Abstract
Synaptic phenotypes in living patients with psychiatric disorders are poorly characterized. Excitatory glutamate α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) is a fundamental component for neurotransmission. We recently developed a positron emission tomography (PET) tracer for AMPAR, [11C]K-2, the first technology to visualize and quantify AMPARs density in living human brain. In this study, we characterized patients with major psychiatric disorders with [11C]K-2. One hundred forty-nine patients with psychiatric disorders (schizophrenia, n = 42; bipolar disorder, n = 37; depression, n = 35; and autism spectrum disorder, n = 35) and 70 healthy participants underwent a PET scan with [11C]K-2 for measurement of AMPAR density. We detected brain regions that showed correlation between AMPAR density and symptomatology scores in each of four disorders. We also found brain areas with significant differences in AMPAR density between patients with each psychiatric disorder and healthy participants. Some of these areas were observed across diseases, indicating that these are commonly affected areas throughout psychiatric disorders. Schizophrenia, bipolar disorder, depression, and autism spectrum disorder are uniquely characterized by AMPAR distribution patterns. Our approach to psychiatric disorders using [11C]K-2 can elucidate the biological mechanisms across diseases and pave the way to develop novel diagnostics and therapeutics based on the synapse physiology.
Subject terms: Neuroscience, Psychiatric disorders
Introduction
Psychiatric disorders such as schizophrenia, bipolar disorder, depression, and autism spectrum disorder (ASD) are common serious diseases which impair social functioning and lower quality of life [1] on an individual level as well as a significant economic loss on a societal level [2, 3]. Despite the grave consequences caused by these illnesses, the biological bases, especially synaptic and circuit substrates, underlying psychiatric disorders still remain elucidated. Due to the lack of biological basis, diagnosis and disease classification of psychiatric disorders are currently based on clinical symptoms [4]. However, patients with different “diagnoses” often show the same clinical symptoms [5–7], while those with the same “diagnosis” are sometimes assumed to have different biological backgrounds [8] raising questions about the validity of the current symptom-based diagnostic system. Thus, it is crucial to establish a new disease classification based on biological characteristics so as to elucidate the pathophysiological mechanisms of psychiatric disorders and develop novel diagnostics and therapeutics. To achieve this goal, a cross-“disease” research with biological modality is needed.
Recent findings from animal models, genetic studies, and post-mortem brain studies suggest that one of the features of psychiatric disorders are considered to be “synapse diseases” [9–14] in which malfunction of synapses can underlie psychiatric disorders. Thus, biological basis of psychiatric disorders could theoretically be identified by monitoring synaptic phenotypes in patients with psychiatric disorders, which however have been poorly characterized due to technical limitations as described below.
Glutamate synapses play essential roles in neuronal function in which fast transmission is mainly mediated by α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) [15–22]. Thus, AMPAR is a principal component of neurotransmission [23]. Therefore, a technology for visualization and quantification of AMPARs in the living human brain and subsequent synaptic characterization of patients with psychiatric disorders has long been desired. We have recently developed the positron emission tomography (PET) tracer for AMPARs, [11C]K-2, the first technology to visualize and quantify the density of AMPARs in the living human brain [24]. [11C]K-2 indicated a good reversible kinetics that is appropriate to quantify AMPA density using PET. We exhibited that Standardized uptake value ratio (SUVR) using the white matter as a reference (quantitative value of absolute AMPARs density in [11C]K-2) showed strong significant positive correlations with local AMPAR density in resected brain tissues of patients with mesial temporal lobe epilepsy [24], and therefore, SUVR obtained from [11C]K-2 PET scan can be used for clinical investigation. Further, PET image with [11C]K-2 depicts cell surface AMPARs, a functionally crucial fraction of AMPARs [25]. Here, we PET-scanned 149 patients with psychiatric disorders, using [11C]K-2, that included schizophrenia (42 patients), bipolar disorder (37 patients), depression (35 patients), and ASD (35 patients). In summary, here we reported synaptic phenotypes of these psychiatric disorders. By using SUVR with the whole brain as the reference region, we characterized changes in the balance of the density of AMPARs among multiple brain areas, potentially providing biological features of major psychiatric disorders.
Patients and methods
Ethics statement
This study comprised five clinical studies that were registered under the following IDs: UMIN000025132, jRCTs031190197, jRCTs031190150, jRCTs031190149, and jRCTs031200083 which targeted multiple diagnoses, schizophrenia, bipolar disorder and depression, ASD, and healthy participants, respectively. CONSORT chart is provided in the Supplementary Figs. 1–5. All studies were approved by Yokohama City University Human Investigation Committee and Yokohama City University Certified Institutional Review Board in accordance with the Ethical guidelines for medical and health research involving human participants by the Japan Ministry of Health, Labour and Welfare and the Clinical Trials Act in Japan. Data from these five studies were combined and analysed with the approval of the Yokohama City University Human Investigation Committee (trial registry number jRCT1030220303). This study was conducted at Yokohama City University Hospital, Keio University Hospital, Kyushu University Hospital, and University of Fukui Hospital between August 2016 and April 2022. All participants provided written informed consent after receiving detailed information about the protocol. Clinical assessments were performed by one of the trained investigators who was blind to the PET and magnetic resonance imaging (MRI) data.
Participants
Patients with schizophrenia
Selection criteria for participants with four psychiatric disorders and healthy participants are detailed in the Supplementary methods. Briefly, in the first study (UMIN000025132) the inclusion criteria were: male in- and outpatients 30–49 years of age; patients who met the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria for schizophrenia [26], using the structured clinical interview for DSM-IV (SCID-I/DSM-IV) [27]. In the second study (jRCTs031190197), the inclusion criteria were the same as those in the first study, other than the age range (i.e., 20–59 years) and sex (i.e., both men and women were included). The demographic and clinical characteristics of the 42 patients (30 men) included in the PET analysis were as follows: age, 38.9 ± 9.1 years; illness duration, 13.5 ± 9.3 years; scores in the Positive and Negative Syndrome Scale (PANSS) [28], 75.7 ± 24.4 (total), 18.1 ± 7.1 (positive symptoms), 20.7 ± 6.7 (negative symptoms) and 37.1 ± 13.4 (general psychopathology) as shown in the Supplementary methods and Supplementary Table 1.
Patients with bipolar disorder
Briefly, in the first study (UMIN000025132) the inclusion criteria were: male in- and outpatients 30–49 years of age; and patients who met the DSM-IV criteria for bipolar disorder using the SCID-I/DSM-IV [27]. In the second study (jRCTs031190150), the inclusion criteria were the same as those in the first study, other than the age range (i.e., 20–59 years) and sex (i.e., both men and women were included). The demographic and clinical characteristics of the 37 patients (22 men) with bipolar disorder included in this analysis are as follows: age, 41.8 ± 8.2 years; illness duration, 15.4 ± 8.1 years; total score in the 17-item Hamilton Depression Rating Scale (HAM-D) [29], 6.2 ± 5.3, the total score in the Young Mania Rating Scale (YMRS) [30], 7.1 ± 7.1 as shown in the Supplementary methods and Supplementary Table 2.
Patients with depression
Briefly, in the first study (UMIN000025132) the inclusion criteria were: male in- and outpatients 30–49 years of age; and patients who met the DSM-IV criteria for major depressive disorder using the SCID-I/DSM-IV [27]. In the second study (jRCTs031190150), the inclusion criteria were the same as those in the first study, other than the age range (i.e., 20–59 years) and sex (i.e., both men and women were included). The demographic and clinical characteristics of the 35 patients (27 men) included in this analysis are as follows: age, 43.0 ± 7.4 years; illness duration, 7.9 ± 7.4 years; total score in the HAM-D, 10.3 ± 7.4 as shown in the Supplementary methods and Supplementary Table 3.
Patients with autism spectrum disorder (ASD)
Briefly, in the first study (UMIN000025132) the inclusion criteria were: male in- and outpatients 30–49 years of age; and patients who met the DSM-5 ASD criteria. In the second study (jRCTs031190149), the inclusion criteria were the same as those in the first study, other than the age range (i.e., 20–59 years), sex (i.e., both men and women were included), and intellectual performance (i.e., full-scale intelligence quotient [FIQ] ≥ 70 according to Wechsler Adult Intelligence Scale third edition [WAIS-III] or Wechsler Adult Intelligence Scale fourth edition [WAIS-IV]). The demographic and clinical characteristics of the 35 patients (28 men) in this analysis are as follows: age, 33.1 ± 7.5 years; illness duration, 19.4 ± 12.5 years; Autism Diagnostic Observation Schedule second edition (ADOS-2) [31] calibrated severity score (CSS), 7.3 ± 2.2 as shown in the Supplementary methods and Supplementary Table 4.
Healthy participants
In the first study (UMIN000025132) the inclusion criteria were: healthy male participants who were 30–79 years of age and did not fulfill any diagnostic criteria for psychiatric conditions according to the DSM-IV [26] using the SCID-I/DSM-IV [27]. Among them, age-matched (i.e., 30–59 years) healthy participants were included. In the second study (jRCTs031200083), the selection criteria were the same as those in the first study, other than the age range (i.e., 20–49 years) and sex (i.e., both men and women were included). The demographic characteristics of the participants are shown in the Supplementary methods.
PET and MRI imaging
The participants underwent a PET scan with [11C]K-2 and an MRI scan. Details of imaging settings and procedures are detailed in the Supplementary methods. Briefly, [11C]K-2 was synthesized locally at Yokohama City University Hospital, Keio University Hospital Kyushu University Hospital, and University of Fukui Hospital in accordance with GMP ordinance. On the day of the PET scan, the patients with psychiatric disorder received the clinical assessments. These assessments were performed by one of the trained investigators who was blind to the PET and MRI data.
Quantification of AMPA receptor density with PET
To quantify receptor density, a non-displaceable binding potential (BPND) that is a quantitative index of receptor density is commonly utilized.
We computed BPND using LGA with reference region (Logan Graphical Analysis [32]). In this study, we introduced a reference region that is the white matter where no expression of AMPARs was detected [24]. LGA is a widely used graphical analysis that uses linear regression to analyze pharmacokinetics of tracers using tissue time activity curves (tTAC; time course of radioactivity in the dynamic scan during 60 min). The plots of relationship between an integrated tTAC in each multiple brain regions and that in the white matter can be obtained by LGA. If these plots show good linear relation, [11C]K-2 achieves at an equilibrium state. Finally, we applied a linear regression 20 min after the administration of K-2 [24] and the slope of the plots provided us BPND in LGA [33, 34].
Standard uptake value ratio (SUVR)
SUVR images of [11C]K-2 were acquired by the followings: the radioactivity values of each brain regions are divided either by the radioactivity value of white matter during 30 min and 50 min after the tracer injection (SUVR30–50 minWM) or whole brain during the same period (SUVR30–50 minWB). SUVR30–50 minWM reflects “absolute” AMPARs density because of no expression of AMPARs in the white matter [24]. SUVR30–50 minWB shows “relative” AMPARs density among brain regions because the radioactivity values of each brain regions are normalized by the radioactivity of whole brain.
For calculation of BPND, subjects needed to be performed a dynamic scan (scan time is 60 min). If we can prove that SUVR30–50 minWM is an appropriate surrogate marker of BPND, we can acquire SUVR using a static PET scan (scan time is 20 min). It is more convenient to conduct the PET scan at the clinical site.
Therefore, we performed regression analysis between BPND and SUVR30–50 minWM-1 [24, 34].
To observe relative regional alterations in the [11C]K-2 signal in all participants, we used SUVR30–50 minWB. We also calculated correlation coefficients between SUVR30–50 minWB and BPND.
The difference in Inter-individual between SUVR30–50 minWM and SUVR30–50 minWB was examined using coefficient of variation (CV).
Normalizing PET images for SPM
A summed PET image at 30–50 min after injection of [11C]K-2 was obtained for each participant. SUVR30-50 min images were normalized by white matter mean value (SUVR30–50 minWM) or whole brain mean value (SUVR30–50 minWB). We created the template for normalization from 3D-T1-weighted images of all participants using the high-dimensional nonlinear warping algorithm DARTEL [35]. SUVR30–50 min images were spatially normalized into MNI standard space and preserved concentration to avoid volume effect using the template with SPM12.
Statistical analysis
The association between SUVR30–50 minWM or SUVR30–50 minWB values and clinical assessment scores of interests was assessed using multiple regression design implemented in SPM12. Statistical significance was set at P < 0.05 (peak-level uncorrected), false discovery rate (FDR) was corrected at P < 0.05 (cluster-level inference, FDRc) for multiple regression across all in-mask voxels. The cluster was extracted for correlation coefficient > 0.4. Comparison of SUVR30-50 min values in patients with each psychiatric disorder (i.e., schizophrenia, bipolar disorder, depression, or ASD) and age-matched healthy participants was performed using two-sample t-test. Age-matched healthy participants were selected from the whole sample of healthy participants to correspond to the mean age of the patients. Therefore, data of the healthy participants in their 30 s, 40 s, and 50 s (n = 49; 30 men and 19 women; and mean age, 42.1 ± 7.1 years), those in their 20 s, 30 s, and 40 s (n = 66; 37 men and 29 women; and mean age, 35.6 ± 9.2 years), and those in their 20 s, 30 s, 40 s, and 50 s (n = 70; 41 men and 29 women; and mean age, 36.8 ± 10.2 years) were used for comparison to bipolar disorder (mean age, 41.8 ± 8.2 years; t-test, P = 0.8654; F-test, P = 0.3346, F = 1.345) and depression (mean age, 43.0 ± 7.4 years; t-test, P = 0.5893; F-test, P = 0.7557, F = 1.098), ASD (mean age, 33.1 ± 7.5 years; t-test, P = 0.1821; F-test, P = 0.2224, F = 1.470), schizophrenia (mean age, 38.9 ± 9.1 years; t-test, P = 0.2709; F-test, P = 0.4467, F = 1.249), respectively. Statistical significance was set at P < 0.05 (peak-level uncorrected), FDRc corrected at P < 0.05 for multiple comparisons across all in-mask voxels. To reduce the number of voxel-wise comparisons that were performed and avoid false positive findings, we used the mask of GM with image values of more than 10% in all above analyses, extracted from standard tissue probability maps equipped in SPM12. All analyses were adjusted for demographic covariates (age and sex) except 3D-rendered brains which were not adjusted covariates. Correlation analysis and t-test were conducted, and scatter plot was created using GraphPad Prism 8 (Graph Pad Software, Massachusetts, USA). All data fulfill the normality assumption.
Detection of commonly affected areas
In the participants with schizophrenia, bipolar disorder, and ASD, we created the binary images from reduction and increase regions (threshold is p < 0.05, FDRc) compared with age-matched healthy participants using SPM12. We identified the overlapped areas showing reduced and increased AMPAR densities, respectively, across these psychiatric disorders.
Results
Characteristics of [11C]K-2 in healthy participants
In order to characterize the distribution of AMPAR in brains of patients with psychiatric disorders, we first PET-scanned 70 healthy participants with [11C]K-2. As was previously observed with tissue time activity curves (tTACs) from multiple brain regions [24], we detected regional heterogeneity of the [11C]K-2 uptake with high AMPAR density in the cortex, putamen, and cerebellum (Supplementary Fig. 6A). To calculate the non-displaceable binding potential (BPND; a quantitative index of AMPAR density utilized in [11C] K-2 imaging), Logan graphical analysis (LGA) was performed. LGA is a widely used graphical analysis that uses linear regression to analyze pharmacokinetics of tracers using tTACs. LGA using the white matter as a reference showed linearity in healthy participants, indicating the reversible binding kinetics of [11C]K-2 (Supplementary Fig. 6B). Based on LGA, we calculated BPND in each brain regions (see details in the method section) (Supplementary Table 5).
We first constructed the regional SUVR using white matter as a reference during 30–50 mins after the tracer injection (SUVR30–50 minWM) (Supplementary Fig. 6C and Supplementary Table 5). To confirm whether SUVR30–50 minWM is a surrogate for BPND, we performed regression analysis between BPND and SUVR30–50minWM-1. As was observed in healthy participants in the previous study [24], SUVR30–50 minWM-1 was compatible with BPND (Supplementary Fig. 6D). The linear relationship between BPND and SUVR30–50 minWM-1 was represented by Y = 0.9434*X +0.0182, indicating that SUVR30–50 minWM-1 is an appropriate surrogate outcome measure for absolute AMPAR density in healthy participants.
We also wondered if unbalanced AMPAR density among brain regions underlies pathogenesis of psychiatric disorder and aimed to examine relative regional changes of AMPAR density across the brain. We constructed the regional SUVR30-50 min using whole brain as a reference (SUVR30–50 minWB) (Supplementary Fig. 6E and Supplementary Table 5). There exists a good linear relationship between BPND and SUVR30–50minWB (Supplementary Fig. 6F and Supplementary Table 6). These results indicate that SUVR30–50 minWB retain the differences of BPND among brain regions.
When we compared SUVR30–50 minWM with SUVR30–50 minWB, the coefficient of variation (CV) among healthy participants were lower with SUVR30–50 minWB than SUVR30–50 minWM, indicating that SUVR30–50minWB has less inter-individual variability and minimize influence of individual factor. (Supplementary Figs. 6C, E and Supplementary Table 7).
Characteristics of [11C]K-2 in schizophrenia
We performed PET scanning of 42 patients with schizophrenia using [11C]K-2. As was previously observed in healthy participants, tTACs from multiple brain regions of [11C]K-2-injected patients with schizophrenia showed rapid radiotracer uptake in the brain and regional heterogeneity, with the lowest radioactivity observed in the white matter, where no AMPARs were detected (Supplementary Fig. 7A) [24]. LGA using the white matter as a reference showed linearity, indicating the reversible binding kinetics of [11C]K-2 in patients with schizophrenia as was observed in healthy participants (Supplementary Fig. 7B). As was observed in healthy participants, there exists a good linear relationship between BPND and SUVR30–50 minWM-1 (Supplementary Fig. 7C), indicating that SUVR30–50 minWM-1 is an appropriate surrogate outcome measure for absolute AMPAR density in patients with schizophrenia. To investigate relative regional alterations in the [11C]K-2 PET signal in patients with schizophrenia, we also constructed the SUVR30–50 minWB. There exists a good linear relationship between BPND and SUVR30–50 minWB (Supplementary Fig. 7D and Supplementary Table 8). The CV among patients with schizophrenia were lower with SUVR30–50 minWB than SUVR30–50 minWM, indicating that SUVR30–50 minWB has less inter-individual variability. (Supplementary Figs. 7E, F and Supplementary Table 7).
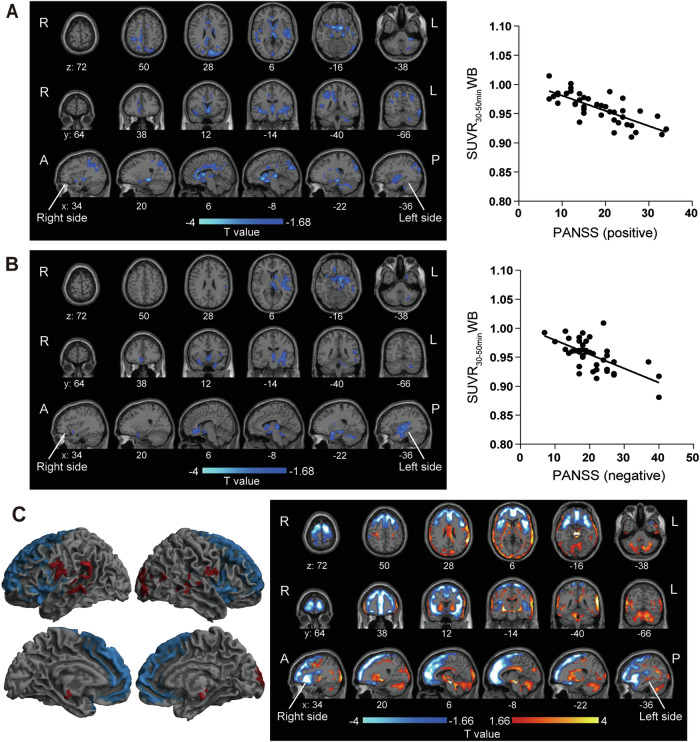
We first performed regression analysis between SUVR30–50 minWM and the PANSS score (symptomatology score for schizophrenia). Voxel-wise analysis with SPM did not detect brain regions which exhibited correlation between them with statistical significance. Next, we examined whether the balance of AMPAR density is disrupted among brain regions, we used SUVR30-50minWB for the analysis. Voxel-wise analysis exhibited a significant negative correlation (P < 0.05, FDR corrected cluster-level inference (FDRc)) between the SUVR30–50 minWB and the PANSS score for positive symptoms in the pregenual and subgenual anterior cingulate cortex (ACC), the cingulate cortex, the hippocampus, the parahippocampal gyrus, the superior temporal gyrus, the left cuneus, the right parietal lobe, the posterior insula including the claustrum, the thalamus and the caudate nucleus (Fig. 1A and Supplementary Fig. 7G). We also found brain regions with significant negative correlation (P < 0.05, FDRc) between the SUVR30–50 minWB of [11C]K-2 PET and PANSS score for negative symptoms in the pregenual and subgenual ACC, the left hippocampus, the parahippocampal gyrus, the temporal lobe, the left posterior insula including the claustrum, the thalamus, and the caudate nucleus (Fig. 1B and Supplementary Fig. 7H). Some regions were overlapped between positive and negative symptoms (the pregenual and subgenual ACC, the left posterior part of insula including the claustrum, the left hippocampus, the parahippocampal gyrus, the superior temporal gyrus, the thalamus, and the caudate nucleus), depicting potential core regions for both symptoms of schizophrenia. We also detected symptom-specific areas (positive symptom: the cingulate cortex and the left cuneus, the right parietal lobe, negative symptom: the inferior to middle temporal gyrus). Thus, we identified state brain regions where dysfunction of glutamate synapses can affect the illness severity of schizophrenia.
Fig. 1. State regions and altered AMPAR distribution in patients with schizophrenia compared with healthy participants.
A Brain regions showing a significant negative correlation between SUVR30–50 minWB and PANSS score for positive symptoms in patients with schizophrenia (P < 0.05, T < −1.68, one-tailed, FDRc). Significant clusters displayed on an axial, coronal and sagittal slices (Left), and scatter plot between averaged SUVR30–50 minWB in significant clusters and PANSS scores for positive symptoms (Right, two-tailed Pearson correlation analysis: correlation coefficient = −0.7514, P < 0.0001). B Brain regions showing a significant negative correlation between SUVR30–50 minWB and PANSS score for negative symptoms in patients with schizophrenia (P < 0.05, T < −1.68, one-tailed, FDRc). Significant clusters displayed on an axial, coronal and sagittal slices (Left), and scatter plot between averaged SUVR30–50 minWB in significant clusters and PANSS score for negative symptoms (Right, two-tailed Pearson correlation analysis: correlation coefficient = −0.6371, P < 0.0001). C Relative reduction (blue) and increase (red) of [11C]K-2 retention in patients with schizophrenia compared to healthy participants (P < 0.05, increase of [11C]K-2 retention: T > 1.66, reduction of [11C]K-2 retention: T < −1.66, one-tailed, FDRc). Significant clusters displayed on a 3D-rendered brain (binary image, Left), and axial, coronal and sagittal slices (T-map, Right). A, B and C (right panel) were adjusted for covariates (age, sex). C (left panel) was not adjusted for covariates.
We performed comparison with SUVR30–50 minWB in healthy participants and patients with schizophrenia. Voxel-wise analysis revealed significantly lower SUVR30–50 minWB of [11C]K-2 PET in patients with schizophrenia in the frontal brain area that consisted of the ACC and the adjacent frontal cortex, and in the lateral brain regions that include the anterior insula and the claustrum, compared to healthy participants (P < 0.05, FDRc) (Fig. 1C and Supplementary Fig. 8A). Further, we found a greater SUVR30–50 minWB of [11C]K-2 PET in the occipital lobe, the putamen, the superior temporal lobe, and the cerebellum of patients with schizophrenia compared to healthy participants (P < 0.05, FDRc) of [11C]K-2 retention (Fig. 1C and Supplementary Fig. 8A). SUVR30–50 minWM of [11C]K-2 PET was low in the extensive cortical area, the insula and the cerebellum of patients with schizophrenia, compared to the healthy participants (P < 0.05, FDRc) (Supplementary Fig. 8B).
Characteristics of [11C]K-2 in bipolar disorder
Next, we imaged 37 patients with bipolar disorder using [11C]K-2. Based on the tTACs (Supplementary Fig. 9A), LGA using the white matter as a reference showed linearity, indicating the reversible binding kinetics of [11C]K-2 in patients with bipolar disorder (Supplementary Fig. 9B). As was observed in patients with schizophrenia, there exists a good linear relationship between BPND and SUVR30–50 minWM-1 in patients with bipolar disorder (Supplementary Fig. 9C), indicating that SUVR30–50 minWM-1 is almost identical to BPND. To investigate relative regional alterations in the [11C]K-2 PET signal in patients with bipolar disorder, we also constructed the SUVR30–50 minWB. There exists a good linear relationship between BPND and SUVR30–50 minWB in patients with bipolar disorder (Supplementary Fig. 9D and Supplementary Table 9). The CV among patients with bipolar disorder were lower with SUVR30–50 minWB than SUVR30–50 minWM, indicating that SUVR30–50 minWB has less inter-individual variability. (Supplementary Fig. 9E, F and Supplementary Table 7).
We first investigated the correlation between SUVR30–50 minWM and symptomatology scores for bipolar disorders (depressive status; the 17-item HAM-D, manic status; YMRS). Voxel-wise analysis did not reveal brain regions which exhibited correlation between them with statistical significance. As in analysis of schizophrenia, we used SUVR30–50 minWB for the analysis. Voxel-wise analysis with SPM exhibited a significant strong negative correlation (P < 0.05, FDRc) between the SUVR30–50 minWB of [11C]K-2 PET (i.e., AMPAR density) and the 17-item HAM-D score in the frontal lobe, while we observed a significant positive correlation between these two in the cerebellum and the part of occipital lobe such as the cuneus (Fig. 2A and Supplementary Fig. 9G). In contrast, we detected a significant positive correlation (P < 0.05, FDRc) between the AMPAR density and YMRS score in the frontal lobe, while there was a significant negative correlation between them in the cerebellum and a part of occipital lobe such as the cuneus (Fig. 2B and Supplementary Fig. 9H). These results demonstrated that relative alteration of glutamatergic synaptic function of brain regions such as the frontal lobe, cerebellum, and the part of the occipital lobe among all brain regions could be related to control illness state of bipolar disorder. Large brain areas with significant strong correlation between the AMPAR density and HAM-D score were overlapped with those for YMRS score, while some areas were distinct between HAM-D and YMRS, suggesting the existence of core regions producing depressive and manic states and areas specifically for the expression of either depressive or manic symptom (Supplementary Fig. 9I).
Fig. 2. State regions and altered AMPAR distribution in patients with bipolar disorder compared with healthy participants.
A Brain regions showing a significant negative (blue) and positive (red) correlation between SUVR30-50minWB and the 17-item HAM-D score in patients with bipolar disorder (P < 0.05, positive correlation: T > 1.69, negative correlation: T < −1.69, one-tailed, FDRc). Significant clusters displayed on an axial, coronal and sagittal slices (Left), and scatter plot between averaged SUVR30–50 minWB in significant clusters and HAM-D score (Right, two-tailed Pearson correlation analysis: positive correlation; correlation coefficient = 0.6841, P < 0.0001, negative correlation; correlation coefficient = −0.5208, P = 0.001). B Brain regions showing a significant negative (blue) and positive (red) correlation between SUVR30–50 minWB and YMRS score in patients with bipolar disorder (P < 0.05, positive correlation: T > 1.69, negative correlation: T < −1.69, one-tailed, FDRc). Significant clusters displayed on an axial, coronal, and sagittal slices (Left), and scatter plot between averaged SUVR30–50 minWB in significant clusters and YMRS score (Right, two-tailed Pearson correlation analysis: positive correlation; correlation coefficient = 0.5842, P = 0.0001, negative correlation; correlation coefficient = −0.7012, P < 0.0001). C Relative reduction (blue) and increase (red) of [11C]K-2 retention in patients with bipolar disorder compared to healthy participants (P < 0.05, increase of [11C]K-2 retention: T > 1.66, reduction of [11C]K-2 retention: T < −1.66, one-tailed, FDRc). Significant clusters displayed on a 3D-rendered brain (binary image, Left), and axial, coronal, and sagittal slices (T-map, Right). A, B and C (right panel) were adjusted for covariates (age, sex). C (left panel) was not adjusted for covariates.
AMPAR density characterized with SUVR30–50 minWB was lower in the patients with bipolar disorder in the frontal lobe, the right anterior insula, and the anterior cingulate cortex, compared to the healthy participants (p < 0.05, FDRc) (Fig. 2C and Supplementary Fig. 10A). We also detected a greater AMPAR density in the occipital lobe and the parietal lobe of patients with bipolar disorder compared to healthy participants (p < 0.05, FDRc) (Fig. 2C and Supplementary Fig. 10A). SUVR30–50 minWM was decreased in the frontal, parietal and temporal lobe, the anterior insula, and the anterior cingulate cortex of patients with bipolar disorder, compared to the healthy participants (p < 0.05, FDRc) (Supplementary Fig. 10B).
Characteristics of [11C]K-2 in depression
We imaged 35 patients with depression using [11C]K-2. Based on the tTACs (Supplementary Fig. 11A), LGA using the white matter as a reference showed linearity, indicating the reversible binding kinetics of [11C]K-2 (Supplementary Fig. 11B). As was observed in patients with schizophrenia and bipolar disorder, there exists a good linear relationship between BPND and SUVR30–50 minWM-1 in patients with depression (Supplementary Fig. 11C), indicating that SUVR30–50 minWM-1 is almost identical to BPND. To investigate relative regional alterations in the [11C]K-2 PET signal in patients with depression, we also constructed the SUVR30–50 minWB. There exists a good linear relationship between BPND and SUVR30–50 minWB in patients with depression (Supplementary Fig. 11D and Supplementary Table 10). The CV among patients with depression were lower with SUVR30–50 minWB than SUVR30–50 minWM, indicating that SUVR30–50 minWB has less inter-individual variability. (Supplementary Figs. 11E, F and Supplementary Table 7).
We first investigated the correlation between SUVR30–50 minWM and symptomatology scores for depression (the 17-item HAM-D). Voxel-wise analysis did not reveal brain regions which exhibited correlation between them with statistical significance. As in analysis of other psychiatric disorders, we used SUVR30–50 minWB for the analysis. Voxel-wise analysis with SPM of the patients with depression exhibited a significant negative correlation (P < 0.05, FDRc) between the SUVR30–50 minWB of [11C]K-2 PET and the 17-item HAM-D score in the frontal and parietal lobe (Fig. 3A and Supplementary Fig. 11G). This demonstrated that brain regions such as the frontal lobe and the parietal lobe can be state regions of depression and the unbalanced synaptic functions among these areas underlie depression. Interestingly, these regions were largely different from the brain areas where we detected a significant negative correlation between the SUVR30–50 min of [11C]K-2 PET and the 17-item HAM-D score in bipolar disorder (Fig. 3B).
Fig. 3. State regions in depression.
A Brain regions showing a significant negative correlation between SUVR30–50 minWB and the 17-item HAM-D score in patients with depression (P < 0.05, T < −1.69, one-tailed, FDRc). Significant clusters displayed on an axial, coronal and sagittal slices (Left), and scatter plot between averaged SUVR30–50 minWB in significant clusters and HAM-D score (Right, two-tailed Pearson correlation analysis: correlation coefficient = −0.5479, P = 0.0007). B Brain regions showing a significant negative correlation between SUVR30–50 minWB and the 17-item HAM-D score in patients with depression as determined above (blue) and bipolar disorder (green) (P < 0.05, T < −1.69, one-tailed, FDRc). Red regions show where the two regions overlap. MNI coordinates for the z-axis (axial slices), y-axis (coronal slices) and x-axis (sagittal slices) were shown bottom of each slice. C No significant difference of SUVR30–50 minWB between the patients with depression and healthy participants. All panels were adjusted for covariates (age, sex).
Interestingly, we detected no significant difference of SUVR30–50 minWM and SUVR30–50 minWB in the brain between the patients with depression and healthy participants (Fig. 3C and Supplementary Fig. 12).
Characteristics of [11C]K-2 in ASD
We next profiled AMPAR in 35 patients with ASD using [11C]K-2. Based on the tTACs (Supplementary Fig. 13A), LGA using the white matter as a reference exhibited linearity, suggesting the reversible binding kinetics of [11C]K-2 (Supplementary Fig. 13B). As was observed in patients with other psychiatric disorders, there exists a good linear relationship between BPND and SUVR30–50 minWM-1 in patients with ASD (Supplementary Fig. 13C), indicating that SUVR30–50 minWM-1 is almost identical to BPND. To investigate relative regional alterations in the [11C]K-2 PET signal in patients with ASD, we also constructed the SUVR30–50 minWB. There exists a good linear relationship between BPND and SUVR30–50 minWB in patients with ASD (Supplementary Fig. 13D and Supplementary Table 11). The CV among patients with ASD were lower with SUVR30–50 minWB than SUVR30–50 minWM, indicating that SUVR30–50 minWB has less inter-individual variability. (Supplementary Fig. 13E, F and Supplementary Table 7).
We first investigated the correlation between SUVR30–50 minWM and symptomatology scores for ASD (ADOS-2 Module 4 CSS). Voxel-wise analysis did not reveal brain regions which exhibited correlation between them with statistical significance. As in analysis of other psychiatric disorders, we used SUVR30–50 minWB for the analysis. We found a significant positive correlation (P < 0.05, FDRc) between the SUVR30–50 minWB of [11C]K-2 PET and the ADOS-2 Module 4 CSS in the large cortical areas such as the orbitofrontal cortex, the ACC, and the frontal and parietal lobe, indicating that these cortical areas are state brain regions for ASD (Fig. 4A and Supplementary Fig. 13G).
Fig. 4. State regions and altered AMPAR distribution in patients with ASD compared with healthy participants.
A Brain regions showing a significant positive correlation between SUVR30–50 minWB and ADOS-2 Module 4 CSS in patients with ASD (P < 0.05, T > 1.69, one-tailed, FDRc). Significant clusters displayed on an axial, coronal, and sagittal slices (Left), and scatter plot between averaged SUVR30–50 minWB in significant clusters and ADOS-2 Module 4 CSS (Right, two-tailed Pearson correlation analysis: correlation coefficient = 0.6027, P = 0.0001). B Relative reduction (blue) and increase (red) of [11C]K-2 retention in patients with ASD compared to healthy participants (P < 0.05, increase of [11C]K-2 retention: T > 1.66, reduction of [11C]K-2 retention: T < −1.66, one-tailed, FDRc). Significant clusters displayed on a 3D-rendered brain (binary image, Left), and axial, coronal and sagittal slices (T-map, Right). A and B (right panel) were adjusted for covariates (age, sex). B (left panel) was not adjusted for covariates.
SUVR30–50 minWB of [11C]K-2 PET was lower in the patients with ASD in the right middle frontal gyrus, the anterior cingulate gyrus, and the left anterior insula (P < 0.05, FDRc) (Fig. 4B and Supplementary Fig. 14A). We also detected a greater SUVR30–50 min of [11C]K-2 in the occipital lobe, the inferior temporal lobe, and the cerebellum of patients with ASD compared to healthy participants (P < 0.05, FDRc) (Fig. 4B and Supplementary Fig. 14A). SUVR30–50 minWM was decreased in the frontal, parietal and temporal lobe, the anterior insula, and the anterior cingulate cortex of patients with ASD, compared to the healthy participants (p < 0.05, FDRc) (Supplementary Fig. 14B).
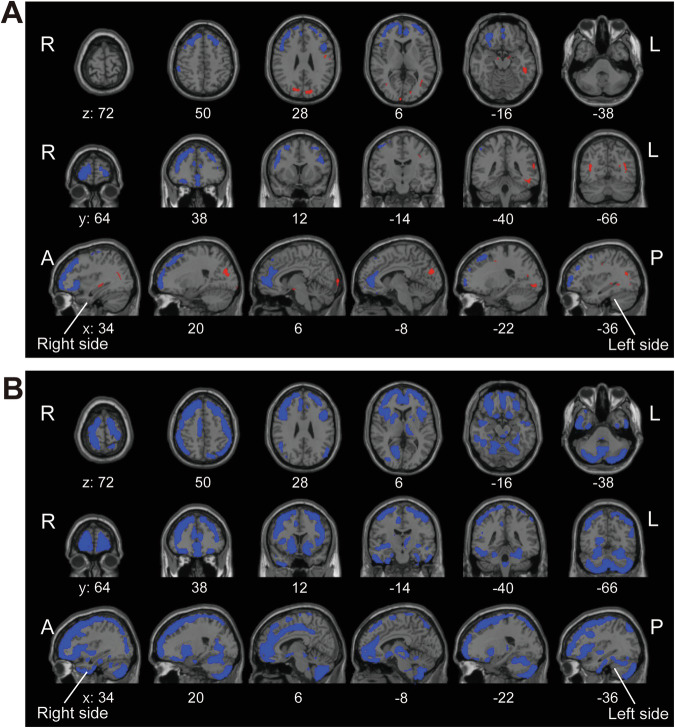
Identification of commonly affected areas in terms of AMPAR distribution across psychiatric disorders
Data of the participants with schizophrenia, bipolar disorder, and ASD that possess brain regions with altered AMPAR distribution compared to healthy participants were used for this analysis. We found that SUVR30–50 minWB was commonly decreased in the ACC, superior frontal gyrus, middle frontal gyrus, the orbital gyrus, the rectus, and the anterior part of insula and increased in the superior temporal gyrus, and the cuneus compared with healthy participants across these disorders (Fig. 5A and Supplementary Fig. 15A). We also found that SUVR30–50 minWM was commonly decreased in the anterior to middle cingulate gyrus, the frontal gyrus, the orbital gyrus, the rectus, the parietal gyrus, the middle occipital gyrus, the right lingual gyrus, the anterior part of insula, the putamen, the caudate, the pallidum, the left thalamus, and the cerebellum (Fig. 5B and Supplementary Fig. 15B).
Fig. 5. Commonly affected areas across psychiatric disorders.
A Common brain regions showing reduced (blue) and increased (red) SUVR30–50 minWB, respectively, in patients with schizophrenia, bipolar disorder and ASD compared with healthy participants. B Common brain regions showing reduced (blue) SUVR30–50 minWM, in patients with schizophrenia, bipolar disorder and ASD compared with healthy participants. MNI coordinates for the z-axis (axial slices), y-axis (coronal slices) and x-axis (sagittal slices) were shown bottom of each slice.
Discussion
The present study has revealed that unbalanced glutamatergic synaptic functions, assessed with AMPAR density, among brain regions can affect distinct and diverse status of psychiatric disorders. While we did not detect brain regions which exhibited significant correlation between SUVR30–50 minWM and symptomatology scores of diseases investigated, we found brain regions with significant correlations between SUVR30–50 minWB and symptomatology scores in these diseases. As was presented in Supplementary Figs. 6C, E, 7E, F, 9E, F, 11E, F, 13E, F and Supplementary Table 7, the CV among individuals were relatively larger in SUVR30–50 minWM than SUVR30–50 minWB. While SUVR30–50 minWM reflects absolute AMPAR density in the brain, SUVR30–50 minWB represents the balance of regional AMPAR density among brain regions. Further, since psychiatric disorders are considered to be the disruption of networks among brain regions whose fundamentals are synaptic function. Therefore, we could detect significant correlations between SUVR30–50 minWB and symptomatology scores but not SUVR30–50 minWM and, thus, SUVR30–50 minWB can be the optimal correction to analyze psychiatric disorders as “synapse diseases” in the network. SUVR30–50 minWM exhibited the reduction of AMPAR density in large brain areas especially in schizophrenia and ASD than healthy participants (Supplementary Figs. 8B and 14B), while SUVR30–50 minWB depicted region specific reduction or increase of AMPAR density in schizophrenia, bipolar disorder, and ASD (Figs. 1C, 2C, 4B and Supplementary Figs. 8A, 10A and 14A). Thus, systemic reduction and unbalanced distribution among brain regions of AMPAR can underlie the pathogenesis of psychiatric disorders.
We observed interesting synaptic features in areas which exhibited significant correlation between symptomatology scores and AMPAR density. In schizophrenia, while some brain areas for positive symptoms were overlapped with those for negative symptoms, there were specific areas either for positive or negative symptoms. The existence of overlapped and non-overlapped areas for these symptoms implies that the common circuit and symptom-specific circuits regulate the expression of positive and negative symptoms (Fig. 1A, B and Supplementary Fig. 7G, H). In bipolar disorder, the balance of synaptic functions between the frontal lobe and occipital lobe/cerebellum can control moods such as depressive and manic state (Fig. 2A, B and Supplementary Fig. 9G, H). Since some regions were overlapped between those correlated with depressive and manic state (Supplementary Fig. 9I), depressive and manic states in bipolar disorder might be biologically related. In depression, we detected a negative correlation between HAM-D score and SUVR30–50 min with [11C]K-2 (Fig. 3A and Supplementary Fig. 11G). While this phenotype in the frontal lobe of depression is similar to that of bipolar disorder, brain regions with this correlation in the frontal lobe were not largely overlapped between these disorders (Fig. 3B). Biological characterization of this observation warrants further investigations. In ASD, we detected a positive correlation between ADOS-2 severity score and SUVR30–50 min with [11C]K-2 in large part of the cortex (Fig. 4A and Supplementary Fig. 13G). This suggests that elevated synaptic activity can disrupt the signal-to-noise ratio during the processing of information such as sensory perception, resulting in the overflow of external inputs which is often observed in patients with ASD [36].
We also detected brain regions exhibiting altered AMPAR density in patients with schizophrenia, bipolar disorder, and ASD, compared to healthy controls; moreover, some regions such as anterior cingulate gyrus, insula, and cuneus were overlapped across these three disorders. Biological characterization of these regions remains to be elucidated. An interesting observation is that we did not detect brain regions with a difference of the average SUVR30–50 min using [11C]K-2 between patients with depression and healthy controls. This finding suggests that there is a continuum between depression and normal condition, unlike schizophrenia, bipolar disorder, and ASD. Our approach to elucidate glutamatergic synaptic functions, using the novel PET tracer, beyond the border of conventional diagnoses can be interpreted in the context of Research Domain Criteria (RDoC) [37]. This line of research will in turn enable the development of a novel diagnostic system coupled with therapeutics based on the biological profiling of psychiatric patients in terms of AMPAR, such as AMPAR blockers for the condition with increased AMPAR density and brain stimulation targeting a specific area that shows decreased AMPAR density.
In schizophrenia, bipolar disorder, and ASD, there was almost no overlap between the regions where clinical scores correlated with AMPAR density (state regions) and those where there were differences in AMPAR density compared to healthy participants. In our hypothesis, the regions with differences compared to healthy participants (but there is no correlation between illness severity and AMPAR density) may exist as ‘trait’ regions of the disease. “Trait” regions can exist regardless of illness severity (can exist even before the onset of the disease). On the other hand, the state regions are brain areas where AMPAR density can directly affect illness severity. We hypothesize that the trait region is formed first potentially due to the genetic factors etc., and then some kind of stress can induce changes in AMPAR in the state region and produces symptoms. It is interesting to know the order of formation of trait regions (ACC, insula, and occipital lobe) and how they generate the state region. However, we cannot rule out the possibility that these regions are secondary effects or not, and this is the limitation of this study. Our hypothesis can be proven by studies with animal models and longitudinal studies in human.
This study is a cross-sectional observation, limiting the causality between the AMPAR expression and the disease entity and behavior. One strength of our approach to psychiatric disorders in human based on the AMPAR is that we can further characterize causal relationship between phenotypes in the distribution of AMPAR and pathogenesis using animal experiments in which we can easily manipulate the expression of AMPAR in specific bran regions. We need further characterization of the above-mentioned brain regions using animal experiments for elucidation of the causal relationship between AMPAR phenotype observed in patients and pathogenesis, circuit dissection responsible for these disorders, and understanding of how commonly affected areas across diseases are generated and regions associated with each psychiatric symptom are created. Our approach to psychiatric disorders using [11C]K-2 can elucidate the biological mechanisms across diseases and pave the way to develop novel diagnostics and therapeutics based on the synapse physiology.
Supplementary information
Acknowledgements
The authors thank H. Ohta (SHOWA University Karasuyama Hospital) and O. Hashimoto (Hashimoto Clinic) for help with patient recruitment, S. Koike (University of Tokyo) for help with MRI imaging, and S. Tsuchimoto (NIPS) for advice with PET and MRI analysis.
Author contributions
TT designed and organized the project and established scientific concepts based on the interpretation of the data in this study. TT wrote the mainframe of the manuscript. TT, HU, MH, WN, and TM wrote the manuscript. HU, TM, TE wrote the clinical protocol based on the scientific concepts and study design. TT, MH and WN analyzed PET imaging data. HU, A Suda, T Asami, AH, MR, and S Tamura organized the recruitment of patients. TT, HU, MH, WN, and TM interpreted the data. T Arisawa and Y T synthesized [11C]K-2. A Sano and KN contributed the patient management on site. HU, TE, HT, NN, TK, SN, SK, YO, KT, YK, TY, YH, and HK assessed patients. MJ organized PET imaging at Keio University. SH organized MRI imaging at University of Tokyo. SB and OT organized PET imaging at Kyushu University. HO organized PET imaging at University of Fukui. YK performed kinetic analysis of the PET data. HA, S Tsugawa and MM discussed the data.
Funding
This project was supported by Ministry of Education, Culture, Sports, Science and Technology (Special Coordination Funds for Promoting Science and Technology) (TT), the Japan Agency for Medical Research and Development (AMED) under grant numbers JP14dm0207023 (TT), JP16dm0107124 (TT), JP24wm0625302 (YH) and the Japan Society for the Promotion of Science KAKENHI under grant numbers 20KK0193 (YH), 21H02851 (YH). This project was partially supported by Takeda Science Foundation (TT), AMED (grant number: JP19dm0207072, JP24wm0625304) (TT), the Japan Society for the Promotion of Science KAKENHI under grant numbers 20H00549 (TT), 20H05922 (TT), 19H03587 (HU), 20K20603 (HU), 22H03001 (HU), 22K15793 (HT), Keio Next-Generation Research Project Program (HU), SENSHIN Medical Research Foundation (HU), and Japan Research Foundation for Clinical Pharmacology (HT).
Data availability
All requests for raw and analyzed data are promptly reviewed by the Yokohama City University Research Promotion Department to determine whether the request is subject to any intellectual property or confidentiality obligations and, further, inspected by the Institutional Review Board of Yokohama City University Hospital. Upon these approvals, derived data will be released via a material transfer agreement from the corresponding author.
Competing interests
TT and TM are the inventors of a patent application for a novel compound that specifically binds to the AMPA receptor (WO 2017006931), including [11C]K-2. TT, TM and TA are the founders and also stockholders of AMPAMETRY, Inc., which holds the exclusive license to use [11C]K-2. No other potential conflicts of interest relevant to this article exist.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mai Hatano, Waki Nakajima, Hideaki Tani.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-024-02785-1.
References
- 1.Nevarez-Flores AG, Sanderson K, Breslin M, Carr VJ, Morgan VA, Neil AL. Systematic review of global functioning and quality of life in people with psychotic disorders. Epidemiol Psychiatr Sci. 2019;28:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin H, Mosweu I. The societal cost of schizophrenia: a systematic review. Pharmacoeconomics. 2017;35:25–42. [DOI] [PubMed] [Google Scholar]
- 3.Bessonova L, Ogden K, Doane MJ, O’Sullivan AK, Tohen M. The economic burden of bipolar disorder in the United States: a systematic literature review. ClinicoEcon Outcomes Res. 2020;12:481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders 5th ed. Wahington, DC: American Psychiatric Publishing; 2013.
- 5.Hantouche EG, Akiskal HS, Azorin JM, Châtenet-Duchêne L, Lancrenon S. Clinical and psychometric characterization of depression in mixed mania: a report from the French National Cohort of 1090 manic patients. J Affect Disord. 2006;96:225–32. [DOI] [PubMed] [Google Scholar]
- 6.Smith DJ, Griffiths E, Kelly M, Hood K, Craddock N, Simpson SA. Unrecognised bipolar disorder in primary care patients with depression. Br J Psychiatry. 2011;199:49–56. [DOI] [PubMed] [Google Scholar]
- 7.Kästner A, Begemann M, Michel TM, Everts S, Stepniak B, Bach C, et al. Autism beyond diagnostic categories: characterization of autistic phenotypes in schizophrenia. BMC Psychiatry. 2015;15:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cumming P, Abi-Dargham A, Gründer G. Molecular imaging of schizophrenia: Neurochemical findings in a heterogeneous and evolving disorder. Behav Brain Res. 2021;398:113004. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki T, Abe H, Uchida H, Takahashi T. Translational medicine of the glutamate AMPA receptor. Proc Jpn Acad Ser B Phys Biol Sci. 2021;97:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasai H, Ziv NE, Okazaki H, Yagishita S, Toyoizumi T. Spine dynamics in the brain, mental disorders and artificial neural networks. Nat Rev Neurosci. 2021;22:407–22. [DOI] [PubMed] [Google Scholar]
- 11.van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010;10:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varghese M, Keshav N, Jacot-Descombes S, Warda T, Wicinski B, Dickstein DL, et al. Autism spectrum disorder: neuropathology and animal models. Acta Neuropathol. 2017;134:537–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berdenis van Berlekom A, Muflihah CH, Snijders G, MacGillavry HD, Middeldorp J, Hol EM, et al. Synapse pathology in schizophrenia: a meta-analysis of postsynaptic elements in postmortem brain studies. Schizophr Bull. 2020;46:374–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diering GH, Huganir RL. The AMPA receptor code of synaptic plasticity. Neuron. 2018;100:314–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–13. [DOI] [PubMed] [Google Scholar]
- 17.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–8. [DOI] [PubMed] [Google Scholar]
- 19.Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, et al. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takemoto K, Iwanari H, Tada H, Suyama K, Sano A, Nagai T, et al. Optical inactivation of synaptic AMPA receptors erases fear memory. Nat Biotechnol. 2017;35:38–47. [DOI] [PubMed] [Google Scholar]
- 21.Mitsushima D, Ishihara K, Sano A, Kessels HW, Takahashi T. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc Natl Acad Sci USA. 2011;108:12503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe H, Jitsuki S, Nakajima W, Murata Y, Jitsuki-Takahashi A, Katsuno Y, et al. CRMP2-binding compound, edonerpic maleate, accelerates motor function recovery from brain damage. Science. 2018;360:50–57. [DOI] [PubMed] [Google Scholar]
- 23.Krystal JH. Imaging the glutamate synapse. Nat Med. 2020;26:165–7. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki T, Nakajima W, Hatano M, Shibata Y, Kuroki Y, Arisawa T, et al. Visualization of AMPA receptors in living human brain with positron emission tomography. Nature Medicine. 2020;26:281–8. [DOI] [PubMed] [Google Scholar]
- 25.Arisawa T, Miyazaki T, Ota W, Sano A, Suyama K, Takada Y, et al. [(11)C]K-2 image with positron emission tomography represents cell surface AMPA receptors. Neurosci Res. 2021;173:106–13. [DOI] [PubMed]
- 26.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
- 27.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, clinician version (SCID-CV). Washington, DC: American Psychiatric Press; 1996.
- 28.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. [DOI] [PubMed] [Google Scholar]
- 31.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. ADOS-2. manual (part I): modules, 1–4. Torrance, CA, USA: Western Psychological Services; 2012.
- 32.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40. [DOI] [PubMed] [Google Scholar]
- 33.Kimura Y, Naganawa M, Shidahara M, Ikoma Y, Watabe H. PET kinetic analysis -pitfalls and a solution for the Logan plot. Ann Nucl Med. 2007;21:1–8. [DOI] [PubMed] [Google Scholar]
- 34.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9. [DOI] [PubMed] [Google Scholar]
- 35.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J Autism Dev Disord. 2009;39:1–11. [DOI] [PubMed] [Google Scholar]
- 37.NIMH. Research Domain Criteria (RDoC). 2013. https://web.archive.org/web/20130609051825/http://www.nimh.nih.gov/research-priorities/rdoc/nimh-research-domain-criteria-rdoc.shtml.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All requests for raw and analyzed data are promptly reviewed by the Yokohama City University Research Promotion Department to determine whether the request is subject to any intellectual property or confidentiality obligations and, further, inspected by the Institutional Review Board of Yokohama City University Hospital. Upon these approvals, derived data will be released via a material transfer agreement from the corresponding author.