Abstract
The supramammillary nucleus (SUM), a dorsal layer of the mammillary body, has recently been implicated in positive reinforcement. The present study examined whether GABAA receptors in the SUM or adjacent regions are involved in primary reinforcement using intracranial self-administration procedures. Rats learned quickly to lever-press for infusions of the GABAA antagonist picrotoxin into the SUM. Although picrotoxin was also self-administered into the posterior hypothalamic nuclei and anterior ventral tegmental area, these regions were less responsive to lower doses of picrotoxin than the SUM. The finding that rats learned to respond selectively on the lever triggering drug infusions is consistent with picrotoxin’s reinforcing effect. Coadministration of the GABAA agonist muscimol disrupted picrotoxin self-administration, and another GABAA antagonist, bicuculline, was also self-administered into the SUM; thus, the reinforcing effect of picrotoxin is mediated by GABAA receptors. Since rats did not self-administer the GABAB antagonist 2-hydroxysaclofen into the SUM, the role of GABAB receptors may be distinct from that of GABAA receptors. Pretreatment with the dopamine receptor antagonist SCH 23390 (0.05 mg/kg, i.p.) extinguished picrotoxin self-administration into the SUM, suggesting that the reinforcing effects of GABAA receptor blockade depend on normal dopamine transmission. In conclusion, the blockade of GABAA receptors in the SUM is reinforcing, and the brain ‘reward’ circuitry appears to be tonically inhibited via supramammillary GABAA receptors and more extensive than the mesolimbic dopamine system.
Keywords: reward, ventral tegmental area, posterior hypothalamic nucleus, picrotoxin, bicuculline, dopamine antagonist SCH 23390
INTRODUCTION
We recently found that the posterior hypothalamic area, particularly the supramammillary nucleus (SUM) and the posterior hypothalamic nucleus (PHN), is involved in primary reinforcement (Ikemoto et al, 2004). Rats learn quickly to lever-press for AMPA injections into these posterior hypothalamic regions, and AMPA injections into the SUM also induce conditioned place preference (Ikemoto et al, 2004). Thus, the SUM and PHN appear to mediate primary reinforcement via AMPA receptors.
The SUM may also mediate reinforcement via GABAA receptors (Ikemoto et al, 1997). We observed that rats self-administered the GABAA receptor antagonist picrotoxin into the SUM (Ikemoto et al, 1997), although we have not yet substantiated this observation with anatomical, pharmacological, and behavioral controls. Since the SUM is localized just anterior to the ventral tegmental area (VTA), it is important to determine whether the reinforcing effect of GABAA receptor antagonists into the SUM is due to diffusion of the drug to the VTA. Indeed, it has been suggested that administration of GABAA receptor antagonists into the VTA is reinforcing (David et al, 1997; Ikemoto et al, 1997; Laviolette and van der Kooy, 2001).
The present study examined the hypothesis that the blockade of GABAA receptors in the SUM is reinforcing. To provide substantial anatomical evidence, the SUM and its surrounding regions including the VTA, PHN, lateral hypothalamic area, and medial mammillary nucleus were examined. The present study expands current understanding of anatomical and transmitter mechanisms for ‘reward,’ which has largely been limited to the mesolimbic dopamine system.
MATERIALS AND METHODS
Subjects
A total of 63 male Wistar rats (Harlan Industries, Dublin, VA; 250–350 g at the time of surgery) were initially housed in groups of two in a colony room kept at a constant temperature (21°C), on a reversed 12-h light–dark cycle (lights on at 2130 hours). After surgery, the animals were individually housed. Food and water were freely available to the animals except during testing. The procedures used were approved by the Animal Care and Use Committee of the NIDA Intramural Research Program and were in accordance with NIH guidelines.
Surgery
Under i.p. sodium pentobarbital (31 mg/kg) and chloral hydrate (142 mg/kg) anesthesia, each animal was implanted with a unilateral guide cannula (24 gauge) that ended 1.0 mm above one of six target sites. Unilateral injections are sufficient to induce drugs’ reinforcing effects (Ikemoto and Wise, 2004), and are more suited to the study of precise relationships between injection sites and drug effects than bilateral injections. Guide cannulae were inserted at a 6° angle from the vertical toward the midline to avoid damaging midline sinuses. Posterior distance from bregma, lateral distance from the midline, and ventral distance from skull surface were measured along the trajectory of the angled cannula. These respective coordinates (in millimeters) were 3.4–4.0, 1.0–1.3, and 7.6 for PHN, 3.8, 2.2, and 8.4 for lateral hypothalamic area, 4.2, 1.4, and 9.2 for medial mammillary nucleus, 4.0–4.5, 1.0–1.3, and 8.3 for SUM, 4.3, 1.5–2.0, and 7.8–8.2 for anteriortip of VTA, 4.8, 1.6, and 7.8 for anterior VTA. The incisor bar was set at 3.3 mm below the interaural line.
Drugs
The GABAA antagonists picrotoxin and bicuculline methiodide, the GABAA agonist muscimol, and the GABAB antagonist 2-hydroxysaclofen (Sigma, St. Louis, MO) were dissolved in an artificial cerebrospinal fluid consisting of 148 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, and 0.85 mM MgCl2 (pH adjusted to 6.5–7.8).
Self-Administration Procedure and Apparatus
Each animal was placed in a 30 × 22 × 24 cm3 chamber. The chamber was equipped with a lever (45 mm wide × 2 mm thick, protruding 19 mm from the wall) and a cue light located just above the lever. The chamber was enclosed in a sound-attenuating box equipped with a ventilating fan. Each rat’s 31-gauge injection cannula was connected by PE tubing to a micropump (Ikemoto and Sharpe, 2001) hanging a few millimeters above the rat’s head. Each pump consisted of a miniature step motor and a small plastic reservoir. When activated, the motor advanced a shaft into the reservoir, displacing drug solution into the injection cannula. A response on the lever caused an intracranial injection (75 nl) over 5 s and turned on the cue light for 1 s; this light served to facilitate contingency learning between lever-pressing and drug delivery. To prevent continuous infusions, the onset of each infusion was followed by a 10-s timeout period during which additional lever-presses were counted but had no experimental consequence. To minimize adverse physiological effects, the number of infusions per session was limited to a total of 60.
Anatomical Substrates of Picrotoxin Self-Administration
The effective regions and concentrations of the GABAA antagonist picrotoxin that support self-administration were determined. Special attention was paid to the anatomical details of the boundary between the posterior hypothalamus and the VTA. This boundary has not been clearly defined, since the area just dorsal to the SUM has been labeled as both the lateral hypothalamic area (Paxinos and Watson, 1997) and the VTA (Paxinos and Watson, 1986; Swanson, 1998). While this area has dopaminergic neurons, they are much sparser than those in more posterior portions of the VTA (Swanson, 1982). In the present study, this area is referred to as anterior tip of the VTA. Unilateral guide cannulae were aimed at six regions (PHN, n = 6; lateral hypothalamic area, n = 4; medial mammillary nucleus, n = 6; SUM, n = 11; anterior tip of VTA, n = 8; anterior VTA, n = 9). Each rat was trained initially with injections of 0.3 mM picrotoxin in two sessions and 0.1 mM in one session; each of these sessions was followed by a vehicle session. Then, a concentration–efficacy relationship was examined in an ascending order; over four sessions, each rat earned vehicle, 0.03, 0.1 and 0.3 mM picrotoxin (in this order). Sessions lasted for 90 min or until 60 infusions were earned.
In the procedures described above, three rats trained to self-administer picrotoxin into the posterior hypothalamic nucleus died immediately after vigorous self-administration sessions in which they earned 60 infusions within 30 min. Death may have been caused by the failure of vital physiological functions due to the diffusion of the drug to nearby regions. Therefore, the self-administration effects of picrotoxin into the posterior hypothalamic nucleus (n = 9) were examined at lower concentrations of picrotoxin (0.01, 0.03, and 0.1 mM). Other than the change in concentration, the procedures for the regions anterior to the SUM were essentially the same as those described for the above experiment. The animals were trained with 0.1 mM picrotoxin instead of 0.3 mM, followed by vehicle and picrotoxin in an ascending order of concentrations over four sessions.
Two-Lever Discrimination Experiment for Picrotoxin Reinforcement
Four rats with no prior operant training were placed in operant conditioning chambers with two retractable levers. The chambers were identical to the one-lever chambers described above except that two levers were placed on the chamber wall. In sessions 1 through 5, a response on the ‘active’ lever resulted in a 5-s infusion (75 nl in volume), turned on a cue light above the lever for 10 s, and retracted both levers for 20 s (ie a fixed-ratio-1 schedule with 20-s timeout). A response on the ‘inactive’ lever did not deliver infusions but retracted both the active and inactive levers for 20 s. In each of sessions 6 through 9, the lever-press requirement for picrotoxin reward increased progressively by an increment of 1 every 10 picrotoxin infusions. Responding on the active lever presented a 0.5-s cue light, and the two levers were retracted only when the lever-press requirement was fulfilled, upon which the same experimental consequence as that described for the FR1 schedule occurred. Responding on the inactive lever also retracted the two levers according to the same partial progressive ratio schedule but did not deliver infusions or cue light. The left lever was active for two rats and inactive for the other two throughout the experiment. The rats received 0.1-mM picrotoxin throughout the experiment, except for the first session, in which they received vehicle. Sessions lasted 90 min or until the rats received a total of 60 infusions. The numbers of responses on each lever and infusions were recorded.
GABAA Receptors and Picrotoxin Reinforcement
The primary role of GABAA receptors in picrotoxin reward was determined in one experiment with the GABAA receptor agonist muscimol, another with the GABAA receptor antagonist bicuculline, and a third with the GABAB receptor antagonist 2-hydroxysaclofen. Operant conditioning procedure and apparatus in these experiments were the same as those used in the first experiment. Six rats that exhibited vigorous picrotoxin self-administration (a rate of one infusion per min or higher with 0.1 mM picrotoxin) in the first experiment were used to determine the effects of coadministration of the GABAA agonist muscimol with picrotoxin. In sessions 1–3, half of the rats earned 0.1 mM picrotoxin, vehicle, and a mixture of 0.3 mM muscimol and 0.1 mM picrotoxin, in this order. The rest received identical treatments except that they received a mixture of muscimol and picrotoxin first and picrotoxin alone last.
Six experimentally-naïve rats were given, in a set of four sessions, vehicle and 0.1, 0.3, and 3 mM 2-hydroxysaclofen in this order, and in another set of four sessions, vehicle, bicuculline (0.1 mM), bicuculline (0.1 mM), and vehicle in this order. Three rats received the 2-hydroxysaclofen treatments first, while the other three received the bicuculline treatments first; thus, the order of testing these drugs was counterbalanced.
Dopamine Receptors and Picrotoxin Reinforcement
Eight rats that were used in the first experiment and self-administered picrotoxin at a rate of one infusion per min or higher (five of which were used for the muscimol experiment as well) received the following treatments. At 30 min prior to picrotoxin (0.1 mM) self-administration test, four of the rats received 0.9% saline (1 ml/kg, i.p.) in session 1 and the dopamine receptor antagonist SCH 23390 (0.05 mg/kg, i.p.) in session 3; the other four rats received these treatments in reverse order. In session 2, they all received i.p. saline injections followed by intracranial vehicle self-administration.
Histology
When each rat completed the experimental procedure, it was anesthetized with a mixture of sodium pentobarbital (31 mg/kg) and chloral hydrate (142 mg/kg) and was perfused transcardially with 50 ml of 0.9% saline with 0.2% sodium nitrite followed by 100 ml of 4% paraformaldehyde in phosphate buffer solution (PB). Its brain was removed and placed in 4% paraformaldehyde PB for a day, then stored in 18% sucrose PB for 1–5 days until sectioning. Coronal sections (40 μm) at the microinjection site were cut with a cryostat. Sections were stained with cresyl violet. The placements of the injection cannulae were confirmed by microscopic examination.
Statistical Analyses
The data were analyzed with ANOVAs followed by Newman–Keuls post hoc tests. Infusion interval data and latency data in the dopamine antagonist experiment highly varied and, thus, were square root transformed to maintain homogeneity of variance. Details of statistical procedures are described below.
RESULTS
Self-Administration of the GABAA Receptor Antagonist Picrotoxin
The distributions of the injection sites are shown in Figure 1a. The rats learned quickly to self-administer picrotoxin into the SUM, and self-administered picrotoxin solutions at significantly higher rates than vehicle (Figure 1b; one-way repeated measures ANOVA on infusion rates with four injection treatments, F3,30 = 19.19, P < 0.0001). As concentrations increased, the rates of self-administration increased. Rats maintained regular rates of self-administration of picrotoxin throughout the session, whereas vehicle self-administration became irregular and less frequent several minutes after the start of the session (Figure 1c). It should be noted that the rats that significantly self-administered picrotoxin were consistently hyperactive; they constantly walked about the cages. Typical cannula placement for the SUM is shown in Figure 2.
Figure 1.
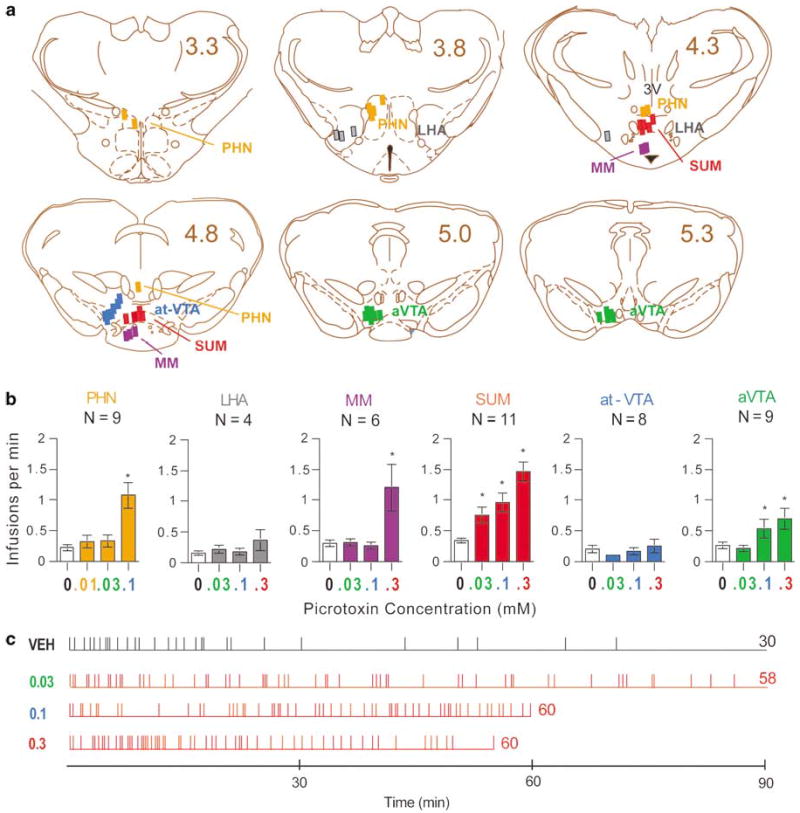
Self-administration of the GABAA receptor antagonist picrotoxin. (a) Injection sites of picrotoxin. Numbers indicate distances (mm) posteriorly from bregma. 3V, third ventricle; at-VTA, anterior tip of ventral tegmental transition area; aVTA, anterior VTA; LHA, lateral hypothalamic area; MM, medial mammillary nucleus; PHN, posterior hypothalamic nucleus; SUM, supramammillary nucleus. Drawings were adapted from the atlas by Paxinos and Watson (1997). (b) Picrotoxin self-administration rates in various brain regions. Each infusion was delivered in a 75 nl volume; therefore, the doses of picrotoxin examined were 0.75, 2.25, 7.5, and 22.5 pmoles per infusion for 0.01, 0.03, 0.1, and 0.3 mM, respectively. *Different from respective vehicle treatment, P < 0.05. (c) Event records of a representative rat self-administering picrotoxin or vehicle (VEH) into the SUM. Each horizontal line indicates the length of session. Each vertical line indicates when an infusion was delivered. The numbers on the right of horizontal lines are total numbers of infusions obtained in sessions.
Figure 2.

Photomicrograph showing a representative injection site in the SUM. The arrowhead indicates the tip of injection cannula track. FR, fasciculus retroflexus; for other abbreviations, see the legend of Figure 1a.
The rats receiving infusions into the posterior hypothalamic nucleus or the anterior ventral tegmental area also learned to self-administer picrotoxin, and showed concentration-dependent self-administration (F3,18 = 3.87, P < 0.05; F3,30 = 8.72, P < 0.001, respectively). The medial mammillary nucleus supported self-administration at the highest concentration (0.3 mM) of picrotoxin, but not at lower concentrations (Newman–Keuls post hoc test following a main concentration effect, F3,15 = 5.19, P < 0.05). The rats did not learn to self-administer picrotoxin into the anterior tip of VTA or lateral hypothalamic area. Differential effects of picrotoxin between regions were confirmed by a significant region-by-treatment interaction (F10,82 = 4.87, P < 0.0001) following a mixed ANOVA with six regions and three injection treatments on infusion rates (Injection treatment included vehicle, 0.03, and 0.1 mM; the highest concentration, 0.3 mM, was not included since it was not systematically examined for the PHN.). Picrotoxin at 0.1 mM was self-administered into the SUM and PHN significantly more than the anterior VTA, anterior tip of VTA, medial mammillary nucleus, and lateral hypothalamic nucleus.
Two Lever Discrimination
To provide stronger evidence for the reinforcing effects of picrotoxin administration, four rats were trained to discriminate between an active lever that rewarded them with picrotoxin administration and an inactive lever that did not. Rats responded on the active lever more than the inactive lever in sessions 2–5 on the FR1 schedule, although repeated training over those sessions did not improve their discrimination between the two levers (Figure 3a). When the rats self-administered picrotoxin on the partial progressive ratio schedule in sessions 6–9, they selectively increased active lever-presses over inactive lever-presses. Interestingly, they failed to increase lever-presses to cope with increased response requirements in session 6 (Figure 3a), and, thus, they obtained fewer infusions than they did in previous sessions (Figure 3b). Over the next three sessions on the progressive ratio schedule, however, they increased active lever-presses and restored infusion frequency to a level comparable to those reached during the FR1 schedule (Figure 3a–c). These observations are confirmed by a significant schedule-by-session-by-lever interaction on response rates (F3,9 = 11.46, P < 0.005) following a 2 × 4 × 2 repeated measures ANOVA with schedule (FR1 vs progressive ratio), session (4), and lever (active vs inactive). In addition, a significant interaction on infusion rates was also found (F3,9 = 4.07, P < 0.05, ANOVAs with schedule and session).
Figure 3.

Two lever discrimination for supramammillary picrotoxin reward. Four rats received vehicle in session 1 and picrotoxin (0.1 mM) in sessions 2–9. They were trained on operant conditioning schedules of a fixed-ratio 1 with a 20-s timeout in sessions 1–5 and a partial progressive ratio (up to 6) in sessions 6–9. (a) Mean response rates (±SEM) of the two levers are summarized over nine sessions. Active lever-presses in each of sessions 7, 8, and 9 were greater than inactive lever-presses in respective sessions, and they were also greater than active lever-presses in each of sessions 2, 3, 4, and 5 (*P < 0.05). (b) Mean infusion rates (±SEM) are shown over nine sessions. The infusions in session 6 were lower than those in sessions 2, 4, and 5 (*P < 0.05). (c) Cumulative response records and infusion event records of a representative rat are shown. Each line moves up a unit vertically every time the rat pressed the active lever. Each perpendicular slit indicates the point of an infusion delivery. Each arrow accompanied by a number indicates the point at which the response requirement was incremented, and the number indicates required lever-presses for each infusion. The horizontal lines in the bottom indicate session length with vertical lines again indicating the points of infusions delivered.
GABAA Receptors Mediate Picrotoxin Reinforcement
Coadministration of the GABAA receptor agonist muscimol markedly decreased self-administration of picrotoxin, as shown by one-way repeated measures ANOVA on infusion rates with three treatments, F2,10 = 30.75, P < 0.0001 (Figure 4a). Figure 4d illustrates typical patterns of self-administration with these treatments. When a rat received picrotoxin alone, the rate of self-administration was high throughout the session. When the rat received the mixture of picrotoxin and muscimol, the rate of self-administration was high at the beginning and then became low and irregular. This pattern of picrotoxin self-administration with muscimol was similar to that of vehicle self-administration.
Figure 4.

GABAA receptors mediate picrotoxin self-administration. Mean infusions per min (±SEM) are shown in (a), (b), and (c). (a) When the GABAA receptor agonist muscimol (MUS; 0.3 mM) was coadministered with picrotoxin (PIC; 0.1 mM), self-administration rates were decreased markedly and were not distinguishable from self-administration rates of vehicle (VEH) (N = 6). *Different from picrotoxin treatment, P < 0.05. Self-administration patterns of a representative rat are shown in (d). (b) Rats (N = 6) learned to self-administer the GABAA receptor antagonist bicuculline (BICUC; 0.1 mM) into the SUM. *Different from vehicle treatments, P < 0.05. (c) The rats that self-administered bicuculline did not self-administer the GABAB receptor antagonist 2-hydroxysacrofen into the SUM. (d) and (e) Each set of event records show self-administration patterns of a representative rat.
Rats learned quickly to self-administer the GABAA antagonist bicuculline into the SUM (Figure 4b; 2 × 2 repeated measures ANOVA on infusion rates with treatment (bicuculline vs vehicle) and trial, F1,7 = 23.50, P < 0.005). Typical acquisition and maintenance of bicuculline self-administration are illustrated in Figure 4e. A rat placed in the operant chamber in session 1 lever-pressed several times, mostly occurring in the first few minutes. These initial spontaneous lever-presses are considered to be triggered by ‘curiosity’ or rat’s tendency to explore a novel environment. The vehicle infusions that the rat received in that session failed to reinforce or maintained lever-pressing. When the rat received bicuculline for the first time in session 2, the rate of lever-pressing was low and irregular at the onset of the session. Over the next few minutes, faster and more constant self-administration emerged. When the rat received the drug for the second time in session 3, the initial phase of slow self-administration was minimal, and was followed by a high and constant rate of self-administration. In session 4 when bicuculline was substituted for vehicle, self-administration rate was high at the onset, and soon became lower and less constant; this pattern suggests a transition toward extinction of self-administration. The same rats did not self-administer the GABAB receptor antagonist 2-hydroxysaclofen more than they did vehicle (Figure 4c).
Role of Dopamine Receptors in Picrotoxin Reinforcement
Pretreatment with the dopamine D1 antagonist SCH 23390 (0.05 mg/kg, i.p.) markedly decreased self-administration of picrotoxin into the SUM (Figure 5a; F2,14 = 26.08, P < 0.001). When the rats were pretreated with vehicle and received picrotoxin, they self-administered picrotoxin at fast rates throughout the session (Figure 5c). When the rats were treated with the dopamine antagonist, they began to self-administer picrotoxin as they did when pretreated with saline; however, after a few minutes, self-administration of picrotoxin became irregular. Similar patterns of self-administration were found when they received vehicle substituted for picrotoxin (Figure 5c). These observations were confirmed by the statistical analyses on latencies to initiate self-administration and infusion intervals. Mean square root latencies (SEM) to initiate self-administration at the onset of sessions were 6.32 (1.55), 6.33 (0.99), and 4.66 (1.57) for picrotoxin infusions with saline treatment, picrotoxin with SCH 23390 treatment, and vehicle with saline treatment, respectively, and there is no difference among the three treatments in the latencies (F2,14 = 0.86, P > 0.1, one-way repeated measures ANOVA with treatment on square root latencies). As shown in Figure 5b, the first two intervals of the self-administration sessions were not different from each other, while the last two intervals of the sessions were significantly longer when the rats earned vehicle or when they earned picrotoxin with the dopamine antagonist treatment than when they earned picrotoxin with saline treatment (a significant interaction between treatment and session-phase (F2,14 = 7.55, P < 0.01) following a 3 × 2 × 2 repeated measures ANOVA with treatment, session phase (first vs last), and trial (2) on square-root infusion intervals).
Figure 5.
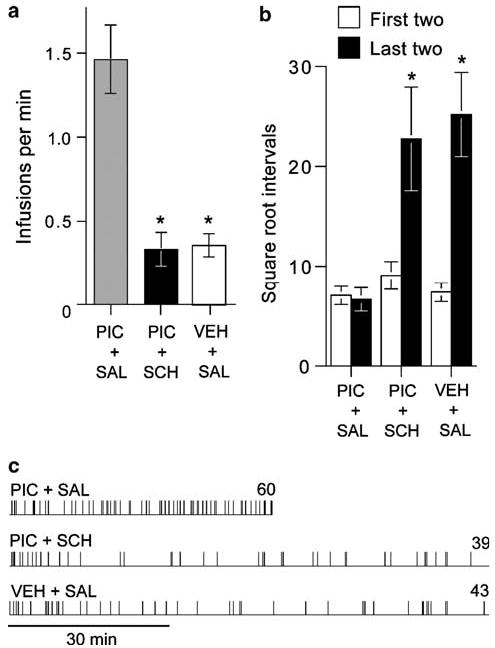
Effects of the dopamine D1 antagonist SCH 23390 on picrotoxin self-administration. (a) When rats (N = 8) were treated with SCH 23390 (SCH; 0.05 mg/kg, i.p.), self-administration rates of picrotoxin (PIC) were markedly higher than when they were treated with saline (SAL) or when they received vehicle (VEH) substituted for picrotoxin. Data are mean infusions per min (SEM). *Different from PIC + SAL, P < 0.001. (b) Data are mean square-root infusion intervals (s) plus SEM with the first two intervals or the last two intervals averaged. *Different from the first intervals of PIC + SAL, PIC + SCH, or VEH + SAL or the last intervals of PIC + SAL, P < 0.01. (c) Event records show typical self-administration patterns of a representative rat.
DISCUSSION
The SUM appears to mediate the reinforcing effects of picrotoxin, since picrotoxin was self-administered into the SUM at a higher rate and at a lower concentration than it was self-administered into any surrounding regions. The medial mammillary nucleus, PHN, and anterior VTA also supported picrotoxin self-administration. However, they did so at higher concentrations than the SUM, raising the possibility that the reinforcing effects of picrotoxin administration into these regions could be explained by diffusion of picrotoxin to the SUM, but not vice versa. This explanation is particularly tempting to use for picrotoxin’s effects at the medial mammillary nucleus, because micro-injected drugs diffuse most effectively along the cannula track (Routtenberg, 1972). Indeed, picrotoxin administration into the medial mammillary nucleus was effective only at the highest concentration, but not the two lower concentrations, which were effective when administered into the SUM. On the other hand, the PHN and anterior VTA may mediate the reinforcing effect of picrotoxin on their own; the anterior tip of the VTA, which is located just laterodorsal to the SUM and is as close to the SUM as the regions, did not support self-administration at any concentration. In any case, the SUM is most responsible for picrotoxin reinforcement.
The unilateral injection method, rather than the bilateral method, was employed in the present study. Bilateral injections exert simultaneous actions at regions in the two hemispheres and should evoke more reliable and powerful self-administration than unilateral injections. However, bilateral injection cannulae may not necessarily be placed perfectly symmetrically between the hemispheres in every surgery, and differences in cannula placements between the hemispheres obscure anatomical acuity. Therefore, the unilateral injection method is more suitable for determining the relationship between sites and effects than the bilateral method.
Additional experiments discussed below substantiated the two important points that supramammillary administration of picrotoxin is reinforcing, and that the reinforcing effect is mediated via GABAA receptors. To substantiate the reinforcing effect of picrotoxin, a two-lever operant conditioning procedure was conducted with a partial progressive ratio schedule. When the rats obtained picrotoxin reward on a progressive ratio schedule, they selectively increased active lever-presses over inactive lever-presses. In addition, the rats exhibited a learning curve, responding more and more effectively to obtain picrotoxin reward over repeated sessions. Such results suggest that picrotoxin administered into the SUM is reinforcing. It remains possible, however, that at least some of the lever-presses could be attributed to motor activation rather than reinforcement, since rats that significantly self-administered picrotoxin were highly aroused and constantly moved about the cages. Possible contribution of hyper-activity to lever-pressing is based on the assumption that hyperactivity induced by picrotoxin reflects a motor process that is entirely independent of reinforcement. An alternative view is that picrotoxin administration into the SUM triggers a coordinated set of changes in mental and bodily states (an instinctive/emotional process), leading to hyperactivity and reinforcement. Thus, the hyperactivity could reflect an arousal of concerted motivational process rather than mere motor process.
The reinforcing effects of picrotoxin administration into the SUM were blocked by coadministration of the GABAA receptor agonist muscimol. Administration of another GABAA receptor antagonist, bicuculline, into the SUM was reinforcing. Therefore, the reinforcing effect of picrotoxin is mediated by GABAA receptors. Interestingly, the GABAB receptor antagonist 2-hydroxysaclofen was not self-administered into the SUM by the rats that self-administered bicuculline. The lack of reinforcing effect of 2-hydroxysaclofen cannot readily be attributed to testing order of the drugs, since the order of testing 2-hydroxysaclofen and bicuculline was counterbalanced. Therefore, it is tempting to suggest that the blockade of GABAB receptors in the SUM is not reinforcing. Since negative data do not prove the absence of effects, additional evidence is needed to determine whether the role of GABAB receptors is distinct from that of GABAA receptors in the SUM. In any case, the present results suggest that GABAergic transmission at GABAA receptors in the SUM plays an important role in primary reinforcement. It appears that supramammillary GABAA receptors tonically inhibit reinforcement circuitry.
Treatment with the dopamine D1 receptor antagonist SCH 23390 attenuated self-administration of picrotoxin into the SUM. This effect appears to be mediated by the blockade of reinforcement rather than a general motor deficit, since rats self-administered picrotoxin at normal rates in session’s beginning and markedly decreased self-administration rates as the session progressed. The patterns of picrotoxin self-administration after the treatment with the dopamine receptor antagonist were similar to those of vehicle self-administration, suggesting an extinction of reinforced responding. Therefore, the results are consistent with the idea that the rewarding effects of picrotoxin into the SUM depend on the activation of the mesolimbic dopamine system. There are other lines of evidence suggesting a close relationship between the SUM and the mesolimbic dopamine system.
We previously found similar effects of dopamine receptor antagonists on self-administration of AMPA into the SUM (Ikemoto et al, 2004). Pretreatment with SCH 23390 extinguishes AMPA self-administration, like it did picrotoxin self-administration. In addition, AMPA administration into the SUM increases extracellular dopamine in the nucleus accumbens. Therefore, the SUM appears to be functionally linked to the mesolimbic dopamine system. Consistently, intracranial reinforcing treatments such as lateral hypothalamic stimulation or intra-VTA administration of carbachol (Ikemoto and Wise, 2002) activate the mesolimbic dopamine system (Westerink et al, 1996; You et al, 2001) and activate supramammillary neurons as shown by marked increases in c-Fos expression in the SUM (Arvanitogiannis et al, 1997; Ikemoto et al, 2003). It remains to be clarified as to how the SUM is physically linked to the dopamine system.
The present findings provide additional evidence for the role of the SUM in primary reinforcement, initially suggested by the self-administration of AMPA into the site (Ikemoto et al, 2004). Together, these findings suggest that the ‘reward’ circuitry is not limited to the mesolimbic dopamine system, but is more extensive. Previous studies (David et al, 1997; Ikemoto et al, 1997; Laviolette and van der Kooy, 2001) have interpreted that the VTA mediates the reinforcing effects of GABAA receptor antagonists, when the drugs were administrated into the vicinity of the VTA and were reinforcing. Such interpretation has to be revaluated in light of SUM’s role in reinforcement.
Acknowledgments
The present work was supported by the NIDA Intramural Research Program. I would like to thank Brian Witkin and Kathleen Donahue for technical assistance, Emily Wentzell for editorial assistance, and Dr Roy Wise for general support.
References
- Arvanitogiannis A, Flores C, Shizgal P. Fos-like immunoreactivity in the caudal diencephalon and brainstem following lateral hypothalamic self-stimulation. Behav Brain Res. 1997;88:275–279. doi: 10.1016/s0166-4328(97)00065-x. [DOI] [PubMed] [Google Scholar]
- David V, Durkin TP, Cazala P. Self-administration of the GABAA antagonist bicuculline into the ventral tegmental area in mice: dependence on D2 dopaminergic mechanisms. Psychopharmacology. 1997;130:85–90. doi: 10.1007/s002130050214. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABAA antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997;111:369–380. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Sharpe LG. A head-attachable device for injecting nanoliter volumes of drug solutions into brain sites of freely moving rats. J Neurosci Methods. 2001;110:135–140. doi: 10.1016/s0165-0270(01)00428-9. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Rewarding effects of the cholinergic agents carbachol and neostigmine in the posterior ventral tegmental area. J Neurosci. 2002;22:9895–9904. doi: 10.1523/JNEUROSCI.22-22-09895.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47:190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Witkin BM, Morales M. Rewarding injections of the cholinergic agonist carbachol into the ventral tegmental area induce locomotion and c-Fos expression in the retrosplenial area and supramammillary nucleus. Brain Res. 2003;969:78–87. doi: 10.1016/s0006-8993(03)02280-7. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Witkin BM, Zangen A, Wise RA. Rewarding effects of AMPA administration into the supramammillary or posterior hypothalamic nuclei but not the ventral tegmental area. J Neurosci. 2004;24:5758–5765. doi: 10.1523/JNEUROSCI.5367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. GABA(A) receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. Eur J Neurosci. 2001;13:1009–1015. doi: 10.1046/j.1460-9568.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986). The Rat Brain in Stereotaxic Coordinates, 2nd edn. Academic Press: Orlando, FL.
- Paxinos G, Watson C (1997). The Rat Brain in Stereotaxic Coordinates, 3rd edn. Academic Press: San Diego.
- Routtenberg A. Intracranial chemical injection and behavior: a critical review. Behav Biol. 1972;7:601–641. doi: 10.1016/s0091-6773(72)80073-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW (1998). Brain Maps: Structure of the Rat Brain, 2nd edn. Elsevier: Amsterdam.
- Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Chen YQ, Wise RA. Dopamine and glutamate release in the nucleus accumbens and ventral tegmental area of rat following lateral hypothalamic self-stimulation. Neuroscience. 2001;107:629–639. doi: 10.1016/s0306-4522(01)00379-7. [DOI] [PubMed] [Google Scholar]


