Abstract
Nuclear spin hyperpolarization utilizing parahydrogen has the potential for broad applications in chemistry, catalysis, biochemistry, and medicine. This review examines recent chemical and biochemical insights gained using parahydrogen-induced polarization (PHIP). We begin with photoinduced PHIP, which allows the investigation of short-lived and photoactivated catalysis. Next, we review the partially negative line effect, in which distinctive line shape helps to reveal information about rapid exchange with parahydrogen and the role of short-lived catalytic species. The NMR signal enhancement of a single proton in oneH-PHIP is discussed, challenging the underpinning concept of the necessity of pairwise hydrogenation. Furthermore, we examine metal-free PHIP facilitated by frustrated Lewis pair molecular tweezers and radicaloids, demonstrating alternative routes to hydrogenation. Although symmetric molecules incorporating parahydrogen are NMR silent, we showcase methods that reveal hyperpolarized states through post-hydrogenation reactions. We discuss chemical exchange processes that mediate polarization transfer between parahydrogen and a molecular target, expanding the reach of PHIP without synthesizing specialized precursors. We conclude this review by highlighting the role of PHIP in uncovering the H2 activation mechanisms of hydrogenases. By providing a detailed review of these diverse phenomena, we aim to familiarize the reader with the versatility of PHIP and its potential applications for mechanistic studies and chemical analysis.
Keywords: parahydrogen, catalysis, hyperpolarization, mechanisms, NMR
1. Introduction
Without hesitation, nuclear magnetic resonance (NMR) is one of the leading and most widely used spectroscopic methods in chemistry. This success is due to its broad analytical capabilities and noninvasive nature, enabled by the minuscule magnetic moments associated with the nuclear spins. Despite its ubiquitous role in molecular characterization and analysis, NMR suffers from low sensitivity, as only a tiny fraction of all available nuclear spins effectively contributes to the total NMR signal. Consequently, combinations of time-consuming signal averaging, concentrated samples (>mM), and elaborate equipment/hardware are often necessary. Addressing these challenges is the focus of much attention, and the scope of NMR applications is constantly expanding through the introduction of more sensitive NMR instruments with higher magnetic fields,1 cryoprobes,2 ultrafast sequences,3 and nuclear spin hyperpolarization methods.4
In this review, we focus on one of many hyperpolarization methods that increase nuclear spin polarization using various physical and chemical effects. Such processes create transient non-Boltzmann population distributions across closely spaced nuclear spin energy levels. This effect is highly beneficial as NMR signal enhancements of several orders of magnitude can be obtained. For example, at a clinical field strength of 1.5 T, only 0.000125%, about one in 800,000 13C nuclei, is effectively detected (thermally polarized under ambient conditions). When the system is fully polarized, all 13C spins contribute to the signal, enhancing the signal—and thus the sensitivity—by more than 5 orders of magnitude. Hence, hyperpolarization is a versatile tool that aids the magnetic resonance detection of molecules at low concentrations or short lifetime, with diverse applications in biomedical imaging,5−8 protein studies,9−11 composition analysis12,13 and catalysis.14−16
Parahydrogen-induced polarization (PHIP), introduced in the 1980s,17−19 exploits the alignment of the nuclear spins in the singlet state spin isomer of molecular hydrogen, parahydrogen (pH2).20 pH2 reflects the lowest energy spin isomer of dihydrogen and can be easily prepared in an almost pure state by cooling hydrogen gas to 20 K or below,21−24 though 77 K is often used for practical simplicity and yields a pH2 enrichment of about 50%.25−27 Para-enriched H2 gas can typically be stored for weeks at ambient temperatures and used on demand to generate nuclear spin hyperpolarization. However, as the para-state is associated with singlet nuclear spin order, it does not yield any NMR signal and is considered “NMR silent”; further chemical reactivity breaking the symmetry of pH2 is required to enhance the NMR signal.17−19
The detailed spin dynamics-based theory underlying PHIP and related phenomena are well described elsewhere.20,28−265 Here, we aim to provide an accessible narrative that, while linking appropriate physicochemical descriptions, does not overburden the reader with overly advanced spin physics. In classical PHIP experiments,17−19 which are also termed hydrogenative PHIP (hPHIP), two protons of pH2 are typically added at vicinal positions to double or triple CC bonds in an unsaturated substrate through catalytic hydrogenation (Figure 1a). As the spin states in the newly formed molecule evolve, the 1H NMR signal can be significantly enhanced. In this regard, two experimental schemes are distinguished: parahydrogen and synthesis allow dramatically enhanced nuclear alignment (PASADENA), where the hydrogenation process takes place at a high magnetic field,18 and adiabatic longitudinal transport after dissociation engenders net alignment (ALTADENA), where hydrogenation occurs at low-field.31 In both cases, the resultant 1H hyperpolarization can be used as is or transferred to another nucleus, such as 13C and 15N, e.g., for observation to benefit from longer relaxation times, no background signals, and greater chemical shift dispersion relative to 1H. While hPHIP has been most commonly achieved by homogeneous Rh or Ru catalysts,32−35 other metals36−41 and heterogeneous catalysts have also exhibited PHIP,42,43 although typically with smaller polarization than homogeneous systems.
Figure 1.
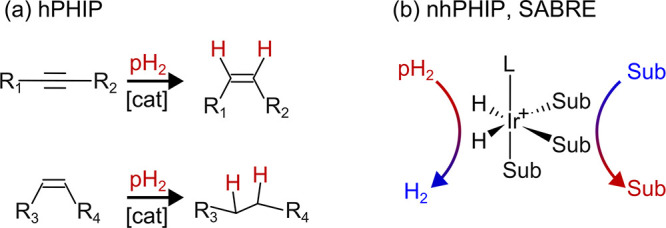
hPHIP and SABRE. Schematics of (a) hPHIP, whereby parahydrogen (pH2) is added to an unsaturated bond in the presence of a catalyst, [cat], and (b) SABRE, where a ligating substrate, Sub, and pH2 undergo reversible exchange at a catalyst. The typical SABRE auxiliary ligand L is IMes = 1,3-bis(2,4,6-trimethylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene.
For alkyne substrates, cis-vicinal hydrogenation is the most common type of hydrogenation achieved by homogeneous metal catalysts, and, therefore, it is a common reaction used in hPHIP (Figure 1a). Recently, however, trans-vicinal hydrogenation was turned into a dominant pathway to unlock direct hyperpolarization of fumarate44,45—an intermediate in the citric acid cycle—and a marker of cell necrosis.46 In contrast, heterogeneous catalysts (e.g., supported metal nanoparticles) typically yield low hydrogenation selectivity as the reaction mechanism can involve multiple metal sites that deliver their hydrogen atoms to various positions in the product, either directly or reversibly.15,43,49,50 The convenience of using heterogeneous catalysts is now facilitating the production of hyperpolarized gases as pH2-enhanced contrast agents for lung MRI.47,48
In general, hPHIP experiments can also be used to quantify the precise stereo and regioselectivity in such processes through the signal amplification effect.49−52 Regardless of the hydrogenation mechanism, a crucial requirement to observe hPHIP is the pairwise addition of H2 to a metal catalyst or unsaturated precursor. In other words, both 1H spins of the same pH2 molecule must end up in the same product molecule to retain their spin correlation. This requirement has provided significant insight into metal-based oxidative addition reactions.53
An alternative to hPHIP is non-hydrogenative PHIP (nhPHIP, Figure 2), with its most popular form called signal amplification by reversible exchange (SABRE, Figure 1b).54 In SABRE, pH2 and a substrate ligate transiently with a metal center. Depending on the experimental conditions, J-couplings or cross-relaxation can then drive the conversion of the spin alignment of the pH2-derived spins into the spin polarization of the transiently bound substrate in the resulting complex. Subsequent dissociation of this ligand results in a chemically unaltered but hyperpolarized free substrate in solution,55 allowing for multiple contacts and continuous polarization of the same molecule.56−58 Typically, the most efficient polarization transfer in SABRE is reached when there is a match between certain energy levels in the SABRE complex spin system, and this can be readily achieved by selecting appropriate DC or AC magnetic fields for the experiment.59−62 Such a matching condition is more precisely termed level anticrossing (LAC), and its theoretical description in the context of hyperpolarization is well-reviewed.63
Figure 2.
Classification of chemical effects that lead to PHIP. The primary scope of this review is to describe the pH2-derived hyperpolarization effects except those of routine hPHIP and SABRE, which are well-reviewed. *Preliminary assignment based on current data. Adapted with permission from Emondts et al.109Copyright 2018 John Wiley and Sons.
Several specific requirements must be met for molecules to become hyperpolarized by PHIP. For hPHIP, these include the presence of an unsaturated functionality (typically alkene or alkyne groups) that can accept pH2. The strategy to hyperpolarize molecules that do not possess an appropriate unsaturated bond64−71 or ligating functionality72,73 is to add such a function on a side arm. The resulting side arm is then released after hydrogenation and hyperpolarization transfer by hydrolysis (e.g., PHIP-SAH).65−71 For SABRE, the substrate and pH2 must ligate to a metal center, form spin–spin interactions, and dissociate. Therefore, such substrates often contain an electron-donating nitrogen site.54,74 However, the ongoing design of new catalysts has significantly expanded the types of molecules amenable to SABRE to include biologically significant O-donor ketoacids.75 Other studies have seen sulfur, phosphorus, and silicon donor sites employed.76−78
Collectively, hPHIP,47,79−84 PHIP-SAH,85−87 and SABRE88,89 have led to in vivo metabolic imaging applications. The translation from optimization of PHIP to in vitro and in vivo studies is not straightforward but has accelerated in recent years, demonstrating PHIP as a viable route to produce preclinical and clinical hyperpolarized agents in liquid and gas phases competitive with other approaches such as dissolution dynamic nuclear polarization (dDNP)85 or spin-exchange optical pumping (SEOP).90−92 Advances in PHIP for in vivo imaging have been well-reviewed.4,93−96 This progress is underpinned by several advancements in the instrumentation associated with PHIP delivery.97
In addition to the relatively well-known protocols of hPHIP and SABRE, there are other exciting effects that, although less well appreciated, more generally illustrate the unique properties of pH2, which in turn provide valuable insight into both chemical and catalytic reactivity.29 For example, one less common type of hPHIP is geminal hydrogenation, where two protons of pH2 bind to the same carbon.98,99 There are other variations of the PHIP effect, such as when the catalyst itself interacts with pH2 and becomes transiently polarized.100−104 Other cases include pairwise replacement of two substrate protons with a proton pair from pH2 with no net hydrogenation,16,49,105,106 the addition of only one proton from a pH2 molecule to the substrate (oneH-PHIP)107 (after the formation of an intermediate by pairwise addition) or even hydrogenation accompanied by oligomerization.108
This review will focus on such more unusual PHIP effects beyond PASADENA, ALTADENA, and SABRE (Figure 2). We will discuss these phenomena and their mechanisms, seeking to promote new analytical applications and provide valuable insight into the underlying chemical interactions. In this regard, we will discuss photoinduced PHIP and SABRE, partially negative line (PNL) effects and their implications for the analysis of short-lived intermediates, oneH-PHIP effects in hydroformylation, the various mechanisms of hyperpolarization of water using pH2, PHIP effects in metal-free hydrogenation (MF-PHIP) reactions, secondary transformation which reveal hidden PHIP, chemically relayed polarization transfer, and PHIP in enzymatically catalyzed hydrogenation reactions.
2. Unconventional Variants of PHIP
2.1. Photo-PHIP and Photo-SABRE
Breaking the bond in pH2 is a critical step in any PHIP-type experiment, as it unlocks the spin order of pH2, ideally without losing the initial spin alignment. This step is most commonly associated with the oxidative addition of pH2 to a metal center. An essential feature of this step, therefore, is the involvement of metal complex species with appropriate electron configurations (preferably diamagnetic) that both prevent rapid nuclear spin relaxation and support the formation of two new metal–hydride bonds. Accordingly, many stable complexes must undergo ligand loss before such a process occurs. This has given rise to studies where light irradiation stimulates ligand loss from a stable metal complex to generate a reactive intermediate that can react rapidly with pH2 (Figure 3a).110−116 Consequently, hyperpolarized metal complexes are formed in a process that is somewhat analogous to hPHIP, which involves pH2 addition to a stable 16-electron transition metal precursor. However, combining photochemistry with PHIP, termed photo-PHIP, can create magnetic states that differ from those created under conventional PASADENA-type PHIP.18,117,118 This photochemical approach has been used to study rapid kinetics111,114 and detect reaction intermediates or products.110,114,119 The examples of photo-PHIP typically involve Ir and Ru carbonyl precursors containing phosphine, diphosphine, or diarsine ligands.115,116,119,120
Figure 3.
Examples of photo-PHIP. (a) The outline of a photo-PHIP experiment in which light-induced ligand dissociation creates a metal complex [M] that can add pH2 to create an inequivalent metal dihydride species with PHIP-enhanced hydride NMR signals. (b) Depiction of laser-pump NMR-probe spectroscopy in which laser irradiation stimulated H2 – pH2 exchange followed by NMR detection (where θ is typically 45° or 90°), with a variable delay between the two (top left). The NMR spectrum at the top right is a single-laser shot, single 90° RF pulse, pH2-enhanced 1H{31P} NMR spectrum of the hydride region of [Ru(PPh3)3(CO)(H)2] with τ = 0.05 ms (broadband 31P decoupling). When the delay is on the millisecond time scale, the zero quantum coherences of pH2 in the chemically inequivalent dihydride complex can evolve due to the chemical shift difference from unobservable ÎxŜx + ÎyŜy to observable ÎyŜx – ÎxŜy. The lower kinetic traces show the hydride signal integral at δ ∼ −6.5 ppm from 1H{31P} NMR spectra of [Ru(PPh3)3(CO)(H)2] (1 × 10–3 M, 3 bar pH2) as a function of τ. Experimental points are shown in blue with fitted red lines. This spin evolution can give information about kinetic hydrogen addition rates. (c) Slow photo-PHIP leading to hyperpolarized styrene and ethylbenzene after [Ru(H)2(PPh3)3(CO)] and an iridium photosensitizer (PS) are irradiated at 420 nm in DCM-d2 at 298 K.127 In these cases, delays between laser irradiation and NMR pulse acquisition are on the order of seconds. Consequently, ZQC coherences are not observed, which is similar to the traditional PASADENA effect. (d) Depiction of photo-SABRE in which a SABRE-catalyst hyperpolarizes cis-azobenzene (ABZ) followed by light irradiation to switch between cis and trans isomers of ABZ (upper). Hyperpolarized molecules are shown with a gray background.128 Example 1H (lower left) and 15N (lower right) NMR spectra were recorded after 10 min of light irradiation of a sample containing 56 mM of 15N2-ABZ in CD3OD with 1 mM of [IrCl(COD)(IMes)] and 200 mM DMSO-d6 at 9.4 T. SABRE polarization transfer to 1H was performed at 200 nT and that to 15N, at 400 nT. Signals for cis-ABZ are shown with a red background, with those of trans-ABZ with a blue background. (a) Adapted with permission from Procacci et al.114Copyright 2016 Royal Society of Chemistry. This publication is licensed under CC BY-NC 3.0. (b) Adapted from Torres et al.113Copyright 2014 American Chemical Society. This publication is licensed under CC-BY. (c) Adapted from Brown et al.127Copyright 2022 American Chemical Society. This publication is licensed under CC-BY 4.0. (d) Adapted with permission from Kiryutin et al.128Copyright 2024 John Wiley and Sons.
To introduce photo-PHIP in further detail, it is convenient to provide theoretical considerations that illustrate the basic differences between traditional and photochemistry-assisted approaches. For reference, pH2 exists as a nuclear spin singlet state that is defined by the density matrix described in eq 1
| 1 |
where Îi and Ŝi (i = x, y, z) are spin operators which refer to identical atoms in pH2. This state consists of the longitudinal two-spin order ÎzŜz, and the in-phase zero-quantum coherence (ZQC) ÎxŜx + ÎyŜy. In hPHIP, numerous catalytic hydrogenation steps, or reversible H2 exchange in SABRE, commonly create weakly coupled metal-dihydrides. In this case, the in-phase ZQC oscillates due to the periodic transformation into the out-of-phase ZQC state, ÎyŜx – ÎxŜy. As a result, it averages to zero over the time of thermally initiated reactions,121 unless strong proton decoupling is applied82,122 or experiments are performed at low magnetic fields when metal-dihydrides are strongly coupled.123
In contrast, the photo-PHIP approach involves short laser-induced photodissociation (on the order of nanoseconds) to create a vacant ligand site such that rapid reaction with pH2 is possible (Figure 3a). As a consequence of the relatively facile pH2 addition (faster than 1/vZQC such that ZQC does not have enough time to oscillate) all terms in eq 1 are preserved through the hydrogenation reaction even though the singlet state is not stationary in the dihydride product complex.116 Due to this, the ZQC oscillation is observable in the subsequent 1H NMR measurements (Figure 3b). For two weakly coupled spins, this oscillation of ZQC occurs at a frequency equal to the difference between the Larmor frequencies of the two nuclei, vZQC = |vI – vS|, where vI and vS are the Larmor frequencies of the respective spins I and S. Typically, in photo-PHIP systems, there are strong interactions between hydride protons and 31P of spectator phosphine ligands, which additionally contribute to vZQC.
At the instant the symmetry of the initial pH2 is broken in forming the newly created hydride ligands (the time after the laser pulse is essentially τ = 0 for a rapid reaction), the newly formed spin system cannot be perturbed by a hard excitation RF pulse as it is instantly formed from the NMR silent singlet state (Figure 3b). Consequently, this situation does not lead to an observable NMR signal. However, as the transverse ZQC term (eq 1) evolves under chemical shift and coupling, an NMR-observable spin state arrangement is created that can be probed directly with hard pulses (typically 45° or 90°, Figure 3b).112,122,124 This approach was used to study the evolution of dihydride singlet order for a wide range of Ru and Ir complexes with a variable τ between laser irradiation and NMR detection.112 This type of laser-pump NMR-probe experiment, conducted over a microsecond-to-millisecond delay time scale, allowed the observation of reactivity in an approach analogous to other laser-based UV and IR time-resolved spectroscopies, which was only possible by marrying laser-induced ligand dissociation with a pH2 addition step (Figure 3b).110−116 Multiple laser pulses or continuous wave laser irradiation over times much longer than 1/vZQC have also been employed.114,116 However, in these cases of slow hyperpolarization preparation, spin order is averaged across the light irradiation time, which leads to an effect analogous to the time-averaged PASADENA effect as the ZQC terms are lost (Figure 3c).
In contrast to hard pulse excitations, the selective RF excitation of one of the chemically inequivalent hydride ligands in the complex can lead to the observation of strong NMR signals without the need for further spin-state evolution after the laser pulse.125 Alternatively, adiabatic RF pulses can convert the singlet spin order into observable magnetization of one or two protons.124,126
The analysis process required to extract information about the kinetics of pH2 addition in photo-PHIP experiments is complex.111,113,124 For example, in cases where the pH2 addition rate is on the same order of magnitude as the frequency of ZQC evolution, vZQC, only partial averaging occurs. This can lead to apparent phase shifts in the signal oscillation that contain quantitative information about the pH2 addition rate.114 However, if product formation is much faster than the rate of ZQC evolution, then only an upper bound for the H2 addition rate can be determined. On the other hand, if product formation is much slower than the characteristic frequencies of the spin system evolution, the ZQC oscillations are no longer observed due to complete averaging. The latter case is similar to traditional PHIP in which thermally controlled reactions (rather than laser-induced) build up the number of H2 addition products over a longer time window (ca. seconds).111
The photo-PHIP method has been applied to measure the H2 addition rate of [Ir(I)(PPh3)2(CO)], which is formed from laser-induced H2 dissociation from [Ir(I)(H)2(PPh3)2(CO)] (Figure 3b).114 Notably, the obtained rate constants were comparable to those measured using flash photolysis coupled with optical spectroscopy.114 However, unlike flash photolysis, NMR has additional chemical resolution that enables a more detailed analysis of chemical reactions, which is especially important for mixtures of photoactive substrates.
Approaches of this type have also been used to study ruthenium arsine complexes that act as alkyne hydrogenation catalysts as catalytic activity is observed after photolysis, and many intermediates and species involved in the catalytic cycle are detected and characterized with the help of photo-PHIP-enhanced signals.115 Due to the lower reaction rate of these catalysts, these types of examples can be called slow photo-PHIP. The delay between laser irradiation and NMR signal acquisition is much longer (on the order of seconds). ZQC completely decays, revealing enhanced antiphase NMR signals analogous to those in traditional PASADENA experiments without amplitude oscillations. Initiating hydrogenation catalysis with a laser pulse allows access to hyperpolarized species inaccessible in thermal reactions (without laser-induced dissociation), as thermal conditions are insufficient to enable ligand dissociation, which must occur before pH2 addition for many metal complexes. One recent example also involved the use of an irradiated iridium photosensitizer to produce excited [Ru(H)2(PPh3)3(CO)], which in turn stimulated H2 dissociation to form [Ru(PPh3)3(CO)]. Subsequent hydrogenation of phenylacetylene with pH2 yielded PHIP-enhanced styrene (single hydrogenation) and ethylbenzene (double hydrogenation) with 1H NMR signals for these organic photoactivated hydrogenation products enhanced by up to 1630 times at 9.4 T (Figure 3c).127
While photoactivated PHIP has several examples in general, few examples of photoactivated SABRE exist. SABRE typically relies on iridium catalysts that reversibly exchange H2 under thermal conditions. Rational catalyst design is, however, often used to improve the efficiency of SABRE by tuning ligand exchange rates or controlling the binding of particular target substrate molecules.16 One example of a type of photo-SABRE involved azobenzene, with light irradiation of the target ligand rather than the metal catalyst. This approach used light irradiation to switch azobenzene between cis and trans conformations, controlling SABRE activity as only cis-azobenzene has appropriate geometry for ligation to the iridium SABRE catalyst (Figure 3d).128 Upon hyperpolarization of its 15N nuclei using a traditional SABRE approach, light-induced isomerization locked the polarization within trans-azobenzene as it does not interact with the metal center, allowing for prolonged polarization lifetimes. Another benefit was that the trans isomer also formed a long-lived spin state with a lifetime of ca. 25 min, which means it can act as a store of polarization. While this example utilized a photoswitchable target, in the future, light-controlled catalysts could be exploited for SABRE, where photoactivation initiates either H2 or substrate exchange within the metal coordination sphere. Indeed, while this review was provisionally accepted, an example of the latter was reported.266 Developing such photo-SABRE approaches would allow the precise tuning of the scalar coupling network within the transient active catalyst to match the ligand exchange rate, thereby optimizing polarization transfer to the bound substrate. By employing photochemical control, such systems could achieve polarization levels closer to the theoretical maximum than those currently achieved through thermally driven exchange. Moreover, rather than requiring a diverse range of catalysts to accommodate different substrates and conditions, a single, photoswitchable catalyst could potentially be used. This would enhance both efficiency and selectivity while simplifying synthetic needs. Given these potential advantages, developing new classes of photo-SABRE catalysts reflects a promising avenue for advancing this hyperpolarization technique.
2.2. Reversible H2 Exchange and the Partial Negative Line (PNL) Effect
Intra- and intermolecular chemical exchange can have a strong influence on the appearance of the resonances in an NMR spectrum, inducing line broadening and line shape distortions. Consequently, NMR line shape analysis can be employed as an analytical tool to monitor the dynamics of transient catalytic species. In the context of PHIP, such behavior is generally relevant to molecular dihydrogen complexes interacting reversibly with pH2 and hence dissolved dihydrogen (Figure 4a), as well as other weakly interacting ligands. At the same time, since these processes involve pH2, unlocking its magnetism introduces novel phenomena that cannot be observed with normal H2. One intriguing effect of this kind was independently observed as an enhanced antiphase signal for dissolved H2 by Zhivonitko et al.129 during the investigation of pH2 activation with ansa-aminoboranes and by Kiryutin and Sauer et al.130 during the investigation of the PHIP enhancement of small oligopeptides (Figure 4b). This phenomenon is now referred to as the partially negative line (PNL) shape of the dihydrogen NMR signal.
Figure 4.
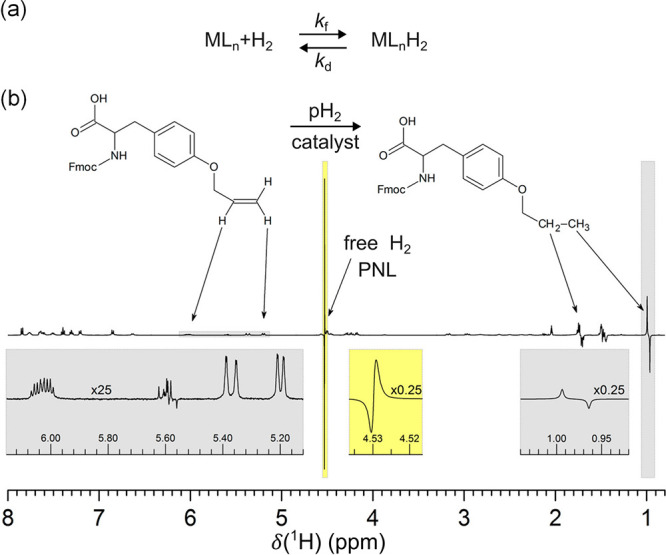
Partial Negative Line effect induced by a catalyst–pH2 interaction. (a) Chemical exchange of pH2 with the metal center MLn of the catalyst results in the PNL effect on free H2. (b) 1H PHIP NMR spectrum recorded during the hydrogenation of Fmoc-O-allyl-tyrosine (left) to Fmoc-O-propyl-tyrosine (right) with pH2, showing a strong PNL signal for free H2 at 4.53 ppm. Adapted with permission from Kiryutin et al.130Copyright 2017 American Chemical Society.
The observation of the PNL effect is unusual because dihydrogen is a symmetric molecule that should exhibit only a single resonance in 1H NMR (A2 spin system). However, the antiphase character of the H2 signal in PHIP experiments implies the presence of two resonances (Figure 4b).130 Kiryutin et al. showed that the PNL effect is independent of the presence of a substrate and occurs via the transient interaction of the pH2 molecules with the catalyst under fast chemical exchange.130 Using the double quantum coherence filter variant of the only parahydrogen spectroscopy (OPSY) NMR pulse sequence,131−134 it was demonstrated that the PNL effect results from the longitudinal two-spin order, ÎzŜz, originating from pH2 (eq 1). Normally, this term is NMR-invisible for a pair of magnetically equivalent protons in H2. However, the rapid exchange between free H2 and two chemically inequivalent hydride ligands in a transient catalyst–dihydrogen complex (Figure 4a) leads to a slight frequency difference for two 1H resonance components of free H2. In addition, the interaction of pH2 with the catalyst promotes so-called singlet–triplet mixing118,135−137 when pH2-originating protons become transiently inequivalent in the complex. This mixing, accompanied by the hydride–H2 exchange, leads to the averaging of the ZQC terms derived from the initial pH2 singlet spin order (eq 1 and discussion thereof) to zero, while the longitudinal term, ÎzŜz, is preserved in both free H2 and bound hydride ligands. Altogether, PASADENA-type exchange broadened antiphase signals are observed for dihydride ligands, while the H2 signal is revealed as a superposition of two enhanced PNL resonance lines with opposite phases and slightly shifted frequencies. In the original work, the former species were difficult to detect directly due to heavy line broadening and their low concentration, while PNL was clearly visible and therefore useful.130
Based on this explanation of the PNL effect, the partially negative line experiment (PANEL)130 was developed (Figure 5a), which employed continuous-wave low-power radio frequency irradiation to detect transient species indirectly by observing the change in the PNL signal of free H2. When the frequency of the continuous radiofrequency pulse is in resonance with one of the hydrogens bound to the short-lived catalyst or free hydrogen, this nucleus is saturated, and the PNL is strongly affected. This experiment is similar to chemical exchange saturation transfer (CEST) NMR experiments, which have also been used to study short-lived exchanging intermediates.138 Using PANEL, a sensitivity gain of at least 3 orders of magnitude, compared to routine NMR experiments, is achievable (Figure 5b). As with CEST, the sensitivity and spectral resolution of PANEL depend on the amplitude of the RF saturation field.139 Since hydride nuclei often resonate at chemical shifts below −10 ppm, while the PNL signal for H2 appears at around 4.5 ppm, the associated resonance separations reach several kHz in high magnetic fields (e.g., 5.8 kHz at 9.4 T). Consequently, RF pulses of 1 kHz amplitude can be applied without intrinsically distorting the H2 signal. Hence, when used to probe the hydride region, the PANEL experiment can detect otherwise invisible intermediates with lifetimes of less than 1 ms. The achievable spectral resolution of the PANEL depends on the RF power used. Resolutions of 1 ppm are achievable by employing RF amplitudes of ca. 100 Hz.
Figure 5.
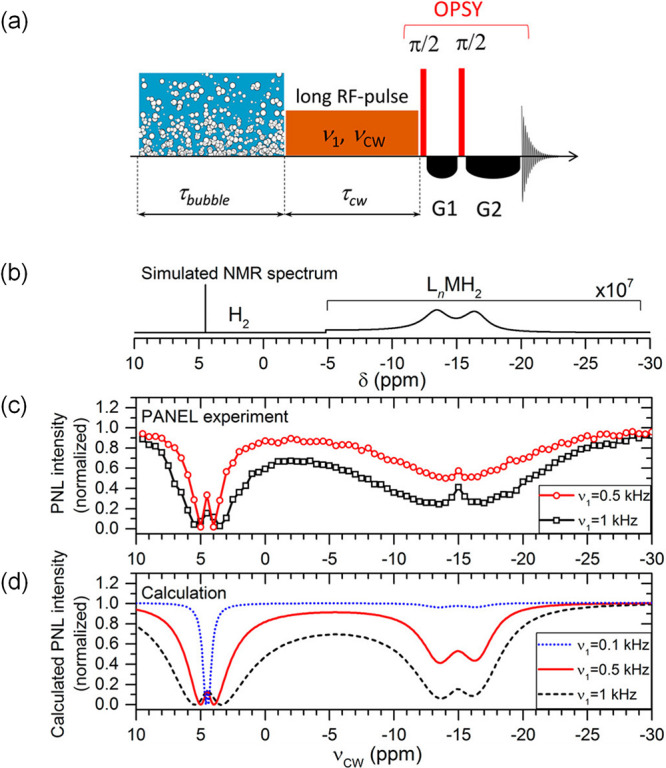
PANEL – A combination of the CEST method and the PNL effect boosts the sensitivity of catalytic intermediate detection. (a) Scheme of the PANEL (partially negative line) experiment for indirectly detecting the hidden hydrogen catalyst complex (LnMH2). (b) Calculated NMR spectrum of the hidden complex, magnified by a factor of 107. (c) Experimental and (d) simulated PANEL spectra showing the signal of the hidden complex (lines at −16.5 ppm and −13.5 ppm). Adapted with permission from Kiryutin et al.130Copyright 2017 American Chemical Society.
The presence of two signals of the same intensity at −16.5 ppm and −13.5 ppm in the PANEL spectrum (Figure 5c) points to the sizable chemical shift difference between two inequivalent hydrides and the corresponding complex asymmetry. In such a weak coupling case (|ωI – ωS|/2π ≫ |JIS|) the H2 resonance splitting that leads to PNL is given by eq 2(130)
| 2 |
where ωI, ωS, ωH are frequencies of the spins in the complex (I and S) and of free dihydrogen (H), JIS is the constant of spin–spin interaction in the complex, Keq = kf[MLn]/kd is the equilibrium constant of the binding of dihydrogen to the complex, kd is the dissociation rate constant, and kf is the formation rate constant.
The same theoretical model of PNL130 reproduced the experimental NMR line-shapes, the nutation angle dependence, and the dependence on the frequency of the resonance position of the PNL. It also permitted the determination of chemical shift values for exchanging protons in the transient complex and the sign of the scalar coupling constant between those protons. Typically, hydride resonances of transient complexes are hardly observable directly in 1H NMR, whereas PNL allows for indirect but sensitive detection of their presence.130 In parallel to that, Johnson et al.140 observed PNL and then managed to detect a hyperpolarized dihydrogen complex by cooling the catalyst sample with pH2 down to 238 K, confirming the presence and rapid exchange of the dihydrogen. The observation of reaction intermediates is highly valuable as such species are difficult to discern in standard 1H NMR experiments due to their low signal intensity and broad line widths. These techniques were later applied to study several exchange pathways with pH2.141
Bernatowicz et al.142 proposed an alternative explanation for the creation of PNL, which suggests it is caused by residual dipolar couplings (RDCs) stemming from the partial ordering of the hydrogen molecules in the external magnetic field. However, further experimental studies into this mechanism are warranted.
Czarnota et al.143 and Alam et al.144 investigated the conditions for a PNL effect employing iridium complexes or metal–organic frameworks (MOFs). PNL effects were found in the catalytic hydrogenation of eptifibatide, a disintegrin derivative based on a protein from the rattlesnake venom,145 or trivinyl orthoacetate,99 as well as in SABRE studies employing Zintl cluster-supported rhodium centers146 or nickel diazadiphosphacyclooctane complexes.141 Finally, PNL effects are also a possible loss channel for hyperpolarization in hPHIP or SABRE applications.118,136,137,147 PNL not only allows for ultrasensitive detection of intermediates but can also serve in the future as a tool to monitor spin dynamics and, thus, chemical kinetics in intermediate complexes.
2.3. OneH-PHIP
Pairwise addition of pH2 to an unsaturated substrate is the typical requirement for generating hyperpolarization in hPHIP (Figure 1), meaning that both hydrogen atoms must end up in the same product molecule and their spins remain correlated. This implies that hydrogens must follow each other throughout all catalytic steps. However, this requirement can be lifted when the pairwise addition of pH2 to an active catalytic center leads to the formation of a dihydride intermediate where the chemical shift difference between the hydride ligands is small, and a strongly coupled spin pair therefore results. Then, if the lifetime of this intermediate dihydride complex is sufficient, the initial singlet spin order of the pH2-derived hydrogen pair (eq 1) can evolve into individual single-spin net polarizations (∓Îz and ∓Ŝz, Zeeman orders) of the hydrogen atoms that will consequently be transferred into the final reaction products. In such cases, the hyperpolarization is associated with each of the two pH2-derived protons separately, without requiring their spin correlation. Therefore, the initial pH2 pair can be separated in subsequent steps of the catalytic cycle, while the hyperpolarization observed in the final product can be derived from only one hydrogen of the pair. This effect is referred to as the oneH-PHIP effect.
OneH-PHIP was first observed by Permin and Eisenberg during their stoichiometric studies of hydroformylation by platinum–tin and iridium carbonyl species.107 Here, trans-PtCl(COEt)(PPh3)2 proved to react with SnCl2 and pH2 to form propanal, where only the slowly relaxing aldehydic proton (Figure 6a) exhibited NMR signal enhancement. This is reflective of the creation of single-spin net polarization associated with an Îz type term (indicated by H in the following products), which differs significantly from the more usual pH2-derived longitudinal two-spin order ÎzŜz term (the last term in eq 1), which is destroyed when the coupling between the spins is lost. As this section will illustrate, such effects are relatively common, although the signal enhancements are relatively low. For example, a signal enhancement of 5-fold at 9.4 T using 50% pH2 was achieved for the aldehydic proton of propanal.107 Hence, oneH-PHIP observation has been limited to slowly relaxing species or high-turnover catalysis.
Figure 6.
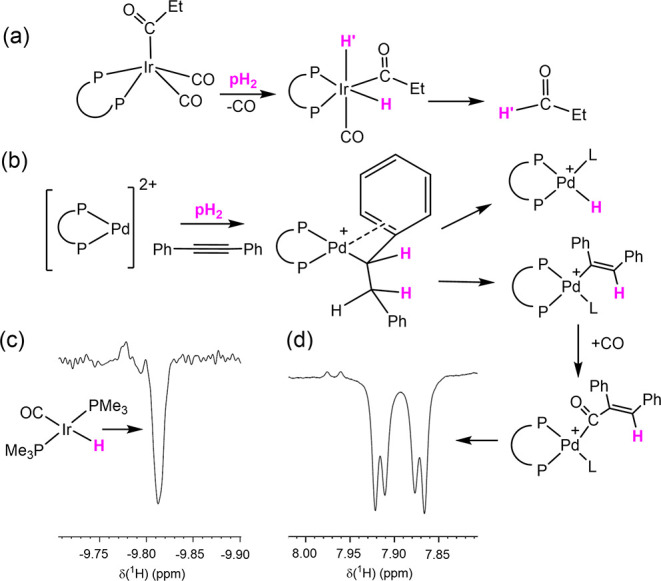
The oneH-PHIP effect has allowed the detection of several single-spin hyperpolarized products. This effect results from the creation of a hyperpolarized AB-type spin system for the pH2-derived protons (indicated with pink H atoms in (a) and (b)), with subsequent further reaction producing single spin hyperpolarized products. Examples shown here include (a) aldehydes,107 (b) and (c) metal hydride complexes,154,155 and (d) vinyl-containing species.156 The NMR traces illustrate the typical appearance of a single spin-polarized resonance under 31P decoupling in the case of (c).
Permin and Eisenberg investigated the catalytic production of propanal (CH3CH2CHO) using PtCl2(CO)(PPh3)–SnCl2107 and [Ir(COEt)(CO)2(dppe)] (dppe is 1,2-bis(diphenylphosphino)ethane) wherein only the aldehydic proton was hyperpolarized (Figure 6a). In the case of Ir systems, hyperpolarized hydride ligand signals were also detected for the intermediate [Ir(H)2(COEt)(CO)(dppe)], where the hydride ligand cis to the phosphines (H′ in the figure) was then found to become the hyperpolarized aldehydic proton in the final product. Hydride signals for this intermediate appear at very close resonances (−8.696 and −8.905 ppm in benzene), forming an AB-type spin system. In Pople notation, the AB-type spin system corresponds to two spins exhibiting a chemical shift difference comparable to their spin–spin coupling. The observed hyperpolarization of only a single proton in the final aldehyde product was called oneH-PHIP.
Subsequent solvent variation of a benzene–acetone mixture led to an inversion in the oneH-PHIP phase of the aldehydic proton as a consequence of the relative change in chemical shifts of the AB spin system of the hydride ligands inverting. Hence, the oneH-PHIP effect was linked to strong coupling. In this case, a rigorous theoretical description, enunciating the role of chemical shift difference and mutual spin–spin interaction in the AB spin system that created the Îz–Ŝz type magnetization was reported, which set Bargon’s earlier description into a firm chemical context.28 However, it does not exclude the possibility of a relaxation-driven polarization transfer mechanism. For instance, in section 2.5, we discuss such mechanisms in the hydrogenation of alkynes and imines with pH2 using ansa-aminoborane catalysts.148−150 To verify the exact mechanism, one can study the magnetic field dependence of the oneH-PHIP effect: cross-correlated relaxation is expected to be stronger at higher fields, while coherent mechanisms dominate at lower fields.28,151,152 Another rationale for the oneH-PHIP effect appears in ref (153), although this time in the context of the hyperpolarization of water and alcohols by either homogeneous or heterogeneous catalysis, as described in section 2.4.
An additional example of a hydride resonance exhibiting oneH-PHIP was observed during catalytic studies of alkyne hydrogenation by [Pd(bcope)(OTf)2] (bcope = (c-C8H14-1,5)PCH2CH2P(c-C8H14-1,5); OTf = CF3SO2O–) where species like [Pd(bcope)(pyridine)(H)](OTf) were detected.155 This study extended into the detection of critical reaction intermediates like [Pd(bcope)(CPh=C(H)Ph)(pyridine)](OTf), where the single vinyl proton exhibited strong hyperpolarization, alongside cis-PhH=CHPh (Figure 6b). During these studies, it was the strongly coupled spin system of the reversibly formed intermediate [Pd(bcope)(CHPhC(H)2Ph)](OTf) that led to this behavior.155 The related complex, the alkene insertion product, [Pd(Ph2PCH2CH2PCy2)(—C(Ph)H—CHPh—CPh=(CH)Ph)]OTf has also been observed thanks to the 1H NMR signal enhancement of oneH-PHIP157 and vinyl ethers have been produced during platinum-catalyzed reactions that exhibit this effect.158
Furthermore, the addition of CO to drive palladium-catalyzed carbonylation has extended the hyperpolarized observations to include the ketone MeOCO(CPh)=CHPh proton resonance, alongside further signals in the acyl bearing reaction intermediate [Pd(bcope)(CO—CPh=C(H)Ph)(CO)](OTf), the novel hydride complex [Pd(bcope)(CO)(H)](OTf), the alkene complex [Pd(bcope)(CHPh=CPh(COOMe)] and free HD (alongside H2) (Figure 6d).156 Here again, detecting a hyperpolarized response for the released H2 reflects the oneH-PHIP that results from strong coupling effects in species that led to it.
Later, Guan et al. reported on studies of related [Ir(η3-C3H5)(CO)(PMe3)2] type species and described how the hydride ligand signal for [HIrI(CO)(PMe3)2] exhibited oneH-PHIP (Figure 6c).154
It should be apparent from these discussions that the sharing of hyperpolarization between species can occur via numerous processes with dramatically different efficiencies. The polarization of a single proton was also reported in other reactions with H2 involving metal-free catalysts or heterogeneous catalysts and led, among others, to a polarization of water, as discussed in the following sections.
2.4. SWAMP and NEPTUN
Hyperpolarized water is an important target molecule as it can be used for angiography and perfusion biomedical imaging159−161 and as a polarization source for heteronuclear signal enhancement in biomolecular NMR spectroscopy.10,162−166 While high polarization levels (>60%) have been shown to result from dDNP,167 pH2-based methods offer an alternate route that is both rapid and less expensive, thus making the approach more widely accessible.
Water had been an elusive target for PHIP until 2017, when it was hyperpolarized in D2O mixtures of l-histidine and a water-soluble iridium complex, [Ir(Cl)(IDEG)(COD)] (IDEG = 1,3-bis(3,4,5-tris(diethylene glycol)benzyl)imidazole-2-ylidene).168 In this system, the hyperpolarized HDO and HD proton signals appear in the emission and absorption phases, respectively (Figure 7a,b). Consistent with the oneH-PHIP theory (see section 2.3),109,153 the enhanced HD and HDO signals have opposite phases and very similar field dependencies of their signal amplitudes, which reach a maximum near 45 mT where the J-coupling and chemical shift difference between the dihydride protons (Figure 7c) are matched. The corresponding oneH-PHIP mechanism, mediated by (i) H/D exchange with coordinated D2O, (ii) dissociation of HDO, and (iii) H–D recombination, was named nuclear exchange polarization by transposing unattached nuclei (NEPTUN).109 The role of l-histidine in this work remains unclear.
Figure 7.
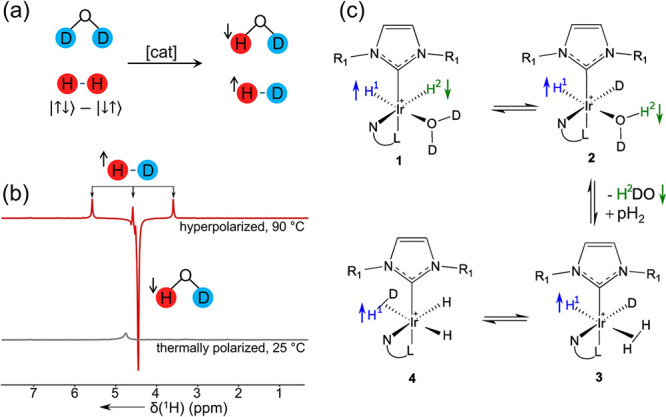
(a) H/D exchange, in the presence of a water-soluble iridium catalyst and histidine, leading to hyperpolarization of HD and HDO and (b) the resulting NMR spectrum, in comparison to the thermally polarized spectrum. (c) The proposed “NEPTUN” mechanism underpins the spectrum. (a, b) Adapted with permission from Lehmkuhl et al.168Copyright 2017 John Wiley and Sons. (c) Adapted with permission from Emondts et al.109Copyright 2018 John Wiley and Sons.
A NEPTUN-type mechanism was also suspected to be active in relayed hyperpolarization experiments where the goal was to further extend the SABRE hyperpolarization to heteronuclei in noncoordinating substrates like alcohols (e.g., methanol, ethanol) via proton exchange with a carrier amine.169 The possible involvement of such a mechanism, in addition to OH/NH exchange, was inferred from magnetic field dependencies of 13C distortionless enhancement by polarization transfer (DEPT) signals, which, in addition to showing a peak at 6.5 mT, as expected for the conventional SABRE matching condition (relayed to the target via NH/OH exchange), there is an even more prominent peak after transfer at 19.2 mT, which is hypothesized to stem from the NEPTUN effect.169 However, attempts to observe the hydride resonances indicative of NEPTUN directly were unsuccessful.
While most PHIP studies utilize dissolved organometallic catalysts, heterogeneous catalysis offers facile separation of the hyperpolarized products from the catalyst and can even be used in a packed-bed flow-reactor configuration.170,171 Supported noble metals are among the most active hydrogenation catalysts. Unfortunately, only a tiny fraction of adducts (ca. 1–5%) are formed by pairwise addition, depending on nanoparticle size and reaction conditions. Rapid H adatom diffusion and facile exchange with gaseous H2 conspire to destroy the singlet order in the nascent H adatom pair.15,50,172,173 Intermetallic phases incorporating an active and inactive metal, such as Pt and Sn, allow for the tuning of molecular adsorption and diffusion dynamics through a combination of geometric and electronic effects.174−176 Thus, as the fraction of Sn increases across the series Pt → Pt3Sn → PtSn, the pairwise selectivity for hydrogenation of propene increases by more than 3 orders of magnitude.174 After bubbling pH2 through a D2O suspension of Pt3Sn@mSiO2 (Pt3Sn nanoparticles encapsulated in mesoporous silica) for 30 s, Zhao et al. observed hyperpolarization of the residual protons of solvent molecules.153 The effect was dubbed surface waters are magnetized from parahydrogen (SWAMP). Proton hyperpolarization in methanol-d4 and ethanol-d6 was also observed. The surface properties of Pt3Sn now balance the necessary facile H2 activation and suppression of diffusion.
The NEPTUN and SWAMP systems share a few similarities: (i) emission phase of the HDO peak. (ii) absence of signal enhancement at zero or high magnetic field, revealing a role of Zeeman interactions; (iii) monotonic growth of [HDO] with total pH2 bubbling time; (iv) emergence of a dissolved HD (triplet) NMR signal. The last two are accounted for by the net isotope exchange reaction described in eq 3.
| 3 |
For Pt surfaces, H/D exchange is mediated by reversible electron transfer from an H adatom to the metal and proton transfer to surface water to yield a hydronium-like species where H/D exchange occurs.177 However, the oneH-PHIP/NEPTUN mechanism was only tentatively excluded by preliminary data revealing the different dependences of the SWAMP signals for exchangeable and nonexchangeable protons of HOCD3 and DOCHD2, respectively, on the total amount of H/D exchange. Plausible mechanisms are illustrated in Figure 8a.
Figure 8.
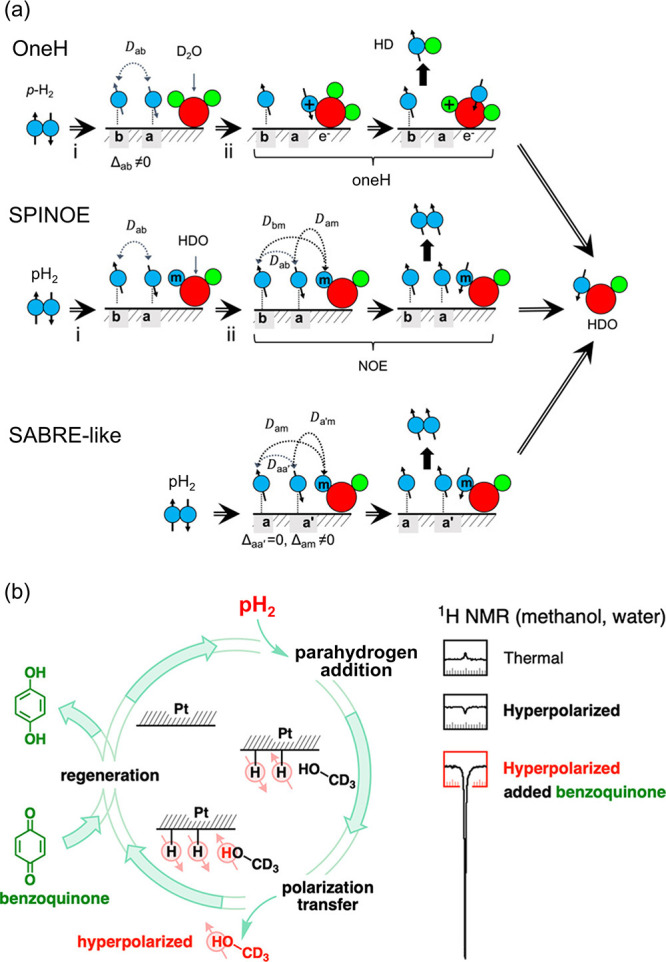
(a) Possible mechanisms underpinning surface-mediated hyperpolarization of liquid water. (b) Mechanism of benzoquinone scavenging of depolarized H adatoms on Pt/C, and its effect on the SWAMP signals. (a) Adapted with permission from Zhao et al.153Copyright 2018 Elsevier. (b) Reproduced from Norcott.178Copyright 2023 American Chemical Society. This publication is licensed under CC-BY-NC-ND 4.0.
While the monometallic Pt@mSiO2 nanoparticles of ref (153) were found to be inactive as SWAMP catalysts, Norcott discovered that one could hyperpolarize water and methanol using a commercially available carbon-supported Pt nanoparticle catalyst when benzoquinone was added to a D2O suspension of the catalyst.178 Maximum 1H NMR signal enhancements (relative to thermal equilibrium at 1.4 T) approaching 45-fold were observed for methanol with ten equivalents of benzoquinone (w/w with respect to Pt/C). Benzoquinone is converted to hydroquinone during this process, which assists in increasing the turnover of fresh pH2 on the surface and thereby increasing the level of polarization of methanol and water (Figure 8b).
While such signal enhancements are likely to increase with further catalyst development and optimization of experimental conditions, it remains to be seen whether the PHIP approach can rival the very high polarization levels achievable by dDNP for water.159,160,162−165 As pH2-based hyperpolarization techniques are inherently rapid, continuous, and low-cost, their use to achieve sufficient levels of water hyperpolarization could provide advantages to dDNP methods and significantly advance the application range of hyperpolarized water in biomedical research.
2.5. Metal-Free PHIP: Molecular Tweezers and pH2 Activators
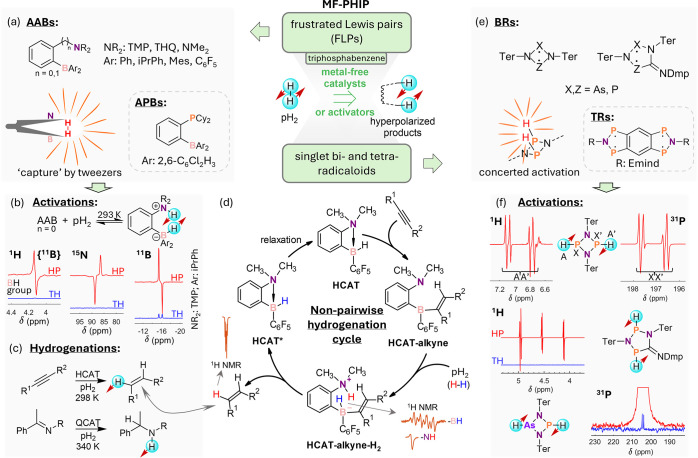
The chemical activation of pH2 is crucial to derive enhanced NMR signals in PHIP. Commonly, transition metal catalysts are employed to mediate such activations and produce hyperpolarized substances. At the same time, the use of metal-free activators and catalysts for pH2-based hyperpolarization, collectively named metal-free PHIP (MF-PHIP), is also documented.129,148,149,179−186 This section focuses on several types of MF catalysts, their structures, hyperpolarization effects, and their unique mechanistic features (Figure 9).
Figure 9.
An overview of metal-free PHIP (MF-PHIP). The central top part highlights that MF-PHIP effects have been demonstrated in activation of pH2 using frustrated Lewis pairs (FLPs) and using bi- and tetraradicaloids (BRs and TRs). Correspondingly, the structures of ansa-aminoborane (AAB) and ansa-phosphinoborane (APB) FLPs are presented in (a). AABs are referred to as molecular tweezers for pH2. pH2 activation under ambient conditions using ortho-phenylene AABs (n = 0) accompanied by the corresponding hyperpolarized NMR spectra is illustrated in (b). See (a) for the definition of n. In addition to 1H, 15N and 11B nuclei are also hyperpolarized spontaneously at high magnetic fields in this process. Alkyne and imine hydrogenation reactions catalyzed by AABs HCAT (n = 0; NR2 = NMe2; Ar = C6F5; R′ = H) and QCAT (n = 1; NR2 = THQ; Ar = C6F5), respectively, are shown in (c). Additionally, the catalytic cycle of alkyne hydrogenation using the HCAT AAB catalyst is presented in (d). Typical 1H NMR signals of the reaction intermediate and the reaction product are shown next to the corresponding structures in the cycle. Structures of BR and TR molecules that demonstrated hyperpolarization effects in pH2 activations are depicted in (e). Corresponding examples of enhanced 1H and 31P NMR signals observed in reactions with BR molecules are shown in (f). The structures of the corresponding BR–H2 adducts are depicted next to the spectra. Abbreviations: TMP = N-2,2,6,6-tetramethylpiperidinyl; THQ = N-tetrahydroquinolinyl; iPrPh = 2-isopropylphenyl; Mes = mesityl; Cy = cyclohexyl; Ter = 2,6-dimesitylphenyl; Dmp = 2,6-dimethylphenyl; Emind = 1,1,7,7-tetraethyl-3,3,5,5-tetramethyl-s-hydrindacenyl. (d) Adapted from Zakharov et al.148Copyright 2022 John Wiley and Sons. This publication is licensed under CC-BY 3.0.
MF activations of H2 are less common than those that rely on transition metal centers. They have recently attracted a lot of attention due to the possibility of using sustainable main-group elements to design less toxic and more environmentally friendly catalysts. MF catalysts for PHIP is an emerging research field that is still in its infancy. In this regard, frustrated Lewis pairs (FLPs)187 are the most studied class of MF activators for pH2. Specifically, various ansa-aminoborane (AAB) FLPs show pronounced hyperpolarization effects (Figure 9a). These compounds are referred to in the literature as “molecular tweezers” that stretch but do not split H2 molecules.188 Recent studies revealed that the stretched H–H bond is relatively weak, making it possible to form various rotomeric forms in solution, including those with large H···H separations.183 Nevertheless, AAB–H2 adducts have motionally averaged J-coupIing constants (2–4 Hz) between the 1H–1H pair, allowing for PHIP effects under high field (PASADENA) conditions.129,183
Unlike homolytic oxidative addition to metal centers, AABs activate pH2 heterolytically with a clear charge separation on the Lewis acidic boron and the Lewis basic nitrogen sites (Figure 9b), although the two protons remain spin correlated. Depending on the AAB structure, hyperpolarization of 1H, 11B, and 15N can be observed in simple pH2 bubbling experiments without harnessing dedicated pulse sequences.36 Signal enhancements as large as 2000-fold at 9.4 T and room temperature have been observed in the resulting 1H NMR spectra for the pH2-originating protons of AAB–H2. The size of this 1H NMR signal gain depends strongly on the experimental conditions and is defined by relaxation and kinetic parameters.183 Density functional theory (DFT) calculations have also revealed various conformational forms of AAB adducts and their transformations.
In addition to simple pH2 activation, ansa-aminoboranes can be used in catalytic hydrogenations of alkynes148 and imines149 with pH2 (Figure 9c). Interestingly, the catalytic cycles that lead to hyperpolarized akenes and amines are nonpairwise, implying that the pH2-derived protons end up in different product molecules (Figure 9d). The hyperpolarization effects in this case are not expected to be observable in PHIP. However, due to the strong chemical shift anisotropy (CSA) of NH protons in the catalytic intermediates, a net negative polarization is generated from the pH2 spin order through CSA–dipole–dipole cross-correlated relaxation.149 For instance, in alkyne hydrogenations, this mechanism is revealed by the negative in-phase resonance of the NH group of HCAT–alkyne–H2 intermediate. As this proton transfers to the final alkene product, a two-orders-of-magnitude enhanced negative signal of one of the added protons at the double bond of the resulting alkene appears in the 1H NMR spectra at 9.4 T. This effect is related to oneH-PHIP in hydroformylation reactions catalyzed by metals (section 2.3), but the underlying mechanisms of hyperpolarization, as well as chemical processes, are different. It is worth noting that the ability of cross-correlated relaxation to transform pH2 spin order to a net polarization was also observed on metal complexes, e.g., by Aime et al. in pH2 activations using Os and Ru clusters.189,190
AABs are reported to be generally water-intolerant, which is a significant obstacle to the wide application of MF-PHIP. Valuable steps have been made to resolve this issue recently.267 In addition, ansa-phosphinoboranes (APBs) were demonstrated to show PHIP effects in the presence of several equivalents of H2O (Figure 9a).182 Other FLPs showing hyperpolarization effects include Sn/P systems,191 though it is strictly not a MF compound and will not be discussed here. In addition, aromatic triphosphabenzene was also shown to reveal hyperpolarization in the reaction with pH2 at elevated temperatures (375 K).179 However, the resulting H2 adduct is prone to decomposition.
Singlet pnictogen radicaloids are another class of MF pH2 activators that demonstrate prominent hyperpolarization effects. Electron spins in these open-shell molecules are coupled into a singlet state that does not possess free electron angular momentum, which excludes the deleterious influence of the radical centers on the nuclear spins. Typical examples include cyclic species with P and/or As radical centers isolated by surrounding bulky substituents for stabilization. This configuration maintains high reactivity toward small molecules, such as H2, while preserving an open-shell structure. In the context of PHIP, biradicaloids (BRs) with four- or five-membered cycles are studied more extensively (Figure 9e), and any observed hyperpolarization effects strongly depend on BR symmetry.185 With symmetric four-membered biradicaloids, pH2 forms symmetric adducts. For instance, four-membered baricaloids form the AA′XX′ spin system, which leads to enhanced 1H and 31P NMR signals in PASADENA experiments (Figure 9f).184,185 Nonsymmetric five-membered species form a system with weakly coupled protons, leading to only 1H hyperpolarization. However, the transfer of 1H hyperpolarization to 31P can be achieved using ESOTHERIC NMR pulse sequences with 31P NMR signal enhancements exceeding 3 orders of magnitude at 9.4 T.185,192 Tetraradicaloids (TRs) are represented by a single example (Figure 9e),186 which showed less pronounced but interesting hyperpolarization for the addition of the first and second equivalents of pH2. Radicaloid systems are generally more reactive than FLPs, which makes them especially interesting for future developments that may lead to active catalysts, e.g., for hydrogenation or hydroformylation reactions.
MF-PHIP represents an exciting frontier in hyperpolarization method development. Using MF compounds such as FLPs and radicaloids introduces new mechanistic pathways and structural features that differentiate them from traditional metal-catalyzed systems. The unique hyperpolarization effects and mechanisms of MF-PHIP, including two-centered activation and nonpairwise hydrogen transfer, offer potential for innovative applications. Future research in this area promises to further enrich our understanding and utilization of these novel catalysts for hyperpolarization.
2.6. Revealing PHIP in Subsequent Chemical Transformations
The involvement of hyperpolarized molecules in subsequent chemical transformations is an interesting application of PHIP that is nicely illustrated using metabolic reactions, such as pyruvate-to-lactate conversion193 and fumarate-to-malate194 conversions. Other examples include oxidation of hyperpolarized pyruvate with H2O2195 or decarboxylation with yttrium polyaminoacarboxylate adducts,196 methylation of N-heterocycles,197 and conversion of hyperpolarized 15NO2– via several reactions to make a range of products with enhanced 15N NMR signals,198 and other transformations.199
Typically, PHIP-enhanced NMR signals are visible without any subsequent reaction and can serve as a tool for kinetic measurements and identifying intermediates or additional products of the following reactions. However, if we consider symmetric molecules produced in reactions with pH2, the product, although in a far from thermodynamic equilibrium nuclear spin state, may not exhibit observable hyperpolarization if the pH2 addition site is at the center of symmetry. In this case, a subsequent symmetry-breaking chemical reaction(s) may be required to reveal otherwise unobservable hyperpolarization (Figure 10a). The quintessential example of this kind is the pH2 molecule, which accommodates singlet nuclear spin order and does not yield NMR signals. As first shown by Bowers and Weitekamp,17,18 one needs to break the symmetry of pH2 in a chemical reaction to observe hyperpolarization. Notably, symmetric molecules can typically host long-lived spin orders.200,201 Such molecules can be synthesized using pH2 in hydrogenative and non-hydrogenative reactions. For example, the syntheses of ethylene from acetylene and pH2 (Figure 10b), dimethyl maleate (DMM) from dimethyl acetylene dicarboxylate (DMAD) (Figure 10c), and para-15N2 using SABRE were described.202−209
Figure 10.

(a) A general scheme of formation of compounds with hyperpolarized spins in chemical reactions of symmetric molecules, such as pH2, ethylene, DMM, and para-15N2, accommodating otherwise unobservable nuclear spin orders. (b) The chemical synthesis of Z- and E-ethylene from acetylene and pH2 over various heterogeneous catalysts, as well as subsequent reactions revealing hyperpolarization. (c) The chemical synthesis of dimethyl maleate (DMM) molecules in hydrogenation with pH2 over a cationic Rh+ catalyst, followed by their subsequent reaction with thiol molecules to reveal hyperpolarization. Abbreviations: DMM - dimethyl maleate.
The case of ethylene is fundamentally interesting since it, like H2, has nuclear spin isomers of molecules (NSIMs) that differ by rotational and spin degrees of freedom due to the coupling of nuclear spin and rotational states through the symmetry properties of their respective wave functions.210 Briefly, there are four NSIMs for ethylene that can be classified according to the symmetries of the nuclear spin state for the D2h molecular point group using Mulliken symbols: Ag (one quintet and two singlets), B1u (triplet), B2u (triplet), and B3g (triplet). Depending on the stereoselectivity of the hydrogenation of acetylene, syn and anti pH2 addition products, Z- and E-ethylene, respectively, can be produced (Figure 10c),202 which is primarily determined by the hydrogenation catalyst employed. For instance, supported Pd nanoparticles are less selective and produce both Z- and E-ethylene products,202 whereas immobilized complexes of Ir are more selective, leading primarily to Z-ethylene.205 Interestingly, the subsequent reactions of ethylene produced using different catalysts with sulfenyl chlorides202 or Br2/D2O205,211 reveal different lifetimes of the nonequilibrium spin states in ethylene. This was rationalized based on the interconversion between different NSIMs of ethylene,204 providing insights that in the gas phase, Z-ethylene has only one long-lived component. In contrast, E-ethylene has two long-lived components due to the imbalance of NSIMs with different inversion symmetry in the latter case.202,205 Lifetime constants of more than 15 min were measured by unlocking the hyperpolarization in the subsequent reactions for gaseous E-ethylene.
Another example of revealing the latent polarization inherited from pH2 is documented for the reaction of thiols with DMM produced from DMAD in a liquid-state hydrogenation over a Rh(I) cationic catalyst (Figure 10c).203 As in the case of ethylene, storage of the nonequilibrium nuclear spin order after the hydrogenation was demonstrated in this study. The thiol reaction allowed for the lifetime measurement of the populated long-lived singlet spin order at a high field of up to 4.7 min. Interestingly, the unique symmetry of the DMM molecule, which induces slight magnetic inequivalence in the added pH2-derived proton pair, allows alternative methods that do not require chemical transformation to reveal hidden singlet spin state populations. Instead, such hidden spin states can be converted into observable magnetization using magnetic field cycling212 or applying RF fields.213−215 These methods do not apply to ethylene, as all its protons are magnetically equivalent.
In addition to ethylene and DMM, para-15N2 has also been reported to form from SABRE-hyperpolarized 15N-labeled tetrazine208 and diazirines209 in chemical reactions involving these agents. However, the successful formation of para-15N2 in these cases was inferred only from the absence of a 15N2 signal in 15N NMR spectra after its production step. Similarly to pH2, para-15N2 is NMR silent. So far, no subsequent symmetry-breaking reaction of para-15N2 that reveals its singlet spin order has been reported to our knowledge.
Overall, the availability of methods that use pH2 to produce symmetric molecules with long-lived nuclear spin orders can expand the range of chemical reactions studied by providing flexible time windows. For example, it can enable one to go beyond conventional hydrogenation studies using pH2 in PHIP, extending to electrophilic additions to double bonds, as demonstrated in the cases of ethylene and DMM. Furthermore, the analysis of the generated spin order lifetimes in molecules such as ethylene and para-15N2 can provide essential insights into the fundamentals of NSIMs and underlying molecular physics.
2.7. Spreading Hyperpolarization via Chemical Exchange: PHIP-X and SABRE-RELAY
In recent years, a new approach has emerged to boost the NMR signal for molecules that do not contain functionalities suitable for direct PHIP or SABRE (natively or on a side arm). Two versions of this approach are based on reversible proton exchange between a hyperpolarized carrier and a to-be-hyperpolarized molecule and are termed PHIP by chemical exchange (PHIP-X)216 and SABRE-Relay.217 In both methods, traditional PHIP or SABRE is used to polarize a “transfer” or “carrier” molecule, whose polarization is then transferred to a secondary target molecule via the exchange of hyperpolarized OH/NH protons (Figures 11 and 12a). These approaches have allowed a significant expansion of the substrate scope of PHIP and SABRE in recent years.
Figure 11.

Schematic view of parahydrogen-induced polarization by chemical exchange (PHIP-X). PHIP-X consists of four essential steps: hydrogenation of the carrier agent (step 1), the polarization of the exchanging protons (step 2), transfer of the exchanging protons from the carrier to the target molecule (step 3), and polarization of the target nucleus (step 4) using RF-induced spin order transfer technique or free evolution at low and ultralow magnetic fields. Adapted from Them et al.219Copyright 2024 Springer Nature. This publication is licensed under CC BY 4.0.
Figure 12.
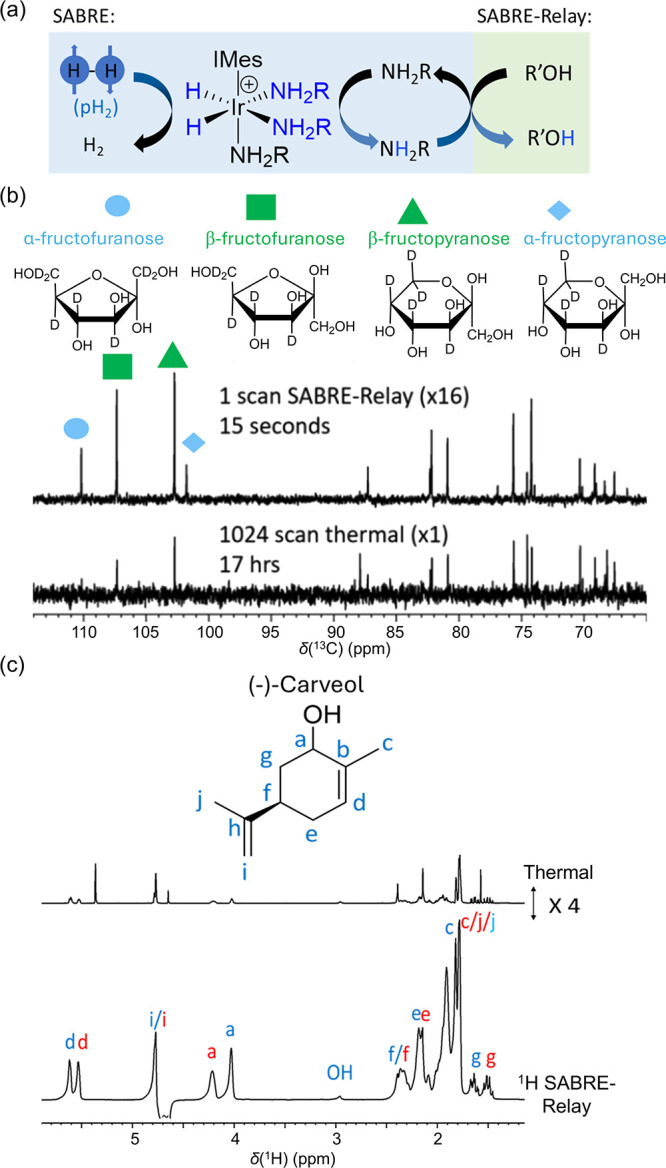
Demonstration of the SABRE-Relay effect. (a) Depiction of the SABRE-Relay method. (b) SABRE-Relay can allow quantification of isomer ratios of fructose in a single scan 13C measurement or (c) quantification of diastereomer ratios of the natural product (−)-carveol from a single scan 1H measurement. Details for (b): 13C{1H} NMR spectra (right) acquired for 40 mM of d-fructose (natural 13C abundance) with 23.8 mM benzyl-d7-amine and 4.8 mM of [Ir(Cl)(COD)(SIMes-d22)] (where COD is cis,cis-cyclooctadiene and SIMes is 1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene) in a 0.65 mL DCM-d2:DMF (1.6:1) mixture measured at 9.4 T. The bottom spectrum shows the result of a thermally polarized signal averaging over 1024 scans (approximately 17 h), and the middle spectrum represents the single scan SABRE-Relay hyperpolarization measurement recorded after shaking the sample with pH2 at 6.5 mT. Details for (c): Exemplar single scan thermally polarized (above) and 1H SABRE-Relay hyperpolarized (lower) 1H NMR spectra for a sample of [IrCl(COD)(IMes)] (5 mM), NH3 (30 mM), (−)-carveol (25 mM) and pH2 (3 bar) in DCM-d2 (0.6 mL). The resonance labels in red and blue correspond to the two diastereomers. The hyperpolarized NMR spectrum is recorded immediately after shaking the sample for 10 s with fresh pH2 at 6.5 mT. (a, c) Adapted from Alshehri et al.225Copyright 2023 Royal Society of Chemistry. This publication is licensed under CC BY 3.0. (b) Adapted from Richardson et al.223Copyright 2019 Royal Society of Chemistry. This publication is licensed under CC BY 3.0.
In the case of PHIP-X216 (also referred to as PHIP-Relay),218 propargyl alcohol, propiolic acid, or propargyl amine were hydrogenated with pH2 using a homogeneous Rh catalyst in an aprotic solvent, such as acetone, to produce a hyperpolarized “transfer” agent (Figure 11).216 Strong spin–spin interactions distribute the polarization among the protons of the transfer agent, including the labile OH (or NH) proton. The polarization of this labile proton is relayed to the spin system of a third molecule by chemical exchange, where, again, spin–spin couplings, low magnetic field, or RF spin order transfer sequences (RF-SOT) facilitate the transfer of the polarization to other nuclei such as 1H, 13C, or 15N.218,219 PHIP-X was shown to spontaneously polarize labile protons to ca. 0.4% for ethanol and water, 0.07% for lactic acid, 0.005% for pyruvic acid, and at least 0.009% 13C polarization for glucose. Using RF-SOTs, 1.2% 15N polarization was achieved for urea, where the 15N coupling to the labile proton is large, 0.024% for 13C glucose,218 0.026% for 13C lactate219 and ca. 0.007% for 13C methanol.219 The balance here is reached when proton exchange is slow enough to allow the J-coupling to transfer polarization to the labile proton of the carrier first and then from the labile proton to other nuclei of the target. At the same time, the exchange must be fast enough such that spin relaxation would not destroy polarization before the target is polarized. Therefore, even higher polarization values are expected to be achievable after thoroughly tuning the exchange parameters. Also, the polarization transfer is faster and more efficient when transferred to nuclei directly bound to the labile proton, such as 15N or 13C, with the strongest J-coupling constants. An advantage of using PHIP to hyperpolarize the transfer agent compared to SABRE is that the molecule can be polarized up to unity by the direct addition of pH2; a disadvantage is that it is irreversible as the addition step can be performed only once (one addition of pH2 per transfer agent).
In SABRE-Relay, classical SABRE is used to hyperpolarize ligating carriers, typically NH3 and amines, with secondary NH/OH exchange effectively relaying polarization to nonligating target substrates.220,221 Consequently, SABRE-Relay has been used to hyperpolarize alcohols,169,222 sugars,223 silanols,78 lactate esters,224 natural products,225 and many other functional groups217,226 that do not interact with the SABRE catalyst directly. As SABRE-Relay, like PHIP-X, depends on transferring proton magnetization, direct polarization of heteronuclei (analogous to SABRE-SHEATH) is impossible. However, the polarization of the exchanging OH group allows the polarization of heteronuclei either spontaneously (i.e., by free evolution), or with the help of RF-SOT. So far, SABRE-Relay has achieved 2.6% 1H,222 2.3% 29Si,78 1.1% 13C,222,223 0.2% 19F,222 and 0.04% 31P222 polarization levels. These NMR signal enhancements are generally lower than typical values achieved by conventional SABRE because the relayed polarization is derived from a finite carrier polarization affected by spin relaxation during the chemical exchange. Current studies have optimized factors such as amine type and concentration ratios to increase the target polarization.222,224,225
A major limitation of PHIP-X and SABRE-Relay is that they cannot be performed in alcohol or aqueous solvents as their exchangeable protons will compete with the ones of the target molecule. Accordingly, they are commonly performed in dry dichloromethane or chloroform, which may pose a significant challenge for insoluble substrates in these media. SABRE-Relay has already shown potential in molecular analysis. It can enhance the NMR signals of sugars223 and natural products225 at concentrations as low as tens of micromolar with a single NMR scan. Notably, it can give single-scan quantification of isomeric ratios for OH-containing molecules, such as α and β forms of glucose and fructose (Figure 12b)223 or diastereomers of natural oils like (−)-carveol (Figure 12c).225 Thanks to the continuous nature of polarization production in SABRE, relayed polarization transfer is expected to become better understood, improved to increase polarization levels, and applied to an ever-increasing scope of target molecules in the years ahead.
Compared to direct pH2 addition (PHIP-X), using reversible exchange (SABRE) to polarize the transfer agent has the advantage that the transfer agent can be continuously repolarized; a disadvantage is that the polarization yield is usually lower. Despite recent progress,219,224,227 the detailed and quantitative description of chemical exchange and the spread of polarization within the target is still not fully understood.
2.8. Perspectives: PHIP in Enzymatically Catalyzed Reactions
The use of pH2 and hydrogen–deuterium scrambling has been explored in a non-NMR context for several decades to study kinetics and intermediates of enzymes thanks to the slow conversion of pH2 to oH2 in pure water of about tens to hundreds of minutes.136 Such scrambling can thereby help to understand the chemisorption and exchange process by forming HD based on a hydrogen and deuterium source.228 Detection of oH2 formation after supplying pH2 yields information on the splitting and recombination of H2.229 This information can then give a clearer kinetic picture of hydrogen-activating enzymes.230 The particular focus, therefore, is on hydrogenases,228−231 an important class of enzymes involved in the hydrogen activation process of, e.g., anaerobic organisms.232−235 Hydrogenases promise to be blueprints for eco-friendly catalysts for hydrogen activation on the way to produce green energy.236 Therefore, understanding the detailed hydrogen activation of hydrogenases could facilitate the design of potent eco-friendly catalysts. Three different types of hydrogenases are currently known: [FeFe]-hydrogenases, [NiFe]-hydrogenases, and the [Fe]-hydrogenase.237 While reaction intermediates of the first two hydrogenases could be well characterized by available methodologies such as EPR, X-ray diffraction, and IR, investigating the [Fe]-hydrogenase has proven to be more challenging. This is because the iron center of the [Fe]-hydrogenase is FeII+ encapsulated in a guanylylpyridinol (FeGP) and remains in a diamagnetic state through the whole catalytic cycle (Figure 13a). Under catalytic conditions, methenyl-tetrahydromethanopterin (methenyl-H4MPT+) is bound by the protein, bringing together FeGP and methenyl-H4MPT+. This is the active site that heterolytically cleaves molecular hydrogen into a proton and a hydride, whereby the hydride is stereospecifically transferred to the Hpro-R position of the methylene carbon of methylene-H4MPT238 (Figure 13b). So far, computational models have predicted several iron–hydrogen species in the catalytic cycle, none of which could be experimentally verified. The use of pH2 in hyperpolarization experiments has recently changed this.151
Figure 13.

Active site of the mono iron hydrogenase and the hydrogenation reaction. (a) The active site of the [Fe]-hydrogenase, including the iron guanylyl pyridonol and methenyl-H4MPT+. (b) Hydride transfer to the Hpro-R position of the substrate forming methylene–H4MPT. Adapted from Kaltschnee et al.151Copyright 2024 Springer Nature. This publication is licensed under CC-BY 4.0.
When pH2 was supplied to an aqueous buffer containing [Fe]-hydrogenase and methenyl-H4MPT+, the appearance of a hyperpolarized PNL (section 2.2) as well as HD and HDO NEPTUN PHIP signals (section 2.4) was observed (Figure 14a).151 The former state is created when pH2 reversibly binds to the enzyme. In addition, hyperpolarized HD signals, first observed with iridium catalysts in the context of the NEPTUN effect,239 were also observed in the presence of hydrogenase when the buffer was partially deuterated. This finding further indicates an isotope exchange with the solvent. In addition, rapid exchange between an enzyme-bound ensemble of hydrogen where both hydrogens are distinguishable, and a state where both hydrogens are indistinguishable on the NMR time scale, needs to occur. An estimate for the lifetime range of 1–100 μs was found, and chemical shifts and 1H–1H J-coupling constants between these hydrogens were estimated. Optimized structural models based on the X-ray crystal structure of the hydrogenase allowed for the computation of 1H chemical shifts and 1H–1H J-coupling constants for the hydrogen atoms within the active site and correlation with the experimental observations. Considering all this, an intermediate could be identified that had only been predicted previously (Figure 14b).240 The optimized structure reveals the presence of an iron hydride and the involvement of the oxopyridine site during the activation process. The determined intermediate supports the previously theoretically predicted process during which an oxo-pyridine moiety serves as a base for hydrogen activation during the catalytic process.
Figure 14.
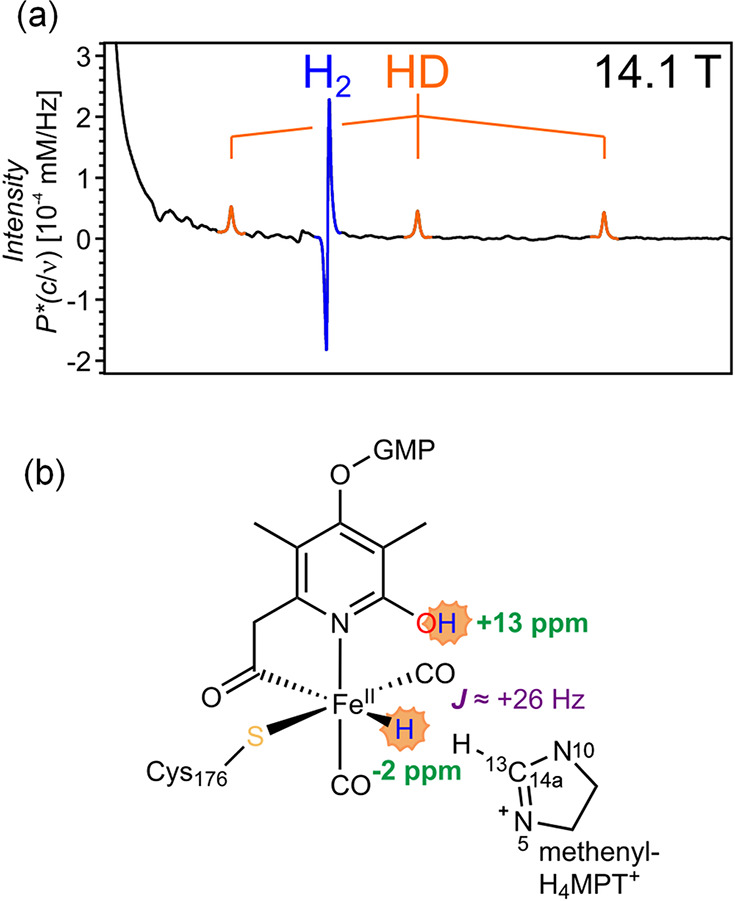
Using PHIP to reveal hitherto unobservable intermediates of [Fe] hydrogenase. (a) Hyperpolarized signals were observed when pH2 was supplied to an enzymatic solution of the hydrogenase containing methenyl-H4MPT+. A PNL effect and oneH-PHIP hyperpolarized HD signals demonstrate the activation of pH2 by hydrogenase. (b) The reaction intermediate was determined using observed NMR parameters and analysis of chemical exchange, and it was derived from a crystal structure quantum mechanical optimization of the structure. Adapted from Kaltschnee et al.151Copyright 2024 Springer Nature. This publication is licensed under CC-BY 4.0.
With this demonstration, the use of pH2-enhanced magnetic resonance has evolved into a tool to study the biochemistry and catalysis of hydrogenases and to investigate so far undetectable reaction intermediates of this important class of enzymes. It is envisioned that the concept can be used to study additional hydrogen-activating enzymes, such as the other two types of hydrogenases and potentially nitrogenases.
3. Outlook
Almost 40 years after its first discovery, PHIP remains a source for ever-surprising novel applications and unique revelations, some of which are discussed in this review. In the wake of biomedical hyperpolarized MRI, recent discoveries of efficient ways to hyperpolarize 13C-labeled pyruvate led to its in vivo imaging demonstrations.85,86,88,89 The high levels of polarization achieved using PHIP led to observations of effects such as radio amplification by stimulated emission of radiation (RASER) and spin diffusion in the solid state that attracted attention beyond the chemical community.58,241−247 Continued exploration and development of PHIP has and will likely continue to yield tools and techniques that complement more traditional methods, which have their shortcomings.
One promising direction lies in the continued development of catalysts and reaction conditions that enable PHIP with catalytic systems previously considered incompatible with PHIP. The ability to perform PHIP in these settings can lead to more sustainable and versatile applications. Hence, the field of applied catalysis will be enriched among others through synergetic development with PHIP as it was when the homogeneous catalysts were tuned to reach exclusive trans hydrogenation for a direct hyperpolarization of fumarate,44 or when PHIP was demonstrated using homogeneous,17−19 heterogeneous,15,42,43,248−268 and metal-free148,149,180, catalytic systems. Contrast agents produced by heterogeneous PHIP are currently progressing toward in vivo lung imaging.47,48
It is worth highlighting the innovative combination of hyperpolarization for boosting NMR sensitivity while maintaining its quantitative nature. Tessari and others proposed two compelling approaches to utilize SABRE in analytical chemistry. In one method, analytes are introduced and hyperpolarized via SABRE, enabling concentration evaluations with near-nanomolar sensitivity.250−254 This demonstrates the potential of SABRE as a powerful quantitative tool. The second approach focuses on hyperpolarizing the hydride signals of the catalyst, exploiting the chemical shifts induced by the coordination of trace analytes to the Ir-complex.104,12,255−269 This method transforms the hyperpolarized hydrides into dynamic probes for chemical analysis, opening avenues for studying even minute concentrations of analytes with precision.
Furthermore, integrating PHIP with advanced NMR techniques, such as ultrafast sequences and ultralow and high-field instrumentation, may allow for detecting and studying transient intermediates and reaction dynamics with unprecedented sensitivity, and spectral and temporal resolution.259−263 This can significantly enhance our understanding of complex chemical processes, lead to the discovery of new reaction pathways, and uncover details of catalytic mechanisms.
All discussed here was made possible by utilizing the correlation of spins in pH2 and its effects on the nuclear spin polarization of other interacting neighboring atomic nuclei. Observing nonequilibrium polarization with NMR provides chemical resolution, enabling chemical analysis on an atomic level. NMR, however, is relatively slow, although, in some cases, like in photo-PHIP, the time resolution was boosted while preserving the chemical resolution. For example, a similar boost in time resolution was achieved when photoinduced radical pairs could be generated with a short UV irradiation, resulting in time-resolved chemically induced dynamic nuclear polarization.264 Many methods discussed here are still under development and require significant expertise that currently limits their wider use. However, it is worth being aware of such effects, as, for example, they could provide invaluable information regarding the mobility of H2 on catalytic centers that are inaccessible to other methods, as was exemplified here with hydrogenase studies.
Acknowledgments
ANP and J-BH acknowledge support from German Federal Ministry of Education and Research (BMBF) within the framework of the e:Med research and funding concept (01ZX1915C, 03WIR6208A hyperquant), DFG (555951950, 527469039, 469366436, HO-4602/2-2, HO-4602/3, HO-4602/4, EXC2167, FOR5042, TRR287). MOIN CC was founded by a grant from the European Regional Development Fund (ERDF) and the Zukunftsprogramm Wirtschaft of Schleswig-Holstein (Project no. 122-09-053). SBD and BJT acknowledge the UK Research and Innovation (UKRI) under the UK government’s Horizon Europe funding guarantee (grant number EP/X023672/1). GB gratefully acknowledges financial support from the DFG (BU 911/22-2). CRB acknowledges NSF grants CHE-2108306, CBET-1933723, and the National High Magnetic Field Laboratory User Collaborative Grants Program, which is supported by NSF DMR-2128556 and the State of Florida. SG acknowledges funding from the DFG (SFB1633 project B03) and the Max Planck Society. VVZ is grateful for the support from the Research Council of Finland (grant number 362959) and the University of Oulu (Kvantum Institute).
The authors declare no competing financial interest.
References
- Luchinat E.; Barbieri L.; Cremonini M.; Banci L. Protein in-cell NMR spectroscopy at 1.2 GHz. J. Biomol. NMR 2021, 75 (2), 97–107. 10.1007/s10858-021-00358-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs H.; Moskau D.; Spraul M. Cryogenically cooled probes—a leap in NMR technology. Prog. Nucl. Magn. Reson. Spectrosc. 2005, 46 (2), 131–155. 10.1016/j.pnmrs.2005.03.001. [DOI] [Google Scholar]
- Tickner B. J.; Singh K.; Zhivonitko V. V.; Telkki V.-V. Ultrafast Nuclear Magnetic Resonance as a Tool to Detect Rapid Chemical Change in Solution. ACS Phys. Chem. Au 2024, 4 (5), 453–463. 10.1021/acsphyschemau.4c00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eills J.; Budker D.; Cavagnero S.; Chekmenev E. Y.; Elliott S. J.; Jannin S.; Lesage A.; Matysik J.; Meersmann T.; Prisner T.; Reimer J. A.; Yang H.; Koptyug I. V. Spin Hyperpolarization in Modern Magnetic Resonance. Chem. Rev. 2023, 123 (4), 1417–1551. 10.1021/acs.chemrev.2c00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aime S.; Dastrù W.; Gobetto R.; Santelia D.; Viale A.. Agents for Polarization Enhancement in MRI; Semmler W., Schwaiger M., Eds.; Springer: Berlin, Heidelberg, 2008; pp 247–272. 10.1007/978-3-540-72718-7_12. [DOI] [PubMed] [Google Scholar]
- Nelson S. J.; Kurhanewicz J.; Vigneron D. B.; Larson P. E. Z.; Harzstark A. L.; Ferrone M.; van Criekinge M.; Chang J. W.; Bok R.; Park I.; Reed G.; Carvajal L.; Small E. J.; Munster P.; Weinberg V. K.; Ardenkjaer-Larsen J. H.; Chen A. P.; Hurd R. E.; Odegardstuen L.-I.; Robb F. J.; Tropp J.; Murray J. A. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1–13C]Pyruvate. Sci. Transl. Med. 2013, 5 (198), 198ra108. 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutte H.; Hansen A. E.; Larsen M. M. E.; Rahbek S.; Henriksen S. T.; Johannesen H. H.; Ardenkjaer-Larsen J.; Kristensen A. T.; Højgaard L.; Kjær A. Simultaneous Hyperpolarized 13C-Pyruvate MRI and 18F-FDG PET (HyperPET) in 10 Dogs with Cancer. J. Nucl. Med. 2015, 56 (11), 1786–1792. 10.2967/jnumed.115.156364. [DOI] [PubMed] [Google Scholar]
- Chung B. T.; Chen H.-Y.; Gordon J.; Mammoli D.; Sriram R.; Autry A. W.; Le Page L. M.; Chaumeil M.; Shin P.; Slater J.; Tan C. T.; Suszczynski C.; Chang S.; Li Y.; Bok R. A.; Ronen S. M.; EZ Larson P.; Kurhanewicz J.; Vigneron D. B. First Hyperpolarized [2–13C]Pyruvate MR Studies of Human Brain Metabolism. J. Magn. Reson. 2019, 309, 106617. 10.1016/j.jmr.2019.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C.; Mankinen O.; Telkki V.-V.; Hilty C. Measuring Protein-Ligand Binding by Hyperpolarized Ultrafast NMR. J. Am. Chem. Soc. 2024, 146 (8), 5063–5066. 10.1021/jacs.3c14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epasto L. M.; Che K.; Kozak F.; Selimovic A.; Kadeřávek P.; Kurzbach D. Toward protein NMR at physiological concentrations by hyperpolarized water—Finding and mapping uncharted conformational spaces. Sci. Adv. 2022, 8 (31), eabq5179 10.1126/sciadv.abq5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham P.; Biswas O.; Hilty C. Parahydrogen Polarization in Reverse Micelles and Application to Sensing of Protein-Ligand Binding. J. Am. Chem. Soc. 2024, 146, 34274. 10.1021/jacs.4c13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermkens N. K. J.; Eshuis N.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Wijmenga S. S.; Tessari M. NMR-Based Chemosensing via p-H2 Hyperpolarization: Application to Natural Extracts. Anal. Chem. 2016, 88 (6), 3406–3412. 10.1021/acs.analchem.6b00184. [DOI] [PubMed] [Google Scholar]
- Dey A.; Charrier B.; Martineau E.; Deborde C.; Gandriau E.; Moing A.; Jacob D.; Eshchenko D.; Schnell M.; Melzi R.; Kurzbach D.; Ceillier M.; Chappuis Q.; Cousin S. F.; Kempf J. G.; Jannin S.; Dumez J.-N.; Giraudeau P. Hyperpolarized NMR Metabolomics at Natural 13C Abundance. Anal. Chem. 2020, 92 (22), 14867–14871. 10.1021/acs.analchem.0c03510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett S. B.; Sleigh C. J. Applications of the parahydrogen phenomenon: A chemical perspective. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34 (1), 71–92. 10.1016/S0079-6565(98)00027-2. [DOI] [Google Scholar]
- Kovtunov K. V.; Zhivonitko V. V.; Skovpin I. V.; Barskiy D. A.; Koptyug I. V.. Parahydrogen-Induced Polarization in Heterogeneous Catalytic Processes. Topics in Current Chemistry; Springer: Berlin, Heidelberg, 2012; Vol. 338, pp 123–180. 10.1007/128_2012_371. [DOI] [PubMed] [Google Scholar]
- Tickner B. J.; Zhivonitko V. V. Advancing homogeneous catalysis for parahydrogen-derived hyperpolarisation and its NMR applications. Chem. Sci. 2022, 13 (17), 4670–4696. 10.1039/D2SC00737A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers C. R.; Weitekamp D. P. Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance. Phys. Rev. Lett. 1986, 57 (21), 2645–2648. 10.1103/PhysRevLett.57.2645. [DOI] [PubMed] [Google Scholar]
- Bowers C. R.; Weitekamp D. P. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 1987, 109 (18), 5541–5542. 10.1021/ja00252a049. [DOI] [Google Scholar]
- Eisenschmid T. C.; Kirss R. U.; Deutsch P. P.; Hommeltoft S. I.; Eisenberg R.; Bargon J.; Lawler R. G.; Balch A. L. Para hydrogen induced polarization in hydrogenation reactions. J. Am. Chem. Soc. 1987, 109 (26), 8089–8091. 10.1021/ja00260a026. [DOI] [Google Scholar]
- Green R. A.; Adams R. W.; Duckett S. B.; Mewis R. E.; Williamson D. C.; Green G. G. R. The theory and practice of hyperpolarization in magnetic resonance using parahydrogen. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 67, 1–48. 10.1016/j.pnmrs.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Feng B.; Coffey A. M.; Colon R. D.; Chekmenev E. Y.; Waddell K. W. A pulsed injection parahydrogen generator and techniques for quantifying enrichment. J. Magn. Reson. 2012, 214, 258–262. 10.1016/j.jmr.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hövener J.-B.; Bär S.; Leupold J.; Jenne K.; Leibfritz D.; Hennig J.; Duckett S. B.; von Elverfeldt D. A continuous-flow, high-throughput, high-pressure parahydrogen converter for hyperpolarization in a clinical setting. NMR Biomed. 2013, 26 (2), 124–131. 10.1002/nbm.2827. [DOI] [PubMed] [Google Scholar]
- Du Y.; Zhou R.; Ferrer M.-J.; Chen M.; Graham J.; Malphurs B.; Labbe G.; Huang W.; Bowers C. R. An Inexpensive Apparatus for up to 97% Continuous-Flow Parahydrogen Enrichment Using Liquid Helium. J. Magn. Reson. 2020, 321, 106869. 10.1016/j.jmr.2020.106869. [DOI] [PubMed] [Google Scholar]
- Nantogma S.; Joalland B.; Wilkens K.; Chekmenev E. Y. Clinical-Scale Production of Nearly Pure (>98.5%) Parahydrogen and Quantification by Benchtop NMR Spectroscopy. Anal. Chem. 2021, 93, 3594. 10.1021/acs.analchem.0c05129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong K.; Min S.; Chae H.; Namgoong S. K. Detecting low concentrations of unsaturated C—C bonds by parahydrogen-induced polarization using an efficient home-built parahydrogen generator. Magn. Reson. Chem. 2018, 56 (11), 1089–1093. 10.1002/mrc.4756. [DOI] [PubMed] [Google Scholar]
- Ellermann F.; Pravdivtsev A.; Hövener J.-B. Open-source, partially 3D-printed, high-pressure (50-bar) liquid-nitrogen-cooled parahydrogen generator. Magn. Reson. 2021, 2 (1), 49–62. 10.5194/mr-2-49-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B.; Joalland B.; Meersman C.; Ettedgui J.; Swenson R. E.; Krishna M. C.; Nikolaou P.; Kovtunov K. V.; Salnikov O. G.; Koptyug I. V.; Gemeinhardt M. E.; Goodson B. M.; Shchepin R. V.; Chekmenev E. Y. Low-Cost High-Pressure Clinical-Scale 50% Parahydrogen Generator Using Liquid Nitrogen at 77 K. Anal. Chem. 2021, 93, 8476. 10.1021/acs.analchem.1c00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natterer J.; Bargon J. Parahydrogen induced polarization. Prog. Nucl. Magn. Reson. Spectrosc. 1997, 31 (4), 293–315. 10.1016/S0079-6565(97)00007-1. [DOI] [Google Scholar]
- Buntkowsky G.; Theiss F.; Lins J.; Miloslavina Y. A.; Wienands L.; Kiryutin A.; Yurkovskaya A. Recent advances in the application of parahydrogen in catalysis and biochemistry. RSC Adv. 2022, 12 (20), 12477–12506. 10.1039/D2RA01346K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buljubasich L.; Franzoni M. B.; Münnemann K. Parahydrogen Induced Polarization by Homogeneous Catalysis: Theory and Applications. Top. Curr. Chem. 2013, 338, 33–74. 10.1007/128_2013_420. [DOI] [PubMed] [Google Scholar]
- Bowers C. R.Sensitivity Enhancement Utilizing Parahydrogen. In eMagRes; Harris R. K., Wasylishen R. L., Eds.; Wiley, 2007. 10.1002/9780470034590.emrstm0489 [DOI] [Google Scholar]
- Pravica M. G.; Weitekamp D. P. Net NMR alignment by adiabatic transport of parahydrogen addition products to high magnetic field. Chem. Phys. Lett. 1988, 145 (4), 255–258. 10.1016/0009-2614(88)80002-2. [DOI] [Google Scholar]
- Imamoto T. New P-chirogenic diphosphines and their use in catalytic asymmetric reactions. Pure Appl. Chem. 2001, 73 (2), 373–376. 10.1351/pac200173020373. [DOI] [Google Scholar]
- Bhattacharya P.; Chekmenev E. Y.; Perman W. H.; Harris K. C.; Lin A. P.; Norton V. A.; Tan C. T.; Ross B. D.; Weitekamp D. P. Towards hyperpolarized 13C-succinate imaging of brain cancer. J. Magn. Reson. 2007, 186 (1), 150–155. 10.1016/j.jmr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hövener J.-B.; Chekmenev E. Y.; Harris K. C.; Perman W. H.; Tran T. T.; Ross B. D.; Bhattacharya P. Quality assurance of PASADENA hyperpolarization for 13C biomolecules. Magn. Reson. Mater. Phys. 2009, 22 (2), 123–134. 10.1007/s10334-008-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh M. T.; Kovacs Z. Homogeneous Catalysts for Hydrogenative PHIP Used in Biomedical Applications. Anal. Sens. 2025, 5, e202400044 10.1002/anse.202400044. [DOI] [Google Scholar]
- Klages S.; Permin A. B.; Petrosyan V. S.; Bargon J. Parahydrogen induced polarization in the Pt(0)-mediated hydrogenation of alkynes and alkenes. J. Org. Chem. 1997, 545–546, 201–205. 10.1016/S0022-328X(97)00265-9. [DOI] [Google Scholar]
- Sulman E.; Deibele C.; Bargon J. Study of homogenous hydrogenation of acetylene compounds withpara-hydrogen and Pd(0) and Pt(0) complexes byin situ NMR spectroscopy. React. Kinet. Catal. Lett. 1999, 67 (1), 117–122. 10.1007/BF02475836. [DOI] [Google Scholar]
- Tokmic K.; Fout A. R. Alkyne Semihydrogenation with a Well-Defined Nonclassical Co-H2 Catalyst: A H2 Spin on Isomerization and E-Selectivity. J. Am. Chem. Soc. 2016, 138 (41), 13700–13705. 10.1021/jacs.6b08128. [DOI] [PubMed] [Google Scholar]
- Zhivonitko V. V.; Skovpin I. V.; Szeto K. C.; Taoufik M.; Koptyug I. V. Parahydrogen-Induced Polarization Study of the Silica-Supported Vanadium Oxo Organometallic Catalyst. J. Phys. Chem. C 2018, 122 (9), 4891–4900. 10.1021/acs.jpcc.7b12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S. R.; Greer R. B.; Ramirez S. B.; Goodson B. M.; Fout A. R. Cobalt-Catalyzed Hyperpolarization of Structurally Intact Olefins. ACS Catal. 2021, 11, 2011–2020. 10.1021/acscatal.0c03727. [DOI] [Google Scholar]
- Najera D. C.; Fout A. R. Iron-Catalyzed Parahydrogen Induced Polarization. J. Am. Chem. Soc. 2023, 145 (38), 21086–21095. 10.1021/jacs.3c07735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koptyug I. V.; Kovtunov K. V.; Burt S. R.; Anwar M. S.; Hilty C.; Han S.-I.; Pines A.; Sagdeev R. Z. para-Hydrogen-Induced Polarization in Heterogeneous Hydrogenation Reactions. J. Am. Chem. Soc. 2007, 129 (17), 5580–5586. 10.1021/ja068653o. [DOI] [PubMed] [Google Scholar]
- Kovtunov K. V.; Salnikov O. G.; Zhivonitko V. V.; Skovpin I. V.; Bukhtiyarov V. I.; Koptyug I. V. Catalysis and Nuclear Magnetic Resonance Signal Enhancement with Parahydrogen. Top. Catal. 2016, 59 (19–20), 1686–1699. 10.1007/s11244-016-0688-6. [DOI] [Google Scholar]
- Ripka B.; Eills J.; Kouřilová H.; Leutzsch M.; Levitt M. H.; Münnemann K. Hyperpolarized fumarate via parahydrogen. Chem. Commun. 2018, 54 (86), 12246–12249. 10.1039/C8CC06636A. [DOI] [PubMed] [Google Scholar]
- Stewart N. J.; Nakano H.; Sugai S.; Tomohiro M.; Kase Y.; Uchio Y.; Yamaguchi T.; Matsuo Y.; Naganuma T.; Takeda N.; Nishimura I.; Hirata H.; Hashimoto T.; Matsumoto S. Hyperpolarized 13C Magnetic Resonance Imaging of Fumarate Metabolism by Parahydrogen-induced Polarization: A Proof-of-Concept in vivo Study. ChemPhysChem 2021, 22 (10), 915–923. 10.1002/cphc.202001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher F. A.; Kettunen M. I.; Hu D.-E.; Jensen P. R.; Zandt R.; Karlsson M.; Gisselsson A.; Nelson S. K.; Witney T. H.; Bohndiek S. E.; Hansson G.; Peitersen T.; Lerche M. H.; Brindle K. M. Production of hyperpolarized [1,4–13C2]malate from [1,4–13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc. Nat. Acad. Sci. 2009, 106 (47), 19801–19806. 10.1073/pnas.0911447106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyasingha N. M.; Samoilenko A.; Birchall J. R.; Chowdhury M. R. H.; Salnikov O. G.; Kovtunova L. M.; Bukhtiyarov V. I.; Zhu D. C.; Qian C.; Bradley M.; Gelovani J. G.; Koptyug I. V.; Goodson B. M.; Chekmenev E. Y. Ultra-Low-Cost Disposable Hand-Held Clinical-Scale Propane Gas Hyperpolarizer for Pulmonary Magnetic Resonance Imaging Sensing. ACS Sens. 2023, 8 (10), 3845–3854. 10.1021/acssensors.3c01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M. R. H.; Oladun C.; Ariyasingha N. M.; Samoilenko A.; Bawardi T.; Burueva D. B.; Salnikov O. G.; Kovtunova L. M.; Bukhtiyarov V. I.; Shi Z.; Luo K.; Tan S.; Gelovani J. G.; Koptyug I. V.; Goodson B. M.; Chekmenev E. Y. Rapid lung ventilation MRI using parahydrogen-induced polarization of propane gas. Analyst 2024, 149 (24), 5832–5842. 10.1039/D4AN01029A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R.; Zhao E. W.; Cheng W.; Neal L. M.; Zheng H.; Quiñones R. E.; Hagelin-Weaver H. E.; Bowers C. R. Parahydrogen-Induced Polarization by Pairwise Replacement Catalysis on Pt and Ir Nanoparticles. J. Am. Chem. Soc. 2015, 137 (5), 1938–1946. 10.1021/ja511476n. [DOI] [PubMed] [Google Scholar]
- Zhou R.; Cheng W.; Neal L. M.; Zhao E. W.; Ludden K.; Hagelin-Weaver H. E.; Bowers C. R. Parahydrogen enhanced NMR reveals correlations in selective hydrogenation of triple bonds over supported Pt catalyst. Phys. Chem. Chem. Phys. 2015, 17 (39), 26121–26129. 10.1039/C5CP04223B. [DOI] [PubMed] [Google Scholar]
- Salnikov O. G.; Pokochueva E. V.; Burueva D. B.; Kovtunova L. M.; Kovtunov K. V.; Koptyug I. V. Parahydrogen-Induced Polarization in Gas-Phase Heterogeneous Hydrogenation of Epoxides. J. Phys. Chem. C 2023, 127 (49), 23634–23644. 10.1021/acs.jpcc.3c05059. [DOI] [Google Scholar]
- Gyton M. R.; Royle C. G.; Beaumont S. K.; Duckett S. B.; Weller A. S. Mechanistic Insights into Molecular Crystalline Organometallic Heterogeneous Catalysis through Parahydrogen-Based Nuclear Magnetic Resonance Studies. J. Am. Chem. Soc. 2023, 145 (4), 2619–2629. 10.1021/jacs.2c12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M.; Duckett S. B.; Eisenberg R. Definitive Evidence for a Pairwise Addition of Hydrogen to a Platinum Bis(phosphine) Complex Using Parahydrogen-Induced Polarization. Organometallics 1996, 15 (13), 2863–2865. 10.1021/om960295i. [DOI] [Google Scholar]
- Adams R. W.; Aguilar J. A.; Atkinson K. D.; Cowley M. J.; Elliott P. I. P.; Duckett S. B.; Green G. G. R.; Khazal I. G.; López-Serrano J.; Williamson D. C. Reversible Interactions with para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323 (5922), 1708–1711. 10.1126/science.1168877. [DOI] [PubMed] [Google Scholar]
- Adams R. W.; Duckett S. B.; Green R. A.; Williamson D. C.; Green G. G. R. A theoretical basis for spontaneous polarization transfer in non-hydrogenative parahydrogen-induced polarization. J. Chem. Phys. 2009, 131 (19), 194505. 10.1063/1.3254386. [DOI] [PubMed] [Google Scholar]
- Hovener J.-B.; Schwaderlapp N.; Lickert T.; Duckett S. B.; Mewis R. E.; Highton L. A. R.; Kenny S. M.; Green G. G. R.; Leibfritz D.; Korvink J. G.; Hennig J.; von Elverfeldt D. A hyperpolarized equilibrium for magnetic resonance. Nat. Commun. 2013, 4, 2946. 10.1038/ncomms3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Yurkovskaya A. V.; Vieth H.-M.; Ivanov K. L. RF-SABRE: A Way to Continuous Spin Hyperpolarization at High Magnetic Fields. J. Phys. Chem. B 2015, 119 (43), 13619–13629. 10.1021/acs.jpcb.5b03032. [DOI] [PubMed] [Google Scholar]
- Suefke M.; Lehmkuhl S.; Liebisch A.; Blümich B.; Appelt S. Para-hydrogen raser delivers sub-millihertz resolution in nuclear magnetic resonance. Nat. Phys. 2017, 13 (6), 568–572. 10.1038/nphys4076. [DOI] [Google Scholar]
- Pravdivtsev A. N.; Yurkovskaya A. V.; Vieth H.-M.; Ivanov K. L.; Kaptein R. Level Anti-Crossings are a Key Factor for Understanding para-Hydrogen-Induced Hyperpolarization in SABRE Experiments. ChemPhysChem 2013, 14 (14), 3327–3331. 10.1002/cphc.201300595. [DOI] [PubMed] [Google Scholar]
- Theis T.; Truong M.; Coffey A. M.; Chekmenev E. Y.; Warren W. S. LIGHT-SABRE enables efficient in-magnet catalytic hyperpolarization. J. Magn. Reson. 2014, 248, 23–26. 10.1016/j.jmr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis T.; Truong M. L.; Coffey A. M.; Shchepin R. V.; Waddell K. W.; Shi F.; Goodson B. M.; Warren W. S.; Chekmenev E. Y. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. 2015, 137 (4), 1404–1407. 10.1021/ja512242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Yurkovskaya A. V.; Zimmermann H.; Vieth H.-M.; Ivanov K. L. Transfer of SABRE-derived hyperpolarization to spin-1/2 heteronuclei. RSC Adv. 2015, 5 (78), 63615–63623. 10.1039/C5RA13808F. [DOI] [Google Scholar]
- Ivanov K. L.; Pravdivtsev A. N.; Yurkovskaya A. V.; Vieth H.-M.; Kaptein R. The role of level anti-crossings in nuclear spin hyperpolarization. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 81, 1–36. 10.1016/j.pnmrs.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Soon P. C.; Xu X.; Zhang B.; Gruppi F.; Canary J. W.; Jerschow A. Hyperpolarization of amino acid precursors to neurotransmitters with parahydrogen induced polarization. Chem. Commun. 2013, 49 (46), 5304–5306. 10.1039/c3cc40426a. [DOI] [PubMed] [Google Scholar]
- Reineri F.; Boi T.; Aime S. ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat. Commun. 2015, 6, 5858. 10.1038/ncomms6858. [DOI] [PubMed] [Google Scholar]
- Cavallari E.; Carrera C.; Di Matteo G.; Bondar O.; Aime S.; Reineri F. In-vitro NMR Studies of Prostate Tumor Cell Metabolism by Means of Hyperpolarized [1–13C]Pyruvate Obtained Using the PHIP-SAH Method. Front. Oncol. 2020, 10, 497. 10.3389/fonc.2020.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera C.; Cavallari E.; Digilio G.; Bondar O.; Aime S.; Reineri F. ParaHydrogen polarized ethyl-[1–13C]pyruvate in water, a key substrate for fostering the PHIP-SAH approach to metabolic imaging. ChemPhysChem 2021, 22, 1042. 10.1002/cphc.202100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahms A.; Pravdivtsev A. N.; Stamp T.; Ellermann F.; Sönnichsen F.; Hövener J.-B.; Herges R. Synthesis of 13C and 2H Labeled Vinyl Pyruvate and Hyperpolarization of Pyruvate. Chem.—Eur. J. 2022, 28, e202201210 10.1002/chem.202201210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.; Korchak S.; Mamone S.; Jagtap A. P.; Stevanato G.; Sternkopf S.; Moll D.; Schroeder H.; Becker S.; Fischer A.; Gerhardt E.; Outeiro T. F.; Opazo F.; Griesinger C.; Glöggler S. Rapidly Signal-enhanced Metabolites for Atomic Scale Monitoring of Living Cells with Magnetic Resonance. Chem. Methods 2022, 2, e202200023 10.1002/cmtd.202200023. [DOI] [Google Scholar]
- Brahms A.; Pravdivtsev A. N.; Thorns L.; Sönnichsen F. D.; Hövener J.-B.; Herges R. Exceptionally Mild and High-Yielding Synthesis of Vinyl Esters of Alpha-Ketocarboxylic Acids, Including Vinyl Pyruvate, for Parahydrogen-Enhanced Metabolic Spectroscopy and Imaging. J. Org. Chem. 2023, 88 (21), 15018–15028. 10.1021/acs.joc.3c01461. [DOI] [PubMed] [Google Scholar]
- Mei R.; Fries L. M.; Hune T. L. K.; Santi M. D.; Rodriguez G. G.; Sternkopf S.; Glöggler S. Hyperpolarization of 15N-Pyridinium by Using Parahydrogen Enables Access to Reactive Oxygen Sensors and Pilot In Vivo Studies. Angew. Chem., Int. Ed. 2024, 63, e202403144 10.1002/anie.202403144. [DOI] [PubMed] [Google Scholar]
- Fernandez Diaz-Rullo F.; Zamberlan F.; Mewis R. E.; Fekete M.; Broche L.; Cheyne L. A.; Dall’Angelo S.; Duckett S. B.; Dawson D.; Zanda M. Synthesis and hyperpolarisation of eNOS substrates for quantification of NO production by 1H NMR spectroscopy. Bioorg. Med. Chem. 2017, 25 (10), 2730–2742. 10.1016/j.bmc.2017.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickner B. J.; Borozdina Y.; Duckett S. B.; Angelovski G. Exploring the hyperpolarisation of EGTA-based ligands using SABRE. Dalton Trans. 2021, 50 (7), 2448–2461. 10.1039/D0DT03839C. [DOI] [PubMed] [Google Scholar]
- Barskiy D. A.; Knecht S.; Yurkovskaya A. V.; Ivanov K. L. SABRE: Chemical kinetics and spin dynamics of the formation of hyperpolarization. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 114–115, 33–70. 10.1016/j.pnmrs.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Iali W.; Roy S. S.; Tickner B. J.; Ahwal F.; Kennerley A. J.; Duckett S. B. Hyperpolarising Pyruvate through Signal Amplification by Reversible Exchange (SABRE). Angew. Chem., Int. Ed. 2019, 58 (30), 10271–10275. 10.1002/anie.201905483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivonitko V. V.; Skovpin I. V.; Koptyug I. V. Strong 31P nuclear spin hyperpolarization produced via reversible chemical interaction with parahydrogen. Chem. Commun. 2015, 51 (13), 2506–2509. 10.1039/C4CC08115C. [DOI] [PubMed] [Google Scholar]
- Shchepin R. V.; Barskiy D. A.; Coffey A. M.; Goodson B. M.; Chekmenev E. Y. NMR Signal Amplification by Reversible Exchange of Sulfur-Heterocyclic Compounds Found In Petroleum. ChemistrySelect 2016, 1 (10), 2552–2555. 10.1002/slct.201600761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner P. J.; Richardson P. M.; Duckett S. B. The Detection and Reactivity of Silanols and Silanes Using Hyperpolarized 29Si Nuclear Magnetic Resonance. Angew. Chem., Int. Ed. 2020, 59 (7), 2710–2714. 10.1002/anie.201915098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P.; Harris K.; Lin A. P.; Mansson M.; Norton V. A.; Perman W. H.; Weitekamp D. P.; Ross B. D. Ultra-fast three dimensional imaging of hyperpolarized 13C in vivo. Magn. Reson. Mater. Phys. 2005, 18 (5), 245–256. 10.1007/s10334-005-0007-x. [DOI] [PubMed] [Google Scholar]
- Olsson L. E.; Chai C.-M.; Axelsson O.; Karlsson M.; Golman K.; Petersson J. S. MR coronary angiography in pigs with intraarterial injections of a hyperpolarized 13C substance. Magn. Reson. Med. 2006, 55 (4), 731–737. 10.1002/mrm.20847. [DOI] [PubMed] [Google Scholar]
- Golman K.; Axelsson O.; Jóhannesson H.; Månsson S.; Olofsson C.; Petersson J. S. Parahydrogen-induced polarization in imaging: Subsecond 13C angiography. Magn. Reson. Med. 2001, 46 (1), 1–5. 10.1002/mrm.1152. [DOI] [PubMed] [Google Scholar]
- Goldman M.; Jóhannesson H.; Axelsson O.; Karlsson M. Design and implementation of 13C hyper polarization from para-hydrogen, for new MRI contrast agents. C. R. Chim. 2006, 9 (3), 357–363. 10.1016/j.crci.2005.05.010. [DOI] [Google Scholar]
- Hövener J.-B.; Chekmenev E. Y.; Harris K. C.; Perman W. H.; Robertson L. W.; Ross B. D.; Bhattacharya P. PASADENA hyperpolarization of 13C biomolecules: equipment design and installation. Magn. Reson. Mater. Phys. 2009, 22 (2), 111–121. 10.1007/s10334-008-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierse M.; Nagel L.; Keim M.; Lucas S.; Speidel T.; Lobmeyer T.; Winter G.; Josten F.; Karaali S.; Fellermann M.; Scheuer J.; Müller C.; van Heijster F.; Skinner J.; Löffler J.; Parker A.; Handwerker J.; Marshall A.; Salhov A.; El-Kassem B.; Vassiliou C.; Blanchard J. W.; Picazo-Frutos R.; Eills J.; Barth H.; Jelezko F.; Rasche V.; Schilling F.; Schwartz I.; Knecht S. Parahydrogen-Polarized Fumarate for Preclinical in Vivo Metabolic Magnetic Resonance Imaging. J. Am. Chem. Soc. 2023, 145 (10), 5960–5969. 10.1021/jacs.2c13830. [DOI] [PubMed] [Google Scholar]
- Nagel L.; Gierse M.; Gottwald W.; Ahmadova Z.; Grashei M.; Wolff P.; Josten F.; Karaali S.; Müller C. A.; Lucas S.; Scheuer J.; Müller C.; Blanchard J.; Topping G. J.; Wendlinger A.; Setzer N.; Sühnel S.; Handwerker J.; Vassiliou C.; van Heijster F. H. A.; Knecht S.; Keim M.; Schilling F.; Schwartz I. Parahydrogen-Polarized [1–13C]Pyruvate for Reliable and Fast Preclinical Metabolic Magnetic Resonance Imaging. Adv. Sci. 2023, 10 (30), 2303441. 10.1002/advs.202303441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hune T. L. K.; Mamone S.; Schmidt A. B.; Mahú I.; D’Apolito N.; Wiedermann D.; Brüning J.; Glöggler S. Hyperpolarized Multi-organ Spectroscopy of Liver and Brain Using 1–13C-Pyruvate Enhanced via Parahydrogen. Appl. Magn. Reson. 2023, 54 (11), 1283–1295. 10.1007/s00723-023-01578-z. [DOI] [Google Scholar]
- Cavallari E.; Carrera C.; Sorge M.; Bonne G.; Muchir A.; Aime S.; Reineri F. The 13C hyperpolarized pyruvate generated by ParaHydrogen detects the response of the heart to altered metabolism in real time. Sci. Rep. 2018, 8 (1), 8366. 10.1038/s41598-018-26583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maissin H.; Groß P. R.; Mohiuddin O.; Weigt M.; Nagel L.; Herzog M.; Wang Z.; Willing R.; Reichardt W.; Pichotka M.; Heß L.; Reinheckel T.; Jessen H. J.; Zeiser R.; Bock M.; von Elverfeldt D.; Zaitsev M.; Korchak S.; Glöggler S.; Hövener J.-B.; Chekmenev E. Y.; Schilling F.; Knecht S.; Schmidt A. B. In Vivo Metabolic Imaging of [1–13C]Pyruvate-d3 Hyperpolarized By Reversible Exchange With Parahydrogen. Angew. Chem., Int. Ed. 2023, 62, e202306654 10.1002/anie.202306654. [DOI] [PubMed] [Google Scholar]
- MacCulloch K.; Browning A.; Guarin Bedoya D. O.; McBride S. J.; Abdulmojeed M. B.; Dedesma C.; Goodson B. M.; Rosen M. S.; Chekmenev E. Y.; Yen Y.-F.; TomHon P.; Theis T. Facile hyperpolarization chemistry for molecular imaging and metabolic tracking of [1–13C]pyruvate in vivo. J. Magn. Reson. Open 2023, 16–17, 100129. 10.1016/j.jmro.2023.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber J.; Cherubini A.; Dzik-Jurasz A. S. K.; Leach M. O.; Bifone A. Spin-lattice relaxation of laser-polarized xenon in human blood. Proc. Natl. Acad. Sci. U.S.A. 1999, 96 (7), 3664–3669. 10.1073/pnas.96.7.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder L.; Lowery T. J.; Hilty C.; Wemmer D. E.; Pines A. Molecular Imaging Using a Targeted Magnetic Resonance Hyperpolarized Biosensor. Science 2006, 314 (5798), 446–449. 10.1126/science.1131847. [DOI] [PubMed] [Google Scholar]
- Khan A. S.; Harvey R. L.; Birchall J. R.; Irwin R. K.; Nikolaou P.; Schrank G.; Emami K.; Dummer A.; Barlow M. J.; Goodson B. M.; Chekmenev E. Y. Enabling Clinical Technologies for Hyperpolarized 129Xenon Magnetic Resonance Imaging and Spectroscopy. Angew. Chem., Int. Ed. 2021, 133 (41), 22298–22319. 10.1002/ange.202015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hövener J.-B.; Pravdivtsev A. N.; Kidd B.; Bowers C. R.; Glöggler S.; Kovtunov K. V.; Plaumann M.; Katz-Brull R.; Buckenmaier K.; Jerschow A.; Reineri F.; Theis T.; Shchepin R. V.; Wagner S.; Bhattacharya P.; Zacharias N. M.; Chekmenev E. Y. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem., Int. Ed. 2018, 57 (35), 11140–11162. 10.1002/anie.201711842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtunov K. V.; Pokochueva E.; Salnikov O.; Cousin S.; Kurzbach D.; Vuichoud B.; Jannin S.; Chekmenev E.; Goodson B.; Barskiy D.; Koptyug I. Hyperpolarized NMR Spectroscopy: d-DNP, PHIP, and SABRE Techniques. Chem.—Asian J. 2018, 13 (15), 1857–1871. 10.1002/asia.201800551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Buntkowsky G.; Duckett S. B.; Koptyug I. V.; Hövener J.-B. Parahydrogen-Induced Polarization of Amino Acids. Angew. Chem., Int. Ed. 2021, 60, 23496. 10.1002/anie.202100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineri F.; Cavallari E.; Carrera C.; Aime S. Hydrogenative-PHIP polarized metabolites for biological studies. Magn. Reson. Mater. Phys. 2021, 34 (1), 25–47. 10.1007/s10334-020-00904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A. B.; Bowers C. R.; Buckenmaier K.; Chekmenev E. Y.; de Maissin H.; Eills J.; Ellermann F.; Glöggler S.; Gordon J. W.; Knecht S.; Koptyug I. V.; Kuhn J.; Pravdivtsev A. N.; Reineri F.; Theis T.; Them K.; Hövener J.-B. Instrumentation for Hydrogenative Parahydrogen-Based Hyperpolarization Techniques. Anal. Chem. 2022, 94 (1), 479–502. 10.1021/acs.analchem.1c04863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagys L.; Ripka B.; Leutzsch M.; Moustafa G. A. I.; Eills J.; Colell J. F. P.; Levitt M. H. Geminal parahydrogen-induced polarization: accumulating long-lived singlet order on methylene proton pairs. Magn. Reson. 2020, 1 (2), 175–186. 10.5194/mr-1-175-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Brahms A.; Kienitz S.; Sönnichsen F. D.; Hövener J.-B.; Herges R. Catalytic Hydrogenation of Trivinyl Orthoacetate: Mechanisms Elucidated by Parahydrogen Induced Polarization. ChemPhysChem 2021, 22 (4), 370–377. 10.1002/cphc.202000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett S. B.; Newell C. L.; Eisenberg R. More than INEPT: parahydrogen and INEPT + give unprecedented resonance enhancement to carbon-13 by direct proton polarization transfer. J. Am. Chem. Soc. 1993, 115 (3), 1156–1157. 10.1021/ja00056a054. [DOI] [Google Scholar]
- Pravdivtsev A. N. SABRE Hyperpolarization of Bipyridine Stabilized Ir-Complex at High, Low and Ultralow Magnetic Fields. Z. Phys. Chem. 2017, 231 (3), 497–511. 10.1515/zpch-2016-0810. [DOI] [Google Scholar]
- Robinson A. D.; Hill-Casey F.; Duckett S. B.; Halse M. E. Quantitative reaction monitoring using parahydrogen-enhanced benchtop NMR spectroscopy. Phys. Chem. Chem. Phys. 2024, 26 (19), 14317–14328. 10.1039/D3CP06221J. [DOI] [PubMed] [Google Scholar]
- Majchrzak R. M.; Norcott P. L. Iridium Pyridylpyrrolides Hyperpolarised by Para-Hydrogen. Chem.—Eur. J. 2024, 30, e202400472 10.1002/chem.202400472. [DOI] [PubMed] [Google Scholar]
- Sellies L.; Reile I.; Aspers R. L. E. G.; Feiters M. C.; Rutjes F. P. J. T.; Tessari M. Parahydrogen induced hyperpolarization provides a tool for NMR metabolomics at nanomolar concentrations. Chem. Commun. 2019, 55 (50), 7235–7238. 10.1039/C9CC02186H. [DOI] [PubMed] [Google Scholar]
- Skovpin I. V.; Zhivonitko V. V.; Koptyug I. V. Parahydrogen-Induced Polarization in Heterogeneous Hydrogenations over Silica-Immobilized Rh Complexes. Appl. Magn. Reson. 2011, 41 (2), 393–410. 10.1007/s00723-011-0255-z. [DOI] [Google Scholar]
- Harthun A.; Giernoth R.; Elsevier C. J.; Bargon J. Rhodium- and palladium-catalysed proton exchange in styrene detected in situ by para-hydrogen induced polarization. Chem. Commun. 1996, (21), 2483–2484. 10.1039/cc9960002483. [DOI] [Google Scholar]
- Permin A. B.; Eisenberg R. One-Hydrogen Polarization in Hydroformylation Promoted by Platinum-Tin and Iridium Carbonyl Complexes: A New Type of Parahydrogen-Induced Effect. J. Am. Chem. Soc. 2002, 124 (42), 12406–12407. 10.1021/ja026698t. [DOI] [PubMed] [Google Scholar]
- Zhivonitko V. V.; Skovpin I. V.; Crespo-Quesada M.; Kiwi-Minsker L.; Koptyug I. V. Acetylene Oligomerization over Pd Nanoparticles with Controlled Shape: A Parahydrogen-Induced Polarization Study. J. Phys. Chem. C 2016, 120 (9), 4945–4953. 10.1021/acs.jpcc.5b12391. [DOI] [Google Scholar]
- Emondts M.; Schikowski D.; Klankermayer J.; Schleker P. P. M. Non-Pairwise Interactions in Parahydrogen Experiments: Nuclear Exchange of Single Protons Enables Bulk Water Hyperpolarization. ChemPhysChem 2018, 19 (20), 2614–2620. 10.1002/cphc.201800521. [DOI] [PubMed] [Google Scholar]
- Procacci B.; Duckett S. B.; George M. W.; Hanson-Heine M. W. D.; Horvath R.; Perutz R. N.; Sun X.-Z.; Vuong K. Q.; Welch J. A. Competing Pathways in the Photochemistry of Ru(H)2(CO)(PPh3)3. Organometallics 2018, 37 (6), 855–868. 10.1021/acs.organomet.7b00802. [DOI] [Google Scholar]
- Halse M. E.; Procacci B.; Perutz R. N.; Duckett S. Towards measuring reactivity on micro-to-millisecond timescales with laser pump, NMR probe spectroscopy. Faraday Discuss. 2019, 220, 28–44. 10.1039/C9FD00039A. [DOI] [PubMed] [Google Scholar]
- Halse M. E.; Procacci B.; Henshaw S.-L.; Perutz R. N.; Duckett S. B. Coherent evolution of parahydrogen induced polarisation using laser pump, NMR probe spectroscopy: Theoretical framework and experimental observation. J. Magn. Reson. 2017, 278, 25–38. 10.1016/j.jmr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Torres O.; Procacci B.; Halse M. E.; Adams R. W.; Blazina D.; Duckett S. B.; Eguillor B.; Green R. A.; Perutz R. N.; Williamson D. C. Photochemical Pump and NMR Probe: Chemically Created NMR Coherence on a Microsecond Time Scale. J. Am. Chem. Soc. 2014, 136 (28), 10124–10131. 10.1021/ja504732u. [DOI] [PubMed] [Google Scholar]
- Procacci B.; Aguiar P. M.; Halse M. E.; Perutz R. N.; Duckett S. B. Photochemical pump and NMR probe to monitor the formation and kinetics of hyperpolarized metal dihydrides. Chem. Sci. 2016, 7 (12), 7087–7093. 10.1039/C6SC01956K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R. W.; John R. O.; Blazina D.; Eguillor B.; Cockett M. C. R.; Dunne J. P.; López-Serrano J.; Duckett S. B. Contrasting Photochemical and Thermal Catalysis by Ruthenium Arsine Complexes Revealed by Parahydrogen Enhanced NMR Spectroscopy. Eur. J. Inorg. Chem. 2022, 2022 (5), e202100991 10.1002/ejic.202100991. [DOI] [Google Scholar]
- Blazina D.; Duckett S. B.; Halstead T. K.; Kozak C. M.; Taylor R. J. K.; Anwar M. S.; Jones J. A.; Carteret H. A. Generation and interrogation of a pure nuclear spin state by parahydrogen-enhanced NMR spectroscopy: a defined initial state for quantum computation. Magn. Reson. Chem. 2005, 43 (3), 200–208. 10.1002/mrc.1540. [DOI] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Ivanov K. L.; Yurkovskaya A. V.; Vieth H.-M.; Sagdeev R. Z. Spontaneous transfer of para-hydrogen induced polarization to 13C spins in symmetric molecules. Dokl. Phys. Chem. 2015, 464 (2), 247–250. 10.1134/S0012501615100073. [DOI] [Google Scholar]
- Berner S.; Schmidt A. B.; Zimmermann M.; Pravdivtsev A. N.; Glöggler S.; Hennig J.; von Elverfeldt D.; Hövener J.-B. SAMBADENA Hyperpolarization of 13C-Succinate in an MRI: Singlet-Triplet Mixing Causes Polarization Loss. ChemistryOpen 2019, 8 (6), 728–736. 10.1002/open.201900139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnip S.; Duckett S. B.; Taylor D. R.; Taylor M. J. Parahydrogen enhanced NMR studies on thermally and photochemically generated products from [IrH3(CO)(PPh3)2]. Chem. Commun. 1998, (8), 923–924. 10.1039/a801224e. [DOI] [Google Scholar]
- Hasnip S. K.; Colebrooke S. A.; Sleigh C. J.; Duckett S. B.; Taylor D. R.; Barlow G. K.; Taylor M. J. Activation of H2 by halocarbonyl bis-phosphine and bis-arsine iridium(I) complexes. The use of parahydrogen induced polarisation to detect species present at low concentration and investigate their reactivity. J. Chem. Soc., Dalton Trans. 2002, (5), 743–751. 10.1039/b107444j. [DOI] [Google Scholar]
- Natterer J.; Schedletzky O.; Barkemeyer J.; Bargon J.; Glaser S. J. Investigating Catalytic Processes with Parahydrogen: Evolution of Zero-Quantum Coherence in AA′X Spin Systems. J. Magn. Reson. 1998, 133 (1), 92–97. 10.1006/jmre.1998.1421. [DOI] [PubMed] [Google Scholar]
- Nasibulov E. A.; Pravdivtsev A. N.; Yurkovskaya A. V.; Lukzen N. N.; Vieth H.-M.; Ivanov K. L. Analysis of Nutation Patterns in Fourier-Transform NMR of Non-Thermally Polarized Multispin Systems. Z. Phys. Chem. 2013, 227 (6–7), 929–953. 10.1524/zpch.2013.0397. [DOI] [Google Scholar]
- Goldman M.; Jóhannesson H.; Axelsson O.; Karlsson M. Hyperpolarization of 13C through order transfer from parahydrogen: A new contrast agent for MRI. Magn. Reson. Imag. 2005, 23 (2), 153–157. 10.1016/j.mri.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Yurkovskaya A. V.; Petrov P. A.; Vieth H.-M. Coherent evolution of singlet spin states in PHOTO-PHIP and M2S experiments. Phys. Chem. Chem. Phys. 2017, 19 (38), 25961–25969. 10.1039/C7CP04122E. [DOI] [PubMed] [Google Scholar]
- Anwar M. S.; Blazina D.; Carteret H. A.; Duckett S. B.; Halstead T. K.; Jones J. A.; Kozak C. M.; Taylor R. J. K. Preparing High Purity Initial States for Nuclear Magnetic Resonance Quantum Computing. Phys. Rev. Lett. 2004, 93 (4), 040501. 10.1103/PhysRevLett.93.040501. [DOI] [PubMed] [Google Scholar]
- Kiryutin A. S.; Yurkovskaya A. V.; Lukzen N. N.; Vieth H.-M.; Ivanov K. L. Exploiting adiabatically switched RF-field for manipulating spin hyperpolarization induced by parahydrogen. J. Chem. Phys. 2015, 143 (23), 234203. 10.1063/1.4937392. [DOI] [PubMed] [Google Scholar]
- Brown E. E.; Mandzhieva I.; TomHon P. M.; Theis T.; Castellano F. N. Triplet Photosensitized para-Hydrogen Induced Polarization. ACS Cent. Sci. 2022, 8 (11), 1548–1556. 10.1021/acscentsci.2c01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryutin A. S.; Kozinenko V. P.; Yurkovskaya A. V. Photo-SABRE: Nuclear Spin Hyperpolarization of cis-trans Photoswitchable Molecules by Parahydrogen**. ChemPhotoChem. 2024, 8 (1), e202300151 10.1002/cptc.202300151. [DOI] [Google Scholar]
- Brown E. E.; Harrison R. J.; Havrylyuk D.; McBride S. J.; Glazer E. C.; Castellano F. N.; Theis T.. Photo-ejected ligands hyperpolarized by parahydrogen in reversible exchange. Chem. Commun. 2025, published online. 10.1039/D4CC06807F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivonitko V. V.; Sorochkina K.; Chernichenko K.; Kótai B.; Földes T.; Pápai I.; Telkki V.-V.; Repo T.; Koptyug I. Nuclear spin hyperpolarization with ansa-aminoboranes: a metal-free perspective for parahydrogen-induced polarization. Phys. Chem. Chem. Phys. 2016, 18 (40), 27784–27795. 10.1039/C6CP05211H. [DOI] [PubMed] [Google Scholar]
- Kiryutin A. S.; Sauer G.; Yurkovskaya A. V.; Limbach H.-H.; Ivanov K. L.; Buntkowsky G. Parahydrogen Allows Ultrasensitive Indirect NMR Detection of Catalytic Hydrogen Complexes. J. Phys. Chem. C 2017, 121 (18), 9879–9888. 10.1021/acs.jpcc.7b01056. [DOI] [Google Scholar]
- Aguilar J. A.; Elliott P. I. P.; López-Serrano J.; Adams R. W.; Duckett S. B. Only para-hydrogen spectroscopy (OPSY), a technique for the selective observation of para-hydrogen enhanced NMR signals. Chem. Commun. 2007, 0 (11), 1183–1185. 10.1039/B616307F. [DOI] [PubMed] [Google Scholar]
- Aguilar J. A.; Adams R. W.; Duckett S. B.; Green G. G. R.; Kandiah R. Selective detection of hyperpolarized NMR signals derived from para-hydrogen using the Only Para-hydrogen SpectroscopY (OPSY) approach. J. Magn. Reson. 2011, 208 (1), 49–57. 10.1016/j.jmr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Kozinenko V. P.; Hövener J.-B. Only Para-Hydrogen Spectroscopy (OPSY) Revisited: In-Phase Spectra for Chemical Analysis and Imaging. J. Phys. Chem. A 2018, 122 (45), 8948–8956. 10.1021/acs.jpca.8b07459. [DOI] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Sönnichsen F.; Hövener J.-B. OnlyParahydrogen SpectrosopY (OPSY) pulse sequences - One does not fit all. J. Magn. Reson. 2018, 297, 86–95. 10.1016/j.jmr.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Buntkowsky G.; Bargon J.; Limbach H.-H. A Dynamic Model of Reaction Pathway Effects on Parahydrogen-Induced Nuclear Spin Polarization. J. Am. Chem. Soc. 1996, 118 (36), 8677–8683. 10.1021/ja954188b. [DOI] [Google Scholar]
- Schmidt A. B.; Worner J.; Pravdivtsev A.; Knecht S.; Scherer H.; Weber S.; Hennig J.; von Elverfeldt D.; Hovener J.-B. Lifetime of Parahydrogen in Aqueous Solutions and Human Blood. ChemPhysChem 2019, 20 (19), 2408–2412. 10.1002/cphc.201900670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markelov D. A.; Kozinenko V. P.; Knecht S.; Kiryutin A. S.; Yurkovskaya A. V.; Ivanov K. L. Singlet to triplet conversion in molecular hydrogen and its role in parahydrogen induced polarization. Phys. Chem. Chem. Phys. 2021, 23 (37), 20936–20944. 10.1039/D1CP03164C. [DOI] [PubMed] [Google Scholar]
- Knecht S.; Hadjiali S.; Barskiy D. A.; Pines A.; Sauer G.; Kiryutin A. S.; Ivanov K. L.; Yurkovskaya A. V.; Buntkowsky G. Indirect Detection of Short-Lived Hydride Intermediates of Iridium N-Heterocyclic Carbene Complexes via Chemical Exchange Saturation Transfer Spectroscopy. J. Phys. Chem. C 2019, 123 (26), 16288–16293. 10.1021/acs.jpcc.9b04179. [DOI] [Google Scholar]
- Ward K. M.; Aletras A. H.; Balaban R. S. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). J. Magn. Reson. 2000, 143 (1), 79–87. 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- Johnson A.; Royle C. G.; Brodie C. N.; Martínez-Martínez A. J.; Duckett S. B.; Weller A. S. η2-Alkene Complexes of [Rh(PONOP-iPr)(L)]+ Cations (L = COD, NBD, Ethene). Intramolecular Alkene-Assisted Hydrogenation and Dihydrogen Complex [Rh(PONOP-iPr)(η-H2)]+. Inorg. Chem. 2021, 60 (18), 13903–13912. 10.1021/acs.inorgchem.0c03687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireev N. V.; Kiryutin A. S.; Pavlov A. A.; Yurkovskaya A. V.; Musina E. I.; Karasik A. A.; Shubina E. S.; Ivanov K. L.; Belkova N. V. Nickel(II) Dihydrogen and Hydride Complexes as the Intermediates of H2 Heterolytic Splitting by Nickel Diazadiphosphacyclooctane Complexes. Eur. J. Inorg. Chem. 2021, 2021 (41), 4265–4272. 10.1002/ejic.202100489. [DOI] [Google Scholar]
- Bernatowicz P.; Ratajczyk T.; Szymański S. Non-uniform orientation of H2 molecules in a magnetic field as a critical condition for the appearance of partially negative NMR lines of o-H2 in hyperpolarization experiments using p-H2. J. Chem. Phys. 2023, 159 (12), 124304. 10.1063/5.0167498. [DOI] [PubMed] [Google Scholar]
- Czarnota M.; Mames A.; Pietrzak M.; Jopa S.; Theiß F.; Buntkowsky G.; Ratajczyk T. A Straightforward Method for the Generation of Hyperpolarized Orthohydrogen with a Partially Negative Line. Angew. Chem., Int. Ed. 2024, 63 (12), e202309188 10.1002/anie.202309188. [DOI] [PubMed] [Google Scholar]
- Alam M. S.; Li X.; Brittin D. O.; Islam S.; Deria P.; Chekmenev E. Y.; Goodson B. M. Anomalously Large Antiphase Signals from Hyperpolarized Orthohydrogen Using a MOF-Based SABRE Catalyst. Angew. Chem., Int. Ed. 2023, 62 (8), e202213581 10.1002/anie.202213581. [DOI] [PubMed] [Google Scholar]
- Fleckenstein M.; Herr K.; Theiß F.; Knecht S.; Wienands L.; Brodrecht M.; Reggelin M.; Buntkowsky G. A disintegrin derivative as a case study for PHIP labeling of disulfide bridged biomolecules. Sci. Rep. 2022, 12 (1), 2337. 10.1038/s41598-022-06327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townrow O. P. E.; Duckett S. B.; Weller A. S.; Goicoechea J. M. Zintl cluster supported low coordinate Rh(I) centers for catalytic H/D exchange between H2 and D2. Chem. Sci. 2022, 13 (25), 7626–7633. 10.1039/D2SC02552C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaneeckhaute E.; De Ridder S.; Tyburn J.-M.; Kempf J. G.; Taulelle F.; Martens J. A.; Breynaert E. Long-Term Generation of Longitudinal Spin Order Controlled by Ammonia Ligation Enables Rapid SABRE Hyperpolarized 2D NMR. ChemPhysChem 2021, 22 (12), 1170–1177. 10.1002/cphc.202100079. [DOI] [PubMed] [Google Scholar]
- Zakharov D. O.; Chernichenko K.; Sorochkina K.; Yang S.; Telkki V.-V.; Repo T.; Zhivonitko V. V. Parahydrogen-Induced Polarization in Hydrogenation Reactions Mediated by a Metal-Free Catalyst. Chem.—Eur. J. 2022, 28 (8), e202103501 10.1002/chem.202103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov D. O.; Chernichenko K.; Sorochkina K.; Repo T.; Zhivonitko V. V. Parahydrogen-induced polarization study of imine hydrogenations mediated by a metal-free catalyst. Dalton Trans. 2022, 51 (36), 13606–13611. 10.1039/D2DT02178A. [DOI] [PubMed] [Google Scholar]
- Zakharov D. O.Parahydrogen-induced polarization in H2 activations and hydrogenations mediated by metal-free catalysts. PhD thesis, University of Oulu Graduate School, Faculty of Science, NMR Research Unit, 2023. [Google Scholar]
- Kaltschnee L.; Pravdivtsev A. N.; Gehl M.; Huang G.; Stoychev G. L.; Riplinger C.; Keitel M.; Neese F.; Hövener J.-B.; Auer A. A.; Griesinger C.; Shima S.; Glöggler S. Sensitivity-enhanced magnetic resonance reveals hydrogen intermediates during active [Fe]-hydrogenase catalysis. Nat. Catal. 2024, 7, 1417–1429. 10.1038/s41929-024-01262-w. [DOI] [Google Scholar]
- Kowalewski J.; Mäler L.. Nuclear spin relaxation in liquids: theory, experiments, and applications; Taylor & Francis: 2006. [Google Scholar]
- Zhao E. W.; Maligal-Ganesh R.; Du Y.; Zhao T. Y.; Collins J.; Ma T.; Zhou L.; Goh T.-W.; Huang W.; Bowers C. R. Surface-Mediated Hyperpolarization of Liquid Water from Parahydrogen. Chem. 2018, 4 (6), 1387–1403. 10.1016/j.chempr.2018.03.004. [DOI] [Google Scholar]
- Guan D.; Godard C.; Polas S. M.; Tooze R. P.; Whitwood A. C.; Duckett S. B. Using para hydrogen induced polarization to study steps in the hydroformylation reaction. Dalton Trans. 2019, 48 (8), 2664–2675. 10.1039/C8DT04723E. [DOI] [PubMed] [Google Scholar]
- López-Serrano J.; Duckett S. B.; Aiken S.; Almeida Leñero K. Q.; Drent E.; Dunne J. P.; Konya D.; Whitwood A. C. A para-Hydrogen Investigation of Palladium-Catalyzed Alkyne Hydrogenation. J. Am. Chem. Soc. 2007, 129 (20), 6513–6527. 10.1021/ja070331c. [DOI] [PubMed] [Google Scholar]
- Guan D.; Holmes A. J.; López-Serrano J.; Duckett S. B. Following palladium catalyzed methoxycarbonylation by hyperpolarized NMR spectroscopy: a parahydrogen based investigation. Catal. Sci. Technol. 2017, 7 (10), 2101–2109. 10.1039/C7CY00252A. [DOI] [Google Scholar]
- López-Serrano J.; Duckett S. B.; Dunne J. P.; Godard C.; Whitwood A. C. Palladium catalysed alkyne hydrogenation and oligomerisation: a parahydrogen based NMR investigation. Dalton Trans. 2008, (32), 4270–4281. 10.1039/b804162h. [DOI] [PubMed] [Google Scholar]
- Boutain M.; Duckett S. B.; Dunne J. P.; Godard C.; Hernández J. M.; Holmes A. J.; Khazal I. G.; López-Serrano J. A parahydrogen based NMR study of Pt catalysed alkyne hydrogenation. Dalton Trans. 2010, 39 (14), 3495–3500. 10.1039/b925196k. [DOI] [PubMed] [Google Scholar]
- Wigh Lipsø K.; Hansen E. S. S.; Tougaard R. S.; Laustsen C.; Ardenkjær-Larsen J. H. Renal MR angiography and perfusion in the pig using hyperpolarized water. Magn. Reson. Med. 2017, 78 (3), 1131–1135. 10.1002/mrm.26478. [DOI] [PubMed] [Google Scholar]
- Lipsø K. W.; Hansen E. S. S.; Tougaard R. S.; Laustsen C.; Ardenkjær-Larsen J. H. Dynamic coronary MR angiography in a pig model with hyperpolarized water. Magn. Reson. Med. 2018, 80 (3), 1165–1169. 10.1002/mrm.27088. [DOI] [PubMed] [Google Scholar]
- Kharbanda Y.; Urbańczyk M.; Zhivonitko V. V.; Mailhiot S.; Kettunen M. I.; Telkki V.-V. Sensitive, Efficient and Portable Analysis of Molecular Exchange Processes by Hyperpolarized Ultrafast NMR. Angew. Chem., Int. Ed. 2022, 61 (28), e202203957 10.1002/anie.202203957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.; Szekely O.; Frydman L. On the Potential of Hyperpolarized Water in Biomolecular NMR Studies. J. Phys. Chem. B 2014, 118 (12), 3281–3290. 10.1021/jp4102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Q.; Milani J.; Vuichoud B.; Bornet A.; Gossert A. D.; Bodenhausen G.; Jannin S. Hyperpolarized Water to Study Protein-Ligand Interactions. J. Phys. Chem. Lett. 2015, 6 (9), 1674–1678. 10.1021/acs.jpclett.5b00403. [DOI] [PubMed] [Google Scholar]
- Kurzbach D.; Canet E.; Flamm A. G.; Jhajharia A.; Weber E. M. M.; Konrat R.; Bodenhausen G. Investigation of Intrinsically Disordered Proteins through Exchange with Hyperpolarized Water. Angew. Chem., Int. Ed. 2017, 56 (1), 389–392. 10.1002/anie.201608903. [DOI] [PubMed] [Google Scholar]
- Hilty C.; Kurzbach D.; Frydman L. Hyperpolarized water as universal sensitivity booster in biomolecular NMR. Nat. Protoc. 2022, 17 (7), 1621–1657. 10.1038/s41596-022-00693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan N.; Hilty C. Cross-Polarization of Insensitive Nuclei from Water Protons for Detection of Protein-Ligand Binding. J. Am. Chem. Soc. 2024, 146, 24754. 10.1021/jacs.4c08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon A. C.; Capozzi A.; Ardenkjær-Larsen J. H. Hyperpolarized water through dissolution dynamic nuclear polarization with UV-generated radicals. Commun. Chem. 2020, 3 (1), 1–9. 10.1038/s42004-020-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmkuhl S.; Emondts M.; Schubert L.; Spannring P.; Klankermayer J.; Blümich B.; Schleker P. P. M. Hyperpolarizing Water with Parahydrogen. ChemPhysChem 2017, 18 (18), 2426–2429. 10.1002/cphc.201700750. [DOI] [PubMed] [Google Scholar]
- Van Dyke E. T.; Eills J.; Picazo-Frutos R.; Sheberstov K. F.; Hu Y.; Budker D.; Barskiy D. A. Relayed hyperpolarization for zero-field nuclear magnetic resonance. Sci. Adv. 2022, 8 (29), eabp9242 10.1126/sciadv.abp9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale W. G.; Zhao T. Y.; Choi D.; Ferrer M.-J.; Song B.; Zhao H.; Hagelin-Weaver H. E.; Bowers C. R. Toward Continuous-Flow Hyperpolarisation of Metabolites via Heterogenous Catalysis, Side-Arm-Hydrogenation, and Membrane Dissolution of Parahydrogen. ChemPhysChem 2021, 22, 822. 10.1002/cphc.202100119. [DOI] [PubMed] [Google Scholar]
- Zhao T. Y.; Lapak M. P.; Behera R.; Zhao H.; Ferrer M.-J.; Weaver H. E. H.; Huang W.; Bowers C. R. Perpetual hyperpolarization of allyl acetate from parahydrogen and continuous flow heterogeneous hydrogenation with recycling of unreacted propargyl acetate. J. Magn. Reson. Open 2022, 12–13, 100076. 10.1016/j.jmro.2022.100076. [DOI] [Google Scholar]
- Zhivonitko V. V.; Kovtunov K. V.; Beck I. E.; Ayupov A. B.; Bukhtiyarov V. I.; Koptyug I. V. Role of Different Active Sites in Heterogeneous Alkene Hydrogenation on Platinum Catalysts Revealed by Means of Parahydrogen-Induced Polarization. J. Phys. Chem. C 2011, 115 (27), 13386–13391. 10.1021/jp203398j. [DOI] [Google Scholar]
- Pokochueva E. V.; Burueva D. B.; Salnikov O. G.; Koptyug I. V. Heterogeneous Catalysis and Parahydrogen-Induced Polarization. ChemPhysChem 2021, 22 (14), 1421–1440. 10.1002/cphc.202100153. [DOI] [PubMed] [Google Scholar]
- Zhao E. W.; Maligal-Ganesh R.; Xiao C.; Goh T.-W.; Qi Z.; Pei Y.; Hagelin-Weaver H. E.; Huang W.; Bowers C. R. Silica-Encapsulated Pt-Sn Intermetallic Nanoparticles: A Robust Catalytic Platform for Parahydrogen-Induced Polarization of Gases and Liquids. Angew. Chem., Int. Ed. 2017, 56 (14), 3925–3929. 10.1002/anie.201701314. [DOI] [PubMed] [Google Scholar]
- Du Y.; Behera R.; Maligal-Ganesh R. V.; Chen M.; Chekmenev E. Y.; Huang W.; Bowers C. R. Cyclopropane Hydrogenation vs. Isomerization over Pt and Pt-Sn Intermetallic Nanoparticle Catalysts: A Parahydrogen Spin Labelling Study. J. Phys. Chem. C 2020, 124 (15), 8304–8309. 10.1021/acs.jpcc.0c02493. [DOI] [Google Scholar]
- Chen M.; Bowers C. R.; Huang W. Silica-Encapsulated Intermetallic Nanoparticles for Highly Active and Selective Heterogeneous Catalysis. Acc. Mater. Res. 2021, 2 (12), 1190–1202. 10.1021/accountsmr.1c00153. [DOI] [Google Scholar]
- Pan M.; Pozun Z. D.; Yu W.-Y.; Henkelman G.; Mullins C. B. Structure Revealing H/D Exchange with Co-Adsorbed Hydrogen and Water on Gold. J. Phys. Chem. Lett. 2012, 3 (14), 1894–1899. 10.1021/jz3007707. [DOI] [PubMed] [Google Scholar]
- Norcott P. L. Benzoquinone Enhances Hyperpolarization of Surface Alcohols with Para-Hydrogen. J. Am. Chem. Soc. 2023, 145 (18), 9970–9975. 10.1021/jacs.3c01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longobardi L. E.; Russell C. A.; Green M.; Townsend N. S.; Wang K.; Holmes A. J.; Duckett S. B.; McGrady J. E.; Stephan D. W. Hydrogen Activation by an Aromatic Triphosphabenzene. J. Am. Chem. Soc. 2014, 136 (38), 13453–13457. 10.1021/ja5077525. [DOI] [PubMed] [Google Scholar]
- Zhivonitko V. V.; Telkki V.-V.; Chernichenko K.; Repo T.; Leskelä M.; Sumerin V.; Koptyug I. V. Tweezers for Parahydrogen: A Metal-Free Probe of Nonequilibrium Nuclear Spin States of H2Molecules. J. Am. Chem. Soc. 2014, 136 (2), 598–601. 10.1021/ja410396g. [DOI] [PubMed] [Google Scholar]
- Sorochkina K.; Zhivonitko V. V.; Chernichenko K.; Telkki V.-V.; Repo T.; Koptyug I. V. Spontaneous 15N Nuclear Spin Hyperpolarization in Metal-Free Activation of Parahydrogen by Molecular Tweezers. J. Phys. Chem. Lett. 2018, 9, 903–907. 10.1021/acs.jpclett.7b03433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorochkina K.; Chernichenko K.; Zhivonitko V. V.; Nieger M.; Repo T. J. Water Reduction and Dihydrogen Addition in Aqueous Conditions With ansa-Phosphinoborane. Chem.—Eur. J. 2022, 28, e202201927 10.1002/chem.202201927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsewicz K.; Laczkó G.; Pápai I.; Zhivonitko V. V. Activation of H2 using ansa-aminoboranes: solvent effects, dynamics, and spin hyperpolarization. Phys. Chem. Chem. Phys. 2024, 26 (4), 3197–3207. 10.1039/D3CP05816F. [DOI] [PubMed] [Google Scholar]
- Zhivonitko V. V.; Bresien J.; Schulz A.; Koptyug I. V. Parahydrogen-induced polarization with a metal-free P-P biradicaloid. Phys. Chem. Chem. Phys. 2019, 21 (11), 5890–5893. 10.1039/C8CP07625A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivonitko V. V.; Beer H.; Zakharov D. O.; Bresien J.; Schulz A. Hyperpolarization Effects in Parahydrogen Activation with Pnictogen Biradicaloids: Metal-free PHIP and SABRE. ChemPhysChem 2021, 22 (9), 813–817. 10.1002/cphc.202100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander E.; Bresien J.; Zhivonitko V. V.; Fessler J.; Villinger A.; Michalik D.; Schulz A. Rational Design of Persistent Phosphorus-Centered Singlet Tetraradicals and Their Use in Small-Molecule Activation. J. Am. Chem. Soc. 2023, 145 (26), 14484–14497. 10.1021/jacs.3c03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan D. W. Frustrated Lewis Pairs. J. Am. Chem. Soc. 2015, 137 (32), 10018–10032. 10.1021/jacs.5b06794. [DOI] [PubMed] [Google Scholar]
- Sumerin V.; Schulz F.; Atsumi M.; Wang C.; Nieger M.; Leskelä M.; Repo T.; Pyykkö P.; Rieger B. Molecular Tweezers for Hydrogen: Synthesis, Characterization, and Reactivity. J. Am. Chem. Soc. 2008, 130 (43), 14117–14119. 10.1021/ja806627s. [DOI] [PubMed] [Google Scholar]
- Aime S.; Gobetto R.; Canet D. Longitudinal Nuclear Relaxation in an A2 Spin System Initially Polarized through Para-Hydrogen. J. Am. Chem. Soc. 1998, 120 (27), 6770–6773. 10.1021/ja973240w. [DOI] [Google Scholar]
- Aime S.; Dastrù W.; Gobetto R.; Russo A.; Viale A.; Canet D. A Novel Application of para H2: the Reversible Addition/Elimination of H2 at a Ru3 Cluster Revealed by the Enhanced NMR Emission Resonance from Molecular Hydrogen. J. Phys. Chem. A 1999, 103 (48), 9702–9705. 10.1021/jp992668+. [DOI] [Google Scholar]
- Konsewicz K.; Repo T.; Zhivonitko V. V.. Towards water-tolerant ansa-aminoboranes for parahydrogen-induced polarization and beyond. Chem. Commun. 2025, published online. 10.1039/D5CC00528K [DOI] [PubMed] [Google Scholar]
- Holtkamp P.; Schwabedissen J.; Neumann B.; Stammler G.; Koptyug I.; Zhivonitko V.; Mitzel N. W. A Zwitterionic Phosphonium Stannate(II) via Hydrogen Split-ting by a Sn/P Frustrated Lewis-Pair and Reductive Elimination. Chem.—Eur. J. 2020, 26, 17381. 10.1002/chem.202004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak S.; Mamone S.; Glöggler S. Over 50% 1H and 13C Polarization for Generating Hyperpolarized Metabolites—A para-Hydrogen Approach. ChemistryOpen 2018, 7 (9), 672–676. 10.1002/open.201800086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineri F.; Daniele V.; Cavallari E.; Aime S. Assessing the transport rate of hyperpolarized pyruvate and lactate from the intra- to the extracellular space. NMR Biomed. 2016, 29 (8), 1022–1027. 10.1002/nbm.3562. [DOI] [PubMed] [Google Scholar]
- Eills J.; Cavallari E.; Kircher R.; Matteo G. D.; Carrera C.; Dagys L.; Levitt M. H.; Ivanov K. L.; Aime S.; Reineri F.; Münnemann K.; Budker D.; Buntkowsky G.; Knecht S. Singlet-Contrast Magnetic Resonance Imaging: Unlocking Hyperpolarization with Metabolism**. Angew. Chem., Int. Ed. 2021, 60 (12), 6791–6798. 10.1002/anie.202014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickner B. J.; Rayner P. J.; Duckett S. B. Using SABRE Hyperpolarized 13C NMR Spectroscopy to Interrogate Organic Transformations of Pyruvate. Anal. Chem. 2020, 92 (13), 9095–9103. 10.1021/acs.analchem.0c01334. [DOI] [PubMed] [Google Scholar]
- Tickner B. J.; Platas-Iglesias C.; Duckett S. B.; Angelovski G. In Situ Ternary Adduct Formation of Yttrium Polyaminocarboxylates Leads to Small Molecule Capture and Activation. Chem.—Eur. J. 2022, 28 (57), e202201780 10.1002/chem.202201780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickner B. J.; Dennington M.; Collins B. G.; Gater C. A.; Tanner T. F. N.; Whitwood A. C.; Rayner P. J.; Watts D. P.; Duckett S. B. Metal-Mediated Catalytic Polarization Transfer from para Hydrogen to 3,5-Dihalogenated Pyridines. ACS Catal. 2024, 14, 994–1004. 10.1021/acscatal.3c05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner P. J.; Fekete M.; Gater C. A.; Ahwal F.; Turner N.; Kennerley A. J.; Duckett S. B. Real-Time High-Sensitivity Reaction Monitoring of Important Nitrogen-Cycle Synthons by 15N Hyperpolarized Nuclear Magnetic Resonance. J. Am. Chem. Soc. 2022, 144 (19), 8756–8769. 10.1021/jacs.2c02619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelovski G.; Tickner B. J.; Wang G. Opportunities and challenges with hyperpolarized bioresponsive probes for functional imaging using magnetic resonance. Nat. Chem. 2023, 15 (6), 755–763. 10.1038/s41557-023-01211-3. [DOI] [PubMed] [Google Scholar]
- Grant A. K.; Vinogradov E. Long-lived states in solution NMR: Theoretical examples in three- and four-spin systems. J. Magn. Reson. 2008, 193 (2), 177–190. 10.1016/j.jmr.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Levitt M. H. Singlet Nuclear Magnetic Resonance. Annu. Rev. Phys. Chem. 2012, 63 (1), 89–105. 10.1146/annurev-physchem-032511-143724. [DOI] [PubMed] [Google Scholar]
- Zhivonitko V. V.; Kovtunov K. V.; Chapovsky P. L.; Koptyug I. V. Nuclear Spin Isomers of Ethylene: Enrichment by Chemical Synthesis and Application for NMR Signal Enhancement. Angew. Chem., Int. Ed. 2013, 52 (50), 13251–13255. 10.1002/anie.201307389. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Soon P. C.; Jerschow A.; Canary J. W. Long-Lived 1H Nuclear Spin Singlet in Dimethyl Maleate Revealed by Addition of Thiols. Angew. Chem., Int. Ed. 2014, 53 (13), 3396–3399. 10.1002/anie.201310284. [DOI] [PubMed] [Google Scholar]
- Chapovsky P. L.; Zhivonitko V. V.; Koptyug I. V. Conversion of Nuclear Spin Isomers of Ethylene. J. Phys. Chem. A 2013, 117 (39), 9673–9683. 10.1021/jp312322f. [DOI] [PubMed] [Google Scholar]
- Sviyazov S. V.; Babenko S. V.; Skovpin I. V.; Kovtunova L. M.; Chukanov N. V.; Stakheev A. Y.; Burueva D. B.; Koptyug I. V. Manipulating stereoselectivity of parahydrogen addition to acetylene to unravel interconversion of ethylene nuclear spin isomers. Phys. Chem. Chem. Phys. 2024, 26 (9), 7821–7829. 10.1039/D3CP04983C. [DOI] [PubMed] [Google Scholar]
- Skovpin I. V.; Sviyazov S. V.; Burueva D. B.; Kovtunova L. M.; Nartova A. V.; Kvon R. I.; Bukhtiyarov V. I.; Koptyug I. V. Nonequilibrium Nuclear Spin States of Ethylene during Acetylene Hydrogenation with Parahydrogen over Immobilized Iridium Complexes. Dokl. Phys. Chem. 2023, 512 (2), 149–157. 10.1134/S0012501623600237. [DOI] [Google Scholar]
- Pokochueva E. V.; Svyatova A. I.; Burueva D. B.; Koptyug I. V. Chemistry of nuclear spin isomers of the molecules: from the past of the Universe to emerging technologies. Russ. Chem. Bull. 2023, 72 (1), 1–19. 10.1007/s11172-023-3711-7. [DOI] [Google Scholar]
- Bae J.; Zhou Z.; Theis T.; Warren W. S.; Wang Q. 15N4–1,2,4,5-tetrazines as potential molecular tags: Integrating bioorthogonal chemistry with hyperpolarization and unearthing para-N2. Sci. Adv. 2018, 4 (3), eaar2978 10.1126/sciadv.aar2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procacci B.; Roy S. S.; Norcott P.; Turner N.; Duckett S. B. Unlocking a diazirine long-lived nuclear singlet state via photochemistry: NMR detection and lifetime of an unstabilized diazo-compound. J. Am. Chem. Soc. 2018, 140 (48), 16855–16864. 10.1021/jacs.8b10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau L. D.; Lifshitz E. M.. Quantum Mechanics: Non-Relativistic Theory, 3rd ed.; Pergamon Press: Oxford; New York, 1977. [Google Scholar]
- Babenko S. V.; Sviyazov S. V.; Burueva D. B.; Koptyug I. V. Hyperpolarized long-lived spin state of methylene protons of 2-bromoethanol obtained from ethylene with non-equilibrium nuclear spin order. J. Magn. Reson. 2024, 360, 107648. 10.1016/j.jmr.2024.107648. [DOI] [PubMed] [Google Scholar]
- Franzoni M. B.; Buljubasich L.; Spiess H. W.; Münnemann K. Long-Lived 1H Singlet Spin States Originating from Para-Hydrogen in Cs-Symmetric Molecules Stored for Minutes in High Magnetic Fields. J. Am. Chem. Soc. 2012, 134 (25), 10393–10396. 10.1021/ja304285s. [DOI] [PubMed] [Google Scholar]
- Franzoni M. B.; Graafen D.; Buljubasich L.; Schreiber L. M.; Spiess H. W.; Munnemann K. Hyperpolarized 1H long lived states originating from parahydrogen accessed by rf irradiation. Phys. Chem. Chem. Phys. 2013, 15 (40), 17233–17239. 10.1039/c3cp52029c. [DOI] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Yurkovskaya A. V.; Lukzen N. N.; Vieth H.-M.; Ivanov K. L. Exploiting level anti-crossings (LACs) in the rotating frame for transferring spin hyperpolarization. Phys. Chem. Chem. Phys. 2014, 16 (35), 18707–18719. 10.1039/C4CP01445F. [DOI] [PubMed] [Google Scholar]
- Graafen D.; Franzoni M. B.; Schreiber L. M.; Spiess H. W.; Münnemann K. Magnetic resonance imaging of 1H long lived states derived from parahydrogen induced polarization in a clinical system. J. Magn. Reson. 2016, 262, 68–72. 10.1016/j.jmr.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Them K.; Ellermann F.; Pravdivtsev A. N.; Salnikov O. G.; Skovpin I. V.; Koptyug I. V.; Herges R.; Hövener J.-B. Parahydrogen-Induced Polarization Relayed via Proton Exchange. J. Am. Chem. Soc. 2021, 143 (34), 13694–13700. 10.1021/jacs.1c05254. [DOI] [PubMed] [Google Scholar]
- Iali W.; Rayner P. J.; Duckett S. B. Using parahydrogen to hyperpolarize amines, amides, carboxylic acids, alcohols, phosphates, and carbonates. Sci. Adv. 2018, 4 (1), eaao6250 10.1126/sciadv.aao6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcicek S.; Van Dyke E.; Xu J.; Pustelny S.; Barskiy D. A. 13C and 15N Benchtop NMR Detection of Metabolites via Relayed Hyperpolarization. Chem. Methods 2023, 3 (7), e202200075 10.1002/cmtd.202200075. [DOI] [Google Scholar]
- Them K.; Kuhn J.; Pravdivtsev A. N.; Hövener J.-B. Nuclear spin polarization of lactic acid via exchange of parahydrogen-polarized protons. Comm. Chem. 2024, 7, 172. 10.1038/s42004-024-01254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravdivtsev A. N.; Yurkovskaya A. V.; Zimmermann H.; Vieth H.-M.; Ivanov K. L. Enhancing NMR of insensitive nuclei by transfer of SABRE spin hyperpolarization. Chem. Phys. Lett. 2016, 661, 77–82. 10.1016/j.cplett.2016.08.037. [DOI] [Google Scholar]
- Iali W.; Rayner P. J.; Alshehri A.; Holmes A. J.; Ruddlesden A. J.; Duckett S. B. Direct and indirect hyperpolarisation of amines using parahydrogen. Chem. Sci. 2018, 9 (15), 3677–3684. 10.1039/C8SC00526E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner P. J.; Tickner B. J.; Iali W.; Fekete M.; Robinson A. D.; Duckett S. B. Relayed hyperpolarization from para-hydrogen improves the NMR detectability of alcohols. Chem. Sci. 2019, 10 (33), 7709–7717. 10.1039/C9SC02765C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P. M.; Iali W.; Roy S. S.; Rayner P. J.; Halse M. E.; Duckett S. B. Rapid 13C NMR hyperpolarization delivered from para-hydrogen enables the low concentration detection and quantification of sugars. Chem. Sci. 2019, 10 (45), 10607–10619. 10.1039/C9SC03450A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickner B. J.; Svensson S. K.-M.; Vaara J.; Duckett S. B. Toward Optimizing and Understanding Reversible Hyperpolarization of Lactate Esters Relayed from para-Hydrogen. J. Phys. Chem. Lett. 2022, 13 (29), 6859–6866. 10.1021/acs.jpclett.2c01442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri A.; Tickner B. J.; Iali W.; Duckett S. B. Enhancing the NMR signals of plant oil components using hyperpolarisation relayed via proton exchange. Chem. Sci. 2023, 14 (36), 9843–9853. 10.1039/D3SC03078D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaneeckhaute E.; Tyburn J.-M.; Kilgour D.; Kempf J. G.; Taulelle F.; Martens J. A.; Breynaert E. Hyperpolarised Magnetic Resonance of Exchangeable Protons Using Parahydrogen and Aminosilane. J. Phys. Chem. C 2020, 124 (27), 14541–14549. 10.1021/acs.jpcc.0c01149. [DOI] [Google Scholar]
- Knecht S.; Barskiy D. A.; Buntkowsky G.; Ivanov K. L. Theoretical Description of Hyperpolarization Formation in the SABRE-relay Method. J. Chem. Phys. 2020, 153, 164106. 10.1063/5.0023308. [DOI] [PubMed] [Google Scholar]
- Inokuchi H. Catalytic activity of organic semiconductors and enzymes. Discuss. Faraday Soc. 1971, 51, 183. 10.1039/df9715100183. [DOI] [PubMed] [Google Scholar]
- Dementin S.; Burlat B.; Lacey A. L. D.; Pardo A.; Adryanczyk-Perrier G.; Guigliarelli B.; Fernandez V. M.; Rousset M. A Glutamate Is the Essential Proton Transfer Gate during the Catalytic Cycle of the [NiFe] Hydrogenase. J. Biol. Chem. 2004, 279 (11), 10508–10513. 10.1074/jbc.M312716200. [DOI] [PubMed] [Google Scholar]
- Yagi T.; Tsuda M.; Inokuchi H. Kinetic studies on hydrogenase. Parahydrogen-Orthohydrogen Conversion and Hydrogen-Deuterium Exchange Reactions. J. Biochem. 1973, 73 (5), 1069–1081. 10.1093/oxfordjournals.jbchem.a130161. [DOI] [PubMed] [Google Scholar]
- Couper A.; Eley D. D.; Hayward A. Hydrogen-activating enzymes of bacteria. Discuss. Faraday Soc. 1955, 20, 174. 10.1039/df9552000174. [DOI] [Google Scholar]
- Shima S.; Thauer R. K. A third type of hydrogenase catalyzing H2 activation. Chem. Record. 2007, 7 (1), 37–46. 10.1002/tcr.20111. [DOI] [PubMed] [Google Scholar]
- Shima S.; Pilak O.; Vogt S.; Schick M.; Stagni M. S.; Meyer-Klaucke W.; Warkentin E.; Thauer R. K.; Ermler U. The Crystal Structure of [Fe]-Hydrogenase Reveals the Geometry of the Active Site. Science 2008, 321 (5888), 572–575. 10.1126/science.1158978. [DOI] [PubMed] [Google Scholar]
- Lubitz W.; Ogata H.; Rüdiger O.; Reijerse E. Hydrogenases. Chem. Rev. 2014, 114 (8), 4081–4148. 10.1021/cr4005814. [DOI] [PubMed] [Google Scholar]
- Wang C.; Lai Z.; Huang G.; Pan H.-J. Current State of [Fe]-Hydrogenase and Its Biomimetic Models. Chem.—Eur. J. 2022, 28 (57), e202201499 10.1002/chem.202201499. [DOI] [PubMed] [Google Scholar]
- Ji H.; Wan L.; Gao Y.; Du P.; Li W.; Luo H.; Ning J.; Zhao Y.; Wang H.; Zhang L.; Zhang L. Hydrogenase as the basis for green hydrogen production and utilization. J. Energy Chem. 2023, 85, 348–362. 10.1016/j.jechem.2023.06.018. [DOI] [Google Scholar]
- Vignais P. M.; Billoud B. Occurrence, Classification, and Biological Function of Hydrogenases: An Overview. Chem. Rev. 2007, 107 (10), 4206–4272. 10.1021/cr050196r. [DOI] [PubMed] [Google Scholar]
- Schleucher J.; Griesinger C.; Schwoerer B.; Thauer R. K. H2-Forming N5,N10-Methylenetetrahydromethanopterin Dehydrogenase from Methanobacterium thermoautotrophicum Catalyzes a Stereoselective Hydride Transfer As Determined by Two-Dimensional NMR Spectroscopy. Biochemistry 1994, 33 (13), 3986–3993. 10.1021/bi00179a027. [DOI] [PubMed] [Google Scholar]
- Glöggler S.; Müller R.; Colell J.; Emondts M.; Dabrowski M.; Blümich B.; Appelt S. Para-hydrogen induced polarization of amino acids, peptides and deuterium-hydrogen gas. Phys. Chem. Chem. Phys. 2011, 13 (30), 13759–13764. 10.1039/c1cp20992b. [DOI] [PubMed] [Google Scholar]
- Huang G.; Wagner T.; Wodrich M. D.; Ataka K.; Bill E.; Ermler U.; Hu X.; Shima S. The atomic-resolution crystal structure of activated [Fe]-hydrogenase. Nat. Catal. 2019, 2 (6), 537–543. 10.1038/s41929-019-0289-4. [DOI] [Google Scholar]
- Pravdivtsev A. N.; Sönnichsen F. D.; Hövener J.-B. Continuous Radio Amplification by Stimulated Emission of Radiation using Parahydrogen Induced Polarization (PHIP-RASER) at 14 T. ChemPhysChem 2020, 21 (7), 667–672. 10.1002/cphc.201901056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak S.; Kaltschnee L.; Dervisoglu R.; Andreas L.; Griesinger C.; Glöggler S. Spontaneous Enhancement of Magnetic Resonance Signals Using a RASER. Angew. Chem., Int. Ed. 2021, 60 (38), 20984–20990. 10.1002/anie.202108306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salnikov O. G.; Trofimov I. A.; Pravdivtsev A. N.; Them K.; Hövener J.-B.; Chekmenev E. Y.; Koptyug I. V. Through-Space Multinuclear Magnetic Resonance Signal Enhancement Induced by Parahydrogen and Radiofrequency Amplification by Stimulated Emission of Radiation. Anal. Chem. 2022, 94 (43), 15010–15017. 10.1021/acs.analchem.2c02929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmkuhl S.; Fleischer S.; Lohmann L.; Rosen M. S.; Chekmenev E. Y.; Adams A.; Theis T.; Appelt S. RASER MRI: Magnetic resonance images formed spontaneously exploiting cooperative nonlinear interaction. Sci. Adv. 2022, 8 (28), eabp8483 10.1126/sciadv.abp8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.; Schmidt A. B.; Adelabu I.; Nantogma S.; Kiselev V. G.; Abdurraheem A.; de Maissin H.; Lehmkuhl S.; Appelt S.; Theis T.; Chekmenev E. Y. Parahydrogen-Induced Carbon-13 Radiofrequency Amplification by Stimulated Emission of Radiation. Angew. Chem., Int. Ed. 2023, 62 (5), e202215678 10.1002/anie.202215678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierse M.; Dagys L.; Keim M.; Lucas S.; Josten F.; Plenio M. B.; Schwartz I.; Knecht S.; Eills J. Hyperpolarizing Small Molecules using Parahydrogen and Solid-State Spin Diffusion. Angew. Chem., Int. Ed. 2024, 63, e202319341 10.1002/anie.202319341. [DOI] [PubMed] [Google Scholar]
- Dagys L.; Korzeczek M. C.; Parker A. J.; Eills J.; Blanchard J. W.; Bengs C.; Levitt M. H.; Knecht S.; Schwartz I.; Plenio M. B. Robust parahydrogen-induced polarization at high concentrations. Sci. Adv. 2024, 10 (30), eado0373 10.1126/sciadv.ado0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard L.-S.; Kovtunov K. V.; Burt S. R.; Anwar M. S.; Koptyug I. V.; Sagdeev R. Z.; Pines A. Para-Hydrogen-Enhanced Hyperpolarized Gas-Phase Magnetic Resonance Imaging. Angew. Chem., Int. Ed. 2007, 46 (22), 4064–4068. 10.1002/anie.200700830. [DOI] [PubMed] [Google Scholar]
- Kovtunov K. V.; Beck I. E.; Bukhtiyarov V. I.; Koptyug I. V. Observation of Parahydrogen-Induced Polarization in Heterogeneous Hydrogenation on Supported Metal Catalysts. Angew. Chem., Int. Ed. 2008, 47 (8), 1492–1495. 10.1002/anie.200704881. [DOI] [PubMed] [Google Scholar]
- Chen S.; Tennakoon A.; You K.-E.; Paterson A. L.; Yappert R.; Alayoglu S.; Fang L.; Wu X.; Zhao T. Y.; Lapak M. P.; et al. Ultrasmall amorphous zirconia nanoparticles catalyse polyolefin hydrogenolysis. Nat. Catal. 2023, 6 (2), 161–173. 10.1038/s41929-023-00910-x. [DOI] [Google Scholar]
- Eshuis N.; Hermkens N.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Wijmenga S. S.; Tessari M. Toward Nanomolar Detection by NMR Through SABRE Hyperpolarization. J. Am. Chem. Soc. 2014, 136 (7), 2695–2698. 10.1021/ja412994k. [DOI] [PubMed] [Google Scholar]
- Eshuis N.; Aspers R. L. E. G.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Wijmenga S. S.; Tessari M. 2D NMR Trace Analysis by Continuous Hyperpolarization at High Magnetic Field. Angew. Chem., Int. Ed. 2015, 54 (48), 14527–14530. 10.1002/anie.201507831. [DOI] [PubMed] [Google Scholar]
- Eshuis N.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Wijmenga S. S.; Tessari M. Quantitative Trace Analysis of Complex Mixtures Using SABRE Hyperpolarization. Angew. Chem., Int. Ed. 2015, 54 (5), 1481–1484. 10.1002/anie.201409795. [DOI] [PubMed] [Google Scholar]
- Reile I.; Eshuis N.; Hermkens N. K. J.; van Weerdenburg B. J. A.; Feiters M. C.; Rutjes F. P. J. T.; Tessari M. NMR detection in biofluid extracts at sub-μM concentrations via para-H2 induced hyperpolarization. Analyst 2016, 141 (13), 4001–4005. 10.1039/C6AN00804F. [DOI] [PubMed] [Google Scholar]
- Tickner B. J.; Komulainen S.; Palosaari S.; Heikkinen J.; Lehenkari P.; Zhivonitko V. V.; Telkki V.-V. Hyperpolarised NMR to aid molecular profiling of electronic cigarette aerosols. RSC Adv. 2022, 12 (3), 1479–1485. 10.1039/D1RA07376A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermkens N. K. J.; Aspers R. L. E. G.; Feiters M. C.; Rutjes F. P. J. T.; Tessari M. Trace analysis in water-alcohol mixtures by continuous p-H2 hyperpolarization at high magnetic field. Magn. Reson. Chem. 2018, 56 (7), 633–640. 10.1002/mrc.4692. [DOI] [PubMed] [Google Scholar]
- Sellies L.; Aspers R.; Feiters M. C.; Rutjes F.; Tessari M. Para-hydrogen hyperpolarization allows direct NMR detection of α-amino acids in complex (bio)mixtures. Angew. Chem., Int. Ed. 2021, 60 (52), 26954–26959. 10.1002/anie.202109588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimets N.; Ausmees K.; Vija S.; Reile I. Developing Analytical Applications for Parahydrogen Hyperpolarization: Urinary Elimination Pharmacokinetics of Nicotine. Anal. Chem. 2021, 93 (27), 9480–9485. 10.1021/acs.analchem.1c01281. [DOI] [PubMed] [Google Scholar]
- Tickner B.; John R. O.; Roy S. S.; Hart S.; Whitwood A. C.; Duckett S. B. Using coligands to gain mechanistic insight into iridium complexes hyperpolarized with para-hydrogen. Chem. Sci. 2019, 10 (20), 5235–5245. 10.1039/C9SC00444K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood N. J.; Brannigan J. A.; Duckett S. B.; Heath S. L.; Wagstaff J. Detection of Picomole Amounts of Biological Substrates by para-Hydrogen-Enhanced NMR Methods in Conjunction with a Suitable Receptor Complex. J. Am. Chem. Soc. 2007, 129 (36), 11012–11013. 10.1021/ja074286k. [DOI] [PubMed] [Google Scholar]
- Barskiy D. A.; Tayler M. C. D.; Marco-Rius I.; Kurhanewicz J.; Vigneron D. B.; Cikrikci S.; Aydogdu A.; Reh M.; Pravdivtsev A. N.; Hövener J.-B.; Blanchard J. W.; Wu T.; Budker D.; Pines A. Zero-field nuclear magnetic resonance of chemically exchanging systems. Nat. Commun. 2019, 10 (1), 3002. 10.1038/s41467-019-10787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barskiy D. A.; Blanchard J. W.; Budker D.; Eills J.; Pustelny S.; Sheberstov K. F.; Tayler M. C. D.; Trabesinger A. H. Zero- to Ultralow-field Nuclear Magnetic Resonance. Prog. Nucl. Magn. Reson. Spectrosc. 2025, 148-149, 101558. 10.1016/j.pnmrs.2025.101558. [DOI] [Google Scholar]
- Myers J. Z.; Bullinger F.; Kempf N.; Plaumann M.; Ortmeier A.; Theis T.; Povolni P.; Romanowski J.; Engelmann J.; Scheffler K.; Hövener J.-B.; Buckenmaier K.; Körber R.; Pravdivtsev A. N. Zero to ultralow magnetic field NMR of [1–13C]pyruvate and [2–13C]pyruvate enabled by SQUID sensors and hyperpolarization. Phys. Rev. B 2024, 109 (18), 184443. 10.1103/PhysRevB.109.184443. [DOI] [Google Scholar]
- Tickner B. J.; Zhivonitko V. V.; Telkki V.-V. Ultrafast Laplace NMR to study metal-ligand interactions in reversible polarisation transfer from parahydrogen. Phys. Chem. Chem. Phys. 2021, 23 (31), 16542–16550. 10.1039/D1CP02383G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryutin A. S.; Sauer G.; Tietze D.; Brodrecht M.; Knecht S.; Yurkovskaya A. V.; Ivanov K. L.; Avrutina O.; Kolmar H.; Buntkowsky G. Ultrafast Single-Scan 2D-NMR Spectroscopic Detection of a PHIP-Hyperpolarized Protease Inhibitor. Chem.—Eur. J. 2019, 25 (16), 4025–4030. 10.1002/chem.201900079. [DOI] [PubMed] [Google Scholar]
- Morozova O. B.; Fishman N. N.; Yurkovskaya A. V. Indirect NMR detection of transient guanosyl radical protonation in neutral aqueous solution. Phys. Chem. Chem. Phys. 2017, 19 (32), 21262–21266. 10.1039/C7CP03797J. [DOI] [PubMed] [Google Scholar]