Abstract
Black tea has been shown to improve endothelial function in patients with coronary artery disease and recent data indicate the polyphenol fraction of black tea enhances endothelial nitric oxide synthase (eNOS) activity through p38 MAP kinase (p38 MAPK) activation. Since the mechanisms for this phenomenon are not yet clear, we sought to elucidate the signaling events in response to black tea polyphenols. Bovine aortic endothelial cells (BAECs) exposed to black tea polyphenols demonstrated eNOS activation that was inhibited by the estrogen receptor (ER) antagonist ICI 182,780 and siRNA-mediated silencing of ER expression. Consistent with this observation, black tea polyphenols induced time-dependent phosphorylation of ERα on Ser-118 that was inhibited by ICI 182,780. Phosphorylation of ERα on Ser-118 was due to p38 MAP kinase (p38 MAPK) as it was inhibited by SB203580 and over-expression of dominant-negative p38 MAPKα. Conversely, constitutively-active MKK6 induced p38 MAPK activation that recapitulated the effects of polyphenols by inducing ERα phosphorylation and downstream activation of Akt, and eNOS. The key role of ERα Ser-118 phosphorylation was confirmed in eNOS-transfected COS-7 cells as polyphenol-induced eNOS activation required co-transfection with ERα subject to phosphorylation at Ser-118. This residue appeared critical for functional association of ERα with p38 MAPK as ERα with Ser-118 mutated to alanine could not form a complex with p38 MAPK. These findings suggest p38 MAP kinase-mediated eNOS activation requires ERα and these data uncover a new mechanism of ERα activation that has broad implications for NO bioactivity and endothelial cell phenotype .
Introduction
Recent evidence indicate that flavonoids, a group of polyphenolic substances found in tea, fruit, vegetables, and wine favorably impact the endothelium (reviewed in 1,2). Normal endothelial function is critical for regulation of vasomotor tone, platelet activity, leukocyte adhesion, and vascular smooth muscle proliferation.3 These actions of the endothelium are mediated via release of several paracrine factors, including nitric oxide (NO).3
These normal functions of the endothelium are impaired in the setting of atherosclerosis and its associated vascular conditions such as hypertension, hypercholesterolemia and diabetes.3 Impaired endothelial function has important consequences as individuals with the poorest endothelial function are at increased risk of cardiovascular events.4,5 Thus, reversing endothelial dysfunction has become a topic of intense investigation. In this regard, endothelial NO bioactivity is enhanced in patients with atherosclerosis by either the acute or chronic administration of black tea.6 In cultured cells, the black tea polyphenolic fraction promotes both eNOS catalytic activity and NO bioactivity.7 This effect is due to activation of phosphoinositol 3-kinase (PI 3-K) and Akt via a p38 MAPK-dependent mechanism.7
Despite observations that black tea polyphenols enhance endothelial cell NO bioactivity, important questions remain. The upstream components of polyphenol-mediated eNOS activation are not well described and the precise signals linking p38 MAPK to eNOS activation are largely unknown. The purpose of this study, therefore, was to investigate the mechanism of p38 MAPK-mediated eNOS activation by black tea polyphenols.
Materials and Methods
Materials
We obtained ICI 182,780 from Tocris (Ellisville, MO). Inhibitors of p38 MAPK (SB203580), ERK1/2 (PD98059) and JNK (SP600125) were obtained from Calbiochem (San Diego, CA). Polyclonal antibodies directed against p38 MAPK, hemagluttinin (HA), ERK1/2, and the phosphorylated forms of p38 MAPK (Thr-180, Tyr-182), ERK1/2 (Thr-202, Tyr-204), JNK (Thr-183, Tyr-185), MAPKAPK-2 (Thr-222), GSK-3α/β (Ser-21/9), and estrogen receptor-alpha (ERα; Ser-118) were obtained from Cell Signaling Technology (Beverly, MA). Polyclonal antibodies directed against phospho-eNOS (Ser1177) were obtained from Upstate Biotechnology, Inc. (Lake Placid, NY). Anti-eNOS monoclonal antibody was obtained from Transduction Laboratories (Lexington, KY). Antibody against ERα (AER 320) was obtained from Lab Vision (Fremont, CA). L-[3H]arginine (1 mCi, 53 mCi/mmol) was obtained from PerkinElmer Life Sciences (Wellesley, MA) and cGMP assay kits were from Cayman (Ann Arbor, MI). Black tea polyphenols and black tea fractions were provided by Unilever, Inc. All other reagents were obtained from Sigma.
Cell Culture
Bovine aortic endothelial cells (BAECs) and human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics and grown on endothelial growth medium (Clonetics, Inc). COS-7 cells (American Type Culture Collection, Rockville, Maryland, USA) were grown in DMEM supplemented with 10% heat inactivated FBS, 50 μg/ml heparin sulfate, 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin as described.8 For experiments, confluent endothelial cells were used between passages 4 and 8 and treated with physiologically relevant concentrations of black tea polyphenols (100 ng/ml) that we previously demonstrated activate eNOS.7 Prior to all experiments, cells were placed in serum- and phenol-free media (opti-MEM, Gibco, NY) for at least 16 hours, washed twice in HEPES-buffered physiologic salt solution (PSS), and experiments carried out in HEPES-buffered PSS as described.7
eNOS Activity Assay
The catalytic activity of eNOS was estimated by the conversion of L-[3H]arginine to L-[3H]citrulline that was sensitive to inhibition by L-nitro-arginine methyl ester (L-NAME). Confluent cells in 100mm culture dishes were washed and incubated in HEPES-buffered PSS for 30 min followed by the addition of 5 μM L-arginine plus 3.3 μCi of L-[3H]arginine. Cells were then treated with the agonist of interest or vehicle for 15 minutes and cells were then lysed and L-[3H]citrulline determined as described.7
Immunoprecipitation and Western Blotting
Cells were washed with PSS and incubated in lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride for 30 min on ice as described.7 For ERα immunoprecipitation, lysates were incubated with ERα monoclonal antibody (4 μg/ml) rotating for 16 h at 4 °C followed by a 1-h incubation with protein A/G-agarose. Following centrifugation, the immunoprecipitates were washed, resuspended in loading buffer, and resolved by SDS-PAGE as described.7 Resolved proteins were transferred to a nitrocellulose membrane (Hybond; Amersham Biosciences, Inc.) and immunoblotting performed as previously described.7 Densitometric analysis of immunoblots was carried out using PDI Imageware System (Huntington Station, NY).
Recombinant Adenoviral Vectors and Transfection
Recombinant adenovirus expressing a HA-tagged constitutively active MKK6 mutant (MKK6bE) was a kind gift from Dr. Jiahuia Han, Scripps Institute.9 Adenoviral construct encoding myristoylated, constitutively active Akt (myr-Akt) was a kind gift of Dr. Kenneth Walsh, Boston University. BAECs were infected in medium without FBS for 2h, washed, and incubated in fresh medium with FBS for 12–24h prior to experimentation.
Plasmid Constructs and Transfection.
The construct for human eNOS cDNA was obtained from Dr. Richard Venema, Medical College of Georgia. Expression plasmids for ERα and ERαs118a have been described previously.10 Transfection of COS-7 cells was performed essentially as previously described.8,11 After transfection, cells were cultured in a phenol red- and estrogen-free media and either vehicle or estrogen was added to effect stimulation of the receptor.
Statistical Analysis
All numerical data are presented as means ± S.E. Western blots shown are representative of three or more independent experiments. For parametric data, comparisons among treatment groups were performed with one-way analysis of variance and an appropriate post hoc comparison. Instances involving only two comparisons were evaluated with a Student’s t test. Statistical significance was accepted if the null hypothesis was rejected with a p < 0.05.
Results
Activation of eNOS by Black Tea Polyphenols involves Estrogen Receptors
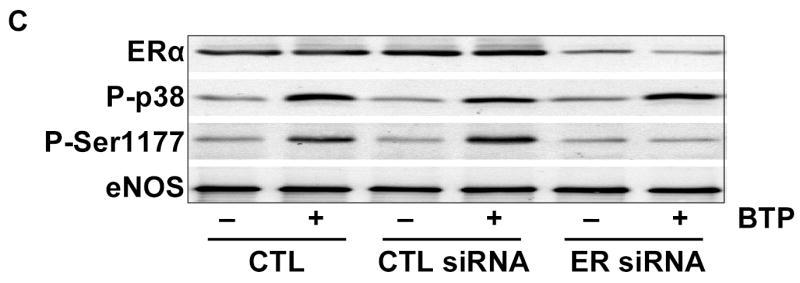
To probe pathways involved in black tea polyphenol-induced eNOS activation, we utilized a pharmacological approach that included the ER antagonist ICI 182,780. As shown in Figs. 1A and B, polyphenol-induced eNOS phosphorylation and catalytic activation were both attenuated by ER antagonism. To confirm this finding, we used siRNA to silence ER expression and found it limited black tea polyphenol-induced eNOS phosphorylation on Ser-1177 (human sequence; Fig. 1C) and eNOS activation (Fig. 1D). Thus, black tea polyphenol-induced eNOS activation involves estrogen receptors.
Figure 1.
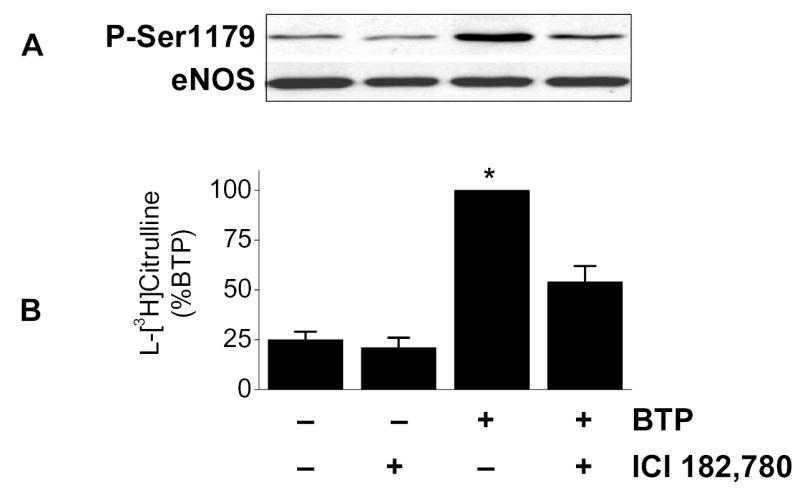
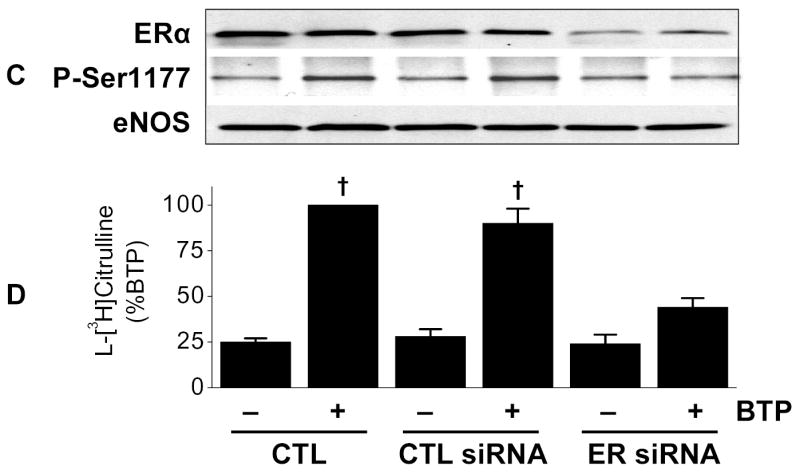
Activation of eNOS by black tea polyphenols involves estrogen receptors. (A) BAECs in HEPES-buffered PSS were incubated with or without the estrogen receptor antagonist ICI 182,780 (100 nM) followed by incubation with 100ng/ml black tea polyphenols (BTP) for 15 min. Cells were then lysed, eNOS immunoprecipitated, and its phosphorylation status at Ser-1179 determined by immunoblotting with antibodies against phosphorylated or total eNOS as indicated. (B) BAECs were incubated as in (A) and eNOS catalytic activity estimated by the conversion of L-[3H]arginine to L-[3H]citrulline as in “Methods.” (C) HUVECs were incubated without (CTL) or with siRNA directed against human ERα/ERβ (ER siRNA) or its scrambled control (CTL siRNA) for 72 hours before treatment with BTP as in (A). The expression of ERα and eNOS phosphorylation were determined by immunoblotting with the indicated antibodies. (D) HUVECs treated as in (C) were assessed for eNOS catalytic activity by the conversion of L[3H]arginine to L-[3H]citrulline as in “Methods.” All immunoblots are representative of 3 independent experiments and composite data are mean ± S.E of 4–8 independent experiments. Legend: *p<0.05 vs no additions, †p<0.05 for the effect of BTP by paired t-test.
Black Tea Polyphenols Induce ERα phosphorylation
Phosphorylation of ERα occurs on multiple residues, including Ser-118.12 To determine if black tea polyphenols induce ERα Ser-118 phosphorylation, BAECs were treated with black tea polyphenols and probed with a phosphorylation-specific antibody. Under resting conditions, BAECs exhibit little ERα Ser-118 phosphorylation, however, exposure to black tea polyphenols resulted in a time-dependent increase in ERα Ser-118 phosphorylation (Fig. 2A). Pharmacological ER inhibition by ICI 182,780 attenuated ERα phosphorylation in response to black tea polyphenols (Figure 2B). These data indicate polyphenols induce ERα phosphorylation in a manner that is inhibited by ICI 182,780.
Figure 2.
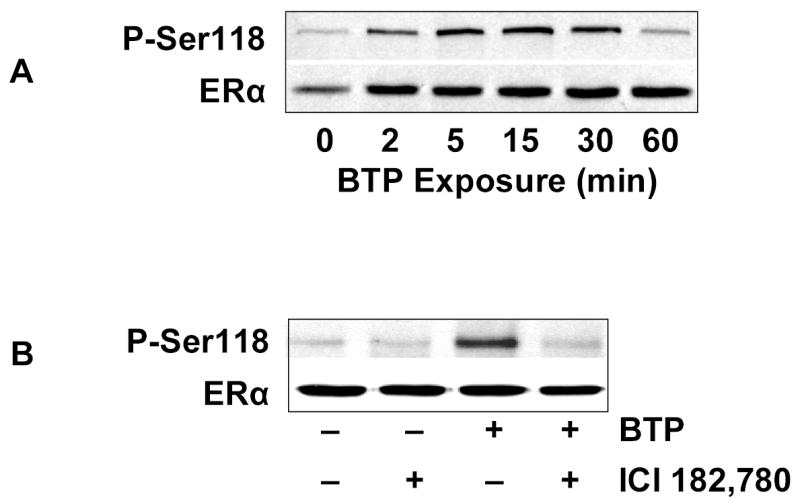
Black tea polyphenols induce ERα phosphorylation. (A) BAECs in HEPES-buffered PSS were incubated with 100 ng/ml black tea polyphenols for the indicated time, lysed, and ERα Ser-118 phosphorylation determined by immunoblotting with antibodies specific for the indicated epitopes. (B) BAECs were incubated with or without ICI 182,780 or BTP as in Fig. 1A. Cells were then lysed and ERα Ser-118 phosphorylation determined by immunoblotting with antibodies against the indicated epitopes. All immunoblots are representative of 3 independent experiments.
Black Tea Polyphenol-Induced ERα phosphorylation involves p38 MAPK
Phosphorylation of ERα on Ser-118 has been linked to activation of extracellular signal-regulated kinase (ERK)12 and we have demonstrated p38 MAPK (p38 MAPK) is required for eNOS activation in response to black tea polyphenols.7 Therefore, to determine the involvement of MAPKs in our system, lysates from black tea polyphenol-treated BAECs were probed for activation of ERK, p38 MAPK, or c-Jun, N-terminal kinase (JNK). As shown in Fig. 3A, p38 MAPK was activated in response to black tea polyphenols with no activation of ERK or JNK. Consistent with this observation, only inhibition of p38 MAPK had a material impact on the polyphenol-induced eNOS stimulation (Fig. 3B).
Figure 3.
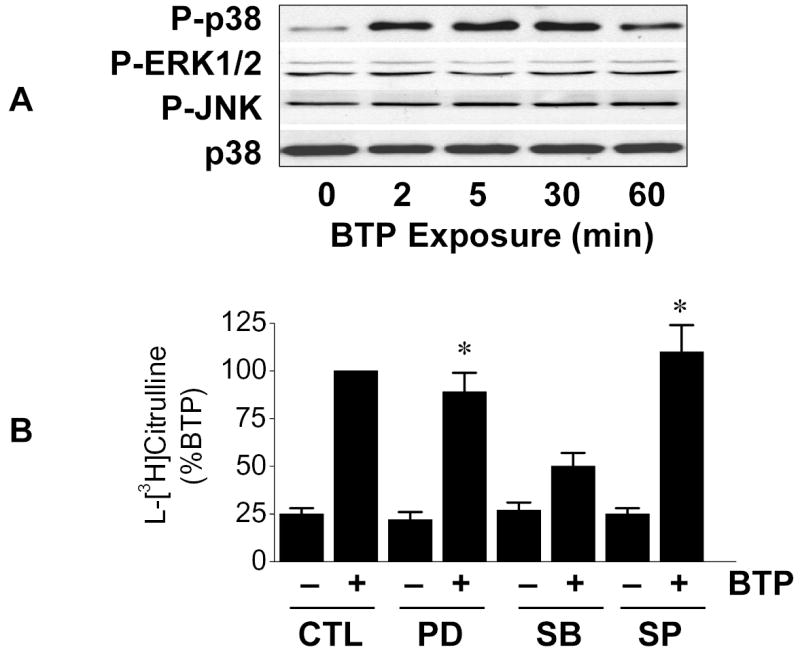
Black tea polyphenol-induced eNOS activation involves p38 MAPK. (A) BAECs in HEPES-buffered PSS were incubated with 100 ng/ml black tea polyphenols (BTP) for the indicated time. Cells were then lysed and immunoblotted with antibodies against the phosphorylated (activated) forms of p38 MAPK, ERK1/2 and JNK as indicated. Loading was assessed by immunoblots for total p38 MAPK. (B) BAECs were incubated for 30 min without (CTL) or with inhibitors for MEK1 (PD98059; 20μM), p38 MAPK (SB203580; 5μM), or JNK (SP600125; 10μM) as indicated. Cells were then treated with BTP (100ng/ml; 15min) and eNOS catalytic activity determined as the conversion of L-[3H]arginine to L-[3H]citrulline as in “Methods.” Immunoblots are representative of 3 independent experiments and composite data are mean ± S.E. Legend: *p<0.05 vs corresponding treatment without BTP.
We next investigated the role of p38 MAPK in ERα phosphorylation. Pharmacological inhibition of p38 MAPK blunted black tea polyphenol-induced ERα Ser-118 phosphorylation and phosphorylation of the p38 MAPK target, MAPKAPK2 (Fig. 4A). We confirmed that ERα phosphorylation was downstream of p38MAPK as adenoviral transfection with a dominant-negative p38 MAPK mutant prevented black tea polyphenol-induced ERα Ser-118 phosphorylation (Fig. 4B). Activation of p38 MAPK proved to be a proximal component of the black tea polyphenol response as dominant-negative p38 MAPK also prevented Akt phosphorylation, eNOS phosphorylation (Fig. 4B), and eNOS activation (Fig 4C) in response to polyphenols. Consistent with this observation, constitutive activation of p38 MAPK with the MKK6bE adenovirus produced the same response as black tea polyphenols with regards to phosphorylation of ERα, Akt, and eNOS as well as eNOS activation (Figs. 4B and C). Combined treatment of BAECs with MKK6bE over-expression and black tea polyphenols was synergistic with regards to eNOS activation (Fig. 4C).
Figure 4.
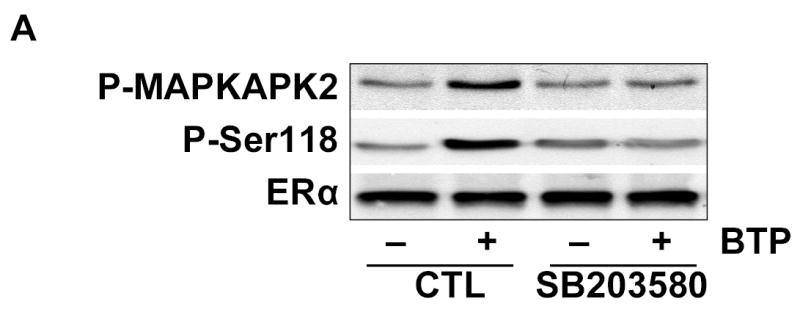
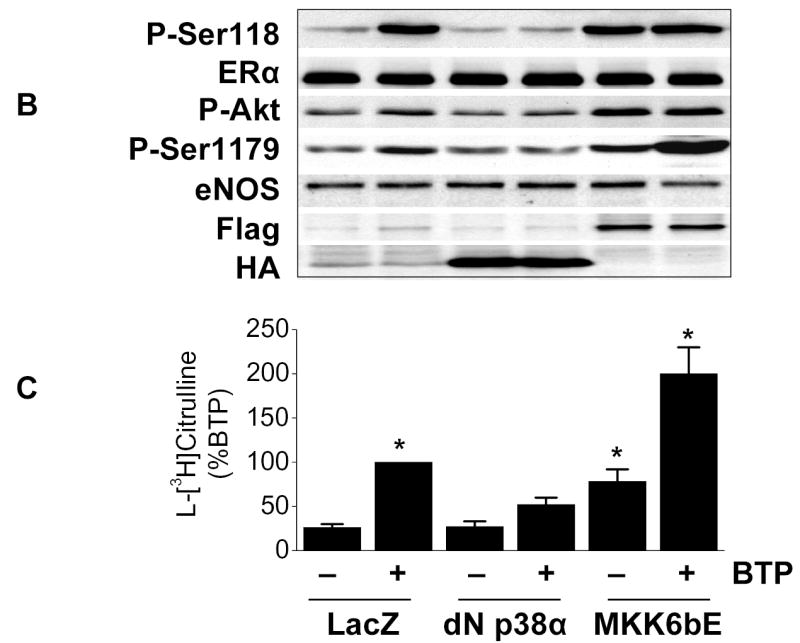
Black tea polyphenol-induced ERα phosphorylation involves p38 MAPK. (A) BAECs were incubated for 30 min with or without the p38 MAPK inhibitor SB203580 (5μM). Cells were then treated with black tea polyphenols (100ng/ml; 15min) or vehicle (CTL), lysed and both Ser-118 phosphorylation and expression of ERα determined with antibodies directed against the indicated epitopes. Activity of p38 MAPK was determined by immunoblotting with antibodies for the phosphorylated (activated) form of the p38 MAPK downstream target, mitogen-activated protein kinase activated protein kinase-2 (MAPKAPK-2). (B) BAECs were transfected with adenoviral vectors expressing LacZ, a dominant-negative p38α mutant (dn p38α), or a constitutively-active MKK6 mutant (MKK6bE) for 24h. Cells were then equilibrated in HEPES-PSS (30 min) followed by treatment with black tea polyphenols (BTP; 100ng/ml) or its vehicle for 15 min. Cells were then lysed and the lysates either collected or immunoprecipitated with ERα or total eNOS antibody. Lysates were probed with antibodies against the indicated epitopes. Cell lysates were also immunoblotted for Flag and hemagluttinin (HA) to assess expression of dn p38α and MKK6bE, respectively. (C) BAECs treated as in B underwent assessment of eNOS catalytic activity determined by the conversion of L-[3H]arginine to L-[3H]citrulline as in “Methods.” All blots are representative of 3 independent experiments and composite data are mean ± S.E derived from of 4–8 independent experiments. Legend: *p<0.05 vs LacZ without BTP treatment.
We next investigated the relation between p38 MAPK, ERα, and Akt in this signaling response. BAECs over-expressing MKK6bE exhibited phosphorylation of p38 MAPK, ERα, Akt, and eNOS as well as eNOS catalytic activation (Figs. 5A and B). Treating MKK6bE over-expressing cells with ICI 182,780 had no impact on p38MAPK activation, but this treatment prevented ERα phosphorylation and downstream signaling to Akt and eNOS. Consistent with this observation, BAECs harboring a constitutively active Akt mutant (Myr-Akt) demonstrated eNOS phosphorylation and activation without any effect on ERα or p38 MAPK (Figs. 5A and B).
Figure 5.
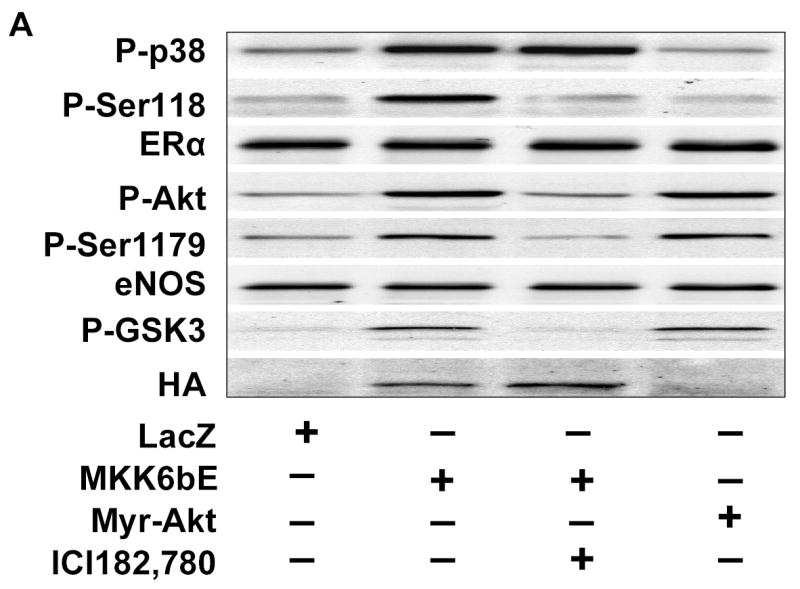
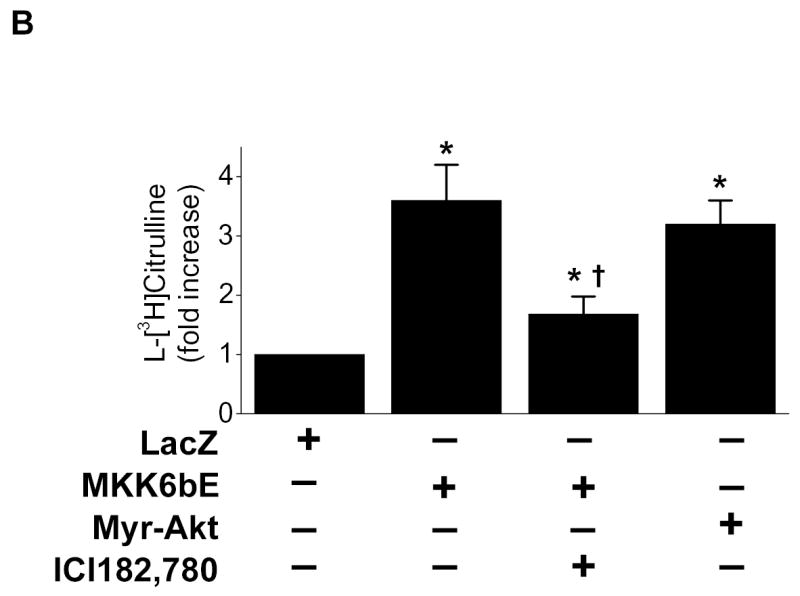
ERα Ser-118 phosphorylation links p38 MAPK to Akt activation. (A) BAECs were transfected with adenoviral vectors expressing LacZ, a constitutively-active MKK6 mutant (MKK6bE) or constitutively-active Akt (Myr-Akt) for 24h. Cells were then equilibrated in HEPES-PSS (30 min) in the presence or absence of ICI 172,780 (100nM) as indicated. Cells were then lysed and the lysates either collected or immunoprecipitated with ERα or eNOS antibody. The phosphorylation status of eNOS or ERα on were assessed in their respective immunoprecipitates by immunoblotting for the indicated epitopes. Lysates were probed for phosphorylation of p38MAPK and Akt as indicated and the expression of MKK6bE or Myr-Akt was estimated by the presence of an HA tag or phosphorylated glycogen synthase kinase 3 (P-GSK3). (B) BAECs treated as in (A) underwent assessment of eNOS catalytic activity determined by the conversion of L-[3H]arginine to L-[3H]citrulline as in “Methods.” (C) HUVECS were subjected to siRNA-mediated ER gene silencing prior to treatment with 100 ng/ml black tea polyphenols (BTP) and then assessed for phosphorylation of p38 MAPK and eNOS as in “Methods.” All blots are representative of 3 independent experiments and composite data are mean ± S.E derived from of 3 independent experiments. Legend: *p<0.05 vs LacZ; †P<0.05 vs MKK6bE alone .
To confirm that p38 MAPK is upstream of ERα, we used siRNA to inhibit ERα expression and found it inhibited black tea polyphenol-induced eNOS Ser-1177 phosphorylation (human sequence) without any effect on p38 MAPK activation (Fig. 5C). Collectively, these data establish a linear signaling cascade from p38 MAPK to ERα, PI 3-K/Akt, and eNOS. Thus, ERα Ser-118 phosphorylation appears critical for signal transduction from p38 MAPK to Akt.
P38 MAPK-Mediated eNOS Activation Requires ERα Ser-118
To establish that black tea polyphenol-induced eNOS activation requires ERα, we conducted experiments in COS-7 cells that lack ERs. In COS-7 cells transfected with eNOS alone, neither estradiol nor black tea polyphenols stimulated eNOS activity, whereas A23187 enhanced eNOS activity by three-fold (Figure 6A). However, in COS-7 cells co-transfected with eNOS and ERα, both estradiol and black tea polyphenols significantly increased eNOS activity and the response to A23187 was not altered (Fig. 6A). Black tea polyphenol-mediated p38 MAPK activation was observed both in the presence and absence of ERα transfection in COS-7 cells (Fig. 6A).
Figure 6.
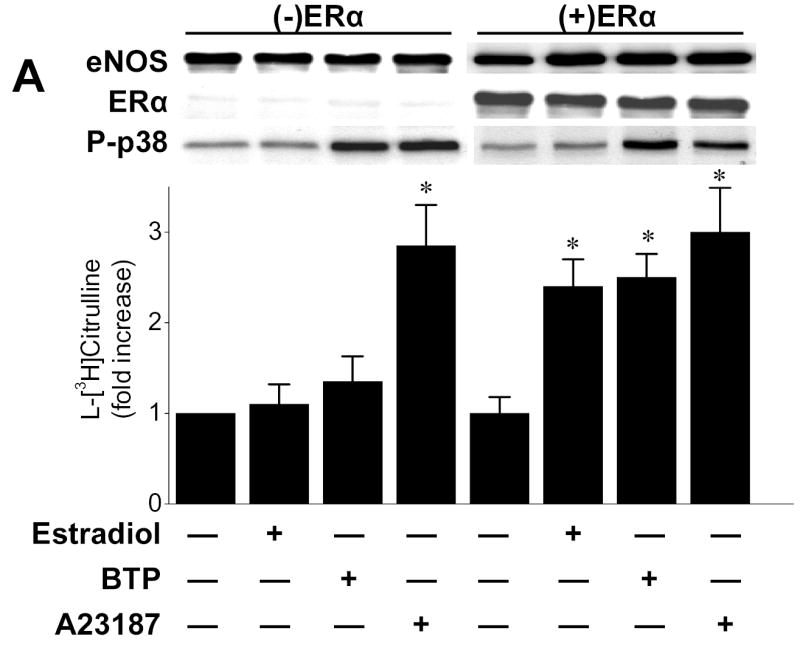
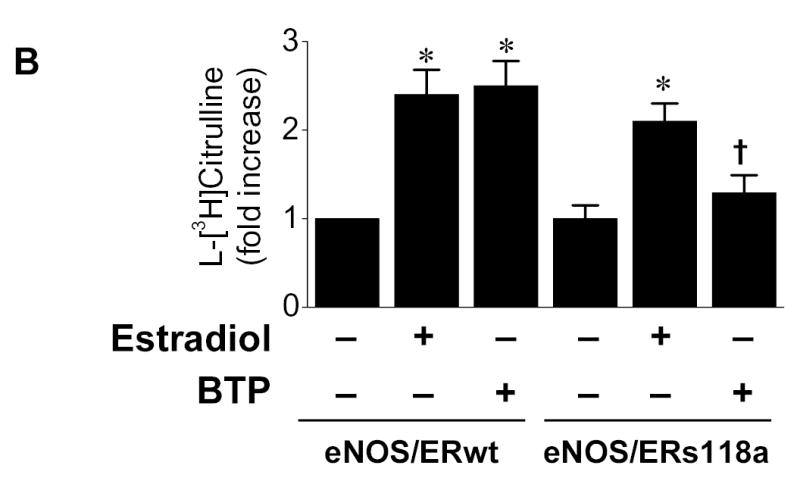
ERα mediates black tea polyphenol-induced eNOS activation in COS-7cells. (A) COS-7 cells were transfected with plasmids expressing eNOS and ERα either alone or in combination. After 36hr, cells were equilibrated in HEPES-PSS (30 min) followed by incubation with buffer alone, black tea polyphenols (BTP; 100ng/ml), estradiol (100nM) or A23187 (1μM) for 15 min. Cells were then assessed for eNOS catalytic activity as described in “Methods.” Cell lysates were probed for eNOS and ERα expression by immunoblotting. (B) COS-7 cells were co-transfected with eNOS and either wild-type (ERwt) or s118a mutant ERα (ERs118a) as in (A). After 36hr, cells were assessed for eNOS catalytic activity as in (A). Blots are representative of 3 independent experiments and composite data are the mean ± S.E of 6–8 independent experiments expressed as the fold-increase compared to vehicle alone. Legend: *p<0.05 vs no additions, †p<0.05 vs ERwt treated with BTP.
To further define the role of ERα Ser-118 in black tea polyphenol-induced eNOS activation, we employed an ERα mutant (ERαs118a) containing an alanine in place of serine at position 118 (ERs118a) that is not subject to phosphorylation.10 As shown in Fig. 6B, COS-7 cells transfected with ERs118a and eNOS showed significantly attenuation of eNOS stimulation in response to black tea polyphenols compared to cells harboring the wild-type ERα. These data indicate that ERα Ser-118 is required for black tea polyphenol-induced eNOS stimulation.
ERα and p38 MAPK Are Functionally Associated in Response to Black Tea Polyphenols
To probe for the existence of a functional complex including both p38 MAPK and ERα, COS-7 cells co-transfected with eNOS and wild-type ERα, were treated with black tea polyphenols and the lysates were immunoprecipitated with antibodies directed against ERα. We then probed the precipitates for p38 MAPK and found a time-dependent increase in the pellet p38 MAPK with a corresponding decrease in supernatant p38MAPK. (Fig. 7A). Similarly, the converse experiment involving immunoprecipitation of p38 MAPK from black tea polyphenol exposed COS-7 cells demonstrated evidence for ERα in the pellet and a corresponding decrease in the supernatant (Fig. 7B). This functional association between p38 MAPK and ERα could not be detected in cells co-transfected with eNOS and ERα(s118a) (Figs. 7C and D). Immunoprecipitation of ERα from BAECs also demonstrated polyphenol-induced complex formation between ERα and p38 MAPK (data not shown). Collectively, these data support the notion that ERα is an upstream mediator of Akt and eNOS activation by black tea polyphenols. Moreover, the data also suggest that p38 MAPK activates ERα in response to black tea polyphenols.
Figure 7.
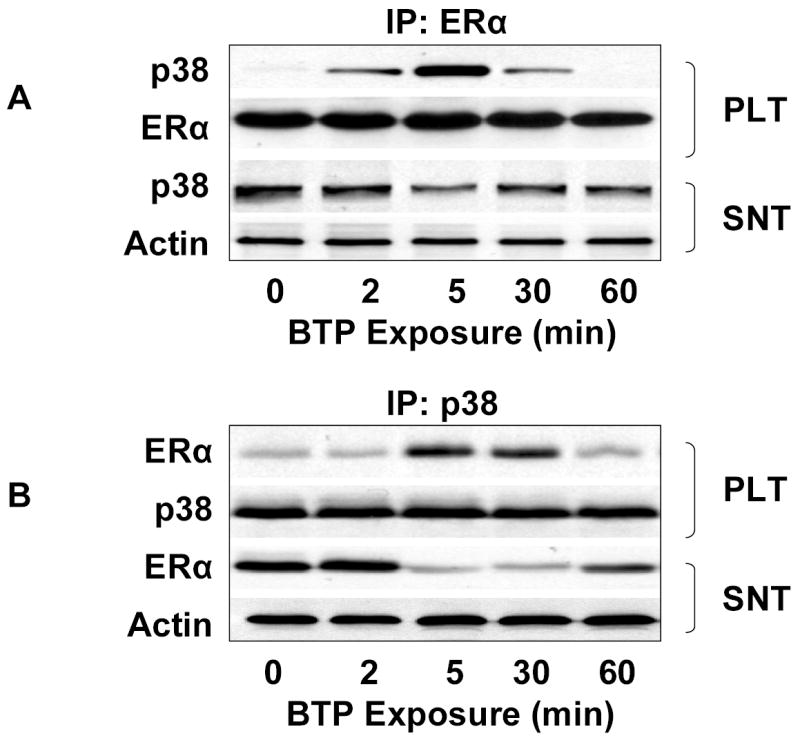
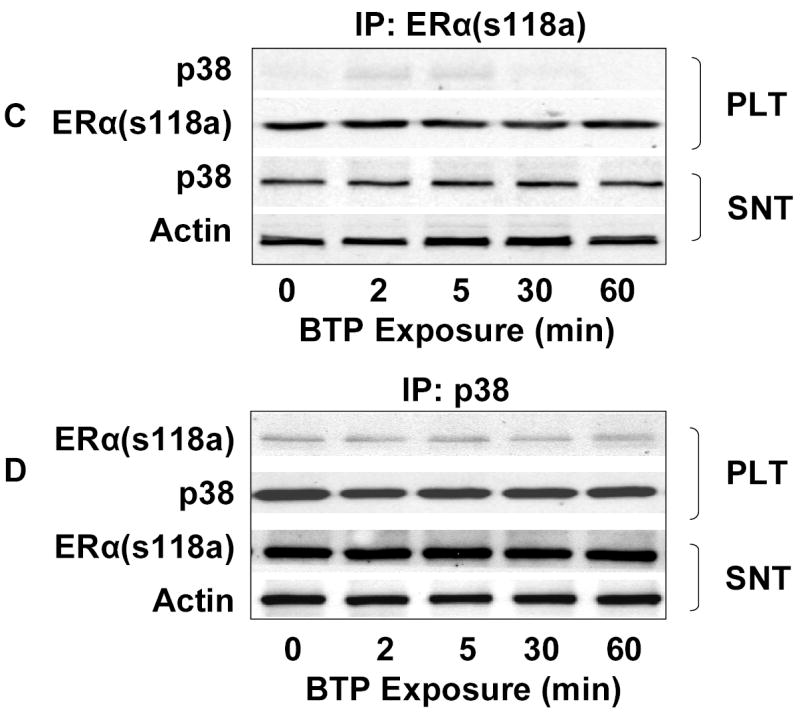
Functional association of p38 MAPK and ERα. (A) COS-7 cells co-transfected with eNOS and ERα were treated with black tea polyphenols (BTP; 100ng/ml) for the indicated times. After treatment, cells were lysed, ERα immunoprecipitated, and both the pellet (PLT) and supernatant (SNT) subjected to immunoblotting as indicated. (B) Cells were treated as in (A) except p38 MAPK and both the pellet and supernatant immunoblotted with antibodies to the indicated epitopes. (C) COS-7 cells were co-transfected with eNOS and mutant ERα (ERαs118a), treated with BTP (100mg/ml) for the indicated times, and subjected to immunoprecipitation and immunoblotting as in (A). (D) COS-7 cells were co-transfected and treated with BTP as in (C) followed by immunoprecipitation and immunoblotting as in (B). All blots are representative of 3 independent experiments.
Discussion
In this study we found that ERα plays a key role in mediating the activation of eNOS in response to black tea polyphenols. In particular, we found that ERα is phosphorylated on Ser-118 in response to black tea polyphenol-mediated p38 MAPK activation. This ERα phosphorylation is required for p38 MAPK downstream signaling to Akt and eNOS that mediates black tea polyphenol-induced eNOS activation. We were able to implicate Ser-118 as a critical residue in this process as we observed ERα Ser-118 phosphorylation in response to black tea polyphenols and mutation of this residue abrogated polyphenol-induced eNOS activation in COS cells. This residue also appeared important for the functional association of p38 MAPK with ERα as mutants lacking this site did not associate with p38 MAPK in response to black tea polyphenols. These data suggest a novel adaptor function of ERα that integrates p38 MAPK signaling with downstream targets.
The rapidity of the response to black tea polyphenols suggests a non-genomic role for ERα in our system. These findings are in agreement with previous studies indicating that ERα mediates non-genomic eNOS activation in response to estradiol.13–15 Ligand engagement of ERα induces its association with the p85α-subunit of phosphoinositol 3-kinase (PI 3-K) and c-Src leading to Akt activation.15,16 This event promotes eNOS phosphorylation at Ser-1177,17 a key event that stimulates enzyme activation18,19 and enhances its sensitivity to calcium.20 The data presented here add to this body of literature by identifying p38 MAPK as an upstream effector of ERα that mediates rapid eNOS activation. Moreover, we have strong evidence that p38 MAPK induces ERα phosphorylation in a ligand-independent manner as molecular activation of p38 MAPK with MKK6bE recapitulated both ERα phosphorylation and eNOS activation in the absence of polyphenols. To our knowledge, this is the first demonstration of rapid, non-genomic effects of ERα stimulation that are ligand-independent. Typically, ligand-independent ERα activation has been restricted to the transcriptional functions of this nuclear hormone receptor in response to stimuli such as epidermal growth factor,21 insulin-like growth factor,21 and the cyclins A22 and D1.23 Thus, the data provided here indicate a novel ERα function as a consequence of ligand-independent activation.
Despite recent reports demonstrating polyphenol-induced ERK activation,24 we did not find a role for ERK in our system (Fig. 3). Possible explanations for this observation might include the fact we used a tea polyphenol extract rather than a red wine extract or authentic resveratrol.24 Moreover, our data fit best with a ligand-independent mechanism rather than polyphenol binding to the estrogen receptor. In this regard, previous data depict ligand-independent ERα activation as an ERK-mediated phenomenon that involves the ERα Ser-118 residue.21 The data presented here add a new facet to this body of literature in that ERα is a target of the p38 MAPK in endothelial cells. There is one prior report of p38 MAPK-mediated ERα phosphorylation in a study that involved endometrial carcinoma cells and Thr-311 as the p38 MAPK target.25 In contrast, our report involves endothelial cells and Ser-118 appears to be the site of phosphorylation in response to black tea polyphenols. This discrepancy could be a function of the different cell types between these studies. Alternatively, it is possible that our study also involved p38 MAPK phosphorylation of ERα on Thr-311 that facilitated ERα Ser-118 phosphorylation by some other, as yet unrecognized kinase. However, we did exclude ERK as mediating Ser-118 phosphorylation in this study, but other kinases do target this site including cdk7.26 Thus, determining the precise molecular events surrounding black tea polyphenol-mediated ERα phosphorylation will require further study.
Evidence from this work does distinguish estradiol-mediated eNOS activation from that observed with black tea polyphenols. Although both agents induce ERα Ser-118 phosphorylation, this event was only critical for polyphenol-induced eNOS activation. In COS-7 cells transfected with ERα harboring an alanine at position 118 (ERαs118a), we observed intact eNOS activation in response to authentic estradiol, whereas the black tea polyphenol response was attenuated compared to wild-type ERα. These data are in keeping with the known role of Ser-118 in mediating ligand-independent ERα function with little role in ligand-dependent responses.10 The results of our study may also be related to the presence of other phosphorylation sites on ERα such as Ser-167, Thr-311 and Ser-522 that were not the subject of this investigation. In this regard, Ser-522 has been proven critical for G-protein-coupled ERα responses whereas Thr-311 appears important for cancer cell-mediated metastasis.25
The data presented here indicate that p38 MAPK has a role in regulating the activity of a nuclear hormone receptor, ERα. Although not described for ERα, there is some precedent for nuclear hormone receptors to be modulated by p38 MAPK. For example, in the setting of cytokine stimulation, PPARγ coactivator-1 is phosphorylated by p38 MAPK and this event regulates the control of genes involved in energy expenditure.27 Phosphorylation of the glucocorticoid receptor by p38 MAPK appears to reduce receptor activity and this may be involved in feedback regulation of the receptor in the setting of inflammation.28 The precise nature of how p38 MAPK interacts with ERα is not clear from this work, but our experiments do suggest these two proteins occur in a common complex in response to polyphenol stimulation. Further investigation will be required to determine the molecular determinants of this event.
In summary, the data presented here indicate that black tea polyphenols stimulates eNOS activity largely through non-genomic ligand-independent activation of ERα in vascular endothelial cells. The mechanisms involve a p38 MAPK-induced phosphorylation of ERα on Ser-118, which in turn leads to the activation of the PI 3-K/Akt pathway and eNOS. Along with the implications regarding estrogen and vascular endothelial function, the present findings are important to the mechanisms underlying the rapid activation of estrogen receptors in vascular endothelium.
Acknowledgments
We thank Nikheil Rau for technical assistance. This work in Dr. Keaney’s laboratory is supported by grants from the National Institutes of Health (DK55656, HL60886, HL67206, HL68758).
References
- 1.Duffy SJ, Vita JA. Effects of phenolics on vascular endothelial function. Curr Opin Lipidol. 2003;14(1):21–7. doi: 10.1097/00041433-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Vita JA. Tea consumption and cardiovascular disease: effects on endothelial function. J Nutr. 2003;133(10):3293S–7S. doi: 10.1093/jn/133.10.3293S. [DOI] [PubMed] [Google Scholar]
- 3.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 4.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. J Am Coll Cardiol. 2003;41(10):1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 5.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long- term outcome of coronary heart disease. Circulation. 2000;101(16):1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 6.Duffy SJ, Keaney JF, Jr, Holbrook M, Gokce N, Swerdloff PL, Frei B, Vita JA. Acute and chronic tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–6. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 7.Anter E, Thomas SR, Schulz E, Shapira OM, Vita JA, Keaney JF., Jr Activation of Endothelial Nitric-oxide Synthase by the p38 MAPK in Response to Black Tea Polyphenols. J Biol Chem. 2004;279(45):46637–43. doi: 10.1074/jbc.M405547200. [DOI] [PubMed] [Google Scholar]
- 8.Chen K, Vita JA, Berk BC, Keaney JF., Jr c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves src-dependent EGF receptor transactivation. J Biol Chem. 2001;276:16045–50. doi: 10.1074/jbc.M011766200. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273(4):2161–8. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 10.Lu Q, Ebling H, Mittler J, Baur WE, Karas RH. MAP kinase mediates growth factor-induced nuclear translocation of estrogen receptor alpha. FEBS Lett. 2002;516(1–3):1–8. doi: 10.1016/s0014-5793(02)02432-8. [DOI] [PubMed] [Google Scholar]
- 11.Chen K, Albano A, Ho A, Keaney JF., Jr Activation of p53 by Oxidative Stress Involves Platelet-derived Growth Factor-{beta} Receptor-mediated Ataxia Telangiectasia Mutated (ATM) Kinase Activation. J Biol Chem. 2003;278(41):39527–33. doi: 10.1074/jbc.M304423200. [DOI] [PubMed] [Google Scholar]
- 12.Joel PB, Traish AM, Lannigan DA. Estradiol and phorbol ester cause phosphorylation of serine 118 in the human estrogen receptor. Mol Endocrinol. 1995;9(8):1041–52. doi: 10.1210/mend.9.8.7476978. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103(3):401–6. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–74. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 15.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407(6803):538–41. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem. 2003;278(4):2118–23. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- 17.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87(8):677–82. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 18.Fulton D, Gratton J, McCabe T, Fontana J, Fujio Y, Walsh K, Franke T, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zelher A. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 20.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem. 2000;275(9):6123–8. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 21.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270(5241):1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 22.Trowbridge JM, Rogatsky I, Garabedian MJ. Regulation of estrogen receptor transcriptional enhancement by the cyclin A/Cdk2 complex. Proc Natl Acad Sci U S A. 1997;94(19):10132–7. doi: 10.1073/pnas.94.19.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuman E, Ladha MH, Lin N, Upton TM, Miller SJ, DiRenzo J, Pestell RG, Hinds PW, Dowdy SF, Brown M, Ewen ME. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17(9):5338–47. doi: 10.1128/mcb.17.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem. 2005;280(9):7460–8. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Bai W. Regulation of estrogen receptor nuclear export by ligand-induced and p38-mediated receptor phosphorylation. Mol Cell Biol. 2002;22(16):5835–45. doi: 10.1128/MCB.22.16.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen D, Washbrook E, Sarwar N, Bates GJ, Pace PE, Thirunuvakkarasu V, Taylor J, Epstein RJ, Fuller-Pace FV, Egly JM, Coombes RC, Ali S. Phosphorylation of human estrogen receptor alpha at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21(32):4921–31. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- 27.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8(5):971–82. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 28.Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol. 2002;109(4):649–57. doi: 10.1067/mai.2002.122465. [DOI] [PubMed] [Google Scholar]


