt DNA samples were digested with HindIII so that the terminal telomere fragments would be large enough for analysis (Tomita et al, 2003); note that the HindIII sites are ∼6 kb proximal to the EcoR1 sites on each chromosome.
3′ overhang processing
The telomeric 3′ overhang is a conserved feature and ranges in length from ∼12 nt in budding yeast during the G1 phase of the cell cycle to ∼50–500 nt in human cells (Makarov et al, 1997; McElligott and Wellinger, 1997; Larrivee et al, 2004). Loss of Taz1 results in deregulation of 3′ end formation, leading to a global elevation in 3′ overhang signal as detected by native in-gel hybridization (Tomita et al, 2003). To determine whether Taz1 acts through Rap1 or Rif1 to control 3′ overhang formation, we electrophoresed telomeric restriction fragments under native conditions and hybridized them to a G-strand-specific telomeric probe. As previously reported, G-strand overhangs were undetectable in asynchronous wt cells while taz1Δ cells displayed an intense, heterogeneous signal (Tomita et al, 2003; Figure 1B). This elevated signal was recapitulated in rap1Δ cells but not in rif1Δ cells (Figure 1B). rif1 deletion did not discernibly affect the 3′ overhang signal seen in rap1Δ or taz1Δ cells (data not shown). We note that in both taz1Δ and rap1Δ mutants, the bulk of the enhanced 3′ overhang signal migrated with higher molecular weight fragments than the bulk of the corresponding double-stranded telomere signal seen upon gel denaturation. This suggests that the longest telomeres in a population are more likely than the shorter telomeres to contain overhangs, or contain longer overhangs than those on the shorter telomeres, or both.
Prevention of chromosome end fusion
Taz1 is required to protect telomeres from NHEJ-mediated telomere fusions (Ferreira and Cooper, 2001). To analyze the roles of Rap1 and Rif1 in this telomere capping function, we subjected rap1Δ and rif1Δ mutants to nitrogen starvation, a condition that results in G1 arrest, leading to upregulation of NHEJ and fusions between taz1Δ telomeres. Pulsed field gel electrophoresis (PFGE) followed by Southern blotting with telomere probes was used to assess telomere fusions. Loss of Rap1, but not Rif1, resulted in the appearance of telomere fusion bands in G1-arrested cells (Figure 2). As has been demonstrated for taz1Δ telomere fusions, rap1Δ telomere fusions were fully dependent on Lig4, indicating that the fusions are formed by NHEJ. We conclude that Rap1 and Taz1 are both required to protect telomeres from NHEJ and may work through the same pathway, while Rif1 is dispensable for this aspect of telomere protection.
Figure 2.

Roles of Rap1 and Rif1 in preventing telomere fusions. (A) Map of fission yeast chromosomes showing telomeric Not1 restriction fragments. Digestion with NotI releases fragments L and I from Chr I and fragments M and C from Chr II. Chr III lacks Not1 sites. (B) PFGE of NotI-digested genomic DNA, blotted and hybridized with a telomere probe. The asterisk denotes the mobility of fragment C as well as any C-containing telomere fusion, which are not resolvable under these conditions.
Protection from cold sensitivity
At 20°C, loss of Taz1 results in chromosomal entanglement and missegregation, cellular elongation due to checkpoint activation and loss of viability (Miller and Cooper, 2003). Surprisingly, rap1Δ and rif1Δ single mutants, as well as rap1Δrif1Δ double mutants, showed normal growth at 20°C (Figure 3A), with both viability and cell morphology being identical to wt cells (Figure 3B and D). Thus, Taz1 acts independently of Rap1 or Rif1 to promote proper chromosome segregation and cell viability in the cold.
Figure 3.
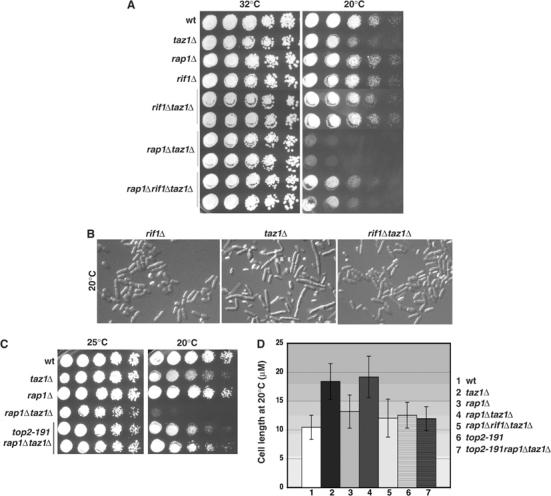
Analysis of fission yeast telomere mutants in the cold. (A) Five-fold serial dilutions of log phase cultures were stamped onto rich medium and incubated at the indicated temperature. (B) rif1+ deletion suppresses the elongation of taz1Δ cells at 20°C. Logarithmically growing cells in rich media at 20°C are compared. (C) A mutation in the gene encoding topoisomerase II, top2-191, suppresses the severe cold sensitivity of rap1Δtaz1Δ mutants. Experiments were performed as in panel A. (D) Cell lengths in the cold. A total of 300 cells of each genotype from asynchronous cultures were analyzed. Error bars represent standard deviations.
Strikingly, however, in taz1Δ cells, Rap1 and Rif1 acquire important and opposing roles in controlling cold sensitivity. rap1Δtaz1Δ double mutants exhibited extreme cold sensitivity, indicating that in the absence of Taz1, Rap1 is important for promoting cellular survival (Figure 3A and B). In marked contrast, deletion of rif1+ suppressed the cold sensitivity of taz1Δ cells (Figure 3A and B). taz1Δrif1Δ cells displayed neither the loss of viability (Figure 3A) nor the cellular elongation (Figure 3B and C) seen in taz1Δ cells at 20°C. Loss of Rif1 also largely suppressed the severe cold sensitivity observed in taz1Δrap1Δ cells (Figure 3A and D).
The cold sensitivity of taz1Δ cells is also suppressed by top2-191, a temperature-sensitive allele of the gene encoding topoisomerase II (Uemura and Yanagida, 1984; Miller and Cooper, 2003). As shown for the rif1 deletion, the top2-191 mutation suppressed the severe cold sensitivity of rap1Δtaz1Δ cells, supporting the notion that the underlying basis for cold sensitivity, presumably a replication defect that leads to telomeric entanglement at 20°C (Miller and Cooper, 2003; Miller et al, manuscript in preparation), is the same in taz1Δ and rap1Δtaz1Δ cells.
Ectopic recruitment of Rap1 to telomeres
The Hiraoka lab previously engineered a pair of chimeric proteins, one in which GFP is N-terminally fused to the C-terminal 167 aa of Taz1, which include the 63 aa DNA binding domain (‘GFP-Taz1C-term'), and another in which Rap1 was fused to the N-terminus of GFP-Taz1C-term to create Rap1-GFP-Taz1C-term (Figure 4A; Chikashige and Hiraoka, 2001). Both constructs were shown to bind telomeres in vivo. Interestingly, Rap1-GFP-Taz1C-term was shown to substantially restore meiotic telomere clustering and spore viability to taz1Δrap1Δ cells, while the GFP-Taz1C-term construct lacked this ability (in a taz1Δrap1+ background; Chikashige and Hiraoka, 2001). These observations demonstrated that Rap1 could function in the context of this fusion construct and suggested that Taz1 was dispensable for meiotic telomere clustering when Rap1 was ectopically tethered to telomeres. However, Rap1-GFP-Taz1C-term lacks the ability to restore telomere length regulation or control 3′ overhang formation in taz1Δrap1Δ strains, as strains harboring this construct have severely elongated telomeres and elevated 3′ overhang signals similar to those seen in taz1Δrap1Δ cells (Figure 4B and C). GFP-Taz1C-term was also unable to confer proper regulation of telomere length or 3′ overhang formation in taz1Δ cells. Thus, ectopic tethering of Rap1 to the telomere cannot confer proper telomere length regulation or 3′ overhang formation.
Figure 4.

Analysis of the effects of ectopically tethering Rap1 to telomeres. (A) Diagram of GFP-Taz1C-term and Rap1-GFP-Taz1C-term. The expressions of both chimeric constructs are driven by the nmt1 promoter. Unless otherwise indicated, all GFP-Taz1C-term experiments were performed in a taz1Δrap1+ background, and all Rap1-GFP-Taz1C-term experiments were performed in a taz1Δrap1Δ background. (B) Effects of GFP-Taz1C-term and Rap1-GFP-Taz1C-term on telomere length regulation. Experiments were performed as in Figure 1A. (C) Telomeric 3′ overhang regulation in GFP-Taz1C-term and Rap1-GFP-Taz1C-term cells. Experiments were performed as in Figure 1B. (D) Analysis of telomere fusions in GFP-Taz1C-term and Rap1-GFP-Taz1C-term cells. Experiments were performed as in Figure 2. The strain represented in lanes 1 and 2 (taz1Δrap1Δ Rap1-GFP-Taz1C-term) displays less ‘I' fragment, and correspondingly less ‘M+I' and ‘L+I' than the other strains, presumably because of mutations in the region containing the terminal Not1 site on the right arm of Chr I. (E) Growth of GFP-Taz1C-term and Rap1-GFP-Taz1C-term at 20°C. Experiments were performed as in Figure 3A.
Rap1-GFP-Taz1C-term was also unable to prevent telomere fusions in G1-arrested taz1Δrap1Δ cells. Surprisingly, however, taz1Δ cells expressing GFP-Taz1C-term remained refractory to telomere fusion during G1, despite having elevated telomere length and 3′ overhang signal (Figure 4B–D). This protection from telomere fusion depended on the presence of Rap1, as taz1Δrap1Δ cells harboring GFP-Taz1C-term display end fusions in G1. These data suggest that the C-terminus of Taz1 carries its capacity to prevent end fusion, and that this capacity is masked in the Rap1-GFP-Taz1C-term fusion construct.
taz1Δ strains harboring GFP-Taz1C-term and taz1Δrap1Δ strains containing Rap1-GFP-Taz1C-term grew normally at 20°C (Figure 4E). Thus, the C-terminal 167 aa of Taz1 fulfill its role in protecting cells from cold-induced defects.
Discussion
Taz1 plays a central role in sustaining a diverse range of telomere functions (Ferreira et al, 2004). Previous work suggested that Taz1 might act by recruiting Rap1 and Rif1, which would themselves fulfill the roles of telomere regulators (Chikashige and Hiraoka, 2001; Kanoh and Ishikawa, 2001). Our data reveal a more complex view of a functional telomere, as we found that Taz1 acts both as a platform for the recruitment of Rap1 and Rif1, and as a Rap1/Rif1-independent regulator of activities at telomeres. This study has also provided insights into which telomere functions are interdependent. For example, as rap1 deletion causes telomere lengthening and deregulation of 3′ overhang formation but not cold sensitivity, we can exclude the possibility that cold sensitivity is a consequence of elongation of the double- or single-stranded telomeric regions.
Telomere length maintenance
Taz1 is a key negative regulator of telomerase, as its loss leads to extremely long telomeres in the presence of telomerase but not in its absence (Nakamura et al, 1998). Interestingly, however, this study provides evidence that Taz1 also plays a positive role in telomere length regulation. rap1Δ and rif1Δ cells both harbor abnormally long telomeres (the former to a much greater extent than the latter), but deletion of either rap1+ or rif1+ has little effect on the length of taz1Δ telomeres, suggesting that each inhibits telomere elongation in a Taz1-dependent manner. The double mutant rap1Δrif1Δ displays longer telomeres than those in rap1Δ, rif1Δ or taz1Δ cells, while deletion of taz1+ in the rap1Δrif1Δ background restores a taz1Δ telomere length. Thus, we speculate that Taz1 inhibits telomerase via two separate pathways, one involving Rap1 and the other involving Rif1. Significantly, however, Taz1 is also required for efficient semiconservative replication of telomeric sequences, as loss of Taz1 leads to replication fork stalling at telomeres (Miller et al, manuscript in preparation). The telomere replication defect seen upon Taz1 loss may limit the extent of lengthening that would otherwise stem from loss of both the Rap1- and Rif1-mediated restraints on telomerase activity, thereby explaining the reduction in telomere length seen upon deletion of taz1+ in the rap1Δrif1Δ background.
Rap1, but not Rif1, is required to regulate 3′ overhang formation and prevent telomere fusions
Taz1 is required for proper 3′ G-strand overhang processing, as its loss leads to a dramatic increase in global 3′ telomeric overhang signal. Elevated overhang signals may stem from overextension of the G-strand by telomerase without compensatory C-strand synthesis, or from excessive degradation of the C-strand. In taz1Δ cells, both these mechanisms appear operative, as the bulk of the overhang signal disappears upon disruption of the end processing ‘MRN' (Mre11/Rad50/Nbs1) complex, but deletion of trt1+ also reduces the signal considerably (Tomita et al, 2003). Deletion of rap1+ also led to deregulated 3′ overhang formation, while loss of Rif1 did not (Figure 1B). Thus, Rap1 and Taz1 orchestrate the replicative and degradative processes that accompany 3′ overhang formation.
A critical function of Taz1 is to protect telomeres from NHEJ reactions that produce lethal telomere fusions (Ferreira and Cooper, 2001, 2004; Tuzon et al, 2004). We found that Rap1 participates in this function while Rif1 does not (Figure 2). Thus, telomere length regulation, 3′ overhang control and protection from NHEJ share a requirement for Taz1 and Rap1. Intriguingly, however, these telomere functions display a striking difference in their Taz1 domain requirement. While GFP-Taz1C-term prevents fusions in a rap1+taz1Δ background, it does not confer proper regulation of telomerase or 3′ overhang formation. This observation suggests that the C-terminus of Taz1 contains its ability to inhibit NHEJ between telomeres but not its capacity to regulate telomerase or end processing. A caveat inherent to this experiment is the GFP moiety within the fusion construct; conceivably, GFP enhances or destroys the activity of the Taz1 C-terminus (although it does not affect the protective function of full-length Taz1; MG Ferreira and JP Cooper, unpublished data). Nonetheless, these results indicate that fusions are not a secondary consequence of single- or double-stranded telomere lengthening. Rather, if long telomeres are bound by GFP-Taz1C-term, they are protected from end-joining reactions.
Roles of telomere proteins in preventing chromosomal entanglement at 20°C
At 20°C, taz1Δ cells suffer chromosomal entanglement, breakage and missegregation, accompanied by checkpoint activation and loss of viability. The appearance of aberrations in chromosome segregation at a given mitosis requires that the preceding S phase occurred at 20°C, indicating that the primary defect arises during S phase (Miller and Cooper, 2003). Indeed, taz1Δ cells accumulate stalled telomeric replication forks, which may constitute the initiating defect (Miller et al, manuscript in preparation). Although rap1Δ and taz1Δ cells share defects in telomere length regulation, 3′ overhang formation and control of NHEJ, the loss of Taz1 is unique in conferring cold sensitivity. Hence, cold sensitivity is not a secondary consequence of elongation of the double- or single-stranded telomere tracts, but rather stems specifically from loss of the Taz1 protein. Interestingly, we found that GFP-Taz1C-term can confer normal growth at 20°C, suggesting that the ability of Taz1 to prevent chromosomal entanglement, like its ability to prevent chromosome end fusion, resides in its C-terminus.
Although rap1Δ cells are not cold sensitive, Rap1 becomes critical for viability in taz1Δ cells at 20°C. Previous work suggested that Rap1 has some residual telomeric localization in cells lacking Taz1, as Taz1 was detected at at least one telomere in one of every three diploid taz1Δ/taz1Δ zygotes (Chikashige and Hiraoka, 2001; Kanoh and Ishikawa, 2001). Hence, the ability of Rap1 to enhance the viability of taz1Δ cells at 20°C may stem from this persisting population of telomere-associated Rap1. Rap1 may limit the replication defect encountered by taz1Δ telomeres or may facilitate resolution of the telomeric entanglements that are seen at 20°C (Miller and Cooper, 2003) and that presumably stem from the replication defect.
Functions of Rif1
While Rif1 plays a modest role in telomerase inhibition and appears dispensable for the control of 3′ overhang formation and protection from telomere fusion, it plays a dramatic and unexpected role in promoting the cold sensitivity of taz1Δ and rap1Δtaz1Δ cells, as rif1+ deletion suppresses these phenotypes. The mode by which Rif1 mediates the demise of taz1Δ cells in the cold is as yet unclear. Rif1 has been detected at telomeres using chromatin immunoprecipitation assays, and this telomeric enrichment depends on the presence of Taz1. However, Rif1 localization is not exclusively telomeric. When viewed by immunofluorescence, Rif1 appears diffusely throughout the nucleus in both wt and taz1Δ cells and only acquires a prominent telomere localization in rap1Δ cells (Kanoh and Ishikawa, 2001). This result could mean that Rif1 localizes to dysfunctional telomeres, like human RIF1 (Silverman et al, 2004). However, its absence from taz1Δ telomeres and from MMS-induced DNA damage foci (data not shown) argues that any targeting of Rif1 to uncapped telomeres or other DNA ‘ends' depends on Taz1. The elevated levels of Rif1 at rap1Δ telomeres may instead indicate that Rif1 and Rap1 compete for binding to Taz1. In either case, it seems likely that actions of nontelomeric Rif1 promote the cold sensitivity of taz1Δ cells.
The suppression of cold sensitivity by rif1+ deletion could be readily understood if Rif1 were a negative regulator of either homologous recombination or topoisomerase II activity, both of which play important roles in the survival of taz1Δ cells at 20°C (Miller and Cooper, 2003). Such actions would be reminiscent of the activity of budding yeast Rif1 in inhibiting the Rad50-dependent recombination-based (‘type II') survival of telomerase-negative cells (Teng et al, 2000). In human cells, Rif1 localizes to midzone microtubules between dividing chromosomes during anaphase, and to chromosomes during telophase (Xu and Blackburn, 2004). This localization is compatible with a role for Rif1 in controlling resolution of entwined chromosomes by topoisomerase II. Alternatively, Rif1 may act indirectly by controlling transcription of telomere maintenance genes (Smith et al, 2003). Current studies aim to delineate the mechanisms underlying Rif1 function.
A model for telomere maintenance in fission yeast
Our data suggest that Taz1 organizes at least three functional telomeric complexes, one involving Rap1, one involving Rif1 and one independent of both Rap1 and Rif1. Furthermore, in the absence of Taz1, Rap1 and Rif1 continue to exert effects on some aspects of telomere metabolism, whether they are acting from telomeric or nontelomeric sites. A summary of these findings is shown in Figure 5. Telomere-bound Taz1 itself prevents telomeric entanglement and cold sensitivity, and also serves as a platform for recruitment of Rap1 and Rif1. Rap1 functions along with Taz1 to control telomerase and 3′ end formation and to prevent telomere fusions. Rif1 mediates a telomerase-inhibitory pathway that is independent of Rap1, and Taz1 plays a Rap1/Rif1-independent positive role in telomere length regulation, probably via its role in promoting semiconservative telomere replication. In the absence of Taz1, Rap1 helps to restrain telomeric entanglement and cold sensitivity while Rif1 promotes it. Given the similarity in telomere organization between fission yeast and humans, these results are likely to be relevant to the functions of human telomere proteins as well.
Figure 5.

Summary of genetic analysis of Rap1, Rif1 and Taz1. (A) Taz1-containing telomere complexes. Taz1 organizes at least three functional telomere complexes. Rif1–Taz inhibits telomere elongation. Rap1–Taz1 inhibits telomere elongation, controls 3′ overhang generation and prevents NHEJ-mediated chromosome end fusions. In addition, Taz1 functions in a Rap1/Rif1-independent manner to protect cells from cold sensitivity and to positively regulate telomere length (see text). (B) Telomere complexes lacking Taz1. In the absence of Taz1, Rap1 restrains cold-specific abnormalities while Rif1 promotes it. In taz1Δ cells, Rif1 may act at nontelomeric sites to affect telomere metabolism.
Materials and methods
Yeast strains and media
The single mutant taz1Δ was previously described (Cooper et al, 1997). Other single mutant strains used in this study were constructed by one-step gene replacement of the entire ORF with a kanMX6 cassette (Bahler et al, 1998). Double and triple mutants were produced by mating, sporulation and selection followed by PCR verification of genotype, except for the taz1Δrap1Δ and taz1rif1Δ double mutants, which were constructed by replacing rap1+ or rif1+ with kanMX6 in a taz1∷ura4 background. The creation of Rap1-GFP-Taz1C-term and GFP-Taz1C-term strains was previously described (Chikashige and Hiraoka, 2001). All media were as described (Moreno et al, 1991). Cultures were grown at either 32 or 20°C in rich medium (YES) as described (Miller and Cooper, 2003), or nitrogen starved by culturing in EMM media without NH4Cl as described (Ferreira and Cooper, 2001).
Cytological analysis
Cellular morphologies were analyzed by collecting log phase cultures grown at the indicated temperatures and visualizing the cells by differential interference contrast (DIC) microscopy. Cell lengths were measured for >100 cells in two independent experiments from cultures maintained at 20°C for 3 days. Quantitation of cell length is presented as the mean±the standard deviation for each strain.
Viability assays
Cold and drug sensitivity analyses were performed as described (Miller and Cooper, 2003). Five-fold serial dilutions from a starting concentration of 2 × 107 cells/ml are shown.
DNA analysis
Telomere lengths were determined as described (Cooper et al, 1997). Blots were hybridized to a random prime labeled telomere fragment from pSNU70 (Nakamura et al, 1998). To detect telomere fusions, cells were embedded in low melting point agarose, digested with NotI and resolved by PFGE as previously described (Ferreira and Cooper, 2001). Telomere 3′ overhangs were detected according to Tomita et al (2003).
Acknowledgments
We thank Yasushi Hiraoka and Yuji Chikashige for generously providing us with strains harboring fusion constructs, Stéphane Marcand for critical reading of the manuscript and sharing unpublished data and Ginger Zakian for discussions and comments on the manuscript. We thank our lab members for discussion and support, and especially Kazunori Tomita for help with strain construction. This work was supported by Cancer Research UK.
References
- Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A III, Steever AB, Wach A, Philippsen P, Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951 [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Hiraoka Y (2001) Telomere binding of the Rap1 protein is required for meiosis in fission yeast. Curr Biol 11: 1618–1623 [DOI] [PubMed] [Google Scholar]
- Conrad MN, Wright JH, Wolf AJ, Zakian VA (1990) RAP1 protein interacts with yeast telomeres in vivo: overproduction alters telomere structure and decreases chromosome stability. Cell 63: 739–750 [DOI] [PubMed] [Google Scholar]
- Cooper JP, Nimmo ER, Allshire RC, Cech TR (1997) Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature 385: 744–747 [DOI] [PubMed] [Google Scholar]
- Ferreira M, Cooper J (2001) The fission yeast Taz1 protein protects chromosomes from Ku-dependent end-to-end fusions. Mol Cell 7: 55–63 [DOI] [PubMed] [Google Scholar]
- Ferreira MG, Cooper JP (2004) Two modes of DNA double-strand break repair are reciprocally regulated through the fission yeast cell cycle. Genes Dev 18: 2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MG, Miller KM, Cooper JP (2004) Indecent exposure: when telomeres become uncapped. Mol Cell 13: 7–18 [DOI] [PubMed] [Google Scholar]
- Hardy CF, Sussel L, Shore D (1992) A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev 6: 801–814 [DOI] [PubMed] [Google Scholar]
- Kanoh J, Ishikawa F (2001) spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr Biol 11: 1624–1630 [DOI] [PubMed] [Google Scholar]
- Kyrion G, Boakye KA, Lustig AJ (1992) C-terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol Cell Biol 12: 5159–5173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrion G, Liu K, Liu C, Lustig AJ (1993) RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev 7: 1146–1159 [DOI] [PubMed] [Google Scholar]
- Larrivee M, LeBel C, Wellinger RJ (2004) The generation of proper constitutive G-tails on yeast telomeres is dependent on the MRX complex. Genes Dev 18: 1391–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, de Lange T (2003) Rap1 affects the length and heterogeneity of human telomeres. Mol Biol Cell 14: 5060–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Oestreich S, de Lange T (2000) Identification of human Rap1: implications for telomere evolution. Cell 101: 471–483 [DOI] [PubMed] [Google Scholar]
- Makarov VL, Hirose Y, Langmore JP (1997) Long G tails at both ends of human chromosomes suggest a C-strand degradation mechanism for telomere shortening. Cell 88: 657–666 [DOI] [PubMed] [Google Scholar]
- McElligott R, Wellinger RJ (1997) The terminal DNA structure of mammalian chromosomes. EMBO J 16: 3705–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Cooper JP (2003) The telomere protein Taz1 is required to prevent and repair genomic DNA breaks. Mol Cell 11: 303–313 [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Nakamura TM, Cooper JP, Cech TR (1998) Two modes of survival of fission yeast without telomerase. Science 282: 493–496 [DOI] [PubMed] [Google Scholar]
- Pardo B, Marcand S (2005) Rap1 prevents telomere fusions by non-homologous end-joining. EMBO J (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D (1994) RAP1: a protean regulator in yeast. Trends Genet 10: 408–412 [DOI] [PubMed] [Google Scholar]
- Shore D (2001) Telomeric chromatin: replicating and wrapping up chromosome ends. Curr Opin Genet Dev 11: 189–198 [DOI] [PubMed] [Google Scholar]
- Silverman J, Takai H, Buonomo SB, Eisenhaber F, de Lange T (2004) Human Rif1, ortholog of a yeast telomeric protein, is regulated by ATM and 53BP1 and functions in the S-phase checkpoint. Genes Dev 18: 2108–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Smith DL, DeRisi JL, Blackburn EH (2003) Telomeric protein distributions and remodeling through the cell cycle in Saccharomyces cerevisiae. Mol Biol Cell 14: 556–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, De Lange T (2004) Regulation of telomerase by telomeric proteins. Annu Rev Biochem 73: 177–208 [DOI] [PubMed] [Google Scholar]
- Sussel L, Shore D (1991) Separation of transcriptional activation and silencing functions of the RAP1-encoded repressor/activator protein 1: isolation of viable mutants affecting both silencing and telomere length. Proc Natl Acad Sci USA 88: 7749–7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MT, Arneric M, Sperisen P, Lingner J (2004) Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 117: 323–335 [DOI] [PubMed] [Google Scholar]
- Teng SC, Chang J, McCowan B, Zakian VA (2000) Telomerase-independent lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, Rif-inhibited recombinational process. Mol Cell 6: 947–952 [DOI] [PubMed] [Google Scholar]
- Tomita K, Matsuura A, Caspari T, Carr AM, Akamatsu Y, Iwasaki H, Mizuno K, Ohta K, Uritani M, Ushimaru T, Yoshinaga K, Ueno M (2003) Competition between the Rad50 complex and the Ku heterodimer reveals a role for Exo1 in processing double-strand breaks but not telomeres. Mol Cell Biol 23: 5186–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzon CT, Borgstrom B, Weilguny D, Egel R, Cooper JP, Nielsen O (2004) The fission yeast heterochromatin protein Rik1 is required for telomere clustering during meiosis. J Cell Biol 165: 759–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Yanagida M (1984) Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J 3: 1737–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JR (1999) The economics of ribosome biosynthesis in yeast. TIBS 24: 437–440 [DOI] [PubMed] [Google Scholar]
- Xu L, Blackburn EH (2004) Human Rif1 protein binds aberrant telomeres and aligns along anaphase midzone microtubules. J Cell Biol 167: 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]


