Abstract
We evaluated a molecular subtyping system for Treponema pallidum for its ability to differentiate between strains obtained from male patients with primary syphilis in South Africa. Of 201 T. pallidum-positive specimens, 161 were typeable, revealing 35 subtypes. The unique subtypes identified in Durban, Cape Town, and Carletonville and the total number of subtypes suggested that the strain population was very diverse and varied geographically.
Syphilis is endemic in South Africa. A recent national survey of antenatal clinic attendees in South Africa revealed a syphilis seroprevalence of 4.9% (1). Although the rates in this group of women have been declining since 1998, the prevalence of the disease in the country’s general population remains unacceptably high by world standards.
In this cross-sectional study, we sought to determine the diversity of T. pallidum strains circulating in a country where syphilis is common and to obtain an indication of the discriminatory ability of a PCR-based typing system for T. pallidum (4) by using genital ulcer specimens. By use of a multiplex PCR assay (3) or a polA PCR assay (2), T. pallidum DNA was detected in 201 of 1,954 consecutive male patients with genital ulcers and attending STD clinics in Durban, KwaZulu-Natal; Cape Town, Western Province; Johannesburg, Gauteng; Carletonville, Gauteng; and Welkom, Orange Free State (Table 1).
TABLE 1.
Clinical specimen collection by site in South Africa (n = 1,954)
| City or town | Collection date | No. of specimens | No. (%) of specimens positive for T. palliduma |
|---|---|---|---|
| Durban | June-August 1996 | 199 | 29 (15) |
| June-August 1997 | 158 | 15 (9.4) | |
| January 1998 | 72 | 6 (8.3) | |
| July-October 1998 | 400 | 33 (8.3) | |
| Cape Town | April-August 1995 | 180 | 58 (32.2) |
| Johannesburg | May-October 1996 | 159 | 19 (12) |
| Carletonville | July-November 1998 | 115 | 18 (16) |
| August 1998–May 2000 | 398 | 21 (5.3) | |
| Welkom | January-November 1996 | 273 | 12 (4.4) |
As determined by a multiplex PCR assay or a polA PCR assay. Typing experiments were not performed on 10 samples because of inadequate or no specimen.
DNA was extracted from a 40- to 200-μl aliquot of the specimen by using a QIAamp blood/tissue kit (Qiagen, Santa Clarita, Calif.) with the following modification: 3 μl of a 1:10 dilution of calf thymus DNA (10.5 μg/μl; Sigma Chemical Co., St. Louis, Mo.) was added to the Eppendorf centrifuge tube before ethanol precipitation to increase the yield of T. pallidum DNA. The repeat region of the arp gene and three members of the tpr gene family were amplified by using the following PCR primer pairs: ARP1A (5′-CAA GTC AGG ACG GAC TGT CC CTT GC-3′) and ARP2A (5′-GGT ATC ACC TGG GGA TGC GCA CG-3′) and B1 (5′-ACT GGC TCT GCC ACA CTT GA-3′) and A2 (5′-CTA CCA GGA GAG GGT GAC GC-3′). All other experimental conditions were as previously described (4, 5).
Of the 201 T. pallidum-positive specimens, 161 (80.1%) contained sufficient DNA for typing. Of the 40 specimens (19.9%) that could not be subtyped, 26 were found negative by both tpr and arp gene PCR assays, while 14 were typeable by the tpr gene PCR method only (data not shown). This result could have been due to the fact that the tpr gene PCR assay is a nested PCR and is therefore more sensitive than the arp gene PCR assay. Other possible reasons for our inability to type T. pallidum from these specimens include (i) insufficient DNA in the specimen, (ii) degradation of DNA during storage, and (iii) false-positive diagnostic PCR results.
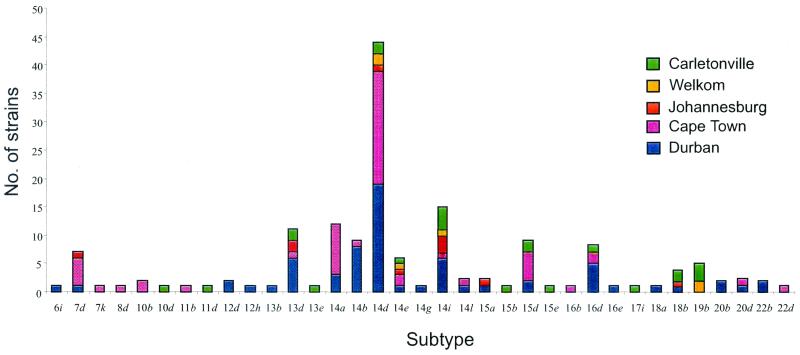
A total of 15 arp repeat sizes (6, 7, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, and 22 repeats) and 10 MseI restriction fragment length polymorphism patterns (a, b, d, e, g, h, i, j, k, and l) were identified in this study. By combining the arp repeat sizes and MseI restriction fragment length polymorphism patterns (4, 5), we observed 35 subtypes (Fig. 1): 22 subtypes among the 67 specimens from Durban, 17 subtypes among the 55 specimens from Cape Town, 14 subtypes among the 23 specimens from Carletonville, 7 subtypes among the 10 specimens from Johannesburg, and 4 subtypes among the 6 specimens from Welkom. The majority of strains (44 of 161, 27.3%) belonged to subtype 14d: 19 of 67 (28.3%) from Durban, 20 of 55 (36.3%) from Cape Town, 2 of 6 (33.3%) from Welkom, 1 of 10 (10%) from Johannesburg, and 2 of 23 (8.7%) from Carletonville. The second most common subtype was 14i, present in 15 of 161 specimens (9.3%), followed by 14a and 13d, found in 12 of 161 specimens (7.4%) and 11 of 161 specimens (6.8%), respectively. Of the remaining specimens, 9 of 161 (5.5%) were subtype 14b, 8 of 161 (4.9%) were subtype 16d, 7 of 161 (4.3%) were subtype 7d, 5 of 161 (3.1%) were subtype 19b, and 4 of 161 (2.4%) were subtype 18b; 2 strains (1.2%) each belonged to subtypes 10b, 12d, 15a, 20b, 20d, and 22b; and the remaining 17 strains had unique subtypes.
FIG. 1.
Distribution of T. pallidum subtypes in South Africa (n = 161).
Subtypes 14d, 14e, and 14i were identified in specimens from all five sites studied, while subtype 13d was found in four sites and subtypes 7d, 15d, 16d, and 18b were each found in three sites. Subtype 14a, which included the third highest number of strains, was identified only in specimens from Durban and Cape Town. A number of subtypes were unique to specimens from Durban, Cape Town, and Carletonville. No unique subtypes were found in specimens from either Johannesburg or Welkom.
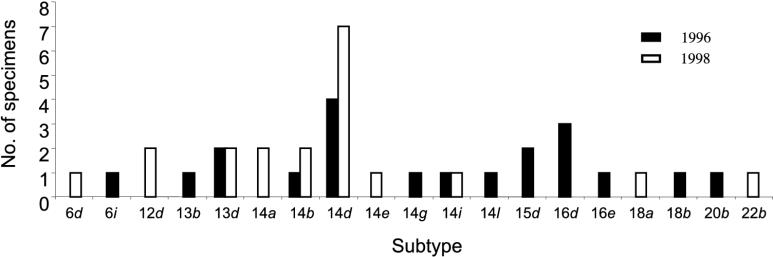
The first 20 typeable specimens obtained from genital ulcer disease patients in Durban beginning in June 1996 were compared with the first 20 typeable specimens collected from patients in July 1998 (Fig. 2). Thirteen subtypes were identified among the 20 specimens collected during 1996, while 10 subtypes were identified among the 20 specimens from 1998. Four subtypes were identified in specimens from both 1996 and 1998; four specimens from 1996 had the 14d subtype, compared with seven in 1998. There were nine unique subtypes among the specimens from 1996 and six unique subtypes among the 1998 specimens.
FIG. 2.
Comparison of T. pallidum strain patterns in Durban in 1996 (n = 20) versus 1998 (n = 20).
The presence of many different T. pallidum subtypes among persons in Durban and Cape Town may reflect the large numbers of T. pallidum-positive specimens from these areas. However, when the total number of strains per total number of subtypes was determined for each site (data not shown), the strain diversity in descending order of sites was Johannesburg, Welkom, Carletonville, Durban, and Cape Town. However, the high strain diversity that was observed in Johannesburg, Welkom, and Carletonville may not be an accurate representation because disproportionately smaller numbers of T. pallidum-positive strains were available for analysis in these areas compared to Cape Town and Durban. Furthermore, if specimens were collected from epidemiologically linked cases, the degree of genetic diversity would appear falsely low.
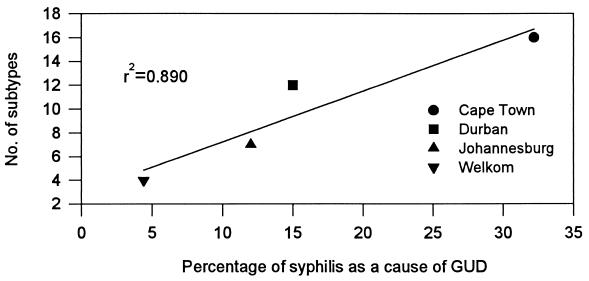
The high strain diversity observed here may reflect long-term endemicity of the disease in South Africa, with many untreated individuals with infectious forms of syphilis, allowing transmission to susceptible contacts. In addition to the genetic diversity of endemic strains of T. pallidum, the introduction of new strains by travelers to and from other neighboring countries may have contributed to the large number of different circulating strains. We examined whether there was a correlation between the number of subtypes and the prevalence of syphilis and observed that the number of subtypes was directly proportional to the relative prevalence of syphilis (the Pearson r2 value was 0.890) in South Africa (Fig. 3). While the correlation was of borderline statistical significance (P = 0.057), it may indicate that the diversity of T. pallidum subtypes is higher when the prevalence of syphilis is high.
FIG. 3.
Number of subtypes versus percentage of syphilis infections as a cause of genital ulcer disease (GUD) by city in South Africa.
The observation of predominant subtypes and the demonstration of strain diversity in this study suggest that the typing system used here may be useful for other countries where syphilis is endemic. However, to validate its usefulness in contributing to an understanding of the epidemiology of syphilis, it will be necessary to demonstrate the same T. pallidum subtype within individual sexual networks.
Acknowledgments
We thank Timothy Green for statistical assistance.
This work was supported in part by an appointment (A.P.) to the Research Participation Program at the CDC, which is administered by the Oak Ridge Institute for Science and Education; a research grant (9227 2277) from the University of Natal; and a Wellcome Trust grant to A.W.S. for research in reproductive health and population studies.
REFERENCES
- 1.Department of Health. 2001. Eleventh national HIV and syphilis seroprevalence survey of women attending public antenatal clinics in South Africa, October 2000. Department of Health, Pretoria, South Africa.
- 2.Liu, H., B. Rodes, C. Y. Chen, and B. Steiner. 2001. New tests for syphilis: rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I gene. J. Clin. Microbiol. 39:1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orle, K. A., C. A. Gates, D. H. Martin, B. A. Body, and J. B. Weiss. 1996. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J. Clin. Microbiol. 34:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillay, A., H. Liu, C. Y. Chen, B. Holloway, A. W. Sturm, B. Steiner, and S. A. Morse. 1998. Molecular subtyping of Treponema pallidum subspecies pallidum. Sex. Transm. Dis. 25:408–414. [DOI] [PubMed] [Google Scholar]
- 5.Sutton, M. Y., H. Liu, B. Steiner, A. Pillay, T. Mickey, L. Finelli, S. Morse, L. E. Markowitz, and M. E. St. Louis. 2001. Molecular subtyping of Treponema pallidum in an Arizona County with increasing syphilis morbidity: use of specimens from ulcers and blood. J. Infect. Dis. 183:1601–1606. [DOI] [PubMed] [Google Scholar]