Summary
Neuronal activity influences myelination of the brain, but the molecular mechanisms involved are largely unknown. Here, we report that oligodendrocyte progenitor cells (OPCs) express functional adenosine receptors, which are activated in response to action potential firing. Adenosine acts as a potent neuron-glial transmitter to inhibit OPC proliferation, stimulate differentiation, and promote the formation of myelin. This neuron-glial signal provides a molecular mechanism for promoting oligodendrocyte development and myelination in response to impulse activity and may help resolve controversy on the opposite effects of impulse activity on myelination in the central and peripheral nervous systems.
Introduction
Rapid impulse conduction in the nervous system is dependent upon electrical insulation from layers of myelin membrane synthesized and wrapped around large diameter axons by glial cells. Myelin formation by oligodendrocytes in the CNS and Schwann cells in the PNS involves a highly regulated sequence of molecular interactions with axons. The signals inducing myelination during development are largely unknown, but there are several lines of evidence from the central and peripheral nervous systems that the onset of neural impulse activity can affect initiation of myelination.
Experiments on CNS neurons have shown that electrical activity can promote myelination via an unknown mechanism. Blockade of sodium-dependent action potentials with tetrodotoxin (TTX) significantly inhibited myelination in vitro and in developing optic nerve in vivo, whereas increasing neuronal firing with α-scorpion toxin enhanced myelination (Demerens et al., 1996). These results are consistent with earlier studies in the optic nerve in which mice reared in the dark developed fewer myelinated axons compared with control mice (Gyllensten and Malmfors, 1963). Hypomyelination is also observed in optic nerve of the naturally blind cape-mole rat (Omlin, 1997), whereas myelination is accelerated by premature eye opening in rabbit optic nerves (Tauber et al., 1980). The molecular mechanisms underlying these activity-dependent effects on myelination are not known. A number of molecules were proposed, including cell adhesion molecules, extracellular potassium, and the release of a soluble axonal signal (Demerens et al., 1996; Zalc and Fields, 2000), but the axonal signals inducing myelination during development are unknown. Indeed, few differentiation factors for OPCs have been described. Thyroid hormone (Barres et al., 1994; Tokumoto et al., 1999; Calza et al., 2002; Baas et al., 2002), neuregulins (Park et al., 2001a; Canoll et al., 1996, 1999; Calaora et al., 2001), growth factors (Park et al., 2001b), and Notch signaling (Wang et al. 1998) are important for controlling OPC differentiation, but they have not been shown to regulate OPC development in an activity-dependent manner.
The opposite effect of neural impulse activity on myelination has been reported in the PNS, and two molecular mechanisms have been identified. Neural impulse activity can inhibit PNS myelination by changing membrane properties of developing axons (Stevens et al., 1998) and via release of a soluble signaling molecule (Stevens and Fields, 2000). Myelination of dorsal root ganglion (DRG) axons by Schwann cells is inhibited following electrical stimulation of axons at a low frequency (0.1 Hz) via downregulating mRNA and protein levels of the cell adhesion molecule L1 on axons (Itoh et al.,1995; Stevens et al., 1998). Homophilic interactions between L1 on axons and Schwann cells is essential for the initiation of myelination (Seilheimer et al., 1989; Wood et al., 1990). Action potential activity can also influence myelination by Schwann cells directly through the release of activity-dependent signals from premyelinated axons. Recent experiments showed that ATP is released by electrically active DRG axons in vitro, and it acts as an activity-dependent axon-glial transmitter to inhibit Schwann cell proliferation, differentiation, and myelination through activation of P2Y receptors on Schwann cells (Stevens and Fields, 2000). This inhibitory mechanism may help coordinate Schwann cell development with the onset of functional activity in the nervous system and prevent premature Schwann cell differentiation (Stevens and Fields, 2000). The functional effects of ATP on myelinating glia of the CNS are unknown. However, both mechanisms (release of ATP and reduction of L1 expression) inhibit myelination in the PNS, suggesting that different signaling molecules and receptors may underlie the positive effects of impulse activity in the CNS.
It is not known if OPCs can respond directly to action potentials in premyelinating axons, or whether ATP or other activity-dependent signaling molecules regulate OPC development and myelination in the CNS. Calcium transients have been reported in optic nerve glia in response to action potentials, but whether these responses derive from astrocytes or oligodendrocytes is unclear, and the activity-dependent signal has not been identified (Kriegler and Chiu, 1993). There are a large number of potential signaling molecules, considering that oligodendroglial lineage cells in vitro and in situ can respond to a wide range of neuroligands linked to calcium mobilization including glutamate, norepinephrine, histamine, carbachol, GABA, serototonin, angiotensin II, bradykinin, substance P, and ATP (e.g., Kastritsis and McCarthy, 1993; Bernstein et al., 1996).
It is not known whether ATP receptor activation can regulate OPC development. Many types of purinergic receptors activated by ATP or its reaction products have been described in other cells (Burnstock, 1997), but the expression of specific purinergic receptor subtypes on OPCs has not been adequately explored (for review see Fields and Stevens, 2000). These questions were studied in OPCs cocultured with dorsal root ganglion (DRG) neurons. DRG neurons have been shown to release ATP in an activity-dependent manner, and in vivo these neurons have bipolar axons that are myelinated by oligodendrocytes in the spinal cord and by Schwann cells in the peripheral nerve. Our results show that ATP does not have the same effects on oligodendrocytes and Schwann cells and that a different signaling molecule, adenosine, promoted differentiation and myelination in the CNS.
Results
OPCs Express Multiple Types of Functional Purinergic Receptors
Purinergic receptors in OPCs are largely unexplored (Fields and Stevens, 2000), although there is evidence that extracellular ATP can induce Ca2+ responses in oligodendroglial cells in vitro and in situ (He and McCarthy, 1994; Kirischuk et al., 1995; Takeda et al., 1995; Bernstein et al., 1996). Using Ca2+ imaging, RT-PCR, and purinergic receptor agonists, our studies revealed the presence of several subtypes of ATP and adenosine receptors on OPCs in culture and in OPCs acutely dissociated from mouse brain. Messenger RNA for all four subtypes of adenosine receptors (A1, A2a, A2b, A3) was detected by RT-PCR in cultures of OPCs (Figure 1Aa).
Figure 1. A Diverse Range of Purinergic Receptors Was Detected in OPCs.
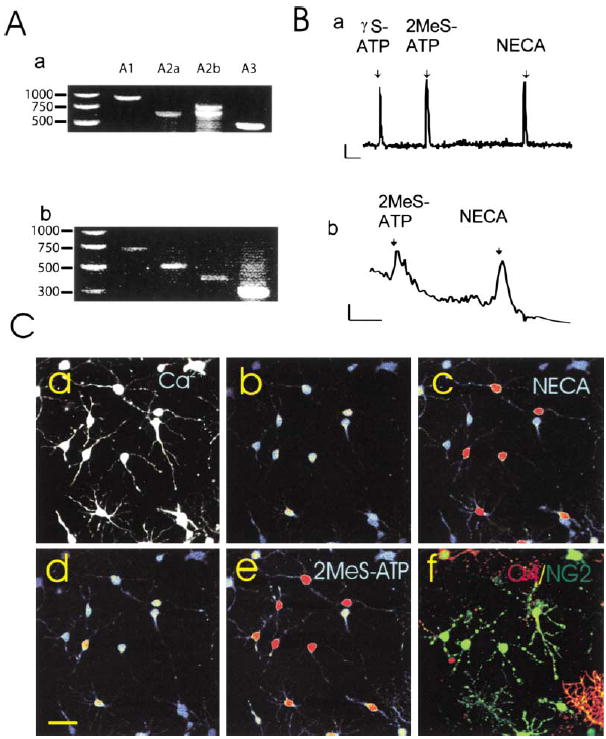
(A) mRNAs for all major classes of adenosine receptors were detected by RT-PCR in rat OPCs after 2 days in culture (a) and in mouse OPCs freshly isolated by fluorescence activated cell sorting (b). Species-specific primers yielded different size products as well as double bands for rat A2b due to alternate splicing (Kreisberg et al., 1997).
(B) Functional purinergic receptors linked to Ca2+ signaling were detected by intracellular Ca2+ imaging in response to ATP receptor agonists (γS-ATP and 2MeS-ATP, 100μM) and adenosine receptor agonists (NECA, 100 μM and adenosine [data not shown]) in individual rat OPCs in culture (a) and OPCs freshly isolated from mice expressing the CNP-GFP transgene (b). Indo-1 was used to measure Ca2+ responses in acutely isolated cells to avoid fluorescence from GFP. Note: Indo-1 is less sensitive than fluo-3. Scale = 10ΔF/F/2 min.
(C) Oligodendroglial cells at both the immature NG2+ stage and more differentiated O4+ stage showed intracellular calcium responses to ATP and adenosine receptor agonists, and often responses to both receptor agonists were seen in the same cell. Cells were filled with fluo-3, a calcium-sensitive fluorescent dye (a), and responses to a specific adenosine receptor agonist (b and c) and an ATP receptor agonist (d and e) were recorded in the same cells using time-lapse confocal microscopy. (Higher concentrations of intra-cellular calcium are indicated in warmer colors.) At the completion of the calcium-imaging experiment, the identical cells were double stained with antibodies against NG2 (green) and O4 (red), and examined by confocal microscopy (f). All the cells in this field were either NG2 and/or O4+ oligodendroglial cells. Of the 17 cells in this microscope field, 11 responded to the adenosine receptor agonist NECA (100 μM), 13/17 responded to the ATP receptor agonist 2MeS-ATP (100 μM), 11/17 cells responded to both agonists, and 4/17 cells showed no measurable response to either agonist.
Functional purinergic receptors were shown in individual OPCs by recording Ca2+ responses using confocal imaging to measure intracellular Ca2+ transients induced by purinergic receptor agonists (Figure 1Ba). Robust responses to ATP and adenosine receptor agonists were observed, often in the same cell (Figure 1Ba). Double immunostaining with antibodies specific for the oligodendrocyte lineage cells following calcium imaging demonstrated that the same cells that responded to the general adenosine agonist NECA (Figure 1Cc) or the ATP receptor agonist 2MeS-ATP (Figure 1Ce) were either NG2+ OPCs or O4+ immature oligodendrocytes (Figure 1Cf).
To determine whether expression of these receptors had been induced artificially by cell culture conditions, OPCs were freshly isolated by fluorescence-activated cell sorting from forebrain of transgenic mice selectively expressing the green fluorescent protein (EGFP) in cells of the oligodendroglial lineage (Yuan et al., 2002; Belachew et al., 2001). RT-PCR (Figure 1Ab) and Ca2+ imaging (Figure 1Bb) showed similar purinergic receptor expression in acutely isolated OPCs and cultured OPCs. The ratio of cells responding to adenosine receptor versus ATP receptor agonists was not significantly different in acutely isolated and cultured OPCs (47.5% versus 35.7%; p > 0.5, χ2; n = 125 cells). It is likely that adenosine receptor expression is higher than revealed with calcium imaging because the A2a and A2b subtypes act through cAMP rather than calcium.
OPCs Detect Action Potentials in Axons
Time-lapse confocal Ca2+ imaging was used to determine whether OPCs could detect action potentials in premyelinated axons (see supplemental movie online at www.neuron.org/cgi/content/full/36/5/855/DC1). Co-cultures of DRG neurons and OPCs were prepared in cell culture chambers equipped with electrodes for stimulating axons. DRG neurons are not spontaneously active in culture (Fields et al., 1992), and only those DRG neurons that extend axons under the high-resistant barriers into the central compartment are electrically stimulated (Li et al., 1996). Within seconds of evoking action potentials in neurons, large increases in intracellular Ca2+ were recorded in OPCs (Figures 2A and 2B). Immunostaining for the O4 antigen in cells loaded with fluo-3 demonstrated that both bipolar O4− and multipolar O4+ cells responded to action potentials in DRG axons (Figure 3).
Figure 2. Communication between DRG Axons and OPCs Is Revealed by Time-Lapse Confocal Ca2+ Imaging (Higher Levels of Ca2+ Are Displayed in Warmer Colors).
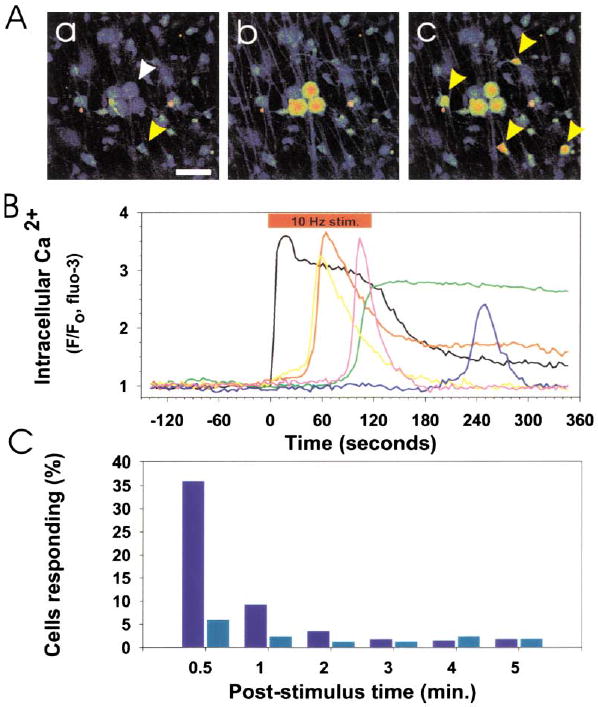
(A) Prior to action potential firing, Ca2+ levels were low in DRG neurons (white arrow) and OPCs (yellow arrow) in coculture (a). Scale bar = 50 μm. Action potentials were induced in DRG axons by electrical stimulation (10 Hz), causing an instantaneous rise in cytoplasmic Ca2+ in the cell body and axons of DRG neurons (b). This was followed by responses in many OPCs (yellow arrows) after several seconds (c). The fluorescence intensity of individual DRG neurons and OPCs in (A) is plotted in (B). 10 Hz electrical stimulation (red bar), cytoplasmic Ca2+ in DRG neurons (black trace), Ca2+ response in OPCs (color traces). No responses to electrical stimulation were seen in OPCs in cultures made without neurons. (C) Incubation with a combination of antagonists of ATP (30 μM suramin) and adenosine receptors (30 μM MRS-1191 and 200 μM DPCPX) inhibited action-potential induced Ca2+ responses in OPCs. A poststimulus time histogram, summarizing the proportion of OPCs responding to action potential firing in the presence (light blue) and absence (dark blue) of these purinergic receptor inhibitors, indicates a significant reduction in short latency (<0.5 min; p < 0.001, χ2, n = 455) and long-latency OPC responses (0.5–5 min; p < 0.02, χ2, n = 455) when purinergic receptors were blocked.
Figure 3. OPCs at O4− and O4+ Stages Respond to Action Potentials in DRG Axons.
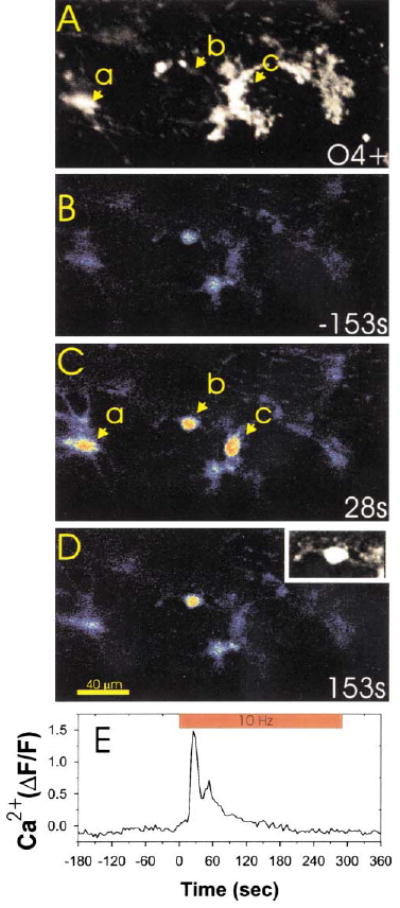
Immunocytochemical staining for the O4 antigen (A) was used after confocal calcium imaging (B–D) to determine the developmental stage at which OPCs responded to action potentials in DRG axons. Three OPCs in this microscope field (a, b, and c) responded to action potentials induced in DRG axons by electrical stimulation. Examination of the same field after O4 staining (A) indicated that OPCs at both the bipolar/O4− stage (cell b and also shown in (D) inset) and O4+ stage (cells a and c in [A] and [C]) responded to axonal firing with large increases in intracellular calcium. Changes in intracellular calcium in cell b are plotted with respect to the time of axonal stimulation in (E). The inset in (D) is an enlargement of cell b filled with the calcium sensitive dye fluo-3, which shows the bipolar cellular morphology more clearly than in the pseudocolor image.
Action potential firing has been shown to release several substances nonsynaptically from axons, including ATP (Stevens and Fields, 2000) and adenosine (Maire et al., 1984; Kuperman et al., 1964), which could potentially mediate neuron-glial communication. Electrical stimulation of axons in the presence of 30 μM suramin, 10 μM MRS-1191, and 200 μM DPCPX, which in combination block metabotropic ATP receptors and all major classes of adenosine receptors, significantly inhibited OPC responses to action potentials (Figure 2C). These antagonists did not affect the number of DRG neurons responding to electrical stimulation, but reduced the number of OPCs responding from 36% in controls to 6% in the presence of the purinergic receptor inhibitors within the first 30 s of axonal stimulation (p < 0.001, n = 455 cells). Responses within 0.5–5 min of axonal stimulation were reduced from 18% in controls to 9% in the presence of the inhibitors (p < 0.009, χ2, n = 455). This indicates that purinergic receptors mediate a substantial portion of activity-dependent axon-OPC communication.
Adenosine, but Not ATP, Inhibits OPC Proliferation
OPCs stop proliferating as they differentiate (Gao et al., 1998), and activity-dependent release of ATP might coordinate OPC proliferation with functional activity in developing axons as shown for Schwann cells (Stevens and Fields, 2000). Tritiated thymidine and 5-bromo-2′-deoxyuridine (BrdU) incorporation assays were used to determine whether activating purinergic receptors could affect OPC proliferation in the presence of the mitogen platelet-derived growth factor (PDGF). Treatment of OPCs in monoculture with ATP for 24 hr produced a relatively weak, concentration-dependent decrease in proliferation (Figure 4A). However, in contrast to studies on Schwann cells (p < 0.002, n = 10), the nonhydrolyzable ATP analog 2MeS-ATP failed to affect OPC proliferation over a wide range of concentrations (Figure 4A). These findings were replicated with a BrdU/Hoechst assay, an independent method of assessing proliferation rate and total cell number, following treatment with two different ATP receptor agonists (2MeS-ATP and γS-ATP). Taken together, these results suggest that the inhibition of OPC proliferation might not be mediated by ATP itself since extracellular ATP is rapidly hydrolyzed to adenosine by ectoenzymes (Zimmermann et al., 1998). Consistent with this hypothesis, treatment with adenosine (1–300 μM) and the general adenosine receptor agonist NECA (1–300 μM) caused a substantial, concentration-dependent decrease in OPC proliferation after 24 hr treatment (Figure 4B). TUNEL assay showed no effect of ATP, 2MeS-ATP, or adenosine on apoptosis.
Figure 4. Adenosine Receptor Activation Selectively Inhibits OPC Proliferation.
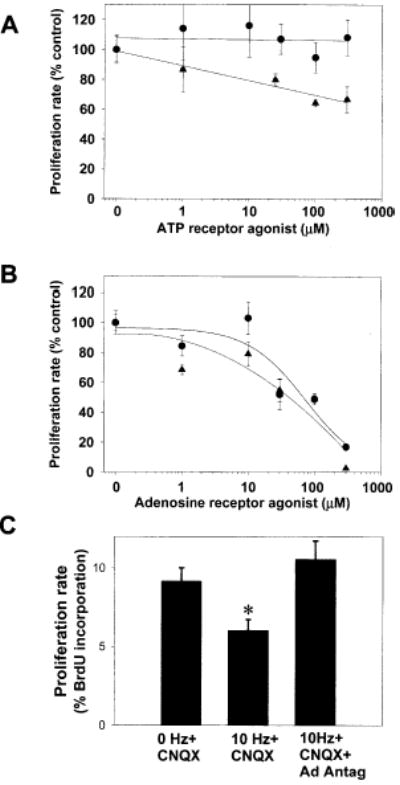
(A) Treatment of OPCs with PDGF+ATP (1–300 μM) for 24 hr resulted in a weak, concentration-dependent inhibition of OPC proliferation (triangles) (p < 0.0001, ANOVA, n = 15 cultures); however, the nonhydrolyzable P2 agonist 2MeS-ATP had no effect (circles)
(B) Adenosine (circles), which can be generated by the breakdown of extracellular ATP, strongly inhibited OPC proliferation in a concentration-dependent manner (p < 0.001; ANOVA; n = 68 cultures). This inhibition was mimicked by the adenosine receptor agonist NECA (triangles) (p < 0.0001; ANOVA; n = 23 cultures)
(C) Electrical stimulation of DRG axons (10 Hz for 24 hr) inhibited the proliferation rate of cocultured OPCs as determined by BrdU incorporation assay (p < 0.005, n = 18 cultures). The activity-dependent inhibition of OPC proliferation occurred in the presence of the non-NMDA glutamate receptor antagonist CNQX (20 μM), but it was blocked when all major adenosine receptor subtypes were inhibited with a combination of adenosine receptor antagonists DPCPX (200 μM) and MRS-1191 (10 μM) (*p < 0.006, ANOVA; n = 27 cultures).
We next determined whether action potential activity could inhibit OPC proliferation, and if so, whether this involved activation of adenosine receptors. Stimulation of DRG axons at 10 Hz for 24 hr significantly reduced the proliferation rate of cocultured OPCs despite the known mitogenic activity of axons (Figure 4C). The inhibitory effect of action potentials on OPC proliferation was blocked when stimulation was performed in the presence of adenosine receptor antagonists inhibiting all major subtypes of adenosine receptors (5 μM MRS 1191 and 200 μM DPCPX). These antagonists do not inhibit ATP receptors and these effects were not blocked by CNQX (20 μM), a non-NMDA glutamate receptor antagonist. This points to a critical role for adenosine receptor activation in the activity-dependent inhibition of OPC proliferation. These results show that action potential firing increases the concentration of extracellular adenosine sufficiently to activate adenosine receptors on OPCs and to inhibit cell cycle progression.
Adenosine Promotes OPC Differentiation and Lineage Progression
Both intrinsic and environmental signals have been implicated in controlling OPC cell cycle exit and lineage progression, but the axonal signals involved in promoting differentiation into mature oligodendrocytes have remained elusive (Barres and Raff, 1999; Rogister et al., 1999). We next addressed whether inhibition of OPC cell cycle progression by adenosine receptor activation was followed by arrest or promotion of OPC differentiation. A sharp increase in the number and relative proportion of OPCs expressing the O4 antigen, a marker of transition from progenitor to a later stage of oligodendroglial development, was seen in cultures treated for 48 hr with adenosine (100 μM) as compared with growth factors alone (Figure 5A). This increase in OPC differentiation was mimicked by the adenosine receptor agonist NECA (300 μM) (280% increase; p < 0.001, t test, n = 6). In contrast, treatment with the ATP analog (100 μM) 2MeS-ATP had no effect, but the effect of adenosine in promoting OPC differentiation was seen in the presence of PDGF and/or bFGF (Figure 5A). Moreover, the number of cells expressing the galactocerebroside O1 antigen, an indicator of a subsequent stage of maturation into a premyelinating oligodendrocyte, was increased after further incubation with adenosine for a total of 72 hr (Figure 5B).
Figure 5. Adenosine Receptor Activation Promotes OPC Lineage Progression and Differentiation.
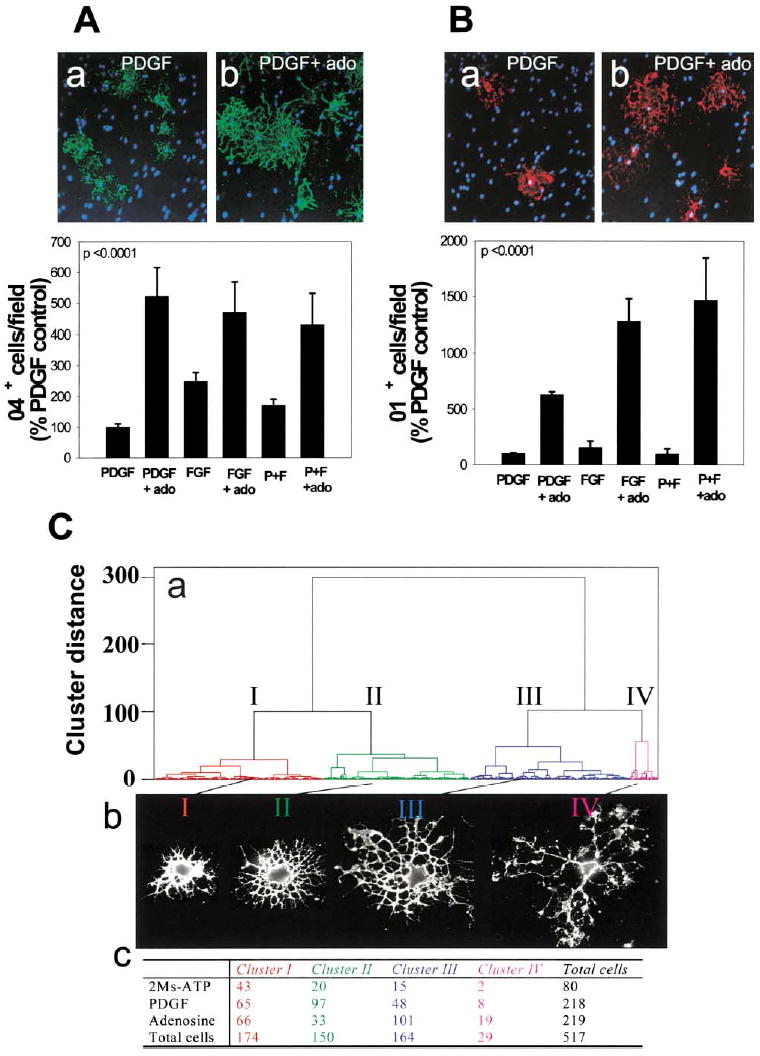
(A) Staining for the O4 antigen (green) in PDGF-treated cultures (a) or after treatment with adenosine (ado) (b) showed a larger proportion of OPCs differentiated to the O4+ stage 48 hr after adenosine treatment (p < 0.0001, t test, n = 12 cultures), regardless of the type of growth factor (PDGF, FGF, or PDGF + FGF) (p < 0.0001, 2-way ANOVA, n = 36 cultures, 39,200 cells)
(B) Adenosine treatment (72 hr) also significantly increased the percentage of oligodendrocytes that expressed the O1 antigen (red), a marker of a more mature premyelinating oligodendrocyte (b), compared with PDGF-treated controls (a) (p < 0.0001; 2-way ANOVA; n = 24 cultures; 21,550 cells). Hoechst dye was used as a counterstain to reveal OPC nuclei (blue).
(C) A quantitative morphometric multivariate cluster analysis was used to compare differences in morphology of OPCs treated with 2MeS-ATP or adenosine for 48 hr. (a) The dendrogram reveals that OPCs could be classified into four morphological clusters (I–IV) with representative examples shown in (b) and the number of cells in each category following treatment tabulated in (c). Adenosine treatment caused a significant decrease in proportion of cells in cluster II and an increase in cells in clusters III and IV. These morphological changes are indicative of lineage progression to a more mature stage of oligodendrocyte development after adenosine treatment.
It is important to note that the inhibitory effect of adenosine on OPC proliferation is not a secondary consequence of promoting differentiation to a postmitotic stage. The majority of OPCs in the proliferation assays were in the bipolar, NG2+ stage of the oligodendrocyte lineage (>90% of the cells), and the assays were performed before the cells could have differentiated substantially. Double staining for BrdU and oligodendrocyte lineage markers confirmed that adenosine significantly inhibited the proliferation rate of OPCs while in the O4−, bipolar stage (7.8% versus 15.7% proliferation rates in adenosine versus PDGF; p < 0.04, n = 4).
Effects of Adenosine on OPC Proliferation and Differentiation in Cerebellar Slice
The inhibitory effect of adenosine on OPC proliferation was confirmed in an intact slice culture preparation (Figure 6A). Chronic treatment (48 hr) of cerebellar slices with adenosine (100 μM) significantly decreased the percentage of LB1+ or NG2+ OPCs that incorporated BrdU as compared with untreated slices (53.1% ± 7.47% versus 80.5% ± 6.46%; p < 0.02, n = 11 slices). Consistent with results in cell culture, adenosine also promoted OPC differentiation in cerebellar slice preparations (Figure 6B). The percentage of O4+ OPCs was increased by 160% in slices treated for 48 hr with adenosine (100 μM), as compared with untreated controls (p < 0.028, n = 15 slices).
Figure 6. The Effects of Adenosine in Cell Culture Are Confirmed in Brain Slice Preparation.
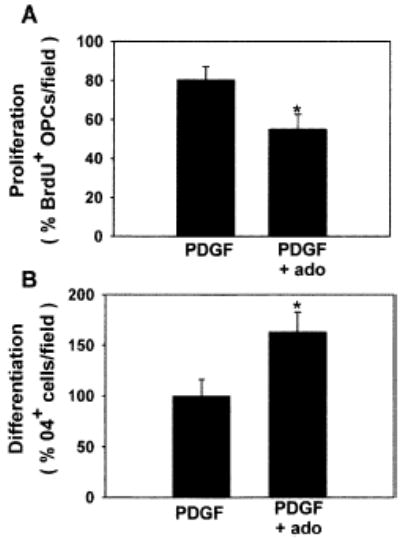
In cerebellar slice culture, adenosine receptor activation inhibited proliferation of LB1+ or NG2+ OPCs (A) and stimulated differentiation (B), consistent with results in cell culture. *p < 0.03, n = 11 and n = 15 slices in (A) and (B), respectively.
Adenosine Receptor Activation Selectively Alters OPC Morphology
Oligodendroglial cells progress through a series of developmental stages characterized by dramatic changes in cell morphology (Baumann and Pham-Dinh, 2001). We observed that OPCs treated with adenosine appeared more mature and highly developed with respect to controls. In order to quantify whether purinergic signaling molecules induce morphological changes in OPCs, we performed a morphometric analysis on OPCs treated for 48 hr with either adenosine or 2MeS-ATP and immunostained for the surface antigen O4. A quantitative morphometric analysis showed statistically significant increases in cell surface area (p < 0.0001), perimeter length (p < 0.0001), outer cell radius (p < 0.0001), and branching (area of holes within cell boundaries) (p < 0.0001) in cells treated with adenosine for 48 hr (ANOVA, n = 517 cells). With the exception of cell perimeter, which was slightly increased (p < 0.05), these morphological features were not affected significantly by 2MeS-ATP treatment. A cluster analysis using a multivariate statistical comparison of five morphometric parameters showed that the cells could be grouped into four major morphological classes (Figure 5C). The frequency distribution of cells in these classes differed significantly after treatment (p < 0.0001, χ2, n = 517) (Figure 5Cc). After adenosine treatment, there was a significant decrease in proportion of cells in cluster II and an increase in proportion of cells in clusters III and IV (p < 0.0001, χ2, n = 306) relative to controls, but not after treatment with 2MeS-ATP (p > 0.5, χ2, n = 190). Cells in clusters III and IV are characterized by numerous, large cellular processes indicative of maturing oligodendrocytes (Figure 5Cb). These pronounced changes in cell morphology following adenosine treatment were indicative of more mature stages of oligodendrocyte development and were consistent with our findings that adenosine promoted OPC lineage progression to the O4+ and O1+ stages.
Adenosine Promotes OPC-Axon Interactions and Myelination
Our results suggest that electrical activity might promote CNS myelination by activating adenosine receptors on OPCs. Our analysis focused on the early phases of myelination since previous work had shown that α-scorpion toxin increased myelination of CNS axons only when used to stimulate action potentials during this narrow developmental period (Demerens et al., 1996). DRG/OPC cocultures were treated with 500 μM adenosine for 14 days and stained for myelin basic protein (MBP), which is highly expressed in the processes of mature myelinating oligodendrocytes (Figure 7Ab versus Figure 7Aa). Adenosine treatment induced a 292% increase in MBP+ oligodendrocytes undergoing myelination (51.4% ± 6.3% versus 17.6% ± 5.3%; p < 0.002, n = 14) (Figure 7B).
Figure 7. Exogenous Adenosine and Adenosine Released from Electrically Stimulated Axons Promote Myelination of DRG Axons by OPCs.
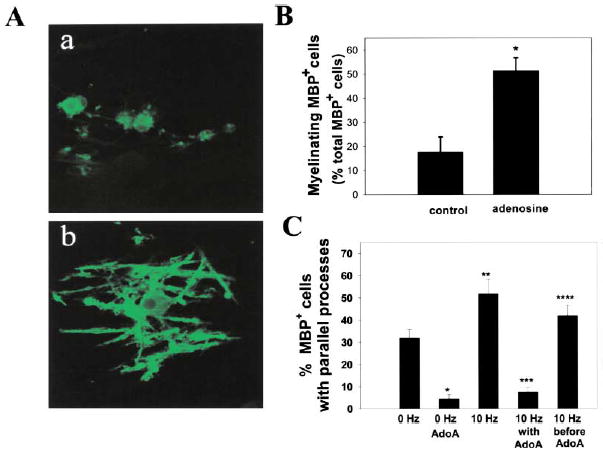
(A) Immunocytochemical staining for MBP was used to compare the effects of chronic adenosine treatment on myelination after 14 days in coculture. In contrast to control cultures (a), OPCs in adenosine-treated cultures displayed multiple parallel processes enriched in MBP (green) and undergoing early stages of myelin formation (b).
(B) The number of MBP+ oligodendrocytes with multiple parallel processes in adenosine-treated cultures increased 292% as compared with controls (p < 0.004, t test, n = 14 cultures). Data shown are normalized with respect to the total number of MBP+ cells/field. Similar results were obtained when expressed as the percentage of total OPCs/field (426% increase with adenosine treatment; 7.8% ± 1.3% versus 1.8% ± 0.69%; p < 0.004, n = 14).
(C) Cocultures treated with adenosine receptor antagonists (AdoA) (5 μM MRS 1191, 500 nM ZM241385, and 10 μM DPCPX) developed significantly fewer MPB+ oligodendrocytes with multiple parallel processes after 3–4 days in coculture (p < 0.0001, n = 26 cultures). Electrical stimulation for 24 hr (10 Hz) increased the number of MBP+ oligodendrocytes with multiple parallel processes as compared with controls (0 Hz) after 3–4 days in coculture (p < 0.01; n = 28), and this effect was blocked by stimulation in the presence of adenosine receptor antagonists (10 Hz with AdoA) (p < 0.00001, n = 31). Adding the antagonists after the 24 hr stimulus (10 Hz before AdoA) resulted in significantly more MBP+ cells with multiple parallel processes than when the antagonists were added during the stimulus (10 Hz with AdoA) (p < 0.0001, n = 27 cultures), indicating an effect of the antagonist in antagonizing an activity-dependent axon-derived signal. All conditions contained the non-NMDA glutamate receptor antagonist CNQX (20 μM), thus excluding the possible involvement of these glutamate receptors. Similar results were obtained in the absence of CNQX (see Results). *p < 0.0001 versus 0 Hz; **p < 0.01 versus 0 Hz; ***p < 0.00001 versus 10 Hz; ****p < 0.0001 versus AdoA.
In addition, endogenous adenosine and adenosine released from electrically stimulated DRG axons promoted differentiation of OPCs into the myelinating phenotype (Figure 7C). We found that the percentage of MBP+ oligodendrocytes with multiple parallel processes was significantly lower in cocultures treated with adenosine receptor antagonists for 3–4 days in coculture (p < 0.0001, n = 26 cultures) (5 μM MRS 1191, 500 nM ZM241385, and 10 μM DPCPX), suggesting that endogenous sources of adenosine are sufficient to promote these biological effects. Electrical stimulation for 24 hr increased the number of OPCs with multiple-parallel processes after 3 days in coculture (p < 0.01, n = 28) compared with controls (Figure 7C). The positive effect of electrical stimulation was blocked by stimulation in the presence of adenosine receptor antagonists (p < 0.00001, n = 31), but applying the antagonists after the 24 hr period of electrical stimulation was not effective (p < 0.0001, n = 27) (Figure 7C). This result is consistent with blockade of receptor activation by adenosine released in an activity-dependent manner from axons. The antagonists applied in the absence of axons had no effect on viability or differentiation of OPCs. After 3 days exposure to the antagonists in monoculture, the number of OPCs was not significantly different from controls (357.6 versus 291.6 cells/field in antagonists versus control; p > 0.2), and differentiation of OPCs to a premyelinating phenotype (O1+) was not affected by the antagonists when applied in the absence of axons (2.82% versus 2.54% in antagonists versus control; p > 0.7).
These results are consistent with the activity-dependent release of adenosine from DRG axons promoting OPC differentiation and early myelination. The effects were not due to activation of non-NMDA glutamate receptors because the inhibitor CNQX (20 μM) was included in all conditions in these experiments. Similar results were obtained when adenosine receptor antagonists were added without CNQX (5 μM MRS 1191; 500 nM ZM241385, and 200 mM DPCPX). Under these conditions, electrical stimulation increased the number of MBP+ oligodendrocytes with multiple parallel processes (p < 0.0001, n = 30 cultures), and adenosine receptor antagonists without CNQX blocked the increase in early myelination due to stimulation (p < 0.00001, n = 46).
We wanted to eliminate unknown effects of adenosine on DRG neurons themselves and determine whether transient exposure to a lower concentration of adenosine could trigger differentiation of OPCs into mature myelinating oligodendrocytes. Therefore, OPCs were cultured without axons for 48 hr in PDGF with 100 μM adenosine, then harvested and replated onto three- week-old DRG cultures in standard culture medium without adenosine. After only 1 day in coculture, adenosine-primed OPCs exhibited striking morphological differences compared to cells primed with PDGF alone (Figure 8B versus Figure 8A). In the initial stages of myelination, oligodendrocytes lose their stellate morphology as multiple glial processes align with axons (Lubetzki et al., 1993; Bunge and Wood, 1987) (Figure 8B versus Figure 8A). The number of oligodendrocytes immunopositive for O1 and with multiple parallel processes increased 454% in cocultures prepared from OPCs primed with adenosine as compared with PDGF (56.4% ± 8.9% versus 12.4% ± 5.1%; p < 0.001, n = 15) (Figure 8C). The effect of adenosine priming persisted after the cells became MBP+, resulting in a 936% increase in MBP+ OPCs with multiple parallel processes 2 days after priming with adenosine versus PDGF (88.0 ± 10.1 versus 9.44 ± 2.06; p < 0.004, n = 8). When such cultures were allowed to develop 10–12 days after transient treatment with adenosine, the number of myelin profiles identified by MBP staining was 633% greater than with PDGF primed OPCs (22.15 profiles ± 2.1 versus 3.5 ± 1.3; p < 0.0001, n = 20) (Figures 8D–8F). Electron microscopy confirmed that our cultures of DRG neurons and adenosine-primed OPCs contained several large diameter axons with multiple wraps of myelin separated by interperiod lines, which is indicative of compact myelin (Figures 9B–9E). Collectively, these findings indicate that relatively brief exposure to adenosine promotes the association of OPCs with axons, their differentiation into mature oligodendrocytes, and substantially increases myelination.
Figure 8. Adenosine Priming Promotes Myelination by OPCs.
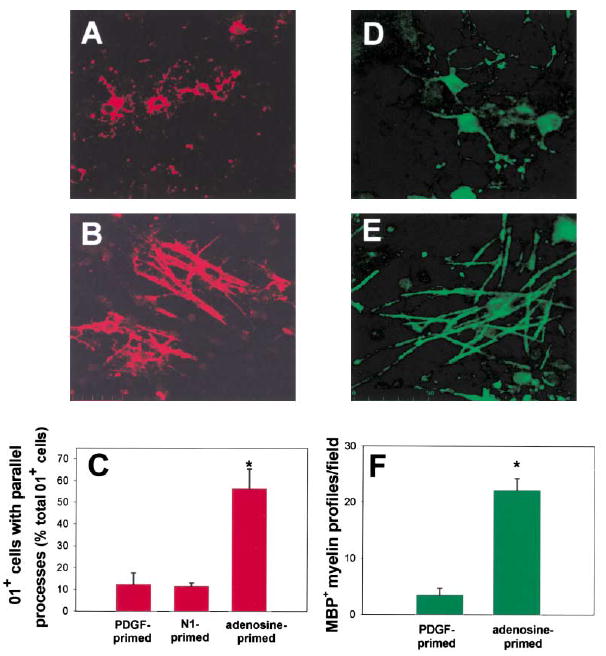
Brief exposure to adenosine was sufficient to promote OPC differentiation and alignment with axons. OPCs were primed by a 48 hr exposure to adenosine, replated onto 3-week-old DRG cultures, and stained for O1. In marked contrast to controls (A), adenosine-primed OPCs expressing the O1 antigen (red) formed multiple parallel processes that were closely associated with axons after only 1 day in co-culture (B). The proportion of O1+ cells with multiple parallel processes increased 402% in cocultures prepared with adenosine-primed OPCs as compared with cocultures of OPCs primed with PDGF or N1, a culture condition known to promote OPC differentiation and increase O1 expression (C) (p < 0.0001; ANOVA, n = 18 cultures). Data shown are normalized to the total number of O1+ cells/field. Similar results were obtained when expressed as the percentage of total OPCs/field (3.5% ± 0.37% versus 0.33% ± 0.12%; adenosine versus control, p < 0.000, n = 15). After 10–12 days in coculture, the degree of myelination was compared in control (D) and cocultures prepared with adenosine-primed OPCs (E) using immunocyto-chemical staining for MBP. (F) A 633% increase in the number of MBP+ myelin profiles/field was seen in cocultures made from adenosine primed OPCs, as compared with controls (E) (p < 0.0001, t test, n = 20 cultures). *Significantly different from control.
Figure 9. Electron Microscopy of Myelin Formation on DRG Axons after 12 Days in Coculture with Oligodendrocytes Derived from Adenosine-Primed OPCs (48 hr Treatment with 100 μM Adenosine).
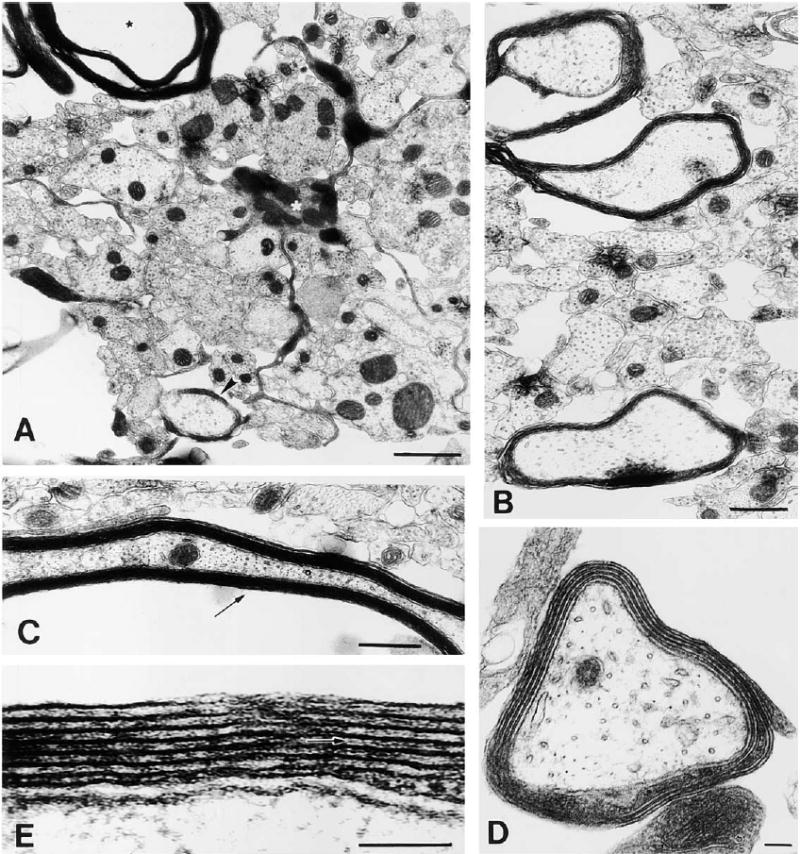
At this stage in culture, oligodendrocytes are actively myelinating large diameter axons. (A) An oligodendrocyte (white asterisk) extending a cellular process between small, unmyelinated axons to ensheath a large-diameter axon (arrowhead), while extending another process forming compact myelin around a different axon (black asterisk). (B) Most large diameter axons are in the process of becoming myelinated. (C) Ultrastructure of compact myelin seen in oblique long section. (D) Higher magnification of an axon undergoing early myelination; four complete wraps of membrane can be seen. (E) A detailed view of the myelin sheath reveals the interperiodal line (arrow) indicative of compact myelin. Scale bars = 1 μm in (A) and (D), 0.5 μm in (B), and 100 nm in (C) and (E).
These effects were not due solely to adenosine increasing the absolute number of differentiated oligodendrocytes expressing the O1 antigen. Although equal numbers of OPCs were plated on axons in all conditions, there were significantly fewer OPCs in adenosine-primed cultures 2 days after coculture compared to PDGF alone, consistent with the antiproliferative effects of adenosine (71.0 ± 2.2 versus 125 ± 2.2; p < 0.0001, n = 8, counts of Hoechst-stained nuclei). Although proportionately higher after adenosine priming, the absolute number of OPCs at the O1+ stage was not significantly different in cocultures made from OPCs primed with adenosine or PDGF (5.2 ± 1.9 versus 4.8 ± 1.4; p = 0.9, n = 15).
Adenosine was more effective in promoting differentiation of OPCs to a myelinating stage than priming cells with N1, a defined medium known to promote differentiation of OPCs (p < 0.0007, n = 11) (Gallo et al., 1996) (Figure 8C). N1-priming resulted in a higher proportion of O1+ oligodendrocytes as compared with adenosine (12.6% versus 7.2%; N1 versus adenosine; p < 0.008, t test, n = 10), but despite this, N1-primed cells failed to align with axons, as seen after adenosine treatment (Figure 8C). This suggests that adenosine promotes differentiation to a stage competent to initiate myelination. This is consistent with changes in MBP expression following adenosine treatment. 1 day after adenosine priming, a significantly higher proportion of cells expressed myelin basic protein (MBP), a marker for terminally differentiated oligodendrocytes (15.2% versus 5.3%; adenosine versus PDGF; n = 4, p < 0.002) (Figure 10A versus Figure 10B), when cultured in the absence of axons. 2 days after adenosine priming, the majority of MBP+ oligodendrocytes in monoculture had reduced MBP expression in the soma and enhanced expression in large rafts of membrane along their processes as compared with PDGF-primed controls (11.5% versus 1.15%; adenosine versus PDGF; n = 6, p < 0.003) (Figure 10C versus Figure 10D), indicative of oligodendrocytes undergoing myelin synthesis. Taken together, our data suggest that adenosine selectively accelerates differentiation of OPCs to the mature oligodendrocyte stage in which they are competent to initiate myelination upon contact with the appropriate axons.
Figure 10. Adenosine Promotes OPC Differentiation into Myelinating Oligodendrocytes.
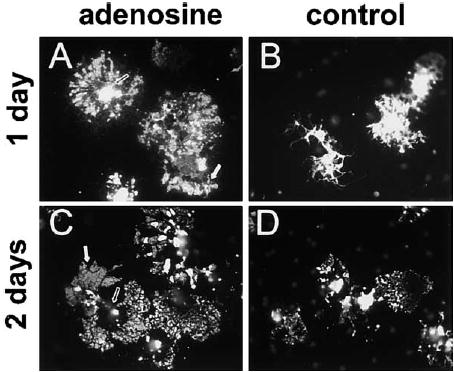
OPCs primed for 48 hr with adenosine (100 μM) or PDGF alone (control) were replated without axons and immunostained for myelin basic protein (MBP) 1 and 2 days later. After 1 day, adenosine-primed OPCs were morphologically distinct from controls ([A] versus [B]). Adenosine-primed OPCs expressed a significantly higher proportion of MBP+ OPCs/field compared with controls (15.2% versus 5.3%; adenosine versus PDGF; n = 4, p < 0.002). After 2 days, the majority of MBP+ oligodendrocytes in adenosine-treated cultures expressed lower levels of MBP in the soma (open arrows, [A] versus [C]) but enhanced expression in large rafts of membrane (white arrows) along their processes as compared to PDGF-primed controls ([C] versus [D]) (11.5% versus 1.15%; adenosine versus PDGF; n = 6, p < 0.003).
Discussion
The present study identifies a novel antiproliferative and differentiating factor for OPCs, which is derived from axons and promotes myelination in an activity-dependent manner. This axon-glial signaling mechanism provides a potent form of communication coordinating oligodendrocyte proliferation, differentiation, and myelination in response to action potentials in axons. Additionally, these findings may help reconcile the current controversy concerning the opposite effects of neural impulse activity on myelination that have been reported in PNS and CNS. Adenosine appears to be the active axonglial signaling molecule in the CNS, where it promotes differentiation and myelination, but ATP is the signaling molecule in the PNS where it arrests differentiation and inhibits myelination (Stevens and Fields, 2000).
OPCs Express a Complex Array of Purinergic Receptors
The present study provides evidence that OPCs express all four subtypes of adenosine receptors, in addition to metabotropic ATP receptors, both in vitro and in acutely isolated cells. This suggests a much more complex range of purinergic receptors on oligodendroglia than previously known. In optic nerve preparations (Kriegler and Chiu 1993) and corpus callosum slices (Bernstein et al., 1996), adenosine was shown to induce calcium responses in white matter glia but the type of glial cell generating this response could not be determined. Also, in these previous studies, it was not possible to distinguish whether the calcium responses were mediated directly by adenosine receptors on glia, or indirectly by adenosine receptors on axons since a large repertoire of neurotransmitter responses linked to calcium signaling have been observed in white matter glia. In cell culture, oligodendrocytes at various stages of development exhibit calcium responses to ATP (Takeda et al., 1995; Kirischuk et al., 1995; He and McCarthy, 1994, Kastritsis and McCarthy, 1993), but adenosine was not tested in these studies or failed to exhibit a calcium response (Kirischuk et al., 1995). Complexity of the purinergic family of receptors and heterogeneity of glial cells, arising from the fact that OPCs are undergoing dramatic developmental changes in the perinatal period, complicate the detection and analysis of adenosine receptors in these cells. This complexity would suggest that purinergic receptors, which may be developmentally regulated on oligodendroglial cells (e.g., He and McCarthy, 1994), could provide a rich neuron-glial signaling system with the potential to interact with several intracellular signaling systems that control many different developmental or functional responses of oligodendrocytes.
This complexity is in contrast to premyelinating Schwann cells, which do not appear to express functional adenosine receptors linked to calcium (Mayer et al., 1998; B.S. and R.D.F., unpublished data). In response to electrical stimulation of DRG axons, the calcium fluxes in Schwann cells were completely blocked by apyrase, an enzyme that degrades extracellular ATP (Stevens and Fields, 2000), but this was not the case for OPCs studied in the present series of experiments. A mixture of antagonists blocking all known classes of adenosine receptors and metabotropic ATP receptors significantly inhibited activity-dependent neuron-glial signaling in OPCs cultured with axons but failed to block all responses. Spontaneous Ca2+ oscillations, incomplete block of all purinergic receptors, or other signaling molecules linked to intracellular Ca2+ may contribute to the remaining Ca2+ transients in the presence of these inhibitors. This implies that the same signaling molecule released by DRG neurons could have selective and independent effects on myelinating glia in the peripheral and central nervous system, thereby conferring greater specificity for activity-dependent plasticity.
Differential Effects of ATP and Adenosine on Development of Central and Peripheral Myelinating Glia
The opposite effects of purinergic signaling in OPC and Schwann cell differentiation are consistent with differences in myelination of CNS and PNS axons of DRG neurons with respect to the onset of impulse activity during development. Myelination of peripheral DRG axons is delayed with respect to the central axons. Although myelination begins at about birth in both the central and peripheral axons, DRG axons do not enter the CNS extensively until after the peripheral axons have reached their peripheral targets and stopped growing (Scott, 1992). The developmental arrest of Schwann cells induced by ATP in the PNS may prevent premature Schwann cell differentiation into a nonmyelinating phenotype while having no apparent developmental effect on OPCs along the central axons at the same time in development. Despite the fact that 2MeS-ATP induced robust Cai 2+ sponses in OPCs (Figure 1B), selective ATP agonists failed to affect OPC proliferation and differentiation. Adenosine, but not ATP, promoted differentiation and myelination of oligodendrocytes in an activity-dependent manner. Adenosine has no known effects on Schwann cell differentiation, and in contrast to ATP (Stevens and Fields, 2000), adenosine did not inhibit O4 expression in Schwann cells cocultured with DRG neurons (B.S. and R.D.F., unpublished data). These different responses of central and peripheral myelinating glia to ATP and adenosine are consistent with the occurrence of myelination in the CNS during a period of development when the nervous system is already functionally active.
Regulation of OPC Proliferation by Purinergic Signaling in the Context of Other Activity-Dependent Signals
Impulse activity is inhibitory for proliferation of both Schwann cells (Stevens and Fields, 2000) and OPCs in coculture with DRG neurons, but this involves activation of different purinergic receptors (ATP versus adenosine, respectively). It would appear that different intracellular signaling pathways inhibit cell cycle in OPCs and Schwann cells because activation of ATP receptors with 2MeS-ATP or γS-ATP caused large increases in intracellular calcium in OPCs but failed to inhibit their mitotic rate. The inhibitory effects of neural impulse activity on both OPCs and SCs are consistent with the requirement that these cells exit the cell cycle prior to differentiation. In the PNS, the onset of high-frequency action potential activity (Fitzgerald, 1987) corresponds to a period of synchronized cell cycle withdrawal (Stewart et al., 1993; Mirsky and Jessen, 1996), which is requisite for myelination to begin. Acting through these distinct receptors, the effect of impulse activity on proliferation could be timed and independently regulated along central and peripheral axons by spatial and developmental regulation of ectoenzymes that synthesize extracellular adenosine from ATP (Zimmermann et al., 1998). In addition, the expression of specific purinergic receptor subtypes on CNS and PNS myelinating glia may be developmentally regulated (e.g., see He and McCarthy 1994; Kirischuk et al., 1995). Although the mechanism for the activity-dependent release of ATP from nonsynaptic regions of DRG axons is unknown, it also may be subject to developmental regulation, limiting the effects to appropriate developmental periods.
There are likely multiple activity-dependent signals regulating OPC development in different regions of the nervous system (Gallo and Ghiani, 2000). For example, activity-dependent release of PDGF in optic nerve has been shown to stimulate OPC proliferation (Barres and Raff, 1993). Conversely, glutamate receptor activation has been shown to inhibit OPC proliferation (Gallo et al., 1996; Yuan et al., 1998). In the present study, the activity-dependent activation of adenosine receptors selectively inhibited OPC proliferation in the presence of several types of growth factors, including PDGF (and/ or bFGF) and when cocultured with axons. This suggests that adenosine is able to overcome the potential effects of these mitogens under the appropriate conditions during development.
Adenosine Is a Novel Differentiation Factor Promoting Myelination
Our results identify adenosine as a potent axonal signal promoting OPC differentiation into myelinating oligodendrocytes. Importantly, our results show that only a brief exposure of OPCs to adenosine was sufficient to induce significant morphological changes in OPCs, which subsequently accelerated OPC-axon interactions and myelination at later stages. The diverse array of adenosine receptors on OPCs identified in this study, which act through multiple intracellular signaling pathways, opens new areas of research on signals regulating oligodendrocyte development and interactions with neurons. Possibly, the ability of adenosine to promote OPC differentiation and increase myelination through activation of adenosine receptors may offer new approaches to the treatment of demyelinating diseases in the CNS, such as multiple sclerosis.
Experimental Procedures
Cell Culture and Electrical Stimulation
DRG neurons were dissected from the spinal cords of embryonic day (E)13.5 mice as described (Stevens et al., 1998). DRG cultures were grown for ~3 weeks in MEM medium supplemented with N3 containing 50 ng/ml of nerve growth factor and 5% horse serum before the addition of OPCs into the side compartments of three-compartment chambers equipped with stimulating electrodes (Fields et al., 1992). Purified cultures of OPCs were prepared from cerebral cortices of E20 rats and postnatal day (P)1 mice as described (Gallo and Armstrong, 1995; Yuan et al., 1998; Ghiani et al., 1999). This method yields a pure population (>99%) of cells of the oligodendrocyte lineage with trace amounts (<0.05%) of GFAP+ astrocytes and undetectable amounts of OX-42+ microglial cells. Immunocytochemical characterization determined that approximately 95% of these freshly isolated cells were NG2+ or LB1+ (OPCs), 4% were O4+ (preoligodendroblasts), and ~1% were O1+ (preoligodendrocytes). OPCs were plated in N1 medium with 0.5% FBS on poly-L-ornithine coated coverslips (200,000 cells), or into the side compartments of DRG cultures (10,000–20,000 cells/side) in the absence of exogenous PDGF or FGF in 10% CO2 at 37°C.
Action potentials were induced in DRG axons by 200 μs 5V biphasic pulses through platinum electrodes in three-compartment chambers (Fields et al., 1992). Axons grew into the central compartment beneath high-resistant barriers. Only those neurons with axons traversing the barrier are stimulated to fire action potentials, and DRG neurons are not spontaneously active and do not form synapses in these cultures ( Li et al., 1996).
Calcium Imaging
After 24 hr in culture, OPCs were treated for 24 hr with PDGF-AB (10 ng/ml), loaded with 10 μg/ml fluo-3AM (Molecular Probes), and imaged on a Bio-Rad MRC1024 as described (Fields and O’Donovan, 1997). Solutions were applied locally through a multibarrel pipette using electronically controlled valves (Harvard Apparatus). Fluorescence intensity from the soma of every cell in the field was plotted as ΔF/Fo. OPCs were acutely isolated from cortices of P0–P14 mice expressing the green fluorescence protein (GFP) under the control of 2′,3′cyclicnucleotide 3′-phosphodiesterase (CNP) oligodendrocyte-specific promoter (Belachew et al., 2001). Cells were analyzed for light forward- and side-scatter using a FACS Vantage SE instrument (Becton Dickinson, San Jose, CA). 100% of the cells collected were GFP+ oligodendroglial cells and were comprised of 45% NG2+, 49% O4+ and 7.5% O1+ cells. The FACS- purified GFP+ fraction did not contain GFAP+ astrocyes or NeuN+ neuronal contaminants (Yuan et al., 2002). Cells isolated from P0–P4 animals were loaded with 20 μg/mL indo-1AM (Molecular Probes) and imaged within 2 hr using UV laser excitation of both GFP (488 nm) and indo-1 (351 nm) through a double dichroic filter with emission collected at >460 nm and 405 ± 35 nm (respectively).
Molecular Biology
RNA was isolated using TRIzol (Invitrogen) from cultured rat OPCs or acutely isolated OPCs from mice carrying the CNP-GFP trans-gene, and RT-PCR was performed using 1 μg RNA in a Retroscript kit (Ambion), and 5 μl of RT product was amplified using SuperTaq (Ambion) in 30 cycles of 94°C (1 min)/60°C (1 min) with 72°C (7 min) after the final cycle. PCR primers for rat A1, A2a, and A2b receptors were as described (Kreisberg et al., 1997) for A3: 5′-TGTGTC CTCCAGGTTATCAGG-3′ and 5′-AGGCATAGAAGTGCATCTGGA-3′ (500 bp). Primers for mouse: A1, 5′-ACCATGTGATTGCTTGAA AGG-3′ and 5′-ACGATGAAGCAGAAGGTAGCA-3′, (700 bp); A2a: 5′-CTCACGCAGAGTTCCATCTTC-3′ and 5′-GAAGCAGTTGATGAT GTGCAG-3′; (500 bp); A2b: 5′-CAGACCCCCACCAACTACTTT-3′ and 5′-TGTCAGAGGACAGCAGCTTTT-3′ (396 bp); A3: 5′-ACCTG CATCCTCCAGGTTAAT-3′ and 5′-TAGGTGATGTTCAGCCAGTCC-3′ (298bp). Products were resolved by electrophoresis on a 2% aga-rose gel. The A1 receptor PCR product was eluted from the gel and sequence verified using an ABI Prism 310 Genetic Analyzer (PE/Applied Biosystems).
Proliferation and Apoptosis
[3H] thymidine assays were performed in OPCs grown in DMEM-N1 medium with 0.5% FBS in 24-well plates (3 × 104 cells/cm2). 24 hr after plating, OPCs were treated with growth factors (10 ng/mL of PDGF and/or bFGF) ± purinergic drugs and incubated with methyl[3H] thymidine for 20 hr. Cells were harvested, and [3H]-Thymidine incorporation was measured by precipitation with 10% trichloracetic acid and scintillation counting.
For the BrDU assay, OPCs were labeled with BrdU (Boehringer Mannheim) for 6 hr, fixed and stained according to manufacturer instructions, and counterstained with Hoechst nuclear stain (Molecular Probes) (1:2000 for 10 min). Proliferation rate was calculated as the ratio of BrdU/Hoechst+ OPC nuclei/microscope field. 10–15 randomly chosen fields per coverslip were sampled to obtain a mean for each culture well. In statistical analysis, n = number of wells. For electrical stimulation experiments, OPCs were cocultured with DRG neurons in standard DRG growth medium without NGF. The following day, DRG neurons were stimulated at 10 Hz for 24 hr and incubated with BrdU during the last 6 hr of stimulation. Cocultures were then fixed and stained for BrdU as described above.
For the apoptosis assay, OPCs were fixed with 4% paraformaldehyde 24 hr after treatment with adenosine, ATP, or 2MeS-ATP (100 and 300 μM). Cells were stained for TUNEL according to manufacturer’s protocol (Roche), and the percentage of TUNEL+ cells was determined from the ratio of TUNEL/Hoechst+ cells/microscope field.
OPC Differentiation
Purified OPCs were plated on poly-L ornithinecoated coverslips (200,000 cells/coverslip) in DMEM-N1 medium with 0.5% FBS and treated 24 hr later with growth factors (10 ng/mL of PDGF and/ or bFGF) ± adenosine or 2MeS-ATP (100 μM). Differentiation was assessed by immunostaining with antibodies against the cell surface antigens O4 and O1, 48 and 72 hr after treatment, respectively. Live cultures were incubated with monoclonal O4 or O1 antibodies (1:10 for 1 hr), and antigens were detected using a fluorescein-conjugated goat anti-mouse IgM antibody (Jackson Immunoresearch). Cultures were counterstained with Hoechst, and the ratio of O4+ or O1+ OPCs/Hoechst nuclei per field was calculated.
Morphometric Analysis
Images of 517 OPCs fluorescently labeled for the O4 antigen were acquired using a digital camera from random fields in three replicate experiments. Eighteen morphological parameters were measured on each cell by automated computerized morphometry (MetaMorph, Universal Imaging Corp.). Five measurements were determined to be most discriminating and used for further analysis including: total cell area, area of holes within cell boundaries, cell perimeter, outer cell radius (distance from centroid to most distant edge of cell), and cell shape factor (4πA/P2); P = perimeter, A = area. Measurements were compared by ANOVA, and a multivariate agglomerative hierarchical cluster analysis using Ward linkage method of Euclidean distance was performed (Minitab, Inc., State College, PA).
Cerebellar Organotypic Slice Cultures
Cerebella were dissected from P2 rats and sagittally sliced (300 μm) using a tissue chopper. Slices were cultured on 0.5 μm LCR sterile membrane filters in 24 mm sterilized mesh sieves as described (Yuan et al., 1998). Adenosine (100 μM) was added to the slices in N1 medium containing 10% FBS for 48 hr. BrdU (50 μM) was added to the slices for the last 24 hr. After 48 hr in culture, slices were treated with protease (1.5 mg/ml) for 5 min at 37° followed by treatment with a trypsin inhibitor (0.65ug/ml) for 5 min at 4°. Cells were then dissociated mechanically (35 times) through a pasteur pipet and plated on poly-D-ornithine coated coverslips at a density of 1–2 million cells/ml. Cells were immunostained for O4, LB1, or NG2 and fixed for BrdU assay.
Myelination
Purified OPCs were cocultured at a low density (100,000 cells/ml) with DRG neurons and treated 1–2 hr later with 500 μM of adenosine in standard DRG culture medium without exogenous PDGF or bFGF. Cocultures were fed every 3–4 days with medium containing adenosine. 14 days later, cultures were fixed and incubated with monoclonal antibodies against MBP (Boehringer Mannheim) as described (Stevens and Fields, 2000; Stevens et al., 1998). The effects of electrical stimulation on early stages of myelination was assessed by MBP immunostaining 2–3 days following electrical stimulation (10 Hz for 24 hr beginning 2–3 hr after coculture). The percentage of premyelinating or myelinating oligodendrocytes was determined by calculating the ratio of MBP+ cells with multiple parallel processes to the total number of oligodendrocytes/microscope field.
In priming experiments, OPCs were first plated on 60 mm dishes precoated with poly-L-ornithine (1 × 106 cells/dish in DMEM-N1 + 0.5% FBS) and treated 24 hr later with 10 ng/mL PDGF ± 100 μM adenosine for 48 hr. OPCs were resuspended by gentle rinsing and brief exposure (5 min) to trypsin (0.05%). Adenosine-, PDGF-, and N1-primed cells were counted, and equal numbers of cells (10,000 cells/side) were replated onto 3-week-old DRG cultures. Cocultures were maintained in DMEM-N1 + 0.5% FBS in the absence of adenosine through the remainder of the experiment.
Electron Microscopy
12 days after coculture of OPCs primed (48 hr with 100 μM adenosine), cultures were rinsed in PBS, fixed in 2.5% gluteraldehyde in 0.015 M sodium cacodylate (pH 7.4) for 60 min, and postfixed for 1 hr in 1% osmium tetroxide and embedded for EM as described (Stevens et al., 1998). After hardening, blocks were cut from cocultures and mounted for cross and longitudinal sectioning. Ultrathin sections were cut with a diamond knife and stained with uranyl acetate and lead citrate, and examined by transmission electron microscopy.
Data Analysis
All experimental treatments were represented in each experimental set, keeping the number of cultures as balanced as possible. For each statistical comparison, the results of replicate experimental sets were pooled from multiple independent trials. Counts were made of cells or myelinated profiles from randomly selected microscope fields (8–15) in each preparation. Results were represented as the mean of each independent culture preparation (dish, cover slip, or side compartment, depending on the experiment). With the exception of the morphometric analysis (see above), the sample size represented the number of independent experimental trials, not microscope fields. The statistical significance of differences was tested by two-sample t test, ANOVA for multiple comparisons data sets, and χ2 for frequency data.
Acknowledgments
We thank X. Yuan for generating the CNP-EGFP mice and for assistance with the slice culture preparations and E.A. Neale for providing full use of her electron microscopy laboratory and offering helpful advice. We also thank S. Belachew and C.A. Ghiani for helpful discussions and reading the manuscript and R.H. Quarles, J.T. Russell, and C.J. McBain for critical comments. We thank B. Weinberg for assistance with confocal microscopy and M. Chelan and J. Novitch for assistance with digital imaging. B.S. is concurrently enrolled in the Neuroscience and Cognitive Science (NACS) doctoral program at the University of Maryland, College Park.
References
- Baas D, Legrand C, Samarut J, Flamant F. Persistence of oligodendrocyte precursor cells and altered myelination in optic nerve associated to retina degeneration in mice devoid of all thyroid hormone receptors. Proc Natl Acad Sci USA. 2002;99:2907–2911. doi: 10.1073/pnas.052482299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Axonal control of oligodendrocyte development. J Cell Biol. 1999;147:1123–1128. doi: 10.1083/jcb.147.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Belachew S, Yuan X, Gallo V. Unraveling oligodendrocyte origin and function by cell-specific transgenesis. Dev Neurosci. 2001;23:287–298. doi: 10.1159/000048712. [DOI] [PubMed] [Google Scholar]
- Bernstein M, Lyons SA, Moller T, Kettenman H. Receptor-mediated calcium signaling in glial cells from mouse corpus collosum slices. J Neurosci Res. 1996;46:152–163. doi: 10.1002/(SICI)1097-4547(19961015)46:2<152::AID-JNR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The past, present and future of purine nucleotides as signaling molecules. Neuropharmacology. 1997;36:127–139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Wood PM. Tissue culture studies of interactions between axons and myelinating cells of the central and peripheral nervous system. Prog Brain Res. 1987;71:143–152. doi: 10.1016/s0079-6123(08)61820-8. [DOI] [PubMed] [Google Scholar]
- Calaora V, Rogister B, Bismuth K, Murray K, Brandt H, Leprince P, Marchionni M, Dubois-Dalcq M. Neuregulin signaling regulates neural precursor growth and the generation of oligodendrocytes in vitro. J Neurosci. 2001;21:4740–4751. doi: 10.1523/JNEUROSCI.21-13-04740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza L, Fernandez M, Giuliani A, Aloe L, Giardino L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc Natl Acad Sci USA. 2002;99:3258–3263. doi: 10.1073/pnas.052704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Canoll PD, Kraemer R, Teng KK, Marchionni MA, Salzer JL. GGF/neuregulin induces a phenotypic reversion of oligodendrocytes. Mol Cell Neurosci. 1999;13:79–94. doi: 10.1006/mcne.1998.0733. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, R.D., and O’Donovan, M.J. (1997). In Current Protocols in Neuroscience (New York: Wiley Press) pp. 2.3.1–2.3.12.
- Fields RD, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 2000;23:625–633. doi: 10.1016/s0166-2236(00)01674-x. [DOI] [PubMed] [Google Scholar]
- Fields, R.D., Yu, C., Neale, E.A., and Nelson, P.G. (1992). Chronic electrical stimulation of multicompartment cell cultures. In Practical Electrophysiological Methods, H. Kettenmann and R. Grantyn eds. (New York: Wiley press), pp. 67–76.
- Fitzgerald M. Spontaneous and evoked activity of fetal primary afferents in vivo. Nature. 1987;326:603–607. doi: 10.1038/326603a0. [DOI] [PubMed] [Google Scholar]
- Gallo V, Armstrong RC. Developmental and growth-factor induced regulation of nestin in oligodendrocyte lineage cells. J Neurosci. 1995;15:394–406. doi: 10.1523/JNEUROSCI.15-01-00394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Ghiani CA. Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci. 2000;21:252–258. doi: 10.1016/s0165-6147(00)01494-2. [DOI] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte cell progenitor proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FB, Apperly J, Raff M. Cell-intrinsic timers and thyroid hormone regulate the probability of cell-cycle withdrawal and differentiation of oligodendrocyte precursor cells. Dev Biol. 1998;197:54–66. doi: 10.1006/dbio.1998.8877. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Eisen AM, Yuan X, DePinho RA, McBain CJ, Gallo V. Neurotransmitter receptor activation triggers p27(Kip1) and p21(CIP1) accumulation and G1 cell cycle arrest in oligodendrocyte progenitors. Development. 1999;126:1077–1090. doi: 10.1242/dev.126.5.1077. [DOI] [PubMed] [Google Scholar]
- Gyllensten L, Malmfors T. Myelination of the optic nerve and its dependence on visual function: a quantitative investigation in mice. J Embryol Exp Morphol. 1963;11:255–256. [PubMed] [Google Scholar]
- He M, McCarthy KD. Oligodendroglial signal transduction systems are regulated by neuronal contact. J Neurochem. 1994;63:501–508. doi: 10.1046/j.1471-4159.1996.67041491.x. [DOI] [PubMed] [Google Scholar]
- Itoh K, Stevens B, Schachner M, Fields RD. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–1372. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- Kastritsis CH, McCarthy KD. Oligodendroglial lineage cells express neuroligand receptors. Glia. 1993;8:106–113. doi: 10.1002/glia.440080206. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Scherer J, Kettenmann H, Verkhratsky A. Activation of P2-purinoreceptors triggered calcium release from InsP3-sensitive internal stores in mammalian oligodendrocytes. J Physiol (Lond) 1995;483:41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg MS, Silldorff EP, Pallone TL. Localization of adenosine-receptor subtype mRNA in rat outer medullary descending vasa recta by RT-PCR. Am J Physiol. 1997;272:H1231–1238. doi: 10.1152/ajpheart.1997.272.3.H1231. [DOI] [PubMed] [Google Scholar]
- Kriegler S, Chiu SY. Calcium signaling of glial cells along mammalian axons. J Neurosci. 1993;13:4229–4245. doi: 10.1523/JNEUROSCI.13-10-04229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman AS, Volpert WA, Okamoto M. Release of adenine nucleotides from nerve axons. Nature. 1964;204:1000–1001. doi: 10.1038/2041000a0. [DOI] [PubMed] [Google Scholar]
- Li M, Jia M, Fields RD, Nelson PG. Modulation of calcium currents by electrical activity. J Neurophysiol. 1996;76:2595–2607. doi: 10.1152/jn.1996.76.4.2595. [DOI] [PubMed] [Google Scholar]
- Lubetzki C, Demerens C, Anglade P, Villarroya H, Frankfurter A, Lee VM, Zalc B. Even in culture, oligodendrocytes myelinate solely axons. Proc Natl Acad Sci USA. 1993;90:6820–6824. doi: 10.1073/pnas.90.14.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire JC, Medilanski J, Straub RW. Release of adenosine, inosine, hypoxanthine from rabbit non-myelinated nerve fibers at rest and during activity. J Physiol (Lond) 1984;357:67–77. doi: 10.1113/jphysiol.1984.sp015489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Quasthoff S, Grafe P. Differences in the sensitivity to purinergic stimulation of myelinating and non-myelinating Schwann cells in peripheral human and rat nerve. Glia. 1998;23:374–382. doi: 10.1002/(sici)1098-1136(199808)23:4<374::aid-glia9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR. Schwann cell development, differentiation, and myelination. Curr Opin Neurobiol. 1996;6:89–96. doi: 10.1016/s0959-4388(96)80013-4. [DOI] [PubMed] [Google Scholar]
- Omlin FX. Optic disc and optic nerve of the blind cape molerat (Georychus capensis): a proposed model for naturally occurring reactive gliosis. Brain Res Bull. 1997;44:627–632. doi: 10.1016/s0361-9230(97)00283-9. [DOI] [PubMed] [Google Scholar]
- Park SK, Miller R, Krane I, Vartanian T. The erbB2 gene is required for the development of terminally differentiated spinal cord oligodendrocytes. J Cell Biol. 2001a;154:1245–1258. doi: 10.1083/jcb.200104025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Solomon D, Vartanian T. Growth factor control of CNS myelination. Dev Neurosci. 2001b;23:327–337. doi: 10.1159/000048716. [DOI] [PubMed] [Google Scholar]
- Rogister B, Ben-Hur T, Dubois-Dalcq M. From neural stem cells to myelinating oligodendrocytes. Mol Cell Neurosci. 1999;14:287–300. doi: 10.1006/mcne.1999.0790. [DOI] [PubMed] [Google Scholar]
- Scott, S.A. (1992). Sensory Neurons (New York: Oxford University Press).
- Seilheimer B, Persohn E, Schachner M. Antibodies to the L1 adhesion molecule inhibit Schwann cell ensheathment of neurons in vitro. J Cell Biol. 1989;109:3095–3103. doi: 10.1083/jcb.109.6.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J Neurosci. 1998;18:9303–9311. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart HJ, Morgan L, Jessen KR, Mirsky R. Changes in DNA synthesis rate in the Schwann cell lineage in vivo are correlated with the precursor-Schwann cell transition myelination. Eur J Neurosci. 1993;5:1136–1144. doi: 10.1111/j.1460-9568.1993.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Takeda M, Nelson DJ, Soliven B. Calcium signaling in cultured rat oligodendrocytes. Glia. 1995;14:225–236. doi: 10.1002/glia.440140308. [DOI] [PubMed] [Google Scholar]
- Tauber H, Waehneldt TV, Neuhoff V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci Lett. 1980;16:235–238. doi: 10.1016/0304-3940(80)90003-8. [DOI] [PubMed] [Google Scholar]
- Tokumoto YM, Durand B, Raff MC. An analysis of the early events when oligodendrocyte precursor cells are triggered to differentiate by thyroid hormone, retinoic acid or PDGF withdrawal. Dev Biol. 1999;213:327–339. doi: 10.1006/dbio.1999.9397. [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Wood PM, Schachner M, Bunge RP. Inhibition of Schwann cell myelination in vitro by antibody to the L1 adhesion molecule. J Neurosci. 1990;10:3635–3645. doi: 10.1523/JNEUROSCI.10-11-03635.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V. Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse model for developmental and physiological studies J. Neurosci Res. 2002;70:529–545. doi: 10.1002/jnr.10368. [DOI] [PubMed] [Google Scholar]
- Zalc B, Fields RD. Do action potentials regulate myelination? The Neuroscientist. 2000;6:1–9. doi: 10.1177/107385840000600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, Braun N, Kegal B, Heine P. New insights into molecular structure and function of ectonucleotidases in the nervous system. Neurochem Int. 1998;32:421–425. doi: 10.1016/s0197-0186(97)00126-5. [DOI] [PubMed] [Google Scholar]


