Abstract
A specific role for the dopamine D3 receptor in behavior has yet to be elucidated. We now report that dopamine D2/D3 agonists elicit dose-dependent yawning behavior in rats, resulting in an inverted U-shaped dose-response curve. A series of experiments was directed toward the hypothesis that the induction of yawning is a D3 receptor-mediated effect, whereas the inhibition of the yawning observed at higher doses is due to competing D2 receptor activity. We compared several dopaminergic agonists with a range of in vitro D3 selectivity, including PD-128,907 [(S)-(+)-(4aR, 10bR)-3,4,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol HCl], PD-128,908 [(R)-(−)-(4aS,10bS)-3,4,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol HCl], quinelorane [(5aR-trans)-5,5a,6,7,8, 9,9a,10-octahydro-6-propylpyrido[2,3-g]quinazolin-2-amine dihydrochloride], pramipexole (N′ -propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine), 7-OH-DPAT [(±)-7-hydroxy-2-dipropylaminotetralin HBr], quinpirole [trans-(−)-(4aR)-4,4a,5,6,7,8, 8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g]quinoline HCl], bromocriptine [(+)-2-bromo-12′-hydroxy-2′-(1-methylethyl)-5′-(2-methylpropyl) ergotaman-3′,6′-18-trione methanesulfonate], and apomorphine [(R)-(−)-5,6,6a,7-tetrahydro-6-methyl-4H-dibenzo-[de,g]quinoline-10,11-diol HCl] with respect to their ability to induce yawning in rats. A series of D2/D3 antagonists differing in selectivity for D3 over D2 receptors were evaluated for their ability to alter the effects of the dopamine agonists. The antagonists L-741,626 (3-[4-(4-chlorophenyl)-4-hydroxypiperidin-l-yl]methyl-1 H-indole), haloperidol (4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-1-butanone HCl), nafadotride (N-[(1-butyl-2-pyrrolidinyl)methyl]-4-cyano-1-methoxy-2-naphthalenecarboxamide), U99194 (2,3-dihydro-5,6-dimethoxy-N,N-dipropyl-1H-inden-2-amine maleate), SB-277011A (trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide), and PG01037 (N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide HCl) were used to determine effects on dose-response curves for D2/D3 agonist-induced yawning. In addition, the potential contribution of cholinergic and/or serotonergic mechanisms to the yawning response was investigated using a series of pharmacological tools including scopolamine [(a,S)-a-(hydroxymethyl)benzeneacetic acid (1a,2b,4b,5a,7b)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]-non7-yl ester hydrobromide], mianserin (1,2,3,4,10,14b-hexahydro-2-methyldibenzo[c,f]pyrazino[1,2-a]azepine HCl), and the D3-preferring antagonists nafadotride, U99194, SB-277011A, and PG01037 to differentially modulate yawning induced by PD-128,907, physostigmine [(3aS)-cis-1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethylpyrrolo[2,3-b]indol-5-ol methylcarbamate hemisulfate], and N-[3-(trifluoromethyl)phenyl]piperazine HCl. The results of these experiments provide convergent evidence that dopamine D2/D3 agonist-induced yawning is a D3 agonist-mediated behavior, with subsequent inhibition of yawning being driven by competing D2 agonist activity. Thus, dopamine agonist-induced yawning may represent an in vivo method for selectively identifying D3 and D2 receptor-mediated activities.
ABBREVIATIONS: PD-128,907, (S)-(+)-(4aR,10bR)-3,4,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol HCl; 7-OH-DPAT, (±)-7-hydroxy-2-dipropylaminotetralin HBr; U99194, 2,3-dihydro-5,6-dimethoxy-N,N-dipropyl-1H-inden-2-amine maleate; SB-277011A, trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide; PG01037, N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide HCl; PD-128,908 [(R)-(−)-(4aS,10bS)-3,4,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol HCl; L-741,626, 3-[4-(4-chlorophenyl)-4-hydroxypiperidin-l-yl]methyl-1H-indole; SCH 23390, (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine HCl; L-745,870, 3-(4-[4-chlorophenyl] piperazin-1-yl)-methyl-1H-pyrrolo[2,3-b]pyridine trihydrochloride; TFMPP,N-[3-(trifluoromethyl)phenyl]piperazine HCl; 5-HT, 5-hydroxytryptamine; ANOVA, analysis of variance
Dopamine D3 receptors have received considerable interest since originally cloned (Sokoloff et al., 1990). The D3 receptor shares significant sequence homology with the dopamine D2 receptor but displays a much more restricted, limbic pattern of distribution compared with that of the D2 receptor in the rat (Levesque et al., 1992) and human brain (Gurevich and Joyce, 1999). Based in large part on this restricted distribution and high sequence homology, it has been hypothesized that the D3 receptor may be of interest as a pharmacological target for antipsychotics and antiparkinsonian therapeutics (for review, see Joyce, 2001). Additionally, the D3 receptor is thought to play a role in reinforcement pathways because the D3 receptor is expressed in high levels within the mesolimbic dopaminergic system and, more specifically, the nucleus accumbens shell (Sokoloff et al., 1990; Stanwood et al., 2000).
However, progress in defining a role for the D3 receptor has been slowed by the inability to identify behavioral effects that can be linked exclusively to a D3 mechanism (Levant, 1997). This is, at least in part, due to the lack of pharmacologically selective compounds acting at either the D3 or D2 receptors, as well as the fact that potentially selective agonists have failed to elicit obvious, direct behavioral changes. Although D2/D3 agonists and antagonists have been shown to produce changes in body temperature, locomotor activity, and other behavioral measures (Millan et al., 1995; Pugsley et al., 1995; Varty and Higgins, 1998), a role for the D3 receptor in the regulation of these effects has typically not been confirmed by studies using D3 receptor-deficient mice (Boulay et al., 1999a,b; Xu et al., 1999). Recently, increases in locomotor activity by dizocilpine maleate (MK-801) (Leriche et al., 2003) and blockade of the convulsant effects of dopamine uptake inhibitors (Witkin et al., 2004) have been proposed as in vivo models of D3 receptor activation. However, systematic replication of those findings or confirmation by other means has not been reported. The studies reported herein provide evidence supporting the contention that yawning induced by D2/D3 agonists is mediated specifically through D3 receptor activation.
The ability of dopaminergic agonists to elicit biphasic yawning resulting in an inverted U-shaped dose-response curve in rats has been a long-studied phenomenon (e.g., Mogilnicka and Klimek, 1977; Holmgren and Urba-Holmgren, 1980; Yamada and Furukawa, 1980). An early hypothesis regarding the biphasic regulation of apomorphine-induced yawning behavior attributed the induction of yawning behavior to a D2 agonist activity, whereas the inhibition seen at higher doses was thought to be due to a competing D1 agonist activation (Yamada and Furukawa, 1980; Urba-Holmgren et al., 1982). The cloning of the dopamine D3 receptor and the development of agonists such as PD-128,907 (Pugsley et al., 1995) and 7-OH-DPAT [(±)-7-hydroxy-2-dipropylaminotetralin HBr] (Levesque et al., 1992; Pugsley et al., 1995), as well as antagonists including U99194 (Cannon et al., 1982; Haadsma-Svensson and Svensson, 1998), SB-277011A (Reavill et al., 2000; Stemp et al., 2000), and PG01037 (Grundt et al., 2005) with greater degrees of in vitro selectivity for the D3 receptor have allowed greater insights into the regulation of dopaminergic agonist-induced yawning behavior to be made. Based on a series of studies examining the unconditioned behavioral effects of 7-OH-DPAT (Daly and Waddington, 1993; Ferrari and Giuliani, 1995; Kurashima et al., 1995), as well as binding studies (Levant et al., 1995), Levant (1997) hypothesized in an extensive review that D2/D3 agonist-induced yawning may be a D3 agonist-mediated effect, whereas the inhibition seen at higher doses was a result of concomitant D2 agonist activation.
This hypothesis was evaluated in the present studies using a host of pharmacological tools. The abilities of a series of compounds with varying in vitro selectivities for the D3 relative to D2 receptors to elicit yawning were examined. A series of antagonists, again defined by binding in vitro selectivity for the D3 and D2 receptors, were evaluated with respect to their modification of dose-response relationships for D2/D3 agonists, with the majority of the studies using PD-128,907 as a prototype D3 agonist.
Finally, in addition to dopaminergic mechanisms, yawning can be induced by cholinergic (Urba-Holmgren et al., 1977; Yamada and Furukawa, 1980) or serotonergic (Stancampiano et al., 1994) compounds. Although the exact mechanisms and neural pathways involved in the regulation of yawning behavior have not been fully elucidated, there is a large set of data that suggests that dopaminergic, serotonergic, and cholinergic induction of yawning occur via distinct mechanisms. In addition, both dopaminergic and serotonergic pathways are thought to eventually feed onto cholinergic neurons, thus allowing for differential regulation of dopaminergic and serotonergic yawning, with a cholinergic component common in all three pathways (for review, see Argiolas and Melis, 1998). Therefore, some D3 antagonists that reduced PD-128,907-induced yawning were also assessed for their capacity to alter nondopaminergic-induced yawning.
The convergent evidence from the agonist and antagonist studies support the hypothesis that dopamine agonist-induced yawning is mediated specifically through activation of D3 receptors. Therefore, yawning in rats may provide a critical model for establishing the in vivo activities of putative D3-selective ligands, a first step toward defining their role in normal and pathological physiological states.
Materials and Methods
Subjects
Male Sprague-Dawley rats (250–400 g) were obtained from Harlan (Indianapolis, IN) and housed three to a cage for the duration of the study. Rats had free access to standard Purina rodent chow and water and were maintained in a temperature- and humidity-controlled environment on a 12-h dark/light cycle with lights on at 7:00 AM. All studies were performed in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health, and all experimental procedures were approved by the University of Michigan Committee on the Use and Care of Animals.
Behavioral Observations
Yawning
Yawning behavior was defined as a prolonged (~1 s), wide opening of the mouth followed by a rapid closure. On the day of testing, rats were transferred from their home cage to a test chamber (19- × 9- × 8-inch clear shoe box rodent cage with standard cob bedding) and allowed to habituate to the chamber for a period of 30 min. Antagonist or vehicle was administered as a 30-min pretreatment prior to the injection of agonist or vehicle. Behavioral observations began 10 min after all injections, and the total number of yawns was recorded for a period of 20 min thereafter. Dose-response curves were first generated for all agonists with a vehicle pretreatment, with antagonists substituted for vehicle pretreatments in subsequent sets of experiments. Each rat was tested multiple times, with separate groups of rats used to establish dose-response curves for each agonist or antagonist × agonist combination. At least 48 h was allowed between experimental sessions to allow for a drug washout period. Food and water were unavailable during individual test sessions, and all experiments were conducted between the hours of 12:00 and 6:00 PM.
Dopamine D2/D3 Agonist-Induced Yawning
A series of dopaminergic agonists with varying degrees of in vitro selectivity for the D3 and D2 receptors were used to assess the ability of D2/D3 agonists to induce yawning behavior in rats. The D2/D3 agonists used in this series of experiments included 7-OH-DPAT (0.0032, 0.01, 0.032, and 0.1 mg/kg), apomorphine (0.001, 0.0032, 0.01, 0.032, 0.1, and 0.32 mg/kg), bromocriptine (0.32, 1.0, 3.2, and 10.0 mg/kg), PD-128,907 (0.0032, 0.01, 0.032, 0.1, and 0.32 mg/kg), PD-128,908 (0.01, 0.032, 0.1, 0.32, and 1.0 mg/kg), pramipexole (0.00032, 0.001, 0.0032, 0.01, 0.032, 0.1, 0.32, and 1.0 mg/kg), quinelorane (0.0001, 0.00032, 0.001, 0.0032, 0.01, and 0.032 mg/kg), and quinpirole (0.0032, 0.01, 0.032, 0.1, and 0.32 mg/kg). All agonists were investigated in separate groups of rats, with doses presented in a random order.
Effects of Dopaminergic Antagonists on D2/D3 Agonist-Induced Yawning Behavior
The effects of dopaminergic antagonists on D2/D3 agonist-induced yawning were examined, with each antagonist × agonist combination determined in separate groups of rats. Agonist and antagonist dose combinations were presented in a random order, with dose-response curves for vehicle × agonist treatments determined before and after antagonist × agonist combinations to insure there were no changes in agonist-induced yawning behavior.
D2-Selective Antagonists
The effects of L-741,626 (0.32 and 1.0 mg/kg) on yawning elicited by PD-128,907 (0.01, 0.032, 0.1, and 0.32 mg/kg) or quinelorane (0.00032, 0.001, 0.0032, 0.01, and 0.032 mg/kg) were determined. Only a dose of 1.0 mg/kg L-741,626 was used in the quinelorane-induced yawning studies.
Nonselective Dopamine Receptor Antagonism
Haloperidol was used to determine the effects of nonselective dopaminergic antagonist activity on PD-128,907 (0.032, 0.1, 0.32, and 1.0 mg/kg)- and quinelorane (0.001, 0.0032, 0.01, 0.032, and 0.1 mg/kg)-induced yawning. Doses of 0.01 and 0.032 mg/kg haloperidol were used in PD-128,907 experiments, whereas only the lowest active dose of 0.032 mg/kg was used in quinelorane studies.
D3-Selective Antagonists
The D3-preferring antagonists nafadotride (0.01, 0.1, and 0.32 mg/kg), U99194 (1.0, 3.2, and 10.0 mg/kg), SB-277011A (3.2, 32.0, and 56.0 mg/kg), and PG01037 (10.0, 32.0, and 56.0 mg/kg) were used to examine their effects on PD-128,907 (0.01, 0.032, 0.1, and 0.32 mg/kg)-induced yawning. In rats treated with 0.32 mg/kg nafadotride, the range of doses used for PD-128,907-induced yawning was extended to 1.0 mg/kg.
D1/D5- and D4-Selective Antagonists
The D1/D5 antagonist SCH 23390 (0.01 mg/kg) and the D4 antagonist L-745,870 (3.2 mg/kg) were used to address the possible involvement of these receptors in yawning elicited by PD-128,907 (0.01, 0.032, 0.1, and 0.32 mg/kg).
Effects of Cholinergic and Serotonergic Agonists and Antagonists on Yawning
Yawning elicited by cholinergic and serotonergic mechanisms was established by administration of physostigmine (0.01, 0.32, 0.1, 0.32, and 1.0 mg/kg i.p.) and TFMPP (0.32, 1.0, 3.2, and 10.0 mg/kg), respectively. Scopolamine (0.0001, 0.001, and 0.01 mg/kg) was used to examine the effects of muscarinic cholinergic antagonism on yawning elicited by physostigmine (0.1 mg/kg i.p.), TFMPP (3.2 mg/kg), and PD-128,907 (0.1 mg/kg). Likewise, the ability of the 5-HT2 receptor antagonist mianserin (0.0032, 0.032, and 0.32 mg/kg) to antagonize yawning induced by TFMPP (3.2 mg/kg), physostigmine (0.1 mg/kg i.p.), and PD-128,907 (0.1 mg/kg) was determined.
D3-Selective Antagonists on Cholinergic and Serotonergic Yawning
The ability of nafadotride (0.01, 0.1, and 1.0 mg/kg), U99194 (1.0, 3.2, and 10.0 mg/kg), SB-277011A (3.2, 32.0, and 56.0 mg/kg), and PG01037 (10.0, 32.0, and 56.0 mg/kg) to modulate yawning behavior induced by PD-128,907 (0.1 mg/kg), TFMPP (3.2 mg/kg), and physostigmine (0.1 mg/kg i.p.) was determined in separate groups of rats.
Drugs
(±)-7-OH-DPAT, (+)-bromocriptine [(+)-2-bromo-12′-hydroxy-2′-(1-methylethyl)-5′-(2-methylpropyl) ergotaman-3′, 6′-18-trione methanesulfonate], haloperidol [4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-1-butanone HCl], mianserin [1,2,3,4,10,14b-hexahydro-2-methyldibenzo[c,f]pyrazino[1,2-a]azepine HCl], PD-128,907, PD-128,908, physostigmine [(3aS)-cis-1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethylpyrrolo[2,3-b]indol-5-ol methylcarbamate hemisulfate], quinelorane [(5aR-trans)-5,5a,6,7,8,9,9a,10-octahydro-6-propylpyrido[2,3-g]quinazolin-2-amine dihydrochloride], (−)-quinpirole [trans-(−)-(4aR)-4,4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g]quinoline HCl], SCH 23390, scopolamine [(a,S)-a-(hydroxymethyl)benzeneacetic acid (1a,2b,4b,5a,7b)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]-non7-yl ester hydrobromide], and TFMPP were obtained from Sigma-Aldrich (St. Louis, MO). L-741,626, L-745,870, nafadotride [N-[(1-butyl-2-pyrrolidinyl)methyl]-4-cyano-1-methoxy-2-naphthalenecarboxamide], and U99194 were obtained from Tocris Cookson Inc. (Ellisville, MO). Pramipexole (N′ -propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine) was generously provided by Dr. Edward F. Domino (University of Michigan Medical School, Ann Arbor, MI), SB-277011A by Dr. Deyi Zhang (Lily Research Labs, Indianapolis, IN), and PG01037 by Dr. Amy H. Newman (Medicinal Chemistry Section–National Institute on Drug Abuse, Baltimore, MD). All drugs were dissolved in sterile water with the exception of haloperidol, which was dissolved in 5% ethanol, L-741,626, which was dissolved in 5% ethanol with 1 M HCl, and SB-277011A, which was dissolved in 10% β-cyclodextrin. All drugs were administered s.c. in a volume of 1 ml/kg, with the exception of physostigmine, which was administered i.p. in a volume of 1 ml/kg. The 56.0 mg/kg doses of SB-277011A and PG01037 were administered in a volume of 3 ml/kg s.c. due to solubility limitations.
Data Analysis
All yawning studies were conducted with eight rats per group, and results are expressed as mean number of yawns during the 20-min observation period ± S.E.M. A one-way, repeated-measures ANOVA with post hoc Dunnett’s tests was used to determine whether agonist-induced yawning was significantly greater compared with vehicle (GraphPad Prism; GraphPad Software Inc., San Diego, CA). Significant differences in the maximal amount of yawning elicited were determined by one-way repeated-measures ANOVA with post hoc Tukey’s honestly significant difference tests. Significant effects of antagonist pretreatment on agonist-induced yawning was determined using an unbalanced, two-way ANOVA with post hoc Bonferroni tests to determine significant differences among antagonist- and vehicle-treated groups (SPSS; SPSS Inc., Chicago, IL). One-way repeated-measures ANOVAs with post hoc Dunnett’s tests were also used to determine whether D3-preferring, cholinergic, or serotonergic antagonists significantly inhibited yawning elicited by the maximal effective dose of D2/D3, cholinergic, or serotonergic agonists (GraphPad Prism).
Results
Dopamine D2/D3 Agonists on Yawning Behavior
D2/D3 agonists generally elicited dose-dependent increases in yawning behavior, with a subsequent inhibition of yawning seen at higher doses resulting in a characteristic inverted U-shaped dose-response curve as shown in Fig. 1. PD-128,907 [F(5,35) = 19.86; p < 0.0001], quinelorane [F(6,42) = 29.68; p < 0.0001], pramipexole [F(8,56) = 14.50; p < 0.0001], 7-OH-DPAT [F(4,28) = 39.68; p < 0.0001], quinpirole [F(5,35) = 42.47; p < 0.0001], and apomorphine [F(6,42) = 3.81; p < 0.01] all elicited significant, dose-dependent increases in yawning behavior compared with vehicle, whereas yawning induced by bromocriptine [F(4,28) = 1.14; p > 0.05] failed to reach significance. PD-128,908, the inactive enantiomer of PD-128,907 (DeWald et al., 1990), did not elicit yawning at any dose tested [F(4,28) = 0.30; p > 0.05]. Significantly greater amounts of yawning compared with vehicle were observed for PD-128,907 (0.032 and 0.1 mg/kg; p < 0.01), quinelorane (0.001 and 0.0032 mg/kg; p < 0.01), pramipexole (0.01, 0.032, and 0.1 mg/kg; p < 0.01; 0.32 mg/kg; p < 0.05), 7-OH-DPAT (0.01 and 0.032 mg/kg; p < 0.01), quinpirole (0.01, and 0.032 mg/kg; p < 0.01), and apomorphine (0.032 mg/kg; p < 0.05).
Fig. 1.
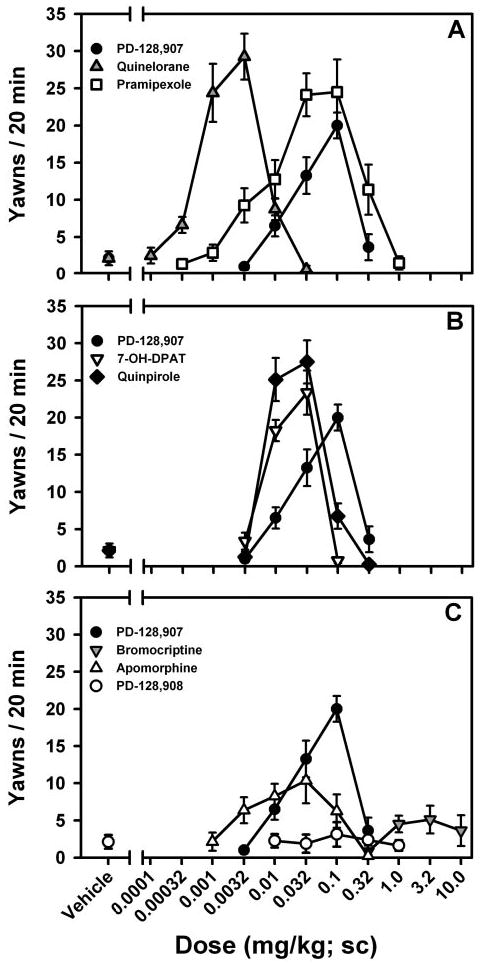
Dose-dependent induction of yawning by dopamine D3-preferring agonists: A, PD-128,907 (0.0032–0.32 mg/kg), quinelorane (0.0001–0.032 mg/kg), and pramipexole (0.00032–1.0 mg/kg); B, PD-128,907 (0.0032–0.32 mg/kg), 7-OH-DPAT (0.0032–0.1 mg/kg), and quinpirole (0.0032–0.32 mg/kg); and C, PD-128,907 (0.0032–0.32 mg/kg), bromocriptine (0.32–10.0 mg/kg), apomorphine (0.001–0.32 mg/kg), and PD-128,908 (0.01–1.0 mg/kg). Data are presented as mean (± S.E.M.), n = 8, number of yawns during a 20-min observation period.
There were no significant differences [F(4,28) = 1.70; p > 0.05] in the amount of yawning elicited by the maximal effective doses of PD-128,907 (0.1 mg/kg; 20.0 ± 1.7), quinelorane (0.0032 mg/kg; 29.3 ± 3.1), pramipexole (0.1 mg/kg; 24.5 ± 4.4), 7-OH-DPAT (0.032 mg/kg; 23.4 ± 3.0), and quinpirole (0.032 mg/kg; 27.5 ± 2.9); however, the maximal effective dose of apomorphine (0.032 mg/kg; 10.4 ± 3.1) [F(5,42) = 4.67; p < 0.01] produced significantly lower levels of yawning compared with all other D2/D3 agonists that elicited significant amounts of yawning.
D2-Selective Antagonism of D2/D3 Agonist-Induced Yawning
The effects of L-741,626, a D2-preferring antagonist approximately 50-fold selective for D2 compared with D3 receptors in vitro (Kulagowski et al., 1996) at behaviorally active doses (Chaperon et al., 2003), on PD-128,907- and quinelorane-induced yawning are shown in Fig. 2, A and B, respectively. An analysis of variance determined that there was an overall significant effect of L-741,626 on PD-128,907-induced yawning and that the effect was dependent on both the dose of L-741,626 and PD-128,907 administered [main antagonist dose effect, F(2,103) = 8.29, p < 0.001; main agonist dose effect, F(4,103) = 20.34, p < 0.001; antagonist dose × agonist dose interaction, F(6,103) = 7.52, p < 0.001]. Likewise, L-741,626 significantly modified quinelorane-induced yawning, an effect that was dependent on both the dose of L-741,626 as well as the dose of quinelorane [main antagonist dose effect, F(1,79) = 11.91, p < 0.001; main agonist dose effect, F(4,79) = 18.64, p < 0.001; antagonist dose × agonist dose interaction, F(4,79) = 11.81, p < 0.001]. L-741,626 significantly increased the amount of yawning elicited by high doses of both PD-128,907 (0.32 mg/kg; p < 0.001) and quinelorane (0.01 mg/kg; p < 0.001), but it did not have any effect on yawning induced by lower doses of either PD-128,907 or quinelorane.
Fig. 2.

Effects of the D2-selective antagonist L-741,626 (0.32 and 1.0 mg/kg) on PD-128,907 (0.0032–1.0 mg/kg)-induced yawning (A) and quinelorane (0.0001–0.032 mg/kg)-induced yawning (B). Effects of the nonselective dopamine receptor antagonist haloperidol (0.01 and 0.032 mg/kg) on PD-128,907 (0.0032–1.0 mg/kg)-induced yawning (C) and quinelorane (0.0001–0.1 mg/kg)-induced yawning (D). Data are presented as mean (± S.E.M.), n = 8, number of yawns during a 20-min observation period.*,p < 0.05;**, p < 0.01;***, p < 0.001. Significant difference from vehicle-treated animals was determined by unbalanced, two-way ANOVA with post hoc Bonferroni tests.
Nonselective Dopaminergic Antagonism of D2/D3 Agonist-Induced Yawning
Haloperidol, a nonselective dopaminergic antagonist with high affinities for all dopamine receptor subtypes (Sokoloff et al., 1992; Kulagowski et al., 1996), was used at behaviorally active doses (e.g., Leriche et al., 2003) to examine the effects of dopaminergic antagonism on yawning induced by PD-128907 and quinelorane (Fig. 2, C and D, respectively). Pretreatment with haloperidol modified PD-128,907-induced yawning in a manner that was dependent on the dose of agonist administered [main antagonist dose effect, F(2,79) = 1.86, p > 0.05; main agonist dose effect, F(3,79) = 12.52, p < 0.001; antagonist dose × agonist dose interaction, F(4,79) = 21.30, p < 0.001]. The effects of haloperidol on quinelorane-induced yawning were similar to those on PD-128,907-induced yawning and were dependent on both the dose of haloperidol and the dose of quinelorane [main antagonist dose effect, F(1,71) = 10.78, p < 0.01; main agonist dose effect, F(4,71) = 13.50, p < 0.001; antagonist dose × agonist dose interaction, F(3,71) = 22.55, p < 0.001]. Unlike L-741,626, haloperidol produced differential effects on D2/D3 agonist-induced yawning. Pretreatment with 0.032 mg/kg haloperidol resulted in significant decreases in yawning elicited by low doses of PD-128,907 (0.032 mg/kg; p < 0.05) and quinelorane (0.001 mg/kg; p < 0.01), whereas it produced significant increases in the amount of yawning elicited by high doses of PD-128,907 (0.32 mg/kg; p = 0.001) and quinelorane (0.01 and 0.032 mg/kg; p < 0.001 and p = 0.001, respectively).
D3-Preferring Antagonists on D2/D3 Agonist-Induced Yawning
Nafadotride, U99194, SB-277011A, and PG01037 have been shown to preferentially bind the D3 receptor over the D2 receptor in vitro, with D3 selectivities of approximately 3-, 30-, 100-, and 133-fold, respectively (Sautel et al., 1995; Audinot et al., 1998; Flietstra and Levant, 1998; Stemp et al., 2000; Grundt et al., 2005), and were used at behaviorally active doses (Waters et al., 1993; Vorel et al., 2002; Di Ciano et al., 2003, Leriche et al., 2003; Millan et al., 2004) to examine their effects on yawning behavior in rats.
The effects of nafadotride (0.01, 0.1, and 0.32 mg/kg) on PD-128,907-induced yawning are shown in Fig. 3A. An analysis of variance revealed that nafadotride altered PD-128,907-induced yawning in a manner that was dependent on the dose of agonist administered [main antagonist dose effect, F(3,135) = 0.34, p > 0.05; main agonist dose effect, F(4,135) = 20.48, p < 0.001; antagonist dose × agonist dose interaction, F(9,135) = 3.92, p < 0.001]. Although slight reductions in yawning elicited by low doses of PD-128,907 were observed with doses of 0.1 and 0.32 mg/kg nafadotride, these effects were not significant at either dose. However, pretreatment with 0.32 mg/kg nafadotride did produce significant increases in yawning elicited by 0.32 mg/kg PD-128,907 (p < 0.001).
Fig. 3.
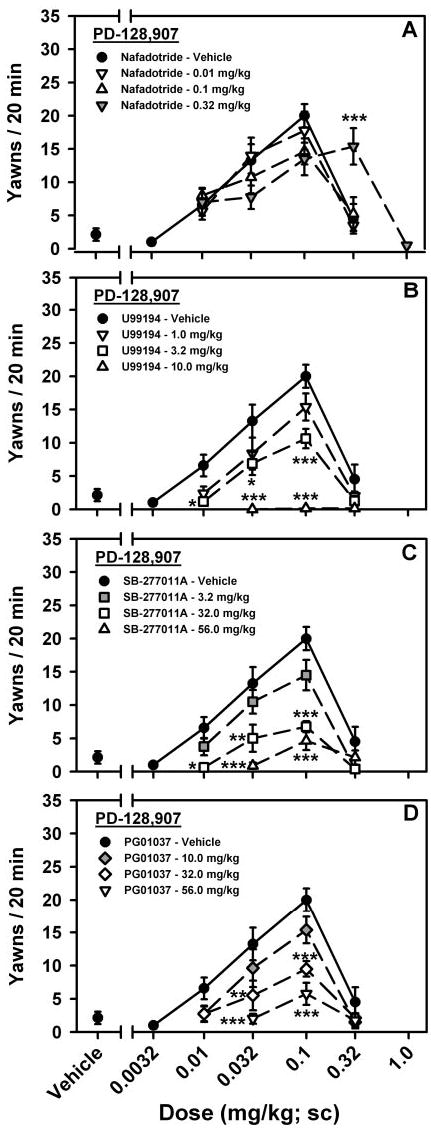
Effects of D3-preferring antagonists on PD-128,907 (0.0032–0.32 mg/kg)-induced yawning in rats. A, nafadotride at doses of 0, 0.001, 0.1, and 0.32 mg/kg; B, U99194 at doses of 0, 1.0, 3.2, and 10.0 mg/kg; C, SB-277011A at doses of 0, 3.2, 32.0, and 56.0 mg/kg; and D, PG01037 at doses of 0, 10.0, 32.0, and 56.0 mg/kg. Data are presented as mean (±S.E.M.), n = 8, number of yawns during a 20-min observation period.*, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant difference from vehicle-treated animals was determined by unbalanced, two-way ANOVA with post hoc Bonferroni tests.
The effects of U99194 (1.0, 3.2, and 10.0 mg/kg) on PD-128,907-induced yawning are shown in Fig. 3B. U99194 modified PD-128,907-induced yawning in a manner that was dependent on both the dose of U99194 and dose of PD-128,907 [main antagonist dose effect, F(3,119) = 40.08, p < 0.001; main agonist dose effect, F(3,119) = 42.26, p < 0.001; antagonist dose × agonist dose interaction, F(8,119) = 4.69, p < 0.001]. At a dose of 3.2 mg/kg, U99194 decreased the amount of yawning elicited by low doses of PD-128,907 (0.032 and 0.1 mg/kg; p < 0.05 for both), whereas there was no effect on yawning elicited by 0.32 mg/kg PD-128,907. At the highest dose of U99194 tested (10.0 mg/kg), PD-128,907-induced yawning was completely inhibited at all doses tested (0.032 mg/kg, p < 0.001; 0.1 mg/kg, p < 0.001; and 0.32 mg/kg, p > 0.05).
The effects of SB-277011A (3.2, 32.0, and 56.0 mg/kg) on PD-128,907-induced yawning are shown in Fig. 3C, and they were dependent on both the dose of SB-277011A as well as the dose of PD-128,907 administered [main antagonist dose effect, F(3,119) = 29.18, p < 0.001; main agonist dose effect, F(3,119) = 37.29, p < 0.001; antagonist dose × agonist dose interaction, F(8,119) = 4.40, p < 0.001]. SB-277011, at a dose of 32.0 mg/kg, significantly inhibited PD-128,907-induced yawning at doses corresponding to the ascending limb of the dose-response curve (0.01 mg/kg, p < 0.05; 0.032 mg/kg, p = 0.001; and 0.1 mg/kg, p < 0.001). Likewise, 56.0 mg/kg SB-277011 further reduced PD-128,907-elicited yawning at both 0.032 (p < 0.001) and 0.1 (p < 0.001) mg/kg. There were no effects of any dose of SB-277011A on yawning induced by a high dose of 0.32 mg/kg PD-128,907.
PG01037, a D3-preferring antagonist with similar in vitro selectivity for the D3 receptor compared with SB-277011A, was administered at doses of 10.0, 32.0, and 56.0 mg/kg, and the effects on PD-128,907-elicited yawning are shown in Fig. 3D. Pretreatment with PG01037 altered PD-128,907-induced yawning in a manner that was dependent on both the dose of PG01037 and dose of PD-128,907 administered [main antagonist dose effect, F(3,119) = 17.68, p < 0.001; main agonist dose effect, F(3,119) = 33.10, p < 0.001; antagonist dose × agonist dose interaction, F(8,119) = 2.69, p < 0.05]. Similar to SB-277011A, PG01037, at a dose of 32.0 mg/kg, significantly reduced yawning elicited by low doses of PD-128,907 (0.032 mg/kg, p < 0.01 and 0.1 mg/kg; p < 0.001). Further decreases in yawning induced by low doses of PD-128,907 (0.032 mg/kg, p < 0.001 and 0.1 mg/kg, p < 0.001) were observed with a dose of 56.0 mg/kg PG01037. There were no effects of any dose of PG01037 on yawning induced by a high dose of 0.32 mg/kg PD-128,907.
Other Dopamine Receptor Antagonists
The D1-like receptor-selective antagonist SCH 23390 (Barnett et al., 1986) and the D4-selective antagonist L-745,870 (Kulagowski et al., 1996) were used at behaviorally active doses (Patel et al., 1997; Chaperon et al., 2003) to assess the ability of D1/D5 and D4 antagonism, respectively, to modulate the dose-response curve for D2/D3 agonist-induced yawning. SCH 23390, at a dose of 0.01 mg/kg, did not produce any significant change in the amount of yawning elicited by any dose of PD-128,907 tested (0.01–0.32 mg/kg; data not shown). Likewise, at a dose of 3.2 mg/kg, the D4-selective antagonist L-745,870 failed to alter PD-128,907-induced yawning at any dose tested (0.01–0.32 mg/kg; data not shown).
Cholinergic- and Serotonergic-Induced Yawning
Both physostigmine [F(4,28) = 7.11; p < 0.001] and TFMPP [F(4,28) = 7.15; p < 0.001] also elicited inverted U-shaped, dose-dependent yawning behavior in rats, as shown in Fig. 4A; however, both the cholinergic and serotonergic agonists were significantly less effective compared with PD-128,907 [F(2,14) = 9.50; p < 0.01]. Maximal amounts of yawning induced by physostigmine and TFMPP occurred at doses of 0.1 and 3.2 mg/kg i.p., respectively and were the only doses to elicit significantly greater amounts of yawning compared with vehicle-treated rats (p < 0.01 for both).
Fig. 4.
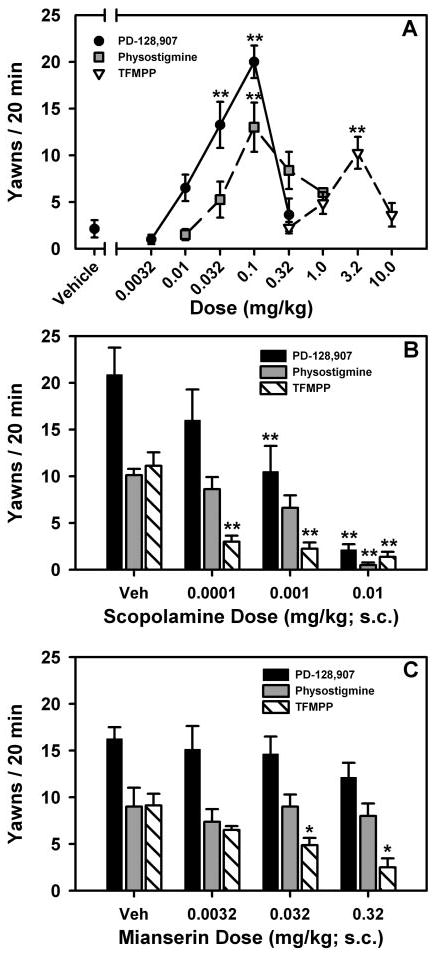
A, dose-response curves for PD-128,907 (0.0032–0.32 mg/kg)-, physostigmine (0.01–1.0 mg/kg i.p.)-, and TFMPP (0.32–10.0 mg/kg)-induced yawning in rats. B, effects of scopolamine (0, 0.0001, 0.001, and 0.01 mg/kg) on yawning induced by PD-128,907 (0.1 mg/kg), physostigmine (0.1 mg/kg i.p.), and TFMPP (3.2 mg/kg). C, effects of mianserin (0, 0.0032, 0.032, and 0.32 mg/kg) on yawning induced by PD-128,907 (0.1 mg/kg), physostigmine (0.1 mg/kg i.p.), and TFMPP (3.2 mg/kg). Data are presented as mean (± S.E.M.), n = 8, number of yawns during a 20-min observation period. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant difference from vehicle-treated rats was determined by one-way repeated-measures ANOVAs with post hoc Dunnett’s tests.
The effects of the nonselective, muscarinic antagonist, scopolamine (0.0001, 0.001, and 0.01 mg/kg), on yawning elicited by physostigmine (0.1 mg/kg i.p.), PD-128,907 (0.1 mg/kg), and TFMPP (3.2 mg/kg) are shown in Fig. 4B. Scopolamine produced significant, dose-dependent antagonism of physostigmine-induced yawning [F(3,21) = 16.89; p < 0.0001], with a dose of 0.01 mg/kg scopolamine significantly inhibiting physostigmine-induced yawning compared with vehicle-treated rats (p < 0.01). In addition, scopolamine dose dependently and significantly inhibited yawning elicited by both PD-128,907 [F(3,21) = 17.25; p < 0.0001] and TFMPP [F(3,21) = 22.40; p < 0.0001]. Significantly lower levels of PD-128,907-induced yawning were observed with doses of 0.001 and 0.01 mg/kg scopolamine (p < 0.01 for both). Scopolamine significantly reduced TFMPP-elicited yawning at all doses tested (p < 0.01 for all).
The effects of the 5-HT2 receptor subtype antagonist, mianserin (0.0032, 0.032, and 0.32 mg/kg), on yawning elicited by TFMPP (3.2 mg/kg), PD-128,907 (0.1 mg/kg), and physostigmine (0.1 mg/kg i.p.) are shown in Fig. 4C. Mianserin produced a dose-dependent and significant inhibition of TFMPP-induced yawning [F(3,21) = 9.85; p < 0.001], with doses of 0.032 and 0.32 mg/kg mianserin significantly inhibiting TFMPP-induced yawning compared with vehicle-treated rats (p < 0.01 for both). Mianserin did not significantly alter yawning elicited by either PD-128,907 [F(3,21) = 0.84; p > 0.05] or physostigmine [F(3,21) = 0.26; p > 0.05] at any dose tested.
D3-Preferring Antagonists on Dopaminergic, Cholinergic, and Serotonergic Agonist-Induced Yawning
Figure 5 shows the effects of the D3-preferring antagonists nafadotride, U99194, SB-277011A, and PG01037 on yawning elicited by PD-128,907 (0.1 mg/kg), physostigmine (0.1 mg/kg i.p.), and TFMPP (3.2 mg/kg). Nafadotride (Fig. 5A) dose dependently and significantly inhibited yawning elicited by PD-128,907 [F(3,21) = 5.36; p < 0.01], with a dose of 1.0 mg/kg significantly reducing yawning compared with vehicle-treated rats (p < 0.01). There were no significant effects of nafadotride on either physostigmine [F(3,21) = 0.32; p > 0.05]- or TFMPP [F(3,21) = 0.60; p > 0.05]-induced yawning. As shown in Fig. 5B, U99194 dose dependently and significantly reduced the amount of yawning elicited by PD-128,907[F(3,21) = 29.78; p < 0.0001], with doses of 3.2 and 10.0 mg/kg U99194 significantly inhibiting yawning compared with vehicle-treated rats (p < 0.01 for both). Unlike nafadotride, U99194 also significantly inhibited the amount of yawning elicited by physostigmine [F(3,21) = 11.91; p < 0.0001] and TFMPP [F(3,21) = 7.07; p < 0.01], with a dose of 10.0 mg/kg U99194 resulting in a significant reductions in the amount of yawning elicited by both physostigmine (p < 0.01) and TFMPP (p < 0.01). The effects of SB-277011A on PD-128,907-, physostigmine-, and TFMPP-induced yawning are shown in Fig. 5C. SB-277011A dose dependently and significantly reduced the amount of yawning elicited by PD-128,907 [F(3,21) = 12.09; p < 0.0001], with doses of 32.0 and 56.0 mg/kg (p < 0.01 for both) significantly inhibiting yawning compared with vehicle-treated rats. No significant effects of SB-277011A were seen on yawning elicited by either physostigmine [F(3,21) = 0.68; p > 0.05] or TFMPP [F(3,21) = 2.20; p > 0.05]. Similarly, PG01037 significantly and dose dependently inhibited yawning elicited by PD-128,907 [F(3,21) = 29.43; p < 0.0001], with doses of 32.0 and 56.0 mg/kg (p < 0.05 for both) PG01037 significantly reducing yawning compared with vehicle-treated rats (Fig. 5D). PG01037 did not significantly alter yawning elicited by either 0.1 mg/kg physostigmine [F(3,21) = 0.16; p > 0.05] or 3.2 mg/kg TFMPP [F(3,21) = 0.07; p > 0.05] at any dose tested.
Fig. 5.

Effects of D3-preferring antagonists on yawning induced by PD-128,907 (0.1 mg/kg), physostigmine (0.1 mg/kg i.p.), and TFMPP (3.2 mg/kg). A, nafadotride at doses of 0, 0.01, 0.1, and 1.0 mg/kg; B, U99194 at doses of 0, 1.0, 3.2, and 10.0 mg/kg; C, SB-277011A at doses of 0, 3.2, 32.0, and 56.0 mg/kg; and D, PG01037 at doses of 0, 10.0, 3.2, and 56.0 mg/kg. Data are presented as mean (±S.E.M.), n = 8, number of yawns during a 20-min observation period. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant difference from vehicle-treated rats was determined by one-way repeated-measures ANOVAs with post hoc Dunnett’s tests.
Discussion
Evidence has been provided in the present study to support the hypothesis that D2/D3 agonist-induced yawning behavior in rats is mediated by agonist activation of the dopamine D3 receptor, whereas the inhibition of yawning is a result of a competing agonist activation of the dopamine D2 receptor. In agreement with the majority of previous studies, all of the D2/D3 agonists tested, with the exception of bromocriptine and PD-128,908 (Fig. 1C), the inactive enantiomer of PD-128,907, elicited significant, dose-dependent increases in yawning behavior with inhibition seen at higher doses, resulting in the characteristic inverted U-shaped dose-response curve for yawning in rats. Evidence is also provided for the selective antagonism of the induction of yawning behavior by D3-preferring antagonists and the inhibition of yawning by D2-preferring antagonists. In addition, the current studies demonstrate that inhibition of D3 agonist-induced yawning by D3-preferring antagonists is a result of their selective antagonist activity at the D3 receptor and not through antagonist effects at D2, serotonergic, or muscarinic cholinergic receptors.
Yawning Is a D3-Mediated Behavior
Several lines of evidence have been provided in support of the hypothesis that yawning is a D3 agonist-mediated behavior. In general, all D3-preferring D2/D3 agonists induced significant amounts of yawning at low doses. Although there were no significant differences in the effectiveness of the agonists with respect to induction of yawning behavior with the exception of apomorphine, there were differences in the potency of the D2/D3 agonists to induce yawning. The rank-order potency of the D2/D3 agonists to elicit yawning behavior was as follows: quinelorane, apomorphine, quinpirole, 7-OH-DPAT, pramipexole, and PD-128,907, whereas bromocriptine and PD-128,908 failed to elicit significant levels of yawning. The stereoselectivity of the yawning response with regard to PD-128,907 (and PD-128,908) is an important finding because dopamine receptors are selective with respect to more rigid agonists (DeWald et al., 1990). Taken together with the findings of Stahle and Ungerstedt (1984), who showed that (+)-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine, but not (−)-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine, will elicit yawning, our current findings provide further evidence that D2/D3 agonists are inducing yawning via dopaminergic agonist mechanisms. Differences in yawning induced by bromocriptine may be a result of pharmacokinetic differences because bromocriptine has been shown to induce significant levels of yawning in studies using a 60-min observation period (Protais et al., 1983; Zarrindast and Jamshidzadeh, 1992).
Antagonists with a high degree of selectivity for the D3 compared with the D2 receptor selectively antagonized the induction of yawning behavior. Three of the four D3-preferring antagonists (U99194, SB-277011A, and PG01037) tested in the current studies possess the ability to dose dependently and selectively antagonize the induction of yawning by PD-128,907 while having no effect on the inhibition of yawning observed at higher doses. As shown in Fig. 3, C and D, respectively, SB-277011A and PG01037, D3-preferring antagonists with similarly high degrees of in vitro D3 selectivity (100- and 133-fold, respectively), produced almost identical effects on PD-128,907-induced yawning; significant, dose-dependent, downward/rightward shifts of the ascending limb of the yawning dose-response curve were observed, whereas the descending limb of the dose-response curve for PD-128,907-induced yawning was not changed. Similar effects were seen with the moderately selective (30-fold) D3-preferring antagonist U99194; however, unlike SB-277011A and PG01037, at the relatively high dose of 10.0 mg/kg, U99194 completely inhibited PD-128,907-induced yawning; however, it should be noted that at this dose, U99194 effectively antagonized not only dopaminergic but cholinergic and serotonergic yawning as well. Nafadotride, the least selective (3-fold) of the D3-preferring antagonists, was the only D3 antagonist to produce a nonselective antagonism of yawning behavior, shifting both the ascending and descending limbs of the dose-response curve for PD-128,907-induced yawning at the highest dose tested. This effect was similar to that observed with haloperidol, a nonselective dopamine antagonist, and suggests that at a dose of 0.32 mg/kg, nafadotride is no longer selective for the D3 receptor, but rather is active at both the D3 and D2 receptors. Taken together, these data provide strong support for the hypothesis that the induction of yawning by D2/D3 agonists is mediated by an agonist activation of the D3 receptor.
Inhibition of Yawning Is a D2-Mediated Effect
We have also provided evidence in support of the hypothesis that inhibition of D2/D3 agonist-induced yawning occurring at higher doses is mediated by an agonist activity at the D2 receptor. As shown in Fig. 2, A and B, the D2-preferring antagonist L-741,626, at the first behaviorally active dose (1.0 mg/kg), selectively antagonized the inhibitory effects of high doses of PD-128,907 and quinelorane, resulting in a rightward shift in the descending limbs, whereas it had virtually no effect on the ascending limbs of the dose-response curves for both PD-128,907- and quinelorane-induced yawning. In addition, L-741,626 produced a rightward shift in the maximal effective dose of PD-128,907 and quinelorane, resulting in an increased effectiveness for both agonists. These data not only suggest that L-741,626, at a dose of 1.0 mg/kg, is an effective D2 antagonist in vivo but that it is also devoid of D3 antagonist activity.
Further support for the differential regulation of yawning behavior by the D3 and D2 receptors was provided by the effects of the nonselective DA antagonist haloperidol. Because D3- and D2-preferring antagonists selectively antagonize the ascending and descending limbs of the dose-response curve for D2/D3 agonist-induced yawning, respectively, it would be expected that antagonists with mixed D2/D3 actions, such as haloperidol, would shift both the ascending and descending limbs of yawning dose-response curves at their initial active doses. Indeed, at the first behaviorally active dose (0.032 mg/kg), haloperidol produced rightward shifts in both the ascending and descending limbs of the dose-response curves for both PD-128,907- and quinelorane-induced yawning (Fig. 2, C and D). This not only suggests that the effects of D3- and D2-preferring antagonists are a result of selective antagonist activity but that nonselective D2/D3 antagonists produce effects distinct from those of other dopaminergic antagonists on D3 agonist-induced yawning.
However, it should be noted that in addition to possessing high affinities for the D3 and D2 receptors, haloperidol also has significant affinities for the D1, D4, and D5 receptors. It is, however, unlikely that activity at these receptors is influencing PD-128,907-induced yawning behavior because the D1/D5-selective antagonist, SCH 23390, and the D4-selective antagonist L-745,870 at behaviorally active doses (Patel et al., 1997; Chaperon et al., 2003) did not alter yawning elicited by either low (0.032–0.1 mg/kg) or high (0.32 mg/kg) doses of PD-128,907. This provides further evidence that D2/D3 agonist-induced yawning behavior is under the direct control of the D3 (induction) and D2 (inhibition) receptors, but not the D1, D4, or D5 receptors. However, the possibility remains that other dopaminergic receptors may modulate D3 agonist-induced yawning elicited by other D2/D3 agonists because several of the agonists tested, such as apomorphine, quinelorane, and quinpirole, possess significant affinities for the D1, D4, and D5 receptors (apomorphine) or D4 receptor (quinelorane and quinpirole) in addition to the D3 and D2 receptors.
Dopaminergic, Serotonergic, and Cholinergic Regulation of Yawning
The findings of the current study confirm and extend those of earlier studies (e.g., Yamada and Furukawa, 1980; Ushijima et al., 1984; Zarrindast and Poursoltan, 1989; Stancampiano et al., 1994) and demonstrate that although scopolamine will dose dependently antagonize yawning induced by cholinergic, serotonergic, and dopaminergic agonists (Fig. 4B), serotonergic and dopaminergic antagonists are able to selectively antagonize yawning elicited by their respective agonists. More specifically, nafadotride, SB-277011A, and PG01037, D3-preferring antagonists with a wide range (3- to 133-fold) of selectivities for the D3 receptor over the D2 receptor in vitro, were able to selectively antagonize PD-128,907-induced yawning, whereas they had no effect on yawning elicited by either physostigmine or TFMPP (Fig. 5). This suggests that SB-277011A and PG01037 are not only selective for the D3 over the D2 receptor but that they are also selective for the D3 receptor over certain serotonergic and cholinergic receptors at doses up to 56.0 mg/kg. Similarly, although nafadotride demonstrated little or no preference for the D3 compared with the D2 receptor in vivo, no serotonergic or cholinergic antagonist activity was detected at doses up to 1.0 mg/kg. However, in contrast to the effects of the other D3-preferring antagonists, U99194, at a dose of 10.0 mg/kg, significantly antagonized yawning elicited by PD-128,907, TFMPP, and physostigmine, suggesting that at higher doses, it is no longer selective for dopaminergic receptors. Although U99194 is unique in this regard within this group of D3-preferring antagonists, clozapine, an antagonist with significant affinities for dopaminergic, serotonergic, and cholinergic receptors, has also been shown to antagonize both dopaminergic and cholinergic yawning (Dubuc et al., 1982), suggesting that antagonism of physostigmine-induced yawning may be a reliable measure of anticholinergic activity. Further evidence of an in vivo antimuscarinic activity of U99194 has been demonstrated by Goudie et al. (2001), who showed in discrimination studies that U99194 generalized to a scopolamine cue, suggesting that U99194 may possess anticholinergic activity at higher doses. Although it has been suggested that U99194 functions as a D3-selective antagonist in vivo at doses ranging from 13.0 to 40.0 mg/kg based on its inability to increase plasma prolactin, to induce catalepsy, and to inhibit the induction of hypothermia by PD-128,907 (Audinot et al., 1998), the results of the current study suggest that while U99194 may be selective for the D3 compared with the D2, a significant anticholinergic effect is apparent at 10.0 mg/kg. Thus, the current studies support the hypothesis that dopaminergic, serotonergic, and cholinergic agonists induce yawning via distinct mechanisms and, furthermore, that yawning induced by D2/D3 agonists is a result of agonist activation of D3 receptors and not serotonergic or cholinergic receptors.
To summarize the results of the studies reported herein, evidence has been provided in support of the hypothesis that the induction of yawning by D2/D3 agonists is mediated through an agonist activity at the D3 receptor, whereas the subsequent inhibition of yawning seen at higher doses is a result of an increasing D2 agonist activity. Based on these findings, several conclusions can be drawn. First, the ascending limb of the dose-response curves corresponds to doses that are selectively activating D3 receptors over D2 receptors, whereas the descending limb corresponds to those activating both the D3 and D2 receptors. Additionally, determinations of in vivo D3 potency and effectiveness may be possible, based on the onset and maximal amount of yawning elicited. Furthermore, inhibition of yawning may provide useful information regarding in vivo D2 potency. Lastly, the shape of the dose-response curves may allow for determinations of in vivo D3 selectivity of D3-preferring D2/D3 agonists to be made. The results of the current set of studies have demonstrated that D3-selective antagonism will only shift the ascending limb of the yawning dose-response curve and that D2-selective antagonism will only shift the descending limb of the yawning dose-response curve, whereas nonselective D2/D3 antagonism will shift both the ascending and descending limbs of the dose-response curve for D2/D3 agonist-induced yawning behavior in rats. In conclusion, as the current studies have provided evidence that the induction of yawning behavior by D2/D3 agonists is mediated by the D3 receptor, yawning may be an important pharmacological effect that can be used in the characterization, classification, and discovery of in vivo D3 agonist and antagonist actions. Thus, it may be possible to relate other behavioral effects of D2/D3 agonists and antagonists to their ability to modulate yawning. Whether the potency and selectivity measures of these compounds can be utilized across behavioral measures will need to be explored in the future.
Acknowledgments
We thank Amy Howard, Josh Gary, Callie Corsa, Angela Marshall, Jeremy Cullio, and Brian Tsay for excellent technical assistance in completion of the studies and Deyi Zhang for supplying SB-277011A.
Footnotes
This research was supported by U.S. Public Health Service National Institute on Drug Abuse Grants DA 09161 and DA 00254 and by the National Institute on Drug Abuse-Intramural Research Program.
References
- Argiolas A, Melis MR. The neuropharmacology of yawning. Eur J Pharmacol. 1998;343:1–16. doi: 10.1016/s0014-2999(97)01538-0. [DOI] [PubMed] [Google Scholar]
- Audinot V, Newman-Tacnredi A, Gobert A, Rivet JM, Brocco M, Lejeune F, Gluck L, Desposte I, Bervoets K, Dekeyne A, et al. A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (1)-S 14297, nafadotride, GR 103,691 and U 99194. J Pharmacol Exp Ther. 1998;287:187–197. [PubMed] [Google Scholar]
- Barnett A, Ahn HS, Billard W, Gold EH, Kohli JD, Glock D, Goldberg LI. Relative activities of SCH 23390 and its analogs in three tests for D1/DA1 dopamine receptor antagonism. Eur J Pharmacol. 1986;128:249–253. doi: 10.1016/0014-2999(86)90772-7. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ. Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology. 1999a;38:1389–1396. doi: 10.1016/s0028-3908(99)00064-7. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Rostene W, Perrault G, Sanger DJ. Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock-out and wild-type mice. Neuropharmacology. 1999b;38:555–565. doi: 10.1016/s0028-3908(98)00213-5. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Perez JA, Bhatnagar RK, Long JP, Sharabi FM. Conformationally restricted congeners of dopamine derived from 2-aminoindan. J Med Chem. 1982;25:1442–1446. doi: 10.1021/jm00354a010. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Tricklebank MD, Unger L, Neijt HC. Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology. 2003;44:1047–1053. doi: 10.1016/s0028-3908(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Daly SA, Waddington JL. Behavioural effects of the putative D-3 dopamine receptor agonist 7-OH-DPAT in relation to other “D-2-like” agonists. Neuropharmacology. 1993;32:509–510. doi: 10.1016/0028-3908(93)90177-5. [DOI] [PubMed] [Google Scholar]
- DeWald HA, Heffner TG, Jaen JC, Lustgarten DM, McPhail AT, Meltzer LT, Pugsley TA, Wise LD. Synthesis and dopamine agonist properties of (+)-trans-3,4,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano [4,3-b]-1,4-oxazin-9-ol and its enantiomers. J Med Chem. 1990;33:445–450. doi: 10.1021/jm00163a068. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Dubuc I, Protais P, Colboc O, Costentin J. Antagonism of the apomorphine-induced yawning by “atypical” neuroleptics. Neuropharmacology. 1982;21:1203–1206. doi: 10.1016/0028-3908(82)90181-2. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Giuliani D. Behavioural effects of the dopamine D3 receptor agonist 7-OH-DPAT in rats. Pharmacol Res. 1995;32:63–68. doi: 10.1016/s1043-6618(95)80010-7. [DOI] [PubMed] [Google Scholar]
- Flietstra RJ, Levant B. Comparison of D2 and D3 dopamine receptor affinity of dopaminergic compounds in rat brain. Life Sci. 1998;62:1825–1831. doi: 10.1016/s0024-3205(98)00148-9. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Baker LE, Smith JA, Prus AJ, Svensson KA, Cortes-Burgos LA, Wong EH, Haadsma-Svensson S. Common discriminative stimulus properties in rats of muscarinic antagonists, clozapine and the D3 preferring antagonist PNU-99194a: an analysis of possible mechanisms. Behav Pharmacol. 2001;12:303–315. doi: 10.1097/00008877-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Haadsma-Svensson SR, Svensson KA. PNU-99194A: a preferential dopamine D3 receptor antagonist. CNS Drug Rev. 1998;4:42–57. [Google Scholar]
- Holmgren B, Urba-Holmgren R. Interaction of cholinergic and dopaminergic influences on yawning behavior. Acta Neurobiol Exp (Wars) 1980;40:633–642. [PubMed] [Google Scholar]
- Joyce JN. Dopamine D3 receptor as a therapeutic target for antipsychotic and antiparkinsonian drugs. Pharmacol Ther. 2001;90:231–259. doi: 10.1016/s0163-7258(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R, Emms F, Freedman SB, Marwood R, Patel S, et al. 3-((4-(4-Chlorophenyl)piperazin-1-yl)-methyl)-1H-pyrrolo-2,3-b-pyridine: an antagonist with high affinity and selectivity for the human dopamine D4 receptor. J Med Chem. 1996;39:1941–1942. doi: 10.1021/jm9600712. [DOI] [PubMed] [Google Scholar]
- Kurashima M, Yamada K, Nagashima M, Shirakawa K, Furukawa T. Effects of putative dopamine D3 receptor agonists, 7-OH-DPAT, and quinpirole, on yawning, stereotypy and body temperature in rats. Pharmacol Biochem Behav. 1995;52:503–508. doi: 10.1016/0091-3057(95)00103-4. [DOI] [PubMed] [Google Scholar]
- Leriche L, Schwartz JC, Sokoloff P. The dopamine D3 receptor mediates locomotor hyperactivity induced by NMDA receptor blockade. Neuropharmacology. 2003;45:174–181. doi: 10.1016/s0028-3908(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- Levant B, Grigoriadis DE, De Souza EB. Relative affinities of dopaminergic drugs at dopamine D2 and D3 receptors. Eur J Pharmacol. 1995;278:243–247. doi: 10.1016/0014-2999(95)00160-m. [DOI] [PubMed] [Google Scholar]
- Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Audinot V, Melon C, Newman-Tancredi A. Evidence that dopamine D3 receptors participate in clozapine-induced hypothermia. Eur J Pharmacol. 1995;280:225–229. doi: 10.1016/0014-2999(95)00250-o. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Seguin L, Gobert A, Cussac D, Brocco M. The role of dopamine D3 compared with D2 receptors in the control of locomotor activity: a combined behavioural and neurochemical analysis with novel, selective antagonists in rats. Psychopharmacology (Berl) 2004;174:341–357. doi: 10.1007/s00213-003-1770-x. [DOI] [PubMed] [Google Scholar]
- Mogilnicka E, Klimek V. Drugs affecting dopamine neurons and yawning behavior. Pharmacol Biochem Behav. 1977;7:303–305. doi: 10.1016/0091-3057(77)90224-6. [DOI] [PubMed] [Google Scholar]
- Patel S, Freedman S, Chapman KL, Emms F, Fletcher AE, Knowles M, Marwood R, Mcallister G, Myers J, Curtis N, et al. Biological profile of L-745,870, a selective antagonist with high affinity for the dopamine D4 receptor. J Pharmacol Exp Ther. 1997;283:636–647. [PubMed] [Google Scholar]
- Protais P, Dubuc I, Costentin J. Pharmacological characteristics of dopamine receptors involved in the dual effect of dopamine agonists on yawning behaviour in rats. Eur J Pharmacol. 1983;94:271–280. doi: 10.1016/0014-2999(83)90416-8. [DOI] [PubMed] [Google Scholar]
- Pugsley TA, Davis MD, Akunne HC, MacKenzie RG, Shih YH, Damsma G, Wikstrom H, Whetzel SZ, Georgic LM, Cooke LW, et al. Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J Pharmacol Exp Ther. 1995;275:1355–1366. [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, et al. Pharmacological actions of a novel, high-affinity and selective human dopamine D(3) receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Sautel F, Griffon N, Sokoloff P, Schwartz JC, Launay C, Simon P, Costentin J, Schoenfelder A, Garrido F, Mann A, et al. Nafadotride, a potent preferential dopamine D3 receptor antagonist, activates locomotion in rodents. J Pharmacol Exp Ther. 1995;275:1239–1246. [PubMed] [Google Scholar]
- Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres MP, Giros B, Schwartz JC. Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur J Pharmacol. 1992;225:331–337. doi: 10.1016/0922-4106(92)90107-7. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature (Lond) 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Stahle L, Ungerstedt U. Assessment of dopamine autoreceptor agonist properties of apomorphine, (+)-3-PPP and (−)-3-PPP by recording of yawning behaviour in rats. Eur J Pharmacol. 1984;98:307–310. doi: 10.1016/0014-2999(84)90608-3. [DOI] [PubMed] [Google Scholar]
- Stancampiano R, Melis MR, Argiolas A. Penile erection and yawning induced by 5-HT1C receptor agonists in male rats: relationship with dopaminergic and oxytocinergic transmission. Eur J Pharmacol. 1994;261:149–155. doi: 10.1016/0014-2999(94)90313-1. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000;295:1223–1231. [PubMed] [Google Scholar]
- Stemp G, Ashmeade T, Branch CL, Hadley MS, Hunter AJ, Johnson CN, Nash DJ, Thewlis KM, Vong AK, Austin NE, et al. Design and synthesis of trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011): a potent and selective dopamine D(3) receptor antagonist with high oral bioavailability and CNS penetration in the rat. J Med Chem. 2000;43:1878–1885. doi: 10.1021/jm000090i. [DOI] [PubMed] [Google Scholar]
- Urba-Holmgren R, Gonzalez RM, Holmgren B. Is yawning a cholinergic response? Nature (Lond) 1977;267:261–262. doi: 10.1038/267261a0. [DOI] [PubMed] [Google Scholar]
- Urba-Holmgren R, Holmgren B, Anias J. Pre- and post-synaptic dopaminergic receptors involved in apomorphine-induced yawning. Acta Neurobiol Exp (Wars) 1982;42:115–125. [PubMed] [Google Scholar]
- Ushijima I, Yamada K, Inoue T, Tokunaga T, Furukawa T, Noda Y. Muscarinic and nicotinic effects on yawning and tongue protruding in the rat. Pharmacol Biochem Behav. 1984;21:297–300. doi: 10.1016/0091-3057(84)90229-6. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Dopamine agonist-induced hypothermia and disruption of prepulse inhibition: evidence for a role of D3 receptors? Behav Pharmacol. 1998;9:445–455. doi: 10.1097/00008877-199809000-00008. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters N, Svensson K, Haadsma-Svensson SR, Smith MW, Carlsson A. The dopamine D3-receptor: a postsynaptic receptor inhibitory on rat locomotor activity. J Neural Transm Gen Sect. 1993;94:11–19. doi: 10.1007/BF01244979. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Dijkstra D, Levant B, Akunne HC, Zapata A, Peters S, Shannon HE, Gasior M. Protection against cocaine toxicity in mice by the dopamine D3/D2 agonist R-(+)-trans-3,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano[4,3-b]-1,4-oxazin-9-ol [(+)-PD 128,907] J Pharmacol Exp Ther. 2004;308:957–964. doi: 10.1124/jpet.103.059980. [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Cooper DC, Tonegawa S, White FJ. Dopamine D3 receptor mutant and wild-type mice exhibit identical responses to putative D3 receptor-selective agonists and antagonists. Synapse. 1999;31:210–215. doi: 10.1002/(SICI)1098-2396(19990301)31:3<210::AID-SYN6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Yamada K, Furukawa T. Direct evidence for involvement of dopaminergic inhibition and cholinergic activation in yawning. Psychopharmacology (Berl) 1980;67:39–43. doi: 10.1007/BF00427593. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Jamshidzadeh A. Inhibitory effect of morphine on yawning induced by cholinoceptor and dopamine D2 receptor activation in rats. Br J Pharmacol. 1992;105:675–678. doi: 10.1111/j.1476-5381.1992.tb09037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Poursoltan M. Interactions of drugs acting on central dopamine receptors and cholinoceptors on yawning responses in the rat induced by apomorphine, bromocriptine or physostigmine. Br J Pharmacol. 1989;96:843–848. doi: 10.1111/j.1476-5381.1989.tb11893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


