Abstract
Purpose
Dietary advanced glycation end products (AGEs) have been implicated in promoting insulin resistance. However, their impact on insulin resistance in a mixed population made up of males and females remains controversial. The aim of this study was to evaluate whether the relationship between dietary AGEs and insulin resistance may be sex-dependent.
Methods
195 males and 239 females were included in this cross-sectional study. Study participants underwent anthropometric and metabolic assessments. AGE intake was estimated using food frequency questionnaires and databases reporting AGE content in individual food items. The relationship between AGE intake and insulin resistance, estimated using HOMA-IR, was assessed using Pearson correlation test. The predictive power of dietary AGEs towards HOMA-IR was investigated using stepwise linear regression.
Results
AGE intake correlated positively with HOMA-IR in females (p < 0.01) but not in male study participants (p > 0.05). Moreover, AGE intake was able to increase the predictive power of BMI towards insulin resistance in females but not males. Instead, anthropometric variables were the only discriminants able to predict insulin resistance in males.
Conclusion
Dietary AGEs exert a sex-dependent effect on insulin resistance as their intake is associated with and able to predict HOMA-IR in females but not males. This suggests that females may be more susceptible to the deleterious impact of these glycotoxins on insulin sensitivity. Nevertheless, considering this study not involving a nutritional intervention to directly elucidate whether the effect of AGEs on insulin resistance is sex-dependent, further studies are warranted to confirm the present findings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-025-03672-3.
Keywords: Advanced glycation end products, Insulin resistance, Sex-dependent effect of diet
Introduction
Developed and increasingly developing countries are facing a diabetes epidemic predominantly driven by the exponential increase in the cases of type 2 diabetes mellitus (T2DM) [1]. Obesity, particularly central adiposity, is a key risk factor for T2DM in light of its close relationship with insulin resistance, the hallmark of T2DM [2]. From a pathogenetic perspective, obesity contributes to insulin resistance by fostering metabolic inflammation and lipotoxicity which, in turn, are two pivotal mechanisms in impairing insulin signaling [3–5]. In this context, the Western diet is crucial, not only because it is the main modifiable and independent risk factor [6], but also because of its role in supporting the chronic low-grade inflammation typical of obesity [7] and in promoting lipotoxicity [8]. The consumption of highly processed foods represents one of the key actors in mediating the metabolic aberrations associated with the Western diet. Indeed, they have been implicated in promoting body weight gain and obesity [9], T2DM [10] and increased cardiovascular risk [11]. Although highly processed foods are high in long-chain saturated fatty acids and sugars and low in dietary fiber, these nutrients may not be the only mediators of their effect on metabolic health and insulin resistance in particular. Indeed, food processing also affects the nutritional quality of foods by promoting the formation of advanced glycation end-products (AGEs) which, in turn, are another link between the Western diet and poor metabolic health [12].
AGEs represent a heterogeneous group of molecules produced non-enzymatically as a consequence of the interaction between reducing sugars and the free amino groups of proteins, nucleic acids, and lipids via the Maillard reaction during dry heat food processing, including cooking [13]. Besides heat processing, AGE formation is dependent on the nutritional composition of food items, with AGE content being higher in foods rich in fat and proteins [13]. Considering their heterogeneity, AGEs include molecules like carboxyethyl lysine, carboxymethyl lysine, glyoxal lysine dimer, 3-deoxyglucosone lysine dimer and pyrroline [12]. AGEs introduced with foods, particularly low-molecular weight AGEs, are absorbed in the small intestine [14] and their biological effects are mediated by the activation of the receptor for AGEs (membrane RAGE, mRAGE; soluble RAGE, sRAGE) [15] which culminate with the induction of oxidative stress and the pro-inflammatory pathways, namely c-Jun N-terminal kinase and the factor nuclear-factor-kappa B (NF-κB) [16–18].
From a metabolic perspective, AGEs have been reported to promote insulin resistance in vivo in rats [16] and mice [19] as well as in vitro in rodent skeletal muscle cells [16] and 3T3-L1 adipocytes [19]. Remarkably, a diet low in AGEs increased insulin sensitivity in overweight but otherwise healthy individuals [20] and in individuals with T2DM [21], further supporting the implications of dietary AGEs on insulin resistance. In humans and animal models, AGEs intake effects on insulin resistance remain controversial; indeed, other studies have not identified a relationship between habitual AGEs intake and insulin sensitivity [22, 23]. The reason for these discrepancies may be related to the fact that females appear to be more susceptible to the metabolically detrimental effects of AGEs. In support of this, while studies only including males did not identify a relationship between serum AGEs and insulin sensitivity [24], in study cohorts only made up of overweight women, AGE restriction improved insulin sensitivity [25]. Additionally, the knock of the RAGE resulted in an improvement in insulin sensitivity in female but not in male mice fed a high-fat diet [26].
Thus, despite dietary AGEs being implicated in promoting insulin resistance in mixed male and female study cohorts, their effects are controversial and may be sex-specific. In light of this, the aim of this study was to evaluate the relationship between AGE intake and insulin resistance and if the ability of these glycotoxins to predict deterioration of insulin sensitivity is sex-dependent.
Subjects and methods
Participants
Participants in the cross-sectional PANGeA (Physical Activity and Nutrition for Quality Aging) project were considered for this study [27]. The study conducted in northern Italy, recruited free-living individuals aged between 55 and 80 years and able to walk for 2 km independently. Subjects with anticoagulant therapy or previous cancer diagnosis or hospitalization in the last year or missing were excluded. After the exclusion of twenty-five PANGeA study participants without food frequency questionnaire data, four hundred and thirty-four participants (195 males and 239 females) were included in this study.
Subjects enrolled gave their written consent to participate in the study and were subjected to anamnestic and nutritional interviews, anthropometric measurements and a blood sampling as previously described [27] and detailed below.
Dietary assessment and semi-quantitative dietary AGEs evaluation
Study participants completed a 90-item food frequency questionnaire with indications of food portion sizes. Dietary AGE intake was estimated for each participant using their food intake frequencies, after adjusting portion sizes for study participant total energy intake, and the AGE content of food samples reported in previously published databases [13, 28]. For some food items, it was not reported the cooking method in the food frequency questionnaire. In this case, the AGE content of the food was expressed as the mean of the most common cooking methods used for that specific item. Then, the AGE intake for each participant was obtained by multiplying the frequency of single food item, referred to as frequency of consumption over a year, by the mean AGE content, expressed as kiloUnits (kU) per serving of each food item consumed by study participants. Finally, the AGEs intake for each participant was expressed as kU AGEs/day.
Supplements if consumed were not considered for the estimation of AGEs intake. Nutrient and energy intake were assessed using 24 h recalls. The adherence to the Mediterranean Diet was computed using a Mediterranean Diet adherence score (MDA) as detailed previously [27].
Biochemical analysis
Blood samples were collected after an overnight fast and centrifuged at 1600 g for 15 min at 4 °C to obtain serum or EDTA plasma. Aliquots were stored at −80 °C until use. Serum triglycerides, total cholesterol, HDL cholesterol (HDL), glucose and insulin concentrations were measured by standard enzymatic-colorimetric methods. LDL cholesterol (LDL) levels were computed by the Friedewald's formula [29]:
Serum High-sensitivity C-reactive protein (hsCRP) was measured by immune-turbidimetry (CRP OSR6147, Beckman Coulter, Brea, CA, USA). Insulin resistance was calculated using the Homeostasis model assessment index (HOMA-IR) formula [30]:
Visceral adipose Index (VAI) was estimated according to Amato et al. [31] as follows:
For female
For male
(BMI = body mass index).
Anthropometric measurements and body composition
Anthropometric measures were performed on participants wearing light clothing and no restrictive underwear nor shoes. Body weight was rounded to the nearest 100 g whereas height and waist circumferences were all rounded to the nearest 0.1 cm. Waist circumference was measured between the lowest rib and iliac crest around the smallest circumference.
Bioelectrical impedance (tetrapolar impedance meter, BIA101, Akern, Florence, Italy) was used to determine body composition: fat mass (FM), fat-free mass (FFM) and muscle mass. Bioimpedance was performed by a trained staff member on subjects in a horizontal position, after 8 h of fasting.
Statistical analysis
Continuous variables were analyzed using Shapiro–Wilk tests. Non-normally distributed variables were expressed as median (Quartile 1–Quartile 3). The comparison of variables between males and females was carried out with Mann–Whitney tests for non-normally distributed parameters. Pearson correlation analysis was used to evaluate the association between AGEs or HOMA-IR and the anthropometric and metabolic parameters. Stepwise multiple regression analysis was performed to assess the predictive power of AGEs and other parameters of interest for HOMA-IR. In these analyses, the variables not normally distributed were log-transformed. Data analysis was performed using SPSS Statistics for Windows, version 26.0 (SPSS, Inc., Chicago, IL) and a p ≤ 0.05 was considered statistically significant.
The missing data for each variable of interest did not exceed 5%.
Results
General, anthropometric, metabolic characteristics and AGE intake of the study participants
The characteristics of the study participants divided by sex are reported in Table 1. As expected, male, compared to female study participants, had a higher BMI, waist circumference, FFM, and systolic and diastolic blood pressure (all p ≤ 0.001) (Table 1). On the contrary, FM in percentage but not in Kg, and VAI (p = 0.007) was higher in females compared to males (p < 0.001) (Table 1). From a metabolic perspective, total, LDL and HDL cholesterol was higher in females than in males (all p < 0.001), whereas fasting blood glucose (p < 0.001), blood insulin (p = 0.012) and HOMA-IR (p < 0.001) were higher in males (Table 1). Finally, the consumption of AGEs adjusted for energy intake was higher in males relative to females (p = 0.003) (Table 1). Furthermore, the number of individuals with hypertension, taking anti-hypertensive drugs or metformin was higher among males compared to females (Table 1).
Table 1.
General characteristics of male and female study participants
| Male (n = 195) | Female (n = 239) | p-value1 | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Age (years) | 66 (63–70) | 65 (63–70) | 0.103 |
| BMI (kg/m2) | 26.8 (24.7–29.3) | 25.7 (23.2–28.2) | 0.001*** |
| Waist circumference (cm) | 96.0 (90.0–103.0) | 89.0 (83.0–96.0) | < 0.001*** |
| FFM (%) | 68.8 (65.1–72.5) | 61.2 (57.1–64.8) | < 0.001*** |
| FM (%) | 31.2 (27.5–34.9) | 38.8 (35.2–42.9) | < 0.001*** |
| FFM (Kg) | 54.7 (51.5–58.6) | 39.2 (36.6–42.3) | < 0.001*** |
| FM (Kg) | 24.5 (20.7–29.7) | 24.82 (20.6–29.5) | 0.907 |
| Muscle Mass (Kg) | 34.9 (32.0–37.3) | 23.9 (21.9–26.0) | < 0.001*** |
| VAI | 0.91 (0.61–1.46) | 1.0 (0.81–1.51) | 0.007** |
| SBP (mmHg) | 143 (132–158) | 133 (120–146) | < 0.001*** |
| DBP (mmHg) | 88 (81–94) | 82 (76.67–90.) | < 0.001*** |
| AGEs (kU/day) | 11,925.2 (8811.6–17,030.5) | 10,492.7 (8103.9–14,692.8) | 0.003** |
| Glucose (mg/dL) | 100 (91–110) | 94 (88–102) | < 0.001*** |
| Insulin (U/L) | 8.8 (6.1–12.4) | 7.50 (5.6–10.0) | 0.012* |
| HOMA-IR index | 2.2 (1.4–3.4) | 1.7 (1.2–2.4) | < 0.001*** |
| Triglycerides (mg/dL) | 94 (70–125) | 91 (71–116) | 0.747 |
| Total Cholesterol (mg/dL) | 200 (179–228) | 226 (204–250) | < 0.001*** |
| Cholesterol HDL (mg/dl) | 57 (48–69) | 72 (61–81) | < 0.001*** |
| Cholesterol LDL (mg/dl) | 122 (100–143) | 136 (113–156) | < 0.001*** |
| hsCRP (mg/L) | 0.11 (0.06–0.21) | 0.10 (0.06–0.26) | 0.792 |
| IL-18 (pg/ml) | 400.9 (319.6—503.5) | 320.9 (263.0—405.8) | < 0.001*** |
| N (%) | N (%) | p-value2 | |
|---|---|---|---|
| Hypertension | 72 (36.9%) | 63 (26.4%) | 0.022* |
| Hypertension therapy | 69 (35.4%) | 62 (26.4%) | 0.036* |
| Hypolipidemic treatment | 43 (22.1%) | 35 (14.6%) | 0.059 |
| Metformin therapy | 13 (6.7%) | 3 (1.3%) | 0.004** |
| Smoke | 16 (8.3%) | 25 (10.6%) | 0.267 |
The comparison between male and female was carried out with Mann–Whitney test1 or Fisher Exact Test2
*0.050 > p-value ≤ 0.010; **0.010 > p-value < 0.001; ***p-value ≤ 0.001
BMI, body mass index; FFM, fat free mass; FM, fat mass; VAI, visceral adiposity index; SBP, systolic blood pressure; DBP, diastolic blood pressure; AGEs, advanced glycation end products; HOMA-IR, homeostatic model assessment for insulin resistance; HDL, high density lipoprotein; LDL, low density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IL-18, interleukin-18
In terms of nutrient and energy intake, males consumed more energy, carbohydrates, proteins and lipids than females (Table S2). However, diet quality expressed as MDA did not differ between the two groups (Table S2).
The relationship between AGE intake, anthropometric and metabolic health-related parameters
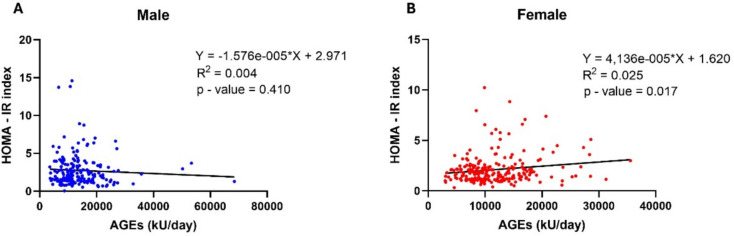
Considering the relationship between dietary AGEs, insulin resistance and obesity [32, 33] which, however, remains controversial [22], it was investigated whether the habitual AGE consumption correlated with anthropometric and metabolic health-related parameters. The intake of AGEs in the whole study cohort correlated positively with FFM and negatively with visceral adiposity index (VAI), fasting triglycerides, total and LDL-cholesterol. Additionally, AGE intake did not correlate with fasting glycemia nor HOMA-IR (Table S3). However, considering that the present study cohort was made up of both males and females, to evaluate whether AGE intake affected differently males and females, the relationship between AGE intake and metabolic parameters was investigated in both sexes separately. While dietary AGEs in male correlated with fasting triglycerides (p = 0.034) but not with insulin resistance (Table 2; Fig. 1A), in females the intake of AGEs correlated positively with fasting blood insulin (p = 0.034) and HOMA-IR (p = 0.022) (Fig. 1B) and negatively with total (p = 0.005) and LDL-cholesterol (p = 0.002) (Table 2). Additionally, in females dietary AGEs tended to correlate positively with fasting blood glucose (p = 0.065).
Table 2.
Pearson’s correlation between AGEs, anthropometric and metabolic parameters of interest
| log AGEs (kU/day) | ||||
|---|---|---|---|---|
| Male (n = 195) | Female (n = 239) | |||
| r | p-value | r | p-value | |
| log BMI (kg/m2) | −0.062 | 0.390 | −0.050 | 0.445 |
| log Waist circumference (cm) | −0.020 | 0.781 | −0.045 | 0.485 |
| log FM (Kg) | −0.133 | 0.065 | −0.023 | 0.724 |
| log FFM (Kg) | −0.002 | 0.976 | 0.034 | 0.597 |
| log VAI | −0.099 | 0.173 | −0.070 | 0.299 |
| log Triglycerides (mg/dL) | −0.153 | 0.034* | −0.064 | 0.339 |
| log Total Cholesterol (mg/dL) | −0.013 | 0.860 | −0.185 | 0.005** |
| log Cholesterol HDL (mg/dl) | 0.002 | 0.973 | 0.056 | 0.403 |
| log Cholesterol LDL (mg/dl) | 0.036 | 0.616 | −0.207 | 0.002** |
| log Glucose (mg/dL) | −0.082 | 0.261 | 0.123 | 0.065 |
| log Insulin (U/L) | −0.026 | 0.726 | 0.142 | 0.034* |
| log HOMA-IR index | −0.047 | 0.518 | 0.153 | 0.022* |
| log IL-18 (pg/ml) | 0.081 | 0.261 | −0.043 | 0.521 |
| log hsCRP (mg/L) | −0.006 | 0.931 | −0.057 | 0.394 |
*0.050 > p-value ≤ 0.010; **0.010 > p-value < 0.001
r, Pearson Correlation Coefficient; BMI, body mass index; FFM, fat free mass; FM, fat mass; VAI, visceral adiposity index; SBP, systolic blood pressure; DBP, diastolic blood pressure; AGEs, advanced glycation end products; HOMA-IR, homeostatic model assessment for insulin resistance; HDL, high density lipoprotein; LDL, low density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IL-18, interleukin-18
Fig. 1.
Correlation between daily AGE intake and insulin resistance in both sexes. On one side, the dot plot chart shows the absence of correlation between daily AGE intake and HOMA-IR index in the male population (A), while, on the other side, it reports a positive correlation between the same parameters in the female population (B). HOMA-IR homeostatic model assessment of insulin resistance, AGEs advanced glycation end product
AGE intake as a predictor of HOMA-IR
Given the relationship between AGE intake and HOMA-IR in female study participants, it was next investigated whether dietary AGEs could predict insulin resistance and how their predictive power compared with known risk factors for insulin resistance [34]. It was first confirmed that in this study cohort existed a relationship between BMI, fat mass, VAI and HOMA-IR. As expected, all these variables correlated positively with HOMA-IR in the whole cohort (Table S4) as well as in males (Table 3) and females (Table 3) separately (all p < 0.001).
Table 3.
Pearson’s correlation between HOMA-IR index and classical predictors for insulin resistance in male and female study participants
| log HOMA-IR index | ||||
|---|---|---|---|---|
| Male (n = 195) | Female (n = 239) | |||
| r | p-value | r | p-value | |
| log BMI (kg/m2) | 0.518 | < 0.001*** | 0.461 | < 0.001*** |
| log VAI | 0.387 | < 0.001*** | 0.417 | < 0.001*** |
| log FM (Kg) | 0.564 | < 0.001*** | 0.439 | < 0.001*** |
***p–value ≤ 0.001
r, Pearson Correlation Coefficient; BMI, body mass index; FM, fat mass; VAI, visceral adiposity index
Considering BMI, FM and VAI all correlated positively with HOMA-IR when considering the whole study cohort, it was next assessed whether AGE intake could increase the predictive power of these variables towards insulin resistance. In the whole study cohort, the BMI was the primary predictor of HOMA-IR (p < 0.001), with its predictive power being increased by VAI (p < 0.001) in model 2, AGE intake (p < 0.005) in model 3 and FM (p = 0.007) in model 4 (Table S5). However, when considering only male study participants AGE intake failed to increase the predictive power of fat mass and VAI towards insulin resistance (Table 4A). On the contrary, in females, dietary AGEs were able to increase the capacity of BMI and VAI to predict variations in HOMA-IR (p = 0.001) (Table 4B).
Table 4.
Stepwise linear regression model indicating predictors of HOMA-IR index in male (A) and female (B) study participants
| (A) Male (n = 195) | |||||
|---|---|---|---|---|---|
| Model | Predictor | R2 | Unstandardized B coefficient | Standard error | p-value |
| 1 | log FM (Kg) | 0.318 | −1.641 | 0.211 | < 0.001*** |
| 1.426 | 0.152 | < 0.001*** | |||
| 2 |
log FM (Kg) log VAI |
0.358 | −1.373 | 0.220 | < 0.001*** |
| 1.238 | 0.157 | < 0.001*** | |||
| 0.229 | 0.067 | 0.001** | |||
| Dependent variable: log HOMA-IR | |||||
|
Model 1 excluded variables: log AGEs (kU/day), log BMI (Kg/mq), log VAI Model 2 excluded variables: log AGEs (kU/day), log BMI (Kg/mq) | |||||
| (B) Female (n = 239) | |||||
|---|---|---|---|---|---|
| Model | Predictor | R2 | Unstandardized B coefficient | Standard Error | p-value |
| 1 | 0.213 | −2.263 | 0.326 | < 0.001*** | |
| log BMI | 1.780 | 0.230 | < 0.001*** | ||
| 2 | 0.307 | −1.825 | 0.317 | < 0.001*** | |
| log BMI | 1.459 | 0.224 | < 0.001*** | ||
| log VAI | 0.327 | 0.060 | < 0.001*** | ||
| 3 | 0.343 | −2.735 | 0.407 | < 0.001*** | |
| log BMI | 1.468 | 0.219 | < 0.001*** | ||
| log VAI | 0.340 | 0.058 | < 0.001*** | ||
| log AGEs | 0.223 | 0.065 | 0.001*** | ||
| Dependent variable: log HOMA-IR | |||||
|
Model 1 excluded variables: log AGEs (kU/day), log FM (Kg), log VAI Model 2 excluded variables: log AGEs (kU/day), log FM (Kg) Model 3 excluded variables: log FM (Kg) | |||||
***p-value ≤ 0.001
BMI, body mass index; FM, fat mass; VAI, visceral adiposity index; AGEs, advanced glycation end products
Discussion
The data reported herein describe a sex-dependent effect of dietary AGEs on insulin resistance. In particular, dietary AGEs are positively associated with and are able to predict HOMA-IR in female but not male study participants. Remarkably, the intake of AGEs was able to increase the predictive power of anthropometric variables known to negatively impact upon insulin sensitivity only in females.
The present data is in agreement with previous studies which reported that a diet low in dietary AGEs is able to improve insulin sensitivity in overweight women [25]. Other studies have also shown an inverse relationship between AGE intake and insulin sensitivity, albeit this relationship was described in mixed cohorts consisting of both males and females [20, 21], which does not allow to discriminate the contribution of each sex. At the same time, in other studies AGE intake did not affect insulin sensitivity [22, 23]. However, none of these studies evaluated whether these glycotoxins elicited a sex-dependent effect on insulin resistance. Thus, to our knowledge, this is the first study dissecting the role of sex on the relationship between AGE intake and insulin resistance. Indeed, despite in the first instance the present result confirming the ability of AGE intake to predict HOMA-IR, this effect disappeared when only males were included in the analysis. This suggests that the impact of habitual AGE intake on insulin resistance in this cohort is driven by females who, compared to males, appear more susceptible to the metabolic effects of dietary AGEs. This sex-dependent response to AGEs was also confirmed in another study, which despite not directly investigating insulin resistance, still reported that circulating AGEs were able to predict all-cause, cardiovascular disease, and coronary heart disease mortality in women but not in men [35]. Additionally, while in study cohorts only made of men serum AGEs were not linked with insulin resistance [24], in women dietary AGE restriction resulted in an improvement in insulin sensitivity [25]. Importantly, the intake of AGEs as part of this study is in line with previously published reports [36, 37] and above (≥ 10,000 kU/day) the value set for a low AGE diet in AGE-restricted dietary intervention studies [21, 38]. As such, the amount of AGEs consumed by both groups should be sufficient to identify a relationship with insulin resistance, further underlining that the effect of AGEs may be sex-specific.
However, the reason underlying the sex-dependent effect of AGEs on insulin resistance in the present and other studies remains to be fully elucidated, even though it may rely on the modulation of RAGE expression by estrogens. Indeed, 17-β-estradiol is able to upregulate mRAGE expression in human vascular endothelial cells [39]. At the same time, menopausal hormone replacement therapy with estradiol and norethisterone acetate led to a decrease in sRAGE [40]. Thus, estrogens, on one hand upregulate mRAGE leading to an increase in AGE-mediated intracellular signal transduction while, on the other hand, also increase AGE bioavailability by downregulating sRAGE [12, 41]. These effects of estrogens, in turn, would amplify the activation of the c-Jun N-terminal kinase and the transcription factor nuclear-factor-kappa B (NF-κB) pro-inflammatory pathways, the induction of oxidative stress and the downregulation of sirtuin1 [16–19, 42] which have all been implicated in the pathogenesis of insulin resistance [5, 43–45]. Nevertheless, the women included in this study were in the post-menopausal state and did not receive any hormone-replacing therapy which may have prevented an oestrogen-dependent modulation of both mRAGE and sRAGE. Despite this, the sex-dependent response to dietary AGEs, described herein, may still be driven by residual endogenous estrogenic milieu, as demonstrated by its effect on bone mass in postmenopausal women [46]. This possibility is supported by data from animal models in which RAGE knock-out in animals fed a high-fat diet was sufficient to improve insulin tolerance in females but not in males [26]. However, it must not be overlooked the fact that RAGE can also recognize ligands other than AGEs. Thus despite the improvement in glucose homeostasis and insulin tolerance upon RAGE knockout in females but not in male mice, it cannot be inferred that this effect is strictly dependent on AGEs, considering RAGE can also bind other ligands [47]. However, these findings still suggest that females, relative to males, are more susceptible to the metabolically detrimental effects of RAGE ligands, including AGEs [26]. Another putative explanation for these sex-dependent effects is that the specific contribution of anthropometric parameters towards insulin resistance may differ between males and females. In keeping with this, not only the anthropometric variables able to predict HOMA-IR are different between males and females but in females the predictive power of these variables is lower compared to males and, most importantly, is increased by dietary AGEs. This suggests that insulin resistance in males, at least in this study cohort, is primarily dependent on anthropometric measures and particularly on fat mass and VAI. On the contrary in females, where the contribution of anthropometric variables is lower, dietary AGEs may further fuel insulin resistance promoted by the BMI and VAI.
From a mechanistic perspective, the relationship between AGE intake and insulin resistance does not appear to be related to NLRP3 inflammasome activation which, in turn, is pivotal for IL-18 production [48]. Indeed, despite this cytokine being associated with insulin resistance [49–51], its circulating levels are not related to dietary AGEs. However, this disagrees with the fact that AGEs have been reported to activate the NLRP3 inflammasome [52]. Nevertheless, there is also evidence that AGEs are able to attenuate NLRP3 inflammasome activation [53], a notion that reflects the lack of relationship between dietary AGEs and IL-18 circulating levels reported herein. Thus, the relationship between AGE intake and insulin resistance may be mediated by the activation of other pathways known to be triggered by AGEs [16–19, 42] even though this was not assessed as part of this study.
AGE intake correlated negatively with total and LDL-cholesterol only in females, whereas no relationship was found between these parameters in males. The negative relationship between dietary AGEs, total and LDL-cholesterol is surprising, particularly considering the tight relationship identified between the intake of AGEs and insulin resistance and the fact that a low intake of AGEs resulted in a decrease in total as well as LDL-cholesterol [54]. In light of this, the relationship between dietary AGEs and the circulating lipid profile in females warrants further studies.
This study has some limitations. In the first instance, circulating AGE levels were not assessed in order to confirm an overlapping between AGE intake and their plasma levels. Additionally, this study, to the same extent as other reports [22], did not discern between low and protein-bound AGE which are absorbed at different rates [55, 56]. Furthermore, this is an observational study which did not directly investigate whether modulating the intake of dietary AGEs via a nutritional intervention would differently impact upon insulin sensitivity in males and females. Always in line with this, the use of food frequency questionnaire may be prone to information bias [57], to reduce this bias, the questionnaire was administered by trained medical doctors with expertise in nutrition. Nevertheless, this study has also some strength in that it is the first study reporting a sex-dependent effect of dietary AGEs on insulin resistance. Additionally, the assessment of AGE intake was conducted using food frequency questionnaires as previously reported [22] which, in combination with published AGE databases [13, 28], have been proposed as the best method to estimate AGE intake in large cohorts [22]. An additional strength of the study is the assessment of habitual dietary habits in a large study cohort which allowed a reduction of information bias associated with food frequency questionnaires.
Conclusion
To conclude dietary AGEs exert a sex-dependent effect on insulin resistance as their intake is associated and able to predict HOMA-IR in females but not males. This suggests that females may be more susceptible to the deleterious impact of these glycotoxins on insulin sensitivity. Nevertheless, considering this study not involving a nutritional intervention in order to directly elucidate whether the effect of AGEs on insulin resistance is sex-dependent, further studies are warranted in order to confirm the present findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the research team of the Pangea study group: Italy—University of Ferrara: Edoardo Dalla Nora (Azienda Ospedaliero-Universitaria di Ferrara, Arcispedale S.Anna, Ferrara, Italy; edoardo.dallanora@unife.it), Gloria Brombo (Azienda Ospedaliero-Universitaria di Ferrara, Arcispedale S.Anna, Ferrara, Italy; gloria.brombo@unife.it), Eleonora Capati (Azienda Ospedaliero-Universitaria di Ferrara, Arcispedale S. Anna, Ferrara, Italy; eleonora.capatti@unife.it), Cecilia Soavi (AUSL Ferrara, Ferrara, Italy; cecilia.soavi@gmail.com), Rosella Colonna (Azienda Ospedaliero-Universitaria di Ferrara, Arcispedale S.Anna, Ferrara, Italy; r.colonna@ospfe.it), Elettra Mantovani (Università di Ferrara, Area Tecnica, Ferrara, Italy; eletra.mantovani@unife.it), Mario Luca Morieri (Department of Medicine, University of Padova, Padua, Italy; Morieri.ml@gmail.com), Maria Agata Miselli (Azienda Ospedaliero-Universitaria di Ferrara, Arcispedale S.Anna, Ferrara, Italy; mariaagate.miselli@unife.it), Alice Omenetto (Department of Translational Medicine, University of Ferrara, Ferrara, Italy; alice.omenetto@edu.unife.it), Sefora Del Mastro (Department of Translational Medicine, University of Ferrara, Ferrara, Italy; sefora.delmastro@edu.unife.it), Gabriella Stifani (Department of Translational Medicine, University of Ferrara, Ferrara, Italy; gabriella.stifani@edu.unife.it) and Daniela Francesconi (Azienda Ospedaliero-Universitaria di Ferrara, Arcispedale S.Anna, Ferrara, Italy; d.francesconi@ospfe.it); University of Udine: Stefano Lazzer (Department of Medicine, University of Udine, Udine, Italy; Stefano.lazzer@uniud.it), Giovanelli Nicola (Department of Medicine, University of Udine, Udine, Italy; Nicola.giovanelli@uniud.it), Mirco Floreani (Department of Medicine, University of Udine, Udine, Italy; Mirco.floreani@uniud.it), Martina Arteni (Department of Medicine, University of Udine, Udine, Italy; Martina.arteni@uniud.it), Alberto Botter (Department of Medicine, University of Udine, Udine, Italy; Alberto.botter@uniud.it), and Desy Salvadego (Experimental Laboratory for Auxo-Endocrinological Research, Piancavallo-Verbania, Italy; salvadegodesy@gmail.com); University of Trieste: Gianni Biolo (Department of Medical, Surgical and Health Sciences, University of Trieste, Trieste, Italy; Gianni.biolo@units.it), Roberta Situlin (Department of Medical, Surgical and Health Sciences, University of Trieste, Trieste, Italy; roberta.situlin@units.it), Filippo Giorgio Di Girolamo (Department of Medical, Surgical and Health Sciences, University of Trieste, Trieste, Italy; Filippo.digirolamo@units.it), Mariella Sturma, Giuseppe Castiglia, Marcello Tence, Greta del Fabbro, Sara Mazzucco, and Paolo de Colle; Slovena—Science and Research Centre of Koper: Boštjan Šimunič (Science and Research Centre Koper, Institute for Kinesiology Research, Koper, Slovenia; Bostjan.Simunic@zrs-kp.si), Rado Pišot (Science and Research Centre Koper, Institute for Kinesiology Research, Koper, Slovenia; Rado.Pisot@zrs-kp.si), Uroš Marušič (Science and Research Centre Koper, Institute for Kinesiology Research, Koper, Slovenia; Uros.Marusic@zrs-kp.si), Matej Plevnik, Saša Pišot, Dorjana Zerbo, Nina Mohorko, and Petra Dolenc; General Hospital of Isola: Mladen Gasparini (Department of General Surgery, Izola General Hospital, Izola, Slovenia); National Institute for Public Health; Mojca Gabrijelčič Blenkuš (Healthy Lifestyle Promotion Department, National Institute for Public Health, Ljubljana, Slovenia).
Abbreviations
- AGEs
Advanced glycation end-products
- FFM
Fat-free mass
- FM
Fat mass
- hsCRP
High-sensitivity C-reactive protein
- RAGE
Receptor for AGEs
- VAI
Visceral adiposity index
Author contributions
DS, JMS and AP developed the hypothesis and designed the study; SA and JMS analyzed the data; DS, SA, JMS and AP wrote the first draft of the manuscript; SA, RS, FC prepared the Figures and Tables; RS, FC and GZ revised the manuscript; AP supervised the project. All authors read and approved the final version of the manuscript.
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement. The study was conducted in the framework of the PANGeA project, CB147—Physical Activity and Nutrition for Quality Ageing, supported by the Cross-border Cooperation Program Slovenia–Italy 2007–2013 and co-financed by European Regional Development Fund Grant 042-2/2009–18/052012.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending. Data are not publicly available, due to the PANGeA study consortium agreement, which regulates the intellectual property of the data.
Declarations
Conflict of interest
The Authors declare no conflict of interest.
Ethical approval
The National Ethical Committee of the Slovenian Ministry of Health approved this study on 17 April 2012 (IR-aging 1200) which was conducted in agreement with the ethical principles for medical research involving human subjects as required by the 2013 Review of the Helsinki Declaration of Helsinki.
Footnotes
Domenico Sergi and Sharon Angelini have contributed equally to the manuscript.
Contributor Information
Juana Maria Sanz, Email: juana.sanz@unife.it.
the PANGEA study group:
Edoardo Dalla Nora, Gloria Brombo, Eleonora Capati, Cecilia Soavi, Rosella Colonna, Elettra Mantovani, Mario Luca Morieri, Maria Agata Miselli, Alice Omenetto, Sefora Del Mastro, Gabriella Stifani, Daniela Francesconi, Stefano Lazzer, Giovanelli Nicola, Mirco Floreani, Martina Arteni, Alberto Botter, Desy Salvadego, Gianni Biolo, Roberta Situlin, Filippo Giorgio Di Girolamo, Mariella Sturma, Giuseppe Castiglia, Marcello Tence, Greta Del Fabbro, Sara Mazzucco, Paolo De Colle, Boštjan Šimunič, Rado Pišot, Uroš Marušič, Matej Plevnik, Saša Pišot, Dorjana Zerbo, Nina Mohorko, Petra Dolenc, and Mojca Gabrijelčič Blenkuš
References
- 1.GBDD Collaborators (2023) Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402:203–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein BJ (2002) Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol 90:3G-10G [DOI] [PubMed] [Google Scholar]
- 3.Sergi D, Naumovski N, Heilbronn LK, Abeywardena M, O’Callaghan N, Lionetti L et al (2019) Mitochondrial (dys)function and Insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Front Physiol 10:532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Fronzo RA (2010) Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H, Ballantyne CM (2020) Metabolic inflammation and insulin resistance in obesity. Circ Res 126:1549–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.How KW (2019) How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes 12:2221–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christ A, Lauterbach M, Latz E (2019) Western diet and the immune system: an inflammatory connection. Immunity 51:794–811 [DOI] [PubMed] [Google Scholar]
- 8.Spaggiari R, Angelini S, Di Vincenzo A, Scaglione G, Morrone S, Finello V et al (2024) Ceramides as emerging players in cardiovascular disease: focus on their pathogenetic effects and regulation by diet. Adv Nutr 15:100252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dicken SJ, Batterham RL (2024) Ultra-processed food and obesity: what is the evidence? Curr Nutr Rep 13:23–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Khandpur N, Desjardins C, Wang L, Monteiro CA, Rossato SL et al (2023) Ultra-processed food consumption and risk of type 2 diabetes: three large prospective U.S. cohort studies. Diabetes Care 46:1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu Y, Hu W, Huang J, Tan B, Ma F, Xing C et al (2024) Ultra-processed food consumption and risk of cardiovascular events: a systematic review and dose-response meta-analysis. EClinicalMedicine 69:102484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sergi D, Boulestin H, Campbell FM, Williams LM (2021) The role of dietary advanced glycation end products in metabolic dysfunction. Mol Nutr Food Res 65:e1900934 [DOI] [PubMed] [Google Scholar]
- 13.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R et al (2010) Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 110(911–16):e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang Z, Chen X, Li L, Li B, Yang Z (2020) The fate of dietary advanced glycation end products in the body: from oral intake to excretion. Crit Rev Food Sci Nutr 60:3475–3491 [DOI] [PubMed] [Google Scholar]
- 15.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S et al (1999) N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 274:31740–31749 [DOI] [PubMed] [Google Scholar]
- 16.Pinto-Junior DC, Silva KS, Michalani ML, Yonamine CY, Esteves JV, Fabre NT et al (2018) Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci Rep 8:8109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A (2014) Role of advanced glycation end products in cellular signaling. Redox Biol 2:411–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du H, Ma Y, Wang X, Zhang Y, Zhu L, Shi S et al (2023) Advanced glycation end products induce skeletal muscle atrophy and insulin resistance via activating ROS-mediated ER stress PERK/FOXO1 signaling. Am J Physiol Endocrinol Metab 324:E279–E287 [DOI] [PubMed] [Google Scholar]
- 19.Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H (2012) Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci U S A 109:15888–15893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Courten B, de Courten MP, Soldatos G, Dougherty SL, Straznicky N, Schlaich M et al (2016) Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: a double-blind, randomized, crossover trial. Am J Clin Nutr 103:1426–1433 [DOI] [PubMed] [Google Scholar]
- 21.Uribarri J, Cai W, Ramdas M, Goodman S, Pyzik R, Chen X et al (2011) Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care 34:1610–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linkens AMA, Eussen S, Houben A, Mari A, Dagnelie PC, Stehouwer CDA et al (2023) Habitual intake of advanced glycation endproducts is not associated with worse insulin sensitivity, worse beta cell function, or presence of prediabetes or type 2 diabetes: the Maastricht Study. Clin Nutr 42:1491–1500 [DOI] [PubMed] [Google Scholar]
- 23.Linkens AM, Houben AJ, Niessen PM, Wijckmans NE, de Goei EE, Van den Eynde MD et al (2022) A 4-week high-AGE diet does not impair glucose metabolism and vascular function in obese individuals. JCI Insight. 10.1172/jci.insight.156950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam JC, Tan KC, Lai AY, Lam DC, Ip MS (2012) Increased serum levels of advanced glycation end-products is associated with severity of sleep disordered breathing but not insulin sensitivity in non-diabetic men with obstructive sleep apnoea. Sleep Med 13:15–20 [DOI] [PubMed] [Google Scholar]
- 25.Mark AB, Poulsen MW, Andersen S, Andersen JM, Bak MJ, Ritz C et al (2014) Consumption of a diet low in advanced glycation end products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care 37:88–95 [DOI] [PubMed] [Google Scholar]
- 26.Du Z, Wu J, Feng Z, Ma X, Zhang T, Shu X et al (2022) RAGE displays sex-specific differences in obesity-induced adipose tissue insulin resistance. Biol Sex Differ 13:65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanz JM, Sergi D, Colombari S, Capatti E, Situlin R, Biolo G et al (2022) Dietary acid load but not Mediterranean diet adherence score is associated with metabolic and cardiovascular health state: a population observational study from Northern Italy. Front Nutr 9:828587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J et al (2004) Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc 104:1287–1291 [DOI] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- 30.Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F et al (2022) Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr 16:102581 [DOI] [PubMed] [Google Scholar]
- 31.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M et al (2010) Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 33:920–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahleova H, Znayenko-Miller T, Uribarri J, Holubkov R, Barnard ND (2023) Dietary advanced glycation products and their associations with insulin sensitivity and body weight: a 16-week randomized clinical trial. Obes Sci Pract 9:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro PVM, Tavares JF, Costa MAC, Mattar JB, Alfenas RCG (2019) Effect of reducing dietary advanced glycation end products on obesity-associated complications: a systematic review. Nutr Rev 77:725–734 [DOI] [PubMed] [Google Scholar]
- 34.Westphal SA (2008) Obesity, abdominal obesity, and insulin resistance. Clin Cornerstone 9:23–29 [DOI] [PubMed] [Google Scholar]
- 35.Kilhovd BK, Juutilainen A, Lehto S, Ronnemaa T, Torjesen PA, Birkeland KI et al (2005) High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: a population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol 25:815–820 [DOI] [PubMed] [Google Scholar]
- 36.Angoorani P, Ejtahed HS, Mirmiran P, Mirzaei S, Azizi F (2016) Dietary consumption of advanced glycation end products and risk of metabolic syndrome. Int J Food Sci Nutr 67:170–176 [DOI] [PubMed] [Google Scholar]
- 37.Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H (2005) Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci 1043:461–466 [DOI] [PubMed] [Google Scholar]
- 38.Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X et al (2009) Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab 94:4483–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H (2000) The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem 275:25781–25790 [DOI] [PubMed] [Google Scholar]
- 40.Pullerits R, d’Elia HF, Tarkowski A, Carlsten H (2009) The decrease of soluble RAGE levels in rheumatoid arthritis patients following hormone replacement therapy is associated with increased bone mineral density and diminished bone/cartilage turnover: a randomized controlled trial. Rheumatology (Oxford) 48:785–790 [DOI] [PubMed] [Google Scholar]
- 41.Tsoporis JN, Hatziagelaki E, Gupta S, Izhar S, Salpeas V, Tsiavou A et al (2020) Circulating ligands of the receptor for advanced glycation end products and the soluble form of the receptor modulate cardiovascular cell apoptosis in diabetes. Molecules 25:5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Portero-Otin M, de la Maza MP, Uribarri J (2023) Dietary advanced glycation end products: their role in the insulin resistance of aging. Cells 12:1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sergi D, Luscombe-Marsh N, Heilbronn LK, Birch-Machin M, Naumovski N, Lionetti L et al (2021) The inhibition of metabolic inflammation by EPA is associated with enhanced mitochondrial fusion and insulin signaling in human primary myotubes. J Nutr 151:810–819 [DOI] [PubMed] [Google Scholar]
- 44.Passaro A, Sanz JM, Naumovski N, Sergi D (2024) The complex interplay between oxinflammation, mitochondrial dysfunction and lipotoxicity: focus on their role in the pathogenesis of skeletal muscle insulin resistance and modulation by dietary fatty acids. Adv Redox Res 11:100100 [Google Scholar]
- 45.Liang F, Kume S, Koya D (2009) SIRT1 and insulin resistance. Nat Rev Endocrinol 5:367–373 [DOI] [PubMed] [Google Scholar]
- 46.Ongphiphadhanakul B, Chanprasertyothin S, Chailurkit L, Chansirikarn S, Puavilai G, Rajatanavin R (2004) Differential associations of residual estradiol levels with bone mineral density and serum lipids in postmenopausal women with osteoporosis. Maturitas 48:193–196 [DOI] [PubMed] [Google Scholar]
- 47.Lee EJ, Park JH (2013) Receptor for advanced glycation endproducts (RAGE), its ligands, and soluble RAGE: potential biomarkers for diagnosis and therapeutic targets for human renal diseases. Genomics Inform 11:224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu S, Li Y, Qian Z, Zhao T, Feng Z, Weng X et al (2023) Role of the inflammasome in insulin resistance and type 2 diabetes mellitus. Front Immunol 14:1052756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sergi D, Sanz JM, Lazzer S, Brombo G, Zuliani G, Biolo G et al (2023) Interleukin-18 is a potential biomarker linking dietary fatty acid quality and insulin resistance: results from a cross-sectional study in Northern Italy. Nutrients 15:1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thorand B, Kolb H, Baumert J, Koenig W, Chambless L, Meisinger C et al (2005) Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984–2002. Diabetes 54:2932–2938 [DOI] [PubMed] [Google Scholar]
- 51.Bruun JM, Stallknecht B, Helge JW, Richelsen B (2007) Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol 157:465–471 [DOI] [PubMed] [Google Scholar]
- 52.Kong X, Lu AL, Yao XM, Hua Q, Li XY, Qin L et al (2017) Activation of NLRP3 inflammasome by advanced glycation end products promotes pancreatic islet damage. Oxid Med Cell Longev 2017:9692546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Son S, Hwang I, Han SH, Shin JS, Shin OS, Yu JW (2017) Advanced glycation end products impair NLRP3 inflammasome-mediated innate immune responses in macrophages. J Biol Chem 292:20437–20448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baye E, Kiriakova V, Uribarri J, Moran LJ, de Courten B (2017) Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: meta-analysis of randomised controlled trials. Sci Rep 7:2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faist V, Erbersdobler HF (2001) Metabolic transit and in vivo effects of melanoidins and precursor compounds deriving from the Maillard reaction. Ann Nutr Metab 45:1–12 [DOI] [PubMed] [Google Scholar]
- 56.Tessier FJ, Niquet-Leridon C, Jacolot P, Jouquand C, Genin M, Schmidt AM et al (2016) Quantitative assessment of organ distribution of dietary protein-bound (13) C-labeled N(varepsilon) -carboxymethyllysine after a chronic oral exposure in mice. Mol Nutr Food Res 60:2446–2456 [DOI] [PubMed] [Google Scholar]
- 57.Naska A, Lagiou A, Lagiou P (2017) Dietary assessment methods in epidemiological research: current state of the art and future prospects. F1000Res 6:926 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending. Data are not publicly available, due to the PANGeA study consortium agreement, which regulates the intellectual property of the data.