Abstract
Ruptured aneurysms or pseudoaneurysms associated with the vasa corona, in the absence of cerebral arteriovenous malformation or dural arteriovenous fistula, are extremely rare but should be recognized as a possible cause of subarachnoid hemorrhage (SAH). We report the case of a 51-year-old female who presented with SAH due to a ruptured vasa corona pseudoaneurysm, with no associated anatomical abnormalities involving the vertebral artery or the posterior inferior cerebellar artery. She recovered after direct surgery. In previous cases, anterior spinal artery dilatation due to posterior inferior cerebellar artery anomalies prompted endovascular treatment. A thorough assessment remains crucial, and direct surgery can be effective.
Keywords: Spinal artery, Subarachnoid hemorrhage, Pseudoaneurysm, Vertebral artery, Posterior inferior cerebellar artery
Introduction
Ruptured aneurysms or pseudoaneurysms associated with the vasa corona, in the absence of cerebral arteriovenous malformations or dural arteriovenous fistulas, are extremely rare [1, 2]. We describe the first case of a ruptured vasa corona pseudoaneurysm, without any other obvious anatomical abnormalities in the vertebral artery (VA) or posterior inferior cerebellar artery (PICA), treated successfully through direct surgery.
Case report
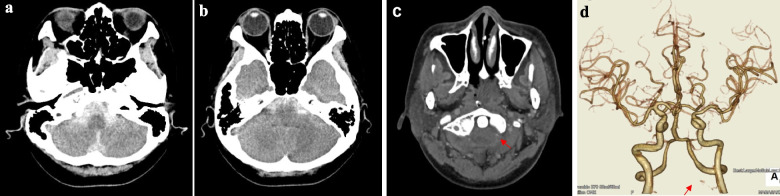
A 51-year-old female with no prior medical history presented with headache and vomiting and sought treatment at another hospital. She was diagnosed with subarachnoid hemorrhage (SAH) and subsequently transferred to our hospital. Upon admission, her Glasgow Coma Scale score was 15, and had no neurological deficits. Computed tomography (CT) revealed a subarachnoid hemorrhage extending from the basilar to the medullary cistern (Fig. 1a, b). She was classified as Grade I on both the World Federation of Neurological Surgeons (WFNS) scale and the Hunt and Kosnik scale. CT angiography (CTA), performed on the day of admission, identified a punctuate contrast on the left anterior surface of the C1 cervical spinal cord and was thought vascular abnormalities, such as aneurysms or pseudoaneurysm, might be present. There was no stenosis or occlusion of the VA or PICA, nor were any other obvious anatomical abnormalities observed (Fig. 2c, d).
Fig. 1.
CT scan revealed subarachnoid hemorrhage (SAH) (a, b). CT angiography (CTA) showed a punctate contrast effect (red arrow) on the left anterior surface of the C1 cervical spinal cord. No stenosis or occlusion of the vertebral artery (VA) or posterior inferior cerebellar artery (PICA) was noted (c, d)
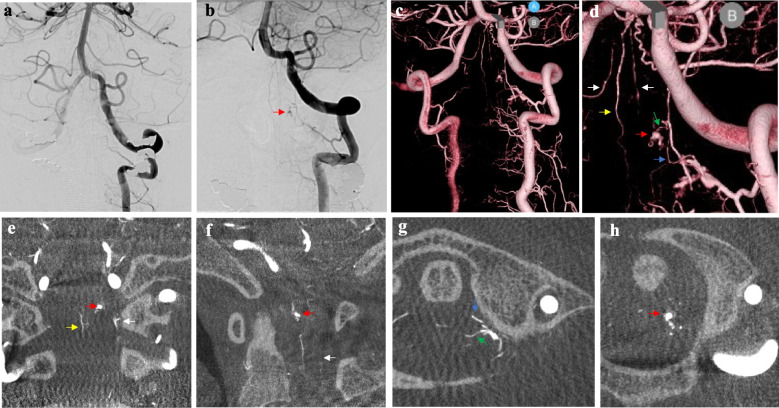
Fig. 2.
Imaging following left vertebral artery (VA) injection on days 1 (a) and 16 (b–d). Red arrows indicate a microaneurysm-like structure on the anterior surface of the C1 cervical spinal cord. The radiculomedullary (blue arrow) and radiculopial arteries (green arrow), running laterally on the left lateral surface of the anterior spinal artery (ASA) (yellow arrow) and lateral spinal artery (LSA) (white arrow), were identified as mother vessels of this aneurysm using Con-beam CT (e–h)
However, the left VA injection performed the day after admission did not show the lesion that was previously revealed by CTA, suggesting that it could have become thrombosed (Fig. 2a). Therefore, we decided to perform follow-ups while managing the spasms. The left VA injection performed on day 16 revealed a microaneurysm-like structure on the left anterior surface of the C1 cervical spinal cord that had been observed using CTA at the time of admission (Fig. 2b–d). Cone beam CT revealed that the mother vessels were the radiculomedullary and radiculopial arteries. Because the aneurysm was located near the anterior surface of the cervical spinal cord, we judged it to be a vasa corona aneurysm as a preoperative diagnosis (Fig. 2e–h).
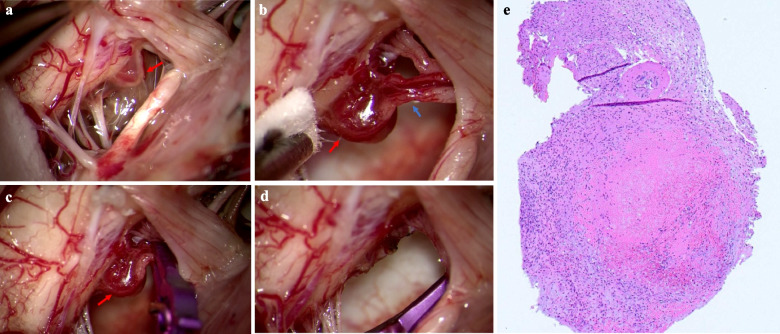
We performed a left suboccipital craniectomy and C1 laminectomy. A microaneurysm-like structure continuous with the radiculomedullary artery was identified (Fig. 3a, b) and clipped (Fig. 3c). These vessels were coagulated and resected along with the vessels draining from the ventral side (Fig. 3d). Pathological diagnosis revealed small muscular vessels, thrombus, granulation tissue formation around the thrombus, and a recanalized thrombus. Based on these findings, it was considered to be a pseudoaneurysm (Fig. 3e).
Fig. 3.
a, b Identification of a microaneurysm-like structure (red arrow) continuous with the radiculomedullary artery (blue arrow). c Localization of clip placement. d Coagulation and resection of the radiculomedullary artery and vessels draining from the ventral side. e Pathological diagnosis revealed pseudoaneurysm diagnosis
The patient progressed well and was discharged with a modified Rankin Scale of 0. Informed consent was obtained from the patient for the publication of this case report and the accompanying images.
Discussion
As shown in Table 1, there have been only two previous reports of vasa corona ruptured aneurysms or pseudoaneurysm without cerebral arteriovenous malformations or dural arteriovenous fistulas, and all previous reports described an anatomical anomaly of the PICA. These anatomical anomalies could not be identified in the present case, and this is the first report of a patient in whom direct surgery was performed.
Table 1.
Reported cases of vasa corona and lateral spinal artery ruptured aneurysms or pseudoaneurysm without arteriovenous malformation or dural arteriovenous fistula
| Case | Author (year) | Age/Sex | Location of the aneurysm | H&H grade | Angiographic findings | Treatment | Pathological diagnosis | Outcome(mRS) |
|---|---|---|---|---|---|---|---|---|
| 1 | Chen et al. (2001) [3] | 72/F | LSA | III | Bilateral VA stenosis | Coil embolization | Not done | 4 |
| 2 | Chen et al. (2001) [3] | 69/F | LSA | III | Lt. VA stenosis | Resection of the aneurysm | Aneurysm | 6 |
| 3 | Kubota et al. (2006) [4] | 59/F | LSA | No data | Rt. PICA occlusion | Resection of the aneurysm | No mention | No mention |
| 4 | Kurita et al. (2009) [5] | 61/M | LSA | I | No obvious anomaly | Clipping | No mention | 0 |
| 5 | Morigaki et al. (2012) [6] | 78/M | LSA | IV | Bilateral VA occlusion | Coil embolization | Not done | 4 |
| 6 | Mizutani et al. (2016) [1] | 42/F | Vasa corona | I | Rt. PICA originating from ASA | Coil embolization | Not done | 1 |
| 7 | Germans et al. (2018) [7] | 49/M | LSA | II | Rt. PICA occlusion | Clipping | Not done | 0 |
| 8 | Matsumoto et al. (2020) [2] | 44/F | Vasa corona | III | Lt. duplication PICA | NBCA embolization | Not done | 0 |
| 9 | Okamoto et al. (2021) [8] | 69/F | LSA | II | Lt.VA occlusion | Resection of the aneurysm | No mention | 2 |
| 10 | Papadimitriou K, et al. (2024) [9] | 74/F | LSA | III | No obvious anomaly | clipping | No mention | 0 |
| 11 | Song Y, et al. (2024) [10] | 55/M | LSA | II | Lt. AICA and PICA stenosis | clipping | No mention | 1 |
| 12 | Song Y, et al. (2024) [10] | 64/M | LSA | I | Bilat. VA stenosis | clipping | No mention | 0 |
| 13 | Song Y, et al. (2024) [10] | 73/F | LSA | I | No obvious anomaly | No mention | No mention | No mention |
| 14 | Jeon YS, et al. (2024) [11] | 51/F | LSA | I | Rt. V4 aplasia | Coil embolization | Not done | 0 |
| 15 | Present case (2024) | 51/F | Vasa corona | II | No obvious anomaly | Resection of the aneurysm | Pseudoaneurysm | 0 |
ASA anterior spinal artery, F Female, H&H grade Hunt and Hess grade, LSA lateral spinal artery LT left, LVA vertebral artery, M Male mRS modified Rankin Scale. NBCA N-butyl cyanoacrylate, PICA posterior inferior cerebellar artery, Rt right
Only 12 LSA [3–8] and 3 vasa corona [1, 2] ruptured aneurysms or pseudoaneurysm in the lateral spinal cord have been described. Of these, nine LSA (75%) and two vasa corona (66.7%) ruptured aneurysm cases had VA or PICA abnormality such as stenosis or occlusion. In addition, severe stenosis was noted, and hemodynamic stress associated with these structural abnormalities was reported as the mechanism of aneurysm occurrence [1–4, 6–8, 12]. However, in this case, no underlying anatomical abnormalities or angiographic findings were confirmed, whereas histopathological findings suggest the possibility of a pseudoaneurysm, which is mostly the main etiology in this case, potentially caused by dissection and fusiform dilation (diffuse and global dilation of the vessel).
Pathological diagnosis was performed in two cases, including our case, which is the first case of a ruptured vasa corona pseudoaneurysm, but we think it is necessary to assess more similar cases for definitive conclusions.
Two previously reported cases of ruptured vasa corona aneurysms were managed endovascularly [1, 2]. In both, an ASA dilatation caused by PICA anomaly allowed microcatheter navigation. Conversely, our case is the first treated via direct surgery; no PICA abnormality or ASA dilatation was identified, making endovascular access difficult. Thus, in the absence of dilated spinal arteries, direct surgery may be necessary.
As for limitations, our report is limited by the small sample size of documented cases and a lack of long-term follow-up data.
In conclusion, we described an extremely rare case in which direct surgery was performed to treat a ruptured pseudoaneurysm in the vasa corona. Even in the absence of anatomical abnormalities or abnormal angiographic findings, it is necessary to conduct a detailed examination of the vasa corona aneurysm. Direct surgery may be effective in such cases.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
H.N.: conceptualization, methodology, software, writing–original draft preparation, visualization, investigation, validation. T.I.: conceptualization, methodology, software, writing–original draft preparation, visualization, investigation, validation, supervision. K.O.: editing. S.Y.:editing. N.U.: editing; S.B.: editing, supervision. K.U.: editing, supervision. Y.M.: editing, supervision. T.H.: editing, supervision. K.Y.: editing, supervision. T.M.: editing, supervision. All authors reviewed the manuscript.
Funding
Open Access funding provided by Gifu University. This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen CC, Bellon RJ, Ogilvy CS, Putman CM (2001) Aneurysms of the lateral spinal artery: report of two cases. Neurosurgery 48(4):949–53; discussion 953–4 [DOI] [PubMed]
- 2.Germans MR, Kulcsar Z, Regli L, Bozinov O (2018) Clipping of ruptured aneurysm of lateral spinal artery associated with anastomosis to distal posterior inferior cerebellar artery: a case report. World Neurosurg 117:186–189 [DOI] [PubMed] [Google Scholar]
- 3.Jeon YS, Park JJ, Chun YI, Roh HG (2024) Lateral spinal artery aneurysm causing subarachnoid hemorrhage: literature review and case report. J Clin Med 13(16):4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubota H, Suehiro E, Yoneda H, Nomura S, Kajiwara K, Fujii M, Fujisawa H, Kato S, Suzuki M (2006) Lateral spinal artery aneurysm associated with a posterior inferior cerebellar artery main trunk occlusion. Case illustration: case illustration. J Neurosurg Spine 4(4):347 [DOI] [PubMed] [Google Scholar]
- 5.Kurita M, Endo M, Kitahara T, Fujii K (2009) Subarachnoid haemorrhage due to a lateral spinal artery aneurysm misdiagnosed as a posterior inferior cerebellar artery aneurysm: case report and literature review. Acta Neurochir (Wien) 151(2):165–169 [DOI] [PubMed] [Google Scholar]
- 6.Lasjaunias P, Vallee B, Person H, Ter Brugge K, Chiu M (1985) The lateral spinal artery of the upper cervical spinal cord. Anatomy, normal variations, and angiographic aspects: anatomy, normal variations, and angiographic aspects. J Neurosurg 63(2):235–241 [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto Y, Takigawa T, Anazawa T et al (2020) A ruptured vasa corona aneurysm treated with NBCA embolization: case report. No Kekkannai Chiryo 5:167–172 [Google Scholar]
- 8.Mizutani K, Akiyama T, Kamamoto D, Nagashima H, Yoshida K (2016) A ruptured aneurysm in the vasa corona at the craniocervical junction with dysgenesis of the posterior inferior cerebellar artery. BJR Case Rep 2(3):20160004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morigaki R, Satomi J, Shikata E, Nagahiro S (2012) Aneurysm of the lateral spinal artery: a case report. Clin Neurol Neurosurg 114(6):713–716 [DOI] [PubMed] [Google Scholar]
- 10.Okamoto A, Uchiyama Y, Maekawa H, Fujimoto K, Hashimoto H (2021) A case of subarachnoid hemorrhage due to ruptured lateral spinal artery aneurysm. Surg Cereb Stroke 49(4):274–277 [Google Scholar]
- 11.Papadimitriou K, Quach ET, Golub D, Patsalides A, Dehdashti AR (2024) Far lateral approach with C1 hemilaminotomy for excision of a ruptured fusiform lateral spinal artery aneurysm: 2-dimensional operative video. Oper Neurosurg (Hagerstown). 10.1227/ons.0000000000001113 [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Lee K, Park H, Hwang SH, Baek HJ, Park IS (2024) Surgical treatment of ruptured aneurysms of lateral spinal artery presenting as intracranial subarachnoid hemorrhage : case series and literature review. J Korean Neurosurg Soc 67(5):586–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.