Abstract
Healthy older men manifest combined declines in testosterone concentrations, LH secretory burst mass (amount of LH released per pulse), and feedback-sensitive regularity of unknown cause. To test a unifying hypothesis of simultaneous reductions in GnRH outflow, gonadotrope responsiveness to GnRH, and androgenic negative feedback, we monitored LH secretion 1) after bolus iv injection of a 1000-fold range of randomly ordered individual doses of GnRH on separate mornings, 2) during unmodified (eugonadal) or testosterone-withdrawn (hypoandrogenemic) negative feedback, and 3) in 16 young (age, 18–35 yr) and 15 older (age, 60–85 yr) healthy men. LH secretory burst mass and pattern regularity were quantitated by intensive blood sampling, high specificity LH β-subunit-directed immunoradiometric assay, deconvolution analysis, and approximate entropy. GnRH dose responsiveness was assessed by four-parameter nonlinear regression analysis. We demonstrated that older men exhibit 1) delayed attainment of GnRH-evoked maximal LH secretion; 2) enhanced potency of GnRH stimulation in both the feedback-intact and feedback-withdrawn states; 3) elevated gonadotrope sensitivity to GnRH, unmasked by experimental testosterone depletion; 4) comparable young adult-like GnRH efficacy, independent of testosterone feedback milieu; and 5) diminished regularity of GnRH-induced LH release evident only during unmodified androgenic feedback. We conclude that a 3-fold interaction among GnRH dose, testosterone concentration, and age governs GnRH action, and age determines both testosterone-modulated and testosterone-independent actions of GnRH.
Abbreviations: ApEn, Approximate entropy; DEX, dexamethasone; IRMA, immunoradiometric assay; KTCZ, ketoconazole; Pl, placebo
Aging in healthy men is accompanied by a significant fall in systemic testosterone concentrations, incremental LH pulse amplitude (a reflection of the amount of LH secreted per burst), and the quantitative orderliness of LH release (an index of feedback control) (1–3). Model-based projections indicate that these ensemble adaptations would be consistent with relative hypogonadotropic hypogonadism (4–6). Available clinical and laboratory data exclude primary gonadotrope failure (7). Thus, assuming that GnRH is the principal peptidyl signal driving high amplitude LH pulses, mechanistic explanations would include decreased secretion of GnRH, impaired action of GnRH, and/or augmented negative feedback by sex steroids (8–11). The first postulate remains conjectural, because hypothalamic GnRH outflow cannot be quantitated directly in the human. The second idea has been examined by stimulating LH release with exogenous GnRH, but remains indeterminate. The third locus, androgenic negative feedback, has not been assessed under controlled GnRH drive. Indeed, LH responses to infused GnRH in elderly men are reportedly normal, accentuated, reduced, and/or delayed (8, 10–17). The bases for such discrepancies are not known, but might reflect 1) non-uniform subject selection; 2) variable GnRH dosimetry; 3) inconsistent sampling schedules; 4) cross-reactivity of certain LH antisera with free α-subunit, which increases in aging; 5) disparate baseline (pre-GnRH injection) LH concentrations; and/or 6) unequal testosterone availability to exert negative feedback on the hypothalamo-pituitary unit (15–31).
In an effort to reconcile the foregoing issues, we have appraised GnRH action in healthy young and older men by a novel composite strategy of 1) separate-day iv infusion of saline and randomly ordered GnRH pulses in individual doses that span a 1000-fold range; 2) frequent (10-min) blood sampling beginning 2 h before and continuing 3 h after each GnRH stimulus; 3) specific LH β-subunit-directed, two-site monoclonal immunoradiometric assay (IRMA); 4) deconvolution analysis to quantitate kinetically adjusted and baseline-corrected saline vs. GnRH-stimulated LH secretion; 5) nonlinear regression of LH secretory burst mass on injected GnRH dose; 6) approximate entropy (ApEn) analysis of the mass-independent regularity of LH release; and 7) response comparisons in the unmodified (intact) and experimentally clamped low androgen feedback milieus (17, 32, 33).
Subjects and Methods
Clinical protocol
A total of 31 healthy men (body mass index, 21–26 kg/m2) participated, 15 older (median age, 68 yr; range, 60–78 yr) and 16 young (median age, 23 yr; range, 18–35 yr) volunteers. Median body mass index values in the young and old men were 23 and 24, respectively (P = NS). Each subject provided written informed consent approved by the local institutional review board. Medical inventory and physical examination (including testis size, libido, and potentia) were normal. There was no history of infertility, systemic disease, recent weight change, hormonal therapy, or psychoactive drug use. Fasting (0800 h) screening biochemical tests of metabolic, hematological, hepatic, and renal function were normal. Baseline endocrine evaluation was unremarkable for age, including serum T4 (normal range, 4–10 μg/dl), total testosterone (≥300 ng/dl or ≥11 nmol/liter), estradiol (<40 pg/ml or <140 pmol/liter), LH (2–12 IU/liter), FSH (2–15 IU/liter), and prolactin (2–15 μg/liter).
An indwelling iv catheter was placed in a forearm vein at 0745 h on the day of study, and blood samples (1.5 ml) were withdrawn every 10 min for 5 h beginning at 0800 h. The first 120 min of blood sampling served as the baseline (pre-GnRH injection). At 1000 h, a single dose of GnRH or saline was given by bolus iv injection. Individual GnRH infusion sessions were scheduled in a randomly assigned order on separate mornings at least 5 d apart. Subjects assigned to the placebo (feedback-intact) intervention received GnRH doses of 0, 2.5, 10, 25, 100, 250, 750, and 2500 ng/kg (eight young and eight older men). Subjects assigned to ketoconazole/dexamethasone (KTCZ/DEX) received the same GnRH doses, except for the 2500 ng/kg dose (eight other young and seven older men; see below). The highest GnRH dose was omitted after KTCZ/DEX administration based on pilot data demonstrating that LH secretory responses are maximal at 250 and 750 ng/kg GnRH in the testosterone feedback-deprived setting.
To block adrenal and testicular steroidogenesis, KTCZ (1000 mg) was given orally with a nondairy snack at 2200 h. To maintain inhibition, a smaller dose of 400 mg KTCZ was administered orally at 0600 h with a light breakfast (17, 32, 33). DEX (0.5 mg, orally) was given concomitantly at 2200 h to obviate confounding of GnRH action by hypocortisolemic drive of the stress-adaptive corticotropic axis. Administration of 4-fold the latter glucocorticoid dose for 14 d does not alter gonadotropin responses to 100 ng/kg GnRH or 24-h LH pulsatility in men (34). A second dose of DEX (1 mg, orally) was given after completion of each sampling session under nursing observation before discharge from the study unit.
Assays
Blood samples were allowed to clot at room temperature, and sera were frozen at −20 C. LH concentrations were assayed by robotics-automated, LH β-subunit-directed, two-site monoclonal IRMA (Nichols Institute, Inc., San Juan Capistrano, CA) (15). The sensitivity of the LH assay is 0.2 IU/liter (First International Reference Preparation), and the median interassay coefficient of variation is 8.5%. Duplicate aliquots of all samples from any given subject were assayed as a single batch, which allows calculation of LH concentration-dependent intraassay variance as an algebraic exponential function of all sample means (217 or 248 pairs) in each subject (9). The median within-assay coefficient of variation was 5.8% (range, 3.9–6.5%). There is less than 0.03% cross-reactivity with free α- or LH β-subunit, FSH, or TSH (35, 36). Concentrations of total testosterone, estradiol, FSH, prolactin, TSH, and SHBG were measured in individual 2-h serum pools (0800–1000 h, baseline) using RIA (sex steroids) and IRMA (protein hormones; Diagnostic Products, Inc., Los Angeles, CA; and Diagnostic Laboratory Systems, Webster, TX) (24).
Data analysis
Multiple-parameter deconvolution analysis was used to quantitate saline vs. GnRH-stimulated LH secretory burst mass (37). The latter was defined as the amount of LH released per unit distribution volume (international units per liter) over the 3-h interval following saline or GnRH injection. Because each time series is relatively brief (5 h), we used a priori estimated biexponential kinetics of highly purified human pituitary LH infused earlier by bolus into hypopituitary men, viz. first and second component half-lives of 18 and 90 min (rapid and slower phases of elimination, respectively) and a fractional contribution of the slower component to total decay of 0.37 (38). Statistically comparable values were obtained after 6-min iv square-wave pulse infusion of recombinant human LH in leuprolide-suppressed men independently of age (39). Kinetically based deconvolution estimates allow mathematically valid distinction among 1) GnRH-stimulated LH secretory burst mass, 2) decay of previously secreted LH, and 3) concomitant basal LH secretion. The entire 5-h (0800–1300 h) LH concentration profile was analyzed first (40). From these data, we tabulated the summed mass of saline vs. GnRH-stimulated LH secretory bursts (international units per liter per 3 h). Response latency was defined as the time delay (minutes) after bolus iv GnRH injection to attain the maximal LH secretion rate (first derivative sign change), as distinguished from the peak serum LH concentration.
Nonlinear regression
The mass of LH secreted after saline or GnRH injection (international units per liter per 3 h) was regressed on GnRH dose (nanograms per kilogram) using a four-parameter logistic (monotonically ascending sigmoidal) function (41). The dose-response model parameters formalize estimates of baseline (zero dose GnRH-stimulated) LH release, GnRH potency (or half-maximally effective dose), gonadotrope sensitivity (maximal slope of the GnRH dose-LH secretory response function), and GnRH efficacy (asymptotically maximal LH secretion). A modified Gauss-Newton iterative procedure was used for simultaneous parameter estimation, followed by a calculation of joint 95% confidence interval via the support plane procedure (37, 41).
ApEn
ApEn analysis was applied to first-differenced (stationarized or epoch-detrended) LH-concentration time series (1000–1300 h) (42, 43). ApEn quantitates the regularity of hormone release, which is a barometer of feedback control that deteriorates in aging men (3, 44). Each LH time series was shuffled 1000 times to generate a distribution of empirically random (null) surrogate ApEn values (45, 46). The primary LH ApEn value (computed on the unshuffled original data) was normalized by computing its distance in standard deviates (sd or z-scores) from mean random ApEn of the matching null distribution. Thus, a more negative (higher absolute) ApEn sd denotes greater orderliness.
Statistical comparisons
One-way ANOVA was used as a preliminary test of the impact of GnRH dose on (log-transformed) LH secretory burst mass and ApEn and as the sole test of differences in baseline hormone concentrations among the four study cohorts. Tukey’s honestly significantly different test was applied post hoc to allow protected comparisons among means. Data are given as the mean ± sem (absolute values) or 95% confidence interval (regression parameters). P < 0.05 was construed as statistically significant.
Results
Figure 1 and Table 1 summarize mean baseline (pre-GnRH injection) hormone concentrations measured in 2-h serum pools (0800–1000 h) in young and older men randomly assigned to the placebo (Pl) or KTCZ/DEX intervention. In the Pl groups, concentrations of LH, estradiol, testosterone, prolactin, and TSH did not differ by age, whereas FSH and SHBG concentrations were higher, and testosterone/SHBG ratios were lower in older individuals.
Fig. 1.
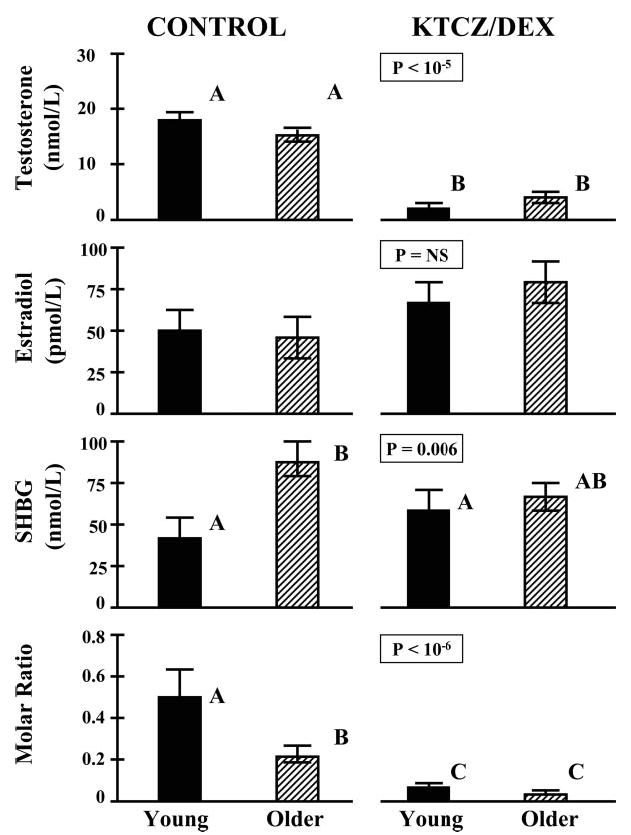
Concentrations of total testosterone, estradiol, and SHBG and the molar ratio of testosterone to SHBG in four cohorts of young and older men randomly assigned to receive oral placebo (control) or KTCZ/DEX overnight (see Subjects and Methods). Measurements were performed on a 2-h pool of serum collected by sampling blood every 10 min before saline or GnRH injection (0800–1000 h). Data are the mean ± sem [n = 8 young and 8 older men (control) and 8 young and 7 older men (KTCZ/DEX)]. Different (unshared) alphabetical superscripts denote significantly different means, as assessed by ANOVA at the corresponding P value (overall interventional effect).
Table 1.
Baseline protein-hormone concentrations
| Control
|
KTCZ/DEX
|
||||
|---|---|---|---|---|---|
| Hormonea | Young (n = 8) | Older (n = 8) | Young (n = 8) | Older (n = 7) | P value |
| LH (IU/liter) | 3.1 ± 0.41b,c | 2.8 ± 0.7b | 9.0 ± 1.1d | 8.5 ± 0.99c,d | <0.001 |
| FSH (IU/liter) | 2.8 ± 0.66b | 9.8 ± 1.5c | 6.3 ± 0.51d | 9.0 ± 2.4c | <0.001 |
| Prolactin (μg/liter) | 6.1 ± 10 | 7.4 ± 1.2 | 6.6 ± 0.97 | 6.6 ± 0.84 | NS |
| TSH (mU/liter) | 1.5 ± 0.28b,c | 1.9 ± 0.26b | 0.82 ± 0.09c | 1.2 ± 0.23b,c | 0.030 |
Data are the mean ± sem. Unshared (unique) alphabetic superscripts (b, c, and d) denote significantly different means as evaluated by ANOVA. Control subjects received placebo. n, Number of volunteers; NS, not significant.
Exposure to KTCZ/DEX compared with Pl increased baseline LH and FSH concentrations (Table 1) and reduced total testosterone concentrations to equivalent values in young and older men. In particular, in both age cohorts, testosterone after KTCZ/DEX treatment was less than 150 ng/dl (5.1 nmol/liter; each P < 0.001 compared with age-matched Pl; Fig. 1). Molar ratios of testosterone/SHBG concentrations fell to equally low values in the two study groups (both P <0.001 vs. Pl). Equilibrium dialysis gave free testosterone concentrations of 0.36 ± 0.04 nmol/liter (young) and 0.23 ± 0.03 nmol/liter (older; P < 0.02) after placebo and 0.04 ± 0.01 nmol/liter (young) and 0.05 ± 0.01 nmol/liter (older; P = NS) after KTCZ/DEX. Estradiol concentrations did not differ significantly by age or intervention. TSH concentrations were slightly lower in young volunteers given KTCZ/DEX than in older controls given Pl (Table 1). Baseline (0800–1000 h) cortisol concentrations were 16 ± 1 μg/dl (young) and 15 ± 1.5 μg/dl (older) in the Pl group and less than 2 μg/dl after active drug treatment (KTCZ/DEX).
Figure 2 shows mean (cohort) plots of GnRH-stimulated LH concentrations in young and older men randomized to receive Pl (Fig. 2A) or KTCZ/DEX (Fig. 2B). Deconvolution analysis followed by ANOVA revealed that the time delay to GnRH-stimulated maximal LH secretion was independent of GnRH dose exceeding 25 ng/kg. An age contrast was tested, therefore, using mean latency values for GnRH doses between 25 ng/kg and the maximum. Elderly subjects exhibited 1.4- and 1.3-fold greater time delays to reach maximal GnRH-induced LH secretion than young volunteers in the Pl and KTCZ/DEX contexts, respectively (both P <0.05; Fig. 3).
Fig. 2.
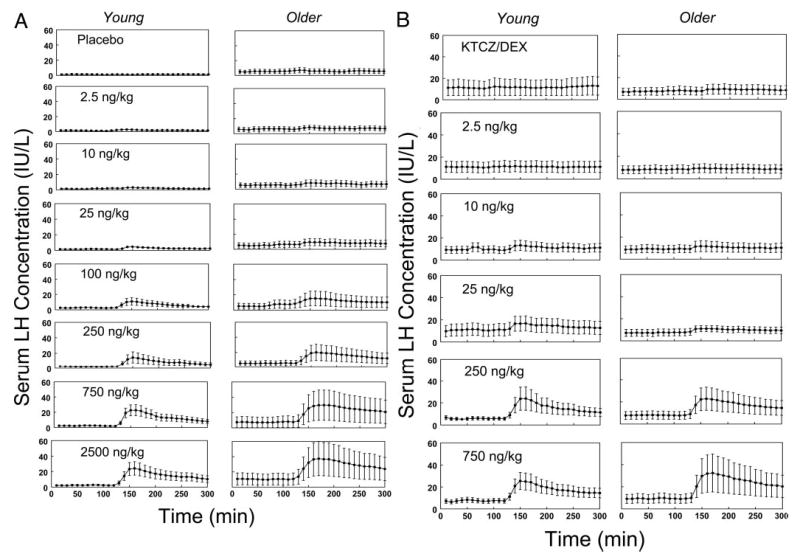
Serum LH concentration (international units per liter)-time series measured by LH β-subunit-directed, double-monoclonal IRMA in blood sampled every 10 min for 5 h in four cohorts of young and older men. Volunteers received either placebo (control; A) or KTCZ/DEX (B) at 2400 and 0800 h to inhibit testicular and adrenal steroidogenesis (see Subjects and Methods). Blood sampling was initiated at 0800 h (x-axis time, +10 min). A bolus of saline or a single randomly ordered dose of GnRH (inset values, nanograms per kilogram) was injected iv at 1000 h (+130 min) on separate mornings. Data comprise the mean ± sem (n is given in the legend of Fig. 1).
Fig. 3.
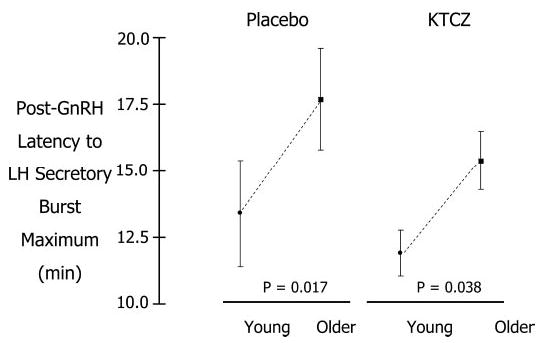
Time latency (minutes) to attain computed maximal LH secretion after iv injection of GnRH in young and older men given placebo (top) or KTCZ/DEX (bottom). Time delays are estimated from secretion rates by deconvolution analysis, rather than as concentration data (see Subjects and Methods). Data are the mean ± sem (see Fig. 1 for n).
Figure 4 depicts nonlinear regression relationships between LH secretory burst mass (international units per liter per 3 h) and GnRH dose (nanograms per kilogram) in young and older men randomized to receive Pl (Fig. 4A) or KTCZ/ DEX (Fig. 4B). ANOVA identified a significant effect of GnRH dose in both age cohorts under both conditions (P < 0.001). In the baseline eugonadal setting (Pl cohorts), older men maintained a 1.8-fold lower half-maximally effective dose of GnRH stimulation than young men (P < 0.05). This difference signifies 1.8-fold higher GnRH potency in the aging individual (Table 2). In contrast, young and older volunteers did not differ in baseline (saline-infused) LH secretory burst mass, the slope of the GnRH dose-response function (gonadotrope sensitivity), or the maximal LH secretory response (GnRH efficacy).
Fig. 4.
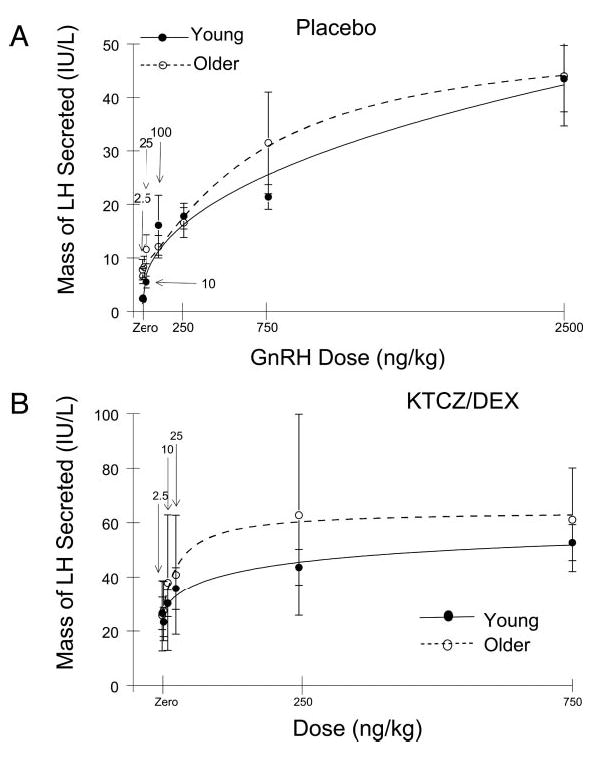
Nonlinear relationship between saline or GnRH dose and LH secretory response in healthy young and older men given placebo (A) or KTCZ/DEX (B). The y-axis depicts the calculated mass of LH secreted (international units per liter per 3 h) exceeding basal (nonpulsatile) and prestimulus LH secretion (see Subjects and Methods). The x-axis gives the injected dose of GnRH (nanograms per kilogram). Continuous curves are cohort-specific, four-parameter, logistic regression plots. Numerical values are the mean and 95% confidence interval.
Table 2.
Dose-response parameters relating the mass of LH secreted to the dose of GnRH injected in young and older men assessed under unmodified (Pl) and feedback-withdrawn (KTCZ/DEX) conditions
| Placebo
|
KTCZ/DEX
|
|||
|---|---|---|---|---|
| Parameter | Young (n = 8) | Older (n = 8) | Young (n = 8) | Older (n = 7) |
| Baseline LH burst massa | 3.3b (1.8–5.5) | 6.4b (2.1–9.3) | 23c (21–25) | 26c (22–23) |
| Sloped | 0.79b,c (0.62–1.02) | 0.73b,c (0.48–1.24) | 0.58b (0.49–0.68) | 1.0c (0.88–1.20) |
| ED50 e | 295b (218–415) | 163b (93–205) | 184b (157–204) | 30f (25–36) |
| Maximal LH releaseg | 42b (40–45) | 39b (37–43) | 64c (61–67) | 64c (63–66) |
Data are cohort mean estimates (and 95% confidence interval) for four-parameter logistic regression analysis (see Subjects and Methods). n, Number of volunteers. Unshared (unique) alphabetic superscripts (b, c, and d) denote significant means as evaluated by ANOVA.
Amount of LH (international units) secreted per unit distribution volume (liters) over the 3 h after saline injection.
International units per liter per 3 h (nanograms per kilogram), maximal slope of the calculated dose-response function, gonadotrope sensitivity.
Half-maximally effective dose of GnRH (nanograms per kilograms), GnRH potency.
Maximal gonadotrope secretory response (international units per liter per 3 h), GnRH efficacy.
Experimental testosterone deprivation achieved by KTCZ/DEX unmasked an additional age-related contrast and accentuated the GnRH potency difference (see above). Specifically, after KTCZ/DEX, the positive slope of the GnRH dose-LH secretory response curve, a measure of pituitary sensitivity, was 1.7-fold greater in older than young individuals (P < 0.01), and GnRH potency became 6.1-fold higher in elderly subjects (P < 0.01, age contrast; Table 2). In addition, compared with Pl, KTCZ/DEX administration 1) elevated unstimulated (after saline injection) pulsatile LH secretion, and 2) doubled GnRH efficacy (maximal GnRH-stimulated LH secretion). Age did not affect either outcome. Thus, combined age-related and hypoandrogenemic enhancements of GnRH potency and sensitivity are selective properties.
The ApEn statistic was applied to quantitate the impact of saline and GnRH on LH secretory regularity in the two androgenic milieus. ApEn provides a measure of feedback control that is independent of burst mass and absolute hormone concentrations (see Subjects and Methods). GnRH increased the orderliness of LH release patterns dose-dependently in both age cohorts (P < 0.001 each, by ANOVA) after both Pl and KTCZ/DEX blockade. Greater regularity was indicated by the higher absolute sd (z-scores) separating observed LH ApEn from mean random (shuffled) ApEn of the same series (Fig. 5). Age comparisons disclosed that in the Pl setting, older men failed to achieve young adult-like enhancement of LH secretory regularity in response to any of the three highest doses of GnRH tested (0.0045 ≥ P > 0.0001 vs. young men; Fig. 5A). The age-related distinction was abolished in cohorts exposed to KTCZ/DEX (Fig. 5B). In particular, hypoandrogenemia increased disorderliness of LH release independently of age stratum.
Fig. 5.
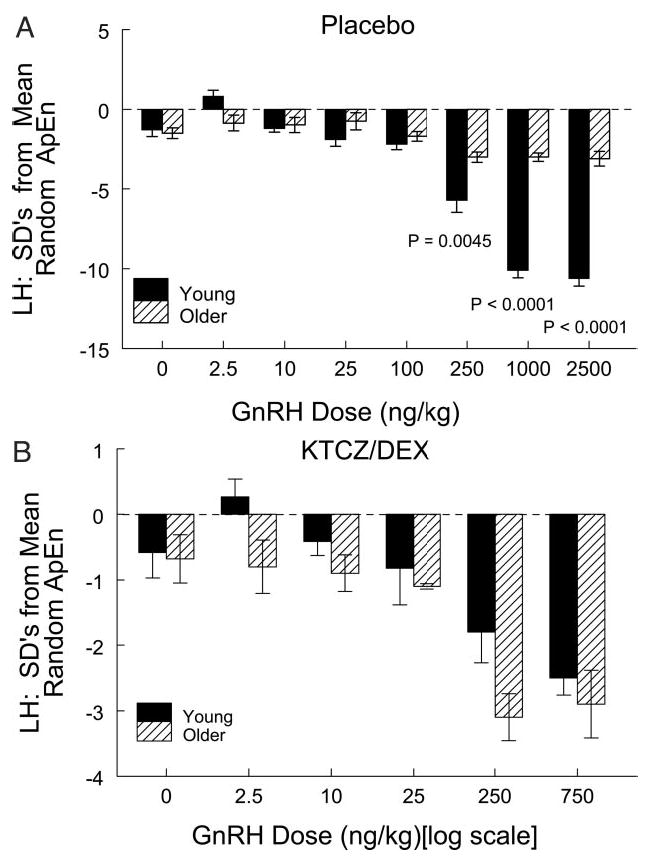
Impact of age stratum, GnRH dose and testosterone availability on the regularity (ApEn; y-axis) of LH release induced by bolus injection of saline or GnRH (nanograms per kilogram; x-axis). More regular LH release is denoted by a higher absolute number of SD separating observed LH ApEn from mean random ApEn determined from 1000 randomly shuffled versions of each time series (see Subjects and Methods). Increasing doses of GnRH enhanced the orderliness of LH release in young and older men given placebo (A) or KTCZ/DEX (B). Data are the mean ± sem (see Fig. 1 for n). Individually stated P values (A) denote post hoc contrasts by age at that GnRH dose.
Discussion
Earlier investigations of GnRH-driven LH secretion in aging men are inconsistent (see introduction). According to the present analytical platform, in the feedback-intact state, older compared with young subjects maintain 1) a 1.4-fold longer delay to attain calculated peak LH secretion, 2) a 1.8-fold greater GnRH potency, and 3) markedly reduced GnRH-stimulated regularity of LH release. In the low testosterone feedback state, older volunteers respond to GnRH with 1) a 1.3-fold prolonged latency to reach computed peak LH secretion rates, 2) a 6.1-fold higher GnRH potency, 3) a 1.7-fold greater pituitary sensitivity, and 4) comparable maximal GnRH-induced orderliness of LH release. GnRH efficacy (asymptotically maximal LH secretion) was independent of age, but increased 2-fold in response to peripheral testosterone depletion. These collective outcomes define a plausible working model for regulation of burst-like LH secretion and concomitantly offer a proximate explanation for prior disparate reports of GnRH action; viz. age, GnRH signal strength, and testosterone concentrations differentially govern gonadotrope sensitivity, GnRH potency, GnRH efficacy, and quantifiable orderliness of LH secretion.
Compared with placebo exposure, KTCZ/DEX administration reduced total testosterone concentrations profoundly in young and older men (to <50 ng/dl, <1.7 nmol/liter), as validated previously (17, 24, 32, 33). The combined regimen also decreased the molar ratio of testosterone/SHBG concentrations markedly and equivalently in the two age cohorts. The effect of the low androgen feedback state was to accentuate maximal GnRH-induced LH secretion (efficacy) by 2-fold independently of age stratum. Because GnRH efficacy also did not differ between young and older men given placebo, we infer that androgen negative feedback, rather than age per se, governs maximal LH secretory responsiveness to GnRH. This distinction is relevant to interpreting earlier studies that reported similar LH secretion after supraphysiological doses of GnRH in older and young men. In corollary, the current data imply that unequal testosterone availability among study volunteers would augment the variability of LH secretion induced by a pharmacological GnRH stimulus.
The hypoandrogenemic milieu enhanced not only GnRH efficacy (above), but also gonadotrope responsiveness to submaximally effective doses of GnRH. Testosterone depletion increased the estimated potency of GnRH in both cohorts, but the degree of increase differed significantly by age category. In particular, compared with placebo conditions, the low androgen feedback state augmented GnRH potency by 5.1-fold in elderly men and by only 1.6-fold in young individuals. Given a directionally similar difference in the placebo groups, the net age discrepancy unmasked by testosterone withdrawal was a 6.1-fold higher GnRH potency in elderly compared with young subjects. From these data, we infer that testosterone (or testosterone/SHBG) concentrations and age stratum in healthy men jointly govern the apparent potency of GnRH pulses.
As a subsidiary finding, time latencies to achieve maximal estimated LH secretion rates were prolonged in older men. The basis for this adaptation is not known. However, the time latency was not significantly affected by testosterone deprivation. Mechanistically, deconvolution analysis thereby accounts for an earlier observation that peak serum LH concentrations after GnRH injection are extended in older men (13).
Systemic testosterone depletion unveiled 1.7-fold higher pituitary sensitivity to GnRH in older than young subjects (greater positive slope of the GnRH dose-LH secretory response curve). The latter age-related distinction was not detectable without muting negative feedback. Thus, we hypothesize that short-term hypoandrogenemia may sensitize gonadotrope responses to GnRH stimulation disproportionately in the elderly male. If this inference is valid, then lesser baseline testosterone availability in older men could explain other reports of accentuated LH release induced by a single submaximally effective GnRH stimulus. Accordingly, we infer that age and testosterone concentrations together determine not only GnRH potency, but also pituitary sensitivity to GnRH.
From a technical vantage, recent kinetic analyses indicate that age does not affect the measured distribution volume and biexponential disappearance rate of recombinant human LH or the estimated half-life of endogenous LH in normal men (1, 39, 47). In contradistinction, incremental LH peak amplitude, the area of LH concentration pulses, and the calculated LH secretory burst mass are each 50% lower in older than young men (1, 6, 15, 17). Thus, to the extent that high amplitude LH bursts are driven by hypothalamic GnRH release (48, 49), we hypothesize that episodic GnRH secretion is reduced in the elderly male. By way of precedence for this inference, laboratory studies have documented diminished in vivo and in vitro hypothalamic GnRH release in the aged male rodent (7, 9, 50–54). This interpretation does not stand alone, because recent biomathematical models of the integrated male gonadal axis predict that combined GnRH and androgen depletion are required to generate both low amplitude LH secretory bursts and disorderly LH release (see below) (4–6).
ApEn or pattern regularity analysis was used as a model-free (burst mass-independent) measure of the capability of increasing doses of GnRH to enhance the orderliness of LH release in young and aging volunteers (3, 42, 43). In all four cohorts, higher doses of GnRH induced quantitatively more regular patterns of LH secretion, as defined objectively by the number of ApEn standard deviates removed from empirically mean random (see Subjects and Methods). From an analytical vantage, more orderly LH release enforced by elevated GnRH drive signifies enhanced synchrony of stimulated gonadotrope secretion (5, 42, 46). Secretory regularity is not a statistical artifact of increased LH concentrations, because normalized ApEn (as used here) is a scale-independent statistic (3, 45). Comparisons by age unveiled that in the testosterone feedback-intact setting, older men fail to achieve maximal (young adult-like) orderliness of LH secretion. This age distinction vanished when testosterone restraint was withdrawn pharmacologically. A unifying explanation is that GnRH stimulus strength, age, and testosterone concentrations all supervise the orderliness of LH release. In support of this hypothesis, we have demonstrated that experimental hypoandrogenemia evokes more irregular patterns of 24-h LH release even in young men (32, 33).
In summary, age and testosterone concentrations jointly regulate the potency and sensitivity of GnRH in driving LH secretory burst mass and orderly patterns of LH secretion in healthy men. Testosterone availability alone determines GnRH efficacy. Determining the relative age at which specific disruption of GnRH feedforward putatively emerges will require prospective cohort analyses.
Acknowledgments
We thank Kris Nunez and Kandace Bradford for assisting in the preparation of the manuscript.
Footnotes
This work was supported in part by Grant MO1-RR-00585 to the Mayo Clinic and Foundation General Clinical Research Center from the National Center for Research Resources (Rockville, MD), Grant R01-AG-23133 from the NIH (Bethesda, MD), and a Merit-Review Grant from the U.S. Department of Veterans Affairs (Washington, D.C.).
References
- 1.Veldhuis JD, Urban RJ, Lizarralde G, Johnson ML, Iranmanesh A. Attenuation of luteinizing hormone secretory burst amplitude is a proximate basis for the hypoandrogenism of healthy aging in men. J Clin Endocrinol Metab. 1992;75:52–58. doi: 10.1210/jcem.75.3.1517359. [DOI] [PubMed] [Google Scholar]
- 2.Veldhuis JD, Iranmanesh A, Mulligan T, Pincus SM. Disruption of the young-adult synchrony between luteinizing hormone release and oscillations in follicle-stimulating hormone, prolactin, and nocturnal penile tumescence (NPT) in healthy older men. J Clin Endocrinol Metab. 1999;84:3498–3505. doi: 10.1210/jcem.84.10.6100. [DOI] [PubMed] [Google Scholar]
- 3.Pincus SM, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc Natl Acad Sci USA. 1996;93:14100–14105. doi: 10.1073/pnas.93.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keenan DM, Veldhuis JD. A biomathematical model of time-delayed feedback in the human male hypothalamic-pituitary-Leydig cell axis. Am J Physiol. 1998;275:E157–E176. doi: 10.1152/ajpendo.1998.275.1.E157. [DOI] [PubMed] [Google Scholar]
- 5.Keenan DM, Veldhuis JD. Hypothesis testing of the aging male gonadal axis via a biomathematical construct. Am J Physiol. 2001;280:R1755–R1771. doi: 10.1152/ajpregu.2001.280.6.R1755. [DOI] [PubMed] [Google Scholar]
- 6.Keenan DM, Veldhuis JD. Disruption of the hypothalamic luteinizing-hormone pulsing mechanism in aging men. Am J Physiol. 2001;281:R1917–R1924. doi: 10.1152/ajpregu.2001.281.6.R1917. [DOI] [PubMed] [Google Scholar]
- 7.Veldhuis JD, Johnson ML, Keenan D, Iranmanesh A 2003 The ensemble male hypothalamo-pituitary-gonadal axis. In: Timiras PS, ed. Physiological basis of aging and geriatrics, 3rd Ed. Boca Raton, FL: CRC Press; 213–231
- 8.Veldhuis JD, Urban RJ, Dufau ML. Differential responses of biologically active LH secretion in older versus young men to interruption of androgen negative feedback. J Clin Endocrinol Metab. 1994;79:1763–1770. doi: 10.1210/jcem.79.6.7989483. [DOI] [PubMed] [Google Scholar]
- 9.Urban RJ, Evans WS, Rogol AD, Kaiser DL, Johnson ML, Veldhuis JD. Contemporary aspects of discrete peak detection algorithms. I. The paradigm of the luteinizing hormone pulse signal in men. Endocr Rev. 1988;9:3–37. doi: 10.1210/edrv-9-1-3. [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen A, Rubens R, Verdonck L. Testosterone secretion and metabolism in male senescence. J Clin Endocrinol Metab. 1972;34:730–735. doi: 10.1210/jcem-34-4-730. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Barcena D, Kastin AJ, Schalch DS, Bermudez JA, Lee D, Arimura A, Ruelas J, Zepeda I, Schally AV. Synthetic LH-releasing hormone (LH-RH) administered to normal men by different routes. J Clin Endocrinol Metab. 1973;37:481–484. doi: 10.1210/jcem-37-3-481. [DOI] [PubMed] [Google Scholar]
- 12.Haug E, Aakvaag A, Sand T, Torjesen PA. The gonadotrophin response to synthetic gonadotrophin-releasing hormone in males in relation to age, dose, and basal serum levels of testosterone, oestradiol-17β and gonadotrophins. Acta Endocrinol (Copenh) 1974;77:625–635. doi: 10.1530/acta.0.0770625. [DOI] [PubMed] [Google Scholar]
- 13.Snyder PJ, Reitano JF, Utiger RD. Serum LH and FSH responses to synthetic gonadotropin-releasing hormone in normal men. J Clin Endocrinol Metab. 1975;41:938–945. doi: 10.1210/jcem-41-5-938. [DOI] [PubMed] [Google Scholar]
- 14.Harman SM, Tsitouras PD, Costa PT, Blackman MR. Reproductive hormones in aging men. II. Basal pituitary gonadotropins and gonadotropin responses to luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. 1982;54:547–551. doi: 10.1210/jcem-54-3-547. [DOI] [PubMed] [Google Scholar]
- 15.Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig-cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol. 1999;141:257–266. doi: 10.1530/eje.0.1410257. [DOI] [PubMed] [Google Scholar]
- 16.Zwart AD, Urban RJ, Odell WD, Veldhuis JD. Contrasts in the gonadotropin-releasing dose-response relationships for luteinizing hormone, follicle-stimulating hormone, and α-subunit release in young versus older men: appraisal with high-specificity immunoradiometric assay and deconvolution analysis. Eur J Endocrinol. 1996;135:399–406. doi: 10.1530/eje.0.1350399. [DOI] [PubMed] [Google Scholar]
- 17.Veldhuis JD, Zwart A, Mulligan T, Iranmanesh A. Muting of androgen negative feedback unveils impoverished gonadotropin-releasing hormone/luteinizing hormone secretory reactivity in healthy older men. J Clin Endocrinol Metab. 2001;86:529–535. doi: 10.1210/jcem.86.2.7200. [DOI] [PubMed] [Google Scholar]
- 18.Madersbacher S, Stulnig T, Huber LA, Schonitzer D, Dirnhofer S, Wick G, Berger P. Serum glycoprotein hormones and their free α-subunit in a healthy elderly population selected according to the SENIEUR protocol. Analyses with ultrasensitive time resolved fluoroimmunoassays. Mech Aging Dev. 1993;71:223–233. doi: 10.1016/0047-6374(93)90086-7. [DOI] [PubMed] [Google Scholar]
- 19.Celani MF, Montanini V, Baraghini GF, Carani C, Marrama P. Effects of acute stimulation with gonadotropin releasing hormone (GnRH) on biologically active serum luteinizing hormone (LH) in elderly men. J Endocrinol Invest. 1984;7:589–595. doi: 10.1007/BF03349491. [DOI] [PubMed] [Google Scholar]
- 20.Rebar R, Yen SSC, Vandenberg G, Naftolin F, Ehara Y, Engblom S, Ryan KJ, Rivier J, Amoss M, Guillemin R. Gonadotropin responses to synthetic LRF: dose-response relationship in men. J Clin Endocrinol Metab. 1973;36:10–16. doi: 10.1210/jcem-36-1-10. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman JM, Giri M, Deslypere JM, Thomas G, Vermeulen A. Influence of age on the responsiveness of the gonadotrophs to luteinizing hormone-releasing hormone in males. J Clin Endocrinol Metab. 1991;72:1255–1260. doi: 10.1210/jcem-72-6-1255. [DOI] [PubMed] [Google Scholar]
- 22.Muta K, Kato K, Akamine Y, Ibayashi H. Age-related changes in the feedback regulation of gonadotrophin secretion by sex steroids in men. Acta Endocrinol (Copenh) 1981;96:154–162. doi: 10.1530/acta.0.0960154. [DOI] [PubMed] [Google Scholar]
- 23.Winters SJ, Troen P. Episodic luteinizing hormone (LH) secretion and the response of LH and follicle-stimulating hormone to LH-releasing hormone in aged men: evidence for coexistent primary testicular insufficiency and an impairment in gonadotropin secretion. J Clin Endocrinol Metab. 1982;55:560–565. doi: 10.1210/jcem-55-3-560. [DOI] [PubMed] [Google Scholar]
- 24.Schnorr JA, Bray MJ, Veldhuis JD. Aromatization mediates testosterone’s short-term feedback restraint of 24-hour endogenously driven and acute exogenous GnRH-stimulated LH and FSH secretion in young men. J Clin Endocrinol Metab. 2001;86:2600–2606. doi: 10.1210/jcem.86.6.7520. [DOI] [PubMed] [Google Scholar]
- 25.Mulligan T, Delemarre-van de Waal HA, Johnson ML, Veldhuis JD. Validation of deconvolution analysis of LH secretion and half-life. Am J Physiol. 1994;267:R202–R211. doi: 10.1152/ajpregu.1994.267.1.R202. [DOI] [PubMed] [Google Scholar]
- 26.Mulligan T, Iranmanesh A, Johnson ML, Straume M, Veldhuis JD. Aging alters feedforward and feedback linkages between LH and testosterone in healthy men. Am J Physiol. 1997;273:R1407–R1413. doi: 10.1152/ajpregu.1997.273.4.R1407. [DOI] [PubMed] [Google Scholar]
- 27.Deslypere JP, Kaufman JM, Vermeulen T, Vogelaers D, Vandalem JL, Vermeulen A. Influence of age on pulsatile luteinizing hormone release and responsiveness of the gonadotrophs to sex hormone feedback in men. J Clin Endocrinol Metab. 1987;64:68–73. doi: 10.1210/jcem-64-1-68. [DOI] [PubMed] [Google Scholar]
- 28.Veldhuis JD 1999 Male hypothalamic-pituitary-gonadal axis. In: Yen SSC, Jaffe RB, Barbieri RL, eds. Reproductive endocrinology, 4th Ed. Philadelphia: Saunders; 622–631
- 29.Shecker CB, Matsumoto AM, Bremner WJ. Testosterone administration inhibits gonadotropin secretion by an effect directly on the human pituitary. J Clin Endocrinol Metab. 1989;68:397–401. doi: 10.1210/jcem-68-2-397. [DOI] [PubMed] [Google Scholar]
- 30.Baker HWG, Burger HG, de Kretser DM, Hudson B, O’Connor S, Wang C, Mirovics A, Court J, Dunlop M, Rennie GC. Changes in the pituitary-testicular system with age. Clin Endocrinol (Oxf) 1976;5:349–372. doi: 10.1111/j.1365-2265.1976.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 31.Tenover JS, Matsumoto AM, Plymate SR, Bremner WJ. The effects of aging in normal men on bioavailable testosterone and luteinizing hormone secretion: response to clomiphene citrate. J Clin Endocrinol Metab. 1987;65:1118–1125. doi: 10.1210/jcem-65-6-1118. [DOI] [PubMed] [Google Scholar]
- 32.Veldhuis JD, Zwart AD, Iranmanesh A. Neuroendocrine mechanisms by which selective Leydig-cell castration unleashes increased pulsatile LH release in the human: an experimental paradigm of short-term ketoconazole-induced hypoandrogenemia and deconvolution-estimated LH secretory enhancement. Am J Physiol. 1997;272:R464–R474. doi: 10.1152/ajpregu.1997.272.2.R464. [DOI] [PubMed] [Google Scholar]
- 33.Zwart A, Iranmanesh A, Veldhuis JD. Disparate serum free testosterone concentrations and degrees of hypothalamo-pituitary-LH suppression are achieved by continuous versus pulsatile intravenous androgen replacement in men: a clinical experimental model of ketoconazole-induced reversible hypoandrogenemia with controlled testosterone add-back. J Clin Endocrinol Metab. 1997;82:2062–2069. doi: 10.1210/jcem.82.7.4035. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuis JD, Lizarralde G, Iranmanesh A. Divergent effects of short-term glucocorticoid excess on the gonadotropic and somatotropic axes in normal men. J Clin Endocrinol Metab. 1992;74:96–102. doi: 10.1210/jcem.74.1.1727834. [DOI] [PubMed] [Google Scholar]
- 35.Veldhuis JD, Iranmanesh A, Evans WS, Lizarralde G, Thorner MO, Vance ML. Amplitude suppression of the pulsatile mode of immunoradiometric LH release in fasting-induced hypoandrogenemia in normal men. J Clin Endocrinol Metab. 1993;76:587–593. doi: 10.1210/jcem.76.3.8445014. [DOI] [PubMed] [Google Scholar]
- 36.Veldhuis JD, Wilkowski MJ, Urban RJ, Lizarralde G, Iranmanesh A, Bolton WK. Evidence for attenuation of hypothalamic GnRH impulse strength with preservation of gonadotropin-releasing hormone (GnRH) pulse frequency in men with chronic renal failure. J Clin Endocrinol Metab. 1993;76:648–654. doi: 10.1210/jcem.76.3.8445020. [DOI] [PubMed] [Google Scholar]
- 37.Veldhuis JD, Carlson ML, Johnson ML. The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci USA. 1987;84:7686–7690. doi: 10.1073/pnas.84.21.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veldhuis JD, Fraioli F, Rogol AD, Dufau ML. Metabolic clearance of biologically active luteinizing hormone in man. J Clin Invest. 1986;77:1122–1128. doi: 10.1172/JCI112411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulligan T, Iranmanesh A, Veldhuis JD. Pulsatile iv infusion of recombinant human LH in leuprolide-suppressed men unmasks impoverished Leydig-cell secretory responsiveness to midphysiological LH drive in the aging male. J Clin Endocrinol Metab. 2001;86:5547–5553. doi: 10.1210/jcem.86.11.8004. [DOI] [PubMed] [Google Scholar]
- 40.Veldhuis JD, Johnson ML. Specific methodological approaches to selected contemporary issues in deconvolution analysis of pulsatile neuroendocrine data. Methods Neurosci. 1995;28:25–92. [Google Scholar]
- 41.Straume M, Veldhuis JD, Johnson ML. Model-independent quantification of measurement error: empirical estimation of discrete variance function profiles based on standard curves. Methods Enzymol. 1994;240:121–150. doi: 10.1016/s0076-6879(94)40046-6. [DOI] [PubMed] [Google Scholar]
- 42.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pincus SM, Hartman ML, Roelfsema F, Thorner MO, Veldhuis JD. Hormone pulsatility discrimination via coarse and short time sampling. Am J Physiol. 1999;277:E948–E957. doi: 10.1152/ajpendo.1999.277.5.E948. [DOI] [PubMed] [Google Scholar]
- 44.Veldhuis JD, Iranmanesh A, Godschalk M, Mulligan T. Older men manifest multifold synchrony disruption of reproductive neurohormone outflow. J Clin Endocrinol Metab. 2000;85:1477–1486. doi: 10.1210/jcem.85.4.6546. [DOI] [PubMed] [Google Scholar]
- 45.Veldhuis JD, Johnson ML, Veldhuis OL, Straume M, Pincus S. Impact of pulsatility on the ensemble orderliness (approximate entropy) of neurohormone secretion. Am J Physiol. 2001;281:R1975–R1985. doi: 10.1152/ajpregu.2001.281.6.R1975. [DOI] [PubMed] [Google Scholar]
- 46.Veldhuis JD, Straume M, Iranmanesh A, Mulligan T, Jaffe CA, Barkan A, Johnson ML, Pincus SM. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol. 2001;280:R721–R729. doi: 10.1152/ajpregu.2001.280.3.R721. [DOI] [PubMed] [Google Scholar]
- 47.Keenan DM, Veldhuis JD. Explicating hypergonadotropism in post-menopausal women: a statistical model. Am J Physiol. 2000;278:R1247–R1257. doi: 10.1152/ajpregu.2000.278.5.R1247. [DOI] [PubMed] [Google Scholar]
- 48.Pavlou SN, Veldhuis JD, Lindner J, Souza KH, Urban RJ, Rivier JE, Vale WW, Stallard DJ. Persistence of concordant LH, testosterone and α subunit pulses following LHRH antagonist administration in normal men. J Clin Endocrinol Metab. 1990;70:1472–1478. doi: 10.1210/jcem-70-5-1472. [DOI] [PubMed] [Google Scholar]
- 49.Kolp LA, Pavlou SN, Urban RJ, Rivier JC, Vale WW, Veldhuis JD. Abrogation by a potent GnRH antagonist of the estrogen/progesterone-stimulated surge-like release of LH and FSH in postmenopausal women. J Clin Endocrinol Metab. 1992;75:993–997. doi: 10.1210/jcem.75.4.1400893. [DOI] [PubMed] [Google Scholar]
- 50.Bourguignon JP, Gerard A, Franchimont P. Age-related difference in the effect of castration upon hypothalamic LHRH content in male rat. Neuroendocrinology. 1984;38:376–381. doi: 10.1159/000123920. [DOI] [PubMed] [Google Scholar]
- 51.Huang HH, Kissane JQ, Hawrylewicz EJ. Restoration of sexual function and fertility by fetal hypothalamic transplant in impotent aged male rats. Neurobiol Aging. 1987;8:465–472. doi: 10.1016/0197-4580(87)90042-x. [DOI] [PubMed] [Google Scholar]
- 52.Jarjour LT, Handelsman DJ, Swerdloff RS. Effects of aging on the in vitro release of gonadotropin-releasing hormone. Endocrinology. 1986;119:1113–1117. doi: 10.1210/endo-119-3-1113. [DOI] [PubMed] [Google Scholar]
- 53.Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J Androl. 2000;21:72–84. [PubMed] [Google Scholar]
- 54.Gruenewald DA, Matsumoto AM. Age-related decreases in serum gonadotropin levels and gonadotropin-releasing hormone gene expression in the medial preoptic area of the male rat are dependent upon testicular feedback. Endocrinology. 1991;129:2442–2450. doi: 10.1210/endo-129-5-2442. [DOI] [PubMed] [Google Scholar]


