Abstract
We examined the distribution and fate of CARTp55–102 immunoreactive (IR) structures in the neonatal and adult rat urinary bladder. Staining for the apoptotic enzyme, caspase-3, and double labeling studies examining CARTp with tyrosine hydroxylase (TH) or neuronal nitric oxide synthase (nNOS) were performed in whole-mount preparations of urothelium or detrusor.
In younger animals (P1, P3), CARTp-IR cell bodies in detrusor smooth muscle were observed in large clusters (~100 cells/cluster) at the ureteral insertion and along thick bundles of nerve fibers at bladder base. The total number of CARTp-IR cells was significantly reduced (5-fold) in older animals, stabilized at P14 and persisted into adulthood. The decrease in number of CARTp expressing cells was complemented with staining for caspase-3 suggestive that apoptosis contributed to this decrease. At birth (P1), all CARTp-IR cells expressed the neuronal marker, Hu. After birth, CARTp was expressed by some neurons (CARTp+, Hu+) that represent intramural ganglion cells and by cells that lacked a neuronal phenotype (CARTp+, Hu−) but did express TH. These cells (CARTp+, Hu−, TH+) may represent paraganglion or small intensely fluorescent (SIF) cells. The percentage of colocalization of CARTp-IR and nNOS or TH was dependent on age and showed an inverse relationship. The suburothelial plexus showed no presence of CARTp-IR nerve fibers until P14 when nerve fibers were observed in urethra and bladder neck.
This study demonstrates that CARTp-IR intramural ganglia and CARTp-IR paraganglion or SIF cells exist in postnatal and adult rat bladder although the role of these cell types remains to be determined.
Indexing Terms: postnatal development, intramural ganglia, SIF cells, apoptosis, nNOS, TH
Introduction
Development of mature micturition reflexes after birth is believed to depend on continuous maturation of the nervous system during the early postnatal period (de Groat and Araki, 1999; de Groat et al., 1998). During the course of postnatal maturation, primitive reflex pathways organized at the spinal level are replaced by a spinobulbospinal reflex leading to emergence of voluntary voiding (de Groat and Araki, 1999; de Groat et al., 1998). The manner in which this is accomplished is not known but it is suggested that postnatal maturation of voiding function involves prominent reorganization of synaptic connections in bladder reflex pathways. This reorganization leads to down regulation of primitive spinal mechanisms and upregulation of mature supraspinal pathways (de Groat and Araki, 1999; de Groat et al., 1998). Previous studies have suggested the importance of neuroactive compounds in the process of maturation of the micturition reflexes during prenatal and early postnatal development (Ekstrom et al., 1994; Iuchi et al., 1994; Sann et al., 1997).
These changes in micturition reflexes during postnatal development may be mediated, in part, by changes in the neurochemical properties of central micturition pathways as well as by similar changes in the urinary bladder during development. It is known that changes in the urinary bladder that result from cyclophosphamide-induced cystitis (Qiao and Vizzard, 2002a; Qiao and Vizzard, 2004; Vizzard, 2000b; Vizzard, 2000c; Vizzard, 2001; Vizzard and de Groat, 1996) or upper motor neuron injury (Qiao and Vizzard, 2002b; Qiao and Vizzard, 2005; Vizzard, 2000a) can influence the neurons that innervate the urinary bladder changing the properties of bladder afferent cells in the dorsal root ganglia as well as properties of central micturition pathways. During development, a similar situation may exist whereby changes in the urinary bladder could drive the development of mature voiding patterns.
The neuropeptide, cocaine- and amphetamine-regulated transcript (CARTp), was originally identified in rat striatum after acute administration of cocaine or amphetamine (Douglass et al., 1995). Subsequent studies have identified CARTp in a number of other locations in brain and spinal cord. Based mainly on this distribution in central nervous system, a number of functions have been proposed for CARTp (for review see (Koylu et al., 1998; Kuhar et al., 2000). During development, neurons expressing CARTp are present as early as embryonic day (E)8 in nervous system of chicken (Ezerman and Forehand, 2004) and E11 of rat (Brischoux et al., 2002). Very little is known about the function of CARTp during postnatal development in general and in particular, no studies have examined CARTp in the context of micturition reflexes during postnatal development.
Postganglionic innervation to the rat urinary bladder is believed to originate from a peripheral ganglion, the major pelvic ganglion (MPG), located in close proximity to the urinary bladder (Dail et al., 1975; Keast et al., 1989; Keast and de Groat, 1989). Although intramural bladder ganglia are known to exist in a number of species including human (Dixon et al., 1992; Dixon et al., 1998), cat (de Groat and Booth, 1993), and guinea pig (Gabella, 1990; Hu et al., 2004), the existence of intramural ganglia in rat bladder has not been widely reported (Alian and Gabella, 1996) and it is still held that rat bladder lacks intramural ganglia (Tuttle, 2003). This study provides evidence of urinary bladder intramural ganglia in rat and begins to characterize the presence, organization and neurochemical properties of these intramural ganglia in early postnatal and adult rat. In addition, we provide evidence of another population of CARTp cells in the bladder that lack a neuronal phenotype but may represent, paraganglia adjacent to the urinary bladder or small intensely fluorescent (SIF) cells. In this study, we investigated the ontogeny of CARTp-immunoreactivity in the rat bladder during early postnatal development using immunohistochemical methods. The aims of the study were to determine the: (1) distribution of CARTp-immunoreactivity in urinary bladder of neonatal (P1–P28) and adult rats; (2) subpopulation(s) of cells and nerve fibers in urinary bladder expressing CARTp-immunoreactivity using multiple-label immunohistochemistry and; (3) fate of CARTp-IR cells in bladder during early postnatal development.
Materials and Methods
Experimental Animals
Wistar rats of either sex of different postnatal ages (Charles River, Canada; P1, P3, P7, P14, P21, P28 n = 67 and P56–63 (adult; 150–200 g) n=27) were used for these studies. Rat pups were born to timed-pregnant rats and several postnatal ages were studied for each litter born. The day of birth is referred to as P1. All animal procedures were approved by the University of Vermont Institutional Animal Care and Use Committee.
Tissue Harvesting
Young animals (P1– P14) were euthanized by decapitation after isoflurane anesthesia (3–4%). Older rats (P21-adult) were euthanized using isoflurane (3–4%) anesthesia followed by exsanguination. Prior to euthanasia in older animals and immediately following decapitation in younger animals, the urinary bladder was dissected and placed into oxygenated (95% O2 and 5% CO2) Krebs solution (119.0 mmol NaCl, 4.7 mmol KCL, 24.0 mmol NaHCO3, 1.2 mmol KH2PO4, 1.2 mmol MgSO4.7H2O, 11.0 mmol glucose, 2.5 mmol CaCl2) at room temperature.
Whole Mount Bladder Preparation
The bladder was cut open through the urethra along the midline and pinned flat on a sylgard-coated dish as previously described (Zvarova et al., 2004). After maximal stretch of the tissue, the bladder was incubated for 1.5 hour at room temperature in cold fixative (2% paraformaldehyde + 0.2% picric acid) and the urothelium was removed with fine forceps and iris scissors under a dissecting microscope. Urothelium and bladder musculature were examined separately for the markers described below by a free-floating technique (Zvarova et al., 2004).
Immunohistochemistry
Following fixation, detrusor and urothelium preparations were processed for CARTp-IR by using a free-floating method (Zvarova et al., 2004). To permeabilize the tissue and to minimize nonspecific binding, the preparations were placed in 0.1M sodium phosphate buffered saline (0.1M PBS) with 1% goat serum and Triton-X 100 for 30 minutes. Tissues were then incubated overnight at room temperature with primary antisera (Table 1) including rabbit anti-CART, mouse anti-neuronal nitric oxide synthase (nNOS), mouse anti-tyrosine hydroxylase (TH), rabbit anti-cleaved caspase-3 (Asp 175). To determine if CARTp-IR cells and fibers in the bladder were neurons and nerve fibers, antiserum directed against the pan neuronal markers, Hu (anti-HuC/HuD) for cell body staining and protein gene product (PGP9.5) for fiber staining was used followed with the appropriate secondary antibody (Table 1). To demonstrate the presence of the suburothelial plexus in P1 and P3 rats prior to CARTp expression, urothelium was stained with rabbit anti-P2X2 and anti-P2X3 receptor antisera (Table 1). After primary antibody incubation, tissues were washed (3 x 15 minutes) with 0.1 M PBS (pH 7.4) and then incubated with species-specific secondary antibodies (Table 1) for 2 hours at room temperature. After three rinses (3 x 15 minutes) in 0.1 M PBS (pH 7.4), tissues were mounted on 0.5% gelled slides and cover slipped using Citifluor (Citifluor, London, UK). Control tissues incubated in the absence of primary or secondary antibody were also processed and evaluated for specificity or background staining levels. In the absence of primary or secondary antibody, no positive immunostaining was observed.
Table 1.
Table of primary and secondary antibodies, working dilutions and sources used.
| Primary Antibody | Source | Working Dilution | Secondary Antibody | Source | Working Dilution |
|---|---|---|---|---|---|
| Rabbit anti-CART | Phoenix Pharmaceuticals, Belmont, CA | 1:16,000 | Cy3 | Jackson Immunoresearch Laboratories, Inc., West Grove, PA | 1:500 |
| Mouse anti-nNOS | Santa Cruz Biotechnology Inc., Santa Cruz, CA | 1:100 | FITC | Jackson Immunoresearch Laboratories, Inc., West Grove, PA | 1:50 |
| Mouse anti-TH | Chemicon International, Inc., Temecula, CA | 1:100 | FITC | Jackson Immunoresearch Laboratories, Inc., West Grove, PA | 1:50 |
| Mouse anti-Hu | Molecular Probes, Inc., Eugene, OR | 1:200 | Cy2 | Jackson Immunoresearch Laboratories, Inc., West Grove, PA | 1:100 |
| Goat anti-ChAT (AB 144P) | Chemicon International Inc., Temecula, CA | 1:50-1:1000 | Cy2 | Jackson Immunoresearch Laboratories, Inc., West Grove, PA | 1:100 |
| Mouse anti-ChAT (MAB5350) | Chemicon International Inc., Temecula, CA | 1:50,1:100 | FITC | Jackson Immunoresearch Laboratories, Inc., West Grove, PA | 1:50 |
| Mouse anti-ChAT (MAB5270) (1.B3.9B3) | Chemicon International Inc., Temecula, CA | 1:50,1:100 | FITC | Jackson Immunoresearch Laboratories, Inc., West Grove, PA | 1:50 |
| Rabbit anti-cleaved caspase-3 (Asp 175) | Cell Signaling Beverly, MA | 1:50 | ABC
DAB |
1.Vectastatin Elite Kit, Vector Labs Burlingame, CA
2.Vector labs Burlingame, CA |
|
| Rabbit anti-PGP 9.5 | Biogenesis Ltd, Poole, England | 1:1000 | Cy3 | Jackson Immunoresearch Laboratories, Inc., West Grove, PA | 1:500 |
Assessment of Positively Stained Structures
Staining observed in experimental tissue was compared to that observed from experiment-matched negative controls. Structures (cell bodies, and nerve fibers) exhibiting immunoreactivity that was greater than the background level observed in experiment-matched negative controls were considered positively stained. Positively counted cells were not further divided into categories of different staining intensities.
Data analysis
Tissues were examined under an Olympus fluorescence photomicroscope (Optical Analysis Corp., Nashua, NH) for visualization of Cy3, Cy2 or FITC. Cy3 was visualized with a filter with an excitation range of 560–596 nm and emission range from 610–655 nm and FITC and Cy2 were visualized with a filter with an excitation range of 470 nm and emission range at 525nm. CARTp-IR cell profiles were counted in whole-mount preparations of bladder musculature from 68 different animals. Individual neurons could be identified and only those with a nucleus were quantified. Numbers of CARTp-IR cell profiles per bladder are presented as mean number of CARTp-IR cells/bladder ± S.E.M. The percentage of CARTp-IR cells expressing Hu, TH, or nNOS is also presented (mean ± S.E.M). Double-labeling was assessed by confocal scanning laser microscopy (Zeiss LSM 510 Meta, Carl Zeiss, Inc., Jena, Germany). For each z-axis interval (1–2 μm), whole-mounts were scanned twice using argon lasers with specific excitation wavelengths and sequential images were captured for computer-generated overlay and analysis. Long axis cell measurements of CARTp-IR cells (mean ± SEM) were also performed using the confocal microscope. Animals, processed and analyzed on the same day, were tested as a block in the analysis of variance (ANOVA). When F ratios exceeded the critical value (p ≤ 0.05), the Newman-Keuls test was used for multiple comparisons among means. Percentage data were arcsin transformed to meet the requirements of the ANOVA. To test for the effect of age on colocalization of CARTp+ and nNOS+ or CARTp+ and TH+, linear regression analysis was performed. Digital images were obtained using a CCD camera (MagnaFire SP; Optronics; Optical Analysis Corp.) and LG-3 frame grabber attached to an Olympus microscope (Optical Analysis Corp). Images were imported in Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, CA) where figure sets were assembled and labeled.
Results
Urothelium
CARTp-IR nerve fibers were first observed on the surface of the urothelium (in the suburothelial plexus) in rats at P14 (Fig. 1). To be certain that nerve fibers existed in the suburothelial plexus at birth but lacked CARTp-immunoreactivity, the urothelium from P1 and P3 rats was stained with antibodies against P2X2 (Fig. 1D) and P2X3 receptor subtypes (data not shown). At P1 and P3, nerve fibers exhibiting P2X2- and P2X3-immunoreactivity were observed but the suburothelial plexus did not express CARTp at these early time points examined (Fig. 1A, D). At P14, CARTp-IR was observed in the region of bladder neck as short, scattered branches that later formed a varicose plexus (Fig. 1B). The greatest density of CARTp fiber staining was observed in the bladder neck region. A less dense distribution was observed elsewhere and continued to decrease toward the bladder body with no staining in bladder dome. Fibers expressing CARTp increased in number and relative density during the first two postnatal weeks and reached adult levels by P21 (Fig. 1C). In adult tissue, CARTp-IR fibers also formed a plexus of suburothelial nerve fibers. As a comparison to the CARTp staining in the suburothelial plexus, PGP9.5 was used to visualize all nerve fibers in the suburothelial plexus (Fig. 1E, F). CARTp-IR nerve fibers represented only a subset of nerve fibers revealed with PGP9.5. For unknown reasons, PGP9.5 staining was not successful in demonstrating the suburothelial plexus at P1 and P3, thus alternative markers were used (i.e., P2X2, P2X3). CARTp-IR nerve fibers also expressed nNOS immunoreactivity (Fig. 2). Extensive colocalization of CARTp-IR and nNOS-IR nerve fibers was observed in the suburothelial plexus (Fig. 2). Some nNOS-IR nerve fibers appeared to lack CARTp-immunoreactivity (Fig. 2G). No CARTp-IR cell bodies were found in the suburothelial plexus during postnatal development or in adult animals. No CARTp-immunoreactivity was observed in urothelial cells during postnatal development or in adult animals.
Figure 1.
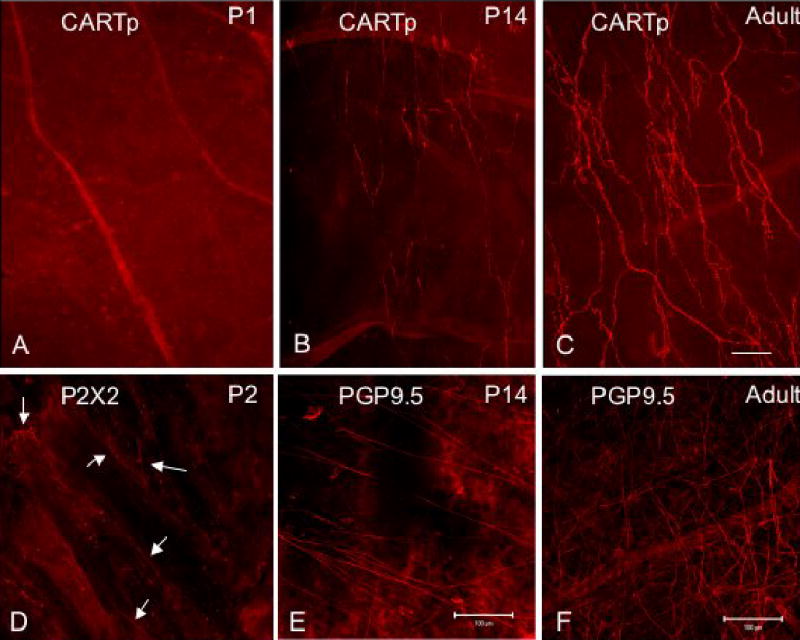
CARTp-IR nerve fibers in suburothelial plexus are not present at birth but appear with increasing postnatal age. Fluorescence images of CARTp-immunoreactivity in whole-mount urothelium from postnatal (P) day P1 (A), P14 (B) and adult rat (C) are shown. For a comparison, nerve fibers in suburothelial plexus were immunostained for the pan neuronal fiber marker, protein gene product (PGP9.5) in P14 (E) and adult (F) rat. Because staining for PGP9.5 in the youngest rat pups examined was not successful, nerve fiber staining (D, arrows) was visualized with immunostaining to P2X2 (D). CARTp-immunoreactivity was not present in urothelium until P14 (B) although nerve fibers were present at early postnatal ages (P2; D). CARTp-IR nerve fibers continued to increase in density with age (C) but only represented a subpopulation of all nerve fibers (B, C vs. E, F). In adult rats, CARTp-IR nerve fibers were most prominent in the bladder neck and proximal urethra with less density in bladder body and absence of staining in bladder dome. Calibration bar in C represents 100 μm for panels A–C, D. Calibration bars in E and F represent 100 μm.
Figure 2.
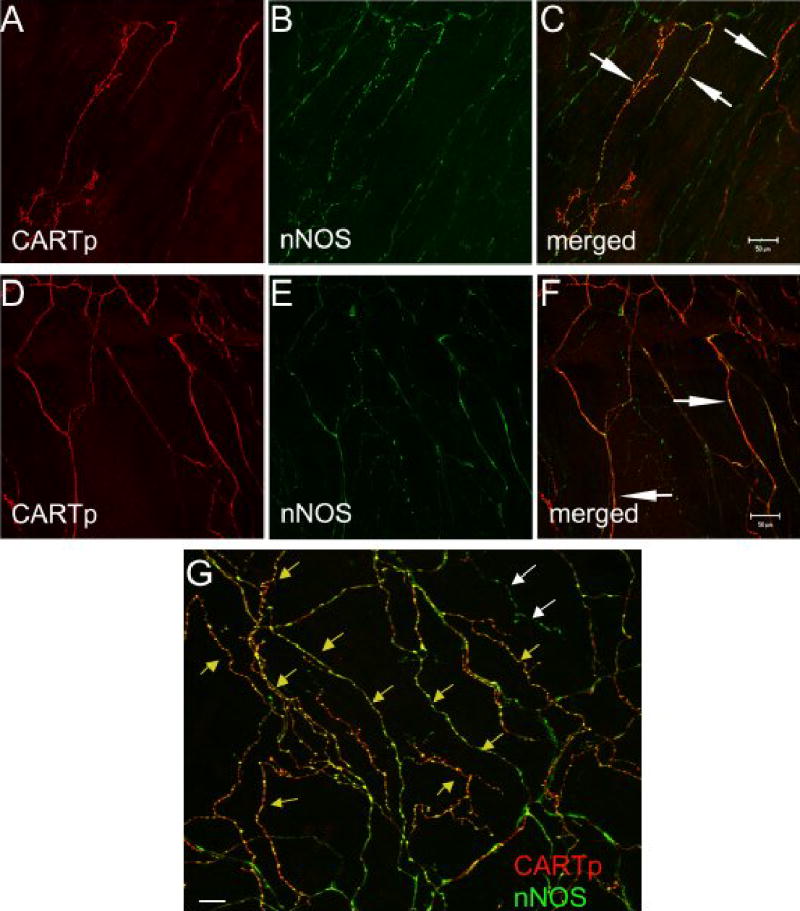
CARTp-IR nerve fibers in suburothelial plexus also express nNOS-immunoreactivity. Confocal fluorescence images of urothelium whole-mounts from adult rats demonstrating CARTp-immunoreactivity (A,D) or nNOS immunoreactivity (B, E) in the same images are shown. Images in C, F and G represent merged images where red staining represents CARTp and green staining represents nNOS. Areas of overlap appear yellow/orange (C, F, white arrows; G, yellow arrows). Some nNOS-IR nerve fibers do not appear to express CARTp-immunoreactivity (G, white arrows) although overlap between CARTp- and nNOS-immunoreactivity is considerable. Calibration bar in C represents 50 μm for A–C. Calibration bar in F represents 50 μm in D–F. Calibration bar represents 100 μm in G.
Detrusor
In all the postnatal and adult rats examined, CARTp-immunoreactivity was expressed in both nerve fibers and cell bodies associated with the detrusor muscle (Fig. 3). No CARTp-immunoreactivity was observed in detrusor smooth muscle itself.
Figure 3.

Montage images of CARTp-IR cell clusters and fibers in detrusor smooth muscle superimposed on schematic representations of urinary bladder at P1 (A) and P7 (B). At birth, CARTp-IR cells and fibers were observed throughout the detrusor. At P1, the size of CARTp-IR cells clusters was large (A, arrows) and clusters consisted of 50–100 cells/cluster. At P7, the size of the CARTp-IR cells clusters was decreased (B, arrows) and clusters were more distributed throughout the bladder. Prominent CARTp-immunoreactivity was associated with ureters at all rat ages examined.
CARTp-IR nerve fibers
CARTp-IR nerve fibers were present at birth (P1) (Fig. 3). CARTp-IR nerve fibers were observed symmetrically around the point of entry of the ureters and extended to all regions of the detrusor smooth muscle (Fig. 3A, B). CARTp-IR nerve fibers were present in large nerve bundles, smaller branches and individual nerve fibers (Fig. 4).
Figure 4.
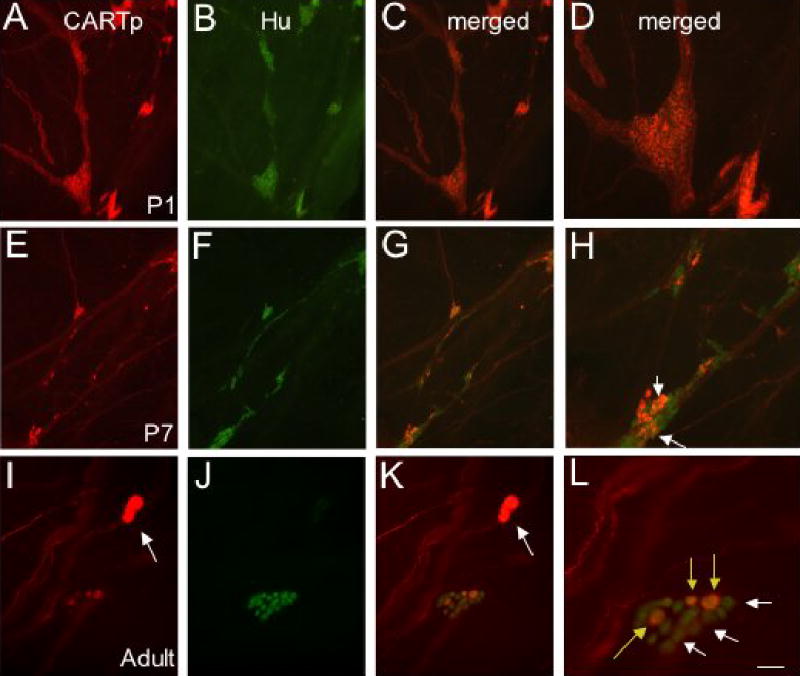
All CARTp-IR cells in detrusor smooth muscle were neurons (Hu-IR) at birth but all CARTp-IR cells did not retain this neuronal phenotype with age and some neurons (Hu-IR) no longer expressed CARTp with age. Fluorescence images of CARTp-IR cells and nerve fibers in detrusor smooth muscle at birth (P1; A). At birth, all CARTp-IR cells expressed the neuronal marker (Hu; B). Panels C and D are merged images of lower (C) and higher (D) power of CARTp+, Hu+ cells. At P7 (E–H), not all CARTp-IR cells expressed a neuronal phenotype (F) but some CARTp-IR cells retained a neuronal phenotype (H, arrows). With increasing postnatal ages, the size of CARTp-IR cell clusters was smaller than at birth (A vs. E). CARTp-IR nerve fibers were still present in larger fiber bundles and individual nerve fibers (A, E, I). Panels G and H are merged images of lower (G) and higher (H) power of CARTp+, Hu+ cells and CARTp-, Hu+ cells (H). In older postnatal rats and adult rats (I–L), CARTp+, Hu+ (L, yellow arrows) and CARTp-, Hu+ (L, white arrows) cells were present. In addition, CARTp-IR cells that lack a neuronal phenotype are also present (I, K, arrows). Calibration bar represents 60 μm in A–C, E–F, I–K and 30 μm in D, H, L.
CARTp-IR cell bodies
As early as P1 (Fig. 3, 4), we observed clusters of numerous, uniformly sized and shaped CART-IR cell bodies in the bladder neck region and along the nerve fibers described above. During the course of postnatal development, these CARTp-IR cell bodies migrated from the level of ureteral insertion toward the bladder body (Fig. 3, 4). Clusters of CARTp-IR cells were generally oval in appearance and contained from 50–100 cell bodies/cluster (Fig. 3, 4). The total number of CARTp expressing cells at birth (P1) was 1007 ± 120 cells/bladder (Fig. 5). At P3, the number of CARTp-IR cells significantly decreased (743.0 ± 99.6 CARTp-IR cells/bladder) (Fig. 5). With increasing postnatal age, CARTp-IR cell clusters dissociated into numerous smaller groups of cells (Fig. 3, 4). CARTp-IR cell groups branched off from clusters into the bladder wall or continued to move along the nerve fibers creating numerous intramural ganglia (Fig. 3, 4). The decrease in the total number of CARTp-IR cell bodies/bladder continued with increasing postnatal age (Fig. 5). By P14, the number of CARTp-IR cells/bladder stabilized and this number was maintained into adulthood (Fig. 5).
Figure 5.
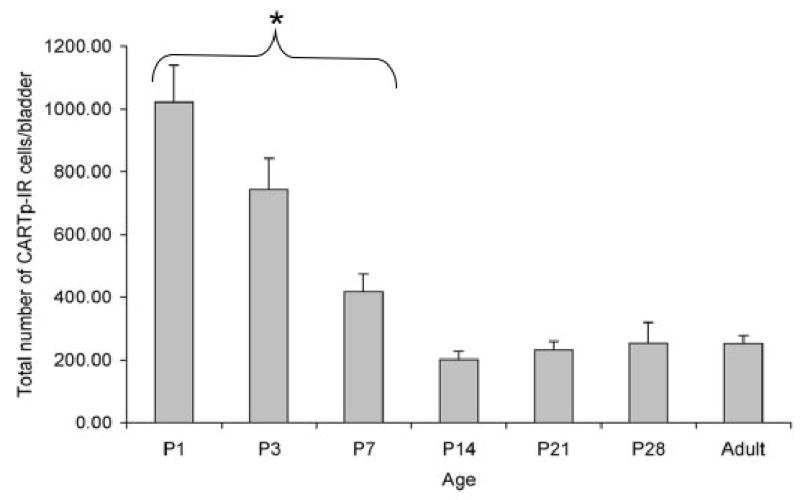
The number of CARTp-IR cells in detrusor smooth muscle significantly decreases after birth (P1). The number of CARTp-IR cells present at P14 is maintained into adulthood. *, P ≤ 0.01 compared to adult numbers of CARTp-IR cells/bladder.
Apoptotic processes may account, in part, for the decrease in CARTp-IR cell bodies in the detrusor of the urinary bladder with postnatal development. This hypothesis was tested by using immunostaining against caspase-3, a key mediator of apoptosis. Cells exhibiting caspase-3-immunoreactivity were observed at the two postnatal ages examined, P1 and P3. Cells expressing caspase-3-immunoreactivity were observed in sites corresponding to the areas described previously (Fig. 6).
Figure 6.
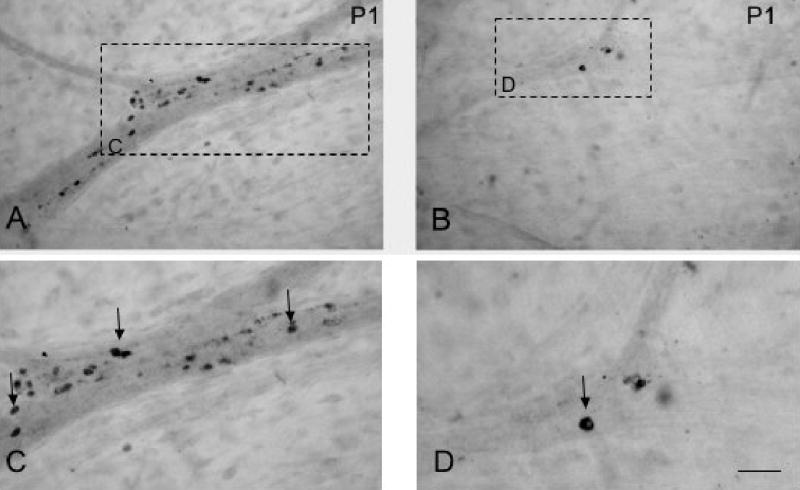
Apoptosis may account, in part, for the decrease in CARTp-IR cells/bladder after birth. Brightfield images of caspase-3-immunoreactivity in detrusor smooth muscle at birth, P1 (A, B). Image in A is from the bladder neck region, a short distance from the ureteral insertion. Image B is from a more cranial area of detrusor. Higher power images (C, D) of caspase-3-IR cells (arrows) in detrusor smooth muscle from regions indicated by rectangles in A, B. Calibration bar represents 150 μm in A, B and 50 μm in C, D.
In addition to changes in the number of CARTp-IR cells observed in the urinary bladder with postnatal development, changes in the neurochemical phenotype of these cells were also observed (Fig. 4). At P1, all (100%) CARTp-IR cells expressed Hu-immunoreactivity demonstrating the initial neuronal character of all CARTp-IR bladder cells (Fig. 4). However, in adult rats, this percentage was significantly (p ≤ 0.01) reduced and only 35.2 ± 6.8% (n = 6 adult rats) of the CARTp-IR cells exhibited Hu-immunoreactivity (Fig. 7). At P1, 1007 ± 120 CARTp-IR neurons were observed in the detrusor smooth muscle. In adults, the number of neurons (Hu-IR) was significantly (p ≤ 0.01) reduced to 146.2 ± 34.6 CARTp-IR neurons (n = 6 adult rats). The size of the CARTp-IR cells changed with postnatal development. In young postnatal rats, CARTp-IR cells with Hu-immunoreactivity measured 14.4 ± 0.6 μm. In adult rats, CARTp-IR cells with Hu-immunoreactivity were significantly (p ≤ 0.01) larger and measured 23.9 ± 0.2 μm. After birth (P1), not all CARTp-IR cells expressed Hu-immunoreactivity; cells examined were divided into three different subgroups based upon neurochemical properties (Fig. 4, 7). Some CARTp-IR cells continued to express the neuronal marker (CARTp+, Hu+) (Fig. 7A) and some CARTp-IR cells lost neuronal character (CARTp+, Hu−) (Fig. 4, 7B). This population of cells, CARTp+, Hu−, was significantly smaller than CARTp+, Hu+ cells and measured 8.9 ± 0.4 μm. A third subset of cell bodies expressed Hu but lacked CARTp (CART−, Hu+) (Fig. 7A). CARTp-IR nerve fibers were observed in close proximity to each of these populations of cells (Fig. 4, 7).
Figure 7.
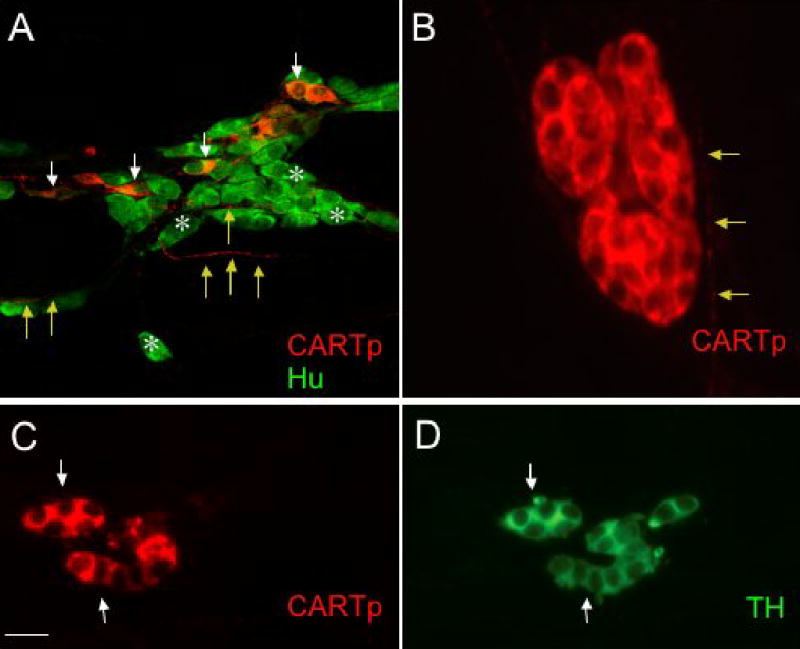
Different populations of CARTp-IR cells are present in detrusor smooth muscle after birth. A. Confocal fluorescence images of cell bodies in detrusor smooth muscle of CARTp+, Hu+ cells (white arrows) as well as numerous neurons (A, Hu+, white asterisks) that lack CARTp-immunoreactivity after birth. CARTp-IR fibers extend among Hu+ cells (A, yellow arrows). In older postnatal and adult rats, CARTp-IR cells that lack a neuronal phenotype are present in detrusor smooth muscle. These CARTp+, Hu- cells (B) are small, rounded cells with large nuclei and a thin rim of cytoplasm. CARTp-IR fibers were observed in close proximity to these cell clusters and (B, yellow arrows). These CARTp+, Hu- cells (C, arrows) express tyrosine hydroxylase (TH) immunoreactivity (D, arrows). Calibration bar represents 30 μm in A, 10 μm in B and 20 μm in C, D.
The neurochemical properties of the CARTp-IR cells with or without Hu-immunoreactivity also changed during the first three postnatal weeks. Double immunostaining with antibodies against CARTp and TH, an immunocytochemical marker for catecholaminergic neurons or CARTp and nNOS (Fig. 8) showed a linear but an inverse trend in percentage of colocalization. The percentage of colocalization of markers examined was compared at different ages during the early postnatal period. At P1, 19.5% of the CARTp-IR cells were TH-immunoreactive whereas at P28 coexpression increased to 72.1% (CARTp+, TH+) (Fig. 9). For CARTp and nNOS colocalization, the results were opposite that found with CARTp and TH. A high percentage of colocalization of CARTp+, nNOS+ initially at P1 (67.1%) was followed by a significant decline (27.9%) at P28 (Fig. 8, 9). The percentage of colocalization for CARTp+, TH+ increased significantly with age and 87% of the variance in this colocalization was explained by age alone (p ≤ 0.01). In contrast, colocalization of CARTp+, nNOS+ showed a significant decrease with age and in this case age accounted for 96% of the variability in the colocalization (p ≤ 0.005). Although attempted with several different antisera, ChAT immunostaining of tissues was not successful and therefore no conclusions can be made about the potential cholinergic phenotype of the CARTp-IR cells.
Figure 8.
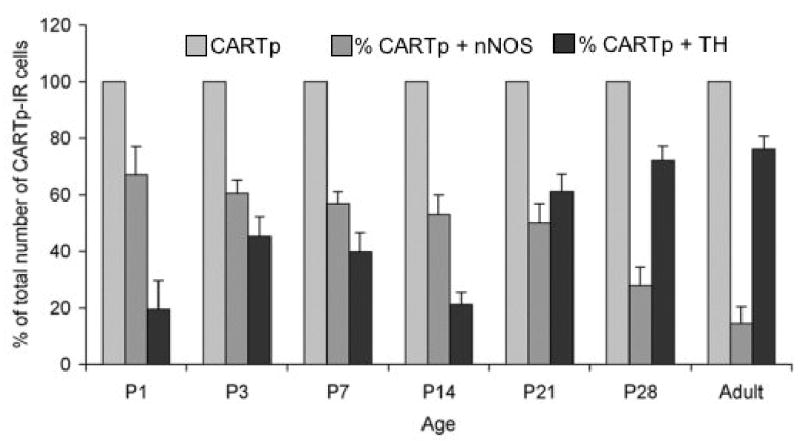
Histogram that demonstrates that the colocalization of CARTp-IR cells with neuronal nitric oxide synthase (nNOS) or tyrosine hydroxylase (TH) is dependent upon postnatal age and the patterns of colocalization show an inverse relationship. The percentage of CARTp-IR cells that also express nNOS is highest at birth and shows an age dependent reduction. Age accounted for 96% of the variability in this colocalization, p ≤ 0.005. In contrast, the percentage of CARTp-IR cells that expressed TH is lowest at birth but shows a significant increase in colocalization with age. Age accounted for 87% of the variability in this colocalization, p ≤ 0.01.
Figure 9.
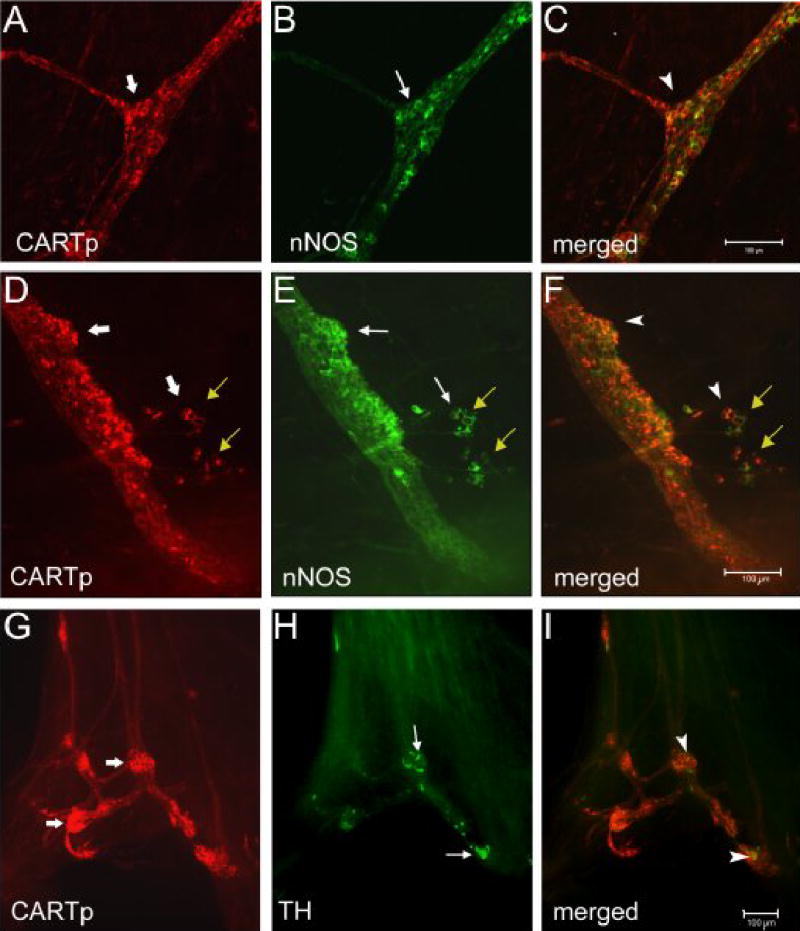
Colocalization of CARTp-IR cells with neuronal nitric oxide synthase (nNOS) or tyrosine hydroxylase (TH) is dependent upon postnatal age. At birth, the majority of CARTp-IR (A, D, wide arrows) cells also express nNOS-immunoreactivity (B, E, arrows). Merged images (C, F) where red staining represents CARTp and green represents nNOS. Cells that express both CARTp and nNOS appear yellow/orange (C, F, arrowheads. Small groups of CARTp-IR cells or individual CARTp-IR cells also appear to migrate away from the larger cell clusters (D–F, yellow arrows). These CARTp-IR cells also show some colocalization with nNOS. In contrast, few CARTp-IR (G, wide arrows) cells express TH-immunoreactivity (H, arrows) at birth. Merged image (I) where red staining represents CARTp and green represents TH. A few cells that express CARTp + TH appear yellow (I, arrowheads). Calibration bar in C represents 100 μm in A–C. Calibration bar in F represents 100 μm in D–F. Calibration bar in I represents 100 μm in G–I.
In adult detrusor, CARTp-immunoreactivity was expressed by two distinct cell types distinguished on the basis of neurochemistry and morphology: (1) neuronal (Hu-IR), larger (23.9 ± 0.2 μm) oval cells (Fig. 7A); and (2) non-neuronal (non Hu-IR), small (8.9 ± 0.4 μm) rounded cells (Fig. 7B) usually closely packed together in groups exhibiting TH-immunoreactivity (Fig. 7C, D). In adult rats, only ~35% of the CARTp-IR cells expressed Hu-immunoreactivity and were therefore neurons. Thus, in adult rats, the majority of detrusor CARTp-IR cells represent non-neuronal cells. In the adult urinary bladder, there were 146.2 ± 35 Hu+ cells.
Other Tissues Expressing CARTp-Immunoreactivity
CART-IR was expressed by a dense network of nerve fibers in ureters and in the wall of bladder blood vessels at the time of birth (P1). This expression of CARTp persisted into adulthood.
Discussion
The present study examined the expression of CARTp-immunoreactivity in the rat urinary bladder during early postnatal development. We observed dynamic changes in expression of CARTp-immunoreactivity during the postnatal period from P1 to P14; a critical period in micturition reflex development (Araki and de Groat, 1995; Araki and de Groat, 1997; de Groat and Araki, 1999; de Groat et al., 1998). There are several novel findings from this study: (1) the presence of large CARTp-IR cell clusters near the ureteral insertion and along the main nerve fibers of the bladder at birth; (2) the first appearance of CARTp-immunoreactivity in the suburothelial nerve plexus between P14-P21; (3) a significant decrease in number of CART-IR cells during the first three postnatal weeks; (4) changes in morphology, cell type and neurochemical phenotype of CARTp-IR cells during the postnatal period.
In neonates of many species, the neural control of micturition undergoes marked changes during early postnatal development (de Groat, 1975; de Groat et al., 1993; Maggi et al., 1988; Thor et al., 1990; Thor et al., 1986). In newborn rats and cats, micturition is dependent upon a spinal reflex pathway that is activated when the mother licks the perineal region of the young animal (de Groat and Kruse, 1993; de Groat et al., 1981; Thor et al., 1986). This reflex pathway consists of a somatic afferent limb in the pudendal nerve and a parasympathetic efferent limb in the pelvic nerve. As the central nervous system matures during the first few postnatal weeks, the spinal micturition reflex is gradually replaced by a spinobulbospinal reflex pathway that is responsible for micturition in adult animals (Capek and Jelinek, 1956; de Groat, 1975; de Groat et al., 1993; Thor et al., 1989). The spinobulbospinal micturition reflex is triggered by tension receptor afferents in the bladder and begins to elicit voiding in the rat between postnatal days (P) 16–18 (Capek and Jelinek, 1956).
All CARTp-IR cells in detrusor smooth muscle at birth (P1) were initially uniform in size, shape and expressed a neuronal phenotype (Hu+). Later in development (P3, P7, P14), some CARTp-IR cells retained this neuronal phenotype (Hu+, CARTp+) and other CARTp-IR cells did not (Hu−, CART+). We also observed a third subset of cells that expressed a neuronal phenotype but were not CARTp-IR (Hu+, CARTp−). The neurochemical phenotype of this neuronal population is not yet known. During the first three postnatal weeks, the total number of cells expressing CARTp-immunoreactivity in the rat urinary bladder significantly decreased. The total number of neurons (Hu+) in the rat bladder similarly decreased (6.7-fold decrease) during the first three postnatal weeks. The decrease in the total number of neurons in the rat detrusor with postnatal development is consistent with a previous report in the postnatal Sprague-Dawley rat (Alian and Gabella, 1996). In contrast, the number of neurons reported in the Sprague-Dawley rat at birth (Alian and Gabella, 1996) was considerably less (~5-fold) than that observed in this study. The reasons for this are not known but may be attributed to species or source differences but may also be attributed to different methodology used to visualize the bladder neurons (Alian and Gabella, 1996). The decrease in the total number of CARTp-IR cells and bladder neurons may involve apoptotic processes as evidenced by the presence of caspase-3-immunoreactivity. Alternatively, decreases in CARTp-IR cells and bladder neurons may be attributed to a shift in the neurochemical phenotype of the CARTp-IR cells that still retain a neuronal phenotype (Hu+) but no longer express CARTp-immunoreactivity after birth (Hu+, CARTp-) or to migration of neurons out of the bladder wall to the major pelvic ganglion (MPG) as previously suggested (Alian and Gabella, 1996). Even with decreases in the number of CARTp-IR cells and associated decreases in bladder neurons with postnatal development, CARTp-IR cells with a neuronal phenotype still persisted into the adult rat as did neurons without CARTp-immunoreactivity (~150 cells/adult bladder).
In the adult rat detrusor, CARTp-IR was expressed in nerve fibers and two distinct cell types: (1) neuronal, larger oval cells (Hu+, CARTp+) that probably represent intrinsic autonomic neurons of intramural ganglia and; (2) non-neuronal, smaller rounded cells with large nuclei and a thin rim of cytoplasm usually closely packed together in groups (TH+, Hu-, CARTp+). Although the efferent innervation of the urinary bladder is described as originating from pelvic ganglia (i.e., MPG in rat) (Dail et al., 1975; Keast et al., 1989; Keast and de Groat, 1989), the distribution of ganglion neurons in the detrusor smooth muscle has been noted for a number of species including human (Dixon et al., 1992; Dixon et al., 1998), cat (de Groat and Booth, 1993), and guinea pig (Gabella, 1990). Although Alian and Gabella, 1996 (Alian and Gabella, 1996) noted the presence of ganglion neurons in postnatal rats and in rats as old as 12–20 weeks of age, there is still the impression that in the rat urinary bladder, only the peripheral fibers of neurons from the MPG are present (Tuttle, 2003). In addition, most in vitro rat bladder studies make the assumption that ganglionic spontaneous activity is absent. However, this study suggests that regulation of rat bladder function may involve integration in the MPG and in intramural bladder ganglia.
In adult rats, the majority of CARTp-IR cells no longer expressed a neuronal phenotype (Hu−) but expressed TH-immunoreactivity. The previous descriptions of catecholamine secreting endocrine cells (i.e., paraganglion cells in urinary bladder) are consistent with the CARTp+, Hu−, TH+ cells described here and thus, these cells may have a potential neuroendocrine function (Dixon et al., 1998; Dixon et al., 1999). Paraganglia have been classified as extra-adrenal chromaffin tissue that contains catecholamines (Coupland, 1965) (Hervonen, 1971). Peripheral catecholamine producing cells are found in close association with the sympathetic nervous system and are often innervated by preganglionic sympathetic nerve fibers (Coupland and Bustami, 1980). A number of sympathetic ganglia contain cells that are small and intensely fluorescent (SIF) and express TH-immunoreactivity (Doupe et al., 1985). A number of ganglia, including cardiac ganglia (Mawe et al., 1996) contain SIF cells that are TH-IR and are unaffected by chemical sympathectomy. Two types of SIF cells have been proposed; an interneuronal form (type I) and an endocrine-like element (type II) (Coupland, 1989; Langley and Grant, 1999; Williams et al., 1976). The role of SIF cells in ganglionic systems is not known but it has been suggested that SIF cells perform endocrine functions (type II) or modulate synaptic output of neurons (intramural ganglion or MPG; type I) (Coupland, 1989; Langley and Grant, 1999). The results of this study suggest that SIF and/or paraganglion cells in the detrusor smooth muscle express CARTp-immunoreactivity. The role of CARTp in the SIF cell population or paraganglion cells is not known but we suggest that CARTp may be released in a paracrine manner to affect neurons (Hu+ with or without CARTp), detrusor smooth muscle and/or blood vessels. Another possibility is that CARTp-IR cells in detrusor smooth muscle at birth may represent undifferentiated precursor cells of sympathoadrenal lineage in which CARTp has been suggested to be a signaling molecule (Dun et al., 2000a). Other functions of CARTp in development are not yet known. CARTp receptors have not yet been identified (Hunter and Kuhar, 2003) so it is not known if the appropriate receptors exist to mediate such effects. A recent study (Okumura et al., 2000) suggests that CARTp exerts its effects through corticotropin releasing factor (CRF) receptor(s). CRF-R1 has been reported in the sacral spinal cord (Bell and de Souza, 1988) but distribution in the urinary bladder is not known.
CARTp-IR neurons and SIF cells have been identified in the rat MPG (Dun et al., 2000b). CARTp-IR neurons in the MPG were of small diameter and lacked TH-immunoreactivity leading to the suggestion that CARTp-IR MPG neurons are cholinergic and are likely to express vasoactive intestinal polypeptide and nNOS (Dun et al., 2000b). In the present study, the percentage of colocalizaton of CARTp-IR cells with nNOS-IR was greatest at birth and this percentage significantly decreased with postnatal age. Attempts to determine if CARTp+, nNOS+ cells were also cholinergic (ChAT+) were not successful despite our attempts using antisera reported to work in the peripheral nervous system (Schemann et al., 1993). Although we cannot prove the cholinergic phenotype of the CARTp+, nNOS+ cells, the literature supports such a suggestion. The majority of intramural ganglia from other species (Pirker et al., 2004; Saffrey et al., 1994; Zhou and Ling, 1998) also demonstrate nNOS-immunoreactivity or the histochemical marker for NOS, NADPH-diaphorase at least at birth. In contrast to the high percentage of colocalization of CARTp+ and nNOS+ at birth, colocalization of CARTp+ and TH+ increased with postnatal age reaching the greatest percentage of colocalization in adult rat. These CARTp+ and TH+ cells could represent intramural ganglion cells, paraganglion or SIF cells. Triple labeling studies were not performed so it is not known if CARTp+, TH+ cells are neurons. Based upon morphology and the findings in the MPG (Dun et al., 2000b), it is likely that the CARTp+, TH+ cells do not represent intramural ganglion cells but are likely to be paraganglion or SIF cells.
Recent studies have demonstrated that the rat urinary bladder exhibits significant changes in contractile properties during the first five postnatal weeks (Szell et al., 2003). Previous studies have demonstrated that the whole rat neonatal bladder preparation in vitro exhibits spontaneous, rhythmic contractions of larger amplitude and slower frequency than the whole adult rat bladder preparation (Sugaya and de Groat, 2000). It has been suggested that these intrinsic contractions reflect pacemaker activity and mechanisms for conducting this activity throughout the bladder (Sugaya and de Groat, 1994; Sugaya and de Groat, 2000). Szell and colleagues (Szell et al., 2003) suggest that the pacemaker in one- to two-week old rat pups is located in the region of the bladder base, presumably in smooth muscle cells, although cells in the bladder dome were also capable of generating coordinated contractions. In the present study, we demonstrate a differential distribution of CARTp-IR nerve fibers and CARTp-IR cell clusters near the ureteral insertion and bladder neck region with decreasing density in the bladder body and absence in bladder dome. Thus, in addition to intrinsic smooth muscle activity (Szell et al., 2003) driving rhythmic contractions in rat neonatal bladders, CARTp-IR intramural ganglion cells may also play a role. Efficient mechanisms for conducting this activity throughout the neonatal bladder may not only involve gap junctions (Kanai et al., 2003) but also neural output from intramural ganglion cells. The coordinated, large-amplitude bladder contractions decline in older animals (Szell et al., 2003). This change in contractile activity coincides with decreases in the number of CARTp-IR cells in the detrusor, changes in the colocalization patterns of CARTp-IR cells in the present study and coincides with the development of mature voiding reflexes in adult animals.
The mechanism(s) underlying the changes in CARTp expression in the neonatal rat bladder in the present study, the development of mature voiding reflexes and changes in neonatal bladder contractility are not known. Previous studies have suggested that maturation of peptidergic afferent pathways in the urinary bladder of the neonatal rat may be involved (Maggi et al., 1988). However, it has also been demonstrated that regional noradrenergic and cholinergic neurochemistry of the rat urinary bladder is relatively stable as the rat matures (Johnson et al., 1988). Thus, maturation of peptidergic afferent pathways may play a greater role in the maturation of micturition reflexes compared to adrenergic and cholinergic systems. Maturation of central pathways is also likely to be a factor in the postnatal development of bladder reflexes. Various transmitters including serotonin (Bregman, 1987), enkephalins (Fitzgerald, 1991), CRF (Kruse et al., 1990), somatostatin (Marti et al., 1987), NADPH-diaphorase and nNOS (Vizzard et al., 1994) appear or increase in prominence in the lumbosacral spinal cord during the second to third postnatal week. In addition to the role that neuroactive compounds in the central and peripheral nervous system may play in the maturation of the micturition reflex, it is hypothesized that neurotrophic factors (NTFs) expressed in the developing urinary bladder may also contribute. Previous studies (Vizzard et al., 2000) from this laboratory demonstrated that NTFs including, nerve growth factor (βNGF), brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), ciliary neurotrophic factor (CNTF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4) mRNA levels increased by postnatal (P) day 10 and reach a maximum by P15. Subsequently, neurotrophic factor mRNA levels declined to adult levels that were less than the earliest postnatal time examined (P5) (Vizzard et al., 2000). NTF mRNA expression was significantly increased from P10–P40 compared to adult levels for each NTF examined except GDNF mRNA. NGF, NT-3 and NT-4 urinary bladder protein levels were similar when compared to the corresponding mRNA expression (Vizzard et al., 2000).
In summary, these studies demonstrate dynamic changes in CARTp-immunoreactivity in postnatal rat urinary bladder. CARTp-IR nerve fibers express nNOS-immunoreactivity, first appear at P14 in the suburothelial plexus and are differentially distributed in the bladder with the greatest density near the bladder neck and ureteral insertion. CARTp-IR nerve fibers are present at birth throughout the detrusor. CARTp-IR cells are present in detrusor smooth muscle and change neurochemical phenotype and cell type (neuron vs. SIF cell) with increasing postnatal age. Decreases in the number of CARTp-IR cells in detrusor smooth muscle with increasing postnatal age may be due, in part, to apoptotic processes. Changes in CARTp expression the rat urinary bladder coincide with maturation of micturition reflexes. The role of CARTp in postnatal and adult rat bladder remains to be determined.
References
- Alian M, Gabella G. Decrease and disappearance of intramural neurons in the rat bladder during postnatal development. Neurosci Lett. 1996;218(2):103–106. doi: 10.1016/s0304-3940(96)13129-3. [DOI] [PubMed] [Google Scholar]
- Araki I, de Groat WC. Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. J Neurophysiol. 1996;76(1):215–226. doi: 10.1152/jn.1996.76.1.215. [DOI] [PubMed] [Google Scholar]
- Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997;17:8402–8407. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JA, de Souza EB. Functional corticotropin-releasing factor receptors in neonatal rat spinal cord. Peptides. 1988;9(6):1317–1322. doi: 10.1016/0196-9781(88)90198-2. [DOI] [PubMed] [Google Scholar]
- Bregman BS. Development of serotonin immunoreactivity in the rat spinal cord and its plasticity after neonatal spinal cord lesions. Dev Brain Res. 1987;34:245–263. doi: 10.1016/0165-3806(87)90213-6. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Griffond B, Fellmann D, Risold PY. Early and transient ontogenetic expression of the cocaine- and amphetamine-regulated transcript peptide in the rat mesencephalon: correlation with tyrosine hydroxylase expression. J Neurobiol. 2002;52(3):221–229. doi: 10.1002/neu.10077. [DOI] [PubMed] [Google Scholar]
- Capek K, Jelinek J. The development of the control of water metabolism. I. The excretion of urine in young rats. Physiol Bohemoslov. 1956;5:91–96. [PubMed] [Google Scholar]
- Coupland RE. (Electron Microscopic Observations on the Structure of the Rat Adrenal Medulla. I. The Ultrastructure and Organization of Chromaffin Cells in the Normal Adrenal Medulla.) J Anat. 1965;99:231–254. [PMC free article] [PubMed] [Google Scholar]
- Coupland RE. The natural history of the chromaffin cell--twenty-five years on the beginning. Arch Histol Cytol. 1989;52(Suppl):331–341. doi: 10.1679/aohc.52.suppl_331. [DOI] [PubMed] [Google Scholar]
- Coupland RE, Bustami F. Influence of drugs affecting the pituitary adrenal axis on chromaffin cells. Adv Biochem Psychopharmacol. 1980;25:175–183. [PubMed] [Google Scholar]
- Dail WGJ, Evan APJ, Eason HR. The major ganglion in the pelvic plexus of the male rat. Cell Tissue Res. 1975;159:49–62. doi: 10.1007/BF00231994. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Nervous control of the urinary bladder of the cat. Brain Res. 1975;87:201–211. doi: 10.1016/0006-8993(75)90417-5. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Araki I. Maturation of bladder reflex pathways during postnatal development. Adv Exp Med Biol. 1999;462:253–263. doi: 10.1007/978-1-4615-4737-2_19. discussion 311–220. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998;92(2):127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Booth AM. 1993. Synaptic transmission in pelvic ganglia. In: Maggi CA, editor. The autonomic nervous system. Switzerland: Harwood. p 291–347.
- de Groat WC, Booth AM, Yoshimura N. 1993. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The Autonomic Nervous System. London: Harwood Academic Publishers. p 227–290.
- de Groat WC, Kruse MN. 1993. Central processing and morphological plasticity in lumbosacral afferent pathways from the lower urinary tract. In: Mayer EA, Raybould HE, editors. Basic and Clinical Aspects of Chronic Abdominal Pain Research and Clinical Management. Amsterdam: Elsevier Science Publishers. p 219–235.
- de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- Dixon JS, Gosling JA, Canning DA, Gearhart JP. An immunohistochemical study of human postnatal paraganglia associated with the urinary bladder. J Anat. 1992;181 (Pt 3):431–436. [PMC free article] [PubMed] [Google Scholar]
- Dixon JS, Jen PY, Gosling JA. Immunohistochemical characteristics of human paraganglion cells and sensory corpuscles associated with the urinary bladder. A developmental study in the male fetus, neonate and infant. J Anat. 1998;192 (Pt 3):407–415. doi: 10.1046/j.1469-7580.1998.19230407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JS, Jen PY, Gosling JA. Tyrosine hydroxylase and vesicular acetylcholine transporter are coexpressed in a high proportion of intramural neurons of the human neonatal and child urinary bladder. Neurosci Lett. 1999;277(3):157–160. doi: 10.1016/s0304-3940(99)00877-0. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15(3 Pt 2):2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Patterson PH, Landis SC. Small intensely fluorescent cells in culture: role of glucocorticoids and growth factors in their development and interconversions with other neural crest derivatives. J Neurosci. 1985;5(8):2143–2160. doi: 10.1523/JNEUROSCI.05-08-02143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Kwok EH, Yang J, Chang J. Cocaine- and amphetamine-regulated transcript-immunoreactivity in the rat sympatho-adrenal axis. Neurosci Lett. 2000a;283(2):97–100. doi: 10.1016/s0304-3940(00)00935-6. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Wong PY, Yang J, Chang J. Cocaine- and amphetamine-regulated transcript peptide in the rat epididymis: an immunohistochemical and electrophysiological study. Biol Reprod. 2000b;63(5):1518–1524. doi: 10.1095/biolreprod63.5.1518. [DOI] [PubMed] [Google Scholar]
- Ekstrom J, Ekman R, Hakanson R. Ontogeny of neuropeptides in the rat urinary bladder. Regul Pept. 1994;50(1):23–28. doi: 10.1016/0167-0115(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Ezerman EB, Forehand CJ. 2004. CART peptide expression in sympathetic preganglionic prevertebral neurons in the chicken embryo. 2004 Abstract Viewer/Itinerary Planner:Program no. 544.546.
- Fitzgerald M. Development of pain mechanisms. Br Med Bull. 1991;47:667–675. doi: 10.1093/oxfordjournals.bmb.a072499. [DOI] [PubMed] [Google Scholar]
- Gabella G. Intramural neurons in the urinary bladder of the guinea-pig. Cell Tissue Res. 1990;261(2):231–237. doi: 10.1007/BF00318664. [DOI] [PubMed] [Google Scholar]
- Hervonen A. Development of catecholamine--storing cells in human fetal paraganglia and adrenal medulla. A histochemical and electron microscopical study. Acta Physiol Scand Suppl. 1971;368:1–94. [PubMed] [Google Scholar]
- Hu J, Chin CM, Png JC, Ng YK, Ling EA. The effect of chronic bladder outlet obstruction on neuronal nitric oxide synthase expression in the intramural ganglia of the guinea pig bladder. J Urol. 2004;172(3):1160–1165. doi: 10.1097/01.ju.0000135047.65089.48. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Kuhar MJ. CART peptides as targets for CNS drug development. Curr Drug Targets CNS Neurol Disord. 2003;2(3):201–205. doi: 10.2174/1568007033482896. [DOI] [PubMed] [Google Scholar]
- Iuchi H, Satoh Y, Ono K. Postnatal development of neuropeptide Y- and calcitonin gene-related peptide-immunoreactive nerves in the rat urinary bladder. Anat Embryol (Berl) 1994;189(4):361–373. doi: 10.1007/BF00190591. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Skau KA, Gerald MC, Wallace LJ. Regional noradrenergic and cholinergic neurochemistry in the rat urinary bladder: effect of age. J Urol. 1988;139:611–615. doi: 10.1016/s0022-5347(17)42543-2. [DOI] [PubMed] [Google Scholar]
- Kanai AJ, Golin F, Roppolo JR, Birder L, Tai C, Meyers S, de Groat WC. Age-induced changes in urinary bladder activity detected using voltage- and calcium-sensitive dyes. FASEB J. 2003:A649. [Google Scholar]
- Keast JR, booth AM, de Groat WC. Distribution of neurons in the major pelvic ganglion of the rat which supply the bladder, colon or penis. Cell Tissue Res. 1989;256:105–112. doi: 10.1007/BF00224723. [DOI] [PubMed] [Google Scholar]
- Keast JR, de Groat WC. Immunohistochemical characterization of pelvic neurons which project to the bladder, colon or penis in rats. J Comp Neurol. 1989;288:387–400. doi: 10.1002/cne.902880303. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391(1):115–132. [PubMed] [Google Scholar]
- Kruse MN, Tanowitz M, Cheng C, de Groat WC. Postnatal development of the sensory innervation of the urinary bladder in the rat. Soc Neurosci Abstr. 1990;16:1064. [Google Scholar]
- Kuhar MJ, Adams LD, Hunter RG, Vechia SD, Smith Y. CART peptides. Regul Pept. 2000;89(1–3):1–6. doi: 10.1016/s0167-0115(00)00096-3. [DOI] [PubMed] [Google Scholar]
- Langley K, Grant NJ. Molecular markers of sympathoadrenal cells. Cell Tissue Res. 1999;298(2):185–206. doi: 10.1007/pl00008810. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Geppeti S, Frilli MG, Spillantini MG, Nediani C, Hunt SP, Meli A. Biochemical, anatomical and functional correlates of postnatal developments of the capsaicin-sensitive innervation of the rat urinary bladder. Dev Brain Res. 1988;43:183–190. doi: 10.1016/0165-3806(88)90098-3. [DOI] [PubMed] [Google Scholar]
- Marti E, Gibson SJ, Polak JM, Facer P, Springall DR, Van Aswegen G, Aithison M, Koltzenburg M. Ontogeny of peptide and amine containing neurones in motor, sensory, and autonomic regions of rat and human spinal cord, dorsal root ganglia and rat skin. J Comp Neurol. 1987;266:332–359. doi: 10.1002/cne.902660304. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Talmage EK, Lee KP, Parsons RL. Expression of choline acetyltransferase immunoreactivity in guinea pig cardiac ganglia. Cell Tissue Res. 1996;285(2):281–286. doi: 10.1007/s004410050645. [DOI] [PubMed] [Google Scholar]
- Okumura T, Yamada H, Motomura W, Kohgo Y. Cocaine-amphetamine-regulated transcript (CART) acts in the central nervous system to inhibit gastric acid secretion via brain corticotropin-releasing factor system. Endocrinology. 2000;141(8):2854–2860. doi: 10.1210/endo.141.8.7588. [DOI] [PubMed] [Google Scholar]
- Pirker ME, Montedonico S, Rolle U, Austvoll H, Puri P. 2004. Regional differences in nitrergic neuronal density in the developing porcine urinary bladder. Pediatr Surg Int. [Epub ahead of print] [DOI] [PubMed]
- Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol. 2002a;454(2):200–211. doi: 10.1002/cne.10447. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Vizzard MA. Up-regulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in lumbosacral dorsal root ganglia after chronic spinal cord (T8–T10) injury. J Comp Neurol. 2002b;449(3):217–230. doi: 10.1002/cne.10283. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Vizzard MA. Up-regulation of phosphorylated CREB but not c-Jun in bladder afferent neurons in dorsal root ganglia after cystitis. J Comp Neurol. 2004;469(2):262–274. doi: 10.1002/cne.11009. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Vizzard MA. Spinal cord injury-induced expression of TrkA, TrkB, phosphorylated CREB, and c-Jun in rat lumbosacral dorsal root ganglia. J Comp Neurol. 2005;482(2):142–154. doi: 10.1002/cne.20394. [DOI] [PubMed] [Google Scholar]
- Saffrey MJ, Hassall CJ, Moules EW, Burnstock G. NADPH diaphorase and nitric oxide synthase are expressed by the majority of intramural neurons in the neonatal guinea pig urinary bladder. J Anat. 1994;185 ( Pt 3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Sann H, Walb G, Pierau FK. Postnatal development of the autonomic and sensory innervation of the musculature in the rat urinary bladder. Neurosci Lett. 1997;236(1):29–32. doi: 10.1016/s0304-3940(97)00752-0. [DOI] [PubMed] [Google Scholar]
- Schemann M, Sann H, Schaaf C, Mader M. Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. Am J Physiol. 1993;265(5 Pt 1):G1005–1009. doi: 10.1152/ajpgi.1993.265.5.G1005. [DOI] [PubMed] [Google Scholar]
- Sugaya K, de Groat WC. Effects of MK-801 and CNQX, glutamate receptor antagonists, on bladder activity in neonatal rats. Brain Res. 1994;640(1–2):1–10. doi: 10.1016/0006-8993(94)91850-3. [DOI] [PubMed] [Google Scholar]
- Sugaya K, de Groat WC. Influence of temperature on activity of the isolated whole bladder preparation of neonatal and adult rats. Am J Physiol. 2000;278(1):R238–R246. doi: 10.1152/ajpregu.2000.278.1.R238. [DOI] [PubMed] [Google Scholar]
- Szell EA, Somogyi GT, de Groat WC, Szigeti GP. Developmental changes in spontaneous smooth muscle activity in the neonatal rat urinary bladder. Am J Physiol. 2003;285(4):R809–816. doi: 10.1152/ajpregu.00641.2002. [DOI] [PubMed] [Google Scholar]
- Thor KB, Blais DP, de Groat WC. Behavioral analysis of the postnatal development of micturition in kittens. Dev Brain Res. 1989;46:137–144. doi: 10.1016/0165-3806(89)90151-x. [DOI] [PubMed] [Google Scholar]
- Thor KB, Hisamitsu T, de Groat WC. Unmasking of a neonatal somatovesical reflex in adult cats by the serotonin autoreceptor agonist 5-methoxy-N,N-dimethyltryptamine. Dev Brain Res. 1990;54:35–42. doi: 10.1016/0165-3806(90)90062-4. [DOI] [PubMed] [Google Scholar]
- Thor KB, Kawatani M, de Groat WC. 1986. Plasticity in the reflex pathways to the lower urinary tract of the cat during postnatal development and following spinal injury. In: Goldberger ME, Gorio A, Murray M, editors. Development and Plasticity of the Mammalian Spinal Cord. Padova: Liviana. p 65–81.
- Tuttle JB. Editorial: Spontaneous contractile activity in rat bladder. J Urol. 2003;170(1):280. doi: 10.1097/01.ju.0000072421.83297.d7. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol. 2000a;279(1):R295–R305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Increased expression of spinal Fos protein in lower urinary tract pathways induced by bladder distension following chronic cystitis. Am J Physiol. 2000b;279:R295–R305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000c;420(3):335–348. [PubMed] [Google Scholar]
- Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21(2):125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, de Groat WC. Increased expression of neuronal nitric oxide synthase (NOS) in bladder afferent pathways following chronic bladder irritation. J Comp Neurol. 1996;370:191–202. doi: 10.1002/(SICI)1096-9861(19960624)370:2<191::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erdman SL, Forstermann U, de Groat WC. Ontogeny of nitric oxide synthase in the lumbosacral spinal cord of the neonatal rat. Dev Brain Res. 1994;81:201–217. doi: 10.1016/0165-3806(94)90307-7. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Wu KH, Jewett IT. Developmental expression of urinary bladder neurotrophic factor mRNA and protein in the neonatal rat. Dev Brain Res. 2000;119(2):217–224. doi: 10.1016/s0165-3806(99)00174-1. [DOI] [PubMed] [Google Scholar]
- Williams TH, Chiba T, Black AC, Bhalla RC, Jew J, editors. 1976. Species variation in SIF cells of superior cervical ganglia: are there two functional types? 1 ed. Washington, D.C.: U.S. Government Printing Office. 143–162 p.
- Zhou Y, Ling EA. Colocalization of nitric oxide synthase and some neurotransmitters in the intramural ganglia of the guinea pig urinary bladder. J Comp Neurol. 1998;394(4):496–505. [PubMed] [Google Scholar]
- Zvarova K, Murray E, Vizzard MA. Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury. J Comp Neurol. 2004;475(4):590–603. doi: 10.1002/cne.20195. [DOI] [PubMed] [Google Scholar]


