Abstract
Background and Objectives
Photodynamic therapy (PDT) mediated with Tookad (Pd-bacteriopheophorbide, WST09) was investigated pre-clinically as part of a program to develop an alternative modality for treating prostate cancer.
Study Design/Materials and Methods
Spontaneous canine prostate cancer and normal canine prostate were used as the animal models. Interstitial PDT was performed by IV infusion of the photosensitizer and irradiating the prostates with a diode laser (763 nm). The prostates were harvested 1-week post-PDT and subjected to histopathologic examinations. The effects of the drug doses and light doses were studied for one- and two-session PDT. Pharmacokinetics were studied using HPLC assay. The feasibility of using perfusing CT scans for assessing PDT lesions was also evaluated.
Results
Tookad is a vascular-acting drug and clears rapidly from the circulation. Tookad-PDT-induced lesions, in both normal and cancerous prostates, were characterized by marked hemorrhagic necrosis.
Conclusions
Tookad-PDT is very effective in ablating prostatic tissue through its vascular effects.
Keywords: photodynamic therapy, prostate cancer, Tookad, pharmacokinetics, vascular effects
INTRODUCTION
Prostate cancer is still a significant health problem mainly due to its high incidence, mortality and cost associated with its diagnosis and treatment, and the lack of effective treatment for advanced stages of the disease. In the United States, approximately 230,110 new cases and 29,900 prostate cancer deaths were predicted for 2004, making it the second leading cause of cancer death in men [1]. Surgery, radiation, and cryotherapy or their combination are conventional forms of focal therapy used to remove or destroy cancerous tissue. Significant side effects, such as impotency, urinary incontinence, and injuries to nearby structures are often associated with these therapies [2,3]. High-risk patients are also unlikely to be cured with these standard therapies and are suitable candidates for clinical trials of novel therapy such as hyperthermia, high intensity focused ultrasound (HIFU), or photodynamic therapy (PDT).
PDT involves administering a photosensitizing drug, then activating the drug with light of a particular wavelength. The interaction of light and photosensitizer initiates production of reactive oxygen species (ROS), which ultimately lead to cell death and tissue ablation [4,5]. PDT can destroy tumor tissue by direct killing of tumor cells (by apoptosis or necrosis), by the destruction of tumor blood supply and tumor vasculature, and/or by activating inflammation and immune responses. The advantages of PDT can be summarized as: comparatively non-invasive; accurate targeting; repeated doses can be given without the total-dose limitations associated with radiotherapy; and the healing process results in little or no scarring. PDT procedures can usually be done in an outpatient or day-case setting, it is convenient for the patient and has few side effects [6,7]. The feasibility of using PDT to treat prostate cancer in animal models has been investigated by our research team and several other groups [8,9]. Because of the difficulty in finding and recruiting spontaneous prostate cancer bearing animals, normal canine prostate has been used as the animal model, due to its resemblance in size and anatomical structure to human prostate. Canine prostate PDT mediated by various photosensitizing drugs has been investigated. Previous studies indicate that interstitial PDT has a great potential in the treatment of prostate cancer [10].
The present work was a pre-clinical study utilizing a second-generation photosensitizer, Tookad® (Pd-bacteriopheophorbide, also known as WST09) to ablate prostatic tissue. Our previous studies have shown that Tookad-mediated PDT can destroy a clinically-significant volume of prostatic tissue in both normal and post-radiotherapy prostates [11,12]. In this study, drug/light dose escalation effects and Tookad pharmacokinetics were investigated in an established canine model. The current Tookad-PDT human clinical studies are drug/light dose escalation trials in subjects who have recurred after radical radiotherapy. Some subjects who received low dose Tookad-PDT are eligible to receive a second full dose treatment to achieve possible therapeutic benefits. Therefore, we also evaluated tissue responses to two-session PDT. Perfusion CT scans were evaluated for assessment of PDT-induced acute lesions in a normal prostate. One dog with spontaneously occurring prostate cancer entered this study and underwent Tookad-PDT with multiple fibers. Although many parameters regarding the optimal photosensitizer and light combination to give maximum ablation of malignant tissue still remain to be elucidated, the results obtained from this and previous translational studies have demonstrated a unique vasculature-targeting property of Tookad-PDT and have provided additional valuable data for the rational design of ongoing human clinical trials.
MATERIALS AND METHODS
Animal Model
A total of 19 healthy adult Beagles (2~9 years old, 10~18 kg) and 1 prostate cancer dog were studied. The healthy animals were obtained from licensed vendors (Marshall Bioresources, North Rose, NY or Harlan Farm, Indianapolis, IN). All studies were performed under the guidance of the Institutional Animal Care and Use Committee at HealthONE Alliance and Colorado State University. The length and the width of the prostate in these animals was typically 2×3 cm. Three animals revealed pre-existing chronic prostatitis and one hyperplasia at necropsy.
Photosensitizer
The photosensitizer Tookad® (Pd-bacteriopheophorbide, molecular weight 715, also known as WST09; STEBA BIOTECH, Toussus-Le-Nobel, France) was prepared in a Cremophor EL-P based vehicle. Tookad® (2.5 mg/ml) and administered at 0.25,0.5,1.0, or 2.0 mg/kg via IV infusion, over a 10 minutes period, through the right cephalic vein under dimmed ambient lighting (Table 1).
TABLE 1.
Treatment Scheme and Number of Treated Lobe(s) for Single-Session PDT
| Drug dose (mg/kg)
|
|||||
|---|---|---|---|---|---|
| Light dose (J/cm) | 0 | 0.25 | 0.5 | 1.0 | 2.0 |
| 0 | 1a | 0 | 0 | 0 | 0 |
| 50 | 0 | 0 | 0 | 1 | 1 |
| 100 | 0 | 1 | 1 | 3 | 4 |
| 200 | 1 | 1 | 1 | 5 | 4 |
| 300 | 0 | 0 | 0 | 1 | 0 |
Number of animals.
Light Source and PDT Procedures
The light source was a portable 763 nm diode laser (Ceralas; CeramOptec GmbH of Biolitec AG, Bonn, Germany). The laser output was directly coupled into a Y-splitter (Fiber Splitter-400 micron, Ocean Optics, Inc., Dunedin, FL) and two optical fibers that allowed both lobes to be treated with an identical light fluence rate (150 mW/cm). The interstitial irradiation was delivered through a diffuser tip of cylindrical fiber (10 mm active length, 1.3 mm outer diameter; Model CD 603-10C; CeramOptec GmbH). The diffuser tip was inserted in the anterior section and approximately 1 cm from the prostatic urethra. Light was applied during drug infusion if the irradiation time was shorter than infusion time (e.g., 50 J/cm) or simultaneously at the onset of drug infusion if the irradiation time was longer than the infusion time (e.g., 100–300 J/cm) (Table 1).
Two-Session PDT
Four animals underwent two separate PDT procedures (Table 2). The first was carried out at a drug dose of 0.25 or 1 mg/kg. Both lobes received an identical light dose in the first session (50 or 100 J/cm). The second was carried out 12–13 weeks later at a drug dose of 2 mg/kg. Both lobes received a different light dose in the second session (100 or 200 J/cm). In both sessions, the diffuser fiber was inserted in the same location.
TABLE 2.
Treatment Scheme of Two-Session PDT
| Treated site | Left | Right | Left | Right | Left | Right | Left | Right |
|---|---|---|---|---|---|---|---|---|
| First session | ||||||||
| Drug dose (mg/kg) | 0.25 | 1.0 | 0.25 | 1.0 | ||||
| Light dose (J/cm) | 50 | 100 | ||||||
| Interval between two treatments (weeks) | 12 | 12 | 13 | 13 | ||||
| Second session | ||||||||
| Drug dose (mg/kg) | 2.0 | |||||||
| Light dose (J/cm) | 200 | 100 | 200 | 100 | 100 | 200 | 100 | 200 |
Pre-Medication
In previous studies, Benadryl (IV, 0.7–1.4 mg/kg) and Dexamethasone (SQ, 2 mg per dog) were given immediately prior to the photosensitizer infusion to counteract the effects of the co-solvent Cremophor EL-P on blood pressure [11]. Alternatively, the same drugs were given 24 hours prior to the photosensitizer infusion to further minimize the side effects on blood pressure.
Surgical Procedures
Standard sterilization procedures were strictly followed. All surgical instruments were autoclaved and invasive probes chemically sterilized. As an extra precaution, the dogs received antibiotics before and after surgery (IM, Ampicillin, 20 mg/kg) to prevent possible infection. Pain control consisted of pre-operative and post-operative injection of morphine with long-term control provided by Fentanyl patches. All dogs were prepared for surgery following a standard canine laparotomy procedure [11]. Immediately following PDT procedures, the abdominal muscles, fascia, and skin were closed with interrupted sutures. After surgery, the dogs were kept in dimmed ambient lighting for 2–4 hours. Urinalysis was performed on all treated animals.
Pharmacokinetics
Blood samples (~3 ml) were taken at timed intervals from the left jugular vein prior to, during, and post-drug infusion. The samples were immediately transferred to tri-sodium citrate pre-treated polypropylene tubes in the dark. Plasma extracts were collected after centrifugation and stored at −70°C until HPLC analysis. Tookad concentrations of the plasma extracts were determined by a reversed phase HPLC system (Waters 600) using an Intersil C8 column (Interchim, Monflucon, France) and a UV detector (Waters 2487).
Treatment of Canine Prostate Cancer
With written owner consent, one dog (Beagle-Hound mix, 13.6 kg, ~12 years old) with spontaneous advanced primary prostate cancer, deemed unsuitable for other treatments, entered this investigational study. The dog was prepared for surgery and PDT followed the standard canine laparotomy procedure described above. The enlarged cancerous prostate (9 × 10 × 8 cm) contained several heterogeneous neoplasm masses and cysts. The left lobe was much larger than the right lobe. Three cylindrical fibers with a diffuser tip of 2,2.5, and 5 cm were placed in the left lobe and one with a diffuser tip of 2.5 cm was placed in the anterior section of the right lobe. The distance between each fiber was approximately 3 cm. Tookad® (2 mg/kg) was administered by slow IV infusion (0.5 ml/minute). Light was applied 5–15 minute after the completion of the infusion. Each treatment area received a total light dose of 100–150 J/cm over a period of 20–40 minutes. Approximately 1/3 of the enlarged prostate was treated. The dog was survived 1-week post-surgery and the prostate was harvested for histopathologic examination. The size of the prostate was measured prior to PDT and at necropsy.
Histopathologic Examination
Treated animals were euthanized at 1 week after PDT, using barbiturate overdose. At necropsy, the prostates were harvested and fixed in 10% neutral buffered formalin for >24 hours. The prostate was dissected into 3 mm blocks, photographed and embedded in paraffin. Sections of 5 μm thickness were stained with standard H&E to examine the histopathological changes. Dyer’s Verhoeff variation stain was performed on two-session PDT specimens to assess fibrosis and collagen damage. Factor VIII related antigen immunostain was performed on the control and one treated prostate for confirmation of microvascular damage. Immunostaining reagents were obtained from Ventana Medical Systems (Tucson, AZ).
Prostate Perfusion Scan
Synchronous vascular injection planning was performed on one healthy dog prior to PDT and at 2 and 7 days post-PDT of 1 mg/kg and 100–200 J/cm at 150 mW/cm. Helical CT images were acquired from the sacral promontory to the ischial tuberosities at 5 mm collimation with 1 second scan time (120 kV and 125 mA; Picker PQ 2000, Philips, Bothell, WA) prior to contrast injection. Following a rapid intravenous administration of non-ionic iodinated contrast medium (600 mg/kg, Hypaque-370, 5 ml/second), high temporal resolution scans were obtained at a single level (5 mm slice thickness) of the prostate every 1.8 seconds for a total of 3 minutes.
RESULTS
Pre-Medication and Blood Pressure Control
It was known that Cremophor EL-P, a co-solvent of Tookad solution, can cause a marked blood pressure drop in dogs. This side effect was easily controlled by pre-medication with an antihistamine (Benadryl) and steroids (Dexamethasone) prior to Tookad® infusion and the adjustment of IV fluid volume and anesthetic dose during PDT. In this study, a total of 16 animals received Benadryl and Dexamethasone 24 hours prior to and immediately prior to Tookad® infusion and only 2 animals (12.5%) showed a mild blood pressure drop during PDT.
Pharmacokinetics
Plasma extracts were collected and analyzed by HPLC to determine the plasma Tookad concentration. The pharmacokinetics data indicate a plasma concentration peak at the end of drug infusion and a subsequent rapid clearance (Fig. 1). The correspondent maximum plasma concentrations were approximately 0.5, 4.6, and 5.9 μg/ml for drug dose levels of 0.25,1.0, and 2.0 mg/kg, respectively.
Fig. 1.
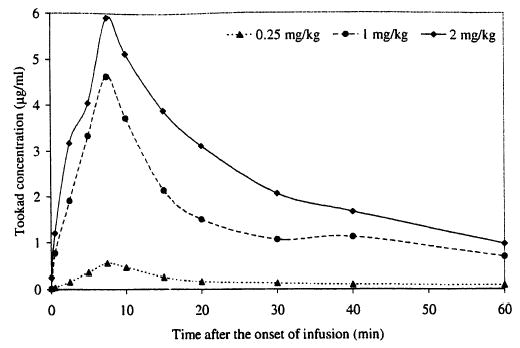
Tookad pharmacokinetics. Data points are averages (0.25 mg/kg, n = 2; 1 mg/kg, n = 6; 2 mg/kg, n = 2).
Post-Surgical Observations
All control animals that received either light only or drug only, and all dogs in the PDT group survived the 1-week post-surgical/PDT period without incident. The surgical wound healed well in all treated dogs, with no post-PDT urethral complications. None of the dogs showed urinary retention and urinary catheterization was not needed. Urinalysis showed trace blood during the first 24–48 hours post-surgery/PDT, but none of the animals required medical attention or treatment.
Histopathologic Findings
At 1-week post-treatment, the single-session PDT-induced lesions were characterized by hemorrhagic necrosis with marked edema. Tookad-PDT-induced lesions were well delineated from the adjacent normal tissue and unaffected tissue. The zone of necrosis increased with the increasing light and drug doses (Fig. 2). Necrotic lesions could be induced at a very low drug dose (e.g., 0.25 mg/kg) at a light dose of 200 J/cm but not at 100 J/cm. On H&E sections examined by light microscopy, marked hemorrhagic necrosis and atrophy of the glandular tissue in the treated areas were seen at 1 week post-PDT. The boundary of the lesions was sharply denned, suggesting a PDT response threshold. The non-glandular tissue (e.g., fibromuscular stroma) appeared to be damaged in the same way as the glandular tissue, characterized by marked degeneration. A total destruction of peripheral glandular structures and capsular layers was observed when the necrotic lesions reached beyond the capsule. For cases in which the PDT lesion extended to the prostatic urethra, only mild submucosa congestion and small areas of epithelial disruption were observed within one tissue block of 3-mm thickness. Similar histopathologic changes were seen in glands with pre-existing chronic prostatitis or hyperplasia. Factor VIII stain indicated a complete destruction of micro-vessels within the necrotic regions of the prostate (Fig. 3).
Fig. 2.
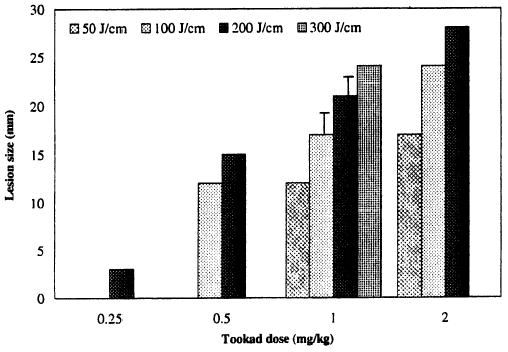
Lesion size as a function of photosensitizer dose and light dose. Note that some data points are based on a single measurement.
Fig. 3.
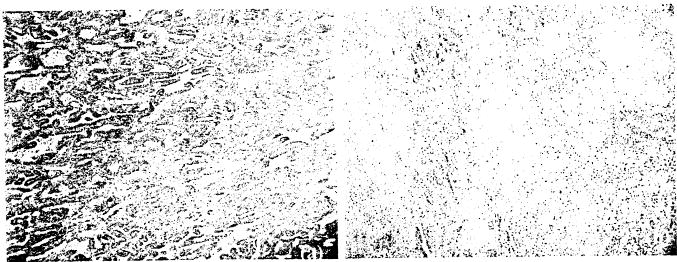
Effect of Tookad-PDT on microvessels. Microvessels are labeled by Factor VIII immunostain. Original red-colored vessels are indicated by the white marks in these black and white photographs, a: Control, b: PDT-treated region. PDT protocol: 2 mg/kg, 150 mW/cm, and 50 J/cm. Note the severe necrosis and the absence of microvessels in the PDT-treated region. (Image sizes ~1 mm2.)
The dissected view at 1-week post the two-session treatment, showed marked hemorrhage and necrosis induced from the second treatment. The lower doses of the first treatment (e.g., 0.25 mg/kg and 50–100 J/cm, or 1 mg/kg and 50 J/cm) did not affect the zone of the necrosis of the second treatment. The higher doses of the first treatment (e.g., 1 mg/kg and 100 J/cm) showed some effects on the second treatment. Gross examination showed a reduction in the zone of the necrosis and residual fibrosis from the first treatment. Microscopic examination showed both patchy and diffused hemorrhage. A mixture of degenerated fibrotic tissue and residual collagen deposition were co-existing in the necrotic region.
Response of Canine Prostate Cancer to Tookad-PDT
One dog suffering from spontaneous prostate cancer was treated with Tookad-PDT. The dog survived 1-week post-surgical and PDT procedures. Gross changes included necrosis and a 25% volume reduction in cancerous nodules of the partially treated area (i.e., 1/3 of the enlarged gland). Unfortunately, the untreated tumor had also spread outside the capsule and had invaded the soft tissues surrounding the prostate. Invasion of the urethra at the neck and into the bladder was responsible for post-surgery urinary leakage. Although 1/3 of the prostate underwent a palliative treatment with multiple diffuser fibers (n = 4) with longer active tips (2–5 cm) in this case, the dog survived only 1 week due to disease progression. H&E stain showed PDT-induced severe necrosis in cancerous glandular tissue, which indicated that the destruction of cancerous prostate could be achieved by Tookad-PDT (Fig. 4).
Fig. 4.
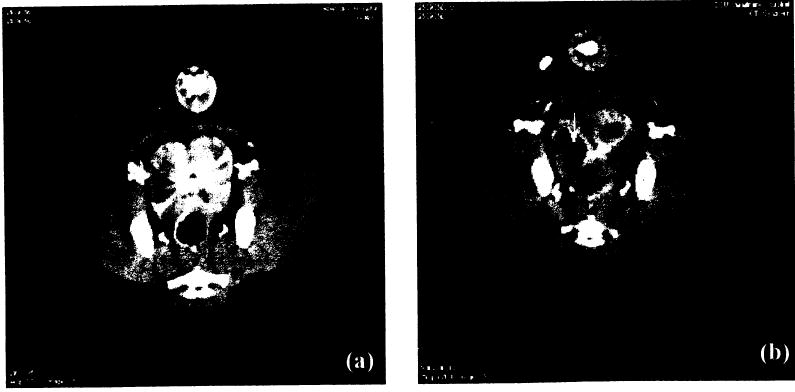
Blood perfusion CT scan, a: Pre-PDT and (b) 48 hours post-PDT, PDT protocol: 2 mg/kg, 150 mW/cm; right lobe −200 J/cm and left lobe −100 J/cm. PDT-induced necrosis is clearly visible and the lesion size correlates well with the light dose. The arrow indicates a cavitationary lesion.
Prostate Perfusion Scan
Post-PDT conventional CT scans showed changes in the prostate shape and size but did not show the PDT-induced tissue damage. The blood perfusion scan performed at 48 hours and 1 week post-PDT clearly revealed PDT-induced necrosis (Fig. 5). Margins of necrotic areas can be determined without further image analysis. Detailed perfusion analysis and mapping were performed with perfusion software. The blood perfusion analysis showed dramatic changes in blood flow, blood volume, and permeability 48-hours post-PDT (data not shown). However, simple image analysis, such as a peak enhancement measurement with threshold, may be adequate for evaluating the changes in blood flow and blood volume after Tookad-PDT. The right lobe (200 J/cm) showed a cavitationary lesion. The cavitation and pre-existing hyperplasia were confirmed by postmortem examination.
Fig. 5.
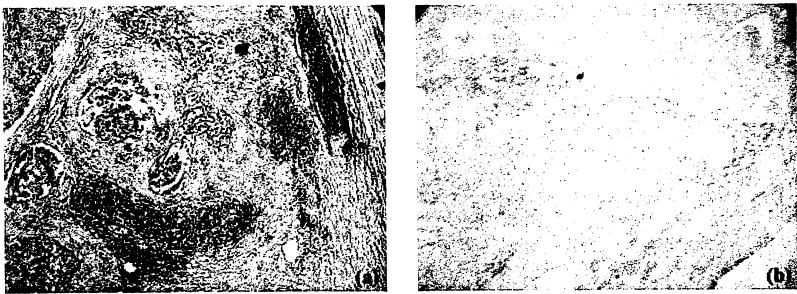
Response of prostate cancer to Tookad-PDT. a: A cancerous area adjacent to the PDT-treated region, and (b) PDT-induced lesions inside the treated region. Tookad-PDT (2 mg/kg, 100 J/cm) induced a complete destruction of cancerous glandular tissue. (Photographs are reduced from 40×.)
DISCUSSION
Standard prostate cancer therapies sometimes fail to provide satisfactory treatment results, while the side effects often significantly degrade the quality of life of the patient. A localized cancer treatment modality, as an alternative in the management of prostate cancer with the maximal protection of surrounding structures, is therefore desired. The application of PDT to treat prostate cancer in animal models as well as human trials has been investigated for the past decade. However, the implementation of PDT in prostate cancer treatment has been limited by the inaccurate or insufficient control of treatment volume, mainly due to inconsistent optical dosimetry and poor pharmacokinetics of currently available photosensitizer(s). Our previous studies suggest that the vascular-acting photosensitizer Tookad might overcome these problems and, thereby, provide an alternative/adjunctive modality to treat primary and recurrent prostate cancer [11,12]. This study investigated Tookad pharmacokinetics and drug/light dose escalation effects in the canine model. The effectiveness of Tookad-PDT for ablating cancerous prostate was further demonstrated in a spontaneously-occurring canine prostate tumor. The observations obtained from this study are directly relevant to designing and optimizing future clinical use of Tookad-PDT for prostate cancer treatment.
Canine prostate tissue-responses to PDT mediated by various photosensitizers have been investigated [13–19] and the general consensus is that, given a fixed optical dose, the value of tissue dynamic light fluence and the volume of tissue damage have been rather unpredictable. In contrast, the value of tissue dynamic light fluence measured during Tookad-PDT is relatively consistent, at least in normal prostate. It is estimated that, with 200 J/cm interstitial irradiation, 1.5 cm radius lesion, 3 mm attenuation depth, and assuming a few mm range for fluence build-up due to backscatter, an upper estimate of the light fluence at the PDT lesion boundary is ~20 J/cm2 [11,12] for large prostates. It is critical that the sensitivity of the adjacent structures (bladder, underlying colon, abdominal muscle, and nerve-vessel bundles, etc) to PDT be carefully evaluated in the process of total ablation of the prostate gland.
Nevertheless, it is also encouraging that the effect of Tookad-PDT on the prostatic urethra was minimal, both functionally and structurally, even when the urethra was within the treatment zone and its adjacent periurethral glandular and stroma tissues were destroyed. This was not the case with Photofrin or other previously-tested photosensitizers [8,13–15,18,19], and may be related to the mechanisms of vascularly-targeted damage. The apparent resistance of prostatic urethra to Tookad-PDT and the feasibility of transurethral PDT need to be further investigated.
In contrast to many photosensitizers being investigated clinically for prostate cancer [20–23], Tookad is believed to be purely vascularly mediated. The clearance of Tookad® from the circulation is very fast and its plasma half-life is less than a few hours in the canine model (see Fig. 1). The results indicate a short period of build-up of Tookad® in the circulation from the onset of drug infusion and also a relatively short PDT treatment time window. Consistent with this, rapid clearance has been observed in whole blood Pd assay using mass spectroscopy [24] and in several other tissues (including tumor and skin) measured in other in vivo animal and human models [25,26], For interstitial prostate PDT, this has the significant practical advantage that the light may be delivered during, or shortly after, photosentitizer drug administration to complete PDT as a single operative session in a short period. The fast clearance also significantly reduces the risk of phototoxicity. Hence, post-treatment management should be considerably simplified for a patient receiving Tookad-PDT. It has been recently demonstrated that there is little uptake of Tookad® by tumor but a gradual accumulation of Tookad ® in the liver of a mouse model [27]. The uptake of Tookad, in the prostate gland and prostatic urethral mucosa needs to be investigated in further studies.
One of the advantages of Tookad-PDT is its capability of being activated at a relatively long wavelength (763 nm), with corresponding greater light attenuation depth (~4 mm) in prostatic tissue [11]. Tookad-PDT induces much larger prostate lesions than other photosensitizers. Figure 2 shows a drug/light dose escalating effect in a simultaneous light irradiation mode, suggesting that Tookad-PDT can be delivered under precise control and, therefore, a predictable treatment outcome can be expected. This may, of course, depend on the heterogeneity of tissue response in the case of tumor-bearing prostate. At the same drug dose and light dose, the simultaneous light irradiation mode produced a similar degree of necrotic lesion as the delayed light irradiation mode (e.g., 5 or 15 minutes after the completion of infusion [11]). The drug infusion-light irradiation interval (DLI) can also be set in a “peaks-overlapping mode,” so that the maximal plasma drug concentration and the mid point of the light irradiation overlap to achieve maximal effectiveness. This overlapping regimen is also now used in the ongoing Phase I/II clinical studies.
In two-session Tookad-PDT, the lesion characteristics of a combination of low drug/light dose of the 1st treatment and a high drug/light dose of the 2nd treatment are identical to those of single, high light dose/high drug dose treatment. The previous low dose treatment has little effect on the second PDT session. However, the typical lesion pattern from a combination of an intermediate drug/light dose in the 1st treatment and a high drug dose/high light dose in the 2nd treatment is a mixture of severe necrosis, patchy, and diffused hemorrhage from the 2nd treatment and some residual fibrosis from the 1st treatment. This indicates that the second treatment can also destroy fibrotic tissue induced by the first treatment. This is consistent with our previous findings that Tookad-PDT can destroy both glandular and non-glandular tissue (e.g., fibromuscular stroma). It is expected that the second session of higher doses will provide a therapeutic effect for patients who undergo two-session PDT trials.
The nature of pure vascular effects of Tookad-PDT on the prostate has been demonstrated in this model for the first time by a simple perfusion CT scan. Tookad-PDT induces rapid and marked changes in blood flow and blood volume (see Fig. 4) inside the treated region. Similarly, diffusion-weighted MRI scans have also been evaluated and preliminary results indicate that they are very useful for early imaging of Tookad-PDT-induced lesions (unpublished data). A recent study of xenograft mouse models has confirmed that the diffusion-weighted MRI is able to detect tumor vascular response to Tookad-PDT within 7 hours post-PDT [28]. These tests demonstrate that Tookad-PDT can severely impair vascular architecture and shut down blood supply to the treatment region. Complete microvessel destruction inside the treatment region has also been confirmed by Factor VIII immunostaining (see Fig. 3).
Although healthy canine prostate is widely used for evaluating PDT feasibility and efficacy for human prostate cancer, it is rare to use PDT to treat spontaneous canine prostate cancer. The first case of treating spontaneous canine prostate cancer with ALA-PDT has been reported (without postmortem examination) [29]. Here, the effectiveness of Tookad-PDT on prostate cancer was evaluated in one dog with spontaneous occurring prostate cancer. Figure 5 shows the response of advanced prostate cancer to Tookad-PDT. Although the palliative treatment with multiple diffuser fibers was intended to relieve symptoms, the dog survived only 1 week due to disease progression. However, a complete destruction of cancerous tissue and reduction of prostate volume within the treated area was achieved with a total light dose of 100–150 J/cm. This demonstrates, for the first time, that Tookad-PDT can be effective in destroying prostate cancer, although a selective destruction of tumor is not a prerequisite for PDT to be applied successfully in prostate cancer patients. Our earlier studies reported mainly the response of normal and pre-radiotherapy prostates to Tookad-PDT. Now extending this, the present study indicates that the normal, preexisting chronic prostatitis or hyperplasia, as well as malignant prostatic tissue, respond in a similar manner. This is consistent with previous reports that, if all other treatment parameters are identical, PDT is likely to be equally effective in destroying normal tissue and the embedded cancerous tissue arising from the same tissue of origin [30–35].
It is known that Cremophor EL-P, the co-solvent of Tookad formulation for intravenous injection, can induce a marked blood pressure drop in canines, due to the anaphylactoid reaction in this species [36]. This side effect can be easily controlled by pre-medication with an antihistamine (Benadryl, 0.7–1.4 mg/kg IV) and steroids (Dexamethasone, 2 mg SQ) and the adjustment of IV fluid volume and anesthetic levels. However, earlier pre-medication, for example 24 hours prior to Tookad-PDT, can significantly reduce the incidence of Cremophor EL-induced blood pressure change. The administration of steroids might also play a role in preventing a potential urethral obstruction after PDT [37].
In conclusion, these results suggest that Tookad-PDT is able to destroy both normal and malignant prostate tissue. This supports the approach being used in current Phase I/II clinical trials of Tookad-PDT for prostate cancer [38,39], in which the intent is to destroy the whole prostate gland, including any cancer within it. However, clearly more extensive studies in spontaneous canine prostate cancer are required in order to quantify any selective response of tumor compared to normal prostate tissues. It is also important that the possible effects of the interstitial PDT on adjacent structures of the prostate gland be further investigated in order to preserve prostate nerve and minimize adverse effect on sexual and urinary functions in the process of total ablation [40].
Acknowledgments
This project was supported in part by NEGMA-LERADS and STEBA BIOTECH (France) and by an NIH grant P01-CA43892. Authors are grateful to Dr. P. Philpott, Dr. J. Sutherland, and Dr. B. Powers for histopathologic analysis, and B. Arcenaux, R. Park, and M. Haider for CT scans and image analysis.
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ American Cancer Society. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Catalona WJ. Management of cancer of the prostate. New Eng J Med. 1994;331:996–1004. doi: 10.1056/NEJM199410133311507. [DOI] [PubMed] [Google Scholar]
- 3.Stamey TA. Irradiation as primary treatment for prostate cancer. Am Urol Assoc Today. 1993;6:14. [Google Scholar]
- 4.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photpbiol. 1992;55:145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 5.Luksiene Z. Photodynamic therapy: Mechanism of action and ways to improve the efficiency of treatment. Medicina (Kaunas) 2003;39:1137–1150. [PubMed] [Google Scholar]
- 6.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 7.Sibata CH, Colussi VC, Oleinick NL, Kinsella TJ. Photodynamic therapy in oncology. Expert Opin Pharmacother. 2001;2:917–927. doi: 10.1517/14656566.2.6.917. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Wilson BC, Shetty SD, Patterson MC, Cerny JC, Hetzel FW. Changes in in vivo optical properties and light distributions in normal canine prostate during photodynamic therapy. Radiat Res. 1997;47:86–91. doi: 10.2307/3579447. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Hetzel FW. Laser dosimetry studies in the prostate. J Clin Laser Med Surg. 1998;16:9–12. doi: 10.1089/clm.1998.16.9. [DOI] [PubMed] [Google Scholar]
- 10.Muschter R. Photodynamic therapy: A new approach to prostate cancer. Current Urol Reports. 2003;4:221–228. doi: 10.1007/s11934-003-0073-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Huang Z, Luck D, Beckers J, Brun P, Wilson B, Scherz A, Salomon Y, Hetzel FW. Preclinical studies in normal canine prostate of a novel palladium-bacteriopheophorbide (WST09) photosensitizer for photodynamic therapy of prostate cancer. Photochem Photobiol. 2002;76:88–95. doi: 10.1562/0031-8655(2002)076<0438:PSINCP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Chen Q, Trncic N, LaRue SM, Brun PH, Wilson BC, Shapiro H, Hetzel FW. Effects of Pd-bacteriopheophorbide (TOOKAD)-mediated photodynamic therapy on canine prostate pretreated with ionizing radiation. Radiat Res. 2004;161:723–731. doi: 10.1667/rr3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang S, Buonaccorsi G, MacRobert A, Bown SG. Interstitial and transurethral photodynamic therapy of the canine prostate using Meso-Tetra-(m-Hydroxyphenyl) Chlorin. Int J Cancer. 1996;67:555–562. doi: 10.1002/(SICI)1097-0215(19960807)67:4<555::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Chang S, Buonaccorsi G, MacRobert A, Bown SG. Interstitial photodynamic therapy in the canine prostate with disulfonated aluminum phthalocyanine and 5-aminolevulinic acid-induced protoporphyrin IX. Prostate. 1997;32:89–98. doi: 10.1002/(sici)1097-0045(19970701)32:2<89::aid-pros3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Hsi RA, Kapatkin A, Strandberg J, Zhu T, Vulcan T, Solonenko M, Rodriguez C, Chang J, Saunders M, Mason N, Hahn S. Photodynamic therapy in the canine prostate using motexafin lutetium. Clin Cancer Res. 2001;7:651–660. [PubMed] [Google Scholar]
- 16.Jankun J, Lilge L, Douplik A, Keck RW, Pestka M, Szkudlarek M, Stevens PJ, Lee RJ, Selman SH. Optical characteristics of the canine prostate at 665 nm sensitized with tin etiopurpurin dichloride: Need for real-time monitoring of photodynamic therapy. J Urol. 2004;172:739–743. doi: 10.1097/01.ju.0000135304.96496.20. [DOI] [PubMed] [Google Scholar]
- 17.Lee LK, Whitehurst C, Pantehdes ML, Moore JV. Interstitial photodynamic therapy in the canine prostate. Br J Urol. 1995;80:898–902. doi: 10.1046/j.1464-410x.1997.00460.x. [DOI] [PubMed] [Google Scholar]
- 18.Selman SH, Keck RW, Hampton JA. Transperineal photodynamic ablation of the canine prostate. J Urol. 1996;156:258–260. [PubMed] [Google Scholar]
- 19.Selman SH, Albrecht D, Keck RW, Brennan P, Kondo S. Studies of Tin Ethyl Etiopurpurin photodynamic therapy of the canine prostate. J Urol. 2001;165:1795–1801. [PubMed] [Google Scholar]
- 20.Nathan TR, Whitelaw DE, Chang SC, Lees WR, Ripley PM, Payne H, Jones L, Parkinson MC, Emberton M, Gillams AG, Mundy AR, Bown SG. Photodynamic therapy for prostate cancer recurrence after radiotherapy: A phase I study. J Urol. 2002;168:1427–1432. doi: 10.1016/S0022-5347(05)64466-7. [DOI] [PubMed] [Google Scholar]
- 21.Whitehurst C, Pantelides ML, Moore JW, Brooman PJ, Blacklock NJ. In vivo laser light distribution in human prpstatic carcinoma. J Urol. 1994;151:1411–1415. doi: 10.1016/s0022-5347(17)35270-9. [DOI] [PubMed] [Google Scholar]
- 22.Windahl T, Andersson SO, Lofgren L. Photodynamic therapy of localized prostatic cancer (letter) Lancet. 1990;336:1139. doi: 10.1016/0140-6736(90)92626-s. [DOI] [PubMed] [Google Scholar]
- 23.Zaak D, Sroka R, Höppner M, Khoder W, Reich O, Tritschler S, Muschter R, Knüchel R, Hofstetter A. Photodynamic therapy by means of 5-ALA induced PPIX in human prostate cancer—preliminary results. Med Laser Appl. 2003;18:91–95. [Google Scholar]
- 24.Huang Z, Chen Q, Brun PH, Wilson BC, Scherz A, Salomon Y, Luck D, Beckers J, Hetzel FW. Studies of a novel photosensitizer Pd-bacteriopheophorbide (Tookad) for the prostate PDT in canine model. SPIE Proc. 2003;5254:83–90. [Google Scholar]
- 25.Zilberstein J, Schreiber S, Bloemers MC, Bendel P, Neeman M, Schechtman E, Kohen F, Scherz A, Salomon Y. Anti vascular treatment of solid melanoma tumors with bacterio-chlorophyll-serine-based photodynamic therapy. Photochem Photobiol. 2001;73:257–263. doi: 10.1562/0031-8655(2001)073<0257:atosmt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Weersink RA, Forbes J, Bisland S, Trachtenberg J, Elhilali M, Brun PH, Wilson BC. Assessment of cutaneous photosensitivity of TOOKAD (WST09) in pre-clinical animal models and in patients. Photochem Photobiol. 2005;81:106–113. doi: 10.1562/2004-05-31-RA-182. [DOI] [PubMed] [Google Scholar]
- 27.Bourre L, Thibaut S, Briffaud A, Rousset N, Elepuet S, Lajat Y, Patrice T. Indirect detection of photosensitizer ex vivo. J Photochem Photobiol B. 2002;67:23–31. doi: 10.1016/s1011-1344(02)00279-8. [DOI] [PubMed] [Google Scholar]
- 28.Plaks V, Koudinova N, Nevo U, Pinthus JH, Kanety H, Eshhar Z, Ramon J, Scherz A, Neeman M, Salomon Y. Photodynamic therapy of established prostatic adenocarcinoma with TOOKAD: A biphasic apparent diffusion coefficient change as potential early MRI response marker. Neoplasia. 2004;6:224–233. doi: 10.1593/neo.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucroy MD, Bowles MH, Higbee RG, Blaik MA, Ritchey JW, Ridgway TD. Photodynamic therapy for prostatic carcinoma in a dog. J Vet Intern Med. 2003;17:235–237. [PubMed] [Google Scholar]
- 30.Barr H, Krasner N, Boulos PB, Chatlani P, Bown SG. Photodynamic therapy for colorectal cancer: A quantitative pilot study. Br J Surg. 1990;77:93–96. doi: 10.1002/bjs.1800770132. [DOI] [PubMed] [Google Scholar]
- 31.Bedwell J, MacRobert AJ, Phillips D, Bown SG. Fluorescence distribution and photodynamic effect of ALA-induced PpIX in the DMH rat colonic tumor model. Br J Cancer. 1992;65:818–824. doi: 10.1038/bjc.1992.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen JY, Mak NK, Wen JM, Leung WN, Chen SC, Fung MC, Cheung NC. A comparison of the photodynamic effects of temoporfin (mTHPC) and MC540 on leukemia cells: Efficacy and apoptosis. Photochem Photobiol. 1998;68:545–554. [PubMed] [Google Scholar]
- 33.Regula J, Ravi B, Bedwell J, MacRobert AJ, Bown SG. Photodynamic therapy using 5-aminolaevulinic acid for experimental pancreatic cancer-prolonged animal survival. Br J Cancer. 1994;70:248–254. doi: 10.1038/bjc.1994.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Geel IP, Oppelaar H, Oussoren YG, van der Valk MA, Stewart FA. Photosensitizing efficacy of MTHPC-PDT compared to Photofrin-PDT in the RIF1 mouse tumour and normal skin. Int J Cancer. 1995;60:388–394. doi: 10.1002/ijc.2910600320. [DOI] [PubMed] [Google Scholar]
- 35.Wagnieres G, Hadjur C, Grosjean P, Braichotte D, Savary JF, Monnier P, van den Bergh H. Clinical evaluation of the cutaneous phototoxicity of 5,10,15,20-tetra(m-hydroxyphenyl)chlorin. Photochem Photobiol. 1998;68:382–387. [PubMed] [Google Scholar]
- 36.Bowers VD, Locker S, Ames S, Jennings W, Corry RJ. The hemodynamic effects of Cremophor-EL. Transplantation. 1991;51:847–850. doi: 10.1097/00007890-199104000-00021. [DOI] [PubMed] [Google Scholar]
- 37.Jackson S, Shepherd A, Brookes S, Abrams P. The effect of oestrogen supplementation on post-menopausal urinary stress incontinence: A double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106:711–718. doi: 10.1111/j.1471-0528.1999.tb08372.x. [DOI] [PubMed] [Google Scholar]
- 38.Weersink RA, Bogaards A, Gertner M, Davidson SRH, Zhang K, Netchev G, Trachtenberg T, Wilson BC. Techniques for delivery and monitoring of TOOKAD (WST09)-mediated photodynamic therapy of the prostate: Clinical experience and practicalities. Photochem Photobiol Sci 2005; (in press). [DOI] [PubMed]
- 39.Gertner MR, Bogaards A, Weersink RA, McCluskey SA, Haider MA, Yue CKK, Savard J, Simpson S, Brun PH, Cohen P, Scherz A, Salomon Y, Aprikian AG, Elhilali MM, Wilson BC, Trachtenberg J. Initial results of a phase I/II trial of WST09-mediated photodynamic therapy (WST09-PDT) for recurrent prostate cancer following failed external beam radiation therapy (EBRT) Eur Urol Suppl. 2004;3:212. [Google Scholar]
- 40.Dole KC, Chen Q, Hetzel FW, Whalen LR, Blanc D, Huang Z. Effects of photodynamic therapy on periphery nerve in situ compound-action potentials study in a canine model. Photomed Laser Surg 2005; (in press). [DOI] [PMC free article] [PubMed]


