Abstract
Purpose
Since fibroblast activation protein (FAP), one predominant biomarker of cancer associated fibroblasts (CAFs), is highly expressed in the tumor stroma of various epidermal-derived cancers, targeting FAP for tumor diagnosis and treatment has shown substantial potentials in both preclinical and clinical studies. However, in preclinical settings, tumor-bearing mice exhibit relatively low absolute FAP expression levels, leading to challenges in acquiring high-quality PET images using radiolabeled FAP ligands (FAPIs) with low molar activity, because of which a saturation effect in imaging is prone to happen. Moreover, how exactly the molar dose of FAPI administered to a mouse influences the targeted PET imaging and radiotherapy remains unclear now. Therefore, this study aims to investigate the impacts of the molar dose of the administered FAPI on FAP-targeted PET imaging and radiotherapy in mouse syngeneic tumor models.
Methods
[68Ga]Ga-FAPI-04 with various molar doses of FAPI-04 was administered to wild-type 4T1 tumor-bearing mice, followed by static PET imaging. Sigmoidal curves were generated to analyze the correlation between the standard uptake value (SUV) and the administered molar doses of FAPI-04. Similarly, [177Lu]Lu-DOTAGA.(SA.FAPi)2 with a consistent dose of radioactivity but containing different moles of DOTAGA.(SA.FAPi)2 were injected into 4T1 tumor-bearing mice to assess the therapeutic effect. [68Ga]Ga-FAPI-04 was also applied to different tumor models for PET/CT imaging.
Results
A gradient blocking effect was observed with increasing FAPI molar dose in [68Ga]Ga-FAPI-04 PET imaging and [177Lu]Lu-DOTAGA.(SA.FAPi)2 treatment, with various imaging and therapeutic outcomes. [68Ga]Ga-FAPI-04 PET exhibit potentials to characterize murine derived FAP expression with low molar dose of administered FAPI-04 using various tumor models.
Conclusion
The molar dose of FAPI in [68Ga]Ga/[177Lu]Lu-FAPI had a substantial impact on FAP-targeted imaging and therapy in mouse syngeneic tumor models. To acquire enhanced reliability and reproducibility in preclinical situation, it is critical to carefully consider the molar dose of the radiotracer when applying radiolabeled FAP ligands to FAP-targeted imaging and radiotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00259-025-07071-y.
Keywords: Molar dose, FAPI, Preclinical PET imaging, Targeted radiotherapy
Introduction
Since 2018, Loktev and Lindner et al. have developed numerous ligands targeting fibroblast activation protein (FAP), known as FAPIs, which have been extensively applied in FAP-based imaging and radiotherapy [1–3]. Radiolabeled FAPIs, such as [68Ga]Ga-FAPI-04, have shown great promise in clinical studies for detecting various tumors [4]. In preclinical settings, tumor cell lines that either naturally highly express FAP or are transfected with the FAP gene (e.g., U87MG or HT1080-FAP) are typically used to develop tumor models with high FAP expression [5–7]. However, in most solid tumors, FAP is primarily expressed by cancer-associated fibroblasts (CAFs) rather than tumor cells [8]. Consequently, there may be discrepancies between preclinical tumor models and human tumors when tumor cell lines that highly express FAP are used.
Our summary of studies referred to FAPI PET revealed that the tumor uptake of radiolabeled FAPIs varied in various tumor models (Table S1). Even when the same radiopharmaceutical, such as [68Ga]Ga-FAPI-04, was used, studies have varied in terms of the apparent molar activity (Am) or specific activity (As) and moles of FAPI-04 administered per mouse, with varying levels of accumulated radioactivity at tumor sites. Currently, the coordination labeling method is the predominant conjugation method for FAPI radiolabeling, in which a C18 light or HLB cartridge is typically used to remove free radionuclides. However, to achieve sufficient precursor concentrations, common radiolabeling practices often involve a substantial excess of precursor (dozens of nanomoles) relative to the radionuclide, while the molar activities of 68Ga, 18F, and 177Lu are approximately 1 × 105 MBq/nmol (0.37 pmol/mCi), 6.33 × 105 MBq/nmol (0.58 pmol/mCi), and 721 MBq/nmol (51.32 pmol/mCi), respectively. The excess cold FAPI precursor cannot be removed by a C18 light or HLB cartridge, leading to a large amount of cold precursor remaining in the final radiolabeled radiopharmaceutical, thereby reducing the apparent Am of the final radiolabeled agent as the cold precursor can also bind to FAP, prone to cause a saturation effect [9].
While blocking assays with cold FAPI precursors is acknowledged to confirm the specific binding of the radiotracer to the FAP protein, no study has assessed the impacts of the administered moles of FAPI on imaging and radiotherapy with FAPI-based radiopharmaceuticals in preclinical tumor-bearing mice, especially in wild-type tumor cell line-derived xenografts in which FAP is expressed mainly in the tumor stroma. To improve the apparent Am of [68Ga]Ga-FAPI-46, Da et al. optimized the existing common radiolabeling process using anion exchange cartridges [10], which enabled a lower administered molar dose of FAPIs for imaging and radiotherapy compared to the typical injection dose.
This work evaluated the feasibility of using the anion exchange cartridge adsorption strategy reported by Da et al. to improve the apparent Am of radiolabeled FAPIs with various radionuclides and chelators through precise amount quantification by High Performance Liquid Chromatography (HPLC). Furthermore, the impacts of the molar dose of FAPI administered on FAP-targeted PET imaging and radiotherapy in wild-type 4T1 tumor-bearing mice were explored to enhance the application of FAP-targeted imaging and radiotherapy in preclinical settings.
Materials and methods
Chemicals
The chemicals used in this study were as follows: FAPI-04 precursor was from Nanchang Tanzhen Biotechnology Co., Ltd (China). DOTAGA.(SA.FAPi)2 is a kind gift from Prof. Frank Roesch (University Mainz, Germany). Sodium acetate, acetic acid, ethanol and NaHCO3 were from Macklin Biochemical Technology Co., Ltd (China). C18 light (WAT023501, 130 mg sorbent, 55 ~ 105 μm) and QMA (WAT023525, 130 mg sorbent, 37 ~ 55 μm) cartridges were purchased from Waters Corporation (USA).
Radiolabeling
The radiolabeling of FAPI-04 with 68Ga was conducted by adding 20 µg (23 nmol) of the FAPI-04 precursor and 0.2 mL of 5 M acetate buffer (pH = 4) to 2 mL of the eluent from a 68Ge-68Ga generator with 0.05 M HCl, followed by incubation at 100 °C for 15 min. After cooling to room temperature, the reaction mixture was diluted with 10 mL of water, and trapped to a C18 light cartridge (preconditioned with 5 mL of ethanol and 10 mL of water in sequence). After washing the C18 light cartridge with 10 mL of water, [68Ga]Ga-FAPI-04 and the cold FAPI-04 precursor were eluted with 1 mL of 50% ethanol. Then, the eluent was passed through a QMA cartridge (preconditioned with 5 mL of 1 M NaHCO3 and 10 mL of water), an anion-exchange cartridge, to adsorb cold precursors and isolate the final radioactive product. The purified radiopharmaceutical was filtered through a 0.22 μm filter and diluted with phosphate buffer solution (PBS) to the desired concentration for administration to the mice.
For 177Lu labeling of DOTAGA.(SA.FAPi)2, 20 µg (13 nmol) of the precursor and 0.1 mL of 5 M acetate buffer (pH = 5) were added to 0.5 mL [177Lu]LuCl3 solution. The subsequent steps were identical to those for the radiolabeling of [68Ga]Ga-FAPI-04.
To assess the feasibility of using a QMA cartridge for adsorbing the cold FAPI precursor in FAPI-based radiopharmaceuticals. Equal volume of the radiolabeling products were analyzed by radio-HPLC both before and after QMA cartridge adsorption. The moles of loaded sample were quantified by HPLC. The radioactivity peak areas were considered relative radioactivity of the loaded samples. Due to a 25-min delay of loading time of samples after QMA adsorption, radioactivity was corrected based on the half-life of the radionuclide.
At the end of synthesis (EOS), the volume and radioactivity of the radiolabeled products were recorded. The dilution ratio and administered volume for every mouse were then documented to precisely determine the moles of FAPI that was administered to each mouse.
The detailed radiosynthesis procedures of [68Ga]Ga-NOTAGA-FAPI-04, [68Ga]Ga-DOTAGA-FAPI-04, [18F]AlF-NOTA-FAPI-04, and [177Lu]Lu-FAPI-04 were reported in supplementary materials.
Cell binding assays
4T1 and U87MG cells were seeded into a 24-well plate and cultured overnight. After gently washing with PBS, 3.7 kBq of [68Ga]Ga-FAPI-04 in 0.5 mL of serum-free medium was added to each well for 1 h of incubation. For blocking assay, 1 nmol/well of cold FAPI-04 precursor was co-incubated. Then, the medium was removed, and the cells were gently washed with PBS. Next, 0.2 M NaOH (0.5 mL/well) was added to lyse the cells before collection. The radioactivity of the cell lysate was measured using a γ counter, normalized to 1 million cells, and calculated as a percentage of the injected dose (%ID).
PET/CT imaging
PET/CT scans were performed one hour after the administration of 1.11 ~ 7.4 MBq of [68Ga]Ga-FAPI-04 or 1.85 ~ 3.7 MBq [18F]FDG to each mouse using bio-EXPLORER PET/CT (United Imaging, Shanghai, China). For [18F]FDG PET, mice were fasted overnight. During the scan, the mice were anesthetized via inhalation of 2% isoflurane. The standard uptake value (SUV) of the tissue was normalized to the total injected radioactivity and the body weight of each mouse. The maximum and mean SUVs of every volume of interest (VOI) are presented as the SUVmax and SUVmean, respectively.
Biodistribution studies and autoradiography
Mice were injected via the tail vein with 0.74 MBq/mouse [68Ga]Ga-FAPI-04 or 1.85 MBq/mouse [177Lu]Lu-DOTAGA.(SA.FAPi)2 with various moles of FAPI (three biological replication for each group). The mice were sacrificed, blood was collected, and the organs were dissected 1 h after [68Ga]Ga-FAPI-04 injection and 8 h after [177Lu]Lu-DOTAGA.(SA.FAPi)2 injection. The distributed radioactivity was measured using a γ counter, and the values were normalized and reported as %ID/g. For autoradiography, tumor and organs were dissected and cut to 1-mm thick slices. A storage phosphor screen was placed onto the slices and exposed 6 h in a dark room and scanned by a biomolecular imager (Amersham Typhoon™ IP).
[177Lu]Lu-DOTAGA.(SA.FAPi)2 treatment of 4T1 xenografts
When the tumors reached to about 50 mm3, the mice were randomly divided into 3 groups: group 1 (vehicle, n = 6), group 2 ([177Lu]Lu-DOTAGA.(SA.FAPi)2 with a high apparent Am, n = 6), and group 3 ([177Lu]Lu-DOTAGA.(SA.FAPi)2 with a low apparent Am, n = 6). Each mouse in group 2 was injected with 18.5 MBq of [177Lu]Lu-DOTAGA.(SA.FAPi)2 with 10 nmol/kg of DOTAGA.(SA.FAPi)2 (determined by HPLC) via the tail vein. Each mouse in group 3 was injected with 18.5 MBq of [177Lu]Lu-DOTAGA.(SA.FAPi)2 with 50 nmol/kg of DOTAGA.(SA.FAPi)2 (coinjected with 40 nmol/kg of the cold DOTAGA.(SA.FAPi)2 precursor) in the same manner. Tumor size was measured using a Vernier caliper every other day, and tumor volumes were calculated using the following formula: long diameter × short diameter2/2. Tumor volumes exceeding 1500 mm3 or a 20% reduction in body weight compared to baseline were considered endpoints for observation.
The detailed methods of Cell Culture, Tumor models establishment, Docking, Immunofluorescence Staining, Immunohistochemical Staining, and Bioinformatics Analysis were reported at Supplementary Materials.
Statistical analyses
All quantitative data are expressed as the mean ± standard deviation. Differences between two or more groups were analyzed using Student’s t test or one-way ANOVA with consecutive LSD-t tests in SPSS 27.0 software. Sigmoidal curve fitting was performed utilizing GraphPad Prism 10 software. A p value of < 0.05 was considered to indicate statistical significance.
Results
Radiolabeling and quality control of radiolabeled FAPI-04
The efficacy of QMA adsorption strategy in increasing apparent Am of radiolabeled FAPI-04 was explored in [68Ga]Ga-FAPI-04, [68Ga]Ga-DOTAGA-FAPI-04, [18F]AlF-NOTA-FAPI-04, [68Ga]Ga-NOTAGA-FAPI-04, [177Lu]Lu-FAPI-04, and [177Lu]Lu-DOTAGA.(SA.FAPi)2. After QMA adsorption, a significant reduction in the ultraviolet absorption peak area was observed for [68Ga]Ga-FAPI-04, [18F]AlF-NOTA-FAPI-04, [68Ga]Ga-NOTAGA-FAPI-04, [177Lu]Lu-FAPI-04, and [177Lu]Lu-DOTAGA.(SA.FAPi)2, while there was only a fractional reduction in radioactivity as a result of delay of loading time for HPLC (Fig. 1B; Supplementary Fig. S1B). Among chelators we applied above, best adsorption efficacy was showed in DOTA, both in [68Ga]Ga-FAPI-04 and [177Lu]Lu-FAPI-04, with more than ten times of magnification of apparent Am (Supplementary Fig. S2 & Table. S3).
Fig. 1.
(A) Radiolabeling scheme of [68Ga]Ga-FAPI-04. (B) Comparison of the ultraviolet absorption peaks and radioactivity of [68Ga]Ga-FAPI-04 with and without QMA adsorption. * There was 25-min delay of loading time of sample post QMA adsorption. HPLC method: Waters e2695 radio-HPLC; Symmetry C18 column, 5.0 μm, 4.6 mm × 250 mm; 0 ~ 2 min 95%/5% water/acetonitrile, 2 ~ 17 min 95%/5% water/acetonitrile to 65%/35% water/acetonitrile, 17 ~ 20 min 65%/35% water/acetonitrile; with 0.1% trifluoroacetic acid; flow rate, 1 mL/min; λ = 254 nm; and column temperature 35 °C)
The radiochemical yields of [68Ga]Ga-FAPI-04/[177Lu]Lu-DOTAGA.(SA.FAPi)2 were 83.0% ± 16.2% (n = 7) and 55.27% (n = 1). The apparent Am of [68Ga]Ga-FAPI-04 varied from 16.50 ~ 513.15 MBq/nmol, resulted from different radioactivity at beginning of synthesis and with or without QMA adsorption. The apparent Am of [177Lu]Lu-DOTAGA.(SA.FAPi)2 was 136.6 MBq/nmol.
The measured pH of reaction mixtures and products were reported in supplementary materials (Supplementary Table S2).
TCGA database analysis and molecular docking results
FAP is significantly upregulated in various tumors compared to normal tissues (Fig. 2A). Molecular docking simulations indicated that muFAP hold similar active pocket to hFAP for FAPI-04 binding, suggesting the feasibility of using [68Ga]Ga-FAPI-04 to characterize CAF-based muFAP expression in tumor-bearing mice (Fig. 2B and C).
Fig. 2.
(A) Expression levels of FAP in various epithelial tumors in TCGA database. (B and C) Molecular docking of FAPI-04 to hFAP and muFAP. (D) Cell immunofluorescence of FAP in 4T1 and U87MG cells. (E) 4T1 and U87MG [68Ga]Ga-FAPI-04 cell binding assay (n = 4 for each group). ****p < 0.0001
Negative FAP expression and [68Ga]Ga-FAPI-04 uptake by 4T1 tumor cells
Immunofluorescence confirmed that FAP was not expressed by 4T1 cells, while positive staining was observed in U87MG cells, a FAP-expressing cell line (Fig. 2D). The results of the cell binding assay revealed that 4T1 cells did not take up [68Ga]Ga-FAPI-04 (Fig. 2E), indicating that 4T1 cell-derived xenografts are expected to constitute a muFAP-expressing tumor model in which muFAP is initially expressed during tumorigenesis after tumor cell plantation, primarily localized to CAFs probablely.
Correlation between the uptake of [68Ga]Ga-FAPI-04 and the moles of FAPI-04 injected
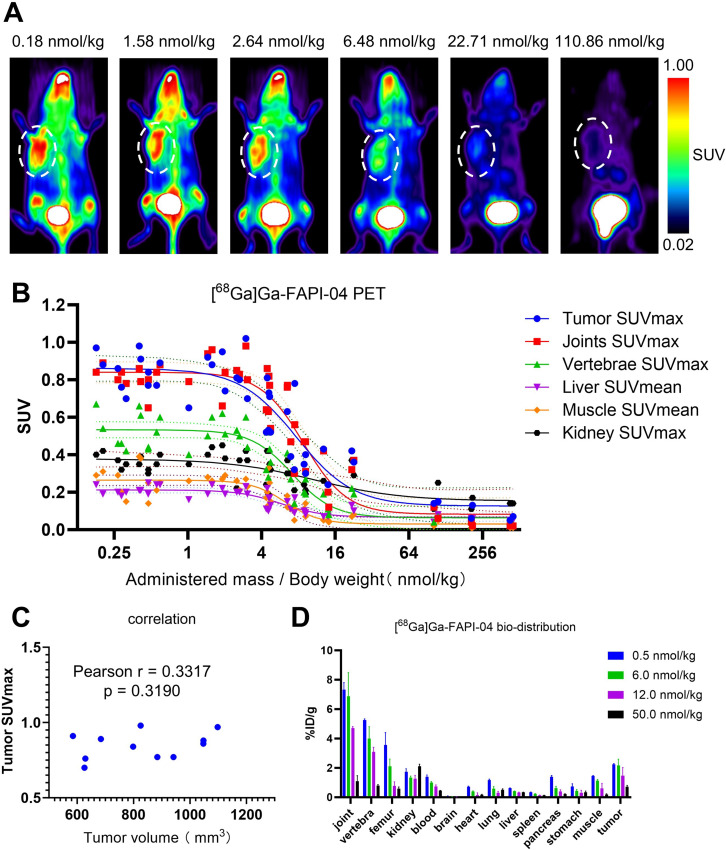
A total of 45 mice bearing 4T1 tumors underwent [68Ga]Ga-FAPI-04 PET imaging after administration of different moles of FAPI-04 and representative maximum intensity projection (MIP) images are presented in Fig. 3A and supplementary Fig. S5. After the simulation, the correlation between the SUV and the administered moles of FAPI-04 fit the standard sigmoidal curves (Fig. 3B; Table 1). The tumors, joints and vertebrae exhibited significant uptake of [68Ga]Ga-FAPI-04 with relatively low molar doses of FAPI-04. As the administered moles of FAPI-04 increased, the uptake in the tumors, joints and vertebrae decreased gradually (Supplementary Fig. S6). Although the liver and muscle showed relatively low [68Ga]Ga-FAPI-04 uptake, a similar blocking phenomenon was also observed in these tissues (Supplementary Fig. S6).
Fig. 3.
(A) Representative [68Ga]Ga-FAPI-04 PET MIP images of 4T1 xenografts after the administration of radiotracers with various molar doses of FAPI-04. The white dotted circles indicate the tumor sites. (B) Sigmoidal model simulation results. A total of 45 mice bearing 4T1 tumors were analyzed. (C) Correlation analysis of tumor volume and tumor SUVmax in [68Ga]Ga-FAPI-04 PET imaging. (D) Biodistribution of [68Ga]Ga-FAPI-04 with various molar doses of FAPI-04 in 4T1 xenografts (n = 3 for each group)
Table 1.
Quantitative simulation results with the sigmoidal model
| Sigmoidal, 4PL | Minimum | Maximum | IC50 | Hillslope | Span |
|---|---|---|---|---|---|
| Tumor SUVmax | 0.1264 | 0.8618 | 7.713 | -1.871 | 0.7353 |
| Joints SUVmax | 0.0825 | 0.8405 | 8.833 | -2.465 | 0.7581 |
| Vertebrae SUVmax | 0.0638 | 0.5333 | 7.120 | -2.589 | 0.4695 |
| Liver SUVmean | 0.0667 | 0.2125 | 5.775 | -2.492 | 0.1458 |
| Muscle SUVmean | 0.0307 | 0.2642 | 6.272 | -3.112 | 0.2335 |
| Kidney SUVmax | 0.1534 | 0.3783 | 10.880 | -1.217 | 0.2249 |
According to the sigmoidal curves, different minimum values were observed in the tumors and organs (Fig. 3B; Table 1). The kidney exhibited the highest minimum value among the studied organs, consistent with the role of the kidney in the excretion and clearance of [68Ga]Ga-FAPI-04. Except kidney, the tumor showed a relatively high minimum value and the joints ranked second.
No significant correlation was observed between the tumor SUVmax and tumor volume for the cases where FAPI-04 was administered at a dose lower than 1 nmol/kg (Fig. 3C).
In biodistribution assay, tumor uptake decreased with an administered FAPI-04 dose of 12 and 50 nmol/kg, while stable accumulation was observed when 0.5 or 6 nmol/kg FAPI-04 was injected into each mouse. Furthermore, [68Ga]Ga-FAPI-04 also strongly accumulated in the osteoarticular system with a lower dose of FAPI-04, consistent with the observed PET imaging trends (Fig. 3D).
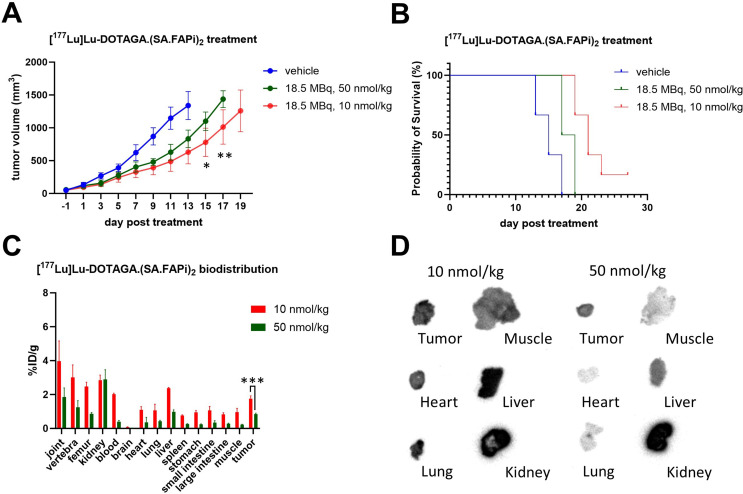
Therapeutic efficacy of [177Lu]Lu-DOTAGA.(SA.FAPi)2 with different molar amounts
Both 18.5 MBq, 50 nmol/kg of [177Lu]Lu-DOTAGA.(SA.FAPi)2 and 18.5 MBq, 10 nmol/kg of [177Lu]Lu-DOTAGA.(SA.FAPi)2 inhibited 4T1 tumor growth. However, a more significant inhibitory rate was observed when 18.5 MBq, 10 nmol/kg of [177Lu]Lu-DOTAGA.(SA.FAPi)2 was used(Fig. 4A and B). Similar differences were observed in the biodistribution assay and autoradiography, explaining that [177Lu]Lu-DOTAGA.(SA.FAPi)2 with 50 nmol/kg and 10 nmol/kg of DOTAGA.(SA.FAPi)2 had different therapeutic effects at the same radioactivity dose (Fig. 4C and D).
Fig. 4.
Treatment with [177Lu]Lu-DOTAGA.(SA.FAPi)2 with the same radioactivity dose but different molar doses of DOTAGA.(SA.FAPi)2. (A) Changes in the tumor volumes of the mice after treatment (n = 6 for each group). *p < 0.05. (B) Survival analysis of the mice after treatment (n = 6 for each group). (C) Biodistribution of [177Lu]Lu- DOTAGA.(SA.FAPi)2 with different molar doses of DOTAGA.(SA.FAPi)2 in mice (n = 3 for each group). (D) Autoradiography image of tumor and organ slices dissected from mice subjected to same radioactivity dose of [177Lu]Lu-DOTAGA.(SA.FAPi)2 but with different molar doses of DOTAGA.(SA.FAPi)2. *p < 0.005, **p < 0.001, ***p < 0.001
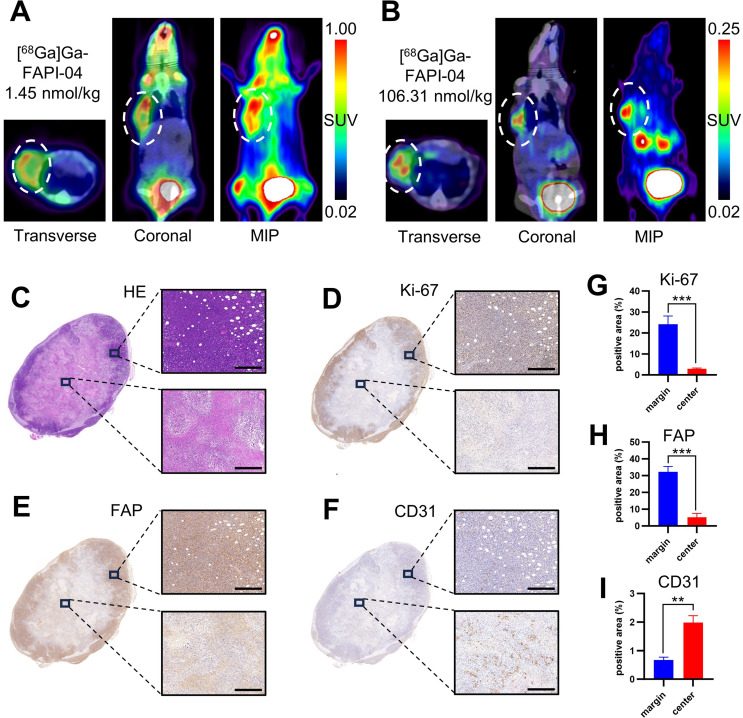
Distinct spatial distributions of radioactivity accumulation at tumor sites after the administration of varying moles of FAPI-04
As shown in Fig. 5A, on adjacent days, the same mouse was injected with 1.45 nmol/kg or 106.31 nmol/kg [68Ga]Ga-FAPI-04, respectively. Notably, marked uptake of radioactivity was observed at the tumor margin after administering a 1.45 nmol/kg dose, while with the 106.31 nmol/kg dose, although the tumor exhibited less resolute uptake, the uptake of radioactivity in the central zone was relatively more pronounced than that in the margin zone (Fig. 5A and B). As noted above, the former pattern represents the specific binding of [68Ga]Ga-FAPI-04 to FAP, while the latter reflects non-specific accumulated radioactivity. Immunochemical staining of FAP, Ki-67, and CD31 in 4T1 tumors validated our hypothesis that non-specific accumulation might be partially attributed to the rich blood supply. Despite the presence of necrotic areas in the tumor center, more CD31-positive sites were observed in the tumor center than in the marginal zone. As expected, the FAP-positive area was predominantly located in the marginal zone, consistent with the PET imaging results obtained after the administration of a relatively low dose of FAPI-04 (Fig. 5C and I).
Fig. 5.
One representative mouse exhibiting the different spatial distributions of accumulated [68Ga]Ga-FAPI-04 radioactivity after administration on adjacent days with different molar doses of FAPI-04. (A and B) [68Ga]Ga-FAPI-04 PET/CT images after the administration of 1.45 nmol/kg and 106.31 nmol/kg FAPI-04. The white dotted circles indicate the tumor sites. (C-F) Images of HE and immunohistochemical staining of Ki-67, FAP, and CD31. (G-I) Semiquantitative analyses of the immunohistochemical staining images. Scale bar = 400 μm. ***p < 0.001, **p < 0.01
[68Ga]Ga-FAPI-04 PET imaging of various tumor bearing mice
Huh7, Hepa1-6, PC3, and MC38 tumor bearing mice—some frequently used preclinical solid tumor models—were subject to [68Ga]Ga-FAPI-04 PET imaging. Considerable tumor and osteoarticular uptake were also observed. Considerable reproducibility of FAPI PET imaging with high apparent Am of radiotracer were realized in multi solid tumor models (Fig. 6A).
Fig. 6.
(A) Representative [68Ga]Ga-FAPI-04 PET MIP images of Huh7, Hepa1-6, PC3, MC38 and U87MG tumor bearing mice (administered FAPI-04 < 1 nmol/kg). (B and C) One representative mouse exhibited different spatial uptake characteristics in [68Ga]Ga-FAPI-04 and [18F]FDG PET/CT imaging at adjacent days (administered FAPI-04 = 4.6 nmol/kg). White dotted circles indicate tumor sites
Unmatched spatial distributions of [68Ga]Ga-FAPI-04 and [18F]F-FDG at tumor sites
For the same mouse, both [68Ga]Ga-FAPI-04 and [18F]FDG PET/CT scans were performed on adjacent days. Interestingly, the accumulation patterns of [68Ga]Ga-FAPI-04 and [18F]FDG at the tumor sites exhibited unmatched and even complementary spatial distribution features (Fig. 6B and C).
Physiological expression of FAP in mice: insights from single-cell RNA sequencing
Two single-cell RNA sequencing databases verified that FAP was highly expressed in bone marrow mesenchymal cells (Fig. 7; Supplementary Fig. S9). Furthermore, at the tissue level, the osteoarticular system contributes significantly to the physiological expression of FAP. By analyzing different stages of growth and development, we found that adult mice (6 to 8 weeks old) exhibited the highest total FAP expression level (Fig. 7).
Fig. 7.
Physiological expression of FAP in MCA single-cell RNA sequence database. FAP expression levels in main cell types, in cell types divided by tissues-lineage, in tissues, and in different periods of mouse life
Discussion
To our knowledge, this is the first study investigating the impacts of the molar amounts of FAPI administered on FAP-targeted PET imaging and therapy in a preclinical setting. Utilizing findings from Da’s study [10], we prepared [68Ga]Ga/[177Lu]Lu-FAPIs with a significantly increased apparent Am, enabling the administration of a low dose of FAPI-04 per mouse for FAPI-based PET imaging and radiotherapy. Although [68Ga]Ga/[177Lu]Lu-FAPIs and the cold FAPI precursors share similar molecular structures, some free carboxylic groups are present in the non-radiolabeled FAPI-04 precursor but are coordinated to [68Ga]Ga3+/[177Lu]Lu3+ ions in [68Ga]Ga/[177Lu]Lu-FAPIs. The anion-exchange QMA cartridge selectively adsorbs the cold FAPI precursors but not [68Ga]Ga/[177Lu]Lu-FAPIs. Using a HPLC external standard method, we measured the actual apparent Am of radiolabeled FAPIs at the EOS and the accurate molar amounts of FAPI injected into each mouse, making it possible to explore the impacts of administered molar dose of FAPI on imaging and therapeutic outcomes.
Recent studies have highlighted the potential of targeting FAP for tumor theranostics [7, 11]. Radiolabeled FAP-targeted ligands, including [68Ga]Ga-FAPI-04, [18F]AlF-FAPI-74, [225Ac]Ac-FAPI-04, [177Lu]Lu-FAP2286, and [177Lu]Lu-DOTAGA.(SA.FAPi)2, have been evaluated in preclinical and clinical studies to detect and treat various types of tumors [12–16]. As demonstrated, the imaging and therapeutic efficacy of [68Ga]Ga/[177Lu]Lu-FAPIs in 4T1 tumors are influenced by the molar dose of FAPI administered, highlighting the importance of considering not only the radioactivity dose but also the molar dose of FAPI in preclinical situations. Our results emphasize the importance of considering the molar dose of FAPI administered in preclinical studies relevant to FAP-targeted radiotherapy. Whether a similar inhibitory effect exists in clinical scenarios remains unclear. It is reasonable to infer that, for PET imaging, much lower doses of FAPI per kilogram body weight are administered to patients in clinical practice (approximately 3.7 MBq/kg) than in preclinical settings (approximately 3.7 ~ 7.4 MBq for a mouse with a weight of approximately 20 g). Nevertheless, maintaining a consistent apparent Am of a radiopharmaceutical during the service life of a 68Ge-68Ga generator, the most broadly used apparatus in nuclear medicine for generating 68Ga, is challenging because of gradual radioactive decay. Moreover, for radiotherapy, radionuclides with longer half-lives and lower molar activities are often administered to patients with higher radioactivity doses than those used for imaging, increasing the likelihood of a blocking effect. Further investigations are necessary to standardize relevant parameters, including apparent Am, administered molar dose, and radioactivity dose, for FAP-targeted imaging and radiotherapy in both preclinical and clinical situations.
A recently published study [17] investigated the correlation between the injected molar dose and the tumor-targeting performance of [177Lu]Lu-OncoFAP-23, a FAP ligand for targeted radiotherapy. The authors recommended an optimal range of 90–250 nmol/kg of [177Lu]Lu-OncoFAP-23, demonstrating a favorable biodistribution profile in SK-RC-52.hFAP tumor bearing mice. In our study, a more pronounced tumor inhibition rate was observed at a dose of 10 nmol/kg compared to 50 nmol/kg with equivalent radioactivity dose. It is well recognized that, in addition to radiation dose at the tumor site, dose-limiting toxicity in normal tissue represents another consideration in targeted radiotherapy. While lower molar dose may enhance radioactivity accumulation in tumors, they can simultaneously increase radiation exposure to normal organs due to physiological uptake. Striking a balance between tumor-targeted radiation and normal tissue protection remains a significant challenge in practical application. In preclinical settings, the optimal dose may be various depending on tumor models and the specific ligands used. Given that only two molar doses of DOTAGA.(SA.FAPi)2 were evaluated in 4T1 tumor models in our study, it’s premature to define the ideal dose range. Further investigations, particularly those incorporating detailed dosimetry analyses, are essential to determine the optimal molar dose.
Some preclinical studies have shown that FAP-targeted PET imaging can be used to predict and evaluate the therapeutic responses of tumors to various FAP-targeted therapies. [18F]AlF-FAPI-74 was employed to monitor the therapeutic response to anti-FAP CAR-T cells in A549-FAP tumor-bearing mice, indicating the potential of FAP-targeted PET imaging for both predictive and therapeutic purposes [18]. Our previous study used [18F]AlF-NOTA-FAPI-04 to monitor CAFs in 4T1 tumor-bearing mice treated with a [177Lu]Lu-FAPI dimer and/or CXCR4 antagonist, and the degree of FAPI uptake in tumors was consistent with the results of immunohistochemical staining for αSMA, a commonly used marker of CAFs [19]. As a novel imaging method that can characterize specific molecules, PET provides a live pathology approach for noninvasively and longitudinally monitoring the expression of specific molecules in vivo. A clinical study demonstrated an ascending correlation between the [68Ga]Ga-FAPI-74 PET parameters (SUVmax, SUVmean, and time to peak) and FAP immunoreactive scores from low- to high-grade dysplasia and pancreatic ductal adenocarcinoma [20]. By improvement of apparent Am and controlling of administered molar dose of FAPI, we reproduced [68Ga]Ga-FAPI-04 PET imaging outcomes in some preclinical solid tumor models. Therefore, by quantifying the molar dose of the FAPI, FAPI PET is expected to be an effective tool for basic preclinical research, allowing noninvasive and longitudinal visualization of muFAP expression levels in CAFs with considerable reproducibility and reliability.
Currently, cell line-derived xenografts are widely used in mouse models to mimic human tumorigenesis. However, FAP is predominantly expressed in the tumor microenvironment rather than in tumor cells [8]. Since it remains challenging to develop mouse models with FAP upregulation specifically in the tumor stroma, tumor cell line-derived xenografts with high FAP expression are often selected for FAP-targeted imaging and radiotherapy in preclinical studies. This approach may not fully replicate the true conditions in which the tumor microenvironment is actually targeted. In our study, sigmoidal curve simulation revealed that the tumors and organs exhibited a gradual decrease in accumulated radioactivity as the molar dose of FAPI-04 administered increased. In the sigmoidal curves, the maximum plateaus represent specific binding of FAPI-04 to the FAP protein, while the minimum plateaus indicate nonspecific accumulated radioactivity, which is partially caused by the blood supply. The tumor and joints showed relatively higher minimum value compared to other organs, perhaps due to the rich blood supply of the tumor tissue and the presence of arterial networks within the joints.
In recent years, numerous novel FAP ligands have been developed for tumor imaging and therapy. However, it remains challengeable to compare the performance of different ligands between different studies. One key issue lies in maintaining consistency in the administered molar doses of different radiolabeled ligands in various studies. Another challenge stems from the complexity of comparing various forms of FAPIs, such as monomers, dimers, and polymers. In our summary (Supplementary Table S1), though for the same tumor model and radiotracer, radio accumulation degrees in tumor site varied from one report to another. There remains a lack of a standard or consensus for this issue. Based on our findings, the maximum plateau of the Sigmoidal curve may best reflect the specific binding between FAP ligand and FAP protein while a high molar dose often induces saturation effect. Therefore, the maximum binding capacity of each tracer should be given priority in comparison.
As shown in Figs. 5, [68Ga]Ga-FAPI-04 PET/CT administration resulted in marked radioactivity distribution at the tumor margin when the dose of FAPI-04 was 1.45 nmol/kg. However, when 106.31 nmol/kg FAPI-04 was administered to the same mouse, more apparent uptake, though to a lesser extent, was observed in the central zone. Immunohistochemical staining of Ki-67 and FAP validated the notable proliferation and FAP expression in the marginal zone of the tumor. Notably, more CD31 staining was observed in the central necrotic zone than in the marginal tumor zone, indicating a richer blood supply in the central zone of the tumor despite the presence of necrosis. Moreover, we speculate that the relatively low interstitial pressure in the necrotic zone compared to that in the marginal zone of the tumor also contributes to the observed distribution. Nevertheless, whether this type of blood perfusion characteristic persists in the advanced necrosis stage remains uncertain. In our experience with the 4T1 tumor model, at low molar doses, [68Ga]Ga-FAPI-04 tends to locate at the tumor margin in most cases, even though there is no necrosis in tumor center zone. This phenomenon, also observed in some previous studies [21, 22], has been validated through immunohistochemical staining of FAP. It may be attributed to the spatial heterogeneity of FAP+ CAFs within tumor stroma, potentially linked to tumor cell invasion and migration. Zhu et al. reported a case of a 29-year-old female patient with a hepatic perivascular epithelioid cell tumor, evaluated using MR imaging, [18F]FDG PET and [68Ga]Ga-FAPI-04 PET imaging [23]. A marginal uptake of [68Ga]Ga-FAPI-04 was observed at the tumor site, whereas arterial enhancement was noted throughout the tumor on contrast-enhanced MRI, with non-increased FDG uptake. Further investigation is warranted to better understand this observation.
In our study, [68Ga]Ga-FAPI-04 and [18F]FDG PET imaging exhibited unmatched and even complementary distribution patterns on the tumor site in the same 4T1 tumor bearing mouse, indicating different functions of [68Ga]Ga-FAPI-04 and [18F]F-FDG in characterizing biological properties of tumors. FAPI PET is typically considered as a visualization tool of tumor stroma while [18F]FDG is a well-known radiotracer for glucose metabolism imaging. The distinct distribution features of these two radiotracers in 4T1 tumors highlight intra-tumoral biological heterogeneity during 4T1 tumorigenesis, raising possibility of combination of them in detecting biological characteristics at different aspects of tumorigenesis.
In addition to the tumor and bladder, the osteoarticular system also exhibited accumulated radioactivity in [68Ga]Ga-FAPI-04 PET in mice. This phenomenon was also observed in some other studies involving [18F]F-FAPI [19, 24, 25] but no similar case has been reported in clinical studies. The physiological expression of FAP in bone marrow mesenchymal cells and the osteoarticular system of mice was confirmed by two single-cell RNA sequencing databases (Fig. 7; Supplementary Fig. S9) [26, 27]. Therefore, the discrepancy in physiological FAP expression between humans and mice should be accounted for during preclinic-to-clinic translation. Moreover, it may be feasible to preliminarily evaluate the molar dose of [68Ga]Ga-FAPI-04 administered by observing its physiological uptake in the osteoarticular system.
Our study has several limitations. The anion-exchange adsorption strategy was verified for only small molecule and one of its dimers. The effect of this strategy on the adsorption of other FAP ligands, peptides for example, remains undetermined. Given that only subcutaneous tumor models were used in this study, further investigations involving other models such as tumor in situ model are necessary to validate our findings. Moreover, quantifying the molar dose of the radiolabeled FAPIs by HPLC before administration is time consuming, while the half-life of 68Ga is as short as approximately 67.8 min. Further progress, not only in synthetic methodology but also in relevant apparatuses, is still needed to enhance the application of radiolabeled FAPIs in preclinical settings.
Conclusion
The molar dose of FAPI in [68Ga]Ga/[177Lu]Lu-FAPIs has a substantial impact on FAP-targeted PET imaging and therapy in preclinical settings. It is critical to carefully consider the molar dose of the administered radiolabeled FAP ligands to acquire enhanced reproducibility and reliability of PET imaging and radiotherapy when applying FAP targeted radiopharmaceuticals to preclinical tumor models.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
This study was conceptualized and designed by Luoxia Liu and Xiaohua Zhu. Data acquisition and interpretation were performed by Luoxia Liu, Yifan Shi, Shujie He, Jingfei Yang, Shuang Song, DongDong Wang, Ziqiang Wang, Huimin Zhou, Xiaoyun Deng, Sijuan Zou and Yuankai Zhu. Luoxia Liu drafted the manuscript, which was polished by Bo Yu and revised and approved by Xiaohua Zhu. Finally, all the authors read and agreed to the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (nos. 82272041, 91959119, 81873903).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Competing interests
The authors have no relevant finacial interests to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bo Yu, Email: yuboxf@hotmail.com.
Xiaohua Zhu, Email: evazhu@vip.sina.com.
References
- 1.Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jäger D, et al. A tumor-imaging Method Targeting Cancer-Associated fibroblasts. J Nucl Med. 2018;59:1423–9. 10.2967/jnumed.118.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of Quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–22. 10.2967/jnumed.118.210443. [DOI] [PubMed] [Google Scholar]
- 3.Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, Kratochwil C, et al. Development of fibroblast activation protein-targeted Radiotracers with Improved Tumor Retention. J Nucl Med. 2019;60:1421–9. 10.2967/jnumed.118.224469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 different kinds of Cancer. J Nucl Med. 2019;60:801–5. 10.2967/jnumed.119.227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindner T, Altmann A, Kramer S, Kleist C, Loktev A, Kratochwil C, et al. Design and development of (99m)Tc-Labeled FAPI Tracers for SPECT Imaging and (188)re therapy. J Nucl Med. 2020;61:1507–13. 10.2967/jnumed.119.239731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backhaus P, Gierse F, Burg MC, Büther F, Asmus I, Dorten P, et al. Translational imaging of the fibroblast activation protein (FAP) using the new ligand [(68)Ga]Ga-OncoFAP-DOTAGA. Eur J Nucl Med Mol Imaging. 2022;49:1822–32. 10.1007/s00259-021-05653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen X, Xu P, Shi M, Liu J, Zeng X, Zhang Y, et al. Evans blue-modified radiolabeled fibroblast activation protein inhibitor as long-acting cancer therapeutics. Theranostics. 2022;12:422–33. 10.7150/thno.68182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: Cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146:895–905. 10.1002/ijc.32193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luurtsema G, Pichler V, Bongarzone S, Seimbille Y, Elsinga P, Gee A, et al. EANM guideline for harmonisation on molar activity or specific activity of radiopharmaceuticals: impact on safety and imaging quality. EJNMMI Radiopharm Chem. 2021;6:34. 10.1186/s41181-021-00149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Da Pieve C, Costa Braga M, Turton DR, Valla FA, Cakmak P, Plate KH, et al. New fully Automated Preparation of High Apparent Molar Activity (68)Ga-FAPI-46 on a Trasis AiO platform. Molecules. 2022;27:675. 10.3390/molecules27030675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Tang H, Song T, Xu M, Chen J, Cui XY, et al. Organotrifluoroborate enhances tumor targeting of fibroblast activation protein inhibitors for targeted radionuclide therapy. Eur J Nucl Med Mol Imaging. 2023;50:2636–46. 10.1007/s00259-023-06230-3. [DOI] [PubMed] [Google Scholar]
- 12.Watabe T, Liu Y, Kaneda-Nakashima K, Shirakami Y, Lindner T, Ooe K, et al. Theranostics Targeting Fibroblast activation protein in the Tumor Stroma: (64)Cu- and (225)Ac-Labeled FAPI-04 in pancreatic Cancer Xenograft Mouse models. J Nucl Med. 2020;61:563–9. 10.2967/jnumed.119.233122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zboralski D, Hoehne A, Bredenbeck A, Schumann A, Nguyen M, Schneider E, et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur J Nucl Med Mol Imaging. 2022;49:3651–67. 10.1007/s00259-022-05842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballal S, Yadav MP, Moon ES, Kramer VS, Roesch F, Kumari S, et al. First-In-Human results on the Biodistribution, Pharmacokinetics, and Dosimetry of [(177)Lu]Lu-DOTA.SA.FAPi and [(177)Lu]Lu-DOTAGA.(SA.FAPi)(2). Pharmaceuticals (Basel). 2021;14:1212. 10.3390/ph14121212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47:1820–32. 10.1007/s00259-020-04769-z. [DOI] [PubMed] [Google Scholar]
- 16.Giesel FL, Adeberg S, Syed M, Lindner T, Jimenez-Franco LD, Mavriopoulou E, et al. FAPI-74 PET/CT using either (18)F-AlF or Cold-Kit (68)Ga labeling: Biodistribution, Radiation Dosimetry, and Tumor Delineation in Lung Cancer patients. J Nucl Med. 2021;62:201–7. 10.2967/jnumed.120.245084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galbiati A, Bocci M, Neri D, Cazzamalli S. Effect of molar dose on the in vivo tissue biodistribution profile of FAP-targeted radioligand therapeutics. Eur J Nucl Med Mol Imaging. 2024. 10.1007/s00259-024-06969-3. [DOI] [PubMed] [Google Scholar]
- 18.Lee IK, Noguera-Ortega E, Xiao Z, Todd L, Scholler J, Song D, et al. Monitoring therapeutic response to Anti-FAP CAR T cells using [18F]AlF-FAPI-74. Clin Cancer Res. 2022;28:5330–42. 10.1158/1078-0432.Ccr-22-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao G, Wang Z, Liu L, Zhang B, Song S, Wang D, et al. Targeting CXCR4/CXCL12 axis via [(177)Lu]Lu-DOTAGA.(SA.FAPi)(2) with CXCR4 antagonist in triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2024;51:2744–57. 10.1007/s00259-024-06704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spektor AM, Gutjahr E, Lang M, Glatting FM, Hackert T, Pausch T, et al. Immunohistochemical FAP expression reflects (68)Ga-FAPI PET Imaging properties of low- and high-Grade Intraductal Papillary Mucinous neoplasms and pancreatic ductal adenocarcinoma. J Nucl Med. 2024;65:52–8. 10.2967/jnumed.123.266393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindeman SD, Mukkamala R, Horner A, Tudi P, Booth OC, Huff R, et al. Fibroblast activation protein-targeted Radioligand Therapy for treatment of solid tumors. J Nucl Med. 2023;64:759–66. 10.2967/jnumed.122.264494. [DOI] [PubMed] [Google Scholar]
- 22.Lindeman SD, Booth OC, Tudi P, Schleinkofer TC, Moss JN, Kearney NB, et al. FAP Radioligand Linker Optimization improves Tumor Dose and Tumor-to-healthy organ ratios in 4T1 syngeneic model. J Med Chem. 2024;67:11827–40. 10.1021/acs.jmedchem.4c00448. [DOI] [PubMed] [Google Scholar]
- 23.Zhu D, Song S, Wang D, Kuang D, Cheng S, Zhou J, et al. Hepatic perivascular epithelioid cell tumor resembling hepatic adenoma and hepatocellular carcinoma on preoperative imaging: a case report. Front Oncol. 2024;14:1292313. 10.3389/fonc.2024.1292313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toms J, Kogler J, Maschauer S, Daniel C, Schmidkonz C, Kuwert T, et al. Targeting fibroblast activation protein: Radiosynthesis and preclinical evaluation of an (18)F-Labeled FAP inhibitor. J Nucl Med. 2020;61:1806–13. 10.2967/jnumed.120.242958. [DOI] [PubMed] [Google Scholar]
- 25.Ge L, Fu Z, Wei Y, Shi D, Geng Y, Fan H, et al. Preclinical evaluation and pilot clinical study of [(18)F]AlF-NOTA-FAPI-04 for PET imaging of rheumatoid arthritis. Eur J Nucl Med Mol Imaging. 2022;49:4025–36. 10.1007/s00259-022-05836-3. [DOI] [PubMed] [Google Scholar]
- 26.Tabula Muris C et al. Overall c, Logistical c, Organ c, processing, Library p,. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018;562:367– 72. 10.1038/s41586-018-0590-4 [DOI] [PMC free article] [PubMed]
- 27.Han X, Wang R, Zhou Y, Fei L, Sun H, Lai S, et al. Mapping the mouse cell atlas by Microwell-Seq. Cell. 2018;172:1091–107. 10.1016/j.cell.2018.02.001. e17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.